Abstract
Atezolizumab plus bevacizumab therapy has high response rates in patients with advanced hepatocellular carcinoma (HCC). It has been reported that HCC with immune exclusion associated with the signal activation of WNT/β-catenin is resistant to immune checkpoint inhibitors; however, to the best of our knowledge, the effectiveness of atezolizumab plus bevacizumab for HCC with WNT/β-catenin signal activation has not been reported. The present study aimed to analyze the efficacy of atezolizumab plus bevacizumab for HCC with WNT/β-catenin signal activation. A total of 24 patients who underwent liver tumor biopsy for HCC were classified into WNT/β-catenin signal activation and inactivation groups according to the expression levels of β-catenin and glutamine synthetase, which are indicative of WNT/β-catenin signal activation. The differences in the clinical responses to treatment between the groups were analyzed. A total of 15 patients had HCC with WNT/β-catenin signal activation, whereas 9 patients had HCC with WNT/β-catenin signal inactivation. There were no significant differences between both groups regarding objective responses (P=0.519) and disease control (P=0.586). In the WNT/β-catenin signal activation group, the median progression-free survival rate was 6.9 months compared with 6.2 months in the WNT/β-catenin signal inactivation group (P=0.674). Although a small number of patients was included in the present study, the present findings suggested that the efficacy of atezolizumab plus bevacizumab might be unaffected by WNT/β-catenin signal activation.
Keywords: hepatocellular carcinoma, atezolizumab plus bevacizumab, WNT/β-catenin signal activation, β-catenin, glutamine synthetase
Introduction
A previous study reported that 85–90% of cases of primary liver cancer are hepatocellular carcinoma (HCC); globally, HCC is the sixth most frequent neoplasm and the third most frequent cause of deaths related to cancer, with approximately 900,000 new cases and 830,000 deaths in 2020 (1,2). Recent advances in systemic chemotherapy for advanced HCC, including immune checkpoint inhibitors (ICIs) and molecular targeted agents, have improved patient prognosis, and the appropriate choice of chemotherapy may further improve the prognosis (3–7) Therefore, it is important to select agents suitable for the personalized treatment of HCC.
Recently, the IMbrave150 trial showed that atezolizumab plus bevacizumab prolongs progression-free survival (PFS) and overall survival (OS) compared with sorafenib in patients with advanced HCC (3). Hence, atezolizumab plus bevacizumab is becoming the preferred first-line systemic chemotherapy.
Modern HCC treatment has focused on disease subclass classification according to WNT/β-catenin mutations. Approximately 40% of HCC cases harbor mutations of WNT/β-catenin that result in the immune microenvironment lacking immune cell filtration, so-called ‘immune exclusion’ or ‘non-inflamed cold’ in HCC (8,9). Furthermore, HCC with immune exclusion associated with WNT/β-catenin signal activation is resistant to ICIs (10–12). Therefore, it is important to identify the subclass of immune condition in HCC induced by WNT/β-catenin signal activation prior to chemotherapy. We previously reported that the efficacy of lenvatinib did not differ between patients with iso-high- or low-intensity HCC, among whom some may have had WNT/β-catenin signal activation, in gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid-enhanced magnetic resonance imaging (EOB-MRI) during the hepatobiliary phase (13). However, the effectiveness of atezolizumab plus bevacizumab for HCC with WNT/β-catenin signal activation has not been reported. Here, we aimed to analyze the efficacy of atezolizumab plus bevacizumab for HCC with WNT/β-catenin signal activation in this study.
Materials and methods
Patients
This prospective single-center study analyzed atezolizumab plus bevacizumab efficacy for HCC with WNT/β-catenin mutations. Fifty-five patients received atezolizumab plus bevacizumab for non-resectable HCC at Iizuka Hospital from December 2020 to December 2021. We excluded 22 patients who did not undergo liver tumor biopsy prior to atezolizumab plus bevacizumab administration and 9 patients who were observed for <6 weeks. In total, we evaluated 24 patients (Fig. 1). The present study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the ethics committee of Iizuka Hospital (approval no. 22008). We applied the opt-out method to obtain consent for this study.
Figure 1.
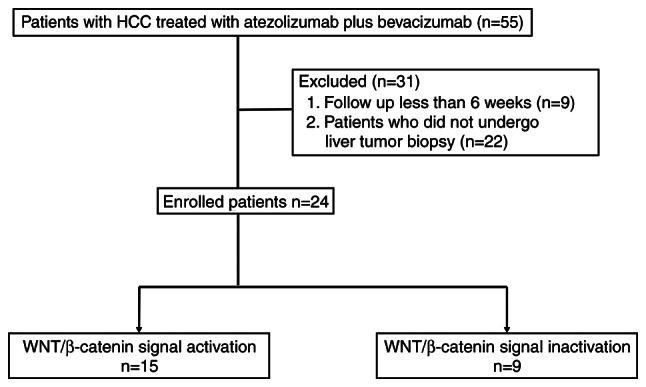
Recruitment flow chart. HCC, hepatocellular carcinoma.
Albumin-bilirubin (ALBI) score
We evaluated liver function using the ALBI score, which was calculated as follows: ALBI score = log10[T-Bil(mg/dl)x17.1]x0.66+[ALB(g/dl)x10]x-0.085, where T-Bil is total bilirubin and ALB is serum albumin level (14).
Treatment protocol
Patients received atezolizumab at a dose of 1,200 mg and bevacizumab at a dose of 7.5 mg/kg intravenously every 3 weeks. The protocols in the IMbrave150 trial were defined by Chugai Co., Ltd. (3). Treatment was continued until disease progression or the development of intolerable adverse events.
Evaluation of effectiveness
Computed tomography (CT) and magnetic resonance imaging (MRI) were used to determine the therapeutic effectiveness every 6–12 weeks after the start of treatment. Antitumor responses were evaluated by the attending physicians according to modified RECIST version 1.1 (15). Complete response (CR), partial response (PR), or stable disease (SD) persisting for ≥4 months were defined as the disease control rate (DCR). The objective response rate (ORR) was defined as PR+CR. Patients were followed up every 3 weeks and treatment was continued until disease progression (PD) or intolerable adverse events.
Immunohistochemical (IHC) analyses
Immunostaining was performed as per the procedure in our previous report (13). Liver tumor biopsy samples fixed with 10% formalin were paraffin-embedded at room temperature for 10–48 h. Serial sections (5-µm) were cut from paraffin blocks and stained with hematoxylin-eosin. The presence of glutamine synthetase (GS) and β-catenin was determined by IHC with the following primary antibodies: monoclonal mouse anti-human β-catenin (#610153; 1:300; BD Biosciences) or monoclonal mouse anti-human GS (#GS-6; 1:500; Millipore). A Bond Polymer System was used to develop reactions (Leica Biosystems) related to secondary antibodies. β-catenin staining in the nucleus is indicative of an activating mutation in the catenin β-1 (CTNNB1) gene (16) and strong GS diffuse staining is indicative of the constitutive activation of WNT/β-catenin signaling, which is associated with β-catenin mutations (17). Therefore, the presence of β-catenin nuclear staining in ≥5% of tumor cells (18) or strong diffuse GS staining in ≥50% of tumor cells were considered to determine the activation of WNT/β-catenin signaling, as previously reported (19,20).
Statistical analysis
JMP Pro Version 11 statistical software was used for all analyses (SAS Institute Inc.). Results were shown as the median (interquartile range). Significant differences between groups were examined by Fisher's exact and Mann-Whitney U test. Kaplan-Meier analysis was performed for the statistical analyses of OS and PFS; significant differences in OS and PFS were determined by log-rank analysis. Statistical significance was determined when P<0.05.
Results
Patient characteristics
The characteristics of 24 patients enrolled in this study are shown in Table I. There were 15 patients with WNT/β-catenin signal activation and 9 patients with no WNT/β-catenin signal inactivation. One patient was Barcelona Clinic Liver Cancer (BCLC) stage A, 8 were stage B, and 12 were stage C in the WNT/β-catenin signal activation group. In addition, there were three patients with stage B and six with stage C in the WNT/β-catenin signal inactivation group (P=0.328). Age, sex, etiology, Child-Pugh grade, ALBI score, tumor size, number of intrahepatic lesions, microvascular invasion, extrahepatic spread, serum α-fetoprotein levels, and vitamin K absence or antagonist-II were similar between groups.
Table I.
Baseline characteristics of patients.
| Characteristics | All | WNT/β-catenin signal activation | WNT/β-catenin signal inactivation | P-value |
|---|---|---|---|---|
| Number | 24 | 15 | 9 | |
| Age, years | 72.0 (63.8-80.8) | 72.0 (63.0-84.0) | 71.0 (64.0-79.0) | 0.8818 |
| Sex, n (male/female) | 20/4 | 13/2 | 7/2 | 0.5700 |
| Max tumor size, cm | 5.0 (3.8-8.1) | 5.0 (3.5-8.3) | 4.9 (1.9-7.4) | 0.3508 |
| Number of intrahepatic lesion >5, n (%) | 11 (45.8) | 9 (60.0) | 2 (22.2) | 0.0721 |
| MVI positive, n (%) | 6 (25.0) | 4 (26.7) | 2 (22.2) | 0.8077 |
| EHS positive, n (%) | 5 (20.8) | 2 (13.3) | 3 (33.3) | 0.2495 |
| Child-Pugh score, n (%) | 0.3941 | |||
| Child-Pugh score 5A | 15 (62.5) | 10 (66.7) | 5 (55.6) | |
| Child-Pugh score 6A | 3 (12.5) | 2 (13.3) | 1 (11.1) | |
| Child-Pugh score >7 | 6 (25.0) | 3 (20.0) | 3 (33.3) | |
| Alb, g/dl | 3.7 (3.1-4.0) | 3.6 (3.1-4.0) | 3.7 (3.2-4.0) | 0.9563 |
| T.Bil, g/dl | 1.0 (0.5-1.4) | 1.0 (0.6-1.2) | 0.8 (0.5-1.7) | 0.8127 |
| ALBI score | −2.36 (−2.60 to −1.93) | −2.33 (−2.61 to −1.91) | −2.39 (−2.63 to −1.79) | 0.9734 |
| BCLC stage, n (%) | 0.3282 | |||
| A | 1 (4.1) | 1 (6.7) | 0 (0.0) | |
| B | 11 (45.8) | 8 (53.3) | 3 (33.3) | |
| C | 12 (50.0) | 6 (40.0) | 6 (66.7) | |
| Treatment lines, n (%) | 0.0767 | |||
| 1 | 19 (79.2) | 12 (80.0) | 7 (77.8) | |
| 2 | 3 (12.5) | 3(20.0) | 0 (0.0) | |
| >3 | 2 (8.3) | 0 (0.0) | 2 (22.2) | |
| Tumor marker | ||||
| AFP, ng/ml | 55.2 (5.1-832.7) | 9.1 (4.1-922.4) | 232.0 (5.2-891.2) | 0.4203 |
| PIVKA-II, mAU/ml | 865.0 (67.0-7,480.0) | 557.0 (88.0-6,676.0) | 1,102.4 (55.5-7,903.5) | 0.4766 |
| Follow-up period, months | 8.4 (6.8-11.7) | 8.9 (6.8-10.7) | 8.4 (6.0-11.9) | 0.9870 |
Data are presented as the median and interquartile range, or number (%). Significant differences between groups were examined by Fisher's exact and Mann-Whitney U-test. MVI, microvascular invasion; EHS, extrahepatic spread; Alb, albumin; T.Bil, total bilirubin; ALBI score, albumin-bilirubin score; BCLC stage, Barcelona Clinic liver cancer stage; AFP, α-fetoprotein; PIVKA-II, vitamin K absence or antagonist-II.
Immunohistochemistry of β-catenin and glutamine synthetase in HCC tissues
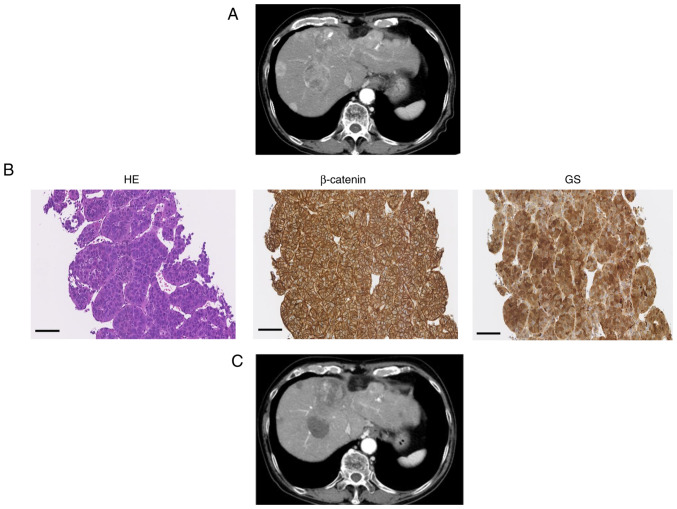
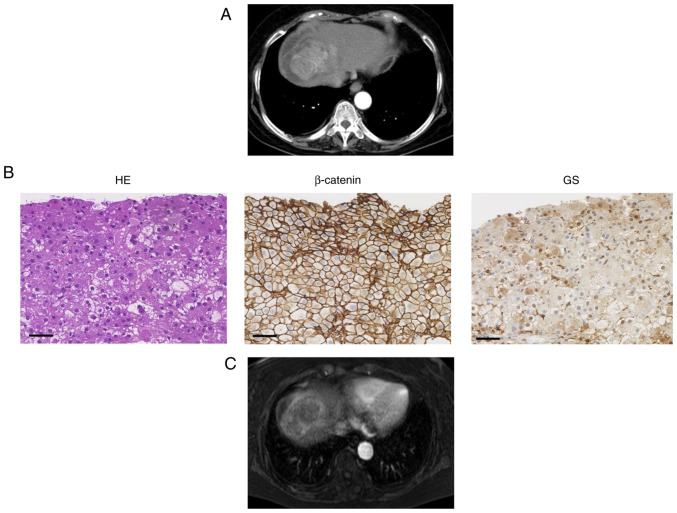
The expression of β-catenin and GS were assessed by IHC before atezolizumab plus bevacizumab therapy. Typical cases are presented in Figs. 2 and 3. Case 1 was a 76-year-old man with hepatitis C virus-related unresectable multiple HCC (Fig. 2). The immunostaining of this moderately differentiated HCC demonstrated WNT/β-catenin signal activation. After administration of atezolizumab plus bevacizumab, CT imaging of liver demonstrated decreased size and enhancement in the arterial phase of tumor, indicating PR. Case 2 was a 76-year-old woman with hepatitis B virus-related advanced HCC (Fig. 3). The specimen was diagnosed as moderately differentiated HCC and immunostaining revealed WNT/β-catenin signal inactivation. MRI imaging demonstrated decreased size and enhancement in the arterial phase, indicating PR.
Figure 2.
Typical case 1 (WNT/β-catenin signal activation). (A) CT image of early arterial phase before treatment. (B) HE staining of liver sections (magnification, ×200; black scale bar, 100 µm). β-catenin and GS staining of liver tissue was performed to evaluate WNT/β-catenin signal activation (magnification, ×200; black scale bar, 100 µm). (C) CT image of the early arterial phase for assessment of treatment efficacy. CT, computed tomography; GS, glutamine synthetase.
Figure 3.
Typical case 2 (WNT/β-catenin signal inactivation). (A) CT image of early arterial phase before treatment. (B) HE staining of liver sections (magnification, ×200; black scale bar, 100 µm). β-catenin and GS staining of liver tissue was performed to evaluate WNT/β-catenin signal activation (magnification, ×200; black scale bar, 100 µm). (C) MRI in the arterial phase for assessment of treatment efficacy. CT, computed tomography; MRI, magnetic resonance imaging; GS, glutamine synthetase.
Effect of atezolizumab plus bevacizumab in the WNT/β-catenin signal activation group vs. the inactivation group
The ORR (CR+PR) was 7/15 (45.7%) in the WNT/β-catenin signal activation group and 3/9 (33.3%) in the inactivation group (P=0.519). The DCR (CR+PR+SD) was 10/15 (66.7%) and 5/9 (55.6%) in the WNT/β-catenin signal activation and inactivation groups, respectively (P=0.586) (Table II). Therefore, WNT/β-catenin signal activation had no effect on the efficacy of atezolizumab plus bevacizumab.
Table II.
Comparison of the response to atezolizumab plus bevacizumab between the WNT/β-catenin signal activation and inactivation groups.
| Response | All, n (%) (n=24) | WNT/β-catenin signal activation, n (%) (n=15) | WNT/β-catenin signal inactivation, n (%) (n=9) | P-value |
|---|---|---|---|---|
| Overall response | 0.4299 | |||
| CR | 1 (4.2) | 0 (0.0) | 1 (11.1) | |
| PR | 9 (37.5) | 7 (46.7) | 2 (22.2) | |
| SD | 5 (20.8) | 3 (20.0) | 2 (22.2) | |
| PD | 9 (37.5) | 5 (33.3) | 4 (44.5) | |
| ORR (CR+PR) | 0.5188 | |||
| CR+PR | 10 (41.7) | 7 (45.7) | 3 (33.3) | |
| SD+PD | 14 (58.3) | 8 (54.3) | 6 (66.7) | |
| DCR (CR+PR+SD) | 0.5862 | |||
| CR+PR+SD | 15 (62.5) | 10 (66.7) | 5 (55.6) | |
| PD | 9 (37.5) | 5 (33.3) | 4 (44.4) |
Significant differences between groups were examined using Fisher's exact test. The ratio of ORR and DCR was compared between the WNT/β-catenin signal activation and inactivation groups using Fisher's exact test. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Progression-free survival (PFS)
The median OS was not reached because the follow-up duration was not long enough. The median PFS in all patients was 6.3 months. There was no significant difference in median PFS between the WNT/β-catenin signal activation group (6.9 months) and inactivation group (6.2 months) by Kaplan-Meier analysis (P=0.674) (Fig. 4).
Figure 4.
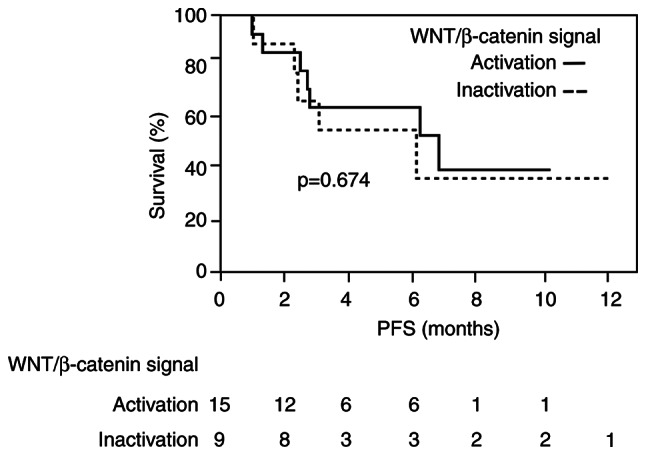
Kaplan-Meier estimates of PFS in patients with hepatocellular carcinoma in the WNT/β-catenin signal activation and inactivation groups. Significant differences in PFS were determined by log-rank analysis. The time zero was defined as the date of atezolizumab plus bevacizumab administration. PFS, progression-free survival.
Discussion
The WNT/β-catenin cascade is a major signaling pathway regulating liver carcinogenesis (21). Approximately 40% of HCC cases were reported to show constitutive activation of WNT/β-catenin signaling induced by relevant gene mutations (8,9). Constitutive WNT/β-catenin activation had negative effects on the DCR and PFS of patients receiving anti-programmed cell death 1 antibody therapy (10–12). Briefly, these studies indicated that WNT/β-catenin may be a biomarker for the response of patients with HCC to ICIs. ICIs are being used in a variety of cancer types and the combination of atezolizumab, an anti-programmed cell death 1 ligand 1 antibody, with bevacizumab has high response rates in patients with advanced HCC (3). However, clinical evidence demonstrating how the responses of HCC patients to atezolizumab plus bevacizumab therapy are affected by WNT/β-catenin activation is still lacking.
WNT/β-catenin activation promotes β-catenin accumulation in the cytoplasm as well as its nuclear translocation, and also diffuse accumulation of GS, a transcriptional target of β-catenin (22–24). The presence of WNT/β-catenin signal activation is determined by immunostaining showing the nuclear expression of β-catenin or cytoplasmic overexpression of GS in HCC tissues, indicating they might be useful biomarkers of WNT/β-catenin signal activation (22–24). The results of the present study demonstrated that the efficacy of atezolizumab plus bevacizumab in terms of treatment response and PFS was unaffected by WNT/β-catenin signal activation.
One of the reasons for these results is that some HCCs with WNT/β-catenin signal activation may consist of immune active tumors (25). Furthermore, bevacizumab may be responsible for these results. Bevacizumab, a monoclonal antibody targeting vascular endothelial growth factor (VEGF), inhibits angiogenesis and tumor growth and had some effect in patients with HCC (26–30). In addition, anti-VEGF therapies reduce VEGF-mediated immunosuppression within the tumor and its microenvironment and may enhance atezolizumab efficacy by reversing VEGF-mediated immunosuppression and promoting T-cell infiltration in tumors (31–33).
Furthermore, Sasaki et al (34) reported that patients with high-intensity HCC in the EOB-MRI hepatobiliary phase that may have WNT/β-catenin signal activation had shorter PFS than patients with low-intensity HCC in the atezolizumab plus bevacizumab group, contrary to our findings. The intensity of the EOB-MRI hepatobiliary phase may be associated with other factors in addition to WNT/β-catenin signal activation.
The limitations of this study included its single-center nature, which restricted the number of HCC patients who underwent liver tumor biopsy. This study included unresectable HCC with different stages. It would have been better if the groups could have been matched by liver function and the stage of HCC, but this was difficult in a small number of cases. In addition, it was unclear whether WNT/β-catenin signal activation of one tumor reflected the signal activation in other tumor masses when considering the heterogeneity of HCC in multiple masses. Furthermore, the presence of WNT/β-catenin signal activation could only be assessed by immunostaining and not gene sequencing.
In conclusion, the efficacy of atezolizumab plus bevacizumab might be unaffected by WNT/β-catenin mutations, although a small number of patients was included in this study. We suggest that more cases need to determine the exact efficacy of atezolizumab plus bevacizumab for HCC with WNT/β-catenin signal activation. Further study for the selection of chemotherapy is desired for the improvement of advanced HCC prognosis.
Acknowledgements
The authors would like to thank Mrs. Yukie Ishibashi (Department of Hepatology, Iizuka Hospital, Iizuka, Japan) for assistance with the manuscript preparation. The authors also thank Dr H. Nikki March for editing a draft of this manuscript.
Glossary
Abbreviations
- HCC
hepatocellular carcinoma
- ICI
immune checkpoint inhibitor
- PFS
progression-free survival
- OS
overall survival
- EOB-MRI
gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid-enhanced magnetic response imaging
- ALBI score
albumin-bilirubin score
- CT
computed tomography
- MRI
magnetic resonance imaging
- CR
complete response
- PR
partial response
- SD
stable disease
- DCR
disease control rate
- ORR
objective response rate
- PD
progression disease
- IHC
immunohistochemical
- GS
glutamine synthetase
- BCLC
Barcelona Clinic liver cancer
- VEGF
vascular endothelial growth factor
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
AK, MY, AM and KM designed the study. AK, KK, SN, KT and KM assisted with the data analyses. FN and YO performed pathological examination including immunostaining. AK wrote the initial draft of the manuscript. MY contributed to the analysis and interpretation of the data. MY and KM assisted in the preparation and critical review of the manuscript. AK and MY confirm the authenticity of all the raw data. All authors agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The research and protocol were in accord with the Declaration of Helsinki principles and the ethical guidelines of the 1975 Declaration of Helsinki, respectively. This study received approval from the Iizuka Hospital Ethics Committee (no. 22008; Iizuka, Japan). The opt-out method was applied to obtain consent for the present study.
Patient consent for publication
Written informed consents were obtained from two patients for publication of this article and accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Caldwell S, Park SH. The epidemiology of hepatocellular cancer: From the perspectives of public health problem to tumor biology. J Gastroenterol. 2009;44((Suppl 19)):S96–S101. doi: 10.1007/s00535-008-2258-6. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 5.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH, Rd, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 7.Eso Y, Marusawa H. Novel approaches for molecular targeted therapy against hepatocellular carcinoma. Hepatol Res. 2018;48:597–607. doi: 10.1111/hepr.13181. [DOI] [PubMed] [Google Scholar]
- 8.Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, Castro de Moura M, Putra J, Camprecios G, Bassaganyas L, Akers N, et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology. 2017;153:812–826. doi: 10.1053/j.gastro.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinyol R, Sia D, Llovet JM. Immune exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies in HCC. Clin Cancer Res. 2019;25:2021–2023. doi: 10.1158/1078-0432.CCR-18-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding JJ, Nandakumar S, Armenia J, Khalil DN, Albano M, Ly M, Shia J, Hechtman JF, Kundra R, El Dika I, et al. Prospective genotyping of hepatocellular carcinoma: Clinical Implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res. 2019;25:2116–2126. doi: 10.1158/1078-0432.CCR-18-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Zhou Q, Liu J, Zhang W. CTNNB1 alternation is a potential biomarker for immunotherapy prognosis in patients with hepatocellular carcinoma. Front Immunol. 2021;12:759565. doi: 10.3389/fimmu.2021.759565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuwano A, Tanaka K, Yada M, Nagasawa S, Morita Y, Masumoto A, Motomura K. Therapeutic efficacy of lenvatinib for hepatocellular carcinoma with iso-high intensity in the hepatobiliary phase of Gd-EOB-DTPA-MRI. Mol Clin Oncol. 2022;16:53. doi: 10.3892/mco.2021.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY. Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol. 2000;157:763–770. doi: 10.1016/S0002-9440(10)64590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zucman-Rossi J, Benhamouche S, Godard C, Boyault S, Grimber G, Balabaud C, Cunha AS, Bioulac-Sage P, Perret C. Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene. 2007;26:774–780. doi: 10.1038/sj.onc.1209824. [DOI] [PubMed] [Google Scholar]
- 18.Tsujikawa H, Masugi Y, Yamazaki K, Itano O, Kitagawa Y, Sakamoto M. Immunohistochemical molecular analysis indicates hepatocellular carcinoma subgroups that reflect tumor aggressiveness. Hum Pathol. 2016;50:24–33. doi: 10.1016/j.humpath.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Ueno A, Masugi Y, Yamazaki K, Komuta M, Effendi K, Tanami Y, Tsujikawa H, Tanimoto A, Okuda S, Itano O, et al. OATP1B3 expression is strongly associated with Wnt/β-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J Hepatol. 2014;61:1080–1087. doi: 10.1016/j.jhep.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen TB, Roncalli M, Di Tommaso L, Kakar S. Combined use of heat-shock protein 70 and glutamine synthetase is useful in the distinction of typical hepatocellular adenoma from atypical hepatocellular neoplasms and well-differentiated hepatocellular carcinoma. Mod Pathol. 2016;29:283–292. doi: 10.1038/modpathol.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He S, Tang S. WNT/β-catenin signaling in the development of liver cancers. Biomed Pharmacother. 2020;132:110851. doi: 10.1016/j.biopha.2020.110851. [DOI] [PubMed] [Google Scholar]
- 22.Nhieu JT, Renard CA, Wei Y, Cherqui D, Zafrani ES, Buendia MA. Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am J Pathol. 1999;155:703–710. doi: 10.1016/S0002-9440(10)65168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cadoret A, Ovejero C, Terris B, Souil E, Lévy L, Lamers WH, Kitajewski J, Kahn A, Perret C. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002;21:8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- 24.Loeppen S, Schneider D, Gaunitz F, Gebhardt R, Kurek R, Buchmann A, Schwarz M. Overexpression of glutamine synthetase is associated with beta-catenin-mutations in mouse liver tumors during promotion of hepatocarcinogenesis by phenobarbital. Cancer Res. 2002;62:5685–5688. [PubMed] [Google Scholar]
- 25.Aoki T, Nishida N, Kudo M. Clinical significance of the duality of Wnt/β-catenin signaling in human hepatocellular carcinoma. Cancers (Basel) 2022;14:444. doi: 10.3390/cancers14020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–235. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 27.Finn RS, Bentley G, Britten CD, Amado R, Busuttil RW. Targeting vascular endothelial growth factor with the monoclonal antibody bevacizumab inhibits human hepatocellular carcinoma cells growing in an orthotopic mouse model. Liver Int. 2009;29:284–290. doi: 10.1111/j.1478-3231.2008.01762.x. [DOI] [PubMed] [Google Scholar]
- 28.Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, Chen H, Clark-Garvey S, Weinberg A, Mandeli J, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992–2998. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boige V, Malka D, Bourredjem A, Dromain C, Baey C, Jacques N, Pignon JP, Vimond N, Bouvet-Forteau N, De Baere T, et al. Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma. Oncologist. 2012;17:1063–1072. doi: 10.1634/theoncologist.2011-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finn RS, Zhu AX. Targeting angiogenesis in hepatocellular carcinoma: Focus on VEGF and bevacizumab. Expert Rev Anticancer Ther. 2009;9:503–509. doi: 10.1586/era.09.6. [DOI] [PubMed] [Google Scholar]
- 31.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roland CL, Dineen SP, Lynn KD, Sullivan LA, Dellinger MT, Sadegh L, Sullivan JP, Shames DS, Brekken RA. Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Mol Cancer Ther. 2009;8:1761–1771. doi: 10.1158/1535-7163.MCT-09-0280. [DOI] [PubMed] [Google Scholar]
- 33.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki R, Nagata K, Fukushima M, Haraguchi M, Miuma S, Miyaaki H, Soyama A, Hidaka M, Eguchi S, Shigeno M, et al. Evaluating the role of hepatobiliary phase of gadoxetic acid-enhanced magnetic resonance imaging in predicting treatment impact of lenvatinib and atezolizumab plus bevacizumab on unresectable hepatocellular carcinoma. Cancers (Basel) 2022;14:827. doi: 10.3390/cancers14030827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.