Abstract
Objective:
To understand predictors of adverse pregnancy outcomes (APOs) among women on antiretroviral treatment (ART).
Design:
Longitudinal cohort
Methods:
Participants from the Mobile WAChX trial were evaluated for APOs, including stillbirth (SB, fetal death at ≥20 weeks’ gestation), preterm birth (PTB, livebirth at <37 weeks’ gestation) and neonatal death (NND, ≤28 days after live birth). Predictors were determined by univariable and multivariable Cox proportional hazards and log-binomial models.
Results:
Among 774 women included, median age was 27 years and 29.0% had unsuppressed HIV viral load (VL >1,000 copies/mL) at enrollment. Half (55.1%) started ART pre-pregnancy, 89.1% on tenofovir-based regimens. Women with depression had higher risk of SB (adjusted hazard ratio [aHR] 2.93, 95%CI 1.04–8.23), and women with lower social support score had higher risk of late SB (aHR 11.74, 2.47–55.86). Among 740 livebirths, 201 (27.2%) were preterm and 22 (3.0%) experienced NND. PTB was associated with unsuppressed maternal VL (adjusted prevalence ratio [aPR] 1.28, 95%CI 1.02–1.61), intimate partner violence (IPV) in pregnancy (aPR 1.94, 1.28–2.94), and history of any sexually transmitted infection (STI) (aPR 1.63, 1.06–2.51). NND was associated with PTB (PR 2.53, 1.10–5.78) and STI history (PR 4.25, 1.39–13.06). Most associations retained significance in the subgroup of women with viral suppression.
Conclusions:
Maternal viremia during pregnancy predicted PTB as did IPV, lower education and STI history, while psychosocial stressors predicted SB. Implementing mental health services, ART adherence, partner support, and routine STI screening and treatment could reduce APOs among women with HIV in sub-Saharan Africa settings.
Keywords: pregnant women, HIV infection, reverse transcriptase inhibitors, stillbirth, preterm birth, neonatal death, viral load
Introduction
An estimated 90% of the 1.4 million pregnancies in women living with HIV (WLWH) annually occur in sub-Saharan Africa (SSA)[1]. There is consistent evidence that WLWH have worse pregnancy outcomes, including preterm birth (PTB), low birth weight (LBW), small for gestational age (SGA), stillbirth (SB), and neonatal death (NND) than women without HIV infection[2]. World Health Organization (WHO) guidelines recommend antiretroviral therapy (ART) for all pregnant WLWH for prevention of mother-to-child HIV transmission (PMTCT) and maternal health benefits[3]. First-line ART regimens for pregnant WLWH include a dual-nucleotide reverse transcriptase inhibitor (NRTI) [tenofovir disoproxil fumarate (TDF) with lamivudine (3TC) or emtricitabine (FTC)], plus a protease inhibitor (PI)-based regimen or a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimen; more recently dolutegravir (DTG)-based regimens are recommended[3]. Global scale-up of ART has resulted in more WLWH in Africa accessing ART and continued decline in new pediatric HIV infections[4].
There is evidence that ART use in pregnancy may affect pregnancy outcomes[5–9], however, ART associations with adverse pregnancy outcomes (APOs) may differ depending on the specific APO evaluated as well as the regimen and duration of treatment. For example, the PROMISE trial reported significantly higher risk of APOs and infant mortality among women receiving TDF-based ART (TDF/FTC/LPV/r) than women receiving zidovudine (ZDV) plus single-dose nevirapine (NVP)[10]. In contrast, the TSEPAMO study in Botswana showed women receiving TDF/FTC/efavirenz (EFV) had the lowest rate of any APO among all women on ART[11], and the DART trial in Uganda/Zimbabwe showed no difference in neonatal mortality between TDF (+NVP) and non-TDF ART[12].
There is also mixed evidence regarding how sociodemographic factors such as food security, stress, sexually transmitted infections (STIs), and mental health status influence pregnancy outcomes among WLWH[13]. Elevated maternal HIV viral load (VL) during pregnancy, even in the context of ART, has been associated with poor perinatal outcomes compared to HIV-negative women[14]. This may be due to immune activation or immune compromise with resultant vulnerability to other infections[15]. Dysregulation of inflammatory cytokine production at the maternal-fetal interface may also contribute to APOs, and this may differ by ART regimen and timing[16–20]. Other HIV-related factors, including clinical stage of disease and CD4 cell count, have been associated with APOs[10,13,21–23]. In this prospective cohort study, we evaluated APOs among WLWH in Kenya and determined potential predictors, with a focus on HIV-related factors as well as mental health, sexual and reproductive history, and partnership.
Methods
Study design and population
This study leveraged data collected from a completed 3-armed randomized clinical trial (RCT) (Mobile WAChX study, ClinicalTrial.gov number NCT02400671, 2015/11/22–2017/05/04). The parent RCT assessed short messaging service (SMS) to improve ART adherence and retention among WLWH in Kenya attending the PMTCT program. Parent study procedures and results have been previously reported[24]. Briefly, the trial enrolled pregnant WLWH from six public maternal-child health (MCH) clinics in Nairobi and Western Kenya if they were aged ≥14 years and had daily access to a mobile phone at the time of enrollment. Women were randomized to receive one-way SMS, two-way SMS or no SMS, and followed-up throughout 2 years postpartum[24]. One woman died before delivery. Gestational age (GA) at delivery was determined based on last menstrual period (LMP) date and delivery date. This study used all available data from the RCT; no additional sample size calculations were conducted prior to secondary analyses. The parent study was approved by the University of Washington (UW) Institutional Review Board (IRB) and the Kenyatta National Hospital/University of Nairobi Ethical Review Committee; no additional IRB approval was required for this analysis.
Data collection
At enrollment in the parent trial, a standardized survey on a tablet using Open Data Kit (ODK) was administered. Data was collected on demographics, family planning, social support (using Medical Outcomes Study [MOS] survey[25]), stigma (using 4-item instrument adapted from the stigma scale for chronic illnesses [SSCI][26]), depression (using Patient Health Questionnaire 9 [PHQ9][27]), intimate partner violence (IPV) (using Abuse Assessment Screen [AAS][28]), food security (using Household Food Insecurity Access Scale [HFIAS][29]), disclosure of HIV status, history of any STI, and ART knowledge (using 15 items from the LifeWindows ART adherence questionnaire[30]). Data on ART use was abstracted from the Mother Child Health (MCH) booklet. HIV VL testing was conducted with maternal plasma samples collected at enrollment[24].
Study outcome
This study assessed three APOs. Stillbirth (SB) was defined as fetal death at ≥20 weeks’ gestation. Analysis of SB was restricted to women enrolled prior to 20 weeks’ gestation, and a second analysis of late SB (defined as SB at 28–36 weeks’ gestation) was conducted among women enrolled prior to 28 weeks’ gestation. Preterm birth (PTB) was defined as live birth at <37 weeks’ gestation. Analysis of PTB was restricted to women enrolled prior to 37 weeks’ gestation, and a secondary analysis of very PTB (defined as live birth at 28–32 weeks’ gestation) was conducted among women enrolled prior to 28 weeks’ gestation. Neonatal death (NND) was defined as an infant death within 28 days. Analysis of NND was conducted among women with live birth.
Statistical analysis
Chi-square tests were used to compare categorical variables and Welch two sample t-tests for continuous variables between women having APOs and women without APOs. Kaplan-Meier survival curves were used to assess time-to-SB and incidence rate (IR) of SB. Cox proportional hazards regression counting time-at-risk from enrollment to delivery was used to identify predictors of SB. Log-binomial regression was used to identify predictors of PTB and NND. Proportions of each APO and an overall proportion of having any APO were compared by TDF- or ZDV-based NRTI ART regimen, EFV- or NVP-based NNRTI ART regimen, and ART initiation time before or during pregnancy using chi-square tests. Study site, dichotomized as Nairobi (Mathare, Riruta) and Western Kenya (Ahero, Bondo, Siaya, Rachuonyo), was identified as an a priori confounder in all regression models to account for potential geographical differences in maternal characteristics and underlying APOs. Covariates with p-value <0.05 in univariate models were included in multivariate analyses. Sub-group stratified analyses were conducted among women who were virally suppressed at enrollment. All models used robust standard errors. All analyses were conducted using RStudio Version 1.2.5042 (RStudio, Inc).
Results
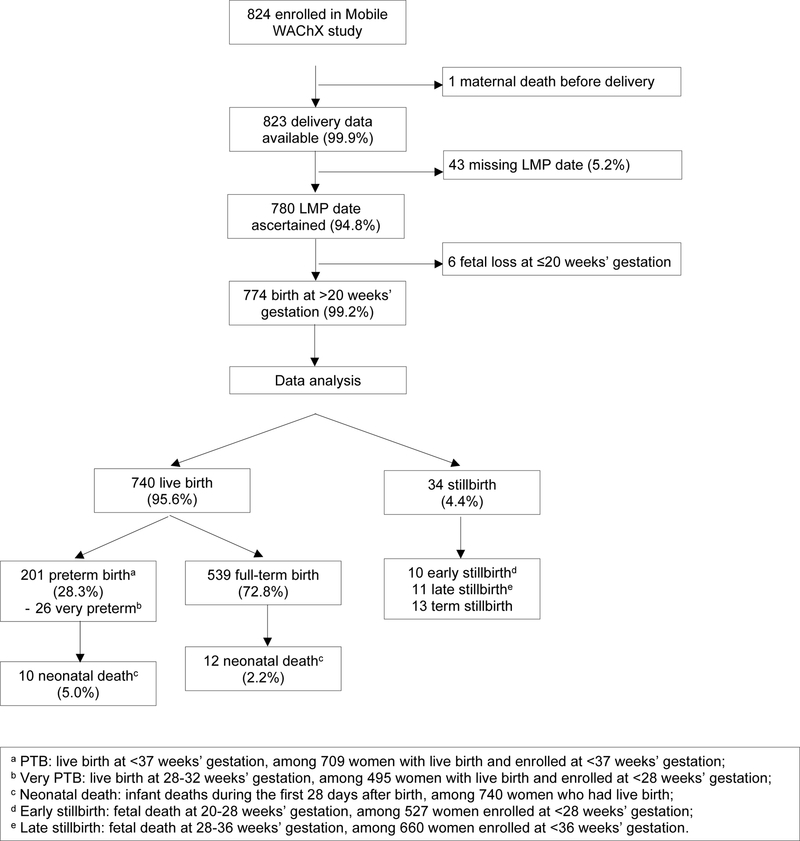
Among 824 women enrolled in the RCT, 1 died prior to delivery, 53 had no LMP data available, 6 had a miscarriage and 774 (93%) women were included in the APO analysis (Figure 1). Among the 774 included women, median age was 27 (interquartile range [IQR] 23–31) years, and median GA of pregnancy at enrollment was 24 (IQR 18–30) weeks (Table 1). Most women completed primary school (77.3%) and were married or cohabiting with a partner (84.2%). About half (46.9%) reported moderate or severe food insecurity (by HFIAS scoring) and 24.4% women had at least moderate depression symptoms (PHQ9 score >5). Seven percent (53/762) of women reported travel time >60 minutes from home to clinic. Among 26 (3.4%) women who reported ever having STI, 57.7% had syphilis. Overall, 21 (2.7%) women reported ever experienced IPV since pregnancy. At enrollment, 28.8% of women had unsuppressed VL. Most women (92.5%) were already on ART at enrollment, 59.6% (461/773) reported diagnosis with HIV before pregnancy, 55.1% (425/771) reported starting ART before pregnancy, and among 723 women who reported ART regimen, most (74.1%) were on TDF+3TC/FTC+EFV as recommended by WHO guidelines.
Figure 1.
Study flowchart
Table 1.
Participant baseline characteristics (N=774)
| N | n (Percent) or median (IQR) | |
|---|---|---|
| Study site | 774 | |
| Western Kenya | 487 (62.9%) | |
| Nairobi | 287 (37.1%) | |
| Sociodemographic | ||
| Age (year) | 774 | 27 (23–31) |
| Education level | 774 | |
| Primary school completed | 598 (77.3%) | |
| Secondary school completed | 198 (25.6%) | |
| Married/cohabiting | 774 | 652 (84.2%) |
| Food insecuritya | 774 | |
| Level 1 (secure) | 326 (42.1%) | |
| Level 2 (mild) | 85 (11.0%) | |
| Level 3 (moderate) | 151 (19.5%) | |
| Level 4 (severe) | 212 (27.4%) | |
| Employed* | 772 | 391 (50.6%) |
| Depressionb | 774 | 189 (24.4%) |
| Social support scorec (percent) | 774 | 64 (50–72) |
| IPV since pregnancy | 774 | 21 (2.7%) |
| Travel time to clinic >60 min* | 762 | 53 (7.0%) |
| Obstetric | ||
| Gestational age at enrollment (week)d | 774 | 24 (18–30) |
| Primigravida | 774 | 109 (14.1%) |
| History of sexually transmitted infection | 774 | 26 (3.4%) |
| Genital infection | 4 (15.4%) | |
| Gonorrhea | 4 (15.4%) | |
| Syphilis | 15 (57.7%) | |
| Chlamydia | 1 (3.8%) | |
| Acute HIV infection | 1 (3.8%) | |
| Cervicitis | 1 (3.8%) | |
| Pregnancy intended* | 771 | 433 (56.2%) |
| HIV/ART | ||
| Diagnosis before pregnancy* | 773 | 461 (59.6%) |
| Disclosure to anyone* | 759 | 623 (82.1%) |
| Unsuppressed viral load (>1,000 copies/mL) | 774 | 226 (29.0%) |
| ART status | 774 | |
| Yes, on ART at enrollment | 716 (92.5%) | |
| No, newly prescribed ART | 43 (5.6%) | |
| No, not on ART | 15 (1.9%) | |
| ART regimen* | 723 | |
| TDF + 3TC/FTC + EFV | 536 (74.1%) | |
| TDF + 3TC + LPV/r | 10 (1.4%) | |
| TDF + 3TC/FTC + NVP | 99 (13.7%) | |
| ZDV + 3TC + NVP | 41 (5.7%) | |
| ZDV + 3TC + EFV | 4 (0.6%) | |
| Other | 33 (4.6%) | |
| Started ART before pregnancy* | 771 | 425 (55.1%) |
| IMB scoree | 716 | 75 (67–80) |
| Partner tested for HIVf | 616 | 479 (77.8%) |
evaluated by Household Food Insecurity Access Scale (HFIAS)
evaluated by Patient Health Questionnaire 9 (PHQ9), a score >5 indicating at least moderate depressive symptoms
evaluated by Medical Outcomes Study (MOS) survey
estimated by self-reported date of last menstruation period (LMP)
Information-Motivation-Behavioral (IMB) score evaluated by 15 items from LifeWindows ART adherence questionnaire
among women who reported having a partner.
denominator <774 due to missing data.
Any adverse pregnancy outcome
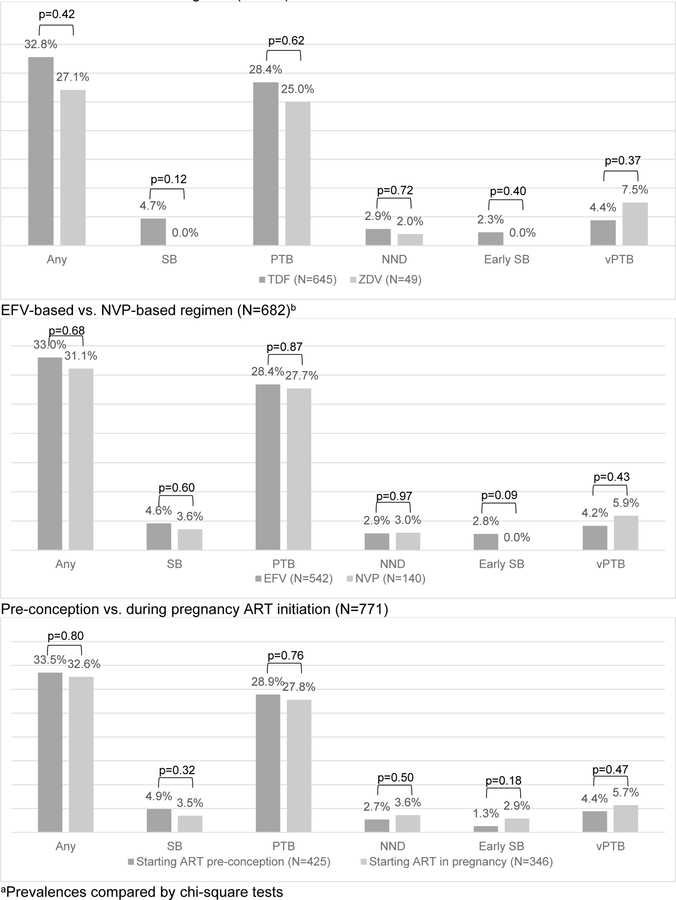
Overall, 34 (4.4%) women experienced a SB, including 10 early SB, 11 late SB and 13 term SB (Figure 1). Among 740 women with live birth, 201 (28.3%) had a PTB (including 26 very PTB) and 22 (3.0%) had a NND (Figure 1). The prevalence of SB, PTB or NND did not significantly differ between TDF-based vs. ZDV-based NRTI regimen, or EFV-based vs. NVP-based NNRTI regimen (Figure 2). Similarly, a combined prevalence of women experiencing any APO did not significantly differ by regimen. There was no difference in prevalence of APOs between women who started ART pre-conception versus those who started ART in pregnancy (Figure 2). In addition, we did not find a difference in APOs between women on NNRTI-based regimens versus TDF/ZDV-based regimens.
Figure 2. Prevalence of adverse pregnancy outcome by ART usea.
TDF-based vs. ZDV-based regimen (N=694)
Incidence and cofactors for stillbirth
Among 235 women who were enrolled at <20 weeks’ gestation, 17 (7.2%) had a SB. The overall IR of SB was 17.2 per 100 person-years (Table 2). Adjusting for site, the risk of SB was significantly higher in women having at least moderate depressive symptoms (HR 2.92, 95%CI 1.09–7.81), and in women reporting a partner had been tested for HIV (HR 0.36, 95%CI 0.13–1.01; p=0.05) (Table 2). To determine if partner HIV testing reflected better social support, we compared social support in women who reported partner HIV testing versus those who did not and found significantly higher prevalence of high social support in women reporting partner HIV testing versus those who did not (53.4% vs. 42.3%, p=0.02). Among 517 women enrolled at <28 weeks’ gestation, 11 (2.1%) had late SB, with the overall IR of late SB of 6.2 per 100 person-years. Adjusting for site, the risk of late SB was significantly higher in women who had social support score below a median of 63 (HR 11.62, 95%CI 1.58–85.5) (Table 2). Adjusting for site and HIV VL at enrollment, the association between SB and depression remained similar (adjusted HR [aHR] 2.93, 95%CI 1.04–8.23), as did the association between late SB and lower social support score (aHR 11.74, 95%CI 2.47–55.86) (Table 2).
Table 2.
Incidence and covariates of stillbirth
| Any SB among women enrolled at <20 weeks’ gestation | Late SB among women enrolled at <28 weeks’ gestation | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| All (N=235)* | VL suppressed (N=170) | All (N=517)* | VL suppressed (N=367) | |||||
|
| ||||||||
| Group | Event/py (IR/100py) | HRa (95%CI); p | Event/py (IR/100py) | HRa (95%CI); p | Event/py (IR/100py) | HRa (95%CI); p | Event/py (IR/100py) | HRa (95%CI); p |
|
| ||||||||
| Overall | 17/99 (17.16) | 13/72.7 (17.89) | 11/176.1 (6.25) | 9/127.2 (7.07) | ||||
|
| ||||||||
| Site | ||||||||
| Western Kenya | 8/66.0 (12.11) | 0.43(0.18–1.07);0.069 | 7/50.4 (13.9) | 0.50(0.18–1.39);0.182 | 7/114.9 (6.09) | 0.94(0.28–3.19);0.918 | 5/85.9 (5.82) | 0.60(0.16–2.22);0.441 |
| Nairobi | 9/32.9 (27.33) | Ref | 6/22.3 (26.88) | Ref | 4/61.1 (6.55) | - | 4/41.3 (9.67) | Ref |
|
| ||||||||
| Age | ||||||||
| AYA (15–24 years) | 5/35.5 (14.08) | 0.87(0.3–2.52);0.794 | 5/24.0 (20.86) | 1.67(0.52–5.34);0.384 | 4/57.3 (6.99) | 1.24(0.36–4.25);0.731 | 3/36.3 (8.25) | 1.34(0.34–5.27);0.677 |
| Older adult (≥25 years) | 12/63.5 (18.91) | Ref | 8/48.7 (16.42) | Ref | 7/118.7 (5.9) | Ref | 6/90.9 (6.6) | Ref |
|
| ||||||||
| Depressionb | ||||||||
| Moderate/severe symptoms | 7/27.0 (25.9) | 2.92(1.09–7.81);0.033 | 5/18.0 (27.84) | 2.41(0.81–7.14);0.112 | 3/43.8 (6.85) | 1.21(0.33–4.39);0.771 | 2/26.1 (7.66) | 1.26(0.26–6.04);0.769 |
| None/mild | 10/71.9 (13.9) | Ref | 8/54.7 (14.62) | Ref | 8/132.2 (6.05) | Ref | 7/101.1 (6.92) | Ref |
|
| ||||||||
| Food insecurityc | ||||||||
| Level 4 | 8/29.7 (26.93) | 1.68(0.63–4.51);0.3 | 7/20.3 (34.55) | 2.33(0.73–7.49);0.155 | 2/48.2 (4.15) | 0.61(0.14–2.75);0.521 | 1/33.7 (2.97) | 0.37(0.05–2.88);0.342 |
| Level 1/2/3 | 9/69.3 (12.99) | Ref | 6/52.4 (11.45) | Ref | 9/127.8 (7.04) | Ref | 8/93.5 (8.55) | Ref |
|
| ||||||||
| Social supportd | ||||||||
| <median | 9/46.2 (19.46) | 1.65(0.68–4.02);0.266 | 9/32.9 (27.36) | 3.15(1.12–8.9);0.03 | 10/81.7 (12.24) | 11.62(1.58–85.54);0.016 | 8/58.0 (13.79) | 10.05(1.33–76);0.025 |
| ≥median | 8/52.7 (15.17) | Ref | 4/39.8 (10.05) | Ref | 1/94.3 (1.06) | Ref | 1/69.2 (1.44) | Ref |
|
| ||||||||
| Travel time to clinic | ||||||||
| >60 min | 2/9.0 (22.21) | 1.63 (0.41–6.53); 0.489 | 2/6.0 (33.1) | 2.05 (0.54–7.74); 0.288 | 1/13.5 (7.39) | 1.28 (0.16–10.06); 0.812 | 0/9.2 (0) | - |
| ≤60 min | 15/88.2 (17.01) | Ref | 11/65.8 (16.71) | Ref | 10/159.7 (6.26) | Ref | 9/116.7 (7.71) | Ref |
|
| ||||||||
| History of STIe | ||||||||
| Yes | 1/4.1 (24.46) | 2.41 (0.33–17.63); 0.385 | 1/3.1 (32.5) | 2.54(0.34–18.74);0.36 | 1/6.9 (14.47) | 2.62(0.31–22.41);0.378 | 1/5.0 (19.86) | 3.49(0.41–29.59);0.251 |
| No | 16/94.4 (16.94) | Ref | 12/69.1 (17.36) | Ref | 10/168.6 (5.93) | Ref | 8/121.7 (6.57) | Ref |
|
| ||||||||
| Diagnosis | ||||||||
| Before pregnancy | 8/67.9 (11.78) | 0.49(0.2–1.22);0.126 | 7/59.3 (11.81) | 0.31(0.11–0.9);0.032 | 6/114.8 (5.23) | 0.88(0.27–2.88);0.838 | 5/100.2 (4.99) | 0.55(0.15–1.95);0.353 |
| During pregnancy | 9/31.1 (28.95) | Ref | 6/13.4 (44.78) | Ref | 4/61.2 (6.53) | Ref | 3/27.0 (11.09) | Ref |
|
| ||||||||
| VL at enroll | ||||||||
| Unsuppressed | 4/26.3 (15.21) | 0.74(0.25–2.15);0.575 | - | - | 2/48.7 (4.1) | 0.57(0.12–2.78);0.487 | - | - |
| Suppressed | 13/72.7 (17.89) | Ref | - | - | 9/127.2 (7.07) | Ref | - | - |
|
| ||||||||
| Partner tested for HIV | ||||||||
|
| ||||||||
| Yes | 10/69.8 (14.34) | 0.36(0.13–1.01);0.052 | 7/56.2 (12.46) | 0.18(0.06–0.55);0.003 | 6/118.7 (5.06) | 0.34(0.09–1.27);0.11 | 6/95.6 (6.28) | 0.28(0.07–1.15);0.076 |
| No | 6/12.9 (46.44) | Ref | 6/7.6 (78.58) | Ref | 4/24.7 (16.17) | Ref | 3/12.3 (24.3) | Ref |
Hazard ratio estimated by Cox proportional hazards regression calculating time-at-risk from enrollment to delivery, and adjusting for site as a covariate
evaluated by Patient Health Questionnaire 9 (PHQ9), a score >5 indicating at least moderate depressive symptoms
level 1,2,3,4 indicating secure, mild, moderate, severe
evaluated by Medical Outcomes Study (MOS) survey, median of 64 among 235 women enrolled at <20 weeks and median of 63 among 517 women enrolled at <28 weeks
including genital infection, gonorrhea, syphilis, chlamydia, candidiasis, cervicitis.
In models adjusting for site and VL at enrollment, the association between SB and depression remained similar (adjusted HR [aHR] 2.93, 95%CI 1.04–8.23), as did the association between late SB and lower social support score (aHR 11.74, 95%CI 2.47–55.86).
Sub-group stratified analysis among women enrolled with viral suppression
In a subset of 170 women enrolled at <20 weeks’ gestation, the association of SB with depression remained but was not statistically significant (HR 2.41, 95%CI 0.81–7.14) while the association with partner HIV testing became more protective (HR 0.18, 95%CI 0.06–0.55, p=0.003). Women with lower social support had higher risk of SB (HR 3.15, 95%CI 1.12–8.90), while women diagnosed before pregnancy had lower risk (HR 0.31, 95%CI 0.11–0.90) (Table 2). The association between late SB and lower social support score also remained in the subgroup (HR 10.05, 95%CI 1.33–76.0) (Table 2).
Prevalence and cofactors for preterm birth
Among 709 women enrolled at <37 weeks’ gestation and delivered a live birth, 201 (28.3%) had PTB (Table 3). In multivariable analyses, prevalence of PTB was significantly higher among women who were virally unsuppressed (adjusted prevalence ratio [aPR] 1.28, 95%CI 1.02–1.61), exposed to IPV since pregnancy (aPR 1.94, 95%CI 1.28–2.94), and with STI history (aPR 1.63, 95%CI 1.06–2.51). Women who reported traveling from home to clinic >1 hour had higher risk of PTB, though this was not statistically significant (PR 1.46, 95%CI 0.98–2.15; p=0.06). Among 619 (87.3%) infants with data available on sex, male infants were more likely to be preterm than female infants (31.2% vs. 23.1%, p=0.02), and the association remained significant when adjusting for site (PR 1.35, 95%CI 1.04–1.76) (Table 3). Among 495 women enrolled at <28 weeks’ gestation, 26 (5.3%) delivered very preterm. No characteristics were significantly related to very PTB (Table 3). Results of PTB, very PTB from sensitivity analyses among women enrolled at <20 weeks’ gestation remained the same (data not shown).
Table 3.
Prevalence and covariates of PTB, very PTB and NND
| PTB among women who enrolled <37 weeks and had live birth (N=709) | Very PTB among women who enrolled <28 weeks and had live birth (N=495)* | NND among women with live birth (N=740)* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (Pr) | PR (95%CI); pa | aPR (95%CI); pb | n (Pr) | PR (95%CI); pa | n (Pr) | PR (95%CI); pa | ||||
| Full-term (n=508) | PTB (n=201) | Birth at ≥32 weeks (n=469) | Very PTB (n=26) | No NND (n=718) | NND (n=22) | |||||
| Western Kenya (ref: Nairobi) | 317(62.4%) | 126(62.7%) | 1.01(0.80–1.28);0.943 | 1.01 (0.80–1.29); 0.917 | 303 (64.6%) | 23 (88.5%) | 3.97(1.24–12.76);0.020 | 451(62.9%) | 15(65.2%) | 1.10 (0.48–2.53); 0.818 |
| AYA (age 15–24 years) | 171 (33.7%) | 72 (35.8%) | 1.07 (0.84–1.36); 0.579 | 152(32.4%) | 12(46.2%) | 1.82(0.86–3.84);0.115 | 27 (23, 31) | 28 (23, 32) | 0.99(0.91–1.08);0.841 | |
| Incomplete secondary school | 366(72.0%) | 161(80.1%) | 1.39 (1.03–1.89); 0.033 | 1.33 (0.99–1.79); 0.055 | 345(73.6%) | 24(92.3%) | 3.38 (0.78–14.62); 0.103 | 530(73.9%) | 20(87.0%) | 2.3 (0.67–7.86); 0.185 |
| Depressionc | 126(24.8%) | 40(19.9%) | 0.81(0.61–1.08);0.157 | 109(23.2%) | 6(23.1%) | 0.90 (0.38–2.15); 0.811 | 172 (24.0%) | 5 (22.7%) | 0.87(0.33–2.32);0.784 | |
| Severe food insecurityd | 134(26.4%) | 57(28.4%) | 1.07(0.83–1.39);0.595 | 124(26.4%) | 9(34.6%) | 1.26 (0.57–2.78); 0.570 | 196(27.3%) | 4(18.2%) | 0.56(0.19–1.63);0.284 | |
| Low social support scoree | 241(47.4%) | 108(53.7%) | 1.20(0.95–1.50);0.120 | 234(49.9%) | 12(46.2%) | 0.73 (0.35–1.51); 0.394 | 349(48.7%) | 14(60.9%) | 1.61(0.68–3.84);0.280 | |
| IPV since pregnancy | 9 (1.8%) | 11 (5.5%) | 2.00 (1.31–3.04);0.001 | 1.94 (1.28–2.94); 0.002 | 16 (3.4%) | 0(0.0%) | - | 19 (2.6%) | 2(8.7%) | 3.41 (0.83–13.99); 0.089 |
| Travel time to clinic >60 min | 27(5.4%) | 18(9.1%) | 1.46(0.98–2.15); 0.060 | 33(7.2%) | 1(3.8%) | 0.47 (0.07–3.43); 0.46 | 46 (6.5%) | 2 (9.1%) | 1.34(0.33–5.49);0.683 | |
| History of STIf | 13(2.6%) | 11(5.5%) | 1.65(1.07–2.53);0.023 | 1.63 (1.06–2.51); 0.025 | 18(3.8%) | 1(3.8%) | 0.84 (0.11–6.64); 0.870 | 22(3.1%) | 3(13.0%) | 4.25(1.39–13.06);0.011 |
| Syphilis | 11(2.2%) | 4(2.0%) | 0.94(0.42–2.11);0.877 | 12(2.6%) | 0(0.0%) | - | 13(1.8%) | 2(8.7%) | 4.57(1.17–17.79);0.029 | |
| Diagnosis before pregnancy | 312(61.4%) | 116(57.7%) | 0.89(0.70–1.13); 0.347 | 298(63.5%) | 17(65.4%) | 0.90 (0.42–1.91); 0.783 | 433 (60.3%) | 10 (45.5%) | 0.50(0.23–1.07);0.073 | |
| Unsuppressed VL at enrollment | 136(26.8%) | 71(35.3%) | 1.33(1.05–1.67); 0.016 | 1.28 (1.02–1.61); 0.036 | 134(28.6%) | 11(42.3%) | 1.92 (0.91–4.06); 0.087 | 206 (28.7%) | 8 (36.4%) | 1.59(0.7–3.62);0.265 |
| Infant sex male (vs. female) | 223(49.6%) | 101(59.8%) | 1.35(1.04–1.76);0.024 | 214(51.6%) | 15(68.2%) | 1.94 (0.82–4.57); 0.130 | 336(52.7%) | 5(50.0%) | 0.89(0.26–3.05);0.856 | |
| Preterm birth | - | - | - | - | - | - | - | 191 (26.6%) | 10 (45.5%) | 2.53 (1.10–5.78); 0.028 |
Prevalence ratio estimated by Log-binomial regression adjusting for site as a covariate
adjusted prevalence ratio estimated by multivariate Log-binomial regression adjusting for covariates with crude-p-value <0.05
evaluated by Patient Health Questionnaire 9 (PHQ9), a score >5 indicating at least moderate depressive symptoms
compared to secure, mild, or moderate level
evaluated by Medical Outcomes Study survey, a score <median of 64 indicating low level
including genital infection, gonorrhea, syphilis, chlamydia, candidiasis, cervicitis.
Adjusting for site and HIV VL at enrollment, results of no characteristics significantly associated with very PTB remained the same, and the association between NND and PTB remained (aPR 2.42, 95%CI 1.03–5.66)
Sub-group stratified analysis among women enrolled with viral suppression
Among 372 women enrolled at <37 weeks’ gestation in this subgroup, 130 (25.9%) had PTB. Site-adjusted associations between PTB and IPV since pregnancy remained (PR 1.97, 95%CI 1.07–3.62), as did maternal STI history (PR 1.87, 95%CI 1.16–3.02). Among 350 women enrolled at <28 weeks’ gestation, 15 (4.3%) delivered very preterm. Primigravida women had a trend for increased risk of very PTB (PR 3.21, 95%CI 0.94–11.0; p=0.06) (Supplementary Table).
Prevalence and cofactors for neonatal death
Among 740 liveborn neonates, 22 (3.0%) died within 28 days after delivery (Table 3). Livebirths resulting in a NND had significantly lower GA at birth than those who survived (median 37 [IQR 30–40] vs. median 39 [IQR 37–40] weeks; p=0.004). After adjusting for site, PTB was significantly associated with NND (PR 2.46, 95%CI 1.10–5.51). Women with any maternal STI history had significantly higher risk of NND (PR 4.25, 95%CI 1.39–13.06), with the association driven by syphilis (PR 4.57, 95%CI 1.17–17.79) in analyses adjusting for each STI separately. Women who were diagnosed with HIV before pregnancy had a trend for a lower risk of NND (PR 0.50, 95%CI 0.23–1.07; p=0.07). Categories of ART regimen, ART initiation time or other maternal sociodemographic did not show significant associations with NND (Table 3). Adjusting for both site and HIV VL at enrollment, the association between NND and PTB remained similar (Table 3). Results of NND from sensitivity analyses among women enrolled at <20 weeks’ gestation remained the same (data not shown).
Sub-group stratified analysis among women enrolled with viral suppression
Risk of NND among neonates in this subgroup of women was 2.66% (14/526). Site-adjusted association between NND and PTB remained (PR 3.35, 95%CI 1.13–9.91), as did maternal STI history (PR 6.90, 95%CI 2.17–21.99) (Supplementary Table).
Discussion
This study evaluated risks and predictors of APOs among pregnant WLWH on ART in Kenya. Compared to published estimates in the general population in sub-Saharan Africa, women in this study experienced a 1.5-fold higher SB risk (4.4% vs. 2.9%[31]) a 2.4-fold higher PTB risk (28.3% vs.12.0%[32]), and a similar NND risk (2.2% vs. 2.7%[33]). We found that SB risk was associated with having at least moderate depressive symptoms, and not having partner been tested for HIV. Late SB was associated with low social support score. PTB risk was associated with IPV during pregnancy, incomplete secondary education, unsuppressed VL at enrollment, and any maternal STI history. Very PTB was significantly higher among women from Western Kenya sites. We found that women previously diagnosed with STIs, particularly syphilis, had increased risk of NND and 45.5% of NND occurred among preterm infants.
Our finding of significant association between depression and SB suggests the importance of emotional stressors as a determinant of SB. Our finding of association between low social support and late SB echoed these results. Depression may reflect persistent stress, and we found women whose partner tested for HIV had a high social support score than women whose partner did not. Our findings are consistent with studies that have reported maternal stress, anxiety, or depression can influence the developing fetus potentially through maternal hormone changes[34,35], inflammation, or placental dysfunction[36]. Almost one quarter of women experienced at least moderate depressive symptoms, similar to rates of antenatal depression among African WLWH in a systematic review[37]. Given the high prevalence of depression and the association with SB, standard depression screening during early pregnancy along with interventions may be important to improve maternal outcomes and ensure fetal survival. We found protective effects of partner HIV testing on SB. It is unclear why women with untested partners had higher risk for SB. Differences in sociodemographic, access to care, quality of partnership, or social stressors could play a role. Among women enrolled with viral suppression, social factors persisted or newly emerged as significant contributors to risk of SB; these data are relevant to increasing numbers of women who are suppressed in early pregnancy. Incorporating more intensive support to women with depression, low social support or those unaware of their partner’s HIV status may be useful within PMTCT programs. Although other studies[38] have noted associations between food insecurity and SB, we did not find a difference, perhaps due to generally high levels of food insecurity in this cohort.
Our finding of associations of unsuppressed HIV VL with PTB demonstrates independent impacts of maternal VL on adverse perinatal outcomes, despite ART use, which is consistent with published evidence[23,39,40]. In addition, we found a significant effect of STI history on PTB risk. Both maternal STIs and viremia are potentially associated with immune activation that could influence likelihood of PTB[15–19]. There is evidence of PTB being associated with syphilis[41–43], chlamydia[44–47], gonorrhea[43,47,48], trichomoniasis[49], and cervicitis[50] during pregnancy in general population, however, there is mixed evidence regarding the association in WLWH[51–55], and not all studies have specified ART use among their study populations, which is a unique contribution of this study. Incorporating STI testing within PMTCT and promoting ART adherence to achieve viral suppression could contribute to lower PTB rates in WLWH.
PTB was associated with IPV during pregnancy and not associated with depression in our study. This is consistent with systematic reviews reporting detrimental impacts of physical violence[56,57] but lower effect of psychosocial stressors on PTB[58]. Studies which demonstrated significant effects of stress on PTB risk were often based on extreme depression[59–61]. Due to the low prevalence of severe depressive symptoms (PHQ9 score >10: 7.6%) in our study population, our analyses may have been underpowered to detect small effects of moderate depression on PTB. Our findings also suggest routine IPV screening among pregnant women is important. Developing evidence-based interventions to involve partners in maternal HIV care may reduce IPV. Our finding of women not completing secondary education contributing to higher risk of PTB is consistent with other studies[62]. Low education may be a proxy for unbalanced partnership, sociodemographic status, or non-adherence to care, all which also contribute to risk for PTB. Infant sex was a risk factor for PTB, with a higher risk for male fetus than female, consistent with prior studies[63,64]. We did not observe a difference in very PTB by fetal sex, in contrast to some studies reporting increased risk for males for very PTB[63,65], probably due to limited statistical power for this analysis.
NND in this cohort was predominantly associated with PTB, consistent with global data[66]. We also found a 6–7-fold increased risk of NND among women with STI history among women with full-term infants, mainly driven by syphilis. This finding is consistent with a systematic review and meta-analysis showing more frequent NNDs among pregnant women with late diagnosed or untreated syphilis[67]. One study in Botswana reported no significant difference in NND between mothers with HIV/syphilis coinfection and mothers with HIV alone, but the authors noted that data on NND was limited to infants who had not been discharged from the hospital after birth[68]. To our knowledge, our study is the first to evaluate the effects of STI history on PTB and NND among WLWH on ART enrolled in a PMTCT program in SSA. WHO guidelines recommend STI screening for women at the first ANC visit[69], and potential risk-based re-screen later in pregnancy[70,71]. While we did not have data on whether women were still having STI or long-term sequelae in pregnancy, our study suggests that WLWH diagnosed with STI may need enhanced follow-up to ensure adequate care. Scaling up routine STI screening and treatment during ANC visits through point-of-care assays will be helpful to decrease the risk of PTB and NND.
We did not find differences in associations of specific ART regimen with risk of any APO, consistent with studies reporting no difference in APOs of TDF-based[11,12,72] and EFV-based regimens[73–75]. Few women in our study received PIs, which have been linked with elevated PTB risk[76–78]. Our findings on ART use provide re-assurance for >90% of pregnant WLWH following WHO recommendations to use TDF/FTC/EFV[3]. It is also important to note that as new ART regimens are expanded, their effectiveness and safety among WLWH and their infants will be important to evaluate.
This study has unique strengths. We assessed several important APOs, and we restricted analyses to women who were truly “at-risk” with assessment of separate outcomes. Baseline data on social factors, HIV VL, and ART use were collected at enrollment; and outcome data including delivery date and adverse events were verified by comparing several data sources. Our study has limitations. Our estimation of GA at delivery was based primarily on self-reported LMP, given the limited availability of ultrasound at clinics. While LMP dating is commonly used in resource-limited settings[79], it can be problematic due to uncertainty of the true LMP[80], recall bias leading to overestimates of GA[81], and therefore underestimates of PTB. Unreliable GA estimation may also lead to misclassification in determining SB. Exclusion of women with missing LMP data may contribute selection bias. However, we observed no differences in prevalence of any APOs between women with and without LMP date. We did not assess history of prior PTB, which is a risk factor of recurrent PTB[82,83]. The predominant use of TDF+3TC/FTC+EFV limited statistical power to evaluate some regimens. STI data was self-reported with unclear timing, limiting precision and validity of this variable. We had limited birthweight data in MCH records which prevented us from assessing SGA as another APO.
Conclusions
This study provides a comprehensive analysis of APOs among WLWH on ART, including social determinants of health. SB was associated with psychosocial stressors, including depression, poor social support and partners not engaged with HIV services. We found PTB associated with IPV during pregnancy, STI history, and NND. Neither ART regimen nor ART initiation timing was associated with APOs, however, maternal viremia during pregnancy predicted PTB. Most associations were retained and some enhanced in the subset of women with viral suppression at enrollment, suggesting broad relevance of these factors as wider ART coverage increases the proportion of women suppressed before conception. Implementation of mental health screening and counselling, IPV screening and prevention, social support (perhaps through peer support), partner support, and routine STI screening and treatment could reduce APOs in PMTCT programs.
Acknowledgements
We would like to acknowledge the significant contributions from study participants and the Mobile WAChX team members. We would also like to acknowledge support from the University of Washington’s Global Center for Integrated Health of Women, Adolescents and Children (Global WACh). This study was funded by the National Institutes of Health [R01 HD080460] (PI: Grace John-Stewart), [K01 AI116298] (PI: Alison L. Drake), [K18MH122978] (PI: Keshet Ronen), and [P30 AI027757] (PI: Connie Celum). The funding institution had no role in study design; collection, analysis and interpretation of data; writing; or in the decision to submit the article for publication.
Source of funding:
This study was funded by the National Institutes of Health [R01HD080460] (PI: Grace John-Stewart), [K01AI116298] (PI: Alison L. Drake), [K18MH122978] (PI: Keshet Ronen) and [P30AI027757] (PI: Connie Celum). The funding institution had no role in study design; collection, analysis, and interpretation of data; writing; or in the decision to submit the article for publication.
Footnotes
Declaration of interests: We declare no competing interests
References
- 1.Yeatman S, Eaton JW, Beckles Z, Benton L, Gregson S, Zaba B. Impact of ART on the fertility of HIV-positive women in sub-Saharan Africa. Tropical Medicine & International Health 2016; 21:1071–1085. [DOI] [PubMed] [Google Scholar]
- 2.Wedi COO, Kirtley S, Hopewell S, Corrigan R, Kennedy SH, Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. The Lancet HIV 2016; 3:e33–48. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS. 90–90-90: An ambitious treatment target to help end the AIDS epidemic. Published Online First: 2014.https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf
- 4.UNAIDS. Global HIV & AIDS statistics — 2019 fact sheet.; 2019. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- 5.Kourtis AP, Schmid CH, Jamieson DJ, Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS 2007; 21. [DOI] [PubMed] [Google Scholar]
- 6.Uthman OA, Nachega JB, Anderson J, Kanters S, Mills EJ, Renaud F, et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. The Lancet HIV 2017; 4:e21–e30. [DOI] [PubMed] [Google Scholar]
- 7.Mofenson LM, Baggaley RC, Mameletzis I. Tenofovir disoproxil fumarate safety for women and their infants during pregnancy and breastfeeding. AIDS (London, England) 2017; 31:213–232. [DOI] [PubMed] [Google Scholar]
- 8.Nachega JB, Uthman OA, Mofenson LM, Anderson JR, Kanters S, Renaud F, et al. Safety of Tenofovir Disoproxil Fumarate-Based Antiretroviral Therapy Regimens in Pregnancy for HIV-Infected Women and Their Infants: A Systematic Review and Meta-Analysis. Journal of Acquired Immune Deficiency Syndromes (1999) 2017; 76:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tshivuila-Matala COO, Honeyman S, Nesbitt C, Kirtley S, Kennedy SH, Hemelaar J. Adverse perinatal outcomes associated with antiretroviral therapy regimens: systematic review and network meta-analysis. AIDS (London, England) 2020; 34:1643–1656. [DOI] [PubMed] [Google Scholar]
- 10.Fowler MG, Qin M, Fiscus SA, Currier JS, Flynn PM, Chipato T, et al. Benefits and Risks of Antiretroviral Therapy for Perinatal HIV Prevention. New England Journal of Medicine Published Online First: 2016. doi: 10.1056/nejmoa1511691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zash R, Jacobson DL, Diseko M, Mayondi G, Mmalane M, Essex M, et al. Comparative Safety of Antiretroviral Treatment Regimens in Pregnancy. JAMA Pediatrics 2017; 171:e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibb DM, Kizito H, Russell EC, Chidziva E, Zalwango E, Nalumenya R, et al. Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial. PLoS Medicine 2012; 9:e1001217–e1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreitchmann R, Li SX, Melo VH, Fernandes Coelho D, Watts DH, Joao E, et al. Predictors of adverse pregnancy outcomes in women infected with HIV in Latin America and the Caribbean: a cohort study. BJOG : an international journal of obstetrics and gynaecology 2014; 121:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebitloane HM, Moodley J. Maternal and obstetric complications among HIV-infected women treated with highly active antiretroviral treatment at a Regional Hospital in Durban, South Africa. Nigerian Journal of Clinical Practice 2017; 20:1360–1367. [DOI] [PubMed] [Google Scholar]
- 15.Arab K, Spence AR, Czuzoj-Shulman N, Abenhaim HA. Pregnancy outcomes in HIV-positive women: a retrospective cohort study. Archives of Gynecology and Bbstetrics 2017; 295:599–606. [DOI] [PubMed] [Google Scholar]
- 16.Thaxton JE, Nevers TA, Sharma S. TLR-mediated preterm birth in response to pathogenic agents. Infectious Diseases in Obstetrics and Gynecology 2010; 2010. doi: 10.1155/2010/378472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivas SK, Ma Y, Sammel MD, Chou D, McGrath C, Parry S, et al. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. American Journal of Obstetrics and Gynecology 2006; 195:797–802. [DOI] [PubMed] [Google Scholar]
- 18.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reproductive Sciences (Thousand Oaks, Calif) 2009; 16:206–215. [DOI] [PubMed] [Google Scholar]
- 19.López M, Figueras F, Coll O, Goncé A, Hernández S, Loncá M, et al. Inflammatory Markers Related to Microbial Translocation Among HIV-Infected Pregnant Women: A Risk Factor of Preterm Delivery. The Journal of Infectious Diseases 2016; 213:343–350. [DOI] [PubMed] [Google Scholar]
- 20.Mdletshe N, Thobakgale C, Malaba TR, Madlala H, Myer L, Muema DM, et al. Low Immune Activation in Early Pregnancy Is Associated With Preterm But Not Small-for-gestational-age Delivery in Women Infected With Human Immunodeficiency Virus Initiating Antiretroviral Therapy in Pregnancy: A Prematurity Immunology in HIV-infected Mothers and their Infants Study (PIMS) Case-control Study in Cape Town, South Africa. Clinical Infectious Diseases Published Online First: 19 February 2021. doi: 10.1093/cid/ciab151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguilar-Zapata D, Piñeirúa-Menéndez A, Volkow-Fernández P, Rodríguez-Zulueta P, Ramos-Alamillo U, Cabrera-López T, et al. Sociodemographic differences among HIV-positive and HIV-negative recently pregnant women in Mexico City: A case-control study. Medicine 2017; 96:e7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Merwe K, Hoffman R, Black V, Chersich M, Coovadia A, Rees H. Birth outcomes in South African women receiving highly active antiretroviral therapy: a retrospective observational study. Journal of the International AIDS Society 2011; 14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JY, Ribaudo HJ, Souda S, Parekh N, Ogwu A, Lockman S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. The Journal of Infectious Diseases 2012; 206:1695–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinuthia J, Ronen K, Unger ID JA, Jiang W, Matemo D, Perrier T, et al. SMS messaging to improve retention and viral suppression in prevention of mother-to-child HIV transmission (PMTCT) programs in Kenya: A 3-arm randomized clinical trial. Published Online First: 2021. doi: 10.1371/journal.pmed.1003650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherbourne CD, Stewart AL. The MOS social support survey. Social Science & Medicine (1982) 1991; 32:705–714. [DOI] [PubMed] [Google Scholar]
- 26.Rao D, Feldman BJ, Fredericksen RJ, Crane PK, Simoni JM, Kitahata MM, et al. A structural equation model of HIV-related stigma, depressive symptoms, and medication adherence. AIDS and Behavior 2012; 16:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine 2001; 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soeken KL, McFarlane J, Parker B, Lominack MC. The Abuse Assessment Screen: A clinical instrument to measure frequency, severity, and perpetrator of abuse against women. In: Empowering survivors of abuse: Health care for battered women and their children. Thousand Oaks, CA, US: Sage Publications, Inc; 1998. pp. 195–203. [Google Scholar]
- 29.Swindale A, Bilinsky P. Development of a universally applicable household food insecurity measurement tool: process, current status, and outstanding issues. The Journal of Nutrition 2006; 136:1449S–1452S. [DOI] [PubMed] [Google Scholar]
- 30.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychology: official journal of the Division of Health Psychology, American Psychological Association 2006; 25:462–473. [DOI] [PubMed] [Google Scholar]
- 31.Blencowe H, Cousens S, Jassir FB, Say L, Chou D, Mathers C, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. The Lancet Global Health 2016; 4:e98–e108. [DOI] [PubMed] [Google Scholar]
- 32.Chawanpaiboon S, Vogel JP, Moller A-B, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. The Lancet Global Health 2019; 7:e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hug L, Alexander M, You D, Alkema L. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. The Lancet Global Health 2019; 7. doi: 10.1016/S2214-109X(19)30163-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herba CM, Glover V, Ramchandani PG, Rondon MB. Maternal depression and mental health in early childhood: an examination of underlying mechanisms in low-income and middle-income countries. The Lancet Psychiatry 2016; 3. doi: 10.1016/S2215-0366(16)30148-1 [DOI] [PubMed] [Google Scholar]
- 35.Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. The Lancet 2014; 384. doi: 10.1016/S0140-6736(14)61277-0 [DOI] [PubMed] [Google Scholar]
- 36.Gentile S Untreated depression during pregnancy: Short- and long-term effects in offspring. A systematic review. Neuroscience 2017; 342. doi: 10.1016/j.neuroscience.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 37.Sowa NA, Cholera R, Pence BW, Gaynes BN. Perinatal Depression in HIV-Infected African Women. The Journal of Clinical Psychiatry 2015; 76. doi: 10.4088/JCP.14r09186 [DOI] [PubMed] [Google Scholar]
- 38.Carmichael SL, Yang W, Herring A, Abrams B, Shaw GM. Maternal Food Insecurity Is Associated with Increased Risk of Certain Birth Defects. The Journal of Nutrition 2007; 137. doi: 10.1093/jn/137.9.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ezechi OC, Gab-Okafor CV, Oladele DA, Kalejaiye OO, Oke BO, Ohwodo HO, et al. Pregnancy, obstetric and neonatal outcomes in HIV positive Nigerian women. African Journal of Reproductive Health 2013; 17:160–168. [PubMed] [Google Scholar]
- 40.Xiao P-L, Zhou Y-B, Chen Y, Yang M-X, Song X-X, Shi Y, et al. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy and Childbirth 2015; 15:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korenromp EL, Rowley J, Alonso M, Mello MB, Wijesooriya NS, Mahiané SG, et al. Global burden of maternal and congenital syphilis and associated adverse birth outcomes—Estimates for 2016 and progress since 2012. PLOS ONE 2019; 14. doi: 10.1371/journal.pone.0211720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Santis M, de Luca C, Mappa I, Spagnuolo T, Licameli A, Straface G, et al. Syphilis Infection during Pregnancy: Fetal Risks and Clinical Management. Infectious Diseases in Obstetrics and Gynecology 2012; 2012. doi: 10.1155/2012/430585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baer RJ, Chambers CD, Ryckman KK, Oltman SP, Rand L, Jelliffe-Pawlowski LL. An Evaluation of Sexually Transmitted Infection and Odds of Preterm or Early-Term Birth Using Propensity Score Matching. Sexually Transmitted Diseases 2019; 46. doi: 10.1097/OLQ.0000000000000985 [DOI] [PubMed] [Google Scholar]
- 44.Olson-Chen C, Balaram K, Hackney DN. Chlamydia trachomatis and Adverse Pregnancy Outcomes: Meta-analysis of Patients With and Without Infection. Maternal and Child Health Journal 2018; 22. doi: 10.1007/s10995-018-2451-z [DOI] [PubMed] [Google Scholar]
- 45.Silva MJPM de A, Florêncio GLD, Gabiatti JRE, Amaral RL do, Eleutério Júnior J, Gonçalves AK da S. Perinatal morbidity and mortality associated with chlamydial infection: a meta-analysis study. Brazilian Journal of Infectious Diseases 2011; 15. doi: 10.1590/S1413-86702011000600006 [DOI] [PubMed] [Google Scholar]
- 46.Rours GIJG, Duijts L, Moll HA, Arends LR, de Groot R, Jaddoe VW, et al. Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population-based prospective cohort study. European Journal of Epidemiology 2011; 26. doi: 10.1007/s10654-011-9586-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu B, Roberts CL, Clarke M, Jorm L, Hunt J, Ward J. Chlamydia and gonorrhoea infections and the risk of adverse obstetric outcomes: a retrospective cohort study. Sexually Transmitted Infections 2013; 89. doi: 10.1136/sextrans-2013-051118 [DOI] [PubMed] [Google Scholar]
- 48.Vallely LM, Egli-Gany D, Wand H, Pomat WS, Homer CSE, Guy R, et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae: systematic review and meta-analysis. Sexually Transmitted Infections 2021; 97. doi: 10.1136/sextrans-2020-054653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silver BJ, Guy RJ, Kaldor JM, Jamil MS, Rumbold AR. Trichomonas vaginalis as a Cause of Perinatal Morbidity. Sexually Transmitted Diseases 2014; 41. doi: 10.1097/OLQ.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 50.Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium Infection and Female Reproductive Tract Disease: A Meta-analysis. Clinical Infectious Diseases 2015; 61. doi: 10.1093/cid/civ312 [DOI] [PubMed] [Google Scholar]
- 51.Burnett E, Loucks TL, Lindsay M. Perinatal Outcomes in HIV Positive Pregnant Women with Concomitant Sexually Transmitted Infections. Infectious Diseases in Obstetrics and Gynecology 2015; 2015. doi: 10.1155/2015/508482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adachi K, Klausner JD, Xu J, Ank B, Bristow CC, Morgado MG, et al. Chlamydia trachomatis and Neisseria gonorrhoeae in HIV-infected Pregnant Women and Adverse Infant Outcomes. Pediatric Infectious Disease Journal 2016; 35. doi: 10.1097/INF.0000000000001199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters R, Klausner J, Vos L, Feucht U, Medina-Marino A. Aetiological testing compared with syndromic management for sexually transmitted infections in HIV-infected pregnant women in South Africa: a non-randomised prospective cohort study. BJOG: An International Journal of Obstetrics & Gynaecology Published Online First: 22 December 2020. doi: 10.1111/1471-0528.16617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Labbe A-C. Mycoplasma genitalium is not associated with adverse outcomes of pregnancy in Guinea-Bissau. Sexually Transmitted Infections 2002; 78. doi: 10.1136/sti.78.4.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ravindran J, Richardson B, Kinuthia J, Unger JA, Drake AL, Osborn L, et al. Chlamydia, gonorrhea, and incident HIV infection during pregnancy predict preterm birth despite treatment. The Journal of Infectious Diseases Published Online First: 23 May 2021. doi: 10.1093/infdis/jiab277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donovan B, Spracklen C, Schweizer M, Ryckman K, Saftlas A. Intimate partner violence during pregnancy and the risk for adverse infant outcomes: a systematic review and meta-analysis. BJOG: An International Journal of Obstetrics & Gynaecology 2016; 123. doi: 10.1111/1471-0528.13928 [DOI] [PubMed] [Google Scholar]
- 57.Hill A, Pallitto C, McCleary-Sills J, Garcia-Moreno C. A systematic review and meta-analysis of intimate partner violence during pregnancy and selected birth outcomes. International Journal of Gynecology & Obstetrics 2016; 133. doi: 10.1016/j.ijgo.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 58.Accortt EE, Cheadle ACD, Dunkel Schetter C. Prenatal Depression and Adverse Birth Outcomes: An Updated Systematic Review. Maternal and Child Health Journal 2015; 19. doi: 10.1007/s10995-014-1637-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoirisch-Clapauch S, Brenner B, Nardi AE. Adverse obstetric and neonatal outcomes in women with mental disorders. Thrombosis Research 2015; 135. doi: 10.1016/S0049-3848(15)50446-5 [DOI] [PubMed] [Google Scholar]
- 60.Jarde A, Morais M, Kingston D, Giallo R, MacQueen GM, Giglia L, et al. Neonatal Outcomes in Women With Untreated Antenatal Depression Compared With Women Without Depression. JAMA Psychiatry 2016; 73. doi: 10.1001/jamapsychiatry.2016.0934 [DOI] [PubMed] [Google Scholar]
- 61.Orr ST. Maternal Prenatal Depressive Symptoms and Spontaneous Preterm Births among African-American Women in Baltimore, Maryland. American Journal of Epidemiology 2002; 156. doi: 10.1093/aje/kwf131 [DOI] [PubMed] [Google Scholar]
- 62.Ruiz M, Goldblatt P, Morrison J, Kukla L, Švancara J, Riitta-Järvelin M, et al. Mother’s education and the risk of preterm and small for gestational age birth: a DRIVERS meta-analysis of 12 European cohorts. Journal of Epidemiology and Community Health 2015; 69. doi: 10.1136/jech-2014-205387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peelen MJCS, Kazemier BM, Ravelli ACJ, de Groot CJM, van der Post JAM, Mol BWJ, et al. Impact of fetal gender on the risk of preterm birth, a national cohort study. Acta Obstetricia et Gynecologica Scandinavica 2016; 95. doi: 10.1111/aogs.12929 [DOI] [PubMed] [Google Scholar]
- 64.Khalil MM, Alzahra E. Fetal gender and pregnancy outcomes in Libya: a retrospective study. Libyan Journal of Medicine 2013; 8. doi: 10.3402/ljm.v8i0.20008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melamed N, Yogev Y, Glezerman M. Fetal gender and pregnancy outcome. The Journal of Maternal-Fetal & Neonatal Medicine 2010; 23. doi: 10.3109/14767050903300969 [DOI] [PubMed] [Google Scholar]
- 66.Howson CP, Kinney M v, McDougall L, Lawn JE. Born Too Soon: Preterm birth matters. Reproductive Health 2013; 10. doi: 10.1186/1742-4755-10-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez GB, Kamb ML, Newman LM, Mark J, Broutet N, Hawkes SJ. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bulletin of the World Health Organization 2013; 91. doi: 10.2471/BLT.12.107623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shava E, Moyo S, Zash R, Diseko M, Dintwa EN, Mupfumi L, et al. Brief Report: High Rates of Adverse Birth Outcomes in HIV and Syphilis Coinfected Women in Botswana. JAIDS Journal of Acquired Immune Deficiency Syndromes 2019; 81. doi: 10.1097/QAI.0000000000002082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson HL, Erbelding EJ, Ghanem KG. Sexually transmitted infections during pregnancy. Current Infectious Disease Reports 2007; 9. doi: 10.1007/s11908-007-0008-1 [DOI] [PubMed] [Google Scholar]
- 70.Rogozińska E, Kara-Newton L, Zamora J, Khan K. On-site test to detect syphilis in pregnancy: a systematic review of test accuracy studies. BJOG: An International Journal of Obstetrics & Gynaecology 2017; 124. doi: 10.1111/1471-0528.14455 [DOI] [PubMed] [Google Scholar]
- 71.Hawkes S, Matin N, Broutet N, Low N. Effectiveness of interventions to improve screening for syphilis in pregnancy: a systematic review and meta-analysis. The Lancet Infectious Diseases 2011; 11. doi: 10.1016/S1473-3099(11)70104-9 [DOI] [PubMed] [Google Scholar]
- 72.Cousens S, Blencowe H, Stanton C, Chou D, Ahmed S, Steinhardt L, et al. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. The Lancet 2011; 377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 73.Ford N, Mofenson L, Shubber Z, Calmy A, Andrieux-Meyer I, Vitoria M, et al. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS (London, England) 2014; 28 Suppl 2:S123–31. [DOI] [PubMed] [Google Scholar]
- 74.World Health Organization. Technical update on treatment optimization: use of efavirenz during pregnancy: a public health perspective. 2012. [Google Scholar]
- 75.Hoffman RM, Brummel SS, Britto P, Pilotto JH, Masheto G, Aurpibul L, et al. Adverse Pregnancy Outcomes Among Women Who Conceive on Antiretroviral Therapy. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America 2019; 68:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Powis KM, Kitch D, Ogwu A, Hughes MD, Lockman S, Leidner J, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. The Journal of InfectiousDdiseases 2011; 204:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. The New England Journal of Medicine 2010; 362:2282–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pasley M v, Martinez M, Hermes A, d’Amico R, Nilius A. Safety and efficacy of lopinavir/ritonavir during pregnancy: a systematic review. AIDS Reviews 2013; 15:38–48. [PubMed] [Google Scholar]
- 79.American College Obstetricians and Gynecologists. Method for estimating due date. Committee opinion No. 611. Obstetrics & Gynecology 2014; 124. doi: 10.1097/01.AOG.0000454932.15177.be [DOI] [PubMed] [Google Scholar]
- 80.Campbell S, Warsof SL, Little D, Cooper DJ. Routine ultrasound screening for the prediction of gestational age. Obstetrics and Gynecology 1985; 65:613–620. [PubMed] [Google Scholar]
- 81.Schonberg D, Wang L-F, Bennett AH, Gold M, Jackson E. The accuracy of using last menstrual period to determine gestational age for first trimester medication abortion: a systematic review. Contraception 2014; 90:480–487. [DOI] [PubMed] [Google Scholar]
- 82.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Pineles BL, Gotsch F, et al. Recurrent Preterm Birth. Seminars in Perinatology 2007; 31. doi: 10.1053/j.semperi.2007.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Phillips C, Velji Z, Hanly C, Metcalfe A. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open 2017; 7. doi: 10.1136/bmjopen-2016-015402 [DOI] [PMC free article] [PubMed] [Google Scholar]