Abstract
Tumor-infiltrating immune cells play an essential role in cancer progression and may help supplement the Tumor, Node, Metastasis (TNM) classification for cancer prognosis. Currently, there are numerous conflicting reports discussing the significance of tumor-associated neutrophils (TANs) in colorectal cancer (CRC). In particular, the role of TANs in the invasive margin is unclear. The present study investigated the prognostic significance of CD66+ TANs and CD8+ tumor-infiltrating lymphocytes (TILs) in the invasive margin of 103 patients with CRC. By using immunohistochemistry, survival analysis was performed on CD8+ TILs and CD66+ TANs individually, as well as models including TILs and TANs simultaneously. The findings indicated that the densities of CD8+ TILs and CD66b+ TANs in the invasive margin may provide significant prognostic value for predicting survival. Moreover, the combined evaluation of CD8+ TILs and CD66b+ TANs in the invasive margin could further improve the validity for the prediction of oncological outcomes. In addition, multivariate analysis revealed that simultaneous low tumor infiltration by CD8+ TILs and CD66b+ was an independent predictive factor for overall survival (HR=4.17, 95% CI, 1.55-12.5; P=0.004) and disease-free survival (HR=2.75, 95% CI, 1.27-6.12; P=0.01). Given the importance of CD8+ TILs and CD66b+ TANs in the tumor microenvironment, the assessment of their densities in the invasive margin may serve as a valuable prognostic marker for CRC.
Keywords: colorectal neoplasms, lymphocytes, neutrophils, prognosis, tumor-infiltrating
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer-related mortality worldwide (1). The prediction of prognosis for cancer depends on the Tumor, Node, Metastasis (TNM) classification system and the features of tumor cell differentiation. This approach serves as a useful model for selecting postoperative treatment options. However, this classification does not provide sufficient information to predict prognosis. Therefore, a better classification system is required to achieve this purpose.
CRC is characterized by the infiltration of immune cells comprising subpopulations of granulocytes, lymphocytes, and macrophages. Accumulating evidence indicates the significant potential of analyzing the presence of tumor-infiltrating immune cells in the tumor microenvironment (TME) as a means for predicting the prognosis of cancer (2–4). In particular, tumor-infiltrating lymphocytes (TILs) and tumor-associated neutrophils (TANs) are essential for the progression of CRC (5–8). Moreover, CD8 is typically used as a marker of cytotoxic T cells that exert antitumor effects on the TME and our previous study indicates that CD8+TILs are useful biomarkers for predicting early relapse of CRC further revealing its potential in this field (9).
Human neutrophils with surface expression of markers such as CD11b, CD16, and CD66b make the identification of mature neutrophils possible (10). Furthermore, CD66b, which is also known as carcinoembryonic antigen-related cell adhesion molecule 8, NCA-95, and CD67, is also a reliable surface marker to identify neutrophils in cancer tissues (11). While, CD66b+ TANs are generally associated with worse prognosis for diverse tumors (12,13). The significance of CD66b+ TANs in CRC is rather controversial (14–16).
Moreover, the type of tumor-infiltrating immune cells, their densities as well as their spatial distributions in the TME, are important for clinical means, and their locations in the different compartments of a tumor are associated with different clinical outcomes. For example, TILs play antitumor roles in the tumor nest and the invasive margin (5), however, the clinical significance of TANs in the invasive margin and the interaction between TILs and TANs in CRC largely remains unclear.
In this study, we hypothesized that TILs and TANs in the invasive margin could provide independent prognostic information for curatively resected patients with stages I–III CRC. We based our study off the definition of the invasive margin which was based on the recommendation by the International Immuno-Oncology Biomarker Working Group (2). We evaluated the densities of TILs and TANs in this area. Furthermore, we determined the clinical importance of CD8+ TILs and CD66b+ TANs in CRC and focused on the combined prognostic significance of these immune-cell subsets in the invasive margin.
Materials and methods
Patients and sample collection
We retrospectively enrolled 131 consecutive patients with stages I–III CRC who underwent curative resection at the Mie University Hospital from 2013 to 2015. 28 patients were excluded, of which, 15 patients received preoperative neoadjuvant therapy, and 13 patients could not be evaluated for immunohistochemical analysis. The patients' median age was 71 years and ranged from 38 to 94 years, and 38.8% of patients were women while 62.2% were male. The clinicopathological characteristics of patients are presented in Table I. The protocol for this research project was approved by the institutional review board of Mie University Hospital (approval no. H2019-187). Informed consent was obtained in the form of opt-out on the web-site. Those who rejected were excluded. The study was conducted in accordance with the Declaration of Helsinki. All patients were classified according to the International Union against Cancer TNM Classification (7th Edition) and underwent resection of the primary tumor.
Table I.
Clinicopathological characteristics of patients with CRC (n=103).
| Variables | N (%) |
|---|---|
| Median age (range), years | 71 (38–94) |
| Gender | |
| Male | 63 (61.2) |
| Female | 40 (38.8) |
| Location | |
| Colon | 59 (57.3) |
| Rectum | 44 (42.7) |
| Differentiation | |
| Differentiated | 95 (92.2) |
| Undifferentiated | 8 (7.8) |
| Pathological T category | |
| T1 | 19 (18.5) |
| T2 | 25 (24.3) |
| T3 | 47 (45.6) |
| T4 | 12 (11.7) |
| Lymph node metastasis | |
| N0 | 70 (67.9) |
| N1 | 33 (32.1) |
| UICC stage classification | |
| Stage I | 38 (36.9) |
| Stage II | 32 (31.1) |
| Stage III | 33 (32.0) |
| MSI | |
| MSI-H | 8 (7.8) |
| MSI-L/MSS | 95 (92.2) |
| KRAS | |
| Wild | 54 (52.4) |
| Mutation | 49 (47.6) |
| BRAF | |
| Wild | 97 (94.2) |
| Mutation | 6 (5.8) |
CRC, colorectal cancer; MSI, microsatellite instability; MSS, microsatellite stability; UICC, Union for International Cancer Control.
After surgery, all patients with stage III CRC received 5-fluorouracil-based chemotherapy, whereas patients with stage I or II CRC were not administered adjuvant chemotherapy. Patients were observed in 3-month intervals for 24 months after the completion of surgery, then every 6 months for the next 3 years, and lastly yearly thereafter. During each annual hospital visit, all patients underwent a chest X-ray, colonoscopy, and abdominal computed tomography. Moreover, at each visit there was a physical examination performed to further document their patient histories. Retrospective clinical data were obtained from medical records and pathological reports, including sex, age, anatomical site, tumor differentiation, depth of invasion, vessel invasion, lymph node metastasis, UICC TNM classification, KRAS status, BRAF status, microsatellite instability, and survival data [disease-free survival (DFS) and overall survival (OS)]. After treatment, formalin-fixed and paraffin-embedded tissue sections were used for further immunohistochemical analysis.
Immunohistochemical analysis
Formalin-fixed and paraffin-embedded specimens were sliced into 5-µm sections and subjected to immunohistochemical analysis to detect the expression of CD8+ TILs and CD66b+ TANs in the invasive tumor margin. The primary antibodies used were a monoclonal rabbit anti-human CD8 (clone: EP1150, dilution 1:1,000; GeneTex, San Antonio, TX, USA) and a monoclonal mouse anti-human CD66b (clone: G10F5, dilution 1:200; Biolegend, San Diego, CA, USA).
Immunohistochemical evaluation of CD8+ TILs and CD66b+ TANs
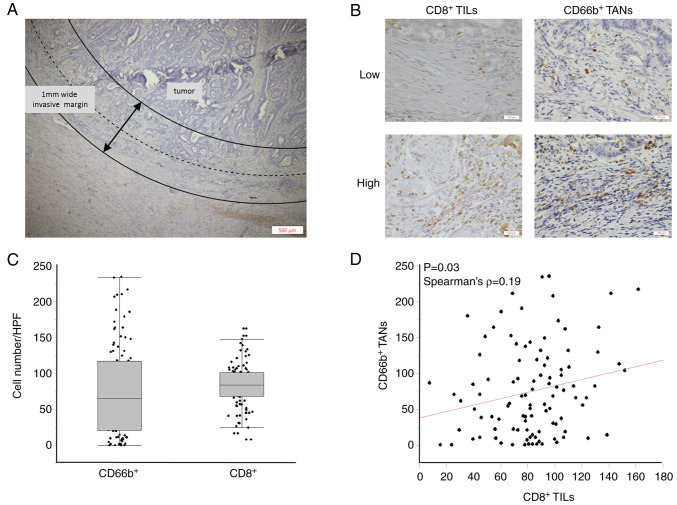
Two independent observers who were uninformed of clinical outcomes evaluated the sections using an inverted research microscope (BX-50, Olympus, Japan). With a magnification of 400×, CD8+ TILs and CD66b+ TANs were photographed in three representative high-power fields (HPFs) at the invasive tumor margin. According to the recommendation by the International Immuno-Oncology Biomarker Working Group, the ‘invasive margin’ was defined as the region centered on the border separating the host tissue from the malignant nets, with an extent of 1 mm (2) (Fig. 1A). Representative images are presented in Fig. 1B.
Figure 1.
Analysis of CD8+ TILs and CD66b+ TANs in the invasive margin of CRC. (A) The ‘invasive margin’ is defined as the region centered on the border separating the host tissue from the malignant nets, with an extent of 1 mm (40×). (B) Representative images of high-density and low-density CD8+ TILs and CD66b+ TANs in the invasive margin of CRC (200×). (C) CD8+ TILs and CD66b+ TANs counts in the invasive margins of all patients (n=103). (D) Correlation between CD8+ TILs and CD66b+ TANs in CRC. Correlation analysis was performed with Spearman's rank correlation coefficient. TILs, tumor-infiltrating lymphocytes; TANs, tumor-associated neutrophils; CRC, colorectal cancer.
Statistical analysis
Statistical analysis was performed using JMP software version 10 (SAS Institute, Cary, NC) and MedCalc Statistical Software version 19.1.2 (MedCalc Software bv, Ostend, Belgium). Spearman's rank correlation analysis was used to determine the relation between non-normally distributed continuous variables. Differences between groups were estimated using the Mann Whitney U, Kruskal-Wallis followed by Steel-Dwass test or one-way ANOVA followed by Tukey's Kramer when appropriate. Shapiro-Wilk tests were performed to evaluate the normality of the data distribution, and Levene's tests were conducted to assess the equality of variance for comparable groups. For time-to-event analyses, survival estimates were calculated using the Kaplan-Meier method, and groups were compared using the log-rank test. Receiver operating characteristic curves with Youden's index was generated to determine the cut-off values for analyzing prognosis. Univariate and multivariate analysis was performed using Cox proportional hazards regression. For multivariate analysis, variables with P-values <0.20 in univariate analysis were included in the multivariate regression model. All P-values were 2-sided, and P<0.05 was considered significant.
Results
Association between clinical characteristics and tumor-infiltrating immune cells in CRC
We first performed histopathological analysis of tissue sections to evaluate the densities of CD8+ TILs and CD66+ TANs in tumor margins as well as evaluated the associations between different clinicopathological factors and the degree of TILs. The median densities of CD8+ TILs and CD66b+ TANS in the tumor margin were 84/HPF (8-162/HPF) and 65/HPF (0-234/HPF), respectively (Fig. 1C). There was a weak positive correlation between the densities of CD8+ TILs and CD66b+ TANs in the tumor margin (Spearman's correlation coefficient: 0.19, P=0.03) (Fig. 1D). Interestingly, the decreased expression of CD8+ TILs in tumor margin was significantly associated with mutated KRAS status (P=0.03), and the decreased expression of CD66+ TANs in the tumor margin was significantly associated with lymph node metastasis (P=0.01) (Table II).
Table II.
Association between the infiltration of immune cells and clinicopathological characteristics of patients with CRC.
| Variables | CD8+ number/HPF mean ± SD | P-value | CD66b+ number/HPF [median (IQR)] | P-value |
|---|---|---|---|---|
| Agea | 0.10 | 0.85 | ||
| Low (<71 years) | 85.9±31.6 | 68.7 (17.9-115.2) | ||
| High (≥71 years) | 81.1±27.9 | 60.8 (21.0-117.5) | ||
| Gender | 0.98 | 0.96 | ||
| Male | 83.5±31.6 | 60.8 (20.7-117.0) | ||
| Female | 83.4±26.9 | 70.0 (19.8-116.5) | ||
| Location | 0.54 | 0.59 | ||
| Colon | 81.9±29.6 | 65.0 (20.7-108.3) | ||
| Rectum | 85.6±30.1 | 67.5 (23.4-127.8) | ||
| Differentiation | 0.15 | 0.22 | ||
| Differentiated | 82.3±29.9 | 67.7 (20.7-121.0) | ||
| Undifferentiated | 97.3±25.0 | 45.8 (15.8-74.1) | ||
| Pathological T category | 0.78 | 0.52 | ||
| pT1/2 | 84.4±31.3 | 67.7 (37.5-106.9) | ||
| pT3/4 | 82.8±28.7 | 60.8 (11.3-129.2) | ||
| Vessel invasion | 0.32 | 0.70 | ||
| Absent | 87.0±34.2 | 65.0 (12.5-115.0) | ||
| Present | 81.1±26.2 | 62.7 (27.5-118.0) | ||
| Lymph node metastasis | 0.73 | 0.01b | ||
| Absent | 82.9±29.6 | 34.0 (9.0-82.7) | ||
| Present | 84.8±30.1 | 79.5 (36.9-126.2) | ||
| UICC stage classification | 0.96 | 0.05b,c | ||
| Stage I | 82.7±31.6 | 73.5 (39.0-109.3) | ||
| Stage II | 83.1±27.4 | 84.5 (15.3-146.3) | ||
| Stage III | 84.8±30.4 | 34.0 (9.0-82.7) | ||
| MSI | 0.40 | 0.75 | ||
| MSI | 92.1±31.4 | 72.7 (55.8-101.7) | ||
| MSS | 82.8±29.6 | 60.8 (19.6-118) | ||
| KRAS | 0.03b | 0.68 | ||
| Mutation | 76.8±31.6 | 69.8 (22.0-110.2) | ||
| Wild | 89.6±26.7 | 55.1 (13.3-120.8) | ||
| BRAF | 0.37 | 0.16 | ||
| Mutation | 94.0±14.0 | 40.3 (7.9-79.1) | ||
| Wild | 82.8±30.3 | 67.7 (20.8-119.5) |
Median age at surgery was 71 years in this cohort.
P<0.05.
Stage I vs. III, P=0.04. CRC, colorectal cancer; MSI, microsatellite instability; MSS, microsatellite stability; UICC, Union for International Cancer Control; SD, standard deviation.
Low densities of CD8+ TILs and CD66+ TANs in the tumor margin correlates with poor prognosis and disease recurrence of patients with stages I–III CRC
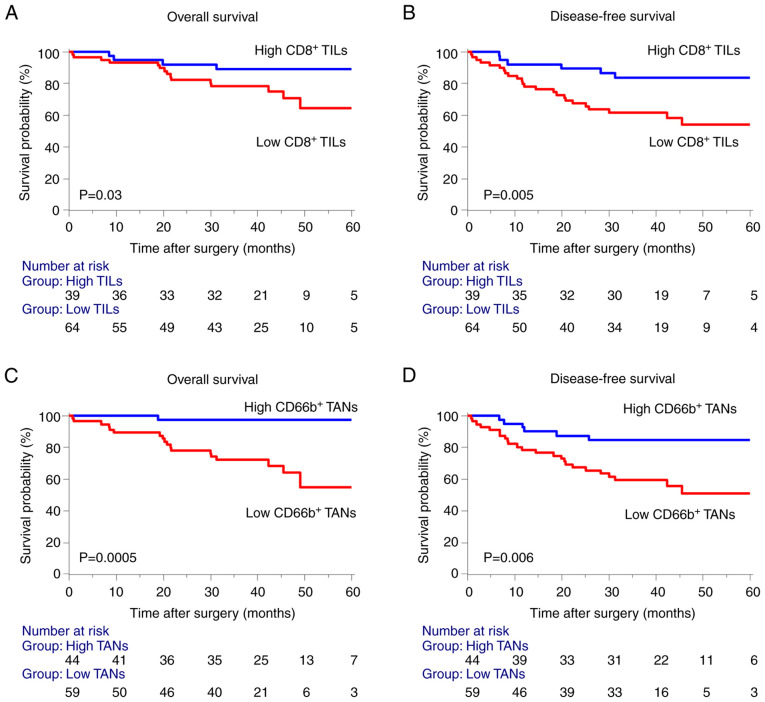
Next, to evaluate the significance of the association between tumor-infiltrating immune cells and survival, we defined the cutoff values of CD8+ TILs and CD66+ TANs, to be 91 and 77 cells per HPF, respectively, according to the receiver operating characteristic analysis and Youden's index. We then conducted Kaplan-Meier survival analysis according to the densities of CD8+ TILs and CD66+ TANs. Consistent with our previous research on CD8+ TILs (9), we found that a low density of CD8+ TILs in the invasive margin significantly correlated with poor prognosis for OS and DFS of patients with stages I–III CRC (Fig. 2A and B). Furthermore, low density of CD66+ TANs in the invasive margin was unexpectedly associated with a poor prognosis in OS and DFS (Fig. 2C and D).
Figure 2.
Prognostic significance of CD8+ TILs and CD66b+ TANs in the invasive margin of stages I–III CRC. (A and B) Kaplan-Meier analysis of OS and DFS designed according to tumor infiltration of CD8+ TILs high/low infiltration. (C and D) Kaplan-Meier analysis of OS and DFS designed according to tumor infiltration of CD66b+ TANs high/low infiltration. Statistical analysis of the survival was performed using the log-rank test. TILs, tumor-infiltrating lymphocytes; TANs, tumor-associated neutrophils; CRC, colorectal cancer; OS, overall survival; DFS, disease-free survival.
Combined assessment of CD8+ TILs and CD66b+ TANs densities (Model 1) in the invasive margin
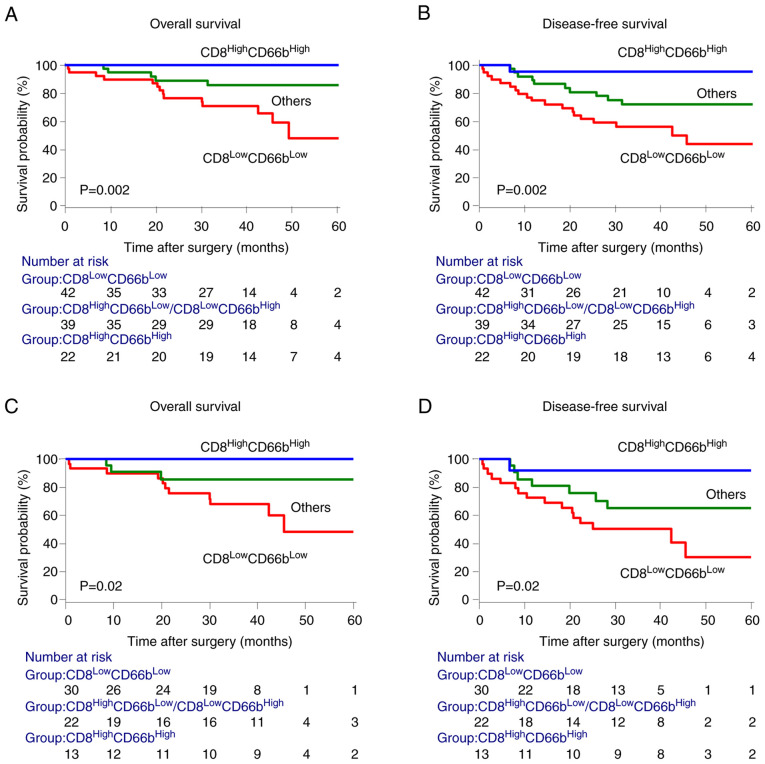
We hypothesized that the immune signature of the invasive margin improves the prognostic impact of established clinicopathological parameters and assumed that the different densities of CD8+ TILs or CD66b+ TANs in the invasive margin could represent different levels of antitumor immunity. We therefore investigated whether the favorable signatures, identified in the patient populations with CRC stages I to III, have prognostic value for the clinical outcomes of subgroups of patients. In the end, the data revealed that cancers of patients infiltrated by CD8+ TILs and CD66b+ TANs were characterized by a favorable prognosis, whereas patients with low densities of CD8+ TILs and CD66b+ TANs had the poorest prognosis (Fig. 3A and B). Similar results were obtained for patients with stages II and III CRC, who may require further post-surgical treatment (Fig. 3C and D).
Figure 3.
Combined assessment of CD8+ TILs and CD66b+ TANs densities (Model 1) in the invasive margin. (A and B) Kaplan-Meier analysis of OS and DFS among subgroups identified by the combination of CD8+ TILs and CD66b+ TANs in patients with stage I–III CRC. (C and D) Kaplan-Meier analysis of OS and DFS among subgroups identified by the combination of CD8+ TILs and CD66b+ TANs in the invasive margin of stages II–III CRC. Statistical analysis of the survival was performed using the log-rank test. TILs, tumor-infiltrating lymphocytes; TANs, tumor-associated neutrophils; CRC, colorectal cancer; OS, overall survival; DFS, disease-free survival.
To further determine whether the potential of tumor-infiltrating immune cells could function as predictive biomarkers for cancer recurrence and prognosis was influenced by other variables, we incorporated an immune feature comprising CD8+ TILs and CD66b+ TANs (CD8LowCD66bLow) into a Cox regression proportional hazard model. Univariate analysis revealed that the pathological T category, lymph node metastasis, and the immune signature were significantly associated with DFS, further revealing that low densities of CD8+ TILs and CD66b+ TANs correlated with poor prognosis. Also, multivariate analysis revealed that the signature of the invasive margin served as an independent prognostic factor for OS (HR=3.80, 95% CI, 1.48-11.1, P=0.005) of patients with CRC (Table III). Furthermore, this signature served as an independent prognostic factor for DFS (HR=2.52, 95% CI, 1.18-5.58, P=0.02) (Table IV).
Table III.
Multivariate analysis for OS of patients with stages I–III CRC.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Gender (Male) | 1.39 | 0.55-3.95 | 0.49 | |||
| Age (≥71 years)a | 1.52 | 0.63-3.91 | 0.36 | |||
| Location (Rectum) | 1.09 | 0.44-2.65 | 0.84 | |||
| Differentiation (Undifferentiated) | 4.22 | 1.20-11.7 | 0.03c | 2.90 | 0.82-8.16 | 0.09 |
| Pathological T category (T3/T4) | 1.84 | 0.74-5.20 | 0.19 | 1.56 | 0.61-4.49 | 0.36 |
| Vessel invasion (Present) | 1.21 | 0.49-3.22 | 0.69 | |||
| Lymph node metastasis (Present) | 1.58 | 0.62-3.85 | 0.32 | |||
| MSI (MSI-L/MSS) | 1.49 | 0.31-26.8 | 0.68 | |||
| KRAS (Wild) | 1.73 | 0.71-4.63 | 0.23 | |||
| BRAF (Wild) | 1.11 | 0.23-19.9 | 0.92 | |||
| CD66b+ (Low) | 8.82 | 2.51-55.9 | 0.0002c | |||
| CD8+ (Low) | 3.15 | 1.14-11.1 | 0.04c | |||
| CD8+CD66b+ (Low-Low)b | 4.41 | 1.75-12.6 | 0.002c | 3.80 | 1.48-11.0 | 0.005c |
The median age at surgery was 71 years in this cohort;
The cutoff value determined by ROC curve analysis for survival;
P<0.05. CRC, colorectal cancer; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Table IV.
Multivariate analysis of DFS of patients with stage I–III CRC.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variables | HR | 95% CI | P-value | HR | 95%CI | P-value |
| Gender (Male) | 1.54 | 0.73-3.53 | 0.26 | |||
| Age (≥71 years)a | 0.83 | 0.41-1.70 | 0.60 | |||
| Location (Rectum) | 1.00 | 0.48-2.04 | 0.99 | |||
| Differentiation (Undifferentiated) | 2.17 | 0.64-5.57 | 0.19 | 1.58 | 0.46-4.21 | 0.43 |
| Pathological T category (T3/T4) | 2.48 | 1.15-5.92 | 0.02c | 1.87 | 0.82-4.71 | 0.14 |
| Vessel invasion (Present) | 1.64 | 0.78-3.78 | 0.19 | 1.32 | 0.59-3.17 | 0.51 |
| Lymph node metastasis (Present) | 2.18 | 1.06-4.44 | 0.04c | 1.22 | 0.54-2.79 | 0.62 |
| MSI (MSI-L/MSS) | 2.51 | 0.54-44.6 | 0.29 | |||
| KRAS (Wild) | 1.19 | 0.59-2.46 | 0.63 | |||
| BRAF (Wild) | 2.02 | 0.43-36.0 | 0.44 | |||
| CD66b+ (Low) | 3.12 | 1.41-7.88 | 0.004 | |||
| CD8+ (Low) | 3.35 | 1.46-9.06 | 0.003 | |||
| CD8+CD66b+ (Low-Low)b | 3.04 | 1.48-6.47 | 0.002c | 2.52 | 1.18-5.58 | 0.02c |
The median age at surgery was 71 years in this cohort;
The cutoff value was determined using ROC curve analysis for survival;
P<0.05. CRC, colorectal cancer; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval.
Low TANs-to-TILs ratio (Model 2) in the invasive margin correlates with poor prognosis of patients with stages I–III
From a statistical standpoint, a continuous variable is more amenable to analysis. Therefore, we evaluated significance of TANs-to-TILs ratio as a prognostic marker in patients with stages I–III CRC. Kaplan-Meier survival analysis showed that patients with low TANs-to-TILs ratio in the invasive margin had a better prognosis (Fig. S1). Multivariate Cox regression analysis indicated TANs-to-TILs ratio was an independent prognostic factor both for OS (P=0.008) of patients with stages I–III CRC, but not for DFS (P=0.07) (Tables SI and SII).
Discussion
To improve the poor prognosis of patients with CRC, we require better stratification analyses to predict the disease development. Accumulating evidence reveals that tumor-infiltrating immune cells may serve as markers for the prognosis of various types of cancer. However, the clinical significance of CD66b+ TANs in CRC remains controversial due to conflicting results as both tumor-promoting and tumor-suppressing roles of CD66b+ TANs in CRC have been reported (14–16).
Inflammatory cells are essential components of the immune microenvironment. More specifically, lymphocytes and neutrophils play crucial roles in the pathogenesis of several diverse diseases including cancer (17). In the TME, surface markers expressed by TILs include CD3, CD8, or FoxP3. CD3+ TILs and CD8+ TILs in the invasive margin play a central role in immunity against CRC (5,18,19). The ‘Immunoscore’ which assesses TILs in CRC can serve as a strong predictive measure of DFS and OS which is superior to the traditional tumor-node-metastasis staging system of the AJCC/UICC (20). Therefore, fully comparing the results from this study to the traditional system, we chose the same definition of tumor margins to assess TANs. However, TANs recruited in tumors exhibit different phenotypes compared with those of circulating peripheral blood neutrophils (21).
Neutrophils recruited into tissues engage in complex bidirectional interactions with macrophages, dendritic cells, natural killer cells, as well as B and T cells (17). In the TME, TANs exhibit complex phenotypic heterogeneity and functional versatility. Neutrophils were classified as antitumorigenic ‘N1’ and protumorigenic ‘N2’ phenotypes (22–24). In humans, CD66b is traditionally defined as a marker expressed on the surface of neutrophils, which are myeloid cells with a short half-life and a specific nuclear morphology (25,26).
Research on TANs generally employs immunohistochemistry to evaluate their densities. However, published studies give conflicting messages regarding the clinical significance of a high density of CD66b+ TANs and are difficult to compare and interpret due to the analyses of diverse tumor types (4,12,13,27,28). These mixed results can be partly explained by inconsistent evaluation areas in the various studies. For example, Zhu et al quantitatively assessed the association between the density of CD66b+ TANs and prognosis using a training cohort of 337 patients and a validation cohort of 245 patients who had CRC. They demonstrated that patients with a low density of CD66b+ TANs experienced better clinical outcomes compared with those with a high density of such cells (14). However, this study did not clarify the areas (tumor center or invasive margin) used to evaluate the densities of CD66b+ cells which is one of the reasons why the results of the study differ from ours.
Moreover, Ye et al conducted tissue microarray (TMA) and immunohistochemical analyses on patients with CRC revealing that patients with CRC with a high density of infiltrating CD66b+ TANs experienced better prognosis (15). We also noticed that many studies used TMAs to evaluate the expression of tumor-infiltrating immune cells (15,16). However, most TMAs only assess the tumor-infiltrating immune cells of the tumor nest and not in the invasive margin which is difficult to evaluate.
To our knowledge, there have only been two studies that have evaluated the role of CD66b+ TANs in the invasive margin of CRC (11,29). One of the two being, Galdiero et al who defined the invasive margin as ‘50% of the entire microscopic field was cancerous tissue’. In their study, all CD66b+ cells in this microscopic area were counted, but the count may increase compared with the true value (11). Moreover, Wikberg et al assessed CD66b+ neutrophil infiltration in the tumor front and center using a semi-quantitative score. However, this study did not specifically define ‘the tumor front’ and chose a semi-quantitative evaluation method because it does not require a precise definition of the evaluation area. In the end, the results revealed that CD66b+ TANs infiltration maintained an independent significance in the multivariable analysis of stage I–II colon cancers, but not of stage III–IV stage colon cancer. All patients (23.8% were stage IV) were divided into 4 groups according to expression of CDd66b+ TANs and CD8+ TILs. The patients with high CD66b+ TANs and high CD8+ TILs in tumor front had the best prognosis in OS. However, in total there was no significant difference between the other three groups (29). We also believe that most stage IV CRC patients will die from metastatic disease and should not be included in the survival analysis for OS discounting some of the data and conclusions gained from this study. More specifically, why the combined assessment of CD8+ TILs and CD66b+ TANs did not show greater potency. At last in our study we defined the invasive margin as ‘the region centered on the border separating the host tissue from the malignant nets with an extent of 1 mm’, which is consistent with the ‘Immunoscore’ (5,20) and the prognostic indicator has been well validated in stages I–III of colon cancer.
In sum, we found in this study that low densities of CD8+ TILs and CD66b+ TANs in the invasive margin of tumor significantly correlated with shorter OS and DFS of patients with stages I–III CRC, suggesting that these tumor-infiltrating immune cells configured the complex microenvironment that influences tumor development. Also, in patients with lymph node metastasis (stage III), the density of CD66b+ TANs decreased significantly, suggesting that density of CD66b+ TANs in invasive margin may be linked to immune escape (30). In addition, low density of CD8+ TILs in the invasive margin was correlated significantly with KRAS mutation. Some evidence suggests that KRAS mutation may not only activate many downstream signaling pathways (31), but can also mediate immune evasion in various tumors (32). Smakman et al reported that silencing KRAS(D12) significantly reduced the tumorigenic potential of C26 cells in mice with intact immune systems. The incidence of tumor formation by KRAS(D12) knockdown cells remained at 100% in immune-deficient hosts (33). Thus, this finding suggests that KRAS-driven tumorigenicity is due to in-part to the suppression of host immunity. Moreover, Zdanov et al reported mutant KRAS conversed conventional T cells into regulatory T cells (Tregs) and enhanced the function of Tregs. In this study, the CD4+CD25− T cells were cocultured with mutant KRAS tumor cells (SW620, SW480), and a high percentage of CD4+CD25− T cells that were converted to Tregs expressed FOXP3 and CTLA-4. In contrast, cocultures established with WT KRAS tumor cells (colo320, widr) contained significantly fewer Tregs (34). Thus, this reveals that KRAS mutation may lead to inhibition of CD8+ TILs activation and proliferation.
A major finding of our present study is that the combined evaluation of CD8+ TILs and CD66b+ TANs in the invasive margin of CRC achieved improved stratification and prognostic accuracy. Also, simultaneous low tumor infiltration by CD8+ TILs and CD66b+ TANs was associated significantly with poorer prognosis compared with that of CD8+ TILs or CD66b+ TANs, suggesting that interaction between CD8+ TILs and CD66b+ TANs enhances the independent effect against CRC. Furthermore, we found that an immune signature with low densities of CD8+ TILs and CD66b+ TANs served as an independent prognostic factor for OS and DFS. Moreover, patients with stages II and III CRC with this signature experienced terrible survival outcomes, suggesting that we should pay more attention to postoperative treatment strategies.
Although there is increasing evidence suggesting that neutrophils are involved in adaptive immunity (17,35), the interplay between these TANs and other immune cells in TME is poorly understood. Furthermore, there is much difficulty associated with research on the mechanism of neutrophils which is due to the effect of various cell separation procedures on assays of neutrophil function. For example, in-vitro experiments that work with isolated neutrophils do not behave normally because they are primed or preactivated during isolation (36). CD8+ T cells play a central role in anti-tumor effect. TGF-β induces neutrophils to acquire N2 phenotype neutrophils (pro-tumor). Moreover, an in vivo study revealed that the antitumor effect of TGF-β receptor kinase inhibitor was lost in mice with CD8+ T cell depletion (treated with anti-CD8 antibody). On the other hand, the depletion of N2 phenotype neutrophils affects the activation of CD8+ T cells (23). Another in vitro study also found that CD66b+ TANs (anti-tumor) frequently co-localize with CD8+ T cells and the co-culture with CD66b+ TANs enhances CD8+ T cell activation and proliferation in CRC (3). Furthermore, simultaneous low tumor infiltration by CD8+ TILs and CD66b+ TANs is associated significantly with poorer prognosis compared with that of CD8+ TILs or CD66b+ TANs, suggesting that the interaction between CD8+ TILs and CD66b+ TANs enhances the independent effect against CRC.
We also tried another combined assessment method, TANs-to-TILs ratio, which is more amenable to analysis as it is a continuous variable. Model 1 and Model 2 yielded similar results since low density of CD66b+ TANs showed the highest hazard ratio for OS (HR: 8.82; 95% CI, 2.51-55.9) in univariate analysis. However, compared to Model 2, Model 1 had a stronger risk stratification ability to identify patients with worse prognosis.
Immunotherapy has changed the treatment strategy for many tumors. Previous studies have shown that PD-1/PD-L1 blockade reinvigorated function of CD8+ cytotoxic T lymphocytes (37), and CTLA-4 mAbs required depletion of regulatory T cells (Tregs) (38). On the other hand, the role of TANs remains unclear in immunotherapy as they can activate or inhibit TILs (3,39). Tumor with the absence of TILs is often referred to as ‘cold tumors’ and associate with initial resistance to immunotherapy. Therefore, CRC patients with low densities of CD8+ TILs and CD66b+ TANs may be related to poor immunotherapy response, requiring a closer follow-up and more aggressive adjuvant therapy. On the other hand, patients with high densities of CD8+ TILs and CD66b+ TANs may benefit from immunotherapy when local recurrence or distant metastasis occurs.
We acknowledge several potential limitations to this study as it is a single instructional and retrospective study with a sample size somewhat smaller. Small number of end-point events limited multivariate Cox regression analysis. Therefore, larger prospective trials and validation cohorts are needed to further confirm the potential of TILs and TANs in the invasive margins as a prognostic marker for patients with CRC.
In conclusion, our present study provides evidence for the clinical significance of CD66b+ TANs in the invasive margin of CRC. Combined assessment of CD8+ TILs and CD66b+ TANs in the invasive margin better stratified high-risk CRC patients. These analyses may help surgeons and oncologists to design more effective postoperative oncological follow-up strategies for managing patients with advanced CRC.
Supplementary Material
Acknowledgements
The authors would like to thank Mr. Arul Goel (La Canada High School, La Canada Flintridge, CA, USA) for editing a draft of this manuscript and English language correction.
Glossary
Abbreviations
- TANs
tumor-associated neutrophils
- CRC
colorectal cancer
- TILs
tumor-infiltrating lymphocytes
- TME
tumor microenvironment
- DFS
disease-free survival
- OS
overall survival
- TMA
tissue microarray
- TNM
Tumor, Node, Metastasis
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CY, YOku and YT conceived and designed the study. YOku, AY, TK, TS, MK, MT, YOki, MO and YT provided the samples and acquired the clinical and prognostic information. CY, YOku and YT analyzed and interpreted the data. CY, YOku and YT performed statistical analysis. CY, YOku, AY, TK, TS, MK, MT, YOki, MO and YT contributed to the writing of the manuscript, and agreed with the manuscript's results and conclusions. YT and CY confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The protocol for this research project was approved by the institutional review board of Mie University Hospital (approval no. H2019-187). Informed consent was obtained in the form of opt-out on the web-site. Those who rejected were excluded. The study was conducted in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: Part 1: Assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017;24:235–251. doi: 10.1097/PAP.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Governa V, Trella E, Mele V, Tornillo L, Amicarella F, Cremonesi E, Muraro MG, Xu H, Droeser R, Däster SR, et al. The interplay between neutrophils and CD8+ T cells improves survival in human colorectal cancer. Clin Cancer Res. 2017;23:3847–3858. doi: 10.1158/1078-0432.CCR-16-2047. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Xie J, Huang W, Chen H, Xi S, Han Z, Huang L, Lin T, Zhao LY, Hu YF, et al. Tumor immune microenvironment and chemosensitivity signature for predicting response to chemotherapy in gastric cancer. Cancer Immunol Res. 2019;7:2065–2073. doi: 10.1158/2326-6066.CIR-19-0311. [DOI] [PubMed] [Google Scholar]
- 5.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 6.Swierczak A, Mouchemore KA, Hamilton JA, Anderson RL. Neutrophils: Important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015;34:735–751. doi: 10.1007/s10555-015-9594-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Ge X, He J, Cheng Y, Wang Z, Wang J, Sun L. The prognostic value of tumor-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: A systematic review and meta-analysis. World J Surg Oncol. 2019;17:85. doi: 10.1186/s12957-019-1621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Idos GE, Kwok J, Bonthala N, Kysh L, Gruber SB, Qu C. The prognostic implications of tumor infiltrating lymphocytes in colorectal cancer: A systematic review and meta-analysis. Sci Rep. 2020;10:3360. doi: 10.1038/s41598-020-60255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori K, Toiyama Y, Saigusa S, Fujikawa H, Hiro J, Kobayashi M, Ohi M, Araki T, Inoue Y, Tanaka K, et al. Systemic analysis of predictive biomarkers for recurrence in colorectal cancer patients treated with curative surgery. Dig Dis Sci. 2015;60:2477–2487. doi: 10.1007/s10620-015-3648-2. [DOI] [PubMed] [Google Scholar]
- 10.Lakschevitz FS, Hassanpour S, Rubin A, Fine N, Sun C, Glogauer M. Identification of neutrophil surface marker changes in health and inflammation using high-throughput screening flow cytometry. Exp Cell Res. 2016;342:200–209. doi: 10.1016/j.yexcr.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Galdiero MR, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, Bonavita E, Barbagallo M, Tartari S, Polentarutti N, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer. 2016;139:446–456. doi: 10.1002/ijc.30076. [DOI] [PubMed] [Google Scholar]
- 12.Carus A, Ladekarl M, Hager H, Nedergaard BS, Donskov F. Tumour-associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Cancer. 2013;108:2116–2122. doi: 10.1038/bjc.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilie M, Hofman V, Ortholan C, Bonnetaud C, Coëlle C, Mouroux J, Hofman P. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;118:1726–1737. doi: 10.1002/cncr.26456. [DOI] [PubMed] [Google Scholar]
- 14.Zhu B, Luo J, Jiang Y, Yu L, Liu M, Fu J. Prognostic significance of nomograms integrating IL-37 expression, neutrophil level, and MMR status in patients with colorectal cancer. Cancer Med. 2018;7:3682–3694. doi: 10.1002/cam4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye L, Zhang T, Kang Z, Guo G, Sun Y, Lin K, Huang Q, Shi X, Ni Z, Ding N, et al. Tumor-infiltrating immune cells Act as a marker for prognosis in colorectal cancer. Front Immunol. 2019;10:2368. doi: 10.3389/fimmu.2019.02368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng YX, Cai MY, Xie D. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients' adverse prognosis. PLoS One. 2012;7:e30806. doi: 10.1371/journal.pone.0030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 18.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 19.Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: A longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 20.Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 21.Eruslanov EB. Phenotype and function of tumor-associated neutrophils and their subsets in early-stage human lung cancer. Cancer Immunol Immunother. 2017;66:997–1006. doi: 10.1007/s00262-017-1976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: Implications for homeostasis and pathogenesis. Blood. 2016;127:2173–2181. doi: 10.1182/blood-2016-01-688887. [DOI] [PubMed] [Google Scholar]
- 23.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’ TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: Friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 25.Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol. 2019;19:255–265. doi: 10.1038/s41577-019-0141-8. [DOI] [PubMed] [Google Scholar]
- 26.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: Similarities and differences. Cell Mol Life Sci. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Posabella A, Köhn P, Lalos A, Wilhelm A, Mechera R, Soysal S, Muenst S, Güth U, Stadlmann S, Terracciano L, et al. High density of CD66b in primary high-grade ovarian cancer independently predicts response to chemotherapy. J Cancer Res Clin Oncol. 2020;146:127–136. doi: 10.1007/s00432-019-03108-6. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Pan Y, Ma J, Kang Z, Xu X, Zhu Y, Chen J, Zhang W, Chang W, Zhu J. Prognostic significance of the infiltration of CD163+ macrophages combined with CD66b+ neutrophils in gastric cancer. Cancer Med. 2018;7:1731–1741. doi: 10.1002/cam4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wikberg ML, Ling A, Li X, Öberg Å, Edin S, Palmqvist R. Neutrophil infiltration is a favorable prognostic factor in early stages of colon cancer. Hum Pathol. 2017;68:193–202. doi: 10.1016/j.humpath.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 30.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 31.Zhu G, Pei L, Xia H, Tang Q, Bi F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol Cancer. 2021;20:143. doi: 10.1186/s12943-021-01441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smakman N, Veenendaal LM, van Diest P, Bos R, Offringa R, Borel Rinkes IH, Kranenburg O. Dual effect of Kras(D12) knockdown on tumorigenesis: Increased immune-mediated tumor clearance and abrogation of tumor malignancy. Oncogene. 2005;24:8338–8342. doi: 10.1038/sj.onc.1208995. [DOI] [PubMed] [Google Scholar]
- 34.Zdanov S, Mandapathil M, Abu Eid R, Adamson-Fadeyi S, Wilson W, Qian J, Carnie A, Tarasova N, Mkrtichyan M, Berzofsky JA, et al. Mutant KRAS conversion of conventional T cells into regulatory T cells. Cancer Immunol Res. 2016;4:354–365. doi: 10.1158/2326-6066.CIR-15-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vono M, Lin A, Norrby-Teglund A, Koup RA, Liang F, Loré K. Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo. Blood. 2017;129:1991–2001. doi: 10.1182/blood-2016-10-744441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glasser L, Fiederlein RL. The effect of various cell separation procedures on assays of neutrophil function. A critical appraisal. Am J Clin Pathol. 1990;93:662–669. doi: 10.1093/ajcp/93.5.662. [DOI] [PubMed] [Google Scholar]
- 37.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du X, Liu M, Su J, Zhang P, Tang F, Ye P, Devenport M, Wang X, Zhang Y, Liu Y, Zheng P. Uncoupling therapeutic from immunotherapy-related adverse effects for safer and effective anti-CTLA-4 antibodies in CTLA4 humanized mice. Cell Res. 2018;28:433–447. doi: 10.1038/s41422-018-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Germann M, Zangger N, Sauvain MO, Sempoux C, Bowler AD, Wirapati P, Kandalaft LE, Delorenzi M, Tejpar S, Coukos G, Radtke F. Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFβ. EMBO Mol Med. 2020;12:e10681. doi: 10.15252/emmm.201910681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.