Abstract
The polymers, chitosan, a polysaccharide, and gelatin, a protein, are crosslinked in different ratios without the aid of a crosslinking agent. Facile chemical reactions were followed to synthesize a chitosan/gelatin nanocomposite in three different ratios (1:1, 1:3, and 3:1). The solubility of chitosan and the stability of gelatin were improved due to the crosslinking. Both the polymers have excellent biodegradability, biocompatibility, adhesion, and absorption properties in a biological environment. The properties of the composite were favorable to be used in drug delivery applications, and the drug dopamine was encapsulated in the composite for all three ratios. The properties of the chitosan/gelatin nanocomposite and dopamine-loaded chitosan/gelatin nanocomposite were examined using XRD, FTIR, SEM, UV, TGA, TEM, and DLS techniques, and the crosslinking was confirmed. Higuchi kinetic release was seen with a cumulative release of 93% within 24 h for the 1:3 nanocomposite in a neutral medium. The peaks at 9 and 20° in the XRD spectrum confirmed the encapsulation of dopamine with the increase in the crystallinity of chitosan, which is also evident from the SAED image. The dopamine functional groups were confirmed from the IR peaks between 500 and 1500 cm–1 and the wide UV absorption maxima between 250 and 290 nm. The particle size of the drug-loaded composite in the ratios 1:1, 1:3, and 3:1 were calculated to be 275, 405, and 355 nm, respectively. The nanocomposite also showed favorable DPPH antioxidant and antibacterial activity againstStaphylococcus aureus. Sustained release of dopamine in a neutral medium using crosslinked chitosan and gelatin without the presence of a crosslinker is the highlight of the work.
Introduction
Biopolymers exhibit poor mechanical properties, chemical resistance, and processability compared to synthetic polymers. To suitably alter them for specific applications, they are reinforced with fillers which drastically improve their inherent properties. Biopolymers that have been reinforced in this way are called biopolymer composites.1 They are combined with chosen materials to reinforce and enhance their desired properties for practical applications.
Chitosan, a polysaccharide, and gelatin, a polypeptide, are unique and possess all the favorable physical, chemical, and biological properties that enhance applications in a biological system. Derivative of chitin, chitosan is an amino polysaccharide molecule with a strong positive electrical charge. This property enables it to bond strongly to negatively charged molecules. Chitosan is used in tissue regeneration, wound healing, as drug delivery vehicles, biosensors, and so forth due to its biocompatibility, biodegradation, antimicrobial, immunogenic, renewable, nontoxic, and bioabsorbable properties.2−4 The solubility of chitosan is a main concern as it is insoluble in organic solvents and H2O. It is soluble only in a slightly acidic medium, for example, with acetic, nitric, hydrochloric, perchloric, and phosphoric acid solutions. The acidic solutions with a pH less than 6.5 are optimum for dissolving chitosan.5−8
In its pure form, chitosan presents low surface area and negligible porosity compared with other available adsorbents. The drawback of chitosan is its weak mechanical strength and insolubility in water. To overcome these shortcomings, chemical and physical modifications should be made to the molecule. Crosslinking and grafting of chitosan are the most common methods employed. Crosslinking means forming a web between polymer strands to form a network. Grafting involves covalent bonding monomer chains to a polymer backbone. In the present work, to improve the properties of chitosan, it is crosslinked with gelatin.9−12
Gelatin has a high solubility in water and easily forms complexes with other molecules because of its gelatinous nature. Gelatin is synthesized by the hydrolytic degradation of protein from collagen, which is the most abundantly present protein in humans and animals. The structure of gelatin is made up of a combination of amino acids. It has high protein content, protective colloids, and is devoid of lipid and cholesterol. Gelatin possesses biodegradability, biocompatibility, and excellent cell adhesion because of the presence of unique amino acid sequences and cell proliferation properties. Gelatin is readily soluble in water. Increasing the temperature of the gelatin solution will lead to the dissolution of gelatin, and on decreasing the temperature, the liquid becomes gelatinous.13,14
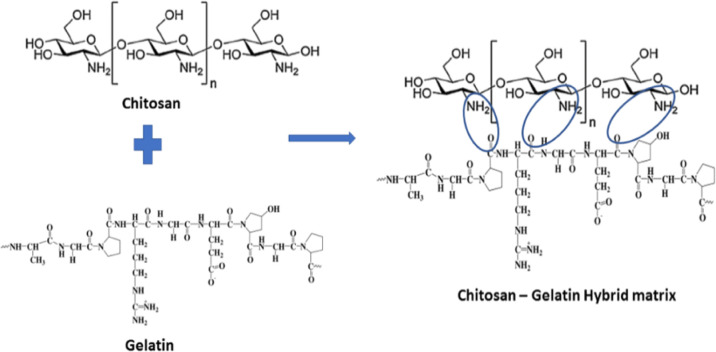
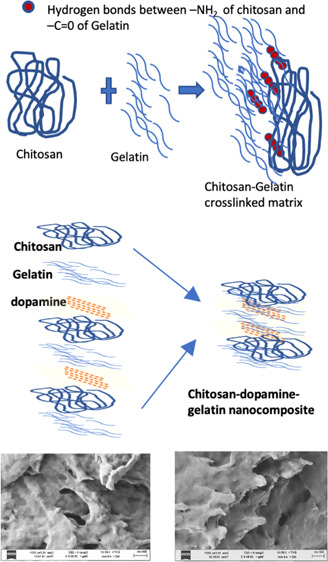
The amino groups and the secondary and primary hydroxyl groups present in chitosan enable the facile modification of inter and intramolecular hydrogen bonds between them. Gelatin comprises of amino acids such as glycine, proline, hydroxyproline, glutamic acid, alanine, arginine, and aspartic acid. Similar to chitosan, it is made up of organic compounds with a mixture of single and double chains. The structure of chitosan, gelatin, and chitosan/gelatin composite is given in Figure 1. The electrostatic attractions between the NH2 group in chitosan and COO groups in gelatin facilitate the crosslinking process.
Figure 1.
Structure of chitosan, gelatin, and chitosan/gelatin hybrid matrix.
There are different methods of crosslinking protein and polysaccharides. Numerous crosslinking agents, such as glutaraldehyde, tannic acid, and so forth, are used in the chemical crosslinking technique. The unreacted aldehyde, however, can become a hindrance to biomedical applications because it reduces biocompatibility. The aim of the present study is to examine the synthesis of the chitosan/gelatin nanocomposite without using any crosslinker. The pH and affinity for the electrophilic and nucleophilic species trigger the crosslinking, and the final product is obtained in the powder form. This crosslinking helps in increasing the solubility, mechanical strength, and drug encapsulation capacity.15−20
Both gelatin and chitosan are used as drug delivery systems to target the drug and improve drug absorption. Manipulation of chitosan and its derivatives for drug delivery toward the CNS has been studied for treatments against many neurological disorders, mainly for Parkinson’s and Alzheimer’s diseases. The advantages of chitosan as a brain-targeted drug carrier are numerous. It has the capacity to penetrate the blood–brain barrier, and it also can control the release of the drug, tightly adhere to mucus linings, and open tight junctions.21,22 However, its solubility in neutral pH is poor, and therefore there have been many attempts to modify this drawback by the addition of a functional group. However, modified chitosan molecules will have alterations in their property, and the toxicity and biocompatibility can pose a problem. Therefore, to maintain the structure of chitosan, we crosslink it with the peptide gelatin to enhance its solubility.
Both chitosan and gelatin have antibacterial and antioxidant properties.23 The oligopeptides present in gelatin have side chains of amino groups responsible for the antimicrobial behavior. In chitosan, the positively charged amino group interacts with negatively charged cell membranes of the bacteria, enhancing its antimicrobial activity.24 Several factors such as pH, molecular weight of chitosan, hydrophilic or hydrophobic nature, and physical state of chitosan influence its antimicrobial property. Complexes of chitosan with other materials substantially improve and modulate the antimicrobial activity.25 Crosslinking chitosan and gelatin, therefore, is favorable and gives a synergistic effect against the antioxidant and antimicrobial activity.
Chitosan and gelatin are versatile because their structure can be modified into microspheres, hydrogels, conjugates, nanocomposites, nanoparticles, films, and so on. Using polymer matrix for drug delivery also increases the therapeutic potential of drugs and safeguard against their degradation. Therefore, in the present work, chitosan/gelatin nanocomposites are synthesized in different aspect ratios (1:1, 1:3, and 3:1). The nanocomposites are then encapsulated with the drug dopamine. Dopamine is a neurotransmitter that is given as a therapeutic against Parkinson’s disease and other CNS-related ailments. Because the composite is ideal for CNS drug delivery, the drug dopamine is used as a model drug to study the encapsulation and drug release properties. Therefore, characteristics of these composites are studied, and the release kinetics of dopamine-loaded chitosan/gelatin nanocomposites are also tabulated. The properties and characteristics of the nanocomposites are discussed in detail.
Materials
Low-molecular-weight chitosan was purchased from Sigma-Aldrich. Gelatin Type A, from porcine skin with gel strength 300, and dopamine hydrochloride were also purchased from Sigma-Aldrich. Sodium tripolyphosphate was purchased from Alfa Aesar and Tween 80 from SRL chemicals. Double distilled water and high-quality ethanol were used for all the synthesis procedures.
Method
Synthesis of Chitosan/Gelatin Nanocomposite
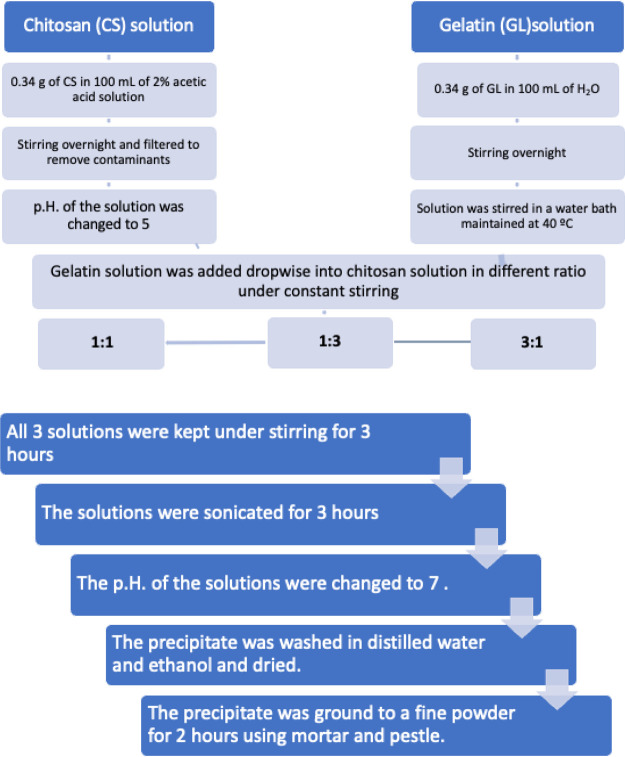
The nanocomposite preparation was done by mixing the gelatin solution with 2% (w/v) chitosan solution in different ratios, that is, 1:1, 1:3, and 3:1. Chitosan solution was prepared by dissolving chitosan in acetic acid. The flowchart of the synthesis procedure is illustrated in Figure 2. An optimal amount of chitosan powder was dissolved in 98 mL of water and 2 mL of acetic acid. This solution was left overnight for the complete dissolution of chitosan and was filtered to remove contaminants. The pH of chitosan was then changed to 5 by adding 0.5 M NaOH into the solution. An optimal amount of gelatin was measured and dissolved in 100 mL of water immersed in a water bath maintained at 40 °C.
Figure 2.
Flowchart of synthesis procedure of chitosan/gelatin nanocomposite in the ratios 1:1, 1:3, and 3:1. Synthesis of dopamine-loaded chitosan/gelatin (dopamine@chitosan/gelatin) nanocomposite (drug loading).
The solution of chitosan and gelatin were then combined in the ratios 1:1, 1:3, and 3:1. This was done by adding an appropriate amount of gelatin solution dropwise into the chitosan solution maintained at constant stirring. The resultant solutions were then allowed to stir for 3 h. After this, the solutions were kept under sonication for 3 h to ensure thorough mixing of the solutions. The pH of the solution was increased to 7. The composites then precipitated when allowed to rest for 1 day. The precipitate was then washed several times with distilled water and ethanol. It was then dried by placing in a desiccator and removing the moisture content. The dried precipitate was then ground into fine particles using mortar and pestle continuously for 2 h, and a fine powder of the composites was obtained.
The synthesis of crosslinked chitosan and gelatin scaffolds without the use of a blending agent was reported previously, and the miscibility of the compounds has been proved.26,27 Sionkowska et al. discuss the hydrogen bond formation between the −OH and NH2 groups of chitosan with the side groups of collagen.28 Because chitosan possesses a large number of −OH groups, it can also form bonds with the −COOH and NH2 end groups of collagen. Thus, in this work, we can conclude that the blends or crosslinking of chitosan and gelatin are formed due to the electrostatic interactions and hydrogen bonds between chitosan and gelatin. Chitosan is dissolved in dilute acetic acid, and the medium is maintained at an acidic pH when gelatin solution is added to it dropwise. In an acidic medium, negatively charged carboxyl groups ionically interact with positively charged amine groups on chitosan chains. These interactions between side chains, end chains, carboxyl, amine, and hydroxy groups lead to the formation of multiple complexes, which results in the blending and crosslinking of chitosan and gelatin.29
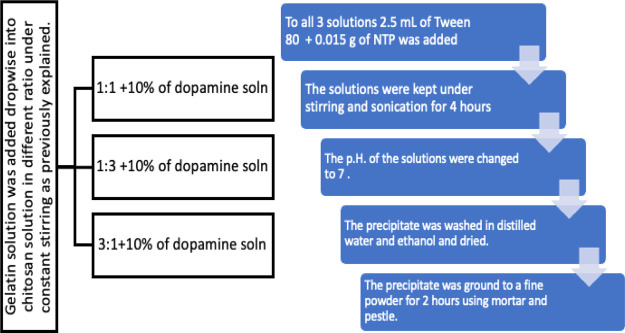
The chitosan and gelatin solutions were prepared identically to the procedure explained above. Gelatin solution was then added dropwise to the chitosan solution in different ratios to prepare the solution in the respective proportions, that is, 1:1, 1:3, and 3:1. The solutions were then left overnight under constant stirring to ensure that the solutions were adequately combined. The solutions were then sonicated for 3 h. 10 wt % of the drug, that is, dopamine solution was added. Tween 80 and sodium triphosphate are added to help in the drug encapsulation. The solutions were kept under constant stirring and sonication for 4 h. The pH was changed to help in the precipitation of the nanocomposite. The precipitate was then collected and given for further characterizations. The synthesis procedure of the drug-encapsulated chitosan/gelatin nanocomposite is given in Figure 3.
Figure 3.
Flowchart of the synthesis procedure of the dopamine-loaded chitosan/gelatin nanocomposite.
Instrumentation
The composite structure was characterized using XRD BRUKER USA D8 ADVANCE, DAVINCI with X-ray source of radiation Cu Kα (α = 1.54 Å). Samples were analyzed from 2θ = 5 to 60°. Jasco FT/IR-6600 Fourier transform infrared (FTIR) spectrometer instrument was used to record the FTIR spectra from the range 400 to 4000 cm–1. Powder samples were used for the analysis. The ultraviolet (UV) spectrum was recorded with the help of the PerkinElmer UV Win Lab 6.3.2.0749/2.02.06 Lambda 650 UV/vis instrument. The particle size was computed from dynamic light scattering (DLS) using the Horiba Scientific Horiba SZ-100 instrument. The zeta potential was also evaluated with the same instrument. The morphology of the samples was studied by imaging the topography of the material with the help of the Gemini scanning electron microscopy (SEM) 300 instrument from ZEISS. The response of the sample to the temperature was analyzed using the NETZSCH STA 2500 STA2500A-0061-N instrument. The analysis was done between the range of 30–300 °C. Transmission electron microscopy (TEM) images were recorded using the FEI-TECNAI G2-20 TWIN 200 kV instrument.
In Vitro Drug Release and Release Kinetics
The drug release behaviors of the nanocomposite were studied in pH 7. Experimentally, 0.2 g of the drug-loaded chitosan/gelatin nanocomposite was immersed in 60 mL of the release medium at room temperature under magnetic stirring. At appropriate time intervals, 5 mL of the solution was extracted, and the amount of dopamine released from the composite was determined using a UV spectrophotometer at 280 nm. The kinetics of dopamine release from chitosan/gelatin was analyzed by fitting the cumulative release with four models, that is, (1) the zero-order model (Qt = K0t), (2) the first-order model with formula (logQt = −K1t/2.303), (3) the Higuchi model (Qt = KHt1/2), and (4) the Korsmeyer–Peppas model with formula (Qt: KKPtn).
DPPH radical scavenging activity (Blois, 1958) also known as the Blois method was employed to determine the DPPH radical scavenging activity. DPPH offers a convenient and accurate method for oxidizable groups of natural or synthetic antioxidants. DPPH solution was prepared at the concentration of 0.1 mM in methanol. For the assay, 1 mL of test solution (20–120 μg/mL) was mixed with 1 mL of DPPH solution. The mixture was placed in the dark for 30 min and incubated at room temperature. The absorbance was recorded at 517 nm by the UV spectrophotometer. The percentage of DPPH-free radical scavenging activity was calculated by the following equation
Method for Antibacterial Assay
Inoculum Preparation
A loopful of bacteria staphylococcus aureus, streptococcus mutants, and actinomyces species was inoculated in the sterile nutrient broth and incubated overnight at 37 °C.
Well Diffusion Assay
The agar well diffusion assay was used to determine the growth inhibition of bacteria by the sample. Muller–Hinton agar was prepared and poured into a sterile Petri plate and allowed to solidify. The overnight nutrient broth culture of Gram-positive bacteria Staphylococcus aureus and Gram-negative bacteria E. coli species was inoculated in Mueller–Hinton agar using a sterile cotton swab. Five wells were made in the agar plate using a sterile cork-borer (8 mm diameter). Standard tetracycline and 250, 375, and 500 μg of extracts were added to each well, and the plates were incubated overnight at 37 °C. Antibacterial activity was determined my measuring the zone of growth inhibition within the well.
Cytotoxicity Studies
96-well plates with SH-SY5Y differentiated cells were plated for 24 h at 37 °C. Nutrient mixture-12 Ham, Kaighn’s modification −HiMedia was used as the culture. The cells were treated with dopamine@chitosan/gelatin after 24 h. In 10 mg mL–1 of the stock solution, the nanocomposite was sonicated. The viability of the cells after 24 h in the presence of the nanocomposite was evaluated.
Results and Discussion
FTIR
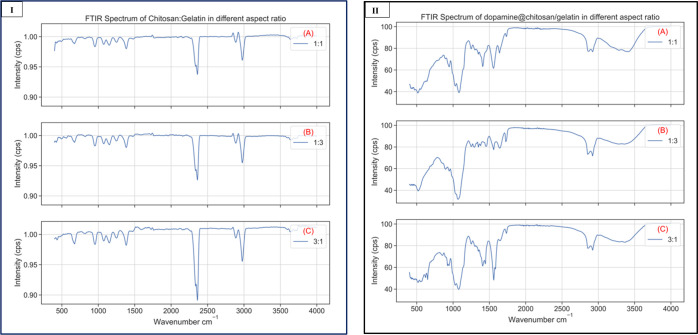
There will be significant changes in the characteristic bands when two or more substances are mixed. Crosslinking or blending of polymers is done to improve the properties of both materials and synthesize a novel composite embodying the characteristics of both constituents. The FTIR spectra analysis enables in studying the possible interaction that are present in the composite. By increasing the amount of gelatin in the composite, we see that there is a shift in the peak position of amide of chitosan. The peaks present between 100 and 1500 cm–1 in the FTIR spectra of the chitosan/gelatin nanocomposite in different ratios as shown in Figure 4I(A–C) indicate that gelatin was thoroughly mixed with chitosan. It also implies strong hydrogen bonding between the chitosan and gelatin polymer structures in the chitosan/gelatin nanocomposite matrix. As denoted in Figure 1, it is due to the interaction between NH3+ ions of chitosan and −COO– groups of gelatin that the crosslinking of the composite is possible. When there is an increase in chitosan composition, there is a decrease in peak intensity at 2400 and 2900 cm–1 because there is a decrease in single helixes and random coils. The decrease in absorbance around 1500 cm–1 indicated a nucleophilic attack by the amino group of chitosan on the carboxylic carbon atom of gelatin. These results show that increasing gelatin concentration increases the folding endurance of the chitosan/gelatin polymer composite. The FTIR of the polymer nanocomposite exhibited a mixture of characteristic absorptions because of amine groups of chitosan and the carboxylic acid group of gelatin30
Figure 4.
(I) (A) FTIR spectrum of CS/GL in the ratios 1:1, (B) 1:3, and (C) 3:1, (II) (A) dopa@chitosan/gelatin 1:1, (B) 1:3, and (C) 3:1.
Figure 4II(A–C) shows dopamine-encapsulated chitosan/gelatin nanocomposite (dopamine@chitosan/gelatin) in the ratios 1:1, 1:3, and 3:1, respectively. The predominant peaks between 500 and 1500 cm–1 indicate the dopamine functional groups. The peak at 1500–1600 cm–1 is due to the aromatic ring. The presence of C–C, C–O, and C–N vibrations are confirmed with peaks between 1200 and 1500 cm–1. The peak at approximately 3000 cm–1 is due to the prescience of C, N, and O vibrations.31,32 Thus, from the FTIR spectra, we can confirm the encapsulation of dopamine within the matrix of chitosan and gelatin.
DLS and Zeta Potential
The particle sizes of the chitosan/gelatin composite synthesized in the ratios 1:1, 1:3, and 3:1 denoted as 1, 2, and 3 are 100, 275, and 260 nm, respectively. This is shown in Figure 5. The particle size of the composites was determined by the DLS technique. These results prove that the composites were successfully synthesized in a nanoscale dimension. Dopamine was encapsulated in the composites, and the particle size of dopamine encapsulated composites in the ratios 1:1, 1:3, and 3:1 denoted as 4,5,6 is calculated to be 275, 405, and 355 nm, respectively.
Figure 5.
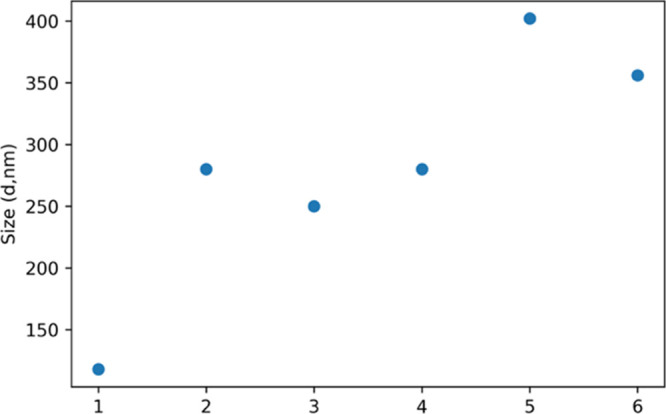
Particle size of chitosan/gelatin and dopamine@chitosan/gelatin 1,2,3—chitosan/gelatin 1:1, 1:3, and 3:1.4,5,6—dopamine@chitosan/gelatin 1:1, 1:3, and 3:1, respectively.
The zeta potential of a molecule is determined by its surface charge. The presence or absence of charges on the functional groups present on the surface alters, that is, increases or decreases the zeta potential. The carboxylic acid groups in gelatin cause a decrease in zeta potential. In chitosan, the positively charged groups such as the amino groups result in net positive zeta potential.33,34
Figure 6 gives the zeta potential for the chitosan/gelatin nanocomposites. For the ratio 1:1, we see that the zeta potential is close to 0 with a net zeta potential of −4 mV. The chitosan/gelatin composite in the ratio 1:3 shows a net negative zeta potential of −49 mV due to the abundance of gelatin. For the ratio 3:1, which has chitosan in excess, the results show a positive shift in the zeta potential with the net surface charge being +15 mV. These results portray that by varying ratios of chitosan and gelatin, there is a difference in the surface charge of the composite. In the drug-loaded composite, an increase in the surface potential is seen because dopamine had a zeta potential of +100 mV.35,36 The zeta potential of dopamine@chitosan/gelatin in the ratio dopamine@1:3, dopamine@1:1, and dopamine@3:1 represented as 4, 5, and 6, respectively, is +40, +15, and −30 mV. These values indicate that the dopamine@chitosan/gelatin nanocomposite is highly stable and can attach well to the inner lining of the membranes.
Figure 6.
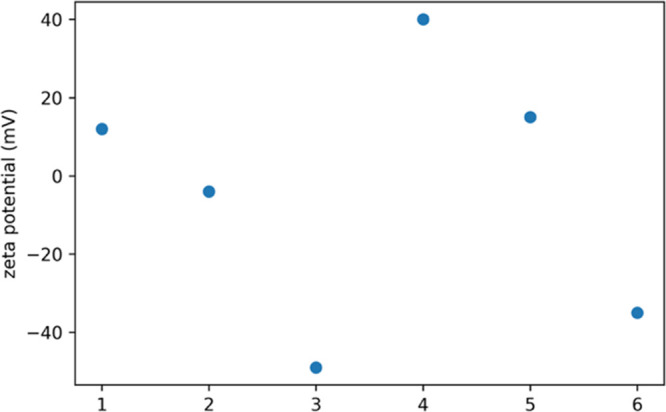
Zeta potential of chitosan/gelatin and dopamine@chitosan/gelatin 1,2,3—chitosan/gelatin 1:1, 1:3, and 3:1.4,5,6—dopamine@chitosan/gelatin 1:1, 1:3, and 3:1.
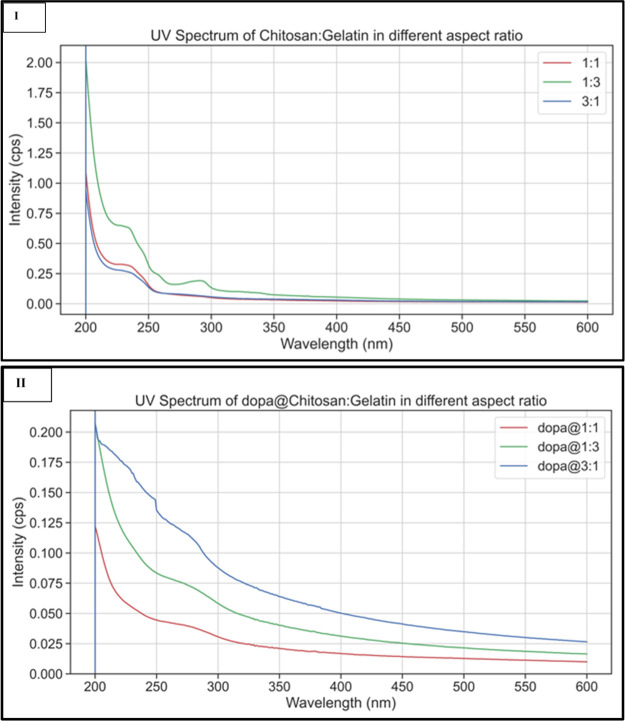
UV Spectroscopy
UV spectra were determined for the wavelengths between 200 and 600 nm. Pure chitosan has a strong absorption peak at 210 nm. The absorption peak of pure gelatin is between 210 and 240 nm.37,38 The amino groups present in gelatin, that is glycine, proline, and argine are responsible for the presence of this absorption peak. Figure 7(I). gives the absorption spectra of the chitosan/gelatin nanocomposites in the ratios 1:1, 1:3, and 3:1. There is a shift in the UV absorption maximum when compared to that of pure chitosan and pure gelatin. The absorption maxima of the chitosan/gelatin nanocomposite are found to be 240 nm. The UV spectra of dopamine encapsulated chitosan/gelatin in different ratios are depicted in Figure 7(II). Dopamine has a strong absorption peak at 280 nm.39 In the spectra, a broad absorption peak from 250 to 290 nm is seen with a maximum absorption at 280 nm. This confirms that the drug is successfully embedded within the matrix.
Figure 7.
(I) UV Spectra of chitosan/gelatin nanocomposite in different ratios, 7 (II). UV spectra of dopamine-encapsulated chitosan/gelatin in different ratios.
X-ray Diffraction
The X-ray diffraction (XRD) spectrum of the chitosan/gelatin nanocomposite in the ratio 1:1 is shown in Figure 8A The diffractogram shows both amorphous nature of gelatin and semicrystalline nature of chitosan.40,41 This is because we have reduced the particle size and synthesized it in nano dimensions. It also indicates that the crystallinity of chitosan was destroyed by gelatin during the synthesis process. Chitosan/gelatin synthesized in ratios 1:3 and 3:1 is given in Figure S2. The absence of crystalline peaks in the chitosan/gelatin nanocomposite also confirms that the constituents are mixed well in the composite. The results prove that the nanocomposite synthesis promoted the formation of the amorphous structure of polymers and hindered crystallization. From the results of XRD, we see that the individual properties of chitosan and gelatin are retained, but due to the presence of strong hydrogen bonds between them, they have successfully formed nanocomposites. Figure 8B shows the XRD spectrum of the dopamine-encapsulated chitosan/gelatin nanocomposite. The sharp peak at 9° indicated the presence of dopamine within the matrix.42 The XRD pattern confirms the crystallinity of the prepared composite due to the encapsulation of dopamine. The (020) plane which showed intensity of 200 cps for pure chitosan as shown in Figure S1, increased in intensity when encapsulated with dopamine. The increase in intensity of this peak proves that the structure of chitosan has become more ordered and crystalline.43−45 The encapsulation of dopamine within the composite has facilitated this process.
Figure 8.
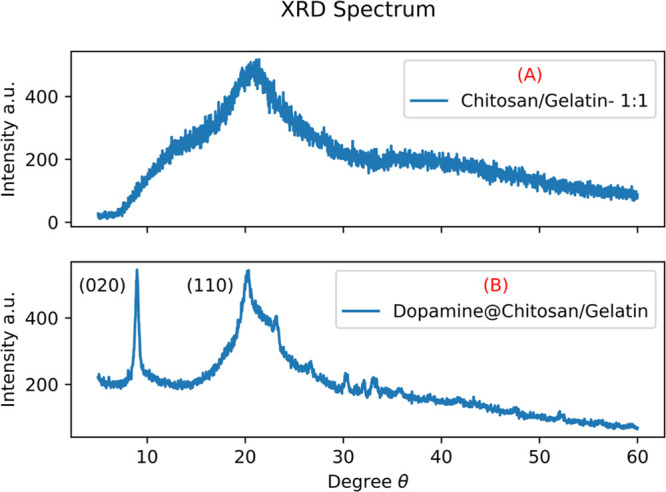
(A) XRD spectrum of chitosan/gelatin in ratio 1:1 (B) dopamine encapsulated chitosan/gelatin in the ratio 1:1.
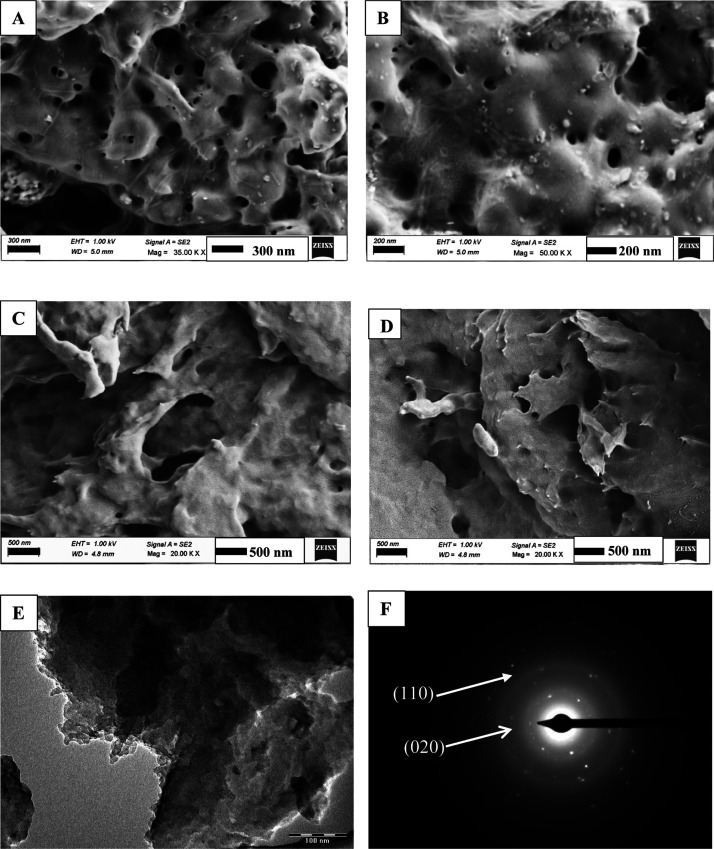
The SEM images of chitosan/gelatin nanocomposite are given in Figure 9A,B. Figure 9C,D shows the morphology of dopamine-encapsulated chitosan/gelatin nanocomposite in the ratio 1:1. The morphologies of chitosan/gelatin and dopamine encapsulated chitosan gelatin are similar. According to the figure, the composite appears as a porous structure. Additionally, there is a roughness of the chitosan/gelatin composite surface. For crosslinked composites, phase separation could not be detected by SEM analysis suggesting good compatibility between chitosan and gelatin.46,47 The morphology appears to be sheets of gelatin and chitosan arranged one above the other to form layers. This also enables efficient encapsulation of the drug within the composite. Figure 9 gives the images of the composite in magnification of 20 and 30 KX. Figure 9E,F shows the TEM image and SAED diffraction pattern of the same nanocomposite. The different layers of chitosan and gelatin formed by the twisting and rotation of polymeric chains are seen in Figure 9E. As seen in the XRD pattern (Figure 8B), there is an increase in crystallinity of chitosan due to the encapsulation of dopamine. Distinct ring patterns and the lattice points are visible in the SAED pattern shown in Figure 9F. The (110) and (020) lattice planes of chitosan are clearly seen in the SAED image, which is evidence of its crystallinity48
Figure 9.
(A,B) FESEM images of the chitosan/gelatin nanocomposite in the ratio 1:1 in different magnifications. (C,D). FESEM images of dopamine@chitosan/gelatin nanocomposite in the ratio 1:1 in different magnifications, (E) TEM image, and (F) SAED diffraction pattern of the dopamine@chitosan/gelatin nanocomposite in the ratio 1:1.
Drug Release Studies
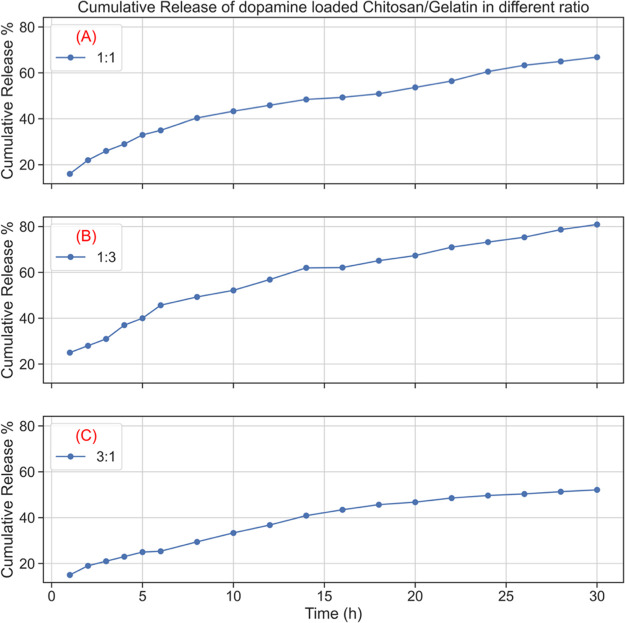
The procedure followed to evaluate the drug release from the composite is described in Mathew et al. 2020.49,50 Similar environments and conditions were followed for the release of the dopamine-encapsulated chitosan/gelatin nanocomposite. The release mechanism was evaluated in a neutral medium, that is, pH 7. This is the pH of a biological environment such as the human body. Mathew et al. 2020 focuses on the release from chitosan in pH 4. However, in the present work, we aim to release the drug at pH 7; therefore, the crosslinking with gelatin was carried out. At room temperature, maintaining the pH at 7, the release of the drug dopamine from the chitosan/gelatin nanocomposite in different ratios was analyzed, and the results are tabulated in Figure 10.
Figure 10.
Cumulative release of dopamine from the dopamine-loaded chitosan/gelatin nanocomposite in the ratios (A) 1:1, (B) 1:3, and (C) 3:1 maintained at pH 7.
The drug dopamine is entrapped within the chitosan/gelatin composite by physical entrapment. As seen in the morphology analysis, the sheets of the chitosan/gelatin matrix hold the dopamine molecule strongly within them. The initial release of the drug from the composite is due to the loosely bound drug molecule entrapped in the composite. The hydrogen bonds responsible for the crosslinking of chitosan and gelatin remain stable in the neutral medium.51,52 Therefore, sustained release of the drug from the composite is seen in the kinetic release profile. Figure 10 gives the cumulative release of the drug from the chitosan/gelatin composite in different ratios. Figure 10A shows the release of the drug from the 1:1 composition of chitosan and gelatin. There is a steady and controlled increase in the release of the drug. At the end of 30 h, a total of 89% of the drug is released into the medium. Figure 10B gives the release from the composite in the ratio 1:3. In this composite, the gelatin content is more compared to chitosan. Therefore, as time progresses, there is more swelling of the composite, and as a result, approximately 93% of the drug is released into the medium. There is also an initial burst release of the drug. This may be because of the discharge of localized drug molecules or due to the dissolution of gelatin in the medium. Figure 10C shows the release of the drug from the 3:1 composition of chitosan/gelatin. The amount of chitosan is greater in this composition. The graph shows a release of 83% at the end of 30 h. This is because the chitosan does not favor release in a neutral medium. The release is seen in chitosan only in an acidic medium. However, due to the presence of gelatin, controlled release is seen from the composite. The drug release is seen in the composite because of the combined diffusion and degradation of both polymers. There is also an increase in the drug release with the increase in gelatin concentration. This is because in the medium, gelatin swells more when compared to chitosan, and this consequently leads to more drug release because of the loose polymeric network. There is a direct correlation between the drug release and swelling of the composite.
Chitosan is a pH-sensitive polymer that triggers the release of the encapsulated drug depending on the pH of the solution. In certain applications such as cancer treatment, this quality is advantageous. To alter this character and increase the release of the drug in a neutral medium, crosslinking of chitosan with gelatin is performed in this work. In Table 1, a comparison is drawn between similar works. The effectiveness and necessity of incorporating gelatin into the chitosan matrix is evident from the comparison. As the release of drug from the chitosan matrix is pH sensitive, crosslinking aids in enhancing the sustained release of the drug in a neutral medium. As seen in Table 1, compared to drug release from pure chitosan, crosslinked chitosan/gelatin shows supreme release in a neutral medium. The comparison drawn from the table shows that a maximum cumulative release is obtained for crosslinked chitosan/gelatin (present work). The high loading efficiency minimizes the amount of unutilized drug. This is correlated to the increase in pore size and crystallinity of chitosan crosslinked gelatin.
Table 1. Comparison of Cumulative Drug Release from Drug-Loaded Chitosan Nanocomposites and Dopamine@Chitosan/Gelatin Nanocomposite (Present Work).
| material | duration (h) | cumulative release in pH 7 (%) | encapsulation efficiency (%) | loading efficiency (%) | references |
|---|---|---|---|---|---|
| dox@chitosan | 30 | 10 | 80 | 4 | (53) |
| resveratrol@chitosan | 30 | 10 | 55 | (54) | |
| dox@chitosan | 30 | 35 | 90 | 9 | (55) |
| vancomycin@chitosan | 30 | 45 | 59 | (56) | |
| floic acid@chitosan | 30 | 27 | (57) | ||
| dopamine@chitosan/carbon dots | 30 | 5 | 82 | 32 | (49) |
| dopamine@CS/GL(1:1) | 30 | 89 | 85 | 39 | present work |
| dopamine@CS/GL(1:3) | 30 | 94 | 87 | 41 | present work |
| dopamine@CS/GL(3:1) | 30 | 83 | 80 | 38 | present work |
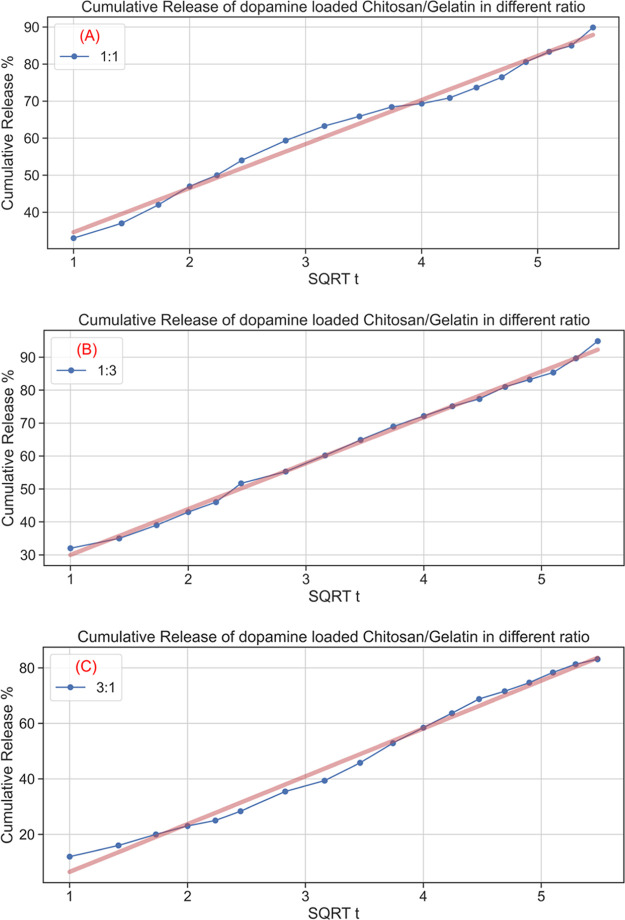
The release kinetics of the dopamine from the dopamine-loaded chitosan/gelatin nanocomposite was studied using zero-order, first-order, Higuchi, and Korsmeyer–Peppas models. The correlation coefficient (R2) was calculated for each model.58,59 The Higuchi model, where the cumulative release is plotted against the square root of time, had the highest correlations, as shown in Figure 11A–C.
Figure 11.
Kinetic release studies of dopamine from the dopamine-encapsulated chitosan/gelatin nanocomposite in the ratios (A) 1:1, (B) 1:3, and (C) 3:1.
There is no burst release, and there is a constant sustained release of the drug. This is the ideal characteristic of any material that acts as a drug carrier. Therefore, we can conclude that chitosan/gelatin is an ideal nano drug delivery carrier when compared to other compositions. The encapsulation efficiencies of the drug onto the chitosan/gelatin polymer are calculated to be 85, 87, and 80% for the 1:1, 1:3, and 3:1 ratios, respectively. The drug-loaded efficiency is approximately 39, 41, and 38%, respectively, as seen in Table 1.
Antioxidant Activity Studies
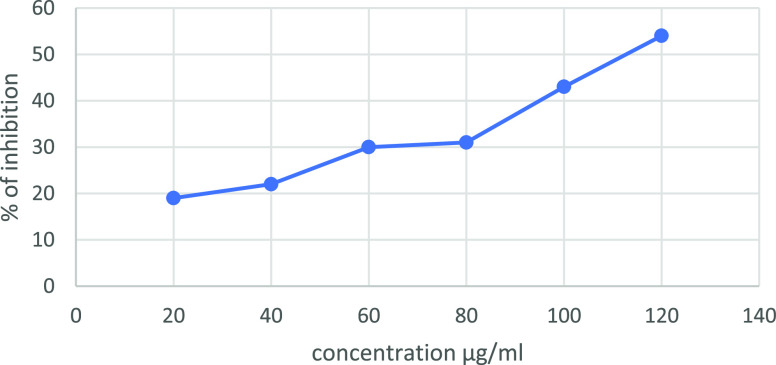
The DPPH scavenging activities of dopamine-encapsulated chitosan/gelatin were studied. The DPPH scavenging percentage of the material increases with the increase in concentration of dopamine from 30 to 120 μg mL–1, as seen in Figure 12. Compared to the standard ascorbic acid, the scavenging activity of the nanocomposite proved to be effective. This study shows that the composite shows extensive DPPH scavenging activity.
Figure 12.
Antioxidant activity of the dopamine@chitosan/gelatin nanocomposite in the ratio 1:3 against DPPH scavenging activity.
Antibacterial Activity Studies
The antibacterial study was performed against a culture of Gram-positive bacteria Staphylococcus aureus (Figure 13B) and Gram-negative bacteria E. coli species (Figure 13A). Against E. coli, the nanocomposite showed intermediate antibacterial activity, as seen in Table 2. The zone of inhibition for Staphylococcus aureus, as seen in Figure 13B, shows an excellent antibacterial property. The growth inhibition at 500 μg was comparable to the standard value. The antibacterial property of chitosan/gelatin blends against several Gram positive and Gram negative bacteria are discussed. Pereda et al. report the antimicrobial activity of gelatin/chitosan solution against E. coli at 24 mm and edible films at 20 mm.60 These nanocomposites are biocompatible and, therefore, an effective and ideal material to be used for antibacterial properties. The antimicrobial property of gelatin/chitosan in the ratios 1:1, 1:3, and 3:1 was examined by Jridi et al. The maximum inhibition halo for S. aureus and E. coli is reported as 17 and 15 mm, respectively, for gelatin/chitosan ratio 1:3.61 Matiacevich et al. report the inhibition of 21 mm against E. coli for bovine gelatin/chitosan films.62 Compared to prior literature, the present work shows excellent inhibition against Gram-positive Staphylococcus aureus with an inhibition hallow of 31 and 16 mm against E. coli.
Figure 13.
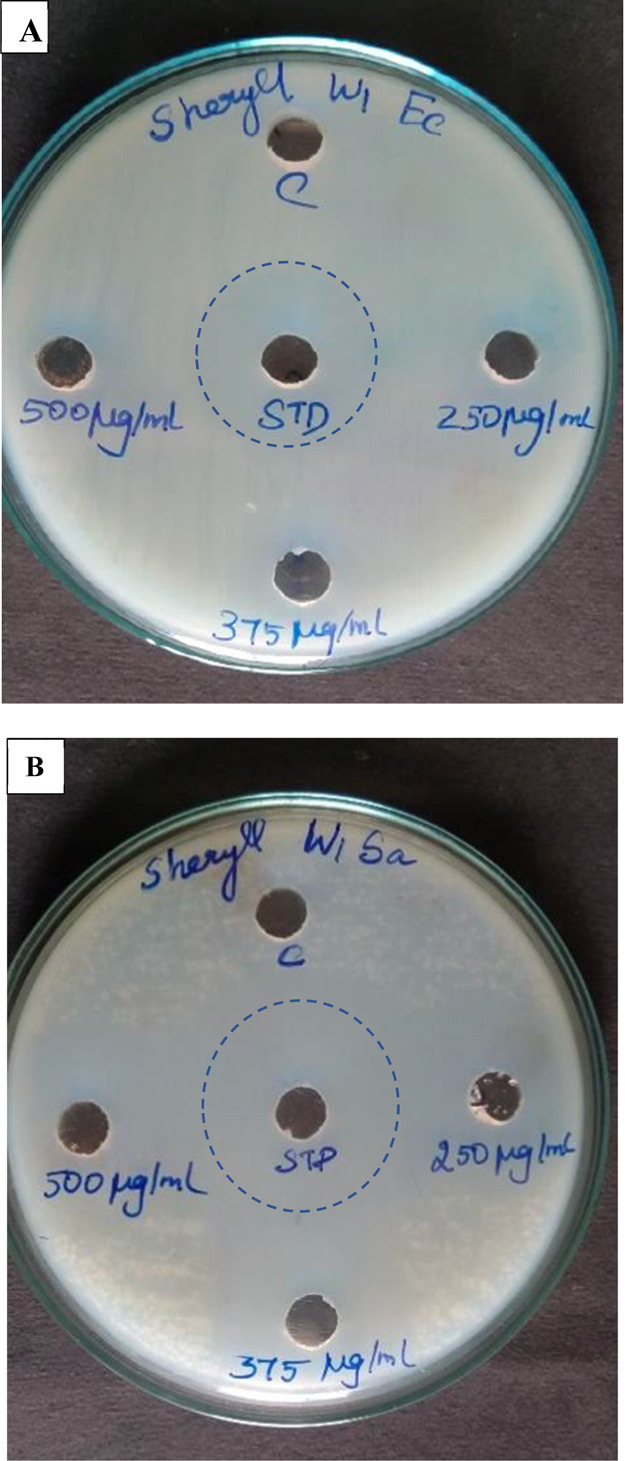
(A) Zone of inhibition of E. coli and (B) zone of inhibition of Staphylococcus aureus.
Table 2. Antibacterial Activity Studies of the Dopamine-Loaded Chitosan/Gelatin Nanocomposite.
| s. no. | organism | STD (mm) | 250 μg (mm) | 375 μg (mm) | 500 μg (mm) |
|---|---|---|---|---|---|
| 1 | E. coli | 27 | 13 | 15 | 16 |
| 2 | Staphyllococcus aureus | 34 | 29 | 30 | 31 |
Cytotoxicity Studies
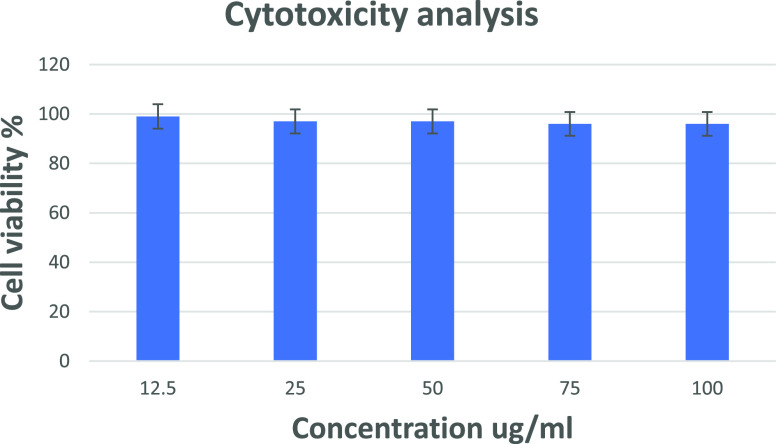
The toxicity analysis of dopamine-loaded chitosan/gelatin toward SH-SY5Y cell culture was examined. As seen in Figure 14, the cell viability of the material in different concentrations toward the cell line was 98%. This proves that the material is biocompatible and non-toxic. Chitosan and gelatin are natural polymers, and their crosslinking was performed without the usage of additives. Hence, the resultant material loaded with dopamine proves to be favorable in a biological environment, as seen in Figure 14.
Figure 14.
Plot of % viability versus sample concentration for dopamine@chitosan/gelatin toward the SH-SY5Y cell line. Values are expressed as mean S.D. of three independent experiments.
Conclusions
The chitosan/gelatin crosslinked polymer matrix and dopamine-encapsulated chitosan/gelatin polymer were synthesized successfully in different ratios 1:1, 1:3, and 3:1 without using a crosslinking agent. The FTIR, DLS, zeta potential, and UV analysis showed that the components chitosan and gelatin were crosslinked successfully and had the characteristic properties attributed to both. The SEM topography showed the presence of layers of matrix decked one above the other to form a network. The XRD and SEM findings were further validated with the TEM images. The properties of the material, such as its biocompatibility, solubility, bioavailability, and superior matrix-forming ability, make it favorable for drug delivery applications. The XRD spectrum provided evidence for the encapsulation of the drug dopamine within the nanocomposite network. The increased crystallinity and improved pore size enhance the drug loading capability of the nanocomposite. The SAED images denote the increase in crystallinity of chitosan due to the encapsulation of dopamine as seen in the XRD spectrum. The release kinetics of all three composites was studied. The composites exhibited sustained release and followed the Higuchi release model with cumulative releases of 89, 94, and 83% at the end of 30 h in a neutral medium. Though chitosan has a pH-responsive drug release with maximum release in an acidic medium, the crosslinking of chitosan with gelatin facilitated for a sustained release in a neutral medium (pH 7). The chitosan/gelatin nanocomposite also exhibited DPPH scavenging activity and proved effective against Gram-positive Staphylococcus aureus. The studies and analysis conclusively prove that the chitosan/gelatin composite is an ideal drug delivery carrier with excellent antioxidant and antibacterial properties. Crosslinking chitosan with gelatin has enhanced its properties to be efficient in a biological medium by increasing its solubility and mechanical strength. Therefore, gelatin is an excellent additive that can be used to modify chitosan to extend its use in biomedical applications.
Acknowledgments
The authors thank ARMATS BIOTECH Research and Training Institute for antioxidant, antibacterial, and cytotoxicity analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01443.
XRD spectrum of pure chitosan and gelatin; diffractogram of chitosan/gelatin in the ratios 1:1, 1:3, and 3:1; thermogravimetric analysis of the chitosan/gelatin nanocomposite in the ratio 1:1; and particle size of the chitosan/gelatin and dopamine-encapsulated chitosan/gelatin composite (PDF)
Author Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript
S.A.M. (IF170624) thanks DST-INSPIRE for the INSPIRE FELLOWSHIP.
The authors declare no competing financial interest.
Supplementary Material
References
- Sharma B.; Malik P.; Jain P. Biopolymer Reinforced Nanocomposites: A Comprehensive Review. Mater. Today Commun. 2018, 16, 353–363. 10.1016/j.mtcomm.2018.07.004. [DOI] [Google Scholar]
- Cheung R.; Ng T.; Wong J.; Chan W. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. 10.3390/md13085156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alipal J.; Mohd Pu’ad N. A. S.; Lee T. C.; Nayan N. H. M.; Sahari N.; Basri H.; Idris M. I.; Abdullah H. Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today: Proc. 2021, 42, 240–250. 10.1016/j.matpr.2020.12.922. [DOI] [Google Scholar]
- Kostag M.; el Seoud O. A. Sustainable Biomaterials Based on Cellulose, Chitin and Chitosan Composites - A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100079. 10.1016/j.carpta.2021.100079. [DOI] [Google Scholar]
- Brasselet C.; Pierre G.; Dubessay P.; Dols-Lafargue M.; Coulon J.; Maupeu J.; Vallet-Courbin A.; de Baynast H.; Doco T.; Michaud P.; Delattre C. Modification of Chitosan for the Generation of Functional Derivatives. Appl. Sci. 2019, 9, 1321. 10.3390/app9071321. [DOI] [Google Scholar]
- Sashiwa H.; Kawasaki N.; Nakayama A.; Muraki E.; Yamamoto N.; Aiba S.-i. Chemical Modification of Chitosan. 14:1 Synthesis of Water-Soluble Chitosan Derivatives by Simple Acetylation. Biomacromolecules 2002, 3, 1126–1128. 10.1021/bm0200480. [DOI] [PubMed] [Google Scholar]
- Kaczmarek M. B.; Struszczyk-Swita K.; Li X.; Szczęsna-Antczak M.; Daroch M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. 10.3389/fbioe.2019.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Castaño C.; Bolaños G. Solubility of Chitosan in Aqueous Acetic Acid and Pressurized Carbon Dioxide-Water: Experimental Equilibrium and Solubilization Kinetics. J. Supercrit. Fluids 2019, 151, 63–74. 10.1016/j.supflu.2019.05.007. [DOI] [Google Scholar]
- Reddy N.; Reddy R.; Jiang Q. Crosslinking Biopolymers for Biomedical Applications. Trends Biotecnol. 2015, 33, 362–369. 10.1016/j.tibtech.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Aryaei A.; Liu J.; Jayatissa A. H.; Jayasuriya A. C. Cross-Linked Chitosan Improves the Mechanical Properties of Calcium Phosphate-Chitosan Cement. Mater. Sci. Eng. C 2015, 54, 14–19. 10.1016/j.msec.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastuti B.; Masykur A.; Hadi S. Modification of Chitosan by Swelling and Crosslinking Using Epichlorohydrin as Heavy Metal Cr (VI) Adsorbent in Batik Industry Wastes. IOP Conf. Ser.: Mater. Sci. Eng. 2016, 107, 012020. 10.1088/1757-899X/107/1/012020. [DOI] [Google Scholar]
- Yang Y.; Chen G.; Murray P.; Zhang H.. Porous Chitosan by Crosslinking with Tricarboxylic Acid and Tuneable Release. Appl. Sci. 2020, 2. DOI: 10.1007/s42452-020-2252-z. [DOI] [Google Scholar]
- Ranasinghe R. A. S. N.; Wijesekara W. L. I.; Perera P. R. D.; Senanayake S. A.; Pathmalal M. M.; Marapana R. A. U. J. Functional and Bioactive Properties of Gelatin Extracted from Aquatic Bioresources–A Review. Food Rev. Int. 2020, 10.1080/87559129.2020.1747486. [DOI] [Google Scholar]
- Gómez-Guillén M. C.; Giménez B.; López-Caballero M. E.; Montero M. P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocolloids 2011, 25, 1813–1827. 10.1016/j.foodhyd.2011.02.007. [DOI] [Google Scholar]
- Fischetti T.; Celikkin N.; Negrini N. C.; Farè S.; Swieszkowski W. Tripolyphosphate-Crosslinked Chitosan/Gelatin Biocomposite Ink for 3D Printing of Uniaxial Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 400. 10.3389/fbioe.2020.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghizadeh Z.; Karkhaneh A.; Khojasteh A. Self-Crosslinking Effect of Chitosan and Gelatin on Alginate Based Hydrogels: Injectable in Situ Forming Scaffolds. Mater. Sci. Eng. C 2018, 89, 256–264. 10.1016/j.msec.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Liu J.; Wang S.; Xu K.; Fan Z.; Wang P.; Xu Z.; Ren X.; Hu S.; Gao Z. Fabrication of Double Crosslinked Chitosan/Gelatin Membranes with Na+ and PH Dual-Responsive Controlled Permeability. Carbohydr. Polym. 2020, 236, 115963. 10.1016/j.carbpol.2020.115963. [DOI] [PubMed] [Google Scholar]
- Shahin A.; Ramazani A.; Mehraji S.; Eslami H. Synthesis and Characterization of a Chitosan/Gelatin Transparent Film Crosslinked with a Combination of EDC/NHS for Corneal Epithelial Cell Culture Scaffold with Potential Application in Cornea Implantation. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 568–578. 10.1080/00914037.2020.1865349. [DOI] [Google Scholar]
- Qian Y.-F.; Zhang K.-H.; Chen F.; Ke Q.-F.; Mo X.-M. Cross-Linking of Gelatin and Chitosan Complex Nanofibers for Tissue-Engineering Scaffolds. J. Biomater. Sci. Polym. Ed. 2011, 22, 1099–1113. 10.1163/092050610X499447. [DOI] [PubMed] [Google Scholar]
- Kim S.; Nimni M. E.; Yang Z.; Han B. Chitosan/Gelatin-Based Films Crosslinked by Proanthocyanidin. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75B, 442–450. 10.1002/jbm.b.30324. [DOI] [PubMed] [Google Scholar]
- Caprifico A. E.; Foot P. J. S.; Polycarpou E.; Calabrese G. Overcoming the Blood-Brain Barrier: Functionalised Chitosan Nanocarriers. Pharmaceutics 2020, 12, 1013. 10.3390/pharmaceutics12111013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio M.; Cirillo G.; Rouaen J. R. C.; Saletta F.; Nicoletta F. P.; Vittorio O.; Iemma F. Natural Polysaccharide Carriers in Brain Delivery: Challenge and Perspective. Pharmaceutics 2020, 12, 1183. 10.3390/pharmaceutics12121183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M. Á. V.; Bertolo M. R. V.; Marangon C. A.; Martins V. d. C. A.; Plepis A. M. d. G. Chitosan and Gelatin Materials Incorporated with Phenolic Extracts of Grape Seed and Jabuticaba Peel: Rheological, Physicochemical, Antioxidant, Antimicrobial and Barrier Properties. Int. J. Biol. Macromol. 2020, 160, 769–779. 10.1016/j.ijbiomac.2020.05.240. [DOI] [PubMed] [Google Scholar]
- Li J.; Zhuang S. Antibacterial Activity of Chitosan and Its Derivatives and Their Interaction Mechanism with Bacteria: Current State and Perspectives. Eur. Polym. J. 2020, 138, 109984. 10.1016/j.eurpolymj.2020.109984. [DOI] [Google Scholar]
- Amankwaah C.; Li J.; Lee J.; Pascall M. A. Antimicrobial Activity of Chitosan-Based Films Enriched with Green Tea Extracts on Murine Norovirus, Escherichia Coli, and Listeria Innocua. Int. J. Food Sci. 2020, 2020, 1. 10.1155/2020/3941924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J. S.; Zhao L. G.; Yin Y. J.; Yao K. D. Structure and Properties of Bilayer Chitosan-Gelatin Scaffolds. Biomaterials 2003, 24, 1067–1074. 10.1016/s0142-9612(02)00442-8. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Onyeri S.; Siewe M.; Moshfeghian A.; Madihally S. v. In Vitro Characterization of Chitosan-Gelatin Scaffolds for Tissue Engineering. Biomaterials 2005, 26, 7616–7627. 10.1016/j.biomaterials.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Sionkowska A.; Wisniewski M.; Skopinska J.; Kennedy C. J.; Wess T. J. Molecular Interactions in Collagen and Chitosan Blends. Biomaterials 2004, 25, 795–801. 10.1016/S0142-9612(03)00595-7. [DOI] [PubMed] [Google Scholar]
- Qiao C.; Ma X.; Zhang J.; Yao J. Molecular Interactions in Gelatin/Chitosan Composite Films. Food Chem. 2017, 235, 45–50. 10.1016/j.foodchem.2017.05.045. [DOI] [PubMed] [Google Scholar]
- Lawrie G.; Keen I.; Drew B.; Chandler-Temple A.; Rintoul L.; Fredericks P.; Grøndahl L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. 10.1021/bm070014y. [DOI] [PubMed] [Google Scholar]
- Zangmeister R. A.; Morris T. A.; Tarlov M. J. Characterization of Polydopamine Thin Films Deposited at Short Times by Autoxidation of Dopamine. Langmuir 2013, 29, 8619–8628. 10.1021/la400587j. [DOI] [PubMed] [Google Scholar]
- Ren Y.; Zhao X.; Liang X.; Ma P. X.; Guo B. Injectable Hydrogel Based on Quaternized Chitosan, Gelatin and Dopamine as Localized Drug Delivery System to Treat Parkinson’s Disease. Int. J. Biol. Macromol. 2017, 105, 1079–1087. 10.1016/j.ijbiomac.2017.07.130. [DOI] [PubMed] [Google Scholar]
- González C.; Reyes L. H.; Muñoz-Camargo C.; Cruz J. C. Synthesis, Characterization and Functionalization of Chitosan and Gelatin Type B Nanoparticles to Develop Novel Highly Biocompatible Cell-Penetrating Agents. Mater. Process. 2021, 4, 30. 10.3390/IOCN2020-07816. [DOI] [Google Scholar]
- Quiroz-Reyes C. N.; Ronquillo-de Jesús E.; Duran-Caballero N. E.; Aguilar-Méndez M. Á. Development and Characterization of Gelatin Nanoparticles Loaded with a Cocoa-Derived Polyphenolic Extract. Fruits 2014, 69, 481–489. 10.1051/fruits/2014034. [DOI] [Google Scholar]
- Pada A.-K.; Desai D.; Sun K.; Govardhanam N. P.; Törnquist K.; Zhang J.; Rosenholm J. M. Comparison of Polydopamine-Coated Mesoporous Silica Nanorods and Spheres for the Delivery of Hydrophilic and Hydrophobic Anticancer Drugs. Int. J. Mol. Sci. 2019, 20, 3408. 10.3390/ijms20143408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood J.; Xu Y.; Lovas K.; Qin Y.; Bao Y. Surface Functionalization of Dopamine Coated Iron Oxide Nanoparticles for Various Surface Functionalities. J. Magn. Magn. Mater. 2017, 427, 220–224. 10.1016/j.jmmm.2016.10.039. [DOI] [Google Scholar]
- Voron’ko N. G.; Derkach S. R.; Kuchina Y. A.; Sokolan N. I. The Chitosan-Gelatin (Bio)Polyelectrolyte Complexes Formation in an Acidic Medium. Carbohydr. Polym. 2016, 138, 265–272. 10.1016/j.carbpol.2015.11.059. [DOI] [PubMed] [Google Scholar]
- Nasreen Z.; Khan M. A.; Mustafa A. I. Improved Biodegradable Radiation Cured Polymeric Film Prepared from Chitosan-Gelatin Blend. J. Appl. Chem. 2016, 2016, 1–11. 10.1155/2016/5373670. [DOI] [Google Scholar]
- El-Zohry A. M.; Hashem E. Y. Environmental Method to Determine Dopamine and Ascorbic Acid Simultaneously via Derivative Spectrophotometry. J. Spectrosc. 2013, 2013, 260376 10.1155/2013/260376. [DOI] [Google Scholar]
- Maji K.; Dasgupta S.; Pramanik K.; Bissoyi A. Preparation and Evaluation of Gelatin-Chitosan-Nanobioglass 3D Porous Scaffold for Bone Tissue Engineering. Int. J. Biomater. 2016, 2016, 9825659 10.1155/2016/9825659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olad A.; Azhar F. F. The Synergetic Effect of Bioactive Ceramic and Nanoclay on the Properties of Chitosan-Gelatin/Nanohydroxyapatite-Montmorillonite Scaffold for Bone Tissue Engineering. Ceram. Int. 2014, 40, 10061–10072. 10.1016/j.ceramint.2014.04.010. [DOI] [Google Scholar]
- Thakur V. K.; Yan J.; Lin M.-F.; Zhi C.; Golberg D.; Bando Y.; Sim R.; Lee P. S. Novel Polymer Nanocomposites from Bioinspired Green Aqueous Functionalization of BNNTs. Polym. Chem. 2012, 3, 962–969. 10.1039/c2py00612j. [DOI] [Google Scholar]
- Facchinatto W. M.; Santos D. M. d.; Fiamingo A.; Bernardes-Filho R.; Campana-Filho S. P.; Azevedo E. R. d.; Colnago L. A. Evaluation of Chitosan Crystallinity: A High-Resolution Solid-State NMR Spectroscopy Approach. Carbohydr. Polym. 2020, 250, 116891. 10.1016/j.carbpol.2020.116891. [DOI] [PubMed] [Google Scholar]
- Ioelovich M. Crystallinity and Hydrophility of Chitin and Chitosan. Res. Rev.: J. Chem. 2014, 3, 7–14. [Google Scholar]
- Jaworska M.; Sakurai K.; Gaudon P.; Guibal E. Influence of Chitosan Characteristics on Polymer Properties. I: Crystallographic Properties. Polym. Int. 2003, 52, 198–205. 10.1002/pi.1159. [DOI] [Google Scholar]
- Cheng Y.; Morovvati M. R.; Huang M.; Shahali M.; Saber-Samandari S.; Niazi Angili S.; Ghadiri Nejad M.; Shakibaie M.; Toghraie D. A Multilayer Biomimetic Chitosan-Gelatin-Fluorohydroxyapatite Cartilage Scaffold Using for Regenerative Medicine Application. J. Mater. Res. Technol. 2021, 14, 1761–1777. 10.1016/j.jmrt.2021.07.052. [DOI] [Google Scholar]
- Azhar F. F.; Olad A.; Salehi R. Fabrication and Characterization of Chitosan-Gelatin/Nanohydroxyapatite- Polyaniline Composite with Potential Application in Tissue Engineering Scaffolds. Des. Monomers Polym. 2014, 17, 654–667. 10.1080/15685551.2014.907621. [DOI] [Google Scholar]
- Guirguis O.; Abdelzaher N.; El-Bassyouni G.; Moselhey M. Structural, Thermal and Optical Modifications of Chitosan Due to UV-Ozone Irradiation. Egypt. J. Chem. 2018, 61, 350–360. 10.21608/EJCHEM.2018.2904.1241. [DOI] [Google Scholar]
- Mathew S. A.; Praveena P.; Dhanavel S.; Manikandan R.; Senthilkumar S.; Stephen A. Luminescent Chitosan/Carbon Dots as an Effective Nano-Drug Carrier for Neurodegenerative Diseases. RSC Adv. 2020, 10, 24386–24396. 10.1039/d0ra04599c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanavel S.; Sivaranjani T.; Sivakumar K.; Palani P.; Gupta V. K.; Narayanan V.; Stephen A. Cross-Linked Chitosan/Hydroxylated Boron Nitride Nanocomposites for Co-Delivery of Curcumin and 5-Fluorouracil towards Human Colon Cancer Cells. J. Iran. Chem. Soc. 2021, 18, 317–329. 10.1007/s13738-020-02031-9. [DOI] [Google Scholar]
- Yokoyama T.; Mizuguchi M.; Nabeshima Y.; Kusaka K.; Yamada T.; Hosoya T.; Ohhara T.; Kurihara K.; Tanaka I.; Niimura N. Hydrogen-Bond Network and PH Sensitivity in Human Transthyretin. J. Synchrotron Radiat. 2013, 20, 834–837. 10.1107/S090904951302075X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanaboni G.; Rossi A.; Onana A. M. T.; Tenni R. Stability and Networks of Hydrogen Bonds of the Collagen Triple Helical Structure: Influence of PH and Chaotropic Nature of Three Anions. Matrix Biol. 2000, 19, 511–520. 10.1016/s0945-053x(00)00096-2. [DOI] [PubMed] [Google Scholar]
- Gooneh-Farahani S.; Naghib S. M.; Naimi-Jamal M. R.; Seyfoori A. A PH-Sensitive Nanocarrier Based on BSA-Stabilized Graphene-Chitosan Nanocomposite for Sustained and Prolonged Release of Anticancer Agents. Sci. Rep. 2021, 11, 17404. 10.1038/s41598-021-97081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma S.; Agarwal S.; Bhuyan P.; Hazarika J.; Ganguly M. Resveratrol-Loaded Chitosan–Pectin Core–Shell Nanoparticles as Novel Drug Delivery Vehicle for Sustained Release and Improved Antioxidant Activities. R. Soc. Open Sci. 2022, 9, 210784. 10.1098/rsos.210784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H.-H.; Hong D.-X.; Guan Y.-X.; Yao S.-J. Preparation of PH-Responsive DOX-Loaded Chitosan Nanoparticles Using Supercritical Assisted Atomization with an Enhanced Mixer. Int. J. Pharm. 2019, 558, 82–90. 10.1016/j.ijpharm.2018.12.077. [DOI] [PubMed] [Google Scholar]
- Kalhapure R. S.; Jadhav M.; Rambharose S.; Mocktar C.; Singh S.; Renukuntla J.; Govender T. PH-Responsive Chitosan Nanoparticles from a Novel Twin-Chain Anionic Amphiphile for Controlled and Targeted Delivery of Vancomycin. Colloids Surf. B Biointerfaces 2017, 158, 650–657. 10.1016/j.colsurfb.2017.07.049. [DOI] [PubMed] [Google Scholar]
- Wang K.; Lin S.; Nune K. C.; Misra R. D. K. Chitosan-Gelatin-Based Microgel for Sustained Drug Delivery. J. Biomater. Sci. Polym. Ed. 2016, 27, 441–453. 10.1080/09205063.2016.1143673. [DOI] [PubMed] [Google Scholar]
- Siepmann J.; Peppas N. A. Higuchi Equation: Derivation, Applications, Use and Misuse. Int. J. Pharm. 2011, 418, 6–12. 10.1016/j.ijpharm.2011.03.051. [DOI] [PubMed] [Google Scholar]
- Paarakh M. P.; Ani Jose P.; Setty C. M.; Christoper G. V. P. Release Kinetics-Concepts and Applications. Int. J. Pharm. Technol. 2018, 8, 12–20. [Google Scholar]
- Pereda M.; Ponce A. G.; Marcovich N. E.; Ruseckaite R. A.; Martucci J. F. Chitosan-Gelatin Composites and Bi-Layer Films with Potential Antimicrobial Activity. Food Hydrocolloids 2011, 25, 1372–1381. 10.1016/j.foodhyd.2011.01.001. [DOI] [Google Scholar]
- Jridi M.; Hajji S.; Ayed H. B.; Lassoued I.; Mbarek A.; Kammoun M.; Souissi N.; Nasri M. Physical, Structural, Antioxidant and Antimicrobial Properties of Gelatin-Chitosan Composite Edible Films. Int. J. Biol. Macromol. 2014, 67, 373–379. 10.1016/j.ijbiomac.2014.03.054. [DOI] [PubMed] [Google Scholar]
- Matiacevich S.; Cofré D. C.; Schebor C.; Enrione J. Physicochemical and Antimicrobial Properties of Bovine and Salmon Gelatin-Chitosan Films. CyTA—J. Food 2013, 11, 366–378. 10.1080/19476337.2013.773564. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.