Abstract
Purpose:
This study aimed at analyzing the prevalence, mortality association, and risk factors for bleeding and thrombosis events (BTEs) among adults supported with venovenous extracorporeal membrane oxygenation (VV-ECMO).
Methods:
We queried the Extracorporeal Life Support Organization registry for adults supported with VV-ECMO from 2010 to 2017. Multivariable logistic regression modeling was used to assess the association between BTEs and in-hospital mortality and the predictors of BTEs.
Results:
Among 7579 VV-ECMO patients meeting criteria, 40.2% experienced ≥ 1 BTE. Thrombotic events comprised 54.9% of all BTEs and were predominantly ECMO circuit thrombosis. BTE rates decreased significantly over the study period (p < 0.001). The inpatient mortality rate was 34.9%. Bleeding events (1.69 [1.49–1.93]) were more strongly associated with in-hospital mortality than thrombotic events (1.23 [1.08–1.41]) p < 0.01 for both. The BTEs most strongly associated with mortality were ischemic stroke (4.50 [2.55–7.97]) and medical bleeding, including intracranial (5.71 [4.02–8.09]), pulmonary (2.02 [1.54–2.67]), and gastrointestinal (1.54 [1.2–1.98]) hemorrhage, all p < 0.01. Risk factors for bleeding included acute kidney injury and pre-ECMO vasopressor support and for thrombosis were higher weight, multisite cannulation, pre-ECMO arrest, and higher PaCO2 at ECMO initiation. Longer time on ECMO, younger age, higher pH, and earlier year of support were associated with bleeding and thrombosis.
Conclusions:
Although decreasing over time, BTEs remain common during VV-ECMO and have a strong, cumulative association with in-hospital mortality. Thrombotic events are more frequent, but bleeding carries a higher risk of inpatient mortality. Differential risk factors for bleeding and thrombotic complications exist, raising the possibility of a tailored approach to VV-ECMO management.
Keywords: Venovenous extracorporeal membrane oxygenation, Bleeding, Thrombosis, Stroke, Survival
Introduction
Venovenous extracorporeal membrane oxygenation (VV-ECMO) is increasingly used for refractory respiratory failure [1], and recent evidence supports benefit in patients who fail conventional treatment [2–5]. However, despite important advances in technology, survival with VV-ECMO remains approximately 60%, likely related to both the severe lung injury necessitating VV-ECMO support as well as ECMO-related complications [1, 6–10]. Bleeding and thrombotic events (BTEs) during VV-ECMO are common, morbid, and potentially lethal [3, 9, 11–14]. The interface between blood and non-biological ECMO circuit elements often leads to activation of coagulation pathways resulting in consumption of both prothrombotic and anti-thrombotic factors, predisposing patients to an increased risk of thrombosis and bleeding [15]. Bleeding events during VV-ECMO, such as intracranial hemorrhage, gastrointestinal hemorrhage, and cannulation site bleeding, are relatively common, with reported rates ranging from 30 to 60% [6, 9, 12, 16–19]. Thrombotic events include circuit thrombosis, oxygenator failure, hemolysis, venous thromboembolism, and ischemic stroke [1, 6, 13, 19–21]. While intracranial hemorrhage has been consistently associated with poor survival [22, 23], the relationship between other BTEs and mortality with VV-ECMO is not well characterized [8, 12].
We recently described the spectrum and impact of BTEs in adult patients supported with VA-ECMO [24]. The existing data regarding BTEs in VV-ECMO are limited by small sample size, single-center setting, or were restricted to the pediatric population [25]. There is little information regarding trends in BTE rates during the modern era of ECMO. We conducted a retrospective analysis of BTEs in adults on VV-ECMO from the Extracorporeal Life Support Organization (ELSO) registry to describe their prevalence and temporal trends, identify risk factors for development, and determine their association with inpatient mortality.
Materials and methods
Data source
The international ELSO registry includes data voluntarily reported from over 400 centers [1] including patient clinical characteristics, circuit components, cannulation configurations, and clinical outcomes. Clinical diagnoses and co-morbidities are reported with International Classification of Diseases, 9th and 10th Revision, Clinical Modification (ICD-9/10-CM) codes. Studies utilizing the ELSO database are exempt from local Institutional Review Board approval as the data are de-identified.
Study population
We included adults (≥ 18 years old) receiving VV-ECMO support from 1/1/2010 through 12/31/2017. We excluded patients with a pre-cannulation diagnosis of coagulopathy or disseminated intravascular coagulation, gastrointestinal or airway bleeding, or stroke (Supplemental Table 1), multiple VV-ECMO runs, and those with missing data for core variables of interest including age, sex, weight, race, cannulation site, and duration and year of ECMO.
Covariates, study groups, and outcomes
Available variables considered in the analysis included baseline demographics, cannulation site strategy, duration of VV-ECMO, year of support, primary indication for ECMO, acute kidney injury (AKI), co-morbid chronic lung disease, pre-ECMO cardiac arrest, vasopressor support prior to ECMO, oxygenator type, gas exchange parameters (pH, PaO2 and PaCO2), and basic ventilator parameters. AKI was defined as the presence of an ICD 9/10 code for acute kidney injury and/or renal replacement therapy within 24 h prior to ECMO initiation. Single-site cannulation was defined as use of a dual-lumen cannula via the right or left internal jugular vein. Primary indications for VV-ECMO were categorized as follows: non-viral pneumonia, viral pneumonia, trauma/burns, aspiration/chemical pneumonitis, asthma, pulmonary hypertension, lung transplant complications, and unspecified acute respiratory distress syndrome (ARDS)/acute respiratory failure (ARF). (Supplemental Table 2). BTEs were classified as surgical bleeding (cardiac tamponade, cannulation, and surgical site bleeding), medical bleeding (pulmonary, gastrointestinal, or intracranial hemorrhage), and thrombotic events including ECMO circuit thrombosis (circuit clotting or oxygenator/pump failure), hemolysis, and ischemic stroke (Supplemental Table 3). Patients were further categorized into the following four subgroups: those without BTE, bleeding only, thrombosis only, and both bleeding and thrombosis. The primary outcome was the association of BTEs with in-hospital mortality. Secondary outcomes included the prevalence and risk factors for BTEs.
Statistical analysis
Categorical variables are presented as counts and percentages and continuous variables as means and standard deviations (SD) or medians and interquartile ranges (IQR), as appropriate. The prevalence of BTEs over time was plotted per 1000 h of ECMO support, and significance was tested using the Mantel–Haenszel trend test. Differences across subgroups were tested by one-way ANOVA or chi-square test as appropriate. Multivariable logistic regression modeling was used to assess the association between the BTEs and in-hospital mortality. Multivariable logistic regression was used to identify risk factors for the following: (1) any bleeding, (2) any thrombosis, (3) medical bleeding, and (4) ECMO circuit thrombosis. Models were adjusted for ECMO duration and the following pre-ECMO characteristics: age, sex, white race, weight, year of support, single-site cannulation, chronic lung disease, AKI, primary diagnoses for VV-ECMO, vasopressor use, cardiac arrest, pH, PaCO2, and the ratio of PaO2 to FiO2 (P/F). To assess for any selection bias resulting from exclusion of patients with missing data on core variables, we compared the clinical features of the total cohort and analytic cohort. Given the moderate rate of missing data for gas exchange parameters, we performed sensitivity analyses excluding these covariates from all models.
Based on previous studies linking changes in gas exchange parameters with neurologic events during VV-ECMO [22, 26], we examined the relationship between the 24-h change in PaO2 (ΔPaO2 = 24-h PaO2 – baseline PaO2) and PaCO2 (ΔPaCO2 = 24-h PaCO2 – baseline PaCO2) with ischemic stroke and intracranial hemorrhage (ICH). To avoid inclusion of extreme, nonsensical values for these parameters, outliers ≥ 99% percentile, and ≤ 1% percentile were excluded. Spline curves were generated to examine the relationship between ΔPaO2 and ΔPaCO2 with the odds of ischemic stroke and ICH (Supplemental Fig. 3). Based on the observed non-linear relationships seen with ΔPaO2, it was included in the multivariable models as a two-level continuous variable with a cutoff point at 80 mmHg. Baseline pH was removed from these models due to significant collinearity with ΔPaCO2. The association between the primary diagnosis for VV-ECMO and the occurrence of any BTE was analyzed with multivariable logistic regression adjusted for the same covariates. A two-sided p value < 0.05 was considered significant. All analyses were performed using SAS version 9.4 (Cary, NC).
Results
Pre-ECMO characteristics
Among 11,880 adults treated with VV-ECMO between 2010 and 2017, 7579 met study criteria (Fig. 1). Patients were predominantly males (60.4%) and of white race (61.6%). Mean age was 46.5 (± 15.4) years and weight was 87.5 (± 28.3) kg. The most common indications for VV-ECMO were non-viral pneumonia (26.9%), viral pneumonia (20.6%), and unspecified ARDS/ARF (18.3%). Prior to ECMO support, 15.7% patients had AKI, 57.2% required vasopressors and 7.2% suffered a cardiac arrest. At ECMO initiation, mean P/F ratio was 75.7 (± 74.3), mean PaCO2 was 60.2 (± 32) mmHg, and mean pH was 7.23 (± 0.15). A single-site cannulation strategy was used in 36.7% of patients. The median duration of VV-ECMO support was 8 (5, 15) days (Table 1). The characteristics of the total cohort were very similar to the analytic cohort (Supplemental Table 4).
Fig. 1.
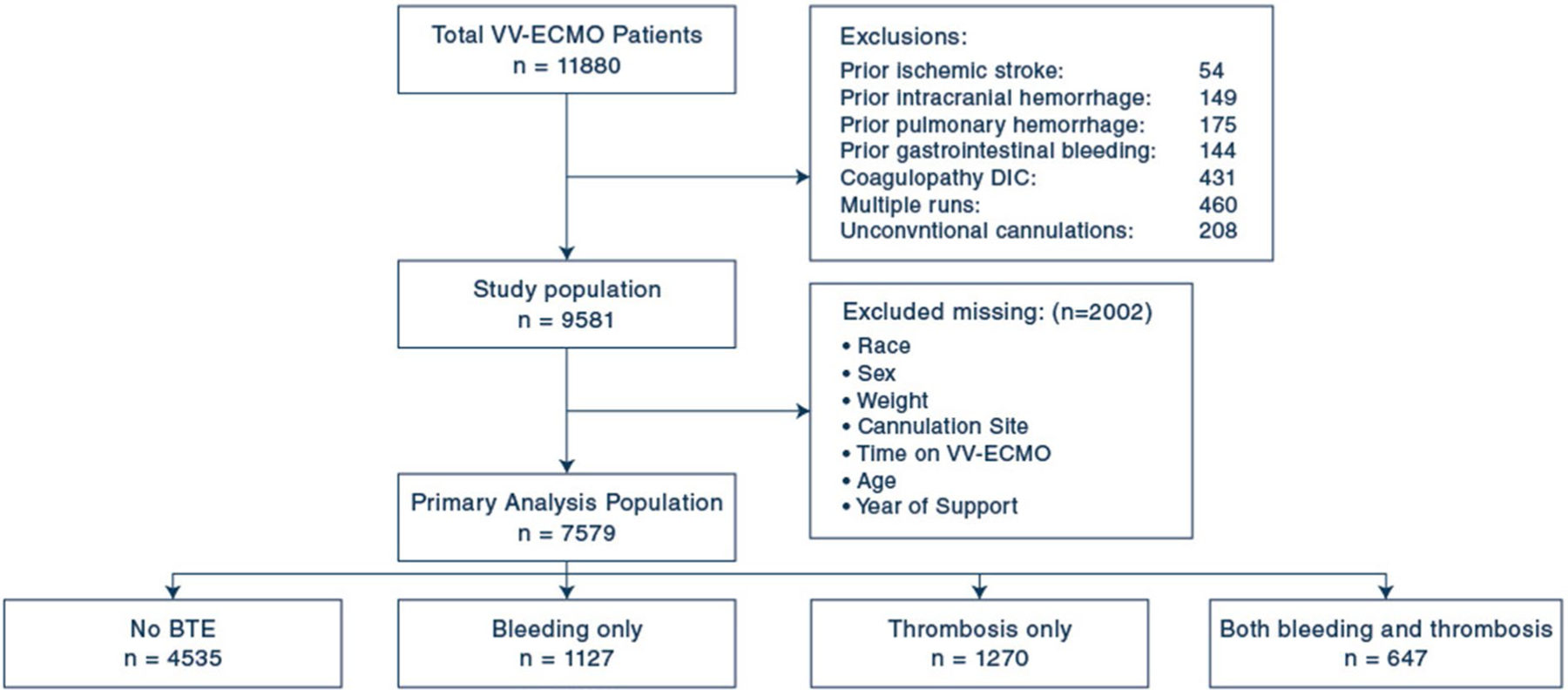
Flow diagram of patient selection for the primary analysis. Flow chart depicting inclusion and exclusion criteria. VV, venovenous; ECMO, extracorporeal life support
Table 1.
Demographics, clinical characteristics and type of event by category of bleeding and thrombotic events
| Characteristic | All patients | Category of BTE | p value | |||
|---|---|---|---|---|---|---|
| None | Bleeding only | Thrombosis only | Both | |||
| n = 7579 | n = 4535 | n = 1127 | n = 1270 | n = 647 | ||
| Age, years—mean ± SD | 46.5 ± 15.4 | 47.3 ± 15.5 | 46 ± 15.1 | 45.6 ± 14.9 | 43.7 ± 15.1 | < 0.001 |
| Female sex—no. (%) | 2999 (39.6) | 1815 (40) | 460 (40.8) | 479 (37.7) | 245 (37.9) | 0.29 |
| Weight, kg—mean ± SD | 87.5 ± 28.3 | 86.9 ± 27.7 | 85.7 ± 27.8 | 90.5 ± 29.9 | 89.1 ± 29.7 | < 0.001 |
| White race—no. (%) | 4668 (61.6) | 2783 (61.4) | 694 (61.6) | 800 (63) | 391 (60.4) | 0.001 |
| Primary diagnosis—no. (%) | ||||||
| Non-viral pneumonia | 1905 (26.9) | 1090 (26.1) | 307 (27.8) | 318 (26.9) | 190 (30.3) | 0.14 |
| Viral pneumonia | 1463 (20.6) | 774 (18.5) | 263 (23.8) | 265 (22.5) | 161 (25.6) | < 0.001 |
| Trauma/burn | 1021 (14.4) | 539 (12.9) | 182 (16.5) | 181 (15.3) | 119 (18.9) | < 0.001 |
| Pneumonitis | 407 (5.7) | 234 (5.6) | 71 (6.4) | 65 (5.5) | 37 (5.9) | 0.73 |
| Asthma | 394 (5.6) | 272 (6.5) | 56 (5.1) | 45 (3.8) | 21 (3.3) | < 0.001 |
| Pulmonary hypertension | 179 (2.5) | 98 (2.3) | 25 (2.3) | 39 (3.3) | 17 (2.7) | 0.28 |
| Lung transplant complications | 170 (2.4) | 104 (2.5) | 28 (2.5) | 30 (2.5) | 8 (1.3) | 0.29 |
| Unspecified ARDS/ARF | 1387 (18.3) | 846 (18.7) | 182 (16.1) | 248 (19.5) | 111 (17.2) | 0.13 |
| Chronic respiratory conditions—no. (%) | ||||||
| ILD | 512 (7.2) | 317 (7.6) | 80 (7.3) | 69 (5.8) | 46 (7.3) | 0.24 |
| COPD | 250 (3.5) | 154 (3.7) | 47 (4.3) | 35 (3) | 14 (2.2) | 0.1 |
| Cystic fibrosis | 147 (2.1) | 87 (2.1) | 18 (1.6) | 27 (2.3) | 15 (2.4) | 0.65 |
| Cannulation strategy—no. (%) | ||||||
| Single cannulation site | 2778 (36.7) | 1677 (37) | 432 (38.3) | 415 (32.7) | 254 (39.3) | 0.007 |
| Pre-ECMO vasopressor—no. (%) | 4336 (57.2) | 2506 (55.3) | 722 (64.1) | 719 (56.6) | 389 (60.1) | < 0.001 |
| Pre-ECMO renal failure—no. (%) | 1192 (15.7) | 666 (14.7) | 230 (20.4) | 190 (15) | 106 (16.4) | < 0.001 |
| Pre-ECMO Cardiac Arrest-no. (%) | 538 (7.2) | 349 (7.9) | 66 (5.9) | 86 (6.8) | 37 (5.8) | 0.04 |
| Pre-ECMO pH—mean ± SD | 7.23 ± 0.15 | 7.22 ± 0.16 | 7.23 ± 0.14 | 7.24 ± 0.14 | 7.25 ± 0.13 | < 0.001 |
| Pre-ECMO PaCO2, mmHg—mean ± SD | 60.2 ± 32 | 60 ± 33 | 58.7 ± 34.1 | 61.9 ± 29.2 | 61 ± 26.5 | 0.11 |
| Pre-ECMO PaO2, mmHg—mean ± SD | 67.6 ± 54.1 | 68.8 ± 57.6 | 65 ± 56.3 | 67.9 ± 45.7 | 64.1 ± 39.7 | 0.08 |
| Respiratory rate, rpm—mean ± SD | 22.9 ± 8.2 | 22.7 ± 8.1 | 22.7 ± 8.3 | 23.1 ± 8.4 | 24 ± 8.6 | 0.005 |
| FiO2, %—mean ± SD | 93.5 ± 14.1 | 93.3 ± 14.4 | 93.5 ± 13.9 | 94 ± 13.6 | 94.2 ± 13.1 | 0.32 |
| P/F ratio—mean ± SD | 75.7 ± 74.3 | 77.1 ± 78.2 | 72.8 ± 73.2 | 76.8 ± 73.7 | 69.9 ± 45.5 | 0.09 |
| PIP, cmH2O—mean ± SD | 34.9 ± 10.6 | 34.4 ± 9.9 | 35.4 ± 12.4 | 35.4 ± 10.9 | 36.4 ± 10.8 | < 0.001 |
| PEEP, cmH2O—mean ± SD | 13 ± 5.6 | 12.8 ± 5.6 | 12.9 ± 5.8 | 13.5 ± 5.4 | 13.4 ± 5.7 | < 0.001 |
| Mean airway pressure, cmH2O—mean ± SD | 24.5 ± 11.4 | 24.2 ± 11.3 | 25.7 ± 12.5 | 24.2 ± 10.6 | 25.5 ± 11.6 | 0.02 |
| Membrane oxygenator material | < 0.001 | |||||
| PMP | 4426 (58.4) | 2547 (56.2) | 693 (61.5) | 749 (59) | 437 (67.5) | |
| PP | 584 (7.7) | 321 (7.1) | 87 (7.7) | 120 (9.4) | 56 (8.7) | |
| Silicone | 6 (0.1) | 4 (0.1) | 0 (0) | 1 (0.1) | 1 (0.2) | |
| Time on ECMO, days—median (IQR) | 8 (5, 15) | 7 (4, 11) | 10 (6, 17) | 12 (7, 20) | 17 (10, 28) | < 0.001 |
| Cannulation site bleeding—no. (%) | 743 (9.8) | – | 466 (41.3) | – | 277 (42.8) | < 0.001 |
| Surgical site bleeding—no. (%) | 460 (6.1) | – | 289 (25.6) | – | 171 (26.4) | < 0.001 |
| Tamponade—no. (%) | 58 (0.8) | – | 36 (3.2) | – | 22 (3.4) | < 0.001 |
| Gastrointestinal bleeding—no. (%) | 397 (5.2) | – | 219 (19.4) | – | 178 (27.5) | < 0.001 |
| Pulmonary bleeding—no. (%) | 291 (3.8) | – | 167 (14.8) | – | 124 (19.2) | < 0.001 |
| Intracranial hemorrhage—no. (%) | 216 (2.8) | – | 161 (14.3) | – | 55 (8.5) | < 0.001 |
| Circuit clotting—no. (%) | 1276 (16.8) | – | – | 841 (66.2) | 435 (67.2) | < 0.001 |
| Oxygenator/pump failure—no. (%) | 575 (7.6) | – | – | 361 (28.4) | 214 (33.1) | < 0.001 |
| Hemolysis—no. (%) | 399 (5.3) | – | – | 255 (20.1) | 144 (22.3) | < 0.001 |
| Ischemic stroke—no. (%) | 92 (1.2) | – | – | 55 (4.3) | 37 (5.7) | < 0.001 |
Values are expressed as mean ± standard deviation or median (interquartile range) for continuous variables, and absolute frequency (percentages) for categorical variables. Continuous variables compared using one-way ANOVA and categorical variables compared using chi-square test
BTE, bleeding and thrombotic event; ARDS, acute respiratory distress syndrome; ARF, acute respiratory failure; ILD, interstitial lung disease; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; PIP, peak inspiratory pressure; PEEP, positive end-expiratory pressure; PMP, polymethylpentene; PP, polypropylene
Prevalence and trends of BTEs
Of 3044 (40.2%) patients who experienced at least one BTE, 41.7% experienced only thrombotic events, 37% had only bleeding events, and 21.2% experienced both. Among 4840 total BTEs, 54.9% were thrombotic events (Fig. 2). The most common thrombotic events were circuit clotting (31.8%) and oxygenator/pump failure (12.7%). The most common bleeding events were cannulation (15.5%) and surgical site (9.6%) bleeding. Medical bleeding comprised 18.7% of BTEs (906/4840). Strokes accounted for 6.4% of events, with intracranial hemorrhage more prevalent than ischemic stroke (4.5% vs 1.9%, respectively). Over the study period, the rates of any bleeding, any thrombosis, and ECMO circuit thrombosis decreased significantly, while medical bleeding decreased more modestly. Rates of both ischemic stroke and intracranial hemorrhage remained relatively stable during the study period (Supplemental Fig. 1).
Fig. 2.
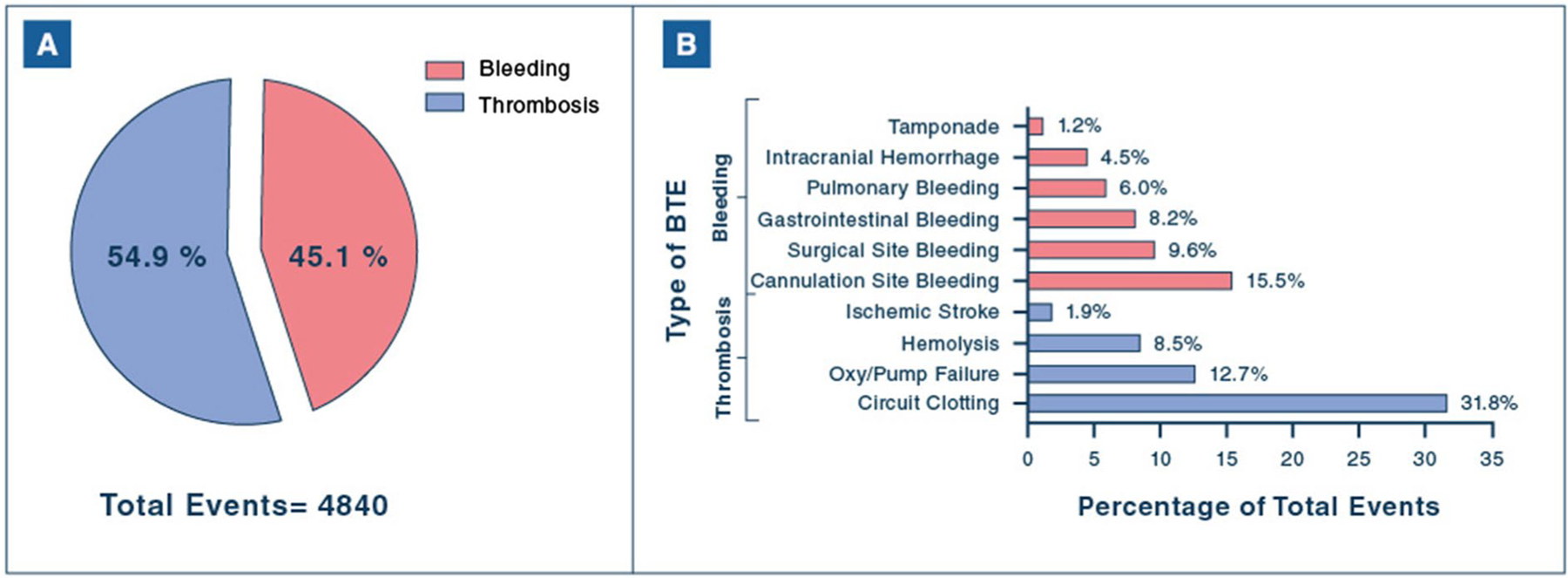
Frequency of bleeding and thrombotic events during VV-ECMO. A Proportion of BTEs comprised of bleeding or thrombotic events. B Frequency of specific types of BTEs. BTEs, bleeding and thrombotic events; Oxy/pump failure, oxygenator/pump failure
BTEs and mortality
In-hospital mortality occurred in 2648 (34.9%) patients. Patients experiencing BTEs had higher mortality compared to those without BTEs as follows: no BTEs 30.6%, thrombosis only 36.1%, bleeding only 46.1%, and both complications 43.7% (p < 0.001). A cumulative increase in mortality was observed in patients experiencing none (1387, 30.6%), 1 (761, 40.5%), 2 (312, 40.7%), and ≥ 3 (188, 47.5%) BTEs (p < 0.001), an effect that persisted after multivariable modeling: 1 BTE: aOR [95% CI] 1.66 [1.45–1.90]; 2 BTEs 1.68 [1.38–2.05]; ≥ 3 BTEs: 2.16 [1.65–2.83], p < 0.001 for all.
The occurrence of any bleeding event was associated with higher mortality than any thrombotic event (Fig. 3). Medical bleeding events were associated with high mortality, whereas none of the surgical bleeding events was significantly associated with mortality. Among thrombotic events, mortality risk was markedly increased with ischemic stroke and modestly increased with oxygenator/pump failure and hemolysis. Circuit clotting was not significantly associated with mortality. The mortality rate associated with each type of BTE were as follows: ischemic stroke 73.9%, intracranial hemorrhage 73.2%, pulmonary bleeding 53.6%, gastrointestinal bleeding 48.6%, tamponade 46.6%, oxygenator/pump failure 45.7%, hemolysis 42.2%, surgical site bleeding 39.6%, cannula site bleeding 39.3%, and circuit clotting 34.7%.
Fig. 3.
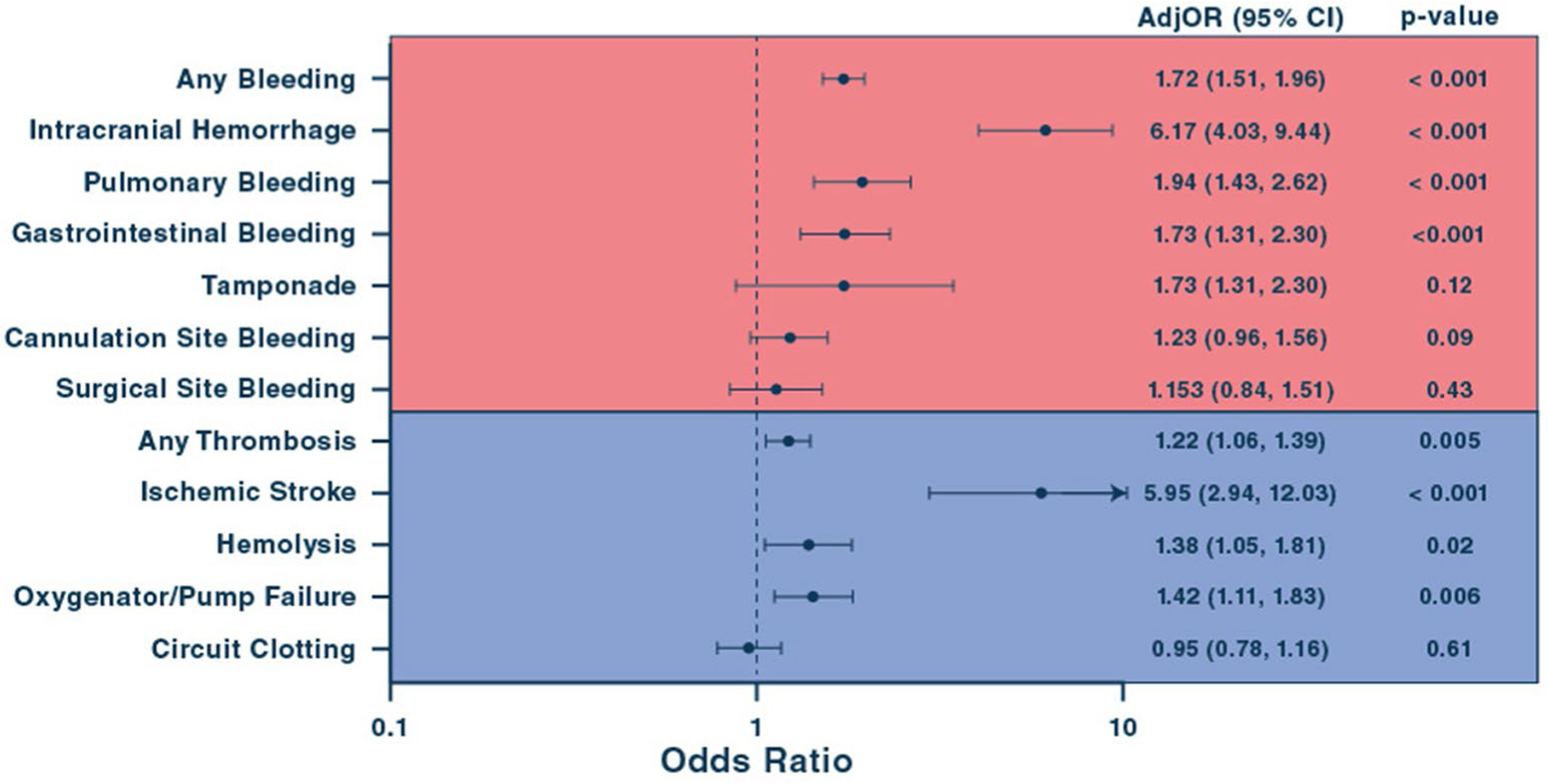
Association of bleeding and thrombotic events during VV-ECMO with in-hospital mortality. Association of BTEs with in-hospital mortality during VV-ECMO. AdjOR, adjusted odds ratio; CI, confidence interval
Predictors of BTEs
Risk factors for any bleeding and medical bleeding are summarized in Fig. 4. Pre-ECMO AKI, vasopressor use, and longer ECMO duration were significantly associated with higher odds of any bleeding and medical bleeding. Later year of ECMO support was associated with a lower risk any bleeding and retained a similar relationship but nominally lost statistical significance for medical bleeding. Older age and lower pH were associated with lower risk of any bleeding but showed no relationship with medical bleeding. There was a weak but significant relationship between higher baseline P/F ratio and lower odds of medical bleeding.
Fig. 4.
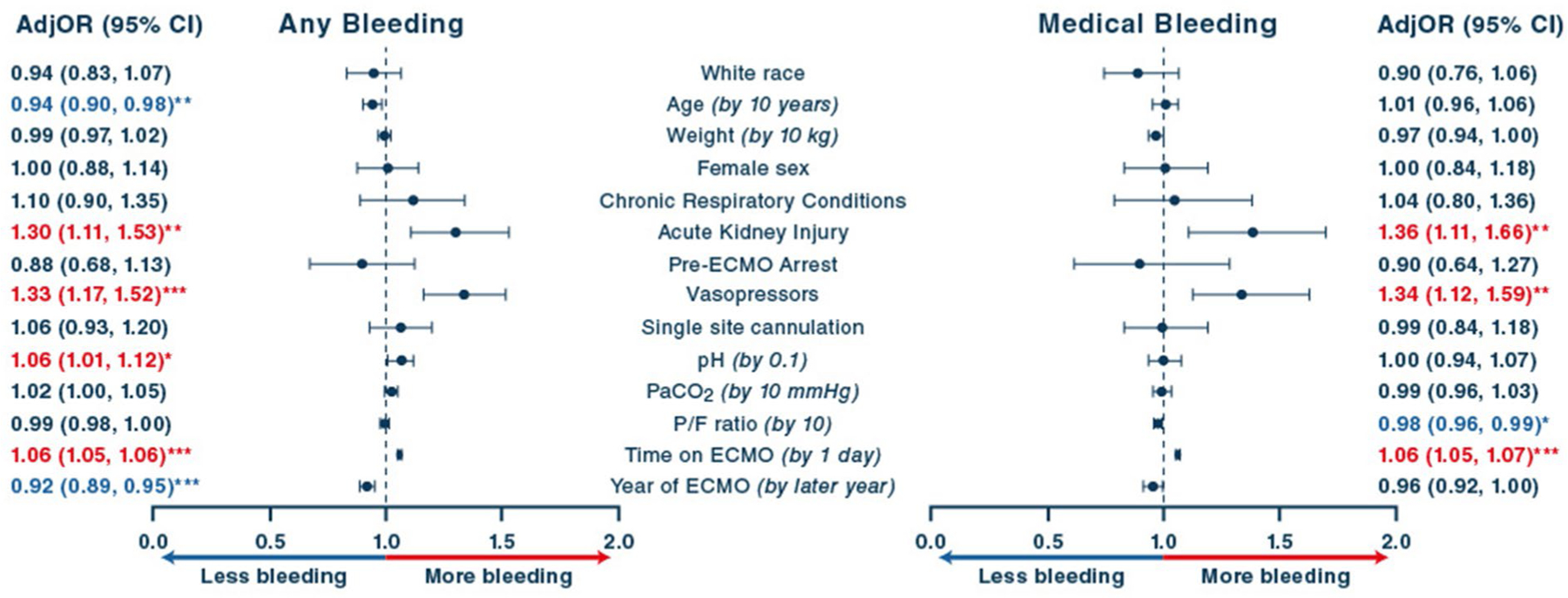
Clinical and circuit characteristics associated with bleeding events. Factors associated with any bleeding events and medical bleeding (including intracranial hemorrhage, pulmonary and gastrointestinal bleeding). AdjOR, adjusted odds ratio; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; PaCO2, arterial partial pressure of carbon dioxide; P/F, PaO2/FiO2 ratio. The unit of observation for all models is the patient. *p < 0.05; **p < 0.01; ***p < 0.001
Risk factors associated with any thrombosis and ECMO circuit thrombosis are summarized in Supplemental Fig. 2. Higher weight, longer ECMO duration, and higher pre-ECMO PaCO2 and pH were all associated with a significantly higher risk of any thrombosis and ECMO circuit thrombosis. Older age, single site cannulation, and later year of ECMO support were all associated with a lower risk of any thrombosis and ECMO circuit thrombosis. Female sex was associated with a modest but significantly lower risk of ECMO circuit thrombosis. Pre-ECMO arrest was a significant risk factor for any thrombosis but not ECMO circuit thrombosis. Sensitivity analyses removing gas exchange parameters from the models did not produce any dramatic changes in these relationships (Supplemental Tables 5 and 6). Notably, AKI became significantly associated with a lower risk of ECMO circuit thrombosis (0.82 [0.69–0.99], p = 0.034).
Risk factors for ischemic stroke included lower P/F ratio prior to ECMO initiation and a ΔPaO2 > 80 mmHg (Fig. 5). For every 10 mmHg increase in ΔPaO2 above 80 mmHg, there was a 15% increased odds of ischemic stroke. Notably, pre-ECMO cardiac arrest had a strong but non-significant association with ischemic stroke, with adj-OR approaching 2.0 for patients with cardiac arrest prior to ECMO. Patients with single-site cannulation had a substantially lower adj-OR of ischemic stroke, but the association did not retain statistical significance in the multivariable model. Risk factors for ICH included non-white race, the need for vasopressors prior to ECMO initiation, and a ΔPaO2 < 80 mmHg. There was no significant association with ΔPaCO2 and ischemic stroke or intracranial hemorrhage (Fig. 5; Supplemental Fig. 3).
Fig. 5.

Clinical and circuit characteristics associated with ischemic stroke and intracranial hemorrhage. Factors associated with ischemic stroke and intracranial hemorrhage. AdjOR, adjusted odds ratio; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen, P/F, PaO2/FiO2 ratio. The unit of observation for all models is the patient. *p < 0.05; **p < 0.01; ***p < 0.001.
The proportion of patients with BTEs varied across the major primary diagnoses for VV-ECMO: highest among patients with viral pneumonia and trauma, and lowest in patients cannulated for asthma (Supplemental Fig. 4, panel A). After multivariable modeling, only lung transplant complications and trauma/burns were significantly associated with any BTEs (Supplemental Fig. 4, panel B). The rates of specific BTEs varied modestly across the primary diagnosis groups (Supplemental Table 7).
Discussion
This is the largest, multi-center study of BTEs among adults supported with VV-ECMO. The major findings of our study are the following:
The prevalence of BTEs is decreasing over time but they remain common complications of VV-ECMO, occurring in ~ 40% of patients.
Thrombotic events are more common than bleeding events.
On balance, the mortality associated with bleeding events exceeded that of thrombotic events, with the caveat that venous thromboembolism is not reported in the registry.
There is a spectrum of severity for BTEs with respect to in-hospital mortality, with medical bleeding events and ischemic stroke associated with the worst outcomes.
There are differential risk factors for BTEs.
Thrombotic events predominated in this VV-ECMO cohort, which contrasts with the spectrum of BTEs observed in VA-ECMO patients where bleeding events were more common [24]. This difference could be related to a variety of factors including longer ECMO runs, lack of arterial cannulation, infrequent need for invasive surgical procedures, and a general tendency toward less aggressive anticoagulation with VV- versus VA-ECMO. The prevalence of both bleeding and thrombotic events steadily decreased over time, which could be explained by several factors. The now widely used hollow-fiber polymethylpentene oxygenators reduce erythrocyte consumption and better preserve coagulation factors and platelets [27–29]. Novel biopassive surfaces have improved hemocompatibility of the extracorporeal circuit [30], and improved pump and cannula designs have led to more favorable laminar blood flow [31]. There is more training and utilization of percutaneous cannulation [32, 33]. Finally, there has been a general trend towards less aggressive anticoagulation strategies, with some centers deferring systemic anticoagulation altogether in VV-ECMO [34–36]. Medical bleeding events decreased more gradually over time, suggesting that the measures above may not be sufficient to mitigate these complications.
We identified a wide range of severity across the different BTEs regarding their association with mortality. Consistent with prior studies, bleeding events were more strongly associated with mortality than thrombotic events, particularly driven by medical bleeding [9, 12, 16, 25]. Surgical bleeding did not confer a substantial impact on mortality, perhaps because these events are more amenable to correction and less likely to provoke organ damage than hemorrhage in the brain, lungs or gastrointestinal tract. As expected, ischemic stroke and intracranial hemorrhage were strongly associated with increased mortality. Given that ischemic stroke is rare and may not be directly related to the ECMO circuit, and the other “thrombotic complications” of VV-ECMO are not as strongly associated with increased mortality, the high mortality associated with medical bleeding supports studies aimed at mitigating bleeding complications such as using less intensive anticoagulation strategies [37, 38]. However, we acknowledge that the majority of thrombotic events captured in our study represent ECMO circuit thrombosis, and we lack data on some important thrombotic events, particularly venous thromboembolism, highlighting the need for additional studies examining the full spectrum of clinical thrombotic events on VV-ECMO outcomes.
Several risk factors for BTEs were identified, including ECMO duration, age, weight, initial pH, PaCO2, AKI, vasopressor use, and single-site cannulation. Prolonged time on ECMO was associated with increased risk for both bleeding and thrombosis. Protracted exposure of blood to the extracorporeal circuit can result in progressive derangements in clotting factors and coagulation, predisposing patients to both bleeding and clotting [15, 24]. While patients with more profound lung injury often require longer VV-ECMO runs and illness severity could increase BTE risk, this finding highlights the paramount importance of minimizing ECMO duration and frequently reassessing patients’ readiness for decannulation.
AKI and vasopressor use prior to ECMO were strong risk factors for overall bleeding and medical bleeding events, and these factors had no association with thrombotic events. Prior studies have identified renal dysfunction as a risk factor for gastrointestinal bleeding in critically ill patients [39, 40] and for intracranial hemorrhage in patients on VV-ECMO [41]. The enhanced bleeding propensity could be related to platelet dysfunction known to occur with renal failure [42]. Hypotension is a risk factor for gastrointestinal bleeding [40] and, combined with vasopressor use, could provoke microcirculatory dysfunction leading to tissue friability and tendency to bleed once ECMO and subsequent anticoagulation are initiated. In our cohort, AKI was present in 15% and vasopressor use in over 50% of patients at ECMO initiation, and these clinical features with significant differential impact on BTEs could be weighed by clinicians when considering the anticoagulation strategy.
Older age was associated with lower risk of both bleeding and thrombosis, although the association with thrombotic risk was more robust and there was no relationship with medical bleeding. The inverse association between age and BTE risk may be partially explained by signals of higher illness acuity and longer ECMO duration in the younger patients. In younger patients with greater physiologic reserve, clinicians are more likely to consider ECMO and offer extended runs to await either lung recovery or transplantation. While inclusion of ECMO duration did not substantially alter the association of age with bleeding or thrombotic events, residual confounding could explain some of the effect of age on BTE risk.
Higher weight was associated with a greater risk of thrombotic events, including ECMO circuit thrombosis. That parallels the finding of higher body mass index as a risk factor for pump thrombosis in patients with durable left ventricular assist devices [43, 44]. This association between weight and thrombosis has been described in other clinical contexts and may be driven by chronic inflammation and impaired fibrinolysis [45, 46].
Higher pH at ECMO initiation was associated with increased risk of both bleeding and thrombosis, but not medical bleeding. There is evidence that acidosis impairs coagulation [47, 48], and pH closer to the normal range at ECMO initiation may enhance clot formation when blood is exposed to an extracorporeal circuit.
We found that lower P/F ratio prior to ECMO and large increases in oxygen tension at 24 h after VV-ECMO initiation were associated with increased risk of ischemic stroke. There was a progressive increase in the risk of ischemic stroke for values of ΔPaO2 above ~ 80 mmHg. These findings suggest that hyperoxemia, particularly following a period of severe hypoxemia, may play a substantial role in ischemic stroke. The mechanism underlying this association requires further study but could be explained by hyperoxemia-associated vasoconstriction or direct free radical-mediated toxicity [49, 50]. Conversely, persistent hypoxemia with minimal or no improvement in PaO2 after 24 h of ECMO was associated with an increased risk of intracranial hemorrhage. Collectively, these results suggest a U-shaped relationship with respect to ΔPaO2 and acute brain injury and that there may be an optimal range for correction of PaO2 within the first 24 h of VV-ECMO support, ranging from 20 to 80 mmHg increase in PaO2 from the pre-ECMO baseline. These findings require validation in prospective studies before recommending changes to clinical practice.
We did not find a strong correlation with 24-h ΔPaCO2 and either ischemic stroke or ICH. In a recent ELSO registry analysis, Cavayas et al. demonstrated that large relative reductions in PaCO2 at 24 h after ECMO support were independently associated with a composite of adverse neurological events. Notably, this study included respiratory failure patients managed with VA-ECMO and hybrid ECMO configurations. Brain death and non-specific “neurological complications” drove the relationship with relative ΔPaCO2, and among patients with large versus smaller relative ΔPaCO2, the rates of ischemic stroke were similar and there was only a modestly higher rate of ICH. In a large single-center analysis, Luyt et al. found that large reductions in PaCO2 (ΔPaCO2 > − 27 mmHg) were associated with increased risk of ICH, but notably they examined very early correction in PaCO2 at 2 h after VV-ECMO initiation. The harm from excessive PaCO2 reduction may occur with very early, rapid corrections of hypercarbia, and PaCO2 assessments in the first few hours after ECMO may be more relevant to the risk of ICH than 24-h values. The results from these prior studies as well as our current analysis suggest that clinicians should avoid excessive correction of both PaO2 and PaCO2 early after initiating VV-ECMO to minimize the risk of adverse neurologic events.
Unexpectedly, single-site cannulation was associated with a lower risk of thrombotic complications, including ECMO circuit thrombosis. A single cannula could have allowed for better patient mobilization or may have been chosen preferentially for patients likely to mobilize early, leading to a reduced risk of thrombosis. Compared to typical femoro-jugular cannulations, dual-lumen single-site cannulas avoid the femoral veins and require less total cannula length in the venous system, potentially reducing the risk of circuit clotting. Future studies should explore the association of cannulation strategy and BTEs.
The primary diagnosis leading to VV-ECMO could influence the risk of BTEs. We found that patients cannulated for lung transplant complications or trauma/burns had significantly higher odds of BTEs. The clinical context surrounding VV-ECMO initiation may influence the risk of BTEs, and additional studies are needed to examine the risk of specific BTEs among key patient subgroups receiving VV-ECMO.
The large size and multi-center, international nature of this cohort support the generalizability of our results and aided in the precise characterization of key risk factors for BTEs. By highlighting the relationships between clinical and circuit parameters with BTEs, we believe this analysis paves the way for future studies aimed at a precision medicine approach with algorithms for anticoagulation in VV-ECMO which incorporate estimations of patient-specific risk. However, our findings should be considered hypothesis-generating, and prospective clinical trials are required before making meaningful changes to clinical practice.
Limitations
Our study has a number of limitations. We excluded patients with missing data on core variables, which could have introduced bias. However, total cohort analyses with multiple imputation for missing data produced similar findings to the analytic cohort. We adjusted models for key available covariates, but residual unmeasured confounders could have affected our results. ELSO does not collect data on certain important BTEs including upper respiratory bleeding, deep vein thrombosis, or pulmonary embolism, and the impact of these events on mortality remains uncertain [51, 52]. Given that venous thromboembolism is not collected in the registry, the influence of thrombotic complications is likely underestimated in our study. Crucial details of the anticoagulation strategy and laboratory coagulation parameters are not available, preventing an assessment of how anticoagulation interacts with clinical factors to influence BTE risk. The ELSO registry does not require secondary diagnoses prior to ECMO support, so co-morbidities and concomitant organ failures are likely underreported. Some parameters of illness severity required to calculate critical care risk scores were not available. Only in-hospital outcomes are available, so we could not assess the impact of BTEs on long-term outcomes. Data on the exact timing of BTEs during VV-ECMO was unavailable, precluding an assessment of time-varying hazard. The duration of ECMO support is a strong risk factor for BTEs and thus we included it as a covariate in all models. While none of the covariates were strongly associated with ECMO duration, we cannot exclude residual confounding from ECMO duration on BTE risk. Finally, this analysis used multivariate regression models on observational data, and causality cannot be inferred.
Conclusion
Despite advances in ECMO technology and management, BTEs remain common during VV-ECMO and have a cumulative association with in-hospital mortality. Bleeding events are less common than thrombotic events but convey a substantially higher risk of death. Our results demonstrate a spectrum of impact for specific BTEs on mortality, with ischemic stroke and medical bleeding events carrying the strongest association with poor outcomes. We identified several clinical and circuit factors that modulate the risk of BTEs during VV-ECMO, and we believe these findings can contribute to future studies examining a more tailored approach to VV-ECMO management.
Supplementary Material
Take-home message.
Bleeding and thrombotic events (BTEs) are common during VV-ECMO, with thrombotic events more prevalent than bleeding. Bleeding events confer a higher risk of mortality, with medical bleeding and ischemic stroke carrying the strongest associations. Differential risk factors exist for bleeding and thrombotic events. Identification of groups at highest risk for bleeding and thrombosis may pave the way for a more tailored approach to anticoagulation in patients undergoing VV-ECMO.
Conflicts of interest
JIN: no relationships with industry. AFG: no relationships with industry. BOG: consulting for Sedana Medical. KFK: no relationships with industry. DA: at-large member of the Steering Committee for the Extracorporeal Life Support Organization (ELSO). DB: receives research support from ALung Technologies. He has been on the medical advisory boards for Abiomed, Xenios, Medtronic, and Cellenkos. He is the President-elect of ELSO. PR: no relationships with industry. SS: No relationships with industry. ARG: receives research support from Abbott. EWG: ELSO Research Grant.
Footnotes
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1007/s00134-021-06593-x.
Ethical approval
Studies utilizing the ELSO database are exempt from Institutional Review Board approval due to the retrospective analysis of de-identified data.
Availability of data and material
The datasets analyzed for this study are available from the Extracorporeal Life Support Organization via data acquisition request.
References
- 1.Thiagarajan RR, Barbaro RP, Rycus PT, McMullan DM, Conrad SA, Forten-berry JD, Paden ML (2017) Extracorporeal life support organization registry international report 2016. ASAIO J 63(1):60–67. 10.1097/mat.0000000000000475 [DOI] [PubMed] [Google Scholar]
- 2.Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A (2018) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 378(21):1965–1975. 10.1056/NEJMoa1800385 [DOI] [PubMed] [Google Scholar]
- 3.Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E (2019) Veno-venous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med 7(2):163–172. 10.1016/s2213-2600(18)30452-1 [DOI] [PubMed] [Google Scholar]
- 4.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374(9698):1351–1363. 10.1016/s0140-6736(09)61069-2 [DOI] [PubMed] [Google Scholar]
- 5.Combes A, Peek GJ, Hajage D, Hardy P, Abrams D, Schmidt M, Dechartres A, Elbourne D (2020) ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med 46(11):2048–2057. 10.1007/s00134-020-06248-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaquer S, de Haro C, Peruga P, Oliva JC, Artigas A (2017) Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann Intensive Care 7(1):51. 10.1186/s13613-017-0275-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramanathan K, Tan CS, Rycus P, MacLaren G (2017) Extracorporeal Membrane Oxygenation for Adult Community-Acquired Pneumonia: Outcomes and Predictors of Mortality*. Crit Care Med 45(5):814–821. 10.1097/ccm.0000000000002320 [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, Scheinkestel C, Cooper DJ, Brodie D, Pellegrino V, Combes A, Pilcher D (2014) Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 189(11):1374–1382. 10.1164/rccm.201311-2023OC [DOI] [PubMed] [Google Scholar]
- 9.Mazzeffi M, Greenwood J, Tanaka K, Menaker J, Rector R, Herr D, Kon Z, Lee J, Griffith B, Rajagopal K, Pham S (2016) Bleeding, transfusion, and mortality on extracorporeal life support: ECLS Working Group on Thrombosis and Hemostasis. Ann Thorac Surg 101(2):682–689. 10.1016/j.athoracsur.2015.07.046 [DOI] [PubMed] [Google Scholar]
- 10.Schmidt M, Hodgson C, Combes A (2015) Extracorporeal gas exchange for acute respiratory failure in adult patients: a systematic review. Crit Care 19:99. 10.1186/s13054-015-0806-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver WC (2009) Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth 13(3):154–175. 10.1177/089253209347384 [DOI] [PubMed] [Google Scholar]
- 12.Aubron C, DePuydt J, Belon F, Bailey M, Schmidt M, Sheldrake J, Murphy D, Scheinkestel C, Cooper DJ, Capellier G, Pellegrino V, Pilcher D, McQuilten Z (2016) Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care 6(1):97. 10.1186/s13613-016-0196-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krueger K, Schmutz A, Zieger B, Kalbhenn J (2017) Venovenous extracorporeal membrane oxygenation with prophylactic subcutaneous anticoagulation only: an observational study in more than 60 patients. Artif Organs 41(2):186–192. 10.1111/aor.12737 [DOI] [PubMed] [Google Scholar]
- 14.Wu MY, Lin PJ, Tseng YH, Kao KC, Hsiao HL, Huang CC (2014) Venovenous extracorporeal life support for posttraumatic respiratory distress syndrome in adults: the risk of major hemorrhages. Scand J Trauma Resusc Emerg Med 22:56. 10.1186/s13049-014-0056-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annich GM (2015) Extracorporeal life support: the precarious balance of hemostasis. J Thromb Haemost 13(Suppl 1):S336–342. 10.1111/jth.12963 [DOI] [PubMed] [Google Scholar]
- 16.Kreyer S, Muders T, Theuerkauf N, Spitzhüttl J, Schellhaas T, Schewe J-C, Guenther U, Wrigge H, Putensen C (2017) Hemorrhage under venovenous extracorporeal membrane oxygenation in acute respiratory distress syndrome patients: a retrospective data analysis. J Thorac Dis 9(12):5017–5029. 10.21037/jtd.2017.11.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu MY, Chang YS, Huang CC, Wu TI, Lin PJ (2017) The impacts of baseline ventilator parameters on hospital mortality in acute respiratory distress syndrome treated with venovenous extracorporeal membrane oxygenation: a retrospective cohort study. BMC Pulm Med 17(1):181. 10.1186/s12890-017-0520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YJ, Kim DJ, Kim JS, Lee JH, Lee CT, Jheon S, Cho YJ (2015) Experience and results with VV-ECMO for severe acute respiratory failure: weaning versus nonweaning. ASAIO J 61(2):184–189. 10.1097/mat.0000000000000174 [DOI] [PubMed] [Google Scholar]
- 19.Shaefi S, Brenner SK, Gupta S, O’Gara BP, Krajewski ML, Charytan DM et al. (2021) Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med 47(2):208–221. 10.1007/s00134-020-06331-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sy E, Sklar MC, Lequier L, Fan E, Kanji HD (2017) Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. J Crit Care 39:87–96. 10.1016/j.jcrc.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 21.Cooper E, Burns J, Retter A, Salt G, Camporota L, Meadows CI, Langrish CC, Wyncoll D, Glover G, Ioannou N, Daly K, Barrett NA (2015) Prevalence of venous thrombosis following venovenous extracorporeal membrane oxygenation in patients with severe respiratory failure. Crit Care Med 43(12):e581–584. 10.1097/ccm.0000000000001277 [DOI] [PubMed] [Google Scholar]
- 22.Luyt CE, Brechot N, Demondion P, Jovanovic T, Hekimian G, Lebreton G, Nieszkowska A, Schmidt M, Trouillet JL, Leprince P, Chastre J, Combes A (2016) Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med 42(5):897–907. 10.1007/s00134-016-4318-3 [DOI] [PubMed] [Google Scholar]
- 23.Lorusso R, Gelsomino S, Parise O, Di Mauro M, Barili F, Geskes G, Vizzardi E, Rycus PT, Muellenbach R, Mueller T, Pesenti A, Combes A, Peek G, Frenckner B, Di Nardo M, Swol J, Maessen J, Thiagarajan RR (2017) Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: findings from the extracorporeal life support organization database. Crit Care Med 45(8):1389–1397. 10.1097/ccm.0000000000002502 [DOI] [PubMed] [Google Scholar]
- 24.Chung M, Cabezas FR, Nunez JI, Kennedy KF, Rick K, Rycus P, Mehra MR, Garan AR, Kociol RD, Grandin EW (2020) Hemocompatibility-related adverse events and survival on venoarterial extracorporeal life support. JACC Heart Fail 8(11):892–902. 10.1016/j.jchf.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalton HJ, Garcia-Filion P, Holubkov R, Moler FW, Shanley T, Heidemann S, Meert K, Berg RA, Berger J, Carcillo J, Newth C, Harrison R, Doctor A, Rycus P, Dean JM, Jenkins T, Nicholson C (2015) Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med 16(2):167–174. 10.1097/pcc.0000000000000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavayas YA, Munshi L, Sorbo Ld, Fan E (2020) The early change in PaCO2 after extracorporeal membrane oxygenation initiation is associated with neurological complications. Am J Respir Crit Care Med 201(12):1525–1535. 10.1164/rccm.202001-0023OC [DOI] [PubMed] [Google Scholar]
- 27.Khoshbin E, Roberts N, Harvey C, Machin D, Killer H, Peek GJ, Sosnowski AW, Firmin RK (2005) Poly-methyl pentene oxygenators have improved gas exchange capability and reduced transfusion requirements in adult extracorporeal membrane oxygenation. ASAIO J 51(3):281–287. 10.1097/01.mat.0000159741.33681.f1 [DOI] [PubMed] [Google Scholar]
- 28.Peek GJ, Killer HM, Reeves R, Sosnowski AW, Firmin RK (2002) Early experience with a polymethyl pentene oxygenator for adult extracorporeal life support. ASAIO J 48(5):480–482. 10.1097/00002480-200209000-00007 [DOI] [PubMed] [Google Scholar]
- 29.Toomasian JM, Schreiner RJ, Meyer DE, Schmidt ME, Hagan SE, Griffith GW, Bartlett RH, Cook KE (2005) A polymethylpentene fiber gas exchanger for long-term extracorporeal life support. Asaio j 51(4):390–397. 10.1097/01.mat.0000169111.66328.a8 [DOI] [PubMed] [Google Scholar]
- 30.Ontaneda A, Annich GM (2018) Novel surfaces in extracorporeal membrane oxygenation circuits. Front Med (Lausanne) 5:321. 10.3389/fmed.2018.00321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohler K, Valchanov K, Nias G, Vuylsteke A (2013) ECMO cannula review. Perfusion 28(2):114–124. 10.1177/0267659112468014 [DOI] [PubMed] [Google Scholar]
- 32.Ganslmeier P, Philipp A, Rupprecht L, Diez C, Arlt M, Mueller T, Pfister K, Hilker M, Schmid C (2011) Percutaneous cannulation for extracorporeal life support. Thorac Cardiovasc Surg 59(2):103–107. 10.1055/s-0030-1250635 [DOI] [PubMed] [Google Scholar]
- 33.Rupprecht L, Lunz D, Philipp A, Lubnow M, Schmid C (2015) Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel 7(4):320–326 [PMC free article] [PubMed] [Google Scholar]
- 34.Kurihara C, Walter JM, Karim A, Thakkar S, Saine M, Odell DD, Kim S, Tomic R, Wunderink RG, Budinger GRS, Bharat A (2020) Feasibility of venovenous extracorporeal membrane oxygenation without systemic anticoagulation. Ann Thorac Surg. 10.1016/j.athoracsur.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamarche Y, Chow B, Bédard A, Johal N, Kaan A, Humphries KH, Cheung A (2010) Thromboembolic events in patients on extracorporeal membrane oxygenation without anticoagulation. Innovations (Phila) 5(6):424–429. 10.1097/IMI.0b013e3182029a83 [DOI] [PubMed] [Google Scholar]
- 36.Hermann A, Schellongowski P, Bojic A, Robak O, Buchtele N, Staudinger T (2019) ECMO without anticoagulation in patients with disease-related severe thrombocytopenia: feasible but futile? Artif Organs 43(11):1077–1084. 10.1111/aor.13514 [DOI] [PubMed] [Google Scholar]
- 37.Agerstrand CL, Burkart KM, Abrams DC, Bacchetta MD, Brodie D (2015) Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg 99(2):590–595. 10.1016/j.athoracsur.2014.08.039 [DOI] [PubMed] [Google Scholar]
- 38.Aubron C, McQuilten Z, Bailey M, Board J, Buhr H, Cartwright B, Dennis M, Hodgson C, Forrest P, McIlroy D, Murphy D, Murray L, Pellegrino V, Pilcher D, Sheldrake J, Tran H, Vallance S, Cooper DJ (2019) Low-dose versus therapeutic anticoagulation in patients on extracorporeal membrane oxygenation: a pilot randomized trial. Crit Care Med 47(7):e563–e571. 10.1097/ccm.0000000000003780 [DOI] [PubMed] [Google Scholar]
- 39.Cook D, Heyland D, Griffith L, Cook R, Marshall J, Pagliarello J (1999) Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. Crit Care Med 27(12):2812–2817. 10.1097/00003246-199912000-00034 [DOI] [PubMed] [Google Scholar]
- 40.MacLaren R, Reynolds PM, Allen RR (2014) Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med 174(4):564–574. 10.1001/jamainternmed.2013.14673 [DOI] [PubMed] [Google Scholar]
- 41.An SJ, Kim TJ, Yoon BW (2017) Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke 19(1):3–10. 10.5853/jos.2016.00864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boccardo P, Remuzzi G, Galbusera M (2004) Platelet dysfunction in renal failure. Semin Thromb Hemost 30(5):579–589. 10.1055/s-2004-835678 [DOI] [PubMed] [Google Scholar]
- 43.Boyle AJ, Jorde UP, Sun B, Park SJ, Milano CA, Frazier OH, Sundareswaran KS, Farrar DJ, Russell SD (2014) Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol 63(9):880–888. 10.1016/j.jacc.2013.08.1656 [DOI] [PubMed] [Google Scholar]
- 44.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Myers S, Acker MA, Rogers J, Slaughter MS, Stevenson LW (2015) Pump thrombosis in the Thoratec HeartMate II device: an update analysis of the INTERMACS Registry. J Heart Lung Transplant 34(12):1515–1526. 10.1016/j.healun.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 45.Blokhin IO, Lentz SR (2013) Mechanisms of thrombosis in obesity. Curr Opin Hematol 20(5):437–444. 10.1097/MOH.0b013e3283634443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, Tang H (2016) Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell Mol Immunol 13(4):432–442. 10.1038/cmi.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engström M, Schött U, Romner B, Reinstrup P (2006) Acidosis impairs the coagulation: a thromboelastographic study. J Trauma 61(3):624–628. 10.1097/01.ta.0000226739.30655.75 [DOI] [PubMed] [Google Scholar]
- 48.Ramaker AJ, Meyer P, van der Meer J, Struys MM, Lisman T, van Oeveren W, Hendriks HG (2009) Effects of acidosis, alkalosis, hyperthermia and hypothermia on haemostasis: results of point of care testing with the thromboelastography analyser. Blood Coagul Fibrinol 20(6):436–439. 10.1097/MBC.0b013e32832dc327 [DOI] [PubMed] [Google Scholar]
- 49.Vincent JL, Taccone FS, He X (2017) Harmful effects of hyperoxia in postcardiac arrest, sepsis, traumatic brain injury, or stroke: the importance of individualized oxygen therapy in critically ill patients. Can Respir J 2017:2834956. 10.1155/2017/2834956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hafner S, Beloncle F, Koch A, Radermacher P, Asfar P (2015) Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care 5(1):42. 10.1186/s13613-015-0084-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parzy G, Daviet F, Persico N, Rambaud R, Scemama U, Adda M, Guervilly C, Hraiech S, Chaumoitre K, Roch A, Papazian L, Forel JM (2019) Prevalence and risk factors for thrombotic complications following venovenous extracorporeal membrane oxygenation: a CT scan study. Crit Care Med. 10.1097/ccm.0000000000004129 [DOI] [PubMed] [Google Scholar]
- 52.Bidar F, Lancelot A, Lebreton G, Pineton de Chambrun M, Schmidt M, Hékimian G, Juvin C, Bréchot N, Schoell T, Leprince P, Combes A, Bouglé A, Luyt CE (2021) Venous or arterial thromboses after venoarterial extracorporeal membrane oxygenation support: frequency and risk factors. J Heart Lung Transplant 40(4):307–315. 10.1016/j.healun.2020.12.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed for this study are available from the Extracorporeal Life Support Organization via data acquisition request.


