Abstract
Background
Patients with heart failure (HF) are at high risk for adverse outcomes when they have COVID-19. Reports of COVID-19 vaccine-related cardiac complications may contribute to vaccine hesitancy in patients with HF.
Methods
To analyze the impact of COVID-19 vaccine status on clinical outcomes in patients with HF, we conducted a retrospective cohort study of the association of COVID-19 vaccination status with hospitalizations, intensive care unit admission and mortality after adjustment for covariates. Inverse probability treatment-weighted models were used to adjust for potential confounding.
Results
Of 7094 patients with HF, 645 (9.1%) were partially vaccinated, 2200 (31.0%) were fully vaccinated, 1053 were vaccine-boosted (14.8%), and 3196 remained unvaccinated (45.1%) by January 2022. The mean age was 73.3 ± 14.5 years, and 48% were female. Lower mortality rates were observed in patients who were vaccine-boosted, followed by those who were fully vaccinated; they experienced lower mortality rates (HR 0.33; CI 0.23, 0.48) and 0.36 (CI 0.30, 0.43), respectively, compared to unvaccinated individuals (P< 0.001) over the mean follow-up time of 276.5 ± 104.9 days, whereas no difference was observed between those who were unvaccinated or only partially vaccinated.
Conclusion
COVID-19 vaccination was associated with significant reduction in all-cause hospitalization rates and mortality rates, lending further evidence to support the importance of vaccination implementation in the high-risk population of patients living with HF.
Key Words: Heart failure, COVID-19, vaccination, mortality
The coronavirus disease 2019 (COVID-19) pandemic constitutes a major public health crisis, especially with the emergence and spread of new variants of the disease. Observational data highlight increased risk of severe complications, including death, of patients with heart failure (HF) who have been hospitalized due to COVID-19.1, 2, 3, 4 Vaccination against COVID-19 has been proven to be highly effective in preventing adverse outcomes in the general population, but concern about potential cardiovascular sequelae may contribute to vaccine hesitancy, particularly among patients with pre-existing cardiac disease.5, 6, 7, 8, 9 We sought to evaluate the impact of COVID-19 vaccination on patients with HF and on all-cause hospitalization and mortality rates across a large New York City health system.
Methods
All patients with diagnoses of HF (identified via electronic health record (EHR) phenotyping and followed in a large NYC health system) who made visits betweenJanuary 1, 2021, and January 24, 2022, were included. Using International Classification of Diseases (ICD-10) codes (hospitalization billing or encounter diagnosis of HF) for the preceding 2 years (January 2019--December 2020), patients with HF were identified by elevated natriuretic peptide levels (NT-proBNP ≥ 500 pg/mL or BNP ≥ 150 pg/mL).10 Patients with histories of mechanical circulatory support or orthotopic heart and other solid-organ transplant were excluded (Supplementary Material).
Demographics (age, sex, self-declared race/ethnicity, insurance status), comorbidities, as well as vaccination status (none, partially vaccinated, fully vaccinated, or vaccine-boosted), and clinical outcomes (hospitalizations, intensive care unit [ICU] admissions, and mortality) were abstracted from EHRs. Comorbidities at study entry were extracted using International Classification of Diseases (ICD-10) codes and included pulmonary disease (asthma, chronic obstructive pulmonary disease, obstructive sleep apnea), other cardiovascular diseases (coronary artery disease, history of myocardial infarction, atrial fibrillation, and peripheral vascular disease), autoinflammatory diseases (ulcerative colitis, Crohn disease, rheumatoid arthritis), history of liver disease, including chronic viral hepatitis or nonalcoholic steatohepatitis, history of cerebrovascular accident, obesity, hypertension, type II diabetes mellitus, and chronic kidney disease. Vaccination status was obtained via 2 workflows: automatic EHR entry upon vaccine order or administration within the MSHS (Mount Sinai Health System), or based on required clinician or staff documentation of COVID-vaccination status based on patients’ reports and demonstration of vaccine card.
To minimize potential systematic differences between patients who elected to or were recommended to receive vaccination and those who were not, inverse probability treatment-weighted models were used. Vaccination statuses of patients changed during the study period, so these inverse probabilities were used as weights in a Cox proportional hazards model with vaccination status as a time-dependent covariate to analyze the outcome of mortality while minimizing potential immortal time bias. Each patient's study time was divided into periods of unvaccinated time, partially vaccinated time (time period between dose 1 and dose 2 of mRNA-based vaccines or end of study period), fully vaccinated time (time period following the second mRNA vaccination until receiving a booster vaccine or end of study period, and vaccine-boosted (time period between receiving the third vaccine until end of the study). Patients who received a viral vector vaccine were considered fully vaccinated after the first dose, and vaccine-boosted if they subsequently received a second dose or an mRNA booster.
To analyze hospitalizations and ICU admissions, negative binomial regression models were constructed with counts of hospitalizations and ICU admissions as the dependent variable. To adjust for differential follow-up between patients who were ultimately vaccinated and patients who were not, an offset term was included in the regression model consisting of the log of the observation period for each patient. The observation period was constructed as the interval between the initiation of the study period and the last contact with the health system. Further details are provided in the Supplementary Material. The Mount Sinai Institutional Review Board approved this research.
Statistical analyses were performed using R version 4.1 (R Foundation, Salt Lake City, UT). Negative binomial models were fit using the glm.nb function from the MASS R package, and Cox proportional hazards models were fit using the Survival R Package.11, 12 Regression coefficients were exponentiated to obtain incidence rate ratios (IRRs, negative binomial models) and hazard ratios (HRs, Cox models). Robust standard errors modeling for clustering by patient were used in all regression models. To ascertain whether the events of hospitalization, ICU admission or mortality were COVID-19-related, we examined SARS-CoV-2 positivity at time of each adverse event encounter as an interaction term in regression models.
Results
Of 7094 patients with histories of HF within the health system, 645 patients (9.1%) were partially vaccinated, 2200 (31.0%) were fully vaccinated, and 1053 (14.8%) were vaccine-boosted (Table 1 ), leaving 3196 patients (45.1%) who were unvaccinated. Patients who received their first vaccination before the beginning of the study period on January 1, 2021 (n = 78) were excluded from analysis.
Table 1.
Baseline Characteristics, Overall and Stratified by Vaccination Period
| Overall | Unvaccinated Period | Partially Vaccinated Period | Vaccinated Period | Vaccine-Boosted Period | P Value* | |
|---|---|---|---|---|---|---|
| Patients in vaccine period, n | 7094 | 7094 | 3898 | 3253 | 1053 | |
| Total patient-days in vaccine period | 19,77,412 | 10,62,752 | 1,85,506 | 6,51,706 | 77,448 | |
| Days in vaccination period: mean (SD) | 276.46 (104.89) | 149.81 (115.44) | 47.59 (62.61) | 200.34 (77.01) | 73.55 (69.64) | |
| Mortality: n (%) | 904 (12.7) | 598 (8.4) | 75 (1.9) | 197 (6.1) | 36 (3.4) | <0.001 |
| Age: mean (SD) | 73.27 (14.48) | 73.27 (14.48) | 73.90 (13.84) | 74.19 (13.58) | 75.91 (12.23) | <0.001 |
| Sex: male (%) | 3719 (52.4) | 3719 (52.4) | 2021 (51.8) | 1671 (51.4) | 559 (53.1) | 0.68 |
| Race/ethnicity: n (%) | <0.001 | |||||
| White | 2360 (33.3) | 2360 (33.3) | 1308 (33.6) | 1106 (34.0) | 432 (41.0) | |
| Asian | 353 (5.0) | 353 (5.0) | 208 (5.3) | 179 (5.5) | 53 (5.0) | |
| Black/African American | 1826 (25.7) | 1826 (25.7) | 998 (25.6) | 821 (25.2) | 237 (22.5) | |
| Hispanic | 1571 (22.1) | 1571 (22.1) | 962 (24.7) | 816 (25.1) | 237 (22.5) | |
| Other/Multiracial | 741 (10.4) | 741 (10.4) | 345 (8.9) | 272 (8.4) | 79 (7.5) | |
| Unknown | 243 (3.4) | 243 (3.4) | 77 (2.0) | 59 (1.8) | 15 (1.4) | |
| Insurance: n (%) | <0.001 | |||||
| Private | 994 (14.0) | 994 (14.0) | 584 (15.0) | 512 (15.7) | 159 (15.1) | |
| Medicaid | 704 (9.9) | 704 (9.9) | 328 (8.4) | 241 (7.4) | 49 (4.7) | |
| Medicare | 4493 (63.3) | 4493 (63.3) | 2568 (65.9) | 2165 (66.6) | 758 (72.0) | |
| Other | 903 (12.7) | 903 (12.7) | 418 (10.7) | 335 (10.3) | 87 (8.3) | |
| Smoking status (n, (%)) | <0.001 | |||||
| Nonsmoker | 4198 (59.2) | 4198 (59.2) | 2145 (55.0) | 1791 (55.1) | 557 (52.9) | |
| Former smoker | 2380 (33.5) | 2380 (33.5) | 1513 (38.8) | 1272 (39.1) | 447 (42.5) | |
| Current smoker | 516 (7.3) | 516 (7.3) | 240 (6.2) | 190 (5.8) | 49 (4.7) | |
| Medical history: n (%) | ||||||
| Pulmonary disease: n (%) | 1255 (17.7) | 1255 (17.7) | 787 (20.2) | 657 (20.2) | 244 (23.2) | <0.001 |
| Immunological disease: n (%) | 169 (2.4) | 169 (2.4) | 123 (3.2) | 102 (3.1) | 38 (3.6) | 0.018 |
| Cerebrovascular disease: n (%) | 958 (13.5) | 958 (13.5) | 568 (14.6) | 476 (14.6) | 165 (15.7) | 0.132 |
| Cardiovascular disease: n (%) | 5529 (77.9) | 5529 (77.9) | 3227 (82.8) | 2707 (83.2) | 923 (87.7) | <0.001 |
| Liver disease: n (%) | 206 (2.9) | 206 (2.9) | 125 (3.2) | 103 (3.2) | 47 (4.5) | 0.06 |
| Obesity: n (%) | 738 (10.4) | 738 (10.4) | 477 (12.2) | 387 (11.9) | 132 (12.5) | 0.008 |
| Hypertension: n (%) | 4139 (58.3) | 4139 (58.3) | 2529 (64.9) | 2122 (65.2) | 739 (70.2) | <0.001 |
| Chronic kidney disease: n (%) | 1789 (25.2) | 1789 (25.2) | 1108 (28.4) | 947 (29.1) | 344 (32.7) | <0.001 |
| Diabetes n (%) | 2272 (32.0) | 2272 (32.0) | 1376 (35.3) | 1149 (35.3) | 399 (37.9) | <0.001 |
P values from comparisons between patients between time periods, not including overall column.
Overall, the mean age was 73.3 ± 14.5 years, 48% were women, and 63.3% of patients had Medicare insurance. Other cardiovascular diseases (as previously defined) (77.9%), hypertension (58.3%), and type II diabetes (32.0%) were the most common comorbidities.
Compared to the unvaccinated and partially vaccinated cohorts, the vaccine-boosted and fully vaccinated cohorts were slightly older, more likely to be white, had Medicare insurance, had higher rates of other cardiovascular disease (P< 0.001), obesity (P= 0.008), hypertension, pulmonary disease, and diabetes, and included more former smokers (P< 0.001).
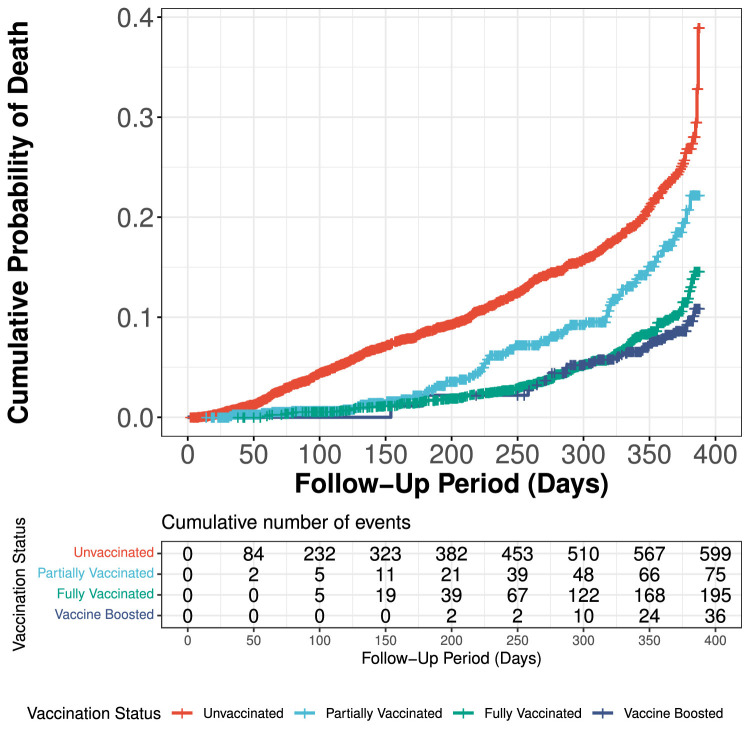
Overall, 904 of 7094 total patients died over the mean follow-up time of 276.5 ± 104.9 days (12.7%). Of these, 73.4% were unvaccinated or only partially vaccinated. Patients in the vaccine-boosted period were observed to experience the lowest mortality rates, followed by patients in the fully vaccinated period (HR 0.36 [0.30, 0.43] and 0.33 [0.23, 0.48] respectively) compared to unvaccinated individuals, all P< 0.001) (Table 2 ). There was no significant associated difference in mortality among patients who were partially vaccinated vs. unvaccinated (HR = 0.87 (0.68, 1.12), P= 0.28) (Fig. 1 ). Fully vaccinated or boosted patients were also significantly less likely to be admitted to the hospital (IRR 0.68 (0.65,0.71); P< 0.001) or admitted to the ICU (IRR 0.63 (0.58, 0.68); P< 0.001) than unvaccinated or partially vaccinated individuals (Table 2).
Table 2.
Summary of Regression Models for Hospitalization, ICU Admission and Mortality
| Statistic | Reference Level | Coefficient | Estimate (95% CI) | P Value | |
|---|---|---|---|---|---|
| Model 1: Mortality* | Hazard ratio (HR) | Unvaccinated | Partially vaccinated | 0.87 (0.68, 1.12) | 0.28 |
| Fully vaccinated | 0.36 (0.30, 0.43) | <0.001 | |||
| Boosted | 0.33 (0.23, 0.48) | <0.001 | |||
| Model 2: Hospitalization Count† | Incidence rate ratio (IRR) | Unvaccinated or partially vaccinated | Fully vaccinated or vaccine-boosted | 0.68 (0.65, 0.71) | <0.001 |
| Model 3: ICU Admission Count‡ | Incidence rate ratio (IRR) | Unvaccinated or partially vaccinated | Fully vaccinated or vaccine-boosted | 0.63 (0.58, 0.68) | <0.001 |
Inverse probability treatment-weighted model with time-dependent covariate for vaccination.
Negative binomial regression model for count of hospitalizations.
Negative binomial regression model for count of ICU admissions.
Adjustment covariates: age, sex, race/ethnicity, insurance, smoking, pulmonary disease, immunological disease, liver disease, obesity, hypertension, chronic kidney disease, diabetes.
Fig. 1.
Cumulative incidence curve for mortality, stratified by vaccination status.
In secondary analyses examining whether adverse events were COVID-19-related, SARS-CoV-2 test positivity was, indeed, associated with higher rates of hospitalization (IRR 1.67 [1.47, 1.89]; P< 0.001), ICU admission (IRR 2.01 [1.65, 2.44]; P< 0.001), and mortality (HR 3.39 [2.54, 4.50]; P< 0.001). Among patients with SARS-CoV-2 positive tests, vaccination status was associated with lower rates of adverse events compared to those with an unvaccinated status: hospitalization (HR 0.83 [0.78, 0.89]; P= 0.02); ICU admission (IRR 0.57 [0.37, 0.86]; P= 0.009), and mortality (HR 0.35 [0.12, 0.97]; P = 0.045.
Discussion
Patients with HF constitute a high-risk population for adverse outcomes with COVID-19. To our knowledge, this is 1 of the largest studies to examine the association between COVID-19 vaccination status and clinical outcomes, specifically among patients with HF, in whom vaccine hesitancy attributed to perceived cardiac side effects may be encountered. Full vaccination and vaccine-boosted status was associated with a significantly lower incidence of hospitalizations, ICU admissions and mortality than partially vaccinated or unvaccinated status. These adverse events were at least in part COVID-19-related (as SARS-CoV-2 test positivity was associated with higher risk) and among patients who tested positive for SARS-CoV-2, fully vaccinated and boosted vaccine status was associated with fewer hospitalizations and lower mortality rates.
There are several limitations to this study related to the rapid, ongoing spread of COVID-19 and its different variants during the assessed study period. Data was restricted to 1, albeit large, hospital system, and, thus, seropositivity for SARS-CoV-2, hospitalization or death in other hospital systems were not captured. Individuals were not distinguished based on type or stage of HF (HF with preserved ejection fraction vs HF with reduced ejection fraction) or cause of hospitalization. Although analyses were adjusted for relevant confounding factors, unmeasured variables, such as social determinants of health, health literacy and access to care may have contributed to between-group differences. Data concerning specific treatment for HF or COVID-19 were not available.
Conclusion
In a large New York City cohort of patients with HF, vaccination against COVID-19 was associated with a lower likelihood of all-cause hospitalizations and mortality. Benefit of vaccination was observed in a graded fashion because vaccine-boosted status was associated with the lowest rates of hospitalization and mortality, followed by fully and partially vaccinated status, with the worst outcome association being observed in unvaccinated individuals. These findings underscore the profound protective effect of vaccination against COVID-19 in patients with HF.
Declaration of Competing Interest
Dr. Lala has received personal fees from Zoll, outside the submitted work. All other authors report no relationships relevant to the contents of this article.
Acknowledgments
Funding
This study was internally funded.
Acknowledgments
We thank the Mount Sinai Data Warehouse for data query, all of the nurses, physicians and providers who contributed to the care of these patients and to the patients and their family members who were affected by this pandemic.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cardfail.2022.05.008.
Appendix. Supplementary materials
References
- 1.Haas EJ, Angulo FJ, Anis E, Singer S, Khan F, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt AS, Jering KS, Vaduganathan M, Claggett B, Cunningham J, Rosenthal N, et al. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Heart Fail. 2021;9:65–73. doi: 10.1016/j.jchf.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roncalli J, Roubille F, Lamblin N, Girerd N, Mouquet F, Chapet N, et al. Coronavirus disease vaccination in heart failure: no time to waste. Arch Cardiovasc Dis. 2021 doi: 10.1016/j.acvd.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu Mouch S, Roguin A, Hellou E, Ishai A, Shoshan U, Mahamid L, et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790–3793. doi: 10.1016/j.vaccine.2021.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosano G, Jankowska EA, Ray R, Metra M, Abdelhamid M, Adamopoulos S, et al. COVID-19 vaccination in patients with heart failure: a position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2021;23:1806–1818. doi: 10.1002/ejhf.2356. PMID: 34612556PMCID: PMC8652673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fazlollahi A, Zahmatyar M, Noori M, Nejadghaderi SA, Sullman MJM, Shekarriz-Foumani R, et al. Cardiac complications following mRNA COVID-19 vaccines: a systematic review of case reports and case series. Rev Med Virol. 2021:e2318. doi: 10.1002/rmv.2318. Epub ahead of print. PMID: 34921468. [DOI] [PubMed] [Google Scholar]
- 8.Bengel CP, Kacapor R. A report of two cases of myocarditis following mRNA coronavirus disease 2019 vaccination. Eur Heart J Case Rep. 2022;6:ytac004. doi: 10.1093/ehjcr/ytac004. PMID: 35169677PMCID: PMC8755378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Vaccine hesitancy for COVID19. April 14, 2021.
- 10.National Library of Medicine (U.S.). Changes in NT-proBNP and outcomes, safety, and tolerability in HFpEF patients with acute decompensated heart failure (ADHF) who have been stabilized during hospitalization and initiated in-hospital or within 30 days post-discharge (PARAGLIDE-HF). 2019. Identifier: NCT03988634. https://clinicaltrials.gov/ct2/show/NCT03988634
- 11.Venables WN, Ripley BD.Modern applied statistics with S-plus. New York, NY: Springer, 2002.
- 12.Therneau TM. The Comprehensive R Archive Network; 2022. Survival analysis [R package survival version 3.3-1]https://CRAN.R-project.org/package=survival Accessed March 7from. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.