Abstract
Background
Dehydroepiandrosterone (DHEA) is a precursor sex hormone with antifibrotic properties. The aims of this study were to investigate antifibrotic mechanisms of DHEA, and to determine the relationship between DHEA-sulfate (DHEAS) plasma levels, disease severity and survival in patients with fibrotic interstitial lung diseases (ILDs).
Methods
Human precision cut lung slices (PCLS) and normal human lung fibroblasts were treated with DHEA and/or transforming growth factor (TGF)-β1 before analysis of pro-fibrotic genes and signal proteins. Cell proliferation, cytotoxicity, cell cycle and glucose-6-phosphate dehydrogenase (G6PD) activity were assessed. DHEAS plasma levels were correlated with pulmonary function, the composite physiologic index (CPI), and time to death or lung transplantation in a derivation cohort of 31 men with idiopathic pulmonary fibrosis (IPF) and in an independent validation cohort of 238 men and women with fibrotic ILDs.
Results
DHEA decreased the expression of pro-fibrotic markers in-vitro and ex-vivo. There was no cytotoxic effect for the applied concentrations, but DHEA interfered in proliferation by modulating the cell cycle through reduction of G6PD activity. In men with IPF (derivation cohort) DHEAS plasma levels in the lowest quartile were associated with poor lung function and higher CPI (adjusted OR 1.15 [95% CI 1.03–1.38], p = 0.04), which was confirmed in the fibrotic ILD validation cohort (adjusted OR 1.03 [95% CI 1.00–1.06], p = 0.01). In both cohorts the risk of early mortality was higher in patients with low DHEAS levels, after accounting for potential confounding by age in men with IPF (HR 3.84, 95% CI 1.25–11.7, p = 0.02), and for age, sex, IPF diagnosis and prednisone treatment in men and women with fibrotic ILDs (HR 3.17, 95% CI 1.35–7.44, p = 0.008).
Conclusions
DHEA reduces lung fibrosis and cell proliferation by inducing cell cycle arrest and inhibition of G6PD activity. The association between low DHEAS levels and disease severity suggests a potential prognostic and therapeutic role of DHEAS in fibrotic ILD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-022-02076-9.
Introduction
Interstitial lung disease (ILD) is a group of inflammatory and fibrotic disorders that damage the lung parenchyma with various disease courses [1]. Especially patients with idiopathic pulmonary fibrosis (IPF) suffer from rapid disease progression and early mortality [2]. Other fibrotic ILDs including hypersensitivity pneumonitis (HP), connective tissue disease (CTD) associated ILD, and unclassifiable ILD can have similar progressive disease behaviour [3], and are characterized by reduced quality of life, frequent comorbidities, and age-related deficits [4]. Disease progression is difficult to predict in the individual patient but impacts treatment decisions.
Underlying mechanisms of progressive pulmonary fibrosis are still insufficiently understood. Biological and functional ageing are suggested to play a pathogenetic and perpetuating role in fibrotic ILDs [4, 5]. Patients with IPF are typically elderly men, an observation that has not been sufficiently explained yet [2]. Levels of dehydroepiandrosterone (DHEA) and its sulfated reservoir form (DHEAS) decrease with ageing, both have weak androgen effects and are precursors for androgens and estrogens, with additional biological roles (including immunosenescence) that have been proposed [6, 7].
Patients with IPF have lower plasma levels of DHEAS in comparison to healthy controls, and DHEA shows antifibrotic properties in-vitro [8]. A regulating role of DHEA in lung fibrosis is suggested by its anti-proliferative effect, regulation of extracellular matrix production [9], and effect on vascular remodelling [10]. In endothelial cells, DHEA inhibits cell growth by modulation of p53 and p21, proteins involved in the control of the cell cycle [6]; however, it is unknown if similar mechanisms apply to other cell types. Other hypothesized mechanisms by which DHEA interferes with cell growth and proliferation include apoptosis, autophagy [11, 12], and glucose-6-phosphate dehydrogenase (G6PD) inhibition [13, 14].
Despite recent advances in the management of patients with IPF and other ILDs with a progressive fibrotic phenotype [15, 16], pharmacological options remain limited and better approaches are urgently needed. In this translational study, we aimed to further investigate the previously described antifibrotic effect of DHEA in primary human lung fibroblasts in vitro and in an ex vivo model of human precision cut lung slices (PCLS) [8]. We hypothesized that DHEAS plasma levels would be associated with disease severity and survival in patients with fibrotic ILDs. We anticipated that these results would confirm the clinical importance of DHEAS for patients with fibrotic ILD and support further investigation into new potential therapeutic targets for this population.
Methods
This translational project consisted of experimental and clinical components. Antifibrotic effects and mechanisms of DHEA were established in normal human lung fibroblasts and in complete human lung tissue (PCLS). Furthermore, the clinical role of DHEAS was explored in men with IPF, and in a second cohort of men and women with various fibrotic ILDs.
In vitro and ex vivo experiments
DHEA treatment in vitro and ex vivo
Normal human lung fibroblasts (Lonza, Switzerland) (n = 3) were cultured in Ham’s F-12K (Kaighn’s) complete medium (Thermos Fisher Scientific, USA). At passage 4–6, cells were treated with 150 µM DHEA (Sigma-Aldrich, USA) and/or 5 ng/ml transforming growth factor (TGF)-β1 (R&D Systems, USA) in resting medium with 0.1% fetal bovine serum (RM). Experiments were performed independently, from different donors and in triplicates.
Gene (Additional file 1: Table S1) and protein expression of several pro-fibrotic markers and signalling proteins related to TGF-β1 signal pathway were evaluated by RT-qPCR and western blot. An ex vivo model for early fibrotic changes in the lung was established as described previously [17]. PCLS (400 µm) were obtained from healthy surrounding human lung tissue obtained during tumour resection and cut in a Compresstome® VF-310-0Z Vibrating Microtome (Precisionary, USA). PCLS were treated with 150 µM DHEA and/or a fibrotic cocktail during 48 h. Several pro-fibrotic markers were evaluated using RT-qPCR and immunofluorescence staining. Tissue sampling was approved by the local Ethics Committee, Bern, Switzerland (KEK-BE_2018-01801).
Proliferation and viability assay
CyQUANT™ XTT Cell Viability (Thermo Fisher Scientific) and LDH Cytotoxicity Assay (Thermo Fisher Scientific) were performed in human lung fibroblasts treated with different concentrations of DHEA (25, 50, 100, 150, 200 µM) at 24, 48 and 72 h, following the manufacturer’s protocol.
Cell cycle distribution and DNA damage
After treatment, lung fibroblasts were fixed with 4% paraformaldehyde and subsequently stained with diamidino-2-phenylindole (DAPI, Sigma-Aldrich) and a directly Alexa Fluor 488 conjugated mouse anti-γH2AX (Ser139) antibody (BioLegend, USA). Lung fibroblasts were analysed by flow cytometry BD LSRII SORP (Becton Dickinson, USA). Cell cycle stages and DNA damage was determined from the percentage of the total singlets (Additional file 1: Fig. S1), as previously described [18].
G6PD activity
G6PD activity assay (Sigma-Aldrich) was performed in normal human lung fibroblasts (n = 3) after 24 h of treatment with or without DHEA (150 µM) and TGF-β1, according to the manufacturer’s protocol. Additional details are provided in Additional file 1.
Patient populations, measurements, and outcome assessment
The derivation cohort included men diagnosed with IPF from an ongoing prospective cohort study (Swiss Ethics Committee, Bern, approval number KEK 246/15 PB_2016-01524) [2].
The validation cohort included patients with a multidisciplinary diagnosis of fibrotic ILD from a Canadian outpatient ILD referral centre (UBC ethics board approval H10-03099). Fibrotic ILDs included IPF [2], fibrotic HP, unclassifiable ILD [1], and CTD-ILD [19]. All patients provided informed written consent.
DHEAS measurement in plasma
The samples from the derivation and validation cohort were analysed locally following the same procedure. Plasma samples were centrifuged and stored in aliquots in a biobank at − 80 °C, and enzyme-linked immunosorbent assay (ELISA) kits were used as per the manufacturer’s protocol (DRG International, Inc., Mountainside, NJ, USA). The assays were run in duplicate, and the mean value of the duplicates were used for analysis.
Outcome assessments
Pulmonary function tests were performed within 3 months of DHEAS measurement using established protocols [20, 21]. The Composite Physiologic Index (CPI) was calculated using forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and diffusing capacity of the lung for carbon monoxide (DLCO). Originally, the CPI was developed to predict radiological severity of fibrosis and predicts mortality in ILD [22, 23]. Time to death, lung transplantation, or censoring was calculated from the date of blood draw for DHEAS measurement.
Statistical analysis
Results from in vitro and ex vivo assays were reported as mean ± standard deviation (SD). Differences between experimental conditions were tested for statistical significance by one- or two-way analysis of variance (ANOVA) with post-hoc analyses, or the corresponding non-parametric tests when appropriate.
Patients were stratified by DHEAS plasma levels in the lowest quartile versus the second to fourth quartiles combined, this cut-off was chosen a-priori based on previous studies [24, 25]. Categorical variables were compared using chi-square or Fisher’s exact test, and continuous variables using two-sample t-test or Wilcoxon rank sum test depending on the distribution of the data. Pearson’s correlation was used for the unadjusted correlation between DHEAS, pulmonary function tests, and CPI. Odds ratios (OR) and corresponding 95% confidence intervals (95% CI) for the association of the lowest DHEAS quartile with pulmonary function tests and CPI were calculated using logistic regression models. Hazard ratios (HR) for the association between DHEAS and time to the composite outcome of death or lung transplantation were determined with Cox proportional hazard models. Models were adjusted for potential confounders with conceptual importance: age for the derivation cohort of men with IPF, and age, sex, IPF diagnosis, and prednisone treatment for the validation cohort (men and women with fibrotic ILDs). Proportional hazards assumption was tested using Schoenfeld residual tests. Unadjusted Kaplan Meier curves were used to illustrate the association of DHEAS with survival. There was no imputation performed for missing data. A two-sided p < 0.05 indicated statistical significance for all comparisons. Data were analysed using GraphPad Prism 9.0 (GraphPad Software Inc., USA) and R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Antifibrotic mechanisms of DHEA
DHEA has strong antifibrotic effects in normal lung fibroblasts and in the fibrotic PCLS model
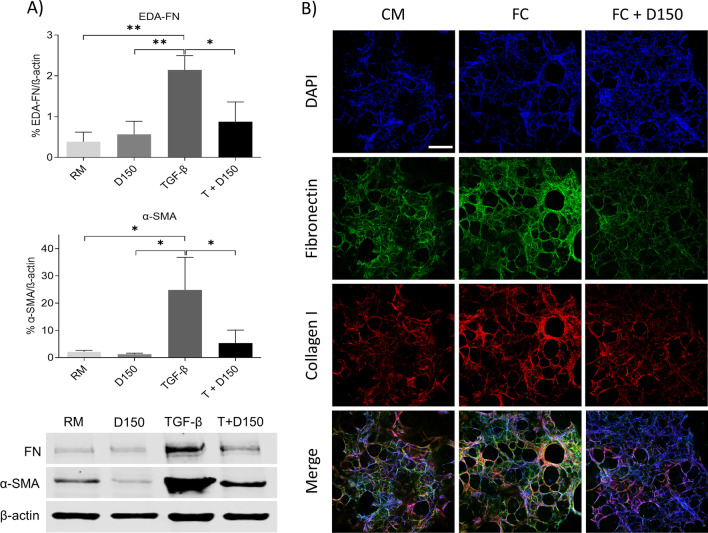
In vitro, DHEA reduced extracellular matrix production such as EDA-fibronectin (EDA-FN) and fibroblast differentiation as shown by alpha smooth muscle actin (α-SMA) expression after 48 h (Fig. 1A). DHEA prevented the pro-fibrotic effect of TGF-β1 after 24 h of treatment, as observed in the reduction of the gene expression of profibrotic markers EDA-FN, ACTA2, COL1A1 and CTGF (Additional file 1: Fig. S2).
Fig. 1.
Antifibrotic effect of DHEA in vitro and ex vivo. A Protein expression of EDA-FN and α-SMA in normal human lung fibroblasts treated with DHEA 150 µM (D150) and/or TGF-β1 (5 ng/ml) (T + D150) for 48 h. Bars represent mean ± SD, (*) p < 0.05, (**) p < 0.01. B Immunofluorescence images of DAPI (blue), Fibronectin (green), collagen I (red), and merged in human PCLS treated with control medium (CM), fibrotic cocktail (FC) and FC plus DHEA (FC + D150). Scale bar = 500 µm. α-SMA: alpha smooth muscle actin; CM: control medium; DAPI: diamidino-2-phenylindole; D150, dehydroepiandrosterone 150 µM; EDA-FN: extra domain A fibronectin; FC: fibrotic cocktail; RM: resting medium; SD: standard deviation; TGF-β1: transforming growth factor β1; T + D150: TGF- β1 + DHEA 150 µM
Ex vivo, immunofluorescence images showed an increase of EDA-FN and Collagen I in PCLS treated with several pro-fibrotic mediators for 48 h. These early fibrotic changes could be prevented by DHEA (Fig. 1B and Additional file 1: Fig. S3). Furthermore, gene expression analyses of EDA-FN, ACTA2, and COL1A1 showed that DHEA prevented the upregulation of fibrotic markers induced by pro-fibrotic treatment in this model (Additional file 1: Fig. S2).
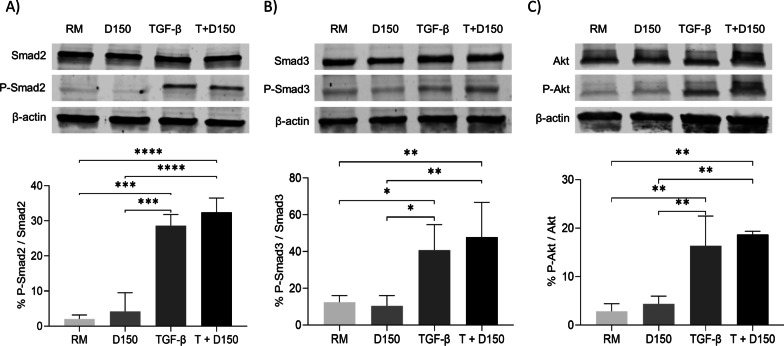
The antifibrotic effect of DHEA is independent of Smad2/3 and Akt phosphorylation
To investigate specific interferences with TGF-β signalling pathway as a potential antifibrotic mechanism, Smad2/3, Akt and their phosphorylated forms were assessed by western blot. Figure 2 illustrates that DHEA did not interfere in Smad2/3 and Akt phosphorylation, suggesting that DHEA effects do not involve Smad or Akt phosphorylation downstream of TGF-β signalling.
Fig. 2.
Effect of DHEA on Smad2/3 and Akt activation. Western blot analyses of P-Smad2/Smad2 (A), P-Smad3/Smad3 (B) and P-Akt/Akt ratio (C) in normal human lung fibroblasts treated for one hour with DHEA 150 µM (D150) and/or TGF-β1 (5 ng/ml) (T + D150). Bars show mean ± SD. (*) p < 0.05, (**) p < 0.01, (***) p < 0.001, (****) p < 0.0001. D150: dehydroepiandrosterone 150 µM; RM: resting medium; SD: standard deviation; TGF-β1: transforming growth factor β1, T + D150: TGF- β1 + DHEA 150 µM
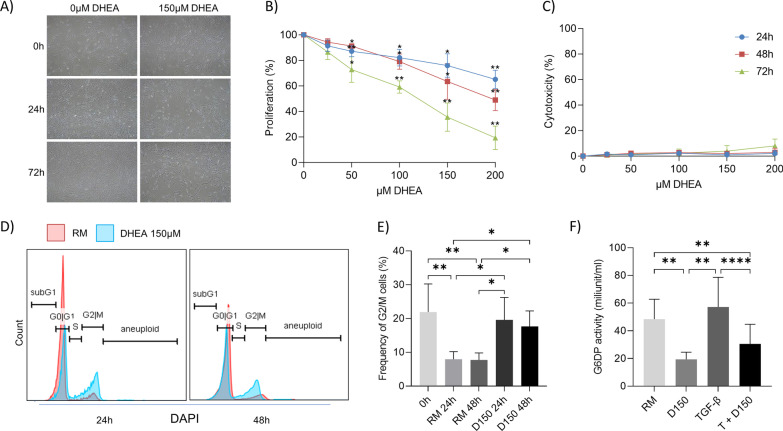
DHEA interferes with cell proliferation by decreasing G6PD activity
DHEA reduced cell proliferation in a time- and dose-dependent manner, without increasing cell cytotoxicity (Fig. 3A). This anti-proliferative effect was linked to interference with the cell cycle regulation by DHEA. Flow cytometry analyses showed that DHEA promoted early cell cycle arrest in the G2/M phase population after 24 h of treatment compared to the control condition (Fig. 3B). The G2/M phase cell cycle arrest was not associated with DNA damage, as demonstrated by the unchanged frequency of γH2AX positive cells in all conditions (Additional file 1: Fig. S4B). To confirm the hypothesis, that DHEA mediates cell cycle arrest by altering the activity of G6PD and thus interfering in the nucleoside generation through pentose phosphate pathway, G6PD activity assay was performed in cell lysate from normal human lung fibroblasts treated with DHEA and TGF-β1. Lung fibroblasts had a reduced enzymatic activity of G6PD after 24 h of DHEA treatment (Fig. 3C).
Fig. 3.
Effect of DHEA on cell growth and cell cycle in normal human lung fibroblasts. A, B DHEA has anti-proliferative effects in normal human lung fibroblasts in resting media (n = 3), C without cytotoxicity at concentrations of 25–200 µM compared to control at 24, 48 and 72 h. D Cell cycle distribution of normal human lung fibroblasts is shown at 24 h and 48 h after treatment with or without 150 µM DHEA (D150). E Frequency of lung fibroblasts in G2/M phase at the beginning of the experiment (0 h) and after 24–48 h of DHEA treatment. F G6PD activity is illustrated in normal human lung fibroblasts treated with 150 µM DHEA (D150), TGF-β or both (T + D150). Bars show mean + SD. (*) p < 0.05, (**) p < 0.01, (****) p < 0.0001. DHEA: dehydroepiandrosterone; D150, DHEA 150 µM; G6PD: glucose-6-phosphate dehydrogenase (G6PD); RM: resting medium; SD: standard deviation; TGF-β1/T: transforming growth factor β1; T + D150: TGF-β1 + DHEA 150 µM
Patient characteristics
The derivation cohort included 31 men with IPF, with a mean (SD) age of 69.8 (10.3) and median (IQR) DHEAS plasma levels of 0.30 (0.19–0.58). Age, body mass index (BMI), smoking status, and antifibrotic treatment did not differ between patients with DHEAS levels in the lowest quartile compared to the remaining patients (Table 1).
Table 1.
Patient characteristics in the derivation cohort (men with IPF) and the validation cohort (men and women with fibrotic ILD) stratified by DHEAS
| Derivation cohort (n = 31) | Validation cohort (n = 238) | |||
|---|---|---|---|---|
| DHEASa Lowest quartile |
DHEASa 2nd–4th quartiles |
DHEASb Lowest quartile |
DHEASb 2nd–4th quartiles |
|
| Sex, men | 8 (100%) | 23 (100%) | 17 (28%) | 89 (50%) |
| Age | 69.1 (13.8) | 70.1 (9.1) | 67.5 (10.6) | 62.7 (13.2) |
| IPF diagnosis | 8 (100%) | 23 (100%) | 5 (8%) | 38 (21%) |
| BMI, kg/m2 | 29.0 (4.4) | 28.0 (4.8) | 28.8 (6.5) | 26.9 (5.0) |
| Ever smoker | 4 (50%) | 15 (65%) | 35 (58%) | 112 (63%) |
| FVC, %-predicted | 54.8 (16.1) | 65.0 (15.5) | 69.0 (21.6) | 78.6 (16.8) |
| FEV1, %-predicted | 65.5 (9.8) | 74.7 (11.7) | 70.1 (21.4) | 80.7 (17.3) |
| DLCO, %-predicted | 30.0 (9.8) | 49.4 (15.5) | 46.0 (18.2) | 52.5 (15.4) |
| CPI | 63.7 (11.9) | 48.9 (12.5) | 46.8 (15.5) | 41.8 (12.8) |
| Nintedanib | 3 (38%) | 12 (52%) | 1 (2%) | 7 (4%) |
| Pirfenidone | 3 (38%) | 4 (17%) | 1 (2%) | 7 (4%) |
Data shown are number (%), mean (SD), or median (IQR)
BMI body mass index, CPI composite physiologic index, DHEAS dehydroepiandrosterone sulfate, DLCO diffusing capacity of the lung for carbon monoxide, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, ILD interstitial lung disease, IPF idiopathic pulmonary fibrosis, IQR interquartile range, SD standard deviation
aLowest quartile DHEAS < 0.19 µg/ml; 2nd–4th quartiles DHEAS ≥ 0.19 µg/ml
bLowest quartile DHEAS < 0.20 µg/ml, 2nd–4th quartiles DHEAS ≥ 0.20 µg/ml
The validation cohort included 238 patients with fibrotic ILD. Forty-seven (20%) had unclassifiable ILD, 43 (18%) IPF, 24 (10%) fibrotic HP, 58 (24%) systemic sclerosis-associated ILD, 48 (20%) ILD associated with other CTDs, and 18 (8%) other ILDs. The 106 men and 132 women had median (IQR) DHEAS plasma levels of 0.50 (0.29–0.80) and 0.29 (0.14–0.58), respectively. Patients in the lowest DHEAS quartile were older, had less frequently IPF, and were more frequently treated with prednisone compared to the remaining patients. BMI, smoking status, and prednisone dose were not different between the two groups. Antifibrotic medications were prescribed in 7% of the entire cohort, with equal distribution across DHEAS quartiles (Table 1). Similarly, treatment with mycophenolate mofetil, azathioprine, rituximab, methotrexate, and other medications previously linked to DHEAS blood levels was not different between the groups (Additional file 1: Table S2).
DHEAS and disease severity
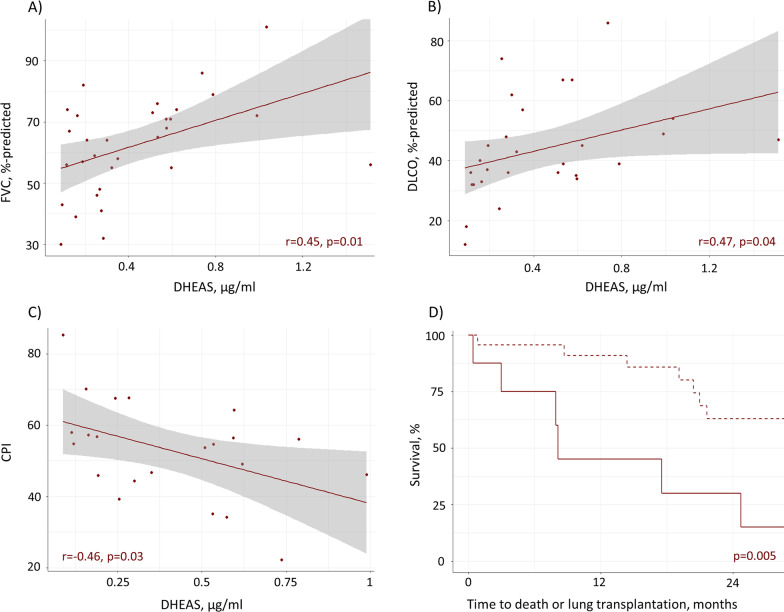
In the derivation cohort, patients with DHEAS levels in the lowest quartile had lower mean FVC (55 versus 65%-predicted, p = 0.14), lower DLCO (30 versus 49%-predicted, p = 0.0008), and higher CPI (64 versus 49, p = 0.03) compared to the remaining patients (Table 1). Correspondingly, there was a moderately strong correlation between DHEAS plasma levels and FVC %-predicted (r = 0.45, p = 0.01), DLCO %-predicted (r = 0.47, p = 0.04), and CPI (r = − 0.46, p = 0.03) (Fig. 4A–C). With adjustment for age, the relationship between low DHEAS and DLCO %-predicted remained statistically significant (OR 0.82 [95% CI 0.64–0.94], p = 0.03), with similar findings for CPI (OR 1.15 [95% CI 1.03–1.38], p = 0.04) (Table 2).
Fig. 4.
Relationship between DHEAS levels, pulmonary function, and survival in the derivation cohort of men with IPF. Correlation between DHEAS plasma levels (µg/ml) with FVC, %-predicted (A), DLCO, %-predicted (B), and CPI (C) in men with IPF. Kaplan Meier curves (D) for time to death or lung transplantation stratified by DHEAS < 0.19 µg/ml (solid line) versus DHEAS ≥ 0.19 µg/ml (dashed line), p-value from log rank test. CPI: composite physiologic index; DHEAS: dehydroepiandrosterone sulfate; DLCO: diffusing capacity of the lung for carbon monoxide; FVC: forced vital capacity; IPF: idiopathic pulmonary fibrosis
Table 2.
Association of DHEAS in the lowest quartile with pulmonary function and survival in the derivation cohort (men with IPF)
| DHEAS lowest quartile | Unadjusted | Adjusted for age | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| FVC, % | 0.96 (0.90–1.01) | 0.13 | 0.96 (0.90–1.01) | 0.16 |
| DLCO, % | 0.84 (0.67–0.95) | 0.03 | 0.82 (0.64–0.94) | 0.03 |
| CPI | 1.12 (1.02–1.30) | 0.05 | 1.15 (1.03–1.38) | 0.04 |
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
|---|---|---|---|---|
| Survivala | 4.35 (1.44–13.1) | 0.009 | 3.84 (1.25–11.7) | 0.02 |
E.g. every one % increase in FVC, %-predicted is associated with a 4% lower odds of a serum DHEAS value in the lowest quartile
CI confidence interval, CPI composite physiologic index, DHEAS dehydroepiandrosterone sulfate, DLCO diffusing capacity of the lung for carbon monoxide, FVC forced vital capacity, HR hazard ratio, IPF idiopathic pulmonary fibrosis, OR odds ratio
aTime to death or lung transplantation e.g. patients with DHEAS in the lowest quartile have a 4.35 higher hazard for early death or lung transplant compared to those with DHEAS values in the second to fourth quartile
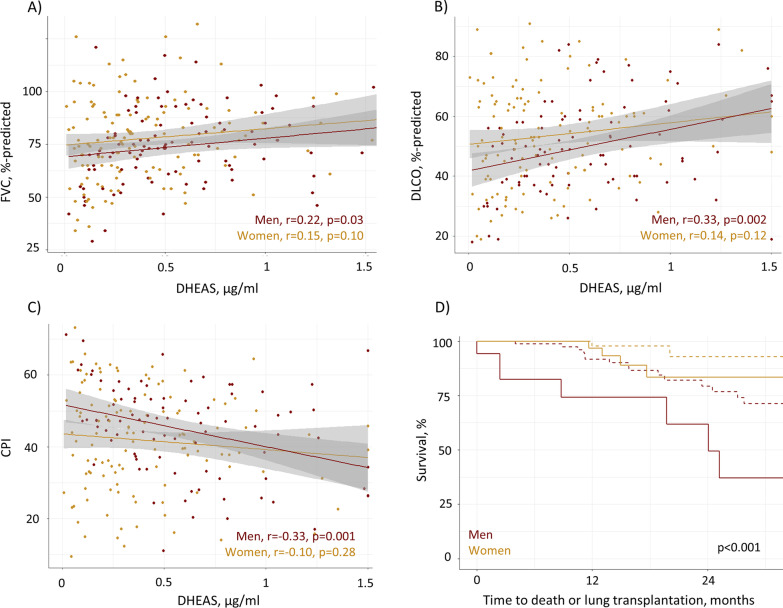
In the validation cohort, patients with DHEAS levels in the lowest quartile had lower mean FVC (69 versus 79%-predicted, p = 0.002), lower DLCO (46 versus 53%-predicted, p = 0.01), and higher CPI (47 versus 42, p = 0.04) compared to the remaining patients (Table 1). DHEAS levels were correlated with pulmonary function in men with fibrotic ILD (FVC %-predicted r = 0.22, p = 0.03; DLCO %-predicted r = 0.33, p = 0.002; CPI r = − 0.34, p < 0.001), with non-significant correlations between DHEAS levels and pulmonary function in women (Fig. 5A–C). After adjustment for confounding by age, sex, IPF diagnosis, and prednisone treatment, the relationship between the lowest DHEAS quartile (< 0.20 µg/ml) and low DLCO and FVC %-predicted remained significant (both OR 0.97 [95% CI 0.95–0.99]; p = 0.004 and p = 0.02, respectively). The association of the lowest DHEAS quartile with high CPI was statistically significant, independent from age and sex (OR 1.03 [95% CI 1.01–1.06], p = 0.01), but not with additional adjustment for IPF and prednisone treatment (p = 0.08) (Table 3).
Fig. 5.
Relationship between DHEAS levels, pulmonary function, and survival in the validation cohort of men and women with fibrotic ILD. Correlation between DHEAS plasma levels (µg/ml) with FVC, %-predicted (A), DLCO, %-predicted (B), and CPI (C) in men and women with fibrotic ILD. Kaplan Meier curves (D) for time to death or lung transplantation stratified by sex and DHEAS < 0.20 µg/ml (solid line) versus DHEAS ≥ 0.20 µg/ml (dashed line), p-value from log rank test. CPI: composite physiologic index; DHEAS: dehydroepiandrosterone sulfate; DLCO: diffusing capacity of the lung for carbon monoxide; FVC: forced vital capacity; ILD: interstitial lung disease
Table 3.
Association of DHEAS in the lowest quartile with pulmonary function and survival in the validation cohort (men and women with fibrotic ILD)
| DHEAS Lowest quartile |
Unadjusted | Adjusted for | ||||
|---|---|---|---|---|---|---|
| Age and sex | Age, sex, IPF and prednisone treatment | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| FVC, % | 0.97 (0.95–0.99) | < 0.001 | 0.96 (0.94–0.98) | < 0.001 | 0.97 (0.95–0.99) | 0.004 |
| DLCO, % | 0.97 (0.95–0.99) | 0.008 | 0.97 (0.95–0.99) | 0.004 | 0.97 (0.95–0.99) | 0.02 |
| CPI | 1.03 (1.00–1.05) | 0.03 | 1.03 (1.01–1.06) | 0.01 | 1.03 (1.00–1.06) | 0.08 |
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Survivala | 1.96 (0.93–4.11) | 0.07 | 2.30 (1.06–5.00) | 0.03 | 3.17 (1.35–7.44) | 0.008 |
E.g. every one % increase in FVC, %-predicted is associated with a 3% lower odds of a serum DHEAS value in the lowest quartile
CI confidence interval, CPI composite physiologic index, DHEAS dehydroepiandrosterone sulfate, DLCO diffusing capacity of the lung for carbon monoxide, FVC forced vital capacity, HR hazard ratio, ILD interstitial lung disease, OR odds ratio
aTime to death or lung transplantation e.g. patients with DHEAS in the lowest quartile have a 1.96 higher hazard for early death or lung transplant compared to those with normal/high DHEAS
DHEAS and survival
Median (IQR) observation time in the derivation cohort was 20.4 (8.4–32.7) months, including 15 deaths and 1 lung transplant. Patients in the lowest DHEAS quartile had higher hazard of early death or lung transplant compared to the rest of the cohort (unadjusted HR 4.35, 95% CI 1.44–13.1, p = 0.009), independent of potential confounding by age (adjusted HR 3.84, 95% CI 1.25–11.7, p = 0.02) (Table 2, Fig. 4D).
In the validation cohort, median (IQR) observation time was 15.4 (7.9–21.7) months, including 30 deaths and 4 lung transplants. Patients in the lowest DHEAS quartile similarly had higher hazard of early death or lung transplantation compared to the rest of the cohort (HR adjusted for age and sex 2.3, 95% CI 1.06–5.0, p = 0.03). This relationship remained significant with additional adjustment for IPF diagnosis and prednisone treatment (HR 3.17, 95% CI 1.35–7.44, p = 0.008) (Table 3, Fig. 5D).
Discussion
This study indicates a role of DHEA in lung fibrosis. Antifibrotic properties of DHEA were confirmed in vitro and shown for the first time ex vivo in a human model. Our findings suggest that DHEA inhibits cell proliferation and induces early cell cycle arrest by suppressing G6PD activity in lung fibroblasts. In men with IPF, higher DHEAS plasma levels correlate with preserved pulmonary function and lower risk for early mortality. These findings were replicated in a diverse validation cohort of men and women with fibrotic ILDs.
Previous studies have shown that DHEA decreases procollagen type I mRNA expression and protein synthesis in cultured cardiac fibroblasts, with prevention of oxidative stress and reduced cardiac fibrosis in animal studies [26, 27]. In lung fibroblasts, DHEA decreases proliferation in a dose- and time-dependent manner [8]. This was confirmed in the current study using normal human lung fibroblasts and, for the first time, in PCLS. We did not find an association of DHEA with Smad2/3 or Akt phosphorylation downstream of TGF-β, which suggests other antifibrotic downstream mechanisms. DHEA increases expression of several proteins involved in apoptosis, with some of these (e.g., p21) also involved in cell cycle regulation [6]. In our study, DHEA induced an early cell cycle arrest by inhibiting G6PD activity which might explain its anti-proliferative effects. Previously, inhibition of G6PD by DHEA has been mainly described in cancer cells [14, 28], in which high G6PD activity is one of the drivers of cell growth and proliferation [29]. In pulmonary fibrosis, controlling the excessive proliferation of fibroblasts and consequently reducing the profibrotic milieu is likely key in preventing progression.
DHEAS has been proposed as a protective factor against atherosclerosis, endothelial dysfunction, immunosenescence, and progression of liver fibrosis [10, 30]. DHEAS blood levels have been associated with overall and cardiovascular mortality in several population-based cohorts, with an overall stronger relationship between DHEAS deficiency and adverse outcomes in men than in women [7, 31]. In our cohort, the correlation between DHEAS levels, pulmonary fibrosis severity, and transplant-free survival was similarly stronger in men than in women. Consequently, the age associated decline in DHEAS might be one possible factor contributing to the increased risk for IPF and progressive pulmonary fibrosis in older men [32]. A previous study has measured levels of circulating DHEAS in pulmonary fibrosis (0.47 and 0.32 µg/ml in men and women with IPF, respectively), these were in line with our findings [8]. Decreased levels of DHEAS have also been observed in pulmonary hypertension [33, 34], and preclinical studies have demonstrated DHEAS to reverse right ventricular hypertrophy and vascular remodelling [10, 35]. Considering the common pathobiological mechanisms of pulmonary hypertension and pulmonary fibrosis, including dysregulated angiogenesis, endothelial dysfunction, and endothelial to mesenchymal transition [36, 37], inhibition of G6PD activity might be a common mechanism of impact of DHEA in pulmonary hypertension and fibrosis [38].
Our study has several strengths and limitations. This study exceeds previously demonstrated antifibrotic properties of DHEA [8] by proposing new underlying mechanisms, using an ex vivo human PCLS model which is considered a substitute for conventional models, and a better substitute model for human lung fibrosis than the classical mouse model with bleomycin. PCLS can be made from human lung and have preserved lung architecture with all pulmonary cell types available, which allows the investigation of changes in the extracellular matrix during fibrosis development [17]. However, this model was not designed to investigate severe fibrosis, reversal of fibrosis or effects of circulating blood cells. We did not exclude patients with subclinical pulmonary hypertension, and the inverse correlation between DHEAS and DLCO might be partly due to concurrent pulmonary vascular disease in patients with low DLCO. A definitive causality between DHEAS and progression of pulmonary fibrosis cannot be established from our clinical data. Nonetheless, the consistent association of DHEAS with FVC and CPI, the robustness of the findings to adjustment for potential confounders, and the antifibrotic effects of DHEAS suggest a pathogenetic role of DHEAS in fibrotic ILD. Lastly, the validation of our findings in two independent cohorts with different patient characteristics, and DHEAS plasma measurement in two different laboratories increase generalizability of the findings to different fibrotic ILD populations.
The safety of short-term DHEA supplementation has been established in several populations [39, 40]. For example, a small, non-controlled pilot study including patients with pulmonary hypertension associated with chronic obstructive pulmonary disease suggested efficacy of DHEA supplementation with a significant improvement in 6-min walk distance [39]. Based on the herein demonstrated antifibrotic effects of DHEA, and the relationship of DHEAS plasma levels with disease severity and adverse outcomes, future studies should investigate the effect of DHEA treatment on the progression of fibrosis in patients with ILD.
In summary, we establish a strong antifibrotic effect of DHEA in vitro and ex vivo with interference of DHEA in the fibroblast cell cycle by suppression of G6PD activity. Lower DHEAS plasma levels are associated with more severe disease and early mortality in men with IPF, and in an independent cohort of patients with fibrotic ILDs. These findings suggest DHEAS as a potential prognostic biomarker and therapeutic target in pulmonary fibrosis.
Supplementary Information
Additional file 1. Table S1. List of qPCR primers. Table S2. Medication stratified by DHEAS in the lowest quartile compared to the second to fourth quartile combined in the validation cohort. Figure S1. Flow cytometry framework. Lung fibroblasts were separated from cell debris and analyses was made based on single cells. Cell cycle distribution was evaluated following DAPI staining. DNA damage was estimated from single cells by the presence of γH2AX (negative control = control medium (CM); positive control = 1mM H2O2). Figure S2. Gene expression of fibrotic markers in vitro and ex vivo after DHEA. A) Fibrotic markers EDA-FN, ACTA2, COL1A1 and CTGF from normal human lung fibroblasts incubated in vitro with TGF-β1 and/or DHEA (D150) (T + D150). B) Ex vivo gene expression of PCLS stimulated with a fibrotic cocktail (FC), DHEA (D150) or both (FC + D150). Bars show mean ± SD. (*) p<0.05, (**) p<0.01, (***) p<0.001, (****) p<0.0001. Figure S3. Immunofluorescence staining of EDA-fibronectin and collagen I in PCLS treated with/without DHEA and the fibrotic cocktail. The signal of EDA-fibronectin (EDA-FN, green) and collagen I (red) in PCLS treated with fibrotic cocktail was reduced after addition of DHEA (FC + D150). Nucleus staining with DAPI (blue). Pictures were taken at 10X magnification. (–) scale represent 500 µm. Figure S4. Effect of DHEA on the cell cycle and DNA damage. A) Cell cycle distribution of control lung fibroblasts (n=3) at the beginning of the experiment (0h) and after treatment with DHEA (D150) or in resting medium (RM) for 24 and 48h. B) Frequency of γH2AX+ cells in lung fibroblasts treated with/without DHEA. (****) p<0.0001. compared to the positive control of DNA damage (H2O2 1mM).
Acknowledgements
We thank Valentin Djonov, Fabian Blank, Sandra Barnowski, and Janice M. Leung for supporting this project.
Abbreviations
- α-SMA
Alpha smooth muscle actin
- ANOVA
Analysis of variance
- BMI
Body mass index
- CI
Confidence interval
- CPI
Composite Physiologic Index
- CTD
Connective tissue disease
- DAPI
Diamidino-2-phenylindole
- DHEA
Dehydroepiandrosterone
- DHEAS
Dehydroepiandrosterone sulfate
- DLCO
Diffusing capacity of the lung for carbon monoxide
- EDA-FN
EDA-fibronectin
- ELISA
Enzyme-linked immunosorbent assay
- FEV1
Forced expiratory volume in 1 s
- FVC
Forced vital capacity
- G6PD
Glucose-6-phosphate dehydrogenase
- HP
Hypersensitivity pneumonitis
- HR
Hazard ratio
- ILD
Interstitial lung disease
- IPF
Idiopathic pulmonary fibrosis
- IQR
Interquartile range
- OR
Odds ratios
- PCLS
Precision cut lung slices
- SD
Standard deviation
- TGF
Transforming growth factor
Author contributions
SAG, CJR and MFC designed the study. SAG, CM, TKG, GK, TMM, BT, VT, CJR and MFC contributed to data acquisition. SAG, CM, CJR and MFC contributed to analysis of the data and drafting of the manuscript. All authors critically revised the manuscript. All authors read and approved the final manuscript.
Funding
Clinical Trial Unit, University of Bern, Switzerland. Lungenliga Bern, Switzerland. British Columbia Lung Association, Canada. Unrestricted grants from Roche and Boehringer Ingelheim.
Availability of data and materials
The datasets used for the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study has been approved by the local ethics committees: Swiss Ethics Committee, Bern, approval number KEK 246/15 PB_2016-01524 and UBC ethics board approval H10-03099.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest related to this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 3.Brown KK, Martinez FJ, Walsh SLF, Thannickal VJ, Prasse A, Schlenker-Herceg R, et al. The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J. 2020;55(6):1–8. doi: 10.1183/13993003.00085-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guler SA, Kwan JM, Leung JM, Khalil N, Wilcox PG, Ryerson CJ. Functional ageing in fibrotic interstitial lung disease: the impact of frailty on adverse health outcomes. Eur Respir J. 2020;55(1):1900647. doi: 10.1183/13993003.00647-2019. [DOI] [PubMed] [Google Scholar]
- 5.Snetselaar R, van Moorsel CHM, Kazemier KM, van der Vis JJ, Zanen P, van Oosterhout MFM, et al. Telomere length in interstitial lung diseases. Chest. 2015;148(4):1011–1018. doi: 10.1378/chest.14-3078. [DOI] [PubMed] [Google Scholar]
- 6.Zapata E, Ventura JL, De la Cruz K, Rodriguez E, Damián P, Massó F, et al. Dehydroepiandrosterone inhibits the proliferation of human umbilical vein endothelial cells by enhancing the expression of p53 and p21, restricting the phosphorylation of retinoblastoma protein, and is androgen- and estrogen-receptor independent. FEBS J. 2005;272(6):1343–1353. doi: 10.1111/j.1742-4658.2005.04563.x. [DOI] [PubMed] [Google Scholar]
- 7.Jia X, Sun C, Tang O, Gorlov I, Nambi V, Virani SS, et al. Plasma dehydroepiandrosterone sulfate and cardiovascular disease risk in older men and women. J Clin Endocrinol Metab. 2020;105(12):e4304–e4327. doi: 10.1210/clinem/dgaa518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendoza-Milla C, Valero Jimenez A, Rangel C, Lozano A, Morales V, Becerril C, et al. Dehydroepiandrosterone has strong antifibrotic effects and is decreased in idiopathic pulmonary fibrosis. Eur Respir J. 2013;42(5):1309–1321. doi: 10.1183/09031936.00027412. [DOI] [PubMed] [Google Scholar]
- 9.Paulin R, Meloche J, Jacob MH, Bisserier M, Courboulin A, Bonnet S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2011;301(5):H1798–H1809. doi: 10.1152/ajpheart.00654.2011. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M, Watson KO, et al. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-{beta}/NFAT axis. Circulation. 2009;120(13):1231–1240. doi: 10.1161/CIRCULATIONAHA.109.848911. [DOI] [PubMed] [Google Scholar]
- 11.Lee MJ, Kim EH, Lee SA, Kang YM, Jung CH, Yoon HK, et al. Dehydroepiandrosterone prevents linoleic acid-induced endothelial cell senescence by increasing autophagy. Metab Clin Exp. 2015;64(9):1134–1145. doi: 10.1016/j.metabol.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Miyazaki T, Honda A, Hirayama T, Yoshida S, Tanaka N, et al. Apoptosis and inhibition of the phosphatidylinositol 3-kinase/Akt signaling pathway in the anti-proliferative actions of dehydroepiandrosterone. J Gastroenterol. 2005;40(5):490–497. doi: 10.1007/s00535-005-1574-3. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez PT, Frolova AI, Chi MM, Grindler NM, Willcockson AR, Reynolds KA, et al. DHEA-mediated inhibition of the pentose phosphate pathway alters oocyte lipid metabolism in mice. Endocrinology. 2013;154(12):4835–4844. doi: 10.1210/en.2012-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Monaco M, Pizzini A, Gatto V, Leonardi L, Gallo M, Brignardello E, et al. Role of glucose-6-phosphate dehydrogenase inhibition in the antiproliferative effects of dehydroepiandrosterone on human breast cancer cells. Br J Cancer. 1997;75(4):589–592. doi: 10.1038/bjc.1997.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behr J, Prasse A, Kreuter M, Johow J, Rabe KF, Bonella F, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med. 2021;5:476–486. doi: 10.1016/S2213-2600(20)30554-3. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 17.Alsafadi HN, Staab-Weijnitz CA, Lehmann M, Lindner M, Peschel B, Königshoff M, et al. An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am J Physiol Lung Cell Mol Physiol. 2017;312(6):L896–l902. doi: 10.1152/ajplung.00084.2017. [DOI] [PubMed] [Google Scholar]
- 18.Tièche CC, Peng RW, Dorn P, Froment L, Schmid RA, Marti TM. Prolonged pemetrexed pretreatment augments persistence of cisplatin-induced DNA damage and eliminates resistant lung cancer stem-like cells associated with EMT. BMC Cancer. 2016;16:125. doi: 10.1186/s12885-016-2117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 21.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 22.Wells AU, Desai SR, Rubens MB, Goh NS, Cramer D, Nicholson AG, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167(7):962–969. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 23.Ryerson CJ, O'Connor D, Dunne JV, Schooley F, Hague CJ, Murphy D, et al. Predicting mortality in systemic sclerosis-associated interstitial lung disease using risk prediction models derived from idiopathic pulmonary fibrosis. Chest. 2015;148(5):1268–1275. doi: 10.1378/chest.15-0003. [DOI] [PubMed] [Google Scholar]
- 24.Maggio M, Lauretani F, Ceda GP, Bandinelli S, Ling SM, Metter EJ, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med. 2007;167(20):2249–2254. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy ME, Waters A, Sen S, Castel AD, Plankey M, Molock S, et al. Psychosocial stress and neuroendocrine biomarker concentrations among women living with or without HIV. PLoS ONE. 2021;16(12):e0261746. doi: 10.1371/journal.pone.0261746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia C, Chen X, Li X, Li M, Miao C, Sun B, et al. The effect of DHEA treatment on the oxidative stress and myocardial fibrosis induced by Keshan disease pathogenic factors. J Trace Elem Med Biol. 2011;25(3):154–159. doi: 10.1016/j.jtemb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki T, Mukasa K, Yoneda M, Ito S, Yamada Y, Mori Y, et al. Marked attenuation of production of collagen type I from cardiac fibroblasts by dehydroepiandrosterone. Am J Physiol Endocrinol Metab. 2005;288(6):E1222–E1228. doi: 10.1152/ajpendo.00370.2004. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida S, Honda A, Matsuzaki Y, Fukushima S, Tanaka N, Takagiwa A, et al. Anti-proliferative action of endogenous dehydroepiandrosterone metabolites on human cancer cell lines. Steroids. 2003;68(1):73–83. doi: 10.1016/S0039-128X(02)00117-4. [DOI] [PubMed] [Google Scholar]
- 29.Yang HC, Wu YH, Yen WC, Liu HY, Hwang TL, Stern A, et al. The redox role of G6PD in cell growth, cell death, and cancer. Cells. 2019;8(9):1055. doi: 10.3390/cells8091055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokushige K, Hashimoto E, Kodama K, Tobari M, Matsushita N, Kogiso T, et al. Serum metabolomic profile and potential biomarkers for severity of fibrosis in nonalcoholic fatty liver disease. J Gastroenterol. 2013;48(12):1392–1400. doi: 10.1007/s00535-013-0766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li R, Zha N. Circulating dehydroepiandrosterone sulfate level and cardiovascular or all-cause mortality in the elderly population: a meta-analysis. Ann Palliat Med. 2020;9(5):3537–3545. doi: 10.21037/apm-20-441. [DOI] [PubMed] [Google Scholar]
- 32.Ryerson CJ, Vittinghoff E, Ley B, Lee JS, Mooney JJ, Jones KD, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest. 2014;145(4):723–728. doi: 10.1378/chest.13-1474. [DOI] [PubMed] [Google Scholar]
- 33.Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, et al. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med. 2016;193(10):1168–1175. doi: 10.1164/rccm.201509-1785OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baird GL, Archer-Chicko C, Barr RG, Bluemke DA, Foderaro AE, Fritz JS, et al. Lower DHEA-S levels predict disease and worse outcomes in post-menopausal women with idiopathic, connective tissue disease- and congenital heart disease-associated pulmonary arterial hypertension. Eur Respir J. 2018 doi: 10.1183/13993003.00467-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonnet S, Dumas-de-La-Roque E, Begueret H, Marthan R, Fayon M, Dos Santos P, et al. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA. 2003;100(16):9488–9493. doi: 10.1073/pnas.1633724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki T, Carrier EJ, Talati MH, Rathinasabapathy A, Chen X, Nishimura R, et al. Isolation and characterization of endothelial-to-mesenchymal transition cells in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2018;314(1):L118–L126. doi: 10.1152/ajplung.00296.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, et al. Endothelial–mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43(2):161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashimoto R, Lanier GM, Dhagia V, Joshi SR, Jordan A, Waddell I, et al. Pluripotent hematopoietic stem cells augment α-adrenergic receptor-mediated contraction of pulmonary artery and contribute to the pathogenesis of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2020;318(2):L386–L401. doi: 10.1152/ajplung.00327.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dumas de La Roque E, Savineau JP, Metivier AC, Billes MA, Kraemer JP, Doutreleau S, et al. Dehydroepiandrosterone (DHEA) improves pulmonary hypertension in chronic obstructive pulmonary disease (COPD): a pilot study. Ann Endocrinol. 2012;73(1):20–25. doi: 10.1016/j.ando.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Jankowski CM, Wolfe P, Schmiege SJ, Nair KS, Khosla S, Jensen M, et al. Sex-specific effects of dehydroepiandrosterone (DHEA) on bone mineral density and body composition: a pooled analysis of four clinical trials. Clin Endocrinol. 2019;90(2):293–300. doi: 10.1111/cen.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. List of qPCR primers. Table S2. Medication stratified by DHEAS in the lowest quartile compared to the second to fourth quartile combined in the validation cohort. Figure S1. Flow cytometry framework. Lung fibroblasts were separated from cell debris and analyses was made based on single cells. Cell cycle distribution was evaluated following DAPI staining. DNA damage was estimated from single cells by the presence of γH2AX (negative control = control medium (CM); positive control = 1mM H2O2). Figure S2. Gene expression of fibrotic markers in vitro and ex vivo after DHEA. A) Fibrotic markers EDA-FN, ACTA2, COL1A1 and CTGF from normal human lung fibroblasts incubated in vitro with TGF-β1 and/or DHEA (D150) (T + D150). B) Ex vivo gene expression of PCLS stimulated with a fibrotic cocktail (FC), DHEA (D150) or both (FC + D150). Bars show mean ± SD. (*) p<0.05, (**) p<0.01, (***) p<0.001, (****) p<0.0001. Figure S3. Immunofluorescence staining of EDA-fibronectin and collagen I in PCLS treated with/without DHEA and the fibrotic cocktail. The signal of EDA-fibronectin (EDA-FN, green) and collagen I (red) in PCLS treated with fibrotic cocktail was reduced after addition of DHEA (FC + D150). Nucleus staining with DAPI (blue). Pictures were taken at 10X magnification. (–) scale represent 500 µm. Figure S4. Effect of DHEA on the cell cycle and DNA damage. A) Cell cycle distribution of control lung fibroblasts (n=3) at the beginning of the experiment (0h) and after treatment with DHEA (D150) or in resting medium (RM) for 24 and 48h. B) Frequency of γH2AX+ cells in lung fibroblasts treated with/without DHEA. (****) p<0.0001. compared to the positive control of DNA damage (H2O2 1mM).
Data Availability Statement
The datasets used for the current study are available from the corresponding author on reasonable request.