Abstract

The most essential issue facing the world today is the provision of energy and sustainable consumption of natural resources. Pretreatment is an essential step to produce biofuels from lignocellulosic biomass. In this study, ammonia fiber explosion (AFEX) combined with NaOH (A-NaOH) pretreatment effects on the characteristics of Pennisetum sinese (herbaceous), oak (hardwood), and camphor wood (softwood) were assessed using enzymatic efficiency analysis, thereby identifying the composition properties of subsequent bio-H2 production. The results show that the lignin removal (84.2%, 59.7%, and 36.7%, respectively) at 5%A-NaOH conditions and enzymatic efficiency (36.2%, 9.7%, and 6.5%, respectively) of Pennisetum sinese (P. sinese), oak, and camphor wood were significantly increased under 4% A-NaOH conditions. Further A-NaOH pretreatment significantly promoted dark fermentation bio-H2 production (152.3, 99.1, and 76.9 mL/g TS, respectively) and volatile acid production (4660.2, 3720.2, and 3496.2 mg/L, respectively) of P. sinese, oak, and camphor wood. These findings show that A-NaOH pretreatment is an effective means of utilization of lignocellulose resources.
1. Introduction
The global economy is growing rapidly, and sustained economic development requires a constant supply of energy.1 Considering the population growth and the attendant demand for food supplies, especially in populous developing countries such as China and India, fossil fuel stocks are being rapidly diminished as the production capacity expands significantly to address the sustainable supply of petroleum-based fossil fuels and the problems associated with their consumption. Moreover, this dependence on fossil fuels is causing substantial environmental problems.2 Therefore, it is necessary to explore clean and renewable energy solutions to address the energy crisis and environmental degradation.
Lignocellulosic biomass is a principal renewable energy source that contains carbon.3 Lignocellulose is mainly derived from two major sectors: agriculture and industry. Bioenergy produced from lignocellulosic biomass is considered a suitable resource for conversion into biofuels and chemicals and has attracted attention globally.4 As an extremely accessible energy source, lignocellulosic biomass has great potential not only to provide energy security but also to minimize greenhouse gas emissions.5 Lignocellulosic substrates are rich in biopolymers, and using them as substrates of different fuel sources to reduce global dependence on fossil fuels is considered one of the most promising solutions.6 The three major components of lignocellulosic biomass are cellulose, hemicellulose, and lignin.7 The robust and solid lignocellulosic cell walls of native lignocellulosic biomass are difficult to break down by enzymes and need to be pretreated in order to improve their sugar release properties; this pretreatment is a key step in the bioconversion of biomass into fuel.8
Pretreatment methods are categorized into four types: physical, chemical, physicochemical, and biological methods.9 They include ultrasonic, grinding, steam explosion, organic solvents, ozonolysis, AFEX, alkali pretreatment, acid pretreatment, ionic liquids, and microwave-assisted methods.10 Acid and alkali treatments are the two main categories of chemical treatments. Compared to acid treatment, alkali treatment has milder reaction conditions, can effectively remove lignin, and greatly improves enzymatic efficiency with few or no major inhibitors produced during the reaction.11 Alkali pretreatment includes three main methods: ammonia, NaOH, and Ca(OH)2.12 Ammonia fiber expansion (AFEX) is the main mode of ammonia treatment, which increases both the surface area of lignocellulosic biomass (LCB) and the hydrolytic accessibility of enzymes, and destroys lignin complex carbohydrates (LCC).13 Increased saccharification efficiency and fermentation product per mass of AFEX-treated LCB have been identified.14 NaOH pretreatment alone can effectively improve the enzymatic degradation of lignocellulose and has been widely used for the conversion of LCB to bioethanol, biomethane, and bio-H2.15 However, the effect of AFEX combined with NaOH on lignocellulosic biomass has not been reported.
Suitable pretreatment can effectively remove lignin from biomass, improve enzymatic efficiency, and promote the production of bio-H2 during fermentation. It was found that the hydrogen production from wheat straw could be significantly increased from 3.4 mL/g TS to 20–35 mL/g TS by the grinding process.16 Jiang et al. found that giant reed pretreated with 20% NaOH had 20% higher hydrogen production and a 1.74 times higher glucose yield than the treatment with Ca(OH)2.17 Dong et al. increased hydrogen production by 161.92% by alkali/urea pretreatment of rice straw at low temperatures.18 However, the effect of AFEX combined with NaOH in lignocellulose has yet to be explored. In particular, it is not clear how this pretreatment method affects the lignocellulosic hydrogen production efficiency.
In this study, the feasibility of A-NaOH pretreatment of lignocellulosic dark fermentation for bio-H2 production was evaluated using P. sinese, oak, and camphor wood as typical representatives of herbaceous, hardwood, and softwood lignocellulosic biomass, respectively. We investigated the effects of different biomass types and NaOH contents on the chemical properties of lignocellulosic biomass pretreated with A-NaOH to improve the enzymatic efficiency and bio-H2 production in dark fermentation. We also investigated the contribution of A-NaOH pretreatment to soluble microbial products (SMPs) during the dark fermentation of lignocellulosic biomass.
2. Experimental Section
2.1. Materials
P. sinese was obtained from Tai’an, Shandong Province, while oak and camphor wood chips were obtained from Jiangxi Province. The outer skin of P. sinese was peeled off, it was rinsed with tap water to remove the black stains until the effluent became clear, and it was dried naturally, cut into small pieces 2–3 cm in length, then crushed with a grinder, and finally sifted through a 20–40 mesh sieve. Oak and camphor wood chips were used without preparation because of their small particle sizes. All materials were dried at 60 °C to constant weight.
2.2. AFEX Pretreatment
The raw materials with a moisture content of 70% (w/w) were loaded into a benchtop autoclave (Nanjing Zhengxin Instruments), with a capacity of 200 mL. Liquid ammonia was slowly added to the reactor at a 1:1 ratio (1 g of ammonia per gram of biomass). The temperature of the reactor was 130 °C (pressure between 35 and 50 bar), and it was held for 10 min followed by the immediate release of pressure. The pretreated biomass was dried overnight in a blast dryer at 60 °C.
2.3. NaOH Pretreatment
To investigate the influence of NaOH concentration on herbaceous plants, softwoods, and hardwoods, various concentration gradients of NaOH were established at 1%, 2%, 3%, 4%, and 5% w/v. Pretreatment was conducted in 100 mL pressure-resistant bottles. The materials treated with AFEX (section 2.2) were treated with different concentrations of sodium hydroxide solution at 80 °C for 40 min in a solid–liquid ratio of 1:10. After pretreatment, the solid residue was separated, cleaned several times with deionized water until the pH was neutral, and dried to a continuous mass at 60 °C.
2.4. Enzymatic Hydrolysis
Enzymatic saccharification of untreated P. sinese, camphor wood chips, oak chips, and treated filtrate was performed using cellulase (15 FPU/g solid residue) in a pH 4.8 citrate buffer solution. The hydrolysates were then placed in a shaker at a constant temperature of 50 °C and 120 rpm for 72 h to obtain hydrolysates for subsequent bio-H2 induction, and the supernatant was used to determine the sugar yield. The sugar concentration in all of the samples after enzymatic degradation was measured using high-performance liquid chromatography (HPLC). The enzymatic efficiency of each sample was determined using eq 1:
| 1 |
1.11 is the transformation of glucan to glucose conversion coefficient, and 1.14 is the transformation of xylan to xylose conversion coefficient.
2.5. Bio-H2 Production by Dark Fermentation
The hydrolysate with the highest sugar yield after enzymatic saccharification in step 2.4 was sequentially transferred to a bioreactor. Each bioreactor was inoculated with 150 mL of domesticated sludge, and the total volume in the fermentation flask was adjusted to 500 mL with deionized water. The top of each bioreactor was rinsed with N2 for 1 min before the rubber stopper was quickly sealed to remove the oxygen. Finally, all bioreactors were heated and incubated in a 37 °C water bath. Gas collection bottles were loaded with 10% NaOH to absorb CO2, H2S, and other gases produced during anaerobic fermentation, whereas untreated raw materials were used as controls. All experiments were repeated thrice, and the average value from the data was taken as the final result.
2.6. Analysis Method
Total solids (TS) and volatile solids (VS) of lignocellulosic biomass were measured using standard methods.19 The lignin content was determined using a two-step acid hydrolysis method based on the NREL laboratory standard protocol.20 The monosaccharide concentrations after acid digestion were analyzed using HPLC (LC-20AT, Shimadzu, Japan) with a refractive index differential detector, Amnex HPX-87H column, and Microguard CationH guard column. Specifications were set at a mobile phase of 0.005 mol/L H2SO4, injection volume of 20 μL, mobile phase flow rate of 0.6 mL/min, column temperature of 65 °C, injection time of 30 min, and residence time of 35 min. Solid recovery, glucan recovery, xylan recovery, and lignin removal from the pretreatment were determined as eq 2, 3, 4, and 5:
| 2 |
| 3 |
| 4 |
| 5 |
The Fourier transform infrared spectroscopy (FTIR) spectra of all samples were measured using an FTIR spectrometer (IRAffinity-1S, Shimadzu, Japan). The samples were mixed with KBr, ground, and then placed in a 2 ton press for approximately 5 min, and the resulting transparent flakes were removed for FTIR analysis. The test parameters were as follows: a scanning wavelength range of 4000–400 cm–1, scanning frequency of 32, and air as background.
The crystallinity of the samples before and after pretreatment was analyzed using an X-ray diffractometer (D8 Advance, Bruker, Germany). The lignocellulose biomass powders were scanned at 5–40° in increments of 0.04°/s. The crystallinity index (CrI) was determined using eq 6:
| 6 |
where I002 is the maximum peak at approximately 22°, and Iam is the minimum peak at approximately 18°.
Scanning electron microscopy (TESCAN MIRA LMS, Czech Republic) was used, microsamples were taken and glued directly to the conductive adhesive and sprayed with gold for 45s using an Oxford Quorum SC7620 sputter coater at 10 mA, and the samples were subsequently photographed using a TESCAN MIRA LMS scanning electron microscope.
NMR (Bruker, Switzerland) spectra were obtained on a Bruker Avance III HD 400 MHz instrument, the MAS spin rate is 10Khz, the recovery time is 4 s, the pulse program for acquisition is cp, and the prescan delay is 6.5 μs.
Samples of the liquid collected during dark fermentation were clarified using centrifugation at 10 000 rpm for 10 min and then strained through a 0.45 μm pore size membrane to obtain the supernatant, in which the SMPs were detected using HPLC. Specifications were set at a mobile phase of 0.005 mol/L H2SO4, injection volume of 20 μL, column temperature of 65 °C, mobile phase flow rate of 0.6 mL/min, injection time of 40 min, and residence time of 45 min.
2.7. Kinetic Model
A modified Gompertz model as shown in eq 7 was applied to perform the kinetic analysis of hydrogen production.
| 7 |
where P(t) denotes the cumulative H2 yield (mL H2/g TS) over the cumulative time (t, h), Pm refers to the maximum H2 yield (mL H2/g TS), Rm denotes the maximum rate of H2 yield (mL H2/g TS/h), e is 2.718, and the lag time is denoted by (h). Origin 2021 software was used to calculate Pm, Rm ,and λ, which are directly related to their correlation coefficient (R2) values.
3. Results and Discussion
3.1. Structural Characterization
3.1.1. Chemical Composition Changes of Lignocellulose after A-NaOH Pretreatment
Table 1 shows the influence of A-NaOH pretreatment on the content and recovery of glucan and xylan from P. sinese, oak, and camphor wood as well as their respective lignin contents and removal.
Table 1. Composition of Untreated Sample and A-NaOH Treated Sample.
| glucan
(%) |
xylan
(%) |
lignin
(%) |
|||||
|---|---|---|---|---|---|---|---|
| materials | solid recovery (%) | content | recovery | content | recovery | content | removal |
| P. sinese | |||||||
| untreated | 47.8 ± 0.5 | 18.8 ± 0.4 | 25.0 ± 1.0 | ||||
| A-1%NaOH | 77.3 ± 1.2 | 55.7 ± 0.4 | 90.1 ± 1.7 | 19.8 ± 0.5 | 81.4 ± 0.9 | 14.7 ± 0.4 | 54.7 ± 1.3 |
| A-2%NaOH | 69.4 ± 0.5 | 67.0 ± 0.9 | 97.3 ± 1.4 | 20.4 ± 0.3 | 75.2 ± 0.9 | 10.7 ± 0.4 | 70.3 ± 1.9 |
| A-3%NaOH | 59.9 ± 1.0 | 64.9 ± 0.2 | 81.4 ± 0.7 | 17.5 ± 0.6 | 55.7 ± 1.8 | 10.3 ± 0.3 | 75.3 ± 1.1 |
| A-4%NaOH | 56.5 ± 1.3 | 68.0 ± 0.3 | 80.5 ± 1.7 | 15.5 ± 0.4 | 46.5 ± 1.3 | 7.0 ± 0.6 | 84.2 ± 1.5 |
| A-5%NaOH | 56.6 ± 1.1 | 74.0 ± 0.4 | 87.7 ± 2.0 | 15.0 ± 0.3 | 45.4 ± 1.0 | 6.9 ± 0.5 | 84.3 ± 1.5 |
| Oak | |||||||
| untreated | 41.3 ± 0.7 | 14.7 ± 0.4 | 32.7 ± 0.3 | ||||
| A-1%NaOH | 75.0 ± 2.6 | 44.6 ± 0.4 | 80.8 ± 3.7 | 19.5 ± 0.5 | 99.5 ± 0.3 | 28.7 ± 0.6 | 34.2 ± 1.3 |
| A-2%NaOH | 74.3 ± 1.0 | 46.5 ± 0.3 | 83.7 ± 2.9 | 18.7 ± 0.4 | 94.4 ± 3.1 | 27.3 ± 0.5 | 37.8 ± 1.7 |
| A-3%NaOH | 72.1 ± 0.6 | 47.4 ± 1.0 | 82.7 ± 1.2 | 17.4 ± 0.4 | 85.1 ± 2.6 | 19.7 ± 0.7 | 56.6 ± 1.6 |
| A-4%NaOH | 70.4 ± 0.6 | 49.0 ± 0.7 | 83.5 ± 2.5 | 14.9 ± 1.0 | 71.4 ± 5.3 | 19.3 ± 0.5 | 58.4 ± 1.3 |
| A-5%NaOH | 68.1 ± 1.0 | 49.6 ± 0.7 | 81.7 ± 2.1 | 14.6 ± 0.4 | 67.4 ± 0.2 | 19.3 ± 0.8 | 59.7 ± 1.2 |
| Camphor Wood | |||||||
| untreated | 44.0 ± 0.5 | 18.5 ± 0.4 | 26.3 ± 0.4 | ||||
| A-1%NaOH | 86.9 ± 0.3 | 48.1 ± 0.6 | 95.0 ± 2.2 | 20.1 ± 0.2 | 94.4 ± 1.2 | 24.3 ± 0.5 | 19.5 ± 1.5 |
| A-2%NaOH | 80.9 ± 0.7 | 45.6 ± 0.3 | 84.0 ± 0.9 | 19.1 ± 0.3 | 83.7 ± 1.9 | 23.7 ± 0.2 | 27.0 ± 0.6 |
| A-3%NaOH | 77.2 ± 1.1 | 48.6 ± 0.4 | 85.2 ± 1.1 | 19.9 ± 0.2 | 82.9 ± 0.8 | 23.0 ± 0.5 | 32.4 ± 0.8 |
| A-4%NaOH | 75.4 ± 0.8 | 48.0 ± 0.2 | 82.1 ± 0.5 | 19.7 ± 0.1 | 80.3 ± 0.4 | 22.4 ± 0.4 | 35.6 ± 0.9 |
| A-5%NaOH | 74.4 ± 1.1 | 46.3 ± 0.7 | 78.4 ± 1.4 | 18.7 ± 0.4 | 75.2 ± 1.1 | 22.3 ± 0.5 | 36.7 ± 1.1 |
As displayed in Table 1, the lignin and cellulose contents of untreated herbaceous biomass were lower than those of woody materials, with the lignin content of softwoods lower than that of hardwoods and the cellulose content of hardwoods lower than that of softwoods, which is consistent with previous reports.21 There was no significant change in the xylan content of P. sinese, oak, and camphor wood after A-NaOH pretreatment, while the relative glucan content increased from 47.8%, 41.3%, and 44.0%, respectively, to 55.7%–74.0%, 44.6%–49.6%, and 45.6%–48.1%, respectively. This finding was mainly attributed to the higher removal of lignin by the A-NaOH pretreatment and the higher retention of glucan. The most significant removal of lignin by A-NaOH pretreatment was attributed to the reaction process of the alkali pretreatment reaction process, including lignin removal and hemicellulose dissolution.22 During AFEX treatment, the breakage of diferulate (cross-linking polysaccharides), lignin-ferulate and lignin-diferulate linkages (cross-linking polysaccharides to lignin), as well as other ester bonds is expected to facilitate lignin removal.23 After A-NaOH pretreatment, the lignin content of P. sinese, oak, and camphor wood decreased with increasing NaOH concentration. After A-5% NaOH treatment, the lignin removal of P. sinese, oak and camphor wood reached 84.3%, 59.7%, and 36.7%, respectively. It can be concluded that the ability of A-NaOH to remove lignin is P. sinese (herbaceous) > oak (hardwood) > camphor (softwood). Lignin is a polymer composed of three types of 4-hydroxyphenyl propane structures: p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S).24 The lignin types of different species of biomass differ, mainly in the amount of methoxy.25 Compared to herbaceous biomass, woody materials contain more lignin and have a greater molecular weight and are more compact. Herbaceous and hardwood biomass generally consist of three main units, H, G, and S, whereas softwoods contain mainly G and a small number of H-units. It has been shown that a higher pH readily removes lignin with a high S-unit content.12 Therefore, A-NaOH pretreatment was more likely to delignify P. sinese (herbaceous) and oak (hardwood). The results proved that the NaOH concentration and lignocellulosic biomass species had significant effects on both solid recovery (p < 0.05) and lignin removal (p < 0.05).
3.1.2. FTIR Analysis
FTIR is a reliable method for the determination of functional group changes in lignin compounds.26 The spectral bands at 3418 and 2920 cm–1 of the pretreated P. sinese were associated with O–H and C–H stretching vibrations in alkanes (Figure 1a). The alkali treatment broke the inter- and intramolecular hydrogen bonds and the methylene and methyl groups of cellulose, which is consistent with previous reports.27 The spectral band at 1045 cm–1 is associated with C–O strapping at cellulose, hemicellulose and lignin, as well as C–O–C strapping in cellulose and hemicellulose.28 The pretreated oak with camphor wood showed a new peak at 1126 cm–1 that belongs to the C–O–C asymmetric valence transition (Figure 1b,c). The spectral band at 1329 cm–1 of P. sinese indicated the presence of an S-unit, the band at 1126 cm–1 of oak was due to the C–H vibration of the S-unit at 1329 and 1127 cm–1, no specific peak of the S-unit was observed in camphor lignin, and 1139 cm–1 was due to the vibration of the G-unit. The spectral bands of oak and camphor after A-NaOH pretreatment were similar to those of untreated oak and camphor (Table 1) because the compositional changes of oak and camphor after pretreatment were not significant.
Figure 1.

FTIR curves of lignocellulose samples: (a) Raw with A-NaOH pretreatment of P. sinese; (b) raw with A-NaOH pretreatment of oak; (c) raw with A-NaOH after camphor wood.
3.1.3. Scanning Electron Microscopy (SEM) Analysis
The morphological changes in P. sinese, oak, and camphor wood before and after pretreatment were observed using SEM (see Supporting Information) It was clearly observed that the surfaces of the untreated samples were smooth, and the biomass structures were neatly arranged, while the surfaces became rough with cracks and fragments after pretreatment. The surfaces of the treated samples became more sparse and exposed more cellulose compared to those of the untreated samples. Therefore, more reaction sites were available for subsequent enzymatic hydrolysis, which increases the accessibility of cellulase. Notably, the degree of exposure of the three treated samples of P. sinese (herbaceous) > oak (hardwood) > camphor wood (softwood) were clearly observed by SEM (see Supporting Information)
3.1.4. XRD Analysis
The lignocellulosic substrate is divided into two parts: the crystalline region consists of crystalline cellulose, and the noncrystalline region mainly consists of noncrystalline zone cellulose, hemicellulose, and lignin. Crystallinity is widely believed to play a major role in the degradation of lignin, and the pretreatment process can also have an important effect on lignocellulose crystallinity. The relative crystallinities of the untreated samples and pretreated P. sinese, oak chips, and camphor chips are shown in Table 2. The intensities of the different pretreated samples varied more than those of the untreated samples, although there was no significant change in the peak positions (Figure 2). This indicates that the pretreatment did not affect the crystalline morphology of cellulose; however, the structure of cellulose was altered to different degrees after pretreatment. It can be seen that the relative crystallinities of untreated P. sinese, oak chips, and camphor wood chips were 47.3%, 53.0% and 55.4%, and the relative crystallinities of the substrates increased continuously with the increase of NaOH concentration; the relative crystallinities of P. sinese, oak, and camphor wood were 70.9%, 62.8%, and 60.0% after the A-5% NaOH treatment. The most significant increase in relative crystallinity was observed for P. sinese, with a maximum increase of 23.6%. The increase in crystallinity after pretreatment may be the result of the removal of amorphous fractions of lignin and hemicellulose.29 This finding is consistent with the results of the changes in the main components of the original samples in Table 1.
Table 2. Comparison of Crystallinity Indices (CrI) of Lignocellulosic Biomass.
| CrI % |
|||
|---|---|---|---|
| materials | P. sinese | oak | camphor wood |
| untreated | 47.3 | 53.0 | 55.4 |
| A-1%NaOH | 66.2 | 59.7 | 56.4 |
| A-2%NaOH | 66.7 | 60.6 | 58.0 |
| A-3%NaOH | 67.4 | 61.9 | 59.2 |
| A-4%NaOH | 68.6 | 62.6 | 59.6 |
| A-5%NaOH | 70.9 | 62.8 | 60.0 |
Figure 2.
XRD curves of lignocellulose samples: (a) Raw with A-NaOH pretreatment of P. sinese; (b) raw with A-NaOH pretreatment of oak; (c) raw with A-NaOH after camphor wood.
3.1.5. Nuclear Magnetic Resonance (NMR) Analysis
Untreated and A-NaOH-pretreated samples had four remarkable signals at approximately 63.3, 73.2, 82.9, and 104.6 ppm and three weak signals at approximately 21.2, 56.0, and 172.6 ppm. Some chemical groups of hemicellulose and cellulose corresponded to signals at 63.3 and 104.6 ppm, while 73.2 and 82.9 ppm are representative of partial chemical groups of cellulose, hemicellulose, and lignin.30 The signal at 21.2 ppm represents −CH3 in the acetyl group in hemicellulose, and the signals at 56.0 and 172.6 ppm represent methoxy and ester groups in lignin, respectively.31 The signals at 22.2 and 172.6 ppm disappeared in all the pretreated samples, indicating that A-NaOH pretreatment led to the deconstruction of acetyl and ester groups. In the untreated samples, the signal at 56.0 ppm was stronger in oak and camphor wood than in P. sinese; however, after A-NaOH pretreatment, the signal at 56.0 ppm disappeared in P. sinese, whereas a change was not observed in oak and camphor wood.
The signals of 105–160 ppm were reported to represent mainly lignin aromatic hydrocarbons and phenols.32 After pretreatment with A-NaOH, the signals at 115.8, 134.0, and 146.7 ppm disappeared and diminished at 152.8 ppm in P. sinese, showing that P. sinese removed most of the lignin (Table 1). After A-NaOH pretreatment, the signal of oak at 117.3 ppm disappeared, and 136.4 and 152.1 ppm decreased to 133.4 and 148.1 ppm and broadened, but the signal intensity did not change. The signal of camphor wood at 133.4 ppm narrowed slightly, and the signal intensity at 148.1 ppm showed no significant change. This finding indicates that the A-NaOH pretreatment had a larger effect on the removal of oak lignin than on camphor lignin (see Supporting Information).
3.2. Enzymatic Digestion of Lignocellulose after A-NaOH Pretreatment
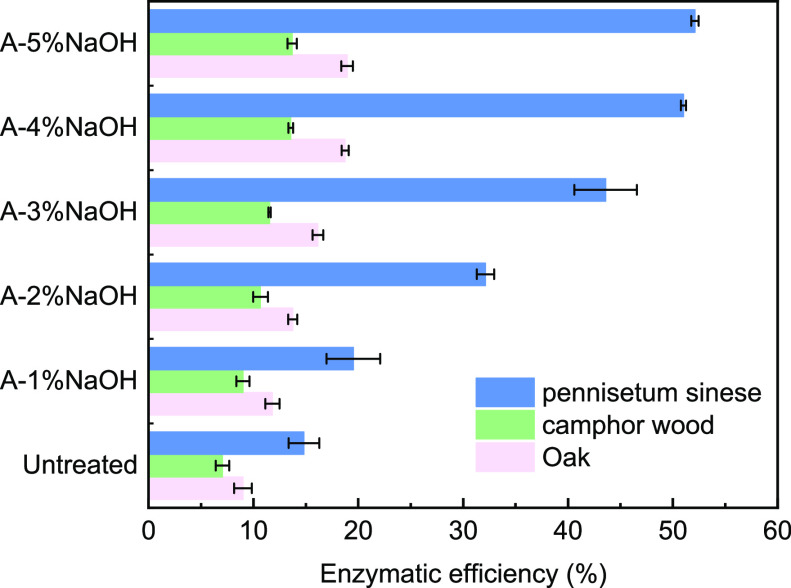
The enzymatic process of lignocellulosic digestion after the A-NaOH treatment is shown in Figure 3. The AFEX during A-NaOH pretreatment did not have a large effect on the lignin removal rate, but it could redistribute the lignin on the cell wall and form a highly porous structure, thus improving the enzymatic efficiency of lignocellulose.33 Additionally, AFEX reduces the adverse effect of lignin on cellulase.34 This alkaline treatment exposes more lignin; since the lignin removal rate affects the enzymatic efficiency, the poor lignin removal rate will adversely affect the enzymatic digestion of lignin.35 After A-NaOH pretreatment, oak and camphor wood maintained a more complete external morphology, and a large amount of lignin remained, which made it difficult for the cellulose to contact the cellulase. It was observed that the enzymatic efficiency of the pretreated samples improved with increasing NaOH concentration, as shown in Figure 3, and the enzymatic efficiency of P. sinese, oak, and camphor wood after A-NaOH pretreatment increased by 36.2%, 9.7%, and 6.5% under 4% NaOH conditions, respectively. This result can also be explained by the composition of A-NaOH pretreated P. sinese, oak, and camphor wood in Table 1, where the glucan, xylan, and lignin contents of P. sinese, oak, and camphor wood were similar at 4–5% NaOH loading. Similar results have been reported in previous studies, where the optimal NaOH of concentration for enzymatic digestion was between 3% and 5%.36 An extremely high NaOH concentration would produce a large amount of black liquor, and both washing of pretreated samples to neutral and treatment of black liquor would result in water waste. It has been reported that the higher the yield of reducing sugars, the more favorable the dark fermentation for bio-H2 production and the higher the bio-H2 production rate.37 Therefore, 4% NaOH was chosen as the optimum concentration for bio-H2 production by enzymatic digestion and dark fermentation.
Figure 3.
Enzymatic efficiency of lignocellulose samples.
3.3. Effect of A-NaOH Pretreatment on Bio-H2 Production
Bio-H2 production by dark fermentation was carried out using untreated P. sinese, oak, and camphor wood and reducing sugar hydrolysates of P. sinese, oak, and camphor wood pretreated with A-4% NaOH as substrates; results are shown in Figure 4. The bio-H2 production of P. sinese, oak, and camphor wood differed significantly, with the difference between the untreated and pretreated samples being more remarkable. As shown in Figure. 4a, under medium temperature conditions, the cumulative bio-H2 production of pretreated P. sinese, oak, and camphor was 152.3, 99.1, and 76.9 mL/g TS, respectively, which was higher than the control group by 88.4, 47.5, and 31.7 mL/g TS, respectively.
Figure 4.

Effect of A-NaOH pretreatment on bio-H2: (a) cumulative hydrogen production of untreated samples and samples after A-NaOH treatment; (b) hydrogen production rate of untreated samples and samples after A-NaOH treatment.
The hydrogen production rate curve exhibited a similar trend (Figure. 4b). The hydrogen production rate increased sharply during the first 18 h and then decreased from 18 to 54 h. The maximum hydrogen production rate increased by 5.3 mL/g TS·h for pretreated P. sinese compared to the control, and by 2.4 mL/g TS·h for both pretreated oak and camphor wood compared to controls.
As shown in Figure 4a, the bio-H2 production and bio-H2 production rates of all three samples increased after pretreatment, and the pretreated herbs produced more bio-H2 than woody ones. This finding may be due to pretreatment increasing the surface area of cellulose and hemicellulose of the sample and the removal of some lignin, both of which stimulate enzymes to cleave the cellulose bonds into sugars, which promotes dark fermentation.38 In contrast, woody species contain more lignin than herbaceous species, and the presence of lignin, which has a significant persistence during biodegradation, is not conducive to dark fermentation.39 The above results further indicate that A-NaOH pretreatment can successfully prepare bio-H2 from lignocellulosic biomass. The direct conversion of raw fibrous biomass to bio-H2 is poor because of its protective structure. Dark fermentation is also affected by metabolic pathways, substrate types, and byproducts (e.g., volatile acids and ethanol).
The dark fermentation of hydrogen production from different biomasses with different pretreatment methods in the literature was compared with the present study. The bio-H2 production of humulus scandens, at a cellulase dosage of 0.203 g/g TS, an inoculum of 42.6%, and an initial pH of 6.59, was 64.08 mL/g TS.40 The bio-H2 production of wood dust mahogany was 84 mL/g TS after ozone pretreatment at pH 11 in 45 min, and at pH 3 in 15 min, the bio-H2 production was 52 mL/g TS.41 The bio-H2 production of the empty fruit bunch of oil palm pretreated with enzyme was 283.91 mL/g TS.42 In comparison with previous studies, P. sinese treated with A-NaOH obtained a higher bio-H2 production (p < 0.05), while the bio-H2 production of oak and camphor wood was at an average level.
3.4. A-NaOH Pretreatment Effects on the Bio-H2 Production Pathway of Dark Fermentation
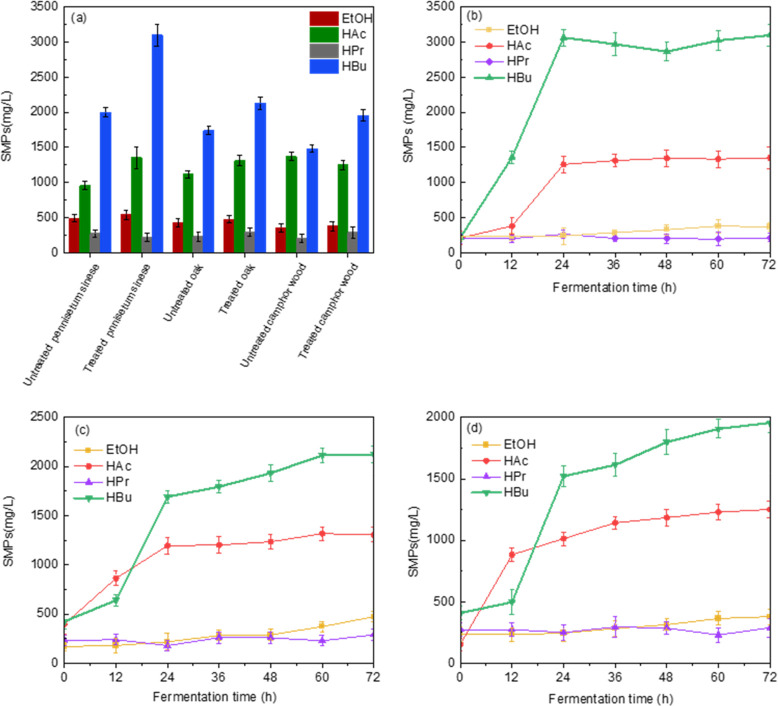
Changes in SMPs are important indicators to evaluate the process of dark fermentation for bio-H2 production.43 SMPs (VFAs and EtOH) were monitored after fermentation to assess the main metabolic pathways of dark fermentation. The reducing sugar hydrolysates of all samples produced bio-H2, but there were significant differences in the distribution and production of SMPs during dark fermentation (Figure 5a). The growth trend of the SMPs was not obvious in any of the samples during the initial 12 h period. The microbial activity increased during 12–36 h, and bio-H2 production and the concentration of SMPs increased significantly. After 36 h, the concentration of SMPs stabilized.
Figure 5.
A-NaOH pretreatment effects on soluble microbial products (SMPs): (a) SMPs of unpretreated samples and A-NaOH pretreated samples; (b) SMPs of A-NaOH pretreated P. sinese; (c) SMPs of A-NaOH pretreated oak; (d) SMPs of A-NaOH pretreated camphor wood.
The major SMPs in the fermentation broth of both the A-NaOH pretreated sample and the original sample were acetate (HAc) and butyrate (HBu), whereas the propionate (HPr) and ethanol (EtOH) contents were lower. This finding indicated that the dark fermentation of all samples was of the HBu type.44 As shown (Figure 5b–d) at 12–72 h, the concentrations of HAc and HBu showed an increasing trend, while the concentrations of HPr and EtOH did not change significantly. It has been reported that the accumulation of volatile fatty acid (VFA) concentration promotes the production of bio-H2.45 It has also been shown that the high production of HAc and HBu promotes the production of bio-H2, whereas HPr consumes bio-H2.46 In addition, A-NaOH helps to maintain a weak alkaline fermentation acid production condition, which also facilitates the production of VFAs. The results showed that the concentrations of HBu and HAc were higher and the concentrations of HPr were lower, and the maximum concentrations of HAc and HBu were significantly higher in pretreated oak and camphor wood, while the concentrations of HPr were lower than those in pretreated oak and camphor wood, and the concentrations of HBu in pretreated oak were significantly higher than those in pretreated camphor wood. It is noteworthy that during dark fermentation, the VFAs of the pretreated P. sinese, oak, and camphor wood increased by 39.9%, 19.5%, and 13.7%, respectively, compared to the untreated samples (Figure. 5a). Therefore, A-NaOH pretreatment significantly promoted the production of total VFAs during dark fermentation for bio-H2 production, and this effect was more pronounced in P. sinese (p < 0.05).
3.5. Bio-H2 Production Kinetic Analysis
A modified Gompertz model (R2 > 0.99) was used to estimate the kinetic parameters (Pm, Rm, λ) of the bio-H2 production process. The fitted curves are shown in Figure 4a, and the kinetic parameters are listed in Table 3. The correlation coefficient (R2) of the modified Gompertz model was 0.9966–0.9988, respectively. The R2 value was greater than 0.99, indicating that the variation in H2 yield could be attributed to the improved Gompertz model, and the improved Gompertz model was suitable for describing the bio-H2 production during dark fermentation.
Table 3. Gompertz Model Fitted to the Parameters of Bio-H2 Production after A-NaOH Pretreatment.
| reactor | materials | P (mL/g) | Rm (mL/g/h) | λ | R2 (%) |
|---|---|---|---|---|---|
| 1 | untreated P. sinese | 64.81 | 3.07 | 7.30 | 99.75 |
| 2 | treated P. sinese | 153.31 | 7.21 | 9.20 | 99.83 |
| 3 | untreated oak | 52.04 | 2.83 | 7.73 | 99.88 |
| 4 | treated oak | 100.70 | 4.22 | 7.46 | 99.70 |
| 5 | untreated camphor wood | 45.90 | 2.14 | 6.72 | 99.66 |
| 6 | treated camphor wood | 77.66 | 4.23 | 8.21 | 99.74 |
4. Conclusion
In this study, P. sinese, oak, and camphor wood were pretreated with A-NaOH to achieve efficient production of bio-H2 from lignocellulosic biomass. FTIR and NMR showed that A-NaOH treatment could effectively break the link between lignin and hemicellulose and remove lignin, and that there were differences in the composition of herbaceous and woody lignin. Meanwhile, XRD and SEM showed that the pretreatment effectively removed amorphous components, and the lignocellulose surface became rough. The enzymatic efficiency was significantly increased by 36.2%, 9.7%, and 6.5%, respectively. In addition, A-NaOH pretreatment significantly increased the bio-H2 production from dark fermentation, which helped to maintain a neutral acid-producing environment, promote the conversion of organic matter to liquid, and improve the yield of VFAs. The Gompertz kinetic model gives good results, as seen from the values of R2 0.9966–0.9988. The results suggest that A-NaOH pretreatment can be an effective biorefinery method.
Acknowledgments
This research was funded by Major Projects of Tackling Key Industrial of Shandong’s New Traditional Kinetic Energy Conversion and Shandong Province Key R&D Program (Major Science and Technology Innovation Project): 2021CXGC010802.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01302.
SEM of lignocellulose samples (Figure S1); NMR of lignocellulose samples Figure S2) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Yadav M.; Paritosh K.; Vivekanand V. Lignocellulose to bio-hydrogen: An overview on recent developments. Int. J. Hydrogen Energy 2020, 45 (36), 18195–18210. 10.1016/j.ijhydene.2019.10.027. [DOI] [Google Scholar]
- Liu C.; Xiao Y.; Xia X. X.; Zhao X. Q.; Peng L.; Srinophakun P.; Bai F. W. J. B. A. Cellulosic ethanol production: Progress, challenges and strategies for solutions. Biotechnology Advances 2019, 37 (3), 491–504. 10.1016/j.biotechadv.2019.03.002. [DOI] [PubMed] [Google Scholar]
- Yogalakshmi K. N.; Devi P.; Sivashanmugam P.; Kavitha S.; Yukesh Kannah R.; Varjani S.; AdishKumar S.; Kumar G.; Rajesh Banu J. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2022, 286, 131824. 10.1016/j.chemosphere.2021.131824. [DOI] [PubMed] [Google Scholar]
- Ahorsu R.; Medina F.; Constantí M. J. E. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. 10.3390/en11123366. [DOI] [Google Scholar]
- Chatalova L.; Balmann A. J. J. o. C. P. The hidden costs of renewables promotion: The case of crop-based biogas. Journal of Cleaner Production 2017, 168, 893–903. 10.1016/j.jclepro.2017.09.031. [DOI] [Google Scholar]
- Lupoi J. S.; Singh S.; Simmons B. A.; Henry R. J. J. B. R. Assessment of Lignocellulosic Biomass Using Analytical Spectroscopy: An Evolution to High-Throughput Techniques. Energies 2014, 7 (1), 1–23. 10.1007/s12155-013-9352-1. [DOI] [Google Scholar]
- Anu; Kumar A.; Rapoport A.; Kunze G.; Kumar S.; Singh D.; Singh B. Multifarious pretreatment strategies for the lignocellulosic substrates for the generation of renewable and sustainable biofuels: A review. Renewable Energy 2020, 160, 1228–1252. 10.1016/j.renene.2020.07.031. [DOI] [Google Scholar]
- Meng X.; Bhagia S.; Wang Y.; Zhou Y.; Pu Y.; Dunlap J. R.; Shuai L.; Ragauskas A. J.; Yoo C. G. Effects of the advanced organosolv pretreatment strategies on structural properties of woody biomass. Industrial Crops and Products 2020, 146, 112144. 10.1016/j.indcrop.2020.112144. [DOI] [Google Scholar]
- Baruah J.; Nath B. K.; Sharma R.; Kumar S.; Deka R. C.; Baruah D. C.; Kalita E.. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Frontiers in Energy Research 2018, 6, 10.3389/fenrg.2018.00141. [DOI] [Google Scholar]
- Hoang A. T.; Nizetic S.; Ong H. C.; Mofijur M.; Ahmed S. F.; Ashok B.; Bui V. T. V.; Chau M. Q. Insight into the recent advances of microwave pretreatment technologies for the conversion of lignocellulosic biomass into sustainable biofuel. Chemosphere 2021, 281, 130878. 10.1016/j.chemosphere.2021.130878. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Shao Q.; Chundawat S. P. S. Recent advances on ammonia-based pretreatments of lignocellulosic biomass. Bioresource Technology 2020, 298, 122446. 10.1016/j.biortech.2019.122446. [DOI] [PubMed] [Google Scholar]
- Kim J. S.; Lee Y. Y.; Kim T. H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresource Technology 2016, 199, 42–48. 10.1016/j.biortech.2015.08.085. [DOI] [PubMed] [Google Scholar]
- Mankar A. R.; Pandey A.; Modak A.; Pant K. K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresource Technology 2021, 334, 125235. 10.1016/j.biortech.2021.125235. [DOI] [PubMed] [Google Scholar]
- Abdul P. M.; Jahim J. M.; Harun S.; Markom M.; Lutpi N. A.; Hassan O.; Balan V.; Dale B. E.; Mohd Nor M. T. Effects of changes in chemical and structural characteristic of ammonia fibre expansion (AFEX) pretreated oil palm empty fruit bunch fibre on enzymatic saccharification and fermentability for biohydrogen. Bioresource Technology 2016, 211, 200–8. 10.1016/j.biortech.2016.02.135. [DOI] [PubMed] [Google Scholar]
- Kumari D.; Singh R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renewable and Sustainable Energy Reviews 2018, 90, 877–891. 10.1016/j.rser.2018.03.111. [DOI] [Google Scholar]
- Motte J. C.; Trably E.; Hamelin J.; Escudie R.; Bonnafous A.; Steyer J. P.; Bernet N.; Delgenes J. P.; Dumas C. Total solid content drives hydrogen production through microbial selection during thermophilic fermentation. Bioresource Technology 2014, 166, 610–5. 10.1016/j.biortech.2014.05.078. [DOI] [PubMed] [Google Scholar]
- Jiang D.; Ge X.; Zhang T.; Chen Z.; Zhang Z.; He C.; Zhang Q.; Li Y. Effect of alkaline pretreatment on photo-fermentative hydrogen production from giant reed: Comparison of NaOH and Ca(OH)2. Bioresource Technology 2020, 304, 123001. 10.1016/j.biortech.2020.123001. [DOI] [PubMed] [Google Scholar]
- Dong L.; Cao G.; Zhao L.; Liu B.; Ren N. Alkali/urea pretreatment of rice straw at low temperature for enhanced biological hydrogen production. Bioresource Technology 2018, 267, 71–76. 10.1016/j.biortech.2018.05.055. [DOI] [PubMed] [Google Scholar]
- Miner G.Standard Methods for the Examination of Water and Wastewater; 2006. (Vol. (1), ), p 130. [Google Scholar]
- Sluiter A.; Hames B.; Ruiz R.; Scarlata C.; Crocker D.. Determination of structural carbohydrates and lignin in biomass determination of structural carbohydrates and lignin in biomass. NREL/TP-510-42618; National Renewable Energy Laboratory: USA, 2012.
- Liao J. J.; Latif N. H. A.; Trache D.; Brosse N.; Hussin M. H. Current advancement on the isolation, characterization and application of lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. 10.1016/j.ijbiomac.2020.06.168. [DOI] [PubMed] [Google Scholar]
- Kim T. H.Pretreatment of Lignocellulosic Biomass. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers; John Wiley & Sons, Inc., 2013. [Google Scholar]
- Chundawat S. P. S.; Donohoe B. S.; da Costa Sousa L.; Elder T.; Agarwal U. P.; Lu F.; Ralph J.; Himmel M. E.; Balan V.; Dale B. E. Multi-scale visualization and characterization of lignocellulosic plant cell wall deconstruction during thermochemical pretreatment. Energy Environ. Sci. 2011, 4 (3), 973. 10.1039/c0ee00574f. [DOI] [Google Scholar]
- Faleva A. V.; Belesov A. V.; Kozhevnikov A. Y.; Falev D. I.; Chukhchin D. G.; Novozhilov E. V. Analysis of the functional group composition of the spruce and birch phloem lignin. Int. J. Biol. Macromol. 2021, 166, 913–922. 10.1016/j.ijbiomac.2020.10.248. [DOI] [PubMed] [Google Scholar]
- Xu C.; Arancon R.; Labidi J.; Luque R. J. C. S. R. Lignin depolymerisation strategies: towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43 (22), 7485–7500. 10.1039/C4CS00235K. [DOI] [PubMed] [Google Scholar]
- Liu C. F.; Sun R. C.; Qin M. H.; Zhang A. P.; Ren J. L.; Ye J.; Luo W.; Cao Z. N. J. B. T. Succinoylation of sugarcane bagasse under ultrasound irradiation. Bioresour. Technol. 2008, 99 (5), 1465–1473. 10.1016/j.biortech.2007.01.062. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Jiang D.; Zhang H.; Lee D. J.; Zhang Z.; Zhang Q.; Jing Y.; Zhang Y.; Xia C. Effects of different pretreatment methods on the structural characteristics, enzymatic saccharification and photo-fermentative bio-hydrogen production performance of corn straw. Bioresource Technology 2020, 304, 122999. 10.1016/j.biortech.2020.122999. [DOI] [PubMed] [Google Scholar]
- Liu C. F.; Sun R. C.; Qin M. H.; Zhang A. P.; Ren J. L.; Ye J.; Luo W.; Cao Z. N. Succinoylation of sugarcane bagasse under ultrasound irradiation. Bioresource Technology 2008, 99 (5), 1465–73. 10.1016/j.biortech.2007.01.062. [DOI] [PubMed] [Google Scholar]
- Zheng J.; Choo K.; Bradt C.; Lehoux R.; Rehmann L. Enzymatic hydrolysis of steam exploded corncob residues after pretreatment in a twin-screw extruder. Biotechnology Reports 2014, 3, 99–107. 10.1016/j.btre.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.; Wang X.; Zhang Y.; Yu Q.; Tan X.; Zhuang X.; Yuan Z. Effect of sodium hydroxide pretreatment on physicochemical changes and enzymatic hydrolysis of herbaceous and woody lignocelluloses. Industrial Crops and Products 2020, 145, 112145. 10.1016/j.indcrop.2020.112145. [DOI] [Google Scholar]
- Wang W.; Tan X.; Yu Q.; Wang Q.; Qi W.; Zhuang X.; Wang Z.; Yuan Z. Effect of stepwise lignin removal on the enzymatic hydrolysis and cellulase adsorption. Industrial Crops and Products 2018, 122, 16–22. 10.1016/j.indcrop.2018.05.053. [DOI] [Google Scholar]
- Davila I.; Remon J.; Gullon P.; Labidi J.; Budarin V. Production and characterization of lignin and cellulose fractions obtained from pretreated vine shoots by microwave assisted alkali treatment. Bioresource Technology 2019, 289, 121726. 10.1016/j.biortech.2019.121726. [DOI] [PubMed] [Google Scholar]
- Karki B.; Muthukumarappan K.; Wang Y.; Dale B.; Balan V.; Gibbons W. R.; Karunanithy C. Physical characteristics of AFEX-pretreated and densified switchgrass, prairie cord grass, and corn stover. Biomass and Bioenergy 2015, 78, 164–174. 10.1016/j.biombioe.2015.04.018. [DOI] [Google Scholar]
- Limayem A.; Ricke S. C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38 (4), 449–467. 10.1016/j.pecs.2012.03.002. [DOI] [Google Scholar]
- Leu S.-Y.; Zhu J. Y. Substrate-Related Factors Affecting Enzymatic Saccharification of Lignocelluloses: Our Recent Understanding. BioEnergy Research 2013, 6 (2), 405–415. 10.1007/s12155-012-9276-1. [DOI] [Google Scholar]
- Shi Z.; Liu Y.; Xu H.; Yang Q.; Xiong C.; Kuga S.; Matsumoto Y. Facile dissolution of wood pulp in aqueous NaOH/urea solution by ball milling pretreatment. Industrial Crops and Products 2018, 118, 48–52. 10.1016/j.indcrop.2018.03.035. [DOI] [Google Scholar]
- Zhang Y.; Yuan J.; Guo L. Enhanced bio-hydrogen production from cornstalk hydrolysate pretreated by alkaline-enzymolysis with orthogonal design method. Int. J. Hydrogen Energy 2020, 45 (6), 3750–3759. 10.1016/j.ijhydene.2019.07.234. [DOI] [Google Scholar]
- Sołowski G.; Konkol I.; Cenian A. Production of hydrogen and methane from lignocellulose waste by fermentation. A review of chemical pretreatment for enhancing the efficiency of the digestion process. Journal of Cleaner Production 2020, 267, 121721. 10.1016/j.jclepro.2020.121721. [DOI] [Google Scholar]
- Kumar G.; Bakonyi P.; Periyasamy S.; Kim S. H.; Nemestóthy N.; Bélafi-Bakó K. Lignocellulose biohydrogen: Practical challenges and recent progress. Renewable and Sustainable Energy Reviews 2015, 44, 728–737. 10.1016/j.rser.2015.01.042. [DOI] [Google Scholar]
- Zhang Y.; Zhang T.; Zhang Z.; Tahir N.; Zhang Q. Biohydrogen production from Humulus scandens by dark fermentation: Potential evaluation and process optimization. Int. J. Hydrogen Energy 2020, 45 (6), 3760–3768. 10.1016/j.ijhydene.2019.07.233. [DOI] [Google Scholar]
- Praptyana I. R.Budiyono, Biohydrogen production from wood dust mahogany (Swietenia mahagony) by dark fermentation using Enterobacter aerogenes: Effect of ozone pretreatment time and pH. Materials Today: Proceedings 2022.
- Gonzales R. R.; Kim J. S.; Kim S.-H. Optimization of dilute acid and enzymatic hydrolysis for dark fermentative hydrogen production from the empty fruit bunch of oil palm. Int. J. Hydrogen Energy 2019, 44 (4), 2191–2202. 10.1016/j.ijhydene.2018.08.022. [DOI] [Google Scholar]
- Zhang J.; Fan C.; Zhang H.; Wang Z.; Zhang J.; Song M. Ferric oxide/carbon nanoparticles enhanced bio-hydrogen production from glucose. Int. J. Hydrogen Energy 2018, 43 (18), 8729–8738. 10.1016/j.ijhydene.2018.03.143. [DOI] [Google Scholar]
- Lo Y. C.; Huang C.-Y.; Fu T.-N.; Chen C.-Y.; Chang J.-S. Fermentative hydrogen production from hydrolyzed cellulosic feedstock prepared with a thermophilic anaerobic bacterial isolate. Int. J. Hydrogen Energy 2009, 34 (15), 6189–6200. 10.1016/j.ijhydene.2009.05.104. [DOI] [Google Scholar]
- Mohan S.; Mohanakrishna G.; Reddy S.; Raju B.; Rao K.; Sarma P. Self-immobilization of acidogenic mixed consortia on mesoporous material (SBA-15) and activated carbon to enhance fermentative hydrogen production. Int. J. Hydrogen Energy 2008, 33 (21), 6133–6142. 10.1016/j.ijhydene.2008.07.096. [DOI] [Google Scholar]
- Zhao W.; Zhang J.; Zhang H.; Yang M.; Zang L. Comparison of mesophilic and thermophilic biohydrogen production amended by nickel-doped magnetic carbon. Journal of Cleaner Production 2020, 270, 122730. 10.1016/j.jclepro.2020.122730. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.