Abstract
Objective:
Longer menstrual cycles have been associated with greater risk of cardiovascular disease, supporting a contribution of abnormal ovarian function. We aimed to characterize trajectories of menstrual cycle length over the menopause transition (MT) and test whether these trajectories are associated with postmenopausal markers of subclinical atherosclerosis.
Methods:
Women from the SWAN Daily Hormone Study were included if they had an observed date of the final menstrual period (FMP), recorded cycle lengths from ≥2 annual menstrual cycles (mean±SD:4.22±1.91 cycles), and had measurements of postmenopausal carotid intima-media thickness (cIMT) and/or brachial-ankle pulse wave velocity (baPWV). Trajectories of cycle length over the MT were identified using Group-based trajectory modeling and linked with cIMT and baPWV using linear regression.
Results:
We studied 428 women who had 1808 cycles over the MT (45.1±2.3 years old at baseline visit), and of whom 263 had cIMT, and 213 had baPWV measured postmenopausally (after 13.88±0.42 and 15.25±0.70 years since baseline visit, respectively). Three distinct trajectories of cycle length were identified: stable (no changes in cycle length over the MT among 62.1% of women), late increase (a late increase 2-years before the FMP among 21.8%), and early-increase (an early increase 5-years before the FMP among 16.2%). Women with the late-increase pattern had significantly lower postmenopausal cIMT (0.72 mm) and baPWV (1392 cm/sec) levels than the stable group (0.77 mm and 1508 cm/sec; respectively) adjusting for race, concurrent age, socioeconomic status, physical activity level, and premenopausal cardiovascular risk profile.
Conclusions:
Patterns of cycle length over the MT appear to be a marker of future vascular health that may help identify groups at greater or lesser risk of atherosclerosis after menopause.
Keywords: women, subclinical measures of vascular health, estrogen, cycle length, climacteric
Introduction
Menstrual cycle length is an important vital sign that can be a key to the diagnosis of potentially serious health conditions.1 Normally, ovulatory menstrual cycles occur at a regular interval of 21 to 35 days in reproductive-aged women.2 Changes in menstrual cycle length in mid-reproductive life are often attributable to hypothalamic-pituitary axis dysfunction, thyroid disorders, and more rarely, primary ovarian failure.
Since reproductive hormone levels vary considerably depending on the timing of ovulation and the length of the cycle, cycle length could also be an important marker for cumulative hormone exposure during reproductive life.3 Interestingly, cumulative estrogen exposure vary by cycle length.3 Compared to women with normal-length cycles, women with short cycles had higher estradiol concentration across the cycle.3 A woman with frequent menstrual cycles (short cycles) will spend relatively more of her reproductive years with higher estrogen levels than a woman with very long cycles, since the early follicular phase of the cycle is marked by relatively less estrogen secretion and is the more variable portion of the cycle.4
Cycle length has been linked to different chronic conditions including breast cancer, osteoporosis and cardiovascular disease (CVD).5,6 Women with irregular or long menstrual cycles have greater CVD risk.7-10 Women with very long cycles may be more likely to have polycystic ovary syndrome,4 which carries with it increased risks for cardiometabolic disease later in life. Moreover, having a long menstrual cycle (>40 days) has been identified as a potential risk factor for the development of type 2 diabetes.11 The reported associations between menstrual cycle irregularities (as measured in term of cycle length or cycle frequency) and CVD and diabetes risks are only restricted to irregularities that were retrospectively–reported during the ages of 20-40 years old.
During the menopause transition (MT), very long cycles become much more likely,12 and it is possible that longer cycles at this time of life could also be linked to future CVD risk. In an analysis of TREMIN data, a historic cohort with menstrual calendars maintained across the entire lifespan, the average menstrual cycle length began to increase rapidly starting at four years prior to the final menstrual period (FMP).13 Other studies that collected menstrual calendar data showed that the menstrual cycle length increased beginning 7.5 years prior to the FMP, with the steepest increase starting 4 years pre-FMP, and increased variability in cycle length beginning 2 years pre-FMP.12 Findings to date assume that all women experience one common trajectory of menstrual cycle length change over the MT. However, as women traverse menopause, they could experience a variety of patterns of changes in menstrual cycle length relative to their FMP. Moreover, these distinct trajectories may have different relationships to future CVD risk.
The Study of Women’s Health Across the Nation (SWAN) Daily Hormone Study (DHS) measured menstrual cycle length and reproductive hormone levels across complete cycles with measures repeated annually for up to 10 years or until post-menopausal. The DHS provides a unique opportunity to test whether women experience different patterns of menstrual cycle length over the MT, and whether these patterns predict early markers of atherosclerosis after menopause. To the best of our knowledge, this question has never been addressed comprehensively before using such a powerful study design as in SWAN DHS.
Methods
Participants
SWAN is an ongoing multi-racial/ethnic longitudinal study of the menopause transition.14 Criteria for entry into SWAN included (A) an intact uterus and at least one ovary, (B) at least one menstrual period in the previous 3 months, (C) no use of sex steroid hormones in the previous 3 months, and (D) not pregnant. At baseline, 3302 women aged 42-52 years old from seven sites (Boston, MA; Detroit, MI; Davis, CA; Los Angeles, CA; Pittsburgh, PA; Chicago, IL; and Newark, NJ) completed a visit. Since then, 16 follow-up visits were completed. The DHS substudy was initiated 2 years after the inception of the SWAN cohort. A volunteer subset of women from all SWAN clinical sites enrolled in the DHS after recruitment of the main cohort. DHS participants collected daily, first-morning voided urine for one entire menstrual cycle or up to 50 days, whichever came first, annually until post-menopausal or for 10 follow-up visits from 1997-2008, coincident with SWAN main follow-up visits 01-10. A total of 953 SWAN participants were initially enrolled in the DHS substudy and 874 of them completed at least one DHS collection. To characterize cycle length trajectories over the MT, women who completed at least two annual DHS collections including measures of cycle length, and for whom date of the final menstrual period (FMP) were observed were included in the final analytical sample of 428 women (1808 cycles).
Early markers of atherosclerosis including carotid intima-media thickness (cIMT) and brachial-ankle pulse wave velocity (baPWV) were measured among active SWAN participants at follow-up visit 12 or 13. The SWAN Los Angeles clinical site did not participate in this protocol. Because all Japanese women in SWAN were from this site, these measures were not available for Japanese participants. Out of 428 women who were included in trajectory analysis of cycle length, 263 women had cIMT and 213 had baPWV measured at visit 12 or 13, by which time all SWAN DHS participants were postmenopausal (6.59±2.55 and 7.79±2.62 years after their final menstrual period, respectively), constituting the final analytical sample sizes for analysis linking cycle length trajectories with early marker of atherosclerosis.
Research protocols were approved by the institutional review board at each study site and all the participants provided a written informed consent prior to enrollment.
Study Measures
Menstrual cycle length
The length of each cycle was prospectively documented and calculated as the number of days from the first day of the cycle to the last day of the cycle. Cycle-related bleeding and urinary hormonal patterns were used to define the start and end of a cycle.
Menopausal status and final menstrual period (FMP) date
Menopausal status was characterized into: 1) premenopausal: bleeding in the past 3 months without menstrual cycle changes during the past year; 2) early perimenopause: bleeding in the past 3 months with some menstrual cycle changes during the past year; 3) late perimenopausal: bleeding at least once between 3 to 12 months prior to the current visit; and 4) naturally postmenopausal: no bleeding in at least 12 months prior to the current visit. FMP was identified as the date of the last menstrual period reported in the visit immediately prior to the first visit that a woman was classified as postmenopausal. At each visit the time preceding FMP was calculated as the difference between the FMP date and the completion date of the visit.
Subclinical atherosclerotic measurements
Carotid intima media thickness (cIMT) was assessed at SWAN visit 12 or 13 using a Terason t3000 Ultrasound System (Teratech Corp, Burlington, MA) equipped with a variable frequency (5-12 MHz) linear array transducer. Four images were obtained for each woman by scanning 2 digitized images at each of the right and left distal common carotid arteries. For each image, the near and far wall cIMT measures were obtained by electronically tracing the lumen-intima interface and media-adventitia interface across a 1-cm segment proximal to the carotid bulb; one measurement was generated for each pixel over the area, for a total of approximately 140 measures for each segment. The average values for these measures were recorded for all 4 locations, with the mean of the average at all 4 locations used in analyses. Reproducibility of cIMT measures was excellent with an intraclass correlation coefficient between sonographers > 0.77 and between readers > 0.89, respectively.
Brachial-ankle pulse wave velocity (baPWV) was measured at SWAN visit 12 or 13 using VP2000 system (Omron Health Care Co., Kyoto, Japan), a non-invasive automated waveform analyzer. Following a 10-minute rest in a supine position, occlusion and monitoring cuffs were placed around both upper arms and ankles, following standardized placement procedures. Arm cuffs were placed on bare arms or over light clothing, and ankle cuffs were placed on bare ankles. ECG electrodes were placed on both wrists and a phonocardiogram was placed on the left edge of the sternum. Cuffs were connected to a plethysmographic sensor to measure pulse volume waveform and an oscillometric pressure sensor to measure blood pressure. Volume waveforms for the arm (brachial artery) and ankle (tibial artery) were stored for a 10-second sampling time with automatic gain analysis and quality adjustment. The baPWV was calculated as the path length between arterial sites divided by the time delay between the foot of the respective waveforms (DTba). The path length was calculated using height-based formulas rather than the actual “above the body” distances which corrects for the opposite direction of blood flow by subtracting the heart-to-brachial distance from the heart-to-tibial distance. The baPWV was calculated by time-phase analysis, for the right and left sides, using the following equation: (La -Lb)/(DTba). Left and right side baPWV values were averaged for use in analyses. Three runs were performed for each participant and the average value was reported.
Study covariates
Except for self-reported race/ethnicity and education level all other covariates were assessed at every follow-up visit. Age, smoking status (ever-smoker or not), physical activity score [generated as the sum of active living index (incorporating time spent in watching television and daily walking), household/childcare activity index (incorporating time spent in housework and childcare), and sports index (incorporating intensity of and time spent in sport and exercise)], difficulty paying for basics (defined as indicating “very hard”, “somewhat hard” or “not at all” to the question “how hard is it to pay for basics?”), and current use of any medication (antihypertensive, anticoagulants, anti-diabetic, or lipid lowering medications) were derived from questionnaires and interviews where medication bottles were reviewed. Diabetic status was defined by meeting at least one of the following criteria: anti-diabetic medication at any visit, fasting glucose ≥126mg/dL (while not on steroids) at 2 consecutive visits or 50% of at least 3 attended visits, or self-reported diabetes at any 2 visits and fasting glucose ≥126mg/dL (while not on steroids) at least one visit.
Body mass index (BMI: weight (kg)/square of height (m2)), systolic blood pressure (SBP: the average of two measurements assessed with at least a 2-minute interval), fasting glucose, fasting insulin, high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), and triglycerides were measured at clinical visits. For this analysis, baseline measures were used. Lipid fractions were determined in EDTA-treated plasma. Fasting HDL-C, triglycerides, and glucose were measured at Medical Research Laboratory, Lexington, KY. Fasting HDL-C was separated within heparin-2M manganese chloride. Fasting triglycerides was evaluated by the Hitachi 747-200 clinical analyzer. LDL-C was calculated by the Friedewald equation when triglycerides were <400 mg/dL15 or set to missing when triglycerides were >400 mg/dL. Glucose levels were measured using a hexokinase-coupled reaction (Boehringer Mannheim Diagnostics, Indianapolis, IN). Serum insulin level was measured using a solid-phase radioimmunoassay (DPC Coat-A-Count Insulin RIA; Diagnostic Products, Los Angeles, CA). Homeostasis model assessment insulin resistance (HOMA-IR) index was calculated by using the equation of [fasting insulin (μIU/mL) × fasting glucose (mmol/L)]/22.5.16
Data analysis
Group-based trajectory model (GBTM),17 was used to identify distinct patterns of cycle length anchored to time to FMP (7.36±2.51 years before FMP) among all eligible participants (n=428). Chi-square test or Fisher’s exact test was applied to determine differences in the frequency of categorical variables by cycle length trajectory group. We performed analysis of variance (ANOVA) to compare differences in the mean of continuous variables by cycle length trajectory group. Non-normally distributed baPWV was natural log transformed for statistical modeling. Linear regression models were used to assess the association between cycle length trajectory group and mean cIMT and baPWV, separately. Multivariable models were adjusted first for race/ethnicity, current age, physical activity and hardship paying for basic life needs. Models were additionally adjusted for SBP, BMI, triglycerides, HOMA-IR, use of medication, and smoking status measured at the baseline visit; corresponding to the first collected menstrual cycle in this substudy. We chose this approach given the literature suggesting CVD traditional risk factors to contribute prospectively to menopause timing,18 and thus possibly to any changes that occur in cycle length over the MT. These same covariates are well known risk factors for subclinical measures of atherosclerosis. We further run sensitivity models that were additionally adjusted for age at FMP and baseline cycle length. Only the most parsimonious models were retained in tables. Statistical analyses were carried out by using SAS software 9.4.
Results
Baseline characteristics of DHS participants with observed FMP, and the subsets with available cIMT and/or baPWV measures are summarized in Table 1. In the overall DHS sample, women were 45 years old [median (Q1: 43, Q3:47)] at baseline and more than 30% were White. On average, women experienced the FMP after a mean of 7.4 years (SD:2.5) since baseline. The average (SD) cycle length was 27.2 (5.2) days when all women were either pre- or early perimenopausal.
Table 1.
Baseline characteristics of overall DHS participants with observed FMP and the subsets with cIMT and/or baPWV
| Characteristics | Overall N=428 |
Subset with cIMT N=263 |
Subset with baPWV N=213 |
|---|---|---|---|
| Age, years, Median (Q1, Q3) | 45 (43, 47) | 45 (43, 47) | 45 (43, 47) |
| Race, n (%) | |||
| Black | 80 (18.7) | 58 (22.1) | 57 (26.8) |
| White | 131 (30.6) | 102 (38.8) | 85 (39.9) |
| Chinese | 92 (21.5) | 89 (33.8) | 58 (27.2) |
| Japanese | 106 (24.8) | 0 | 0 |
| Hispanic | 19 (4.4) | 14 (5.3) | 13 (6.1) |
| Difficulty paying for basics, n (%) | |||
| Very hard | 27 (6.3) | 14 (5.4) | 8 (3.8) |
| Somewhat hard | 129 (30.3) | 77 (29.5) | 82 (35.1) |
| Not at all | 270 (63.4) | 170 (65.1) | 129 (61.1) |
| Menopausal status, n (%) | |||
| Premenopause | 286 (67.0) | 177 (67.6) | 140 (66.0) |
| Early Perimenopause | 141 (33.0) | 85 (32.4) | 72 (34.0) |
| Education, n (%) | |||
| ≤ High school/some college | 223 (52.1) | 137 (52.1) | 113 (53.1) |
| College degree/post college | 205 (47.9) | 126 (47.9) | 100 (47.0) |
| BMI, kg/m2, Median (Q1, Q3) | 24.5 (21.6, 29.1) | 25.0 (21.8, 29.9) | 25.1 (22.0, 30.6) |
| SBP, mmHg, Median (Q1, Q3) | 110 (102, 120) | 112 (92, 132) | 111 (103, 122) |
| HDL-C, mg/dL, Mean (SD) | 57.2 (13.4) | 57.4 (13.7) | 57.0 (13.2) |
| LDL-C, mg/dL, Mean (SD) | 111.5 (28.3) | 112.4 (29.2) | 111.2 (28.4) |
| Triglycerides, mg/dL, Median (Q1, Q3) | 89.0 (64.0, 122.0) | 89.0 (65.0, 120.5) | 84.5 (63.0, 117.0) |
| HOMA-IR index, Median (Q1, Q3) | 1.6 (1.2, 2.5) | 1.8 (1.3, 2.6) | 1.8 (1.2, 2.6) |
| Total physical activity score a, Mean (SD) | 7.7 (1.7) | 7.7 (1.6) | 7.8 (1.6) |
| Diabetes, n (%) | 17 (4.0) | 11 (4.2) | 7 (3.3) |
| Ever-smoker, n (%) | 136 (31.9) | 77 (29.3) | 69 (32.4) |
| Use of medications b, yes, n (%) | 46 (10.8) | 24 (9.1) | 17 (8.0) |
| Age at FMP, Mean (SD) | 53.0 (2.5) | 52.8 (2.6) | 52.9 (2.6) |
| First cycle length, Mean (SD) | 27.2 (5.2) | 27.1 (5.2) | 27.2 (5.2) |
| Time preceding FMP c, year, Mean (SD) | 7.4 (2.5) | 7.3 (2.6) | 7.5 (2.6) |
Higher score indicates more physical activity
Antihypertensive, antidiabetic, lipid lowering, or anticoagulant medications
Years preceding FMP was the time difference between the completion date of baseline visit and the final menstrual period date
Abbreviations: DHS: Daily Hormone Study; FMP: final menstrual period; baPWV: brachial-ankle pulse wave velocity; cIMT: carotid intima-media thickness; BMI: body mass index; SBP: systolic blood pressure; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; HOMA-IR: homeostasis model assessment insulin resistance.
Trajectory of cycle length over the MT
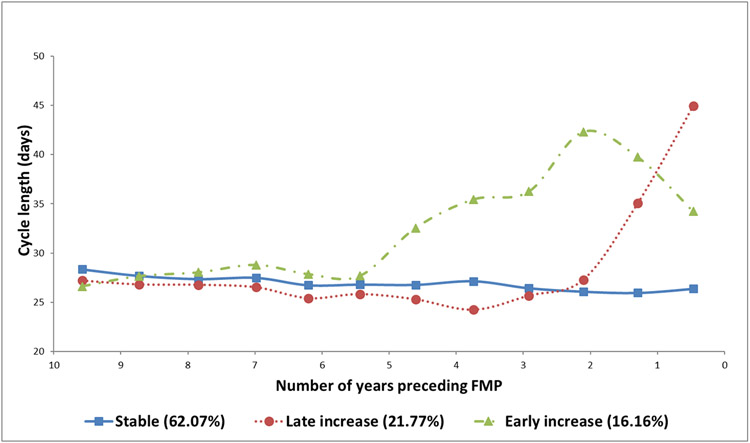
On average, participants had 4.22±1.9 observed cycles over 7.36±2.51 years before reaching menopause. Three distinct trajectories of cycle length were identified relative to time to FMP (Figure 1): 1) Stable: 62.1% of study participants followed a stable cycle length trajectory up to their FMP, 2) Late increase: 21.8% of the participants experienced a late increase in cycle length (as early as 2 years before the FMP), 3) Early increase: 16.2% of the participants experienced an early increase in cycle length (as early as 5 years before the FMP).
Figure 1. Trajectories of cycle length over the menopausal transition.
Abbreviations: FMP: final menstrual period
Baseline characteristics, mean cIMT, and baPWV by cycle length trajectory groups are presented in Table 2. Among DHS participants, there were significant differences in baseline age, BMI, SBP, HDL-C, triglycerides, HOMA-IR, cycle length, age at FMP and time preceding FMP. Women who experienced the late increase trajectory had the earliest age at menopause compared to the other two groups. In addition, significant differences in mean cIMT and baPWV were also observed. In general, women who experience a late increase in their cycle length over the MT were more likely to have a better cardiovascular risk factor profile and vascular health measures than the other two groups.
Table 2.
Overall DHS participants baseline characteristics and subclinical measures of atherosclerosis by cycle length trajectory groups
| Characteristics | Stable N=328 |
Late increase N=41 |
Early increase N=59 |
P valued |
|---|---|---|---|---|
| Age, years, Median (Q1, Q3) | 45 (43, 47) | 45 (44, 46) | 46 (44, 47) | 0.03 |
| Race, n (%) | 0.07 | |||
| Black | 65 (19.8) | 5 (12.2) | 10 (17.0) | |
| White | 100 (30.5) | 16 (39.0) | 15 (25.4) | |
| Chinese | 67 (20.4) | 14 (34.2) | 11 (18.6) | |
| Japanese | 83 (25.3) | 6 (14.6) | 7 (28.8) | |
| Hispanic | 13 (4.0) | 0 (0.0) | 6 (10.2) | |
| Difficulty paying for basics, n (%) | 0.0003 | |||
| Very hard | 17 (5.2) | 1 (2.5) | 9 (15.5) | |
| Somewhat hard | 112 (34.2) | 4 (10.0) | 13 (22.4) | |
| Not at all | 199 (60.7) | 35 (87.5) | 36 (62.1) | |
| Menopausal status, n (%) | 0.77 | |||
| Premenopause | 222 (67.9) | 26 (63.4) | 38 (64.4) | |
| Early Perimenopause | 105 (32.1) | 15 (36.6) | 21 (35.6) | |
| Education, n (%) | 0.47 | |||
| ≤ High school/some college | 168 (51.2) | 20 (48.8) | 35 (59.3) | |
| College degree/post college | 160 (48.8) | 21 (51.2) | 24 (40.7) | |
| BMI, kg/m2, Median (Q1, Q3) | 24.8 (21.6, 29.2) | 22.9 (20.8, 24.3) | 26.1 (22.3, 31.4) | 0.0003 |
| SBP, mmHg, Median (Q1, Q3) | 110 (102, 120) | 107 (101, 113) | 114 (107, 126) | 0.02 |
| HDL-C, mg/dL, Mean (SD) | 57.3 (13.1) | 63.4 (13.5) | 52.2 (13.4) | 0.0002 |
| LDL-C, mg/dL, Mean (SD) | 110.9 (28.3) | 106.0 (24.0) | 119.3 (30.3) | 0.06 |
| Triglycerides, mg/dL, Median (Q1, Q3) | 86.0 (64.0, 119.0) | 73.5 (54.5, 98.0) | 128.0 (87.0, 193.0) | <0.0001 |
| HOMA-IR index, Median (Q1, Q3) | 1.6 (1.2, 2.4) | 1.4 (1.2, 1.8) | 1.9 (1.3, 3.9) | 0.001 |
| Total physical activity score a, Mean (SD) | 7.7 (1.7) | 8.1 (1.6) | 7.6 (1.7) | 0.51 |
| Diabetes, n (%) | 13 (4.0) | 1 (2.4) | 3 (5.1) | 0.83 |
| Ever-smoker, n (%) | 108 (33.1) | 11 (26.8) | 17 (28.8) | 0.62 |
| Use of medications b, n (%) | 36 (11.0) | 2 (4.9) | 8 (13.6) | 0.38 |
| Age at FMP, Mean (SD) | 53.1 (2.5) | 51.5 (2.0) | 53.5 (2.3) | 0.0002 |
| First cycle length, Mean (SD) | 27.0 (4.5) | 24.5 (4.3) | 30.9 (7.1) | <.0001 |
| Time preceding FMP, year, Mean (SD) | 7.6 (2.5) | 6.1 (2.2) | 7.2 (2.5) | 0.001 |
| Mean cIMT, mm, Mean (SD) c | 0.8 (0.1) | 0.7 (0.1) | 0.8 (0.1) | 0.007 |
| baPWV, cm/sec, Median (Q1, Q3) c | 1442 (1316, 1616) | 1324 (1164, 1457) | 1424 (1367, 1630) | 0.02 |
Higher score indicating more physical activity
Antihypertensive, antidiabetic, lipid lowering, or anticoagulants medications
Sample size for cIMT by groups (stable, late increase, early increase): 195, 33, 35; for baPWV: 161, 23, 29, respectively
We applied Chi-square test or Fisher’s exact test to determine differences in the frequency of categorical variables and ANOVA to compare differences in the mean of continuous variables.
Abbreviations: FMP: final menstrual period; baPWV: brachial-ankle pulse wave velocity; cIMT: carotid intima-media thickness; BMI: body mass index; SBP: systolic blood pressure; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; HOMA-IR: homeostasis model assessment insulin resistance.
Trajectory of cycle length over the MT and postmenopausal subclinical measures of atherosclerosis
In the unadjusted models, there were significant differences in mean cIMT (p=0.007) and baPWV (p=0.02) by trajectory groups. Women in the late increase group had 0.07 mm lower mean cIMT (p=0.003) and 129 cm/sec lower baPWV (p=0.01) compared with women in the stable group. Adjusting for study covariates attenuated the overall association, however differences between women in the late increase group and the stable group remained significant with very similar effect sizes (Table 3). Results from sensitivity models that were additionally adjusted for age at FMP and baseline cycle length provided similar effect sizes although the P-values for differences between the late increase group and the stable group were attenuated, potentially due to small cell sizes and large number of covariates in these models. No significant differences were found between the stable and early increase groups.
Table 3.
Adjusted means of cIMT and baPWV by cycle length trajectory groups
| Model Cycle Length Trajectory Group |
cIMT, mm | baPWV, cm/seca | ||
|---|---|---|---|---|
| Mean (95% CI) | P value | Geometric Mean (95% CI) | P value | |
| Unadjusted model | 0.007 | 0.02 | ||
| Stable | 0.80 (0.78 to 0.81) | ref | 1462 (1427 to 1498) | ref |
| Late increase | 0.73 (0.68 to 0.77) | 0.003 | 1333 (1250 to 1421) | 0.008 |
| Early increase | 0.81 (0.77 to 0.85) | 0.58 | 1486 (1404 to 1573) | 0.60 |
| Model 1 b | 0.049 | 0.14 | ||
| Stable | 0.80 (0.77 to 0.84) | ref | 1545 (1471 to 1622) | ref |
| Late increase | 0.75 (0.69 to 0.80) | 0.02 | 1445 (1338 to 1560) | 0.047 |
| Early increase | 0.81 (0.76 to 0.86) | 0.65 | 1537 (1437 to 1644) | 0.88 |
| Model 2 c | 0.10 | 0.07 | ||
| Stable | 0.77 (0.72 to 0.80) | ref | 1508 (1418 to 1598) | ref |
| Late increase | 0.72 (0.67 to 0.77) | 0.03 | 1392 (1279 to 1514) | 0.02 |
| Early increase | 0.76 (0.71 to 0.82) | 0.81 | 1496 (1381 to 1621) | 0.84 |
Natural log transformed baPWV analyzed, antilog of mean and 95% confidence interval (CI) presented as geometric mean and 95% CI.
Adjusted for race, age, total physical activity score, and difficulty paying for basics measured at cIMT or baPWV visit.
Adjusted for covariates listed with Mode 1 + baseline SBP, triglyceride, HOM-IR index, BMI, use of medication, and ever smoking status.
Abbreviations: baPWV: brachial-ankle pulse wave velocity; cIMT: carotid intima-media thickness.
Discussion
The current study showed that not all women experienced the same trajectory of cycle length over the MT. Almost 62% of women experienced a stable trajectory of cycle length with no changes in length until they approached menopause, while the rest experienced either an early increase in the lengths of their menstrual cycles that began as early as 5 years (16.2%) prior to the FMP, or a later increase in their cycle length that began as late as 2 years before the FMP (21.8%). The current study showed that pattern of cycle length trajectory over the MT could be an independent marker of vascular health after menopause. We reported that women who experienced a late increase in their cycle length over the MT had more favorable subclinical atherosclerotic measures compared to women who experienced the stable trajectory. This finding was independent of premenopausal CVD risk profile including BMI, race/ethnicity, and concurrent age, socioeconomic status and physical activity level. Interestingly, women who experienced an early increase trajectory had the worst cardiometabolic risk profile.
In an earlier analysis from the SWAN DHS study, we showed that menstrual cycle length remained stable up to 4 years before the FMP, after which cycles become longer.19 These earlier findings were in agreement with findings from the TREMIN cohort, one of few data sets providing women’s menstrual calendar data across nearly all of the adult reproductive life span, showing the average menstrual cycle length increased as early as 4 years prior to the FMP.13 Interestingly, another work from SWAN focusing on between-participants’ variabilities showed cycle length to increase around 7.5 years before the FMP with the steepest increase occurring between 4 and 1.5 years before the FMP. However, these estimates were for women who were White, normal or underweight, did not smoke, had at least some post high school education, and had no moderate or physical activity during the average week.12 The wide range in the estimated timing of cycle length changes relative to the FMP suggest heterogeneity in cycle length trajectories over the MT, however, these patterns cannot be characterized using traditional statistical approaches. Our current work, using an anlytical method that accounts for heterogenity, showed that women indeed experience different patterns of cycle length change as they transition through menopause. This reported heterogeneity appears to be critical when it comes to CVD risk after menopause.
Several studies assessed the link between menstrual cycle irregularities and cardiometabolic disease risk.5,10,20,21 However, few focused on cycle length. Additionally, previous work targeted cycle irregularities at a younger age and none assessed patterns of change in cycle length as women approach menopause. Among 101,073 women (baseline age 24-43 years) from the Nurses’ Health Study II with no history of diabetes, women with long (≥40 days) or highly irregular menstrual cycles at age 18-22 had a significantly increased risk for developing type 2 diabetes independent of obesity or other potential confounders compared to women with a usual cycle length of 26-31 days.10 Interestingly, reporting menstrual irregularity during the ages of 20-35 was also linked to a higher risk of non-fatal or fatal coronary heart disease independent of traditional CVD risk factors compared to reporting a history of very regular menstrual cycle among 82,439 female nurses (age 36-61 years old at baseline).5 Our findings of a link between cycle length trajectories and early markers of atherosclerosis later in life are in agreement with previous work. Moreover, our current research extends the previous body of literature by focusing on patterns of change in cycle length over the MT and showing that the timing of increases in cycle length matters when it comes to CVD risk.
In the current study, women who experienced an increase in their cycle length for a longer time, the early increase group, had the worst cardiometabolic profile at baseline. These findings were consistent with other studies linking menstrual irregularities to CVD risk.5,10,20,21 Cycle length over the MT was related to having cycles with no evidence of luteal activity in an earlier work from the SWAN DHS;19 suggesting that longer cycle length could be a marker of fewer ovulations and thus a lower mean of estrogen levels.10 Higher endogenous estradiol levels before menopause has been related to a lower risk of subclinical atherosclerosis after menopause.22 Alternatively, menstrual irregularities and experiencing longer cycle could be a marker of insulin resistance and metabolic abnormalities A direct effect of insulin on ovarian steroidogenesis has been suggested.23 Moreover, women with a history of long cycles (≥30 days) during reproductive life (age of 30-40 years old) had higher triglycerides compared to women with a history of shorter cycles (≤26 days).24
Interestingly, women who experienced an increase in their cycle length later in the transition had a better cardiometabolic profile than women who did not have any changes in their cycle length. This group also had better postmenopausal subclinical measures of atherosclerosis than the stable group, independent of traditional CVD risk factors and other important confounders. The explanation of why the late increase group had a better CVD risk profile than the stable group is not obvious. Cycle quality as measured by the underlying hormonal milieu may differ in these two groups. As discussed above, longer cycles may be hypoestrogenic 10 so the less time a woman spends with long cycles prior to menopause the more protected her cardiovascular system would be. However, having as much estrogen on board after a certain age may not be as cardioprotective as during reproductive life. Higher estrogen levels after menopause have been found to be associated with greater insulin resistance,25 inflammatory marker levels,26 and a more pro-atherogenic lipid profile.27 It is plausible that the late increase group is protected by not having too much estrogen at a critical time during the MT. In the current study, women who experienced the late increase trajectory had the youngest age at menopause compared to the other two groups supporting the argument that differences in hormone production in these cycles across the three groups might exist and requires additional investigation.
Major strengths of this study are the well-characterized cohort, the inclusion of women from different race/ethnic groups, the prospective collection of annual menstrual cycle data until women reached menopause enabling a comprehensive characterization of cycle length changes over the MT. The current results should also be viewed in light of some limitations including the small sample size for women who classified into the early increase group which may limit our ability to detect significant differences across groups and the lack of data from Japanese limiting study generalizability. We reported that 62% of the included women had a stable cycle length trajectory until they approached menopause. The reported prevalence of a stable cycle length is much higher than what has been previously reported in TREMIN cohort of about 28%.28 The higher prevalence in the current study could be a result of the DHS study design requiring all women to be included to have a regular menstrual cycle suggesting an oversampling of this category. Other studies should conduct similar analysis to confirm our findings. Whether the reported differences in subclinical measures of atherosclerosis among the three cycle length groups are clinically significant would require further research to assess their relationship with hard events later in life.
Conclusions:
We identified 3 trajectories of cycle length over MT: stable, late increase, and early increase. Among the three groups of women, those who experienced an early increase trajectory had the worst cardiometabolic risk profile. Women who experience expected changes, a pattern of late increases in cycle length close to the FMP, had less evidence of subclinical atherosclerosis than did those with no changes in cycle length preceding the FMP. Patterns of cycle length over the MT appear to be a marker of future vascular health that may help identify groups at greater risk of atherosclerosis after menopause.
Acknowledgments:
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Sherri-Ann Burnett-Bowie, PI 2020 – Present; Joel Finkelstein, PI 1999 – 2020; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Imke Janssen, PI 2020 – Present; Howard Kravitz, PI 2009 – 2020; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Elaine Waetjen and Monique Hedderson, PIs 2020 – Present; Ellen Gold, PI 1994 - 2020; University of California, Los Angeles – Arun Karlamangla, PI 2020 – Present; Gail Greendale, PI 1994 - 2020; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Rebecca Thurston, PI 2020 – Present; Karen Matthews, PI 1994 - 2020.
NIH Program Office: National Institute on Aging, Bethesda, MD – Rosaly Correa-de-Araujo 2020 - present; Chhanda Dutta 2016- present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor – Siobán Harlow 2013 - 2018; Dan McConnell 2011 - 2013; MaryFran Sowers 2000 – 2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Sources of funding:
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Footnotes
Financial Disclosures/Conflict of Interests: Dr. Thurston: Consultant/advisory board member for Astellas, Procter & Gamble, and Pfizer, Consultant fees from Virtue Health. Dr. Santoro: Consultant for Ansh labs, advisory board member for Astellas, and scientific advisory board and grant support to her institution: Menogenix, Inc. The other authors have nothing to disclose.
Previous presentation: This abstract has been previously presented at the 2018 NAMS annual meeting; October 3-6, 2018, in San Diego, CA.
References
- 1.Diaz A, Laufer MR, Breech LL. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics. 2006;118(5):2245–2250. [DOI] [PubMed] [Google Scholar]
- 2.Popat VB, Prodanov T, Calis KA, Nelson LM. The menstrual cycle: a biological marker of general health in adolescents. Ann N Y Acad Sci. 2008;1135:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mumford SL, Steiner AZ, Pollack AZ, et al. The utility of menstrual cycle length as an indicator of cumulative hormonal exposure. J Clin Endocrinol Metab. 2012;97(10):E1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landgren BM, Undén AL, Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol (Copenh). 1980;94(1):89–98. [DOI] [PubMed] [Google Scholar]
- 5.Solomon CG, Hu FB, Dunaif A, et al. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87(5):2013–2017. [DOI] [PubMed] [Google Scholar]
- 6.Whelan EA, Sandler DP, Root JL, Smith KR, Weinberg CR. Menstrual cycle patterns and risk of breast cancer. Am J Epidemiol. 1994;140(12):1081–1090. [DOI] [PubMed] [Google Scholar]
- 7.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause. 2012;19(10):1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol. 1998;8(4):229–235. [DOI] [PubMed] [Google Scholar]
- 9.de Kleijn MJ, van der Schouw YT, van der Graaf Y. Reproductive history and cardiovascular disease risk in postmenopausal women: a review of the literature. Maturitas. 1999;33(1):7–36. [DOI] [PubMed] [Google Scholar]
- 10.Solomon CG, Hu FB, Dunaif A, et al. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. Jama. 2001;286(19):2421–2426. [DOI] [PubMed] [Google Scholar]
- 11.Shim U, Oh JY, Lee HJ, Hong YS, Sung YA. Long menstrual cycle is associated with type 2 diabetes mellitus in korean women. Diabetes Metab J. 2011;35(4):384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paramsothy P, Harlow SD, Elliott MR, et al. Influence of race/ethnicity, body mass index, and proximity of menopause on menstrual cycle patterns in the menopausal transition: the Study of Women's Health Across the Nation. Menopause. 2015;22(2):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrell RJ, Simon JA, Pincus SM, et al. The length of perimenopausal menstrual cycles increases later and to a greater degree than previously reported. Fertil Steril. 2006;86(3):619–624. [DOI] [PubMed] [Google Scholar]
- 14.Sowers M, Crawford S, Sternfeld B, et al. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. 2000. [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 17.NAGIN D Group-Based Modeling of Development. Harvard University Press; 2009. [Google Scholar]
- 18.Zhu D, Chung HF, Pandeya N, et al. Premenopausal cardiovascular disease and age at natural menopause: a pooled analysis of over 170,000 women. Eur J Epidemiol. 2019;34(3):235–246. [DOI] [PubMed] [Google Scholar]
- 19.Santoro N, Crawford SL, El Khoudary SR, et al. Menstrual Cycle Hormone Changes in Women Traversing Menopause: Study of Women’s Health Across the Nation. J Clin Endocrinol Metab. 2017;102(7):2218–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertuccio P, Tavani A, Gallus S, Negri E, La Vecchia C. Menstrual and reproductive factors and risk of non-fatal acute myocardial infarction in Italy. Eur J Obstet Gynecol Reprod Biol. 2007;134(1):67–72. [DOI] [PubMed] [Google Scholar]
- 21.Rostami Dovom M, Ramezani Tehrani F, Djalalinia S, Cheraghi L, Behboudi Gandavani S, Azizi F. Menstrual Cycle Irregularity and Metabolic Disorders: A Population-Based Prospective Study. PLoS One. 2016;11(12):e0168402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Khoudary SR, Santoro N, Chen HY, et al. Trajectories of estradiol and follicle-stimulating hormone over the menopause transition and early markers of atherosclerosis after menopause. Eur J Prev Cardiol. 2016;23(7):694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poretsky L, Kalin MF. The gonadotropic function of insulin. Endocr Rev. 1987;8(2):132–141. [DOI] [PubMed] [Google Scholar]
- 24.Rubba F, Mattiello A, Chiodini P, et al. Menstrual cycle length, serum lipids and lipoproteins in a cohort of Italian Mediterranean women: findings from Progetto ATENA. Nutr Metab Cardiovasc Dis. 2008;18(10):659–663. [DOI] [PubMed] [Google Scholar]
- 25.Golden SH, Dobs AS, Vaidya D, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92(4):1289–1295. [DOI] [PubMed] [Google Scholar]
- 26.Folsom AR, Golden SH, Boland LL, Szklo M. Association of endogenous hormones with C-reactive protein, fibrinogen, and white blood count in post-menopausal women. Eur J Epidemiol. 2005;20(12):1015–1022. [DOI] [PubMed] [Google Scholar]
- 27.Lambrinoudaki I, Christodoulakos G, Rizos D, et al. Endogenous sex hormones and risk factors for atherosclerosis in healthy Greek postmenopausal women. Eur J Endocrinol. 2006;154(6):907–916. [DOI] [PubMed] [Google Scholar]
- 28.Gorrindo T, Lu Y, Pincus S, et al. Lifelong menstrual histories are typically erratic and trending: a taxonomy. Menopause. 2007;14(1):74–88. [DOI] [PubMed] [Google Scholar]