Abstract
Totality of evidence indicates menopausal hormone replacement therapy (HRT) effects are determined by timing of initiation according to age and/or time-since-menopause, underlying health of target tissue and duration of therapy. Initiated in women <60 years of age and/or at or near menopause, HRT significantly reduces all-cause mortality and cardiovascular disease (CVD) whereas other primary CVD prevention therapies such as lipid-lowering fail to do so. Magnitude and type of HRT-associated risks, including breast cancer, stroke and venous thromboembolism are rare (<10 events/10,000 women), not unique to HRT and comparable with other medications. HRT is a sex-specific and time dependent primary CVD prevention therapy that concomitantly reduces all-cause mortality as well as other aging-related diseases with an excellent risk profile. Keeping in mind that prevention strategies must be personalized, health-care providers and patients can use cumulated HRT data in making clinical decisions concerning chronic disease prevention including CVD and mortality reduction.
Keywords: Hormone replacement therapy, estrogen, menopause, prevention, cardiovascular disease, all-cause mortality, randomized trials, observational studies, meta-analysis
Introduction
Causing 1 in 3.2 deaths in women each year in the U.S., cardiovascular disease (CVD) remains the number one killer of women, accounting for approximately one death every 80 seconds (1). Differences exist between women and men in presentation, outcomes and pathophysiological mechanisms making CVD a more severe disease for women than men (2). For example, 64% of women versus 50% of men who die suddenly of coronary heart disease (CHD) have no previous symptoms (3). In addition, more women than men die within one year (23% of women versus 18% of men) and within five years (47% of women versus 36% of men) after a first myocardial infarction (MI), and more women than men develop heart failure (22% of women versus 16% men) and suffer a stroke (7% of women versus 4% of men) within five years of a first MI (1,2). Further, more women than men have a recurrent MI or fatal CHD (21% of women versus 17% of men) within five years after a first MI (1).
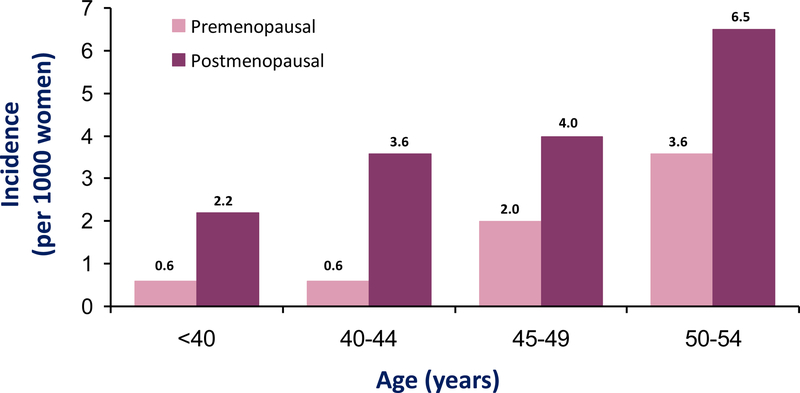
Pathophysiologically, incidence of CHD in women lags behind men by 10 years and incidence of MI and sudden death in women lags behind men by 20 years (1). This delay in onset of CVD appears due to the cardioprotective effects of endogenous estrogen where women exhibit two patterns of cardiovascular risk during their life span. While premenopausal, women are protected from clinical manifestations of CVD relative to men, whereas, after menopause, CVD complications exceed those of men. Although there is an age-associated increase in CVD incidence for women as there is for men, age-specific CVD incidence is two- to six-fold greater for postmenopausal than premenopausal women across the age range <40–54 years (Fig. 1) (4).
Figure 1.
Incidence of cardiovascular disease in women is both age-associated and age-specific as reported from the Framingham Study (4). In addition to an increase in cardiovascular disease incidence by age in women, postmenopausal women have a two- to six-fold greater incidence of cardiovascular disease than premenopausal women across the age range <40–54 years.
Development of substantial CVD risk after menopause provides a window of opportunity for extension of cardioprotection from endogenous estrogen in postmenopausal women with hormone replacement therapy (HRT) as a sex-specific primary preventive therapy for CVD and reduction of all-cause mortality.
The Premise of the Timing Hypothesis
The “timing hypothesis” posits that the effects of menopausal HRT on atherosclerosis and clinical events are dependent upon when HRT is initiated in relation to age and/or menopause. The timing hypothesis has been supported by randomized controlled atherosclerosis imaging trials, animal studies as well as randomized controlled clinical event trials and observational studies taken together.
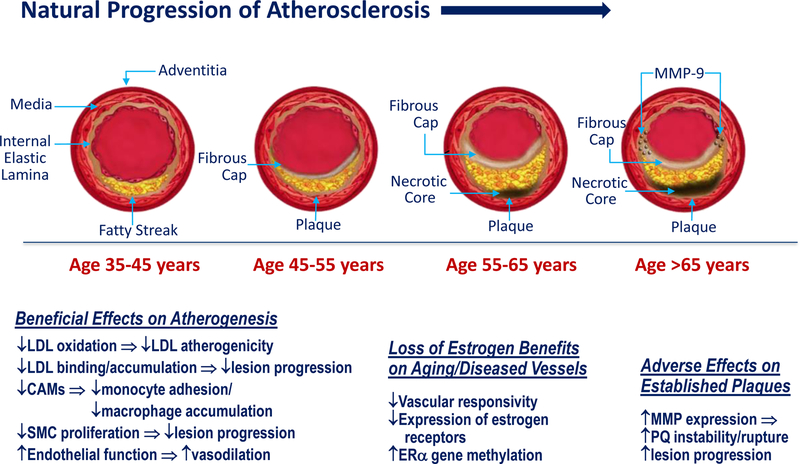
The timing hypothesis was built on the premise of the “healthy endothelium hypothesis” accounting for a duality of estrogen effects on the natural progression of atherosclerosis. According to the hypothesis, estrogen exerts early beneficial effects of HRT on healthy endothelium, but adverse effects on established plaques (Fig. 2) (5).
Figure 2.
The “healthy endothelium hypothesis” accounts for the duality of estrogen on the natural progression of atherosclerosis by taking in account the early beneficial effects of HRT on healthy endothelium at younger ages and the adverse effects on established plaques typically at older ages. MMP = matrix metalloproteinase; LDL = low-density lipoprotein; CAMs = cell adhesion molecules; SMC = smooth muscle cell; ERα = estrogen receptor alpha; PQ = plaque
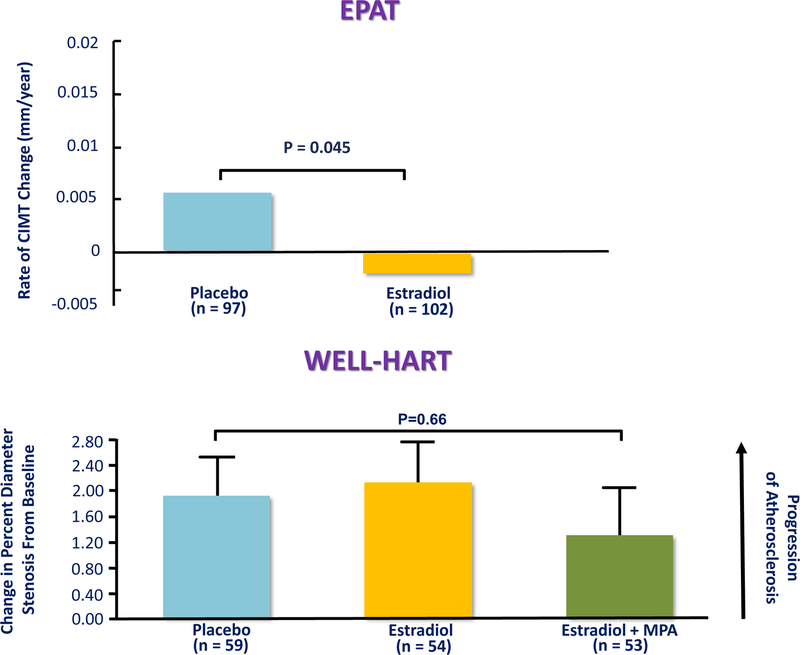
Sister randomized, double-blinded, placebo-controlled trials were designed to determine HRT effects on atherosclerosis progression in postmenopausal women without and with pre-existing clinical vascular disease, the Estrogen in the Prevention of Atherosclerosis Trial (EPAT) and the Women’s Estrogen-Progestin Lipid-Lowering Hormone Atherosclerosis Regression Trial (WELL-HART), respectively (Fig. 3) (5,6). Taken together, the different outcomes in the two trials of an estrogen-related reduction in atherosclerosis progression in EPAT (postmenopausal women without vascular disease) and no estrogen effect on atherosclerosis progression in WELL-HART (postmenopausal women with vascular disease) support the healthy endothelium hypothesis according to the stage of atherosclerosis as reflected by the imaging methods used in these two trials (Fig. 4). Carotid artery wall thickness was used in EPAT to evaluate asymptomatic early subclinical atherosclerosis, while quantitative coronary angiography was used in WELL-HART to measure symptomatic late-stage atherosclerosis lesions (7). While confirming EPAT and WELL-HART results, other lines of evidence support the healthy endothelium hypothesis across multiple animal species including rabbits (8), rats (9), mice (10) and non-human primates (11–15); these animal studies clearly show that the antiatherosclerosis action of estrogen is dependent on a healthy intact endothelium. For example, in apolipoprotein E deficient mice, estrogen therapy prevented formation of new lesions when initiated at the time of atherogenesis but had no effect on established lesions (Fig. 5) (10).
Figure 3.
The Estrogen in the Prevention of Atherosclerosis Trial (EPAT) showed a reduction in the progression of early subclinical atherosclerosis measured by carotid artery wall intima-media thickness (CIMT) in healthy asymptomatic postmenopausal women treated with estradiol replacement therapy relative to placebo (5). In contrast, the Women’s Estrogen-Progestin Lipid-Lowering Hormone Atherosclerosis Regression Trial (WELL-HART) showed no effect of hormone replacement therapy on serial quantitative coronary angiography used to measure late-stage atherosclerosis lesions in symptomatic women with preexisting coronary artery disease (6). MPA = medroxyprogesterone acetate
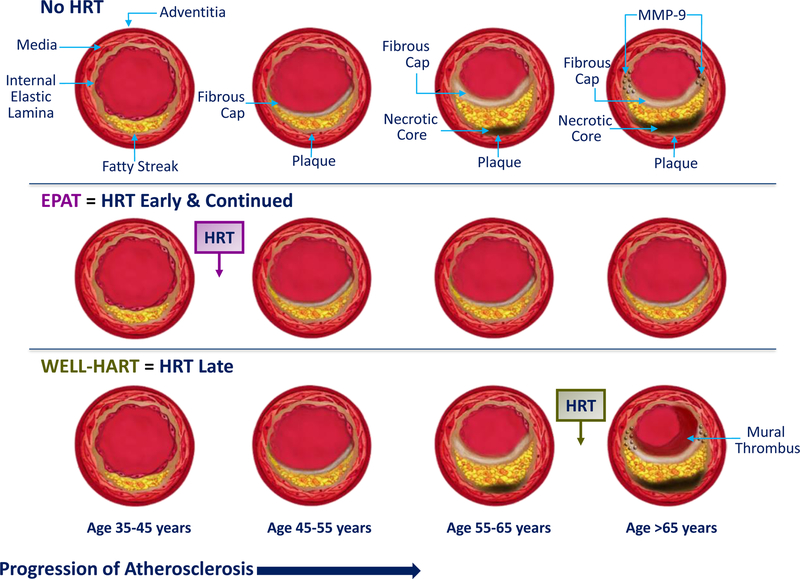
Figure 4.
Results of the Estrogen in the Prevention of Atherosclerosis Trial (EPAT) (5) and the Women’s Estrogen-Progestin Lipid-Lowering Hormone Atherosclerosis Regression Trial (WELL-HART) (6) in relation to the pathogenic sequence of vascular aging substantiating the “healthy endothelium hypothesis.” Initiation of hormone replacement therapy (HRT) early and continued while endothelium is intact, maintains vascular health, and reduces vascular aging and progression of atherosclerosis (5). On the other hand, HRT initiated during late-stage atherosclerosis has no effect on established vascular disease (6). MMP = matrix metalloproteinase
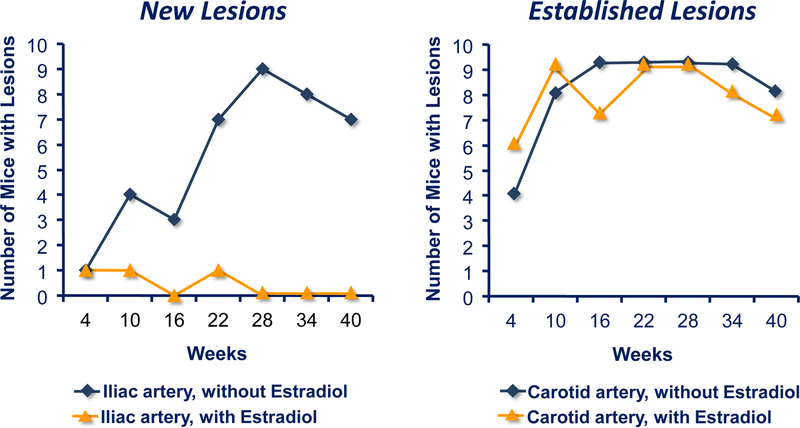
Figure 5.
As an example of animal studies supporting the “healthy endothelium hypothesis,” apolipoprotein E deficient mice show that estrogen therapy prevents formation of new atherosclerosis lesions when initiated at the time of atherogenesis but has no effect on established lesions (10).
Taken together, randomized controlled atherosclerosis imaging trials and animal studies strongly support the healthy endothelium hypothesis showing that HRT is more effective in maintaining vascular health rather than treating established vascular disease manifested as atherosclerosis lesions.
The Timing Hypothesis - Cardioprotection and Reduction in All-Cause Mortality According to Timing of Initiation of Menopausal HRT
Soon after publication of the initial Women’s Health Initiative (WHI) trial results (16), discordance between the HRT association with CHD outcome in randomized controlled trials (RCTs) contrasted to observational studies was recognized to likely be due to the considerably different populations of women studied across the two study designs (Table 1) (17). More than 40 observational studies show a consistent 30%–50% reduction in CHD in HRT users versus nonusers (18,19). On the other hand, RCTs have shown a null effect of HRT on CHD in analyses of women over all ages, typically 45–90 years old when randomized to HRT versus placebo. Considering the background of arterial imaging trials and animal studies supporting the healthy endothelium hypothesis, comparison of the characteristics of the cohorts studied in the two study designs, RCTs and observational studies, provides further support for timing of HRT initiation in relation to age and/or time-since-menopause.
Table 1.
Characteristics of Women in Observational Studies and Randomized Trials
| Observational Studies | Randomized Trials | |
|---|---|---|
|
| ||
| Mean age or age range at enrollment (years) | 30–55 | >63 |
| Time-since-menopause at HRT initiation (years) | <2 | >10 |
| Menopausal symptoms (flushing) | predominant | excluded |
| Duration of therapy (years) | >10–40 | <7 |
| Body mass index (mean, kg/m2) | 25.1 | 28.5 |
HRT = hormone replacement therapy
Examination of Table 1 shows that women selected for RCTs were considerably different from women enrolled in observational studies. Observational studies represent the typical woman who initiates HRT. As shown in Table 1, the typical woman initiating HRT is relatively young (30–55 years of age), close in proximity to menopause (predominantly initiating HRT within 2 years of menopause), relatively lean (body mass index (BMI) of 25.1 kg/m2) and symptomatic with flushing and other menopausal symptoms as these are the primary reasons for seeking HRT. Many of the women in observational studies who chose to use HRT did so for decades (10–40 years). On the other hand, relative to women in observational studies, women randomized to clinical trials were considerably older (average age >63 years) with more than 90% >55 years old and on average >10 years-since-menopause (range 13–23 years). Women with menopausal symptoms, mainly flushing were excluded from RCTs because of the concern of unblinding. Mean <7 years duration of HRT (range 1–6.8 years) was also considerably less in RCTs than duration of HRT use in observational studies. Additionally, women in RCTs were, on average, overweight to obese (average BMI 28.5 kg/m2) with some RCTs including large numbers of obese women. For example, 34% of the women in the WHI-conjugated equine estrogen + medroxyprogesterone acetate (CEE+MPA) trial (16) and 45% of the women in the WHI-CEE trial (20) were obese with BMI >30 kg/m2. BMI may be an important determinant of the effectiveness of HRT on CVD (21).
Clearly, comparison of characteristics of women from cumulated RCTs and observational studies shows that women selected for RCTs were markedly different than women studied from the general population in observational studies. The latter women represent the typical woman initiating HRT when younger and close in proximity to menopause. In this regard, the Danish Osteoporosis Prevention Study (DOPS) is the only randomized clinical event trial specifically designed to study HRT in a cohort of recently postmenopausal women with similar characteristics to women in observational studies from which the cardioprotective hypothesis was developed (22). Similarly, the Early versus Late Intervention Trial with Estradiol (ELITE) is the only RCT specifically designed to formally test the HRT timing hypothesis in women who were randomized to HRT when <6 years and >10 years-since-menopause (23).
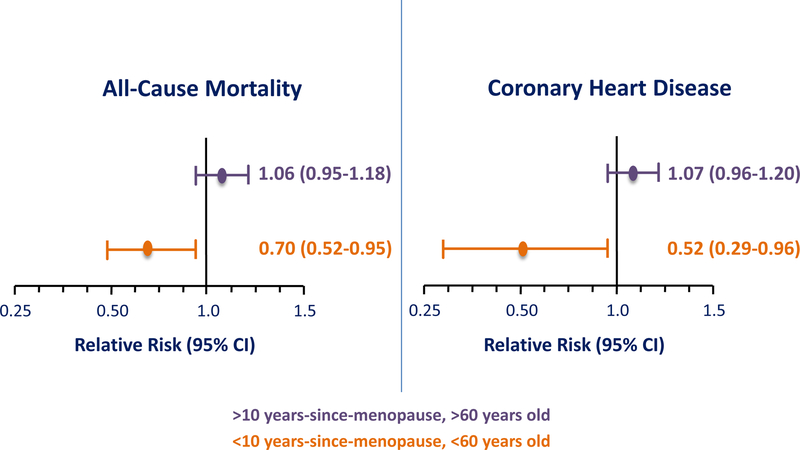
Although the effect of HRT on CHD over all ages is null in RCTs, these trials also show that there are beneficial effects of HRT on CHD according to timing of initiation of HRT relative to age and/or menopause. Over the last 20 years, there has been a substantial cumulation of RCT data that strongly support the timing hypothesis (23). In two meta-analyses, Salpeter, et al. showed that relative to placebo, HRT significantly reduced all-cause mortality by 39% (95% confidence interval (CI), 5%–61%) across 30 RCTs and reduced CHD by 32% (95% CI, 4%–52%) across 23 RCTs when initiated in women <60 years of age and/or <10 years-since-menopause (Fig. 6) (24,25). On the other hand, the effect of HRT on all-cause mortality and CHD was null when initiated in women >60 years old and/or >10 years-since-menopause. Importantly, these cumulated data showed that when the effect of HRT on all-cause mortality and CHD were analyzed across all women regardless of time-since-menopause with all ages combined, the outcomes were null, obfuscating the beneficial effect of HRT on all-cause mortality and CHD in women who initiated HRT at a younger age close in proximity to menopause. The estimates of HRT-associated reduction in all-cause mortality and CHD reported in the Salpeter, et al. meta-analyses are congruent with estimates from observational studies completed in populations of younger women who initiated HRT at or near the time of menopause (18,19).
Figure 6.
Salpeter, et al., meta-analyses show that initiation of hormone replacement therapy in women less than 60 years (y) of age and/or less than 10 years-since-menopause (ysm), statistically significantly reduces both all-cause mortality (24) and coronary heart disease (25) relative to placebo. The all-cause mortality meta-analysis included 30 randomized controlled trials of 119,188 women-years and the coronary heart disease meta-analysis included 23 randomized controlled trials of 191,340 women-years. CI = confidence interval
The Salpeter, et al. meta-analyses were validated and confirmed by the Cochrane group, who also showed similar reductions in all-cause mortality (30%; 95% CI, 5% – 48%) and CHD (48%; 95% CI, 4% – 71%) in women initiating HRT <60 years old and/or <10 years-since-menopause (Fig. 7) (26).
Figure 7.
Cochrane meta-analysis validates the Salpeter et al., meta-analyses (Fig. 6) showing similar reductions in all-cause mortality and coronary heart disease in women initiating HRT <60 years old and/or <10 years-since-menopause relative to placebo (26). Nineteen randomized controlled trials of 40,410 women comparing hormone replacement therapy of estrogen with or without progestogen with placebo. CI = confidence interval
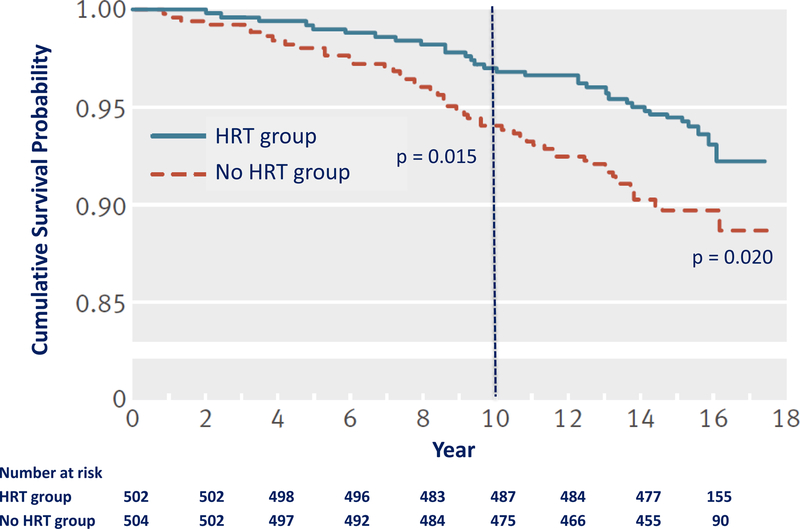
DOPS is the only randomized clinical event trial to closely replicate the populations of women in observational studies (22). Women in DOPS were on average 50 years of age and 7 months past menopause with an average BMI of 25.2 kg/m2 when randomized to HRT or no HRT. In addition, DOPS provides the longest duration of randomized HRT than any other RCT to date. CVD was reduced by 52% (hazard ratio (HR), 0.48; 95% CI, 0.27–0.89) after 10 years of randomized HRT compared with no HRT and reduced by 39% (HR, 0.61; 95% CI, 0.39–0.94) after 16 years of total follow-up (10 years of randomized treatment and 6 years of postintervention follow-up) (Fig. 8). All-cause mortality was reduced by 43% (HR, 0.57; 95% CI, 0.30–1.08) after 10 years of randomized HRT and by 34% (HR, 0.66; 95% CI, 0.41–1.08) after 16 years of total follow-up (22).
Figure 8.
Survival curve from the Danish Osteoporosis Study showing a statistically significant reduction of cardiovascular disease by 52% (HR, 0.48; 95% CI, 0.27–0.89) after 10 years of randomized hormone replacement therapy (HRT) (estrogen with or without progestogen) relative to no HRT and reduction by 39% (HR, 0.61; 95% CI, 0.39–0.94) after 16 years of total follow-up (10 years of randomized treatment and 6 years of postintervention follow-up) (22).
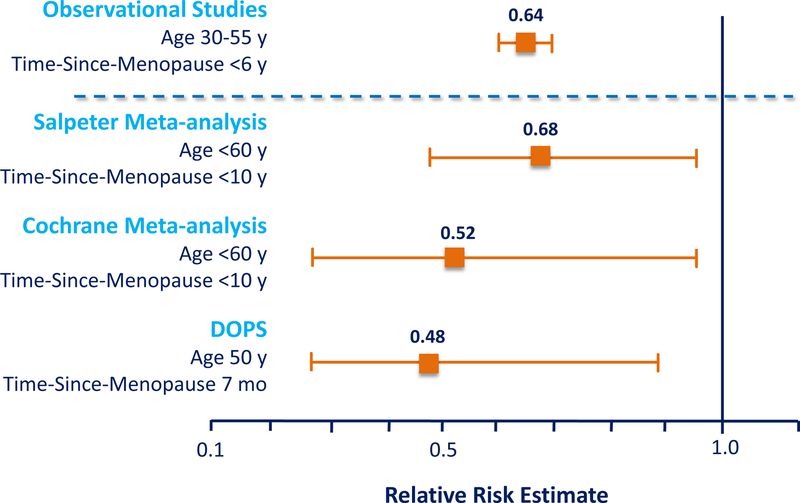
Observational studies, RCT meta-analyses of women initiating HRT <60 years and/or <10 years-since-menopause, and DOPS with the closest cohort to the population of women in observational studies, show similar statistical reductions of CHD (Fig. 9).
Figure 9.
Comparison of meta-analyses of randomized trials (25,26) and the Danish Osteoporosis Study (DOPS) (22) with observational studies (18,19) show that when hormone replacement therapy is initiated in women <60 years (y) and/or <10 years-since-menopause there is a consistent reduction of cardiovascular disease across randomized trials and observational studies in similar populations of women. DOPS included the closest cohort to the population of women in observational studies with an average age of 50 years and time-since-menopause of 7 months and average body mass index of 25.2 kg/m2. Mo = months
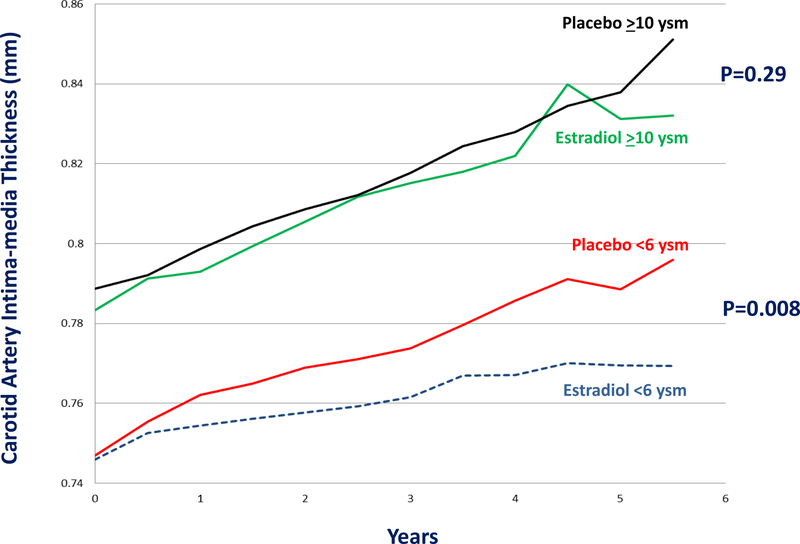
Designed to specifically test the HRT timing hypothesis, ELITE showed that HRT initiated in women close in proximity to menopause reduced progression of subclinical atherosclerosis relative to placebo. In contrast, when initiated more distant from menopause, HRT had no effect on atherosclerosis progression relative to placebo (Fig. 10) (27). ELITE was a randomized, double-blinded, placebo-controlled noninvasive serial arterial imaging trial. Healthy postmenopausal women without clinical evidence of CVD were randomized to HRT or placebo in two strata according to time-since-menopause, <6 years (early postmenopausal stratum) or >10 years (late postmenopausal stratum) (23). In the early postmenopausal stratum, the median time-since-menopause was 3.5 years, and the mean participant age was 55.4 years. In the late postmenopausal stratum, the median time-since-menopause was 14.3 years, and the mean participant age was 65.4 years. The difference in atherosclerosis progression between treatment groups (HRT vs. placebo) in both postmenopausal strata (early vs. late) was highly statistically different (p-value for interaction=0.007) (27). In addition to confirming the timing hypothesis, ELITE provides a pathophysiological mechanism by which HRT reduces CVD through reduction of atherosclerosis progression, the primary underlying pathogenesis of CVD (7).
Figure 10.
The Early versus Late Intervention Trial with Estradiol (ELITE) was specifically designed to test the hormone replacement therapy timing hypothesis comparing oral estradiol with or without vaginal progesterone with placebo in two strata of women who were at the time of randomization <6 years-since-menopause (<6 ysm) or ≥10 years-since-menopause (≥10 ysm) (23). After median 5-year intervention, women in the early postmenopause stratum (<6 ysm) showed a statistically significant reduction in progression of subclinical atherosclerosis measured by carotid artery intima-media thickness with hormone replacement therapy relative to placebo; 0.0044 versus 0.0078 mm per year (p=0.008), respectively (27). Among women in the late postmenopause stratum (≥10 ysm), rates of atherosclerosis progression were similar in the hormone replacement therapy and placebo groups; 0.0088 versus 0.0100 mm per year (p=0.29), respectively. The effect of hormone replacement therapy on subclinical atherosclerosis progression significantly differed between the early postmenopause stratum and the late postmenopause stratum (P=0.007 for interaction) (27).
Reduction of Mortalities with HRT
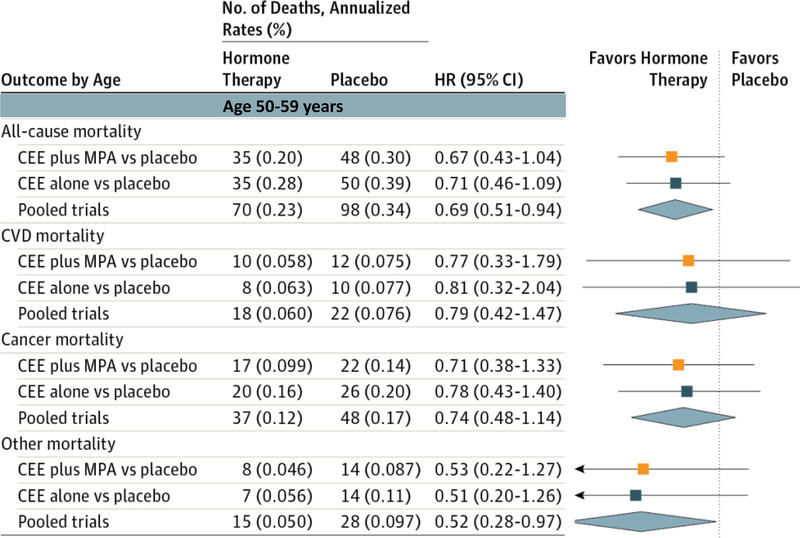
An important contribution of the WHI was publication of mortality data showing statistically significant reductions in all-cause and other mortalities in women who were <60 years of age when randomized to HRT; CVD and cancer mortalities were non-significantly reduced (Fig. 11) (28). Magnitude of mortality reductions was similar across both WHI trials of CEE alone and continuous combined CEE+MPA.
Figure 11.
Mortality outcomes during the Women’s Health Initiative trials of hormone replacement therapy for women aged 50–59 years (y) at the time of randomization (28). Magnitude of mortality reductions was similar across both Women’s Health Intervention trials of continuous combined conjugated equine estrogen plus medroxyprogesterone acetate (CEE plus MPA) and conjugated equine estrogen alone (CEE). For the CEE plus MPA trial, median intervention was 5.6 years [interquartile range, 4.9–6.5 years]. For the CEE alone trial, median intervention was 7.2 years [interquartile range, 6.5–8.2 years]. For the pooled trials (CEE plus MPA and CEE alone) median intervention was 6.3 years [interquartile range, 5.3–7.3 years]. HR = hazard ratio; No. = number; CI = confidence interval
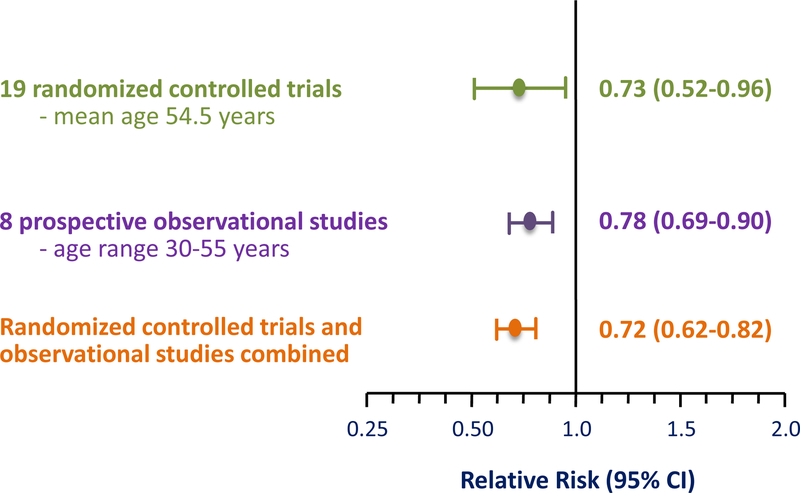
Reduction of all-cause mortality in women who initiate HRT <60 years old and/or <10 years-since-menopause is consistent across RCTs and observational studies (Table 2). This observation is confirmed in a Bayesian meta-analysis that shows all-cause mortality in postmenopausal women initiating HRT with an average age of 54.5 years in RCTs is very similar to all-cause mortality in observational studies (Fig. 12) (29).
Table 2.
Consistent Reduction in All-Cause Mortality in Women Initiating Hormone Replacement Therapy Before Age 60 years and/or Within 10 Years of Menopause Across Cumulated Data From Randomized Trials and Observational Studies
| Studies | Age; Time-Since-Menopause | Therapy | % Reduction (Risk Ratio; 95% Confidence Interval) |
|---|---|---|---|
| WHI-CEE (28) | <60 y | CEE alone | ↓ 29% (0.71; 0.46–1.09) |
| WHI-CEE+MPA (28) | <60 y | CEE+MPA | ↓ 33% (0.67; 0.43–1.04) |
| WHI-CEE/CEE+MPA Pooled Trials (28) | <60 y | CEE and CEE+MPA | ↓ 31% (0.69; 0.51–0.94) |
| DOPS – 10-year follow-up (22) | 50 y | E2/E2+NE | ↓ 43% (0.57; 0.30–1.08) |
| DOPS – 16-year follow-up (22) | 50 y | E2/E2+NE | ↓ 34% (0.66; 0.41–1.08) |
| Salpeter meta-analysis, 2004 (24) | 54 y | HRT | ↓ 39% (0.61; 0.39–0.95) |
| Salpeter meta-analysis, 2009 (29) | 55 y | HRT | ↓ 27% (0.73; 0.52–0.96) |
| Cochrane meta-analysis (26) | <60 y; <10 ysm | HRT | ↓ 30% (0.70; 0.52–0.95) |
| Observational studies (18,19) | 30–55 y; <5 ysm | HRT | ↓ 20–60% |
CEE = conjugated equine estrogen alone; CEE+MPA = continuous combined conjugated equine estrogen plus medroxyprogesterone acetate; E2/E2+NE = estradiol with or without norethisterone acetate; HRT = hormone replacement therapy; y = years; ysm = years-sincemenopause
Figure 12.
Bayesian meta-analysis of 19 randomized controlled trials and 8 prospective observational studies showing the consistency of reduction of all-cause mortality between both study types in women who initiate hormone replacement therapy before age 60 years (29). Randomized controlled trials comprised of 16,283 women with mean age of 54.5 years at time of initiation of hormone replacement therapy; mean trial intervention was 5.1 years (1–6.8 years) with 83,043 women-years follow-up. Prospective observational studies comprised of 212,717 women with age range of 30–55 years at time of initiation of hormone replacement therapy; mean observation was 13.8 years (6–22 years) with 2,935,495 women-years of follow-up. CI = confidence interval
Meta-analyses of the cumulated RCT data show that HRT significantly reduces all-cause mortality and CVD when initiated in women <60 years of age and/or <10 years-since-menopause. Specifically designed to test the HRT timing hypothesis, ELITE confirms the hypothesis as well as RCT event data and provides a pathophysiological mechanism through atherosclerosis by which HRT reduces CVD events.
Stroke
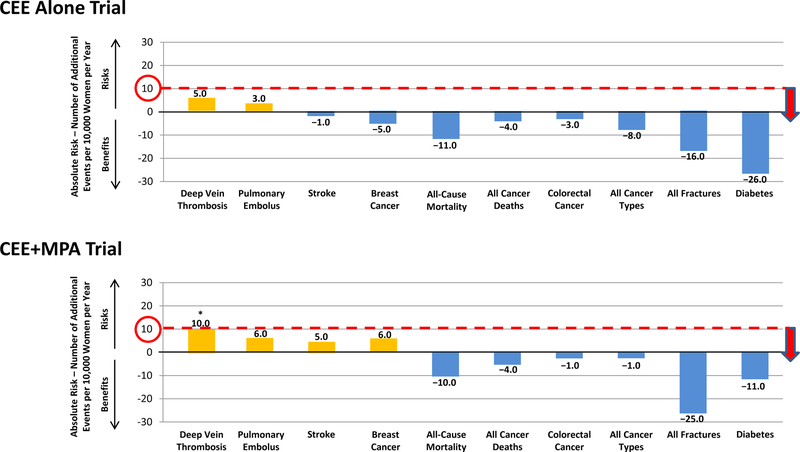
The Women’s Estrogen for Stroke Trial (WEST), the only RCT designed with stroke as the primary trial outcome showed that HRT had a null effect on secondary stroke prevention after 3-years of randomized treatment in high-risk women with non-disabling stroke or transient ischemic attack 90 days before randomization; women were on average 71 years old and 20 years past menopause (30). Although no RCTs of primary CVD prevention have been conducted with stroke as the primary trial outcome, several RCTs have analyzed stroke as a secondary or other outcome. Importantly, as shown by WHI, stroke is statistically nonsignificant and rare (<10 additional events/10,000 women) relative to placebo when initiated in women <60 years of age (Fig. 13) (31). The Cochran meta-analysis of RCTs shows that HRT has a null effect on stroke when initiated in women <60 years of age and/or <10 years-since-menopause (HR, 1.37; 95% CI, 0.8–2.34) (26). This is consistent with majority of observational data that show that menopausal HRT either reduces or has no association with stroke (21). As the only randomized cohort similar to populations from observational studies, DOPS showed that HRT did not significantly affect stroke risk after 10 years (HR, 0.77; 95% CI, 0.35–1.70) of randomized treatment and after 16 years (HR, 0.89; 95% CI, 0.48–1.65) of follow-up in women with average age 50 years and 7 months postmenopausal when randomized; 6 fewer strokes/10,000 women/year of HRT at 10 years and 2 fewer strokes/10,000 women/year of HRT at 16 years (22). The association of menopausal HRT with stroke is predominantly reported with initiation of HRT in older women distant from menopause, >60 years of age and/or >10 years-since-menopause (HR, 1.21; 95% CI, 1.06–1.38) (26).
Figure 13.
Absolute benefits and risks of hormone replacement therapy in women 50–59 years of age when randomized in the Women’s Health Initiative trials of conjugated equine estrogen alone (CEE) and continuous combined conjugated equine estrogen plus medroxyprogesterone acetate (CEE+MPA) (31). Absolute benefits and risks expressed as difference between number of events in hormone replacement therapy group and number of events in placebo group per 10,000 women per year. Absolute risk of 10/10,000 is delineated to show relation of number of additional events with the category of rare frequency of events (Table 4). *Deep vein thrombosis is the only statistically significant adverse event.
Primary CVD Prevention with HRT in Clinical Perspective
Comparison of medications is a common approach to understanding clinical utility as well as benefits and risks among therapies (32). Accordingly, comparison of benefits/risks on an absolute rather than a relative scale, permits understanding of true magnitude of benefits/risks as well as provides the ability to compare magnitude of benefits/risks across different medications using a common standard metric. Placing HRT into clinical perspective requires comparison with other medications used in primary CVD prevention. As the mainstay for primary CVD prevention in women, lipid-lowering medications, predominantly HMG-CoA reductase inhibitors (statins), are an appropriate comparator to HRT for understanding the role of HRT in primary CVD prevention.
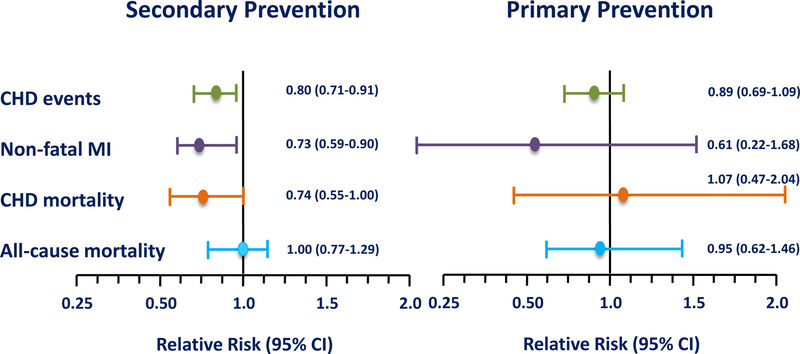
In the first large meta-analysis of RCTs of lipid-lowering therapy including statin therapy in which primary and secondary CVD prevention trials were analyzed separately in women, cumulated data show that lipid-lowering therapy does not statistically significantly reduce CHD (RR, 0.89; 95% CI, 0.69–1.09), nonfatal MI (RR, 0.61; 95% CI, 0.22–1.68) or CHD mortality (RR, 1.07; 95% CI, 0.47–2.40) when used for primary prevention in women (Fig. 14) (33). On the other hand, lipid-lowering therapy is effective in reducing CHD, nonfatal MI and CHD mortality by 20–30% when used for secondary CVD prevention in women. Cumulated data across RCTs show that when lipid-lowering therapy is used in women, all-cause mortality is neither reduced in primary CVD prevention (RR, 0.95; 95% CI, 0.62–1.46) nor secondary CVD prevention (RR, 1.00; 95% CI, 0.77–1.29) (33).
Figure 14.
Meta-analysis of randomized controlled trials of lipid-lowering therapy in which secondary (8 trials of 8,272 women) and primary (6 trials of 11,435 women) cardiovascular disease prevention trials were analyzed separately in women (33). Although lipid-lowering therapy statistically significantly reduces cardiovascular events in secondary prevention in women, lipid-lowering does not statistically significantly reduce such events in primary cardiovascular disease prevention. All-cause mortality is unaffected by lipid-lowering therapy in women in secondary and primary cardiovascular disease prevention. CHD = coronary heart disease; MI = myocardial infarction; CI = confidence interval
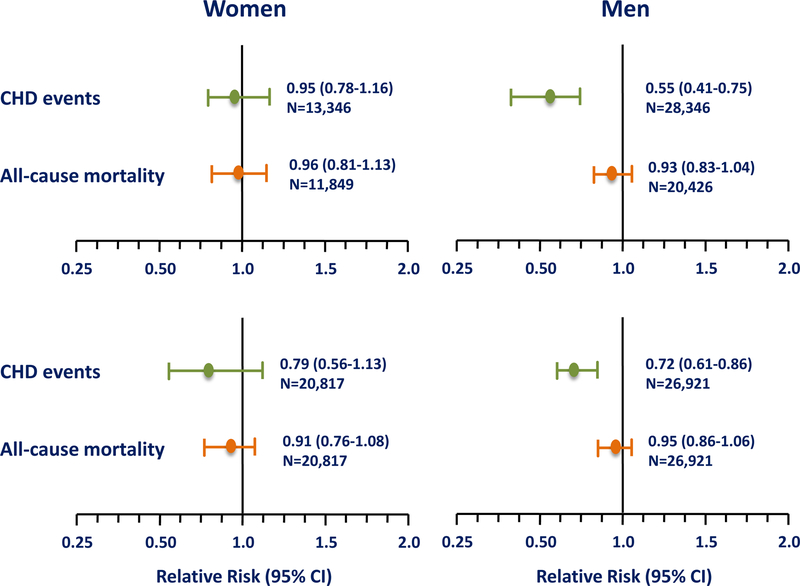
The null effect of statin therapy on primary CVD prevention in women contrasts with that of men. With careful separation of women from men and primary from secondary prevention trials, the first sex-specific meta-analysis to analyze primary CVD prevention trials showed that statin therapy reduced CHD in men but not in women (Fig. 15, top panel) (34). All-cause mortality was neither reduced in men nor women in primary CVD prevention RCTs. This latter meta-analysis included the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) trial in which 5,356 women were treated for more than 5 years (35).
Figure 15.
Sex-specific meta-analyses with careful separation of women from men and primary from secondary prevention trials to analyze primary cardiovascular disease prevention trials show that statin therapy does not statistically significantly reduce coronary heart disease (CHD) in women as it does in men. In both women and men in primary cardiovascular disease prevention, statin therapy does not reduce all-cause mortality. Upper panel from Petretta M, et al. (34). Lower Panel from Brugts JJ, et al. (36). CI = confidence interval
A second sex-specific meta-analysis that also provides careful separation of women from men and primary from secondary prevention trials confirms the first sex-specific meta-analysis showing that statin therapy significantly reduces CHD in men but does not statistically significantly reduce CHD in women in primary CVD prevention RCTs; all-cause mortality was unchanged in men and women (Fig. 15, bottom panel) (36). This meta-analysis included RCTs conducted in individuals with diabetes mellitus without a history of CVD, MEGA and the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER), a primary CVD prevention RCT of statin therapy that included 6,801 women (37). Although this meta-analysis included a larger number of women at greater CVD risk than the first sex-specific meta-analysis, lack of statistically significant reductions of all-cause mortality and CHD with statin therapy in primary CVD prevention was the same as the first meta-analysis.
The most recent primary CVD prevention RCT with statin therapy conducted in a large cohort of 5,874 women, the Heart Outcomes Prevention Evaluation-3 (HOPE-3 trial), is consistent with the sex-specific meta-analyses showing that statin therapy significantly reduces CHD in men but does not statistically significantly reduce CHD in women in primary CVD prevention and has no effect on all-cause mortality in either sex (Fig. 16) (38).
Figure 16.
The Heart Outcomes Prevention Evaluation-3 trial was a randomized, double-blinded, placebo-controlled primary cardiovascular disease prevention trial of statin therapy versus placebo conducted in 5,874 women and 6,831 men. After median 5.6 years of randomized treatment, cardiovascular disease was significantly reduced in men but not statistically significantly reduced in women. All-cause mortality was unaffected by statin therapy in both women and men (n=12,705) (38). CI = confidence interval
The consistency across individual primary CVD prevention RCTs and sex-specific meta-analyses shows that statin therapy does not statistically significantly reduce all-cause mortality or CHD in women when administered for primary CVD prevention. Sex-specific benefits of CVD predominantly restricted to men under primary prevention conditions is noteworthy for other interventions such as aspirin (39–46) and angiotensin-converting enzyme (ACE) inhibitor therapy where women show no statistically significant benefits on heart failure or on CVD or all-cause mortality whereas men show significant reductions (47,48).
Comparison of HRT initiated in women <60 years of age and/or <10 years-since-menopause with other primary CVD prevention therapies used in women reveals a stark difference between cumulated data. Whereas HRT significantly reduces all-cause mortality and CHD 30%–50%, lipid-lowering therapy, aspirin and ACE inhibitor therapy have null effects on CHD with all-cause mortality hovering around unity with HRs ranging from 0.91 to 1.09 (Table 3).
Table 3.
Comparison of Primary Cardiovascular Disease Prevention Therapies in Women1
| Outcome | HRT2 | Lipid-Lowering | Aspirin | ACE-I |
|---|---|---|---|---|
|
| ||||
| CHD | 0.68 (0.48–0.96) (25) | 0.89 (0.69–1.09) (33) | 1.01 (0.84–1.21) (39) | |
| 0.52 (0.29–0.96) (26) | 0.95 (0.78–1.16) (34) | 0.99 (0.83–1.19) (40) | ||
| 0.79 (0.56–1.13) (36) | 0.91 (0.80–1.03) (40) | |||
| 0.83 (0.64–1.09) (38) | 0.88 (0.53–1.44) (41) | |||
| 0.85 (0.60–1.20) (42) | ||||
| 0.92 (0.78–1.09) (43) | ||||
| All-cause morality | 0.61 (0.39–0.95) (24) | 0.95 (0.62–1.46) (33) | 0.94 (0.74–1.19) (39) | 1.00 (0.83–1.21) (47) |
| 0.73 (0.52–0.96) (29) | 0.96 (0.81–1.13) (34) | 1.09 (0.91–1.30) (44–46) | 0.92 (0.81–1.04) (48)3 | |
| 0.70 (0.52–0.95) (26) | 0.91 (0.76–1.08) (36) | |||
| 0.93 (0.80–1.08) (38) | ||||
Risk ratio (95% confidence interval)
Women <60 years old and/or <10 years-since-menopause at time of randomization
Mortality from heart failure
HRT = hormone replacement therapy; ACE-I = angiotensin converting enzyme inhibitor; CHD = coronary heart disease
HRT Risks in Clinical Perspective
Importantly, HRT risks, especially when initiated in women <60 years of age and/or <10 years-since-menopause are rare in magnitude and no greater than other commonly used therapies (32). Across both WHI trials of CEE alone and CEE+MPA, the primary statistically significant risk in women <60 years of age when started on HRT was deep vein thrombosis with continuous combined CEE+MPA with benefits of HRT relative to placebo ranging from 1–26 fewer events per 10,000 women per year of HRT (Fig. 13). As shown in WHI, risks of HRT on an absolute scale are small and rare (<10 additional events/10,000 women) (Table 4) (49).
Table 4.
World Health Organization (WHO) Council for International Organizations of Medical Sciences (CIOMS) Classification of Frequency of Drug Reactions
| Very common | >1/10 (>10%) |
| Common (frequent) | >1/100 and <1/10 (>1% and <10%) |
| Uncommon (infrequent) | >1/1,000 and <1/100 (>0.1% and <1%) |
| Rare | >1/10,000 and <1/1,000 (0.01% and <0.1%) |
| Very rare | <1/10,000 (<0.01%) |
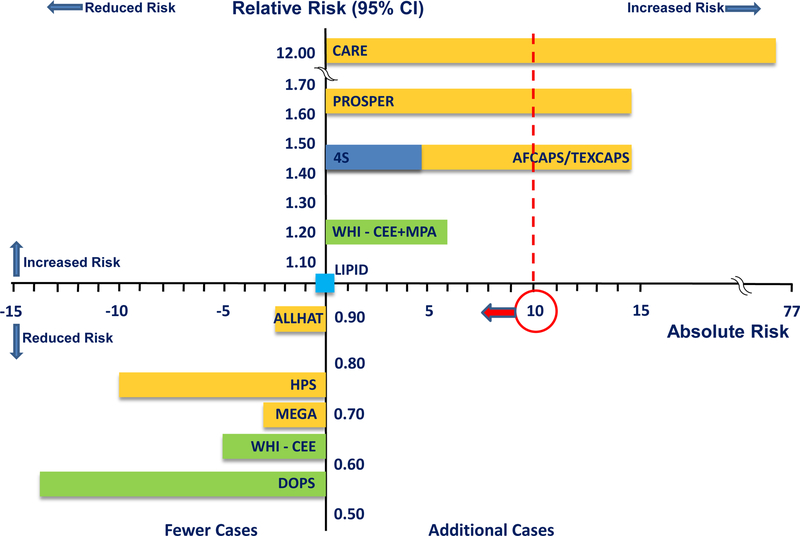
Compared with other primary CVD prevention therapies, the risk profile of HRT is excellent, especially when HRT is initiated in typical women, before age 60 years and close in proximity to menopause. Cumulated data show for example, similar magnitudes of breast cancer risk for statin therapy and continuous combined CEE+MPA initiated in women <60 years of age as reported from WHI (Fig. 17) (31,32,50–58). In three independent meta-analyses of statin RCTs, incidence of breast cancer was 9%–33% greater with statin therapy than with placebo accounting for 2–7 additional breast cancers per 10,000 women per year of statin therapy (59–61). Other non-hormonal medications are also associated with breast cancer risk such as calcium channel blockers, which are associated with more than two-fold increased risk for ductal breast cancer (relative risk (RR), 2.4; 95% CI, 1.2–4.5) and lobular breast cancer (RR, 2.6; 95% CI, 1.3–5.3) (62).
Figure 17.
Comparison of breast cancer incidence from hormone replacement therapy in women <60 years of age when randomized and statin therapy randomized trials. On relative and absolute risk scales, breast cancer incidence from hormone replacement therapy trials (14 fewer to 6 additional breast cancer cases/10,000 women/year of hormone replacement therapy) is similar or less than statin therapy trials (10 fewer to 77 additional breast cancer cases/10,000 women/year of statin therapy). Whereas incidence of breast cancer from hormone replacement therapy is rare, breast cancer incidence from statin therapy exceeds the rare threshold in certain statin therapy trials. Absolute risk of 10 cases/10,000 women is delineated to show relation of breast cancer incidence to the category of rare frequency of events (Table 4). CARE = Cholesterol and Recurrent Events [Pravastatin] (52); PROSPER = Prospective Study of Pravastatin in the Elderly at Risk [Pravastatin] (53); 4S = Scandinavian Simvastatin Survival Study [Simvastatin] (54); AFCAPS/TEXCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study [Lovastatin] (55); WHI-CEE+MPA = Women’s Health Initiative Conjugated Equine Estrogen plus Medroxyprogesterone Acetate trial (31); LIPID = Long-Term Intervention with Pravastatin in Ischemic Disease [Pravastatin] (56); ALLHAT = Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial [Pravastatin] (57); HPS = Heart Protection Study [Simvastatin] (58); MEGA = Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese [Pravastatin] (35); WHI-CEE = Women’s Health Initiative Conjugated Equine Estrogen trial (31); DOPS = Danish Osteoporosis Study (22); CI = confidence interval
In April 2012, the United States Food and Drug Administration (FDA) mandated safety label changes to statin drugs to carry warnings for increased diabetes mellitus and cognitive impairment based on post-marketing data (63). Data reported from individual statin studies, meta-analyses of statin RCTs and observational studies show that risk of new onset diabetes mellitus with statin therapy exceeds the rare level of risk (Fig. 18) (37,64–67). Women appear to be particularly susceptible to statin-induced diabetes mellitus. In JUPITER, incident diabetes mellitus was significantly increased in women randomized to statin therapy relative to placebo (HR, 1.49; 95% CI, 1.11–2.01) whereas in men, diabetes mellitus was non-significantly elevated (HR, 1.14; 95% CI, 0.91–1.43) (37). Although studies show a greater risk of statin-induced diabetes mellitus in women than men, the FDA safety label warning applies to both sexes.
Figure 18.
Comparison of incidence of new onset diabetes mellitus from hormone replacement therapy and statin therapy. Data reported from individual statin randomized controlled trials and meta-analyses of statin randomized controlled trials show increased risk of new onset diabetes mellitus that exceeds rare level of risk with statin therapy. In contrast, individual hormone replacement randomized controlled trials and meta-analyses of hormone replacement randomized controlled trials show decreased risk of new onset diabetes mellitus with hormone replacement therapy. Absolute risk of 10 cases/10,000 women is delineated to show relation of new onset diabetes mellitus to the category of rare frequency of events (Table 4). RCT = randomized controlled trial; meta = meta-analysis; JUPITER = Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin; WHI = Women’s Health Initiative; WHI-CEE = Women’s Health Initiative-conjugated equine estrogen; WHI-CEE+MPA = Women’s Health Initiative-conjugated equine estrogen plus medroxyprogesterone acetate; HERS = Heart and Estrogen/progestin Replacement Study; HRT = hormone replacement therapy
On the other hand, HRT is well-known to reduce new onset diabetes mellitus by 20%–30% (Fig. 18). The anti-diabetogenic effect of HRT has been shown across many independent RCTs and meta-analyses including an analysis of 107 RCTs (68–71). Medications including statins that increase risk of new onset diabetes mellitus must be carefully considered prior to initiation for primary CVD prevention especially for women since the risk to benefit ratio is not known.
Diabetes Mellitus, Women and HRT
Diabetes has immense health implications, both morbidity and mortality, especially for women as menopause serves a marker of increased risk for insulin resistance and glucose intolerance as well as new onset diabetes mellitus (72). Lifetime residual risk of diabetes mellitus for women aged 50 years is 3,000 cases per 10,000 women (30%) and for Hispanic and Black women, 4,000 cases per 10,000 women (40%) (73). Approximately 70% of individuals with diabetes mellitus die from CVD and morbidity associated with diabetes is greater for women than for men. For example, men with diabetes mellitus have a 2.4-fold greater risk of CHD than men without diabetes, whereas women with diabetes have a 5.1-fold greater risk of CHD than women without diabetes (74).
Since aging and menopause are inextricably linked, it remains unclear whether menopause is an independent cause of diabetes mellitus (72). What is clear is that when women transition to menopause, many metabolic and biochemical as well as phenotypic changes occur that increase diabetes mellitus risk. Hormonal changes during the transition to menopause contribute to insulin resistance, decreased glucose tolerance and increased incidence of diabetes mellitus (75). Further, HRT improves insulin resistance, increases glucose tolerance, and reduces new onset diabetes mellitus by 20%–30% (68–71,76). Whether statin-induced diabetes mellitus contributes to the null effect of statin therapy on all-cause mortality and CVD reduction in women or whether HRT reduction of new onset diabetes mellitus contributes to reduction in all-cause mortality and CVD in women when initiated <60 years of age and/or <10 years-since-menopause need to be determined.
Discussion
As the number one cause of death of women who also suffer greater morbidity than men, primary CVD prevention is fundamentally a high priority for women that requires substantial improvement. Similar with lipid-lowering therapy and aspirin, cumulated data show that over all ages, menopausal HRT has a null effect on the incidence of all-cause mortality and CVD in women. However, analyses using age stratification and randomized trials specifically conducted in young women close in proximity to menopause along with meta-analyses, show that menopausal HRT is unique in that it reduces all-cause mortality and CVD when initiated in women <60 years of age and/or <10 years-since-menopause. These cumulated findings from RCTs are consistent with the large body of observational studies that consistently show a reduction of all-cause mortality and CVD in young women who initiate HRT at the time of menopause. When initiated in women in their 50s and continued for 5–30 years, HRT reduces all-cause mortality and cost-effectively increases quality adjusted life-years (QALYs) (77). As such, menopausal HRT has been calculated to substantially lower the economic burden from menopausal symptoms and chronic diseases (77–80).
Menopausal HRT must be considered differently than other medications since no other single primary CVD preventive therapy offers diverse systemic-wide benefits. Timing of initiation of HRT has significant biological and clinical consequences for women since clinical consequences of most aging-related diseases manifest in women on average 10 years after menopause (1,81). Timing of initiation of HRT before tissue damage appears and clinical consequences manifest, appears to be the key for successful prevention and amelioration of further tissue and organ deterioration due to aging (81). Thus, an important window of opportunity is afforded by intervening with prevention strategies at onset of menopause creating a new paradigm for primary CVD prevention in women. Reduction of all-cause mortality and CVD in younger women with menopausal HRT and lack of effectiveness of other primary prevention strategies for reduction of all-cause mortality and CVD in women provide compelling arguments for primary CVD prevention with HRT. Additional benefits beyond reduction of all-cause mortality including reduction in menopausal symptoms (82), potential reductions in CVD, cancer and other mortalities (28), prevention of new onset diabetes mellitus (68–71), osteoporosis and bone fracture prevention (83), improved quality of life (77) as well as demonstration of cost-effectiveness and reduced economic burden (77–80) provide further compelling arguments for primary CVD prevention with initiation of HRT at onset of menopause (81). Prevention of new onset diabetes mellitus with menopausal HRT is a particularly important primary CVD preventive strategy since metabolic syndrome and insulin and glucose dyscrasias as well as new onset diabetes mellitus are common manifestations following menopause and represent major causes of CVD and morbidity in women (73,74).
Evidence-based data from RCTs are reassuring in that compared with placebo, risks associated with menopausal HRT are rare (<10 cases/10,000 women) when initiated in the typical women requiring HRT (<60 years of age and/or <10 years-since-menopause). Magnitude and types of HRT risks, including breast cancer, stroke and venous thromboembolism are rare and not unique to menopausal HRT as well as comparable with or less than other commonly used medications in women, including those used for primary CVD prevention such as statins, aspirin and calcium channel blockers (32).
In conclusion, a large body of randomized trial data converge with results from observational studies, animal studies and basic science supporting that HRT health outcomes vary by time-since-menopause. ELITE provided direct evidence for the timing hypothesis. DOPS provided direct randomized trial evidence in women (average age 50 years and 7 months postmenopausal) similar with populations from observational studies for the beneficial effects of HRT on CVD with low risk when initiated at or near menopause and continued long-term. Accordingly, HRT is a sex-specific and time-dependent primary CVD preventive therapy that concomitantly reduces all-cause mortality as well as a diversity of other aging-related diseases with an excellent risk profile. Health-care providers and patients can use the cumulated data in making clinical decisions concerning chronic disease prevention including CVD and downstream reduction in mortality keeping in mind that any prevention strategy must be personalized. Data not only provide strong consistent evidence for beneficial effects of menopausal HRT when initiated close in proximity to menopause but also reassurance of long-term safety. It is time to recognize that HRT reduces all-cause mortality and CVD, and that it is all about timing.
Funding:
National Institutes of Health, National Institute on Aging, R01-AG058691, R01-AG059690, R01-AG024154, R01-AG18798 and National Institutes of Health, National Heart, Lung, and Blood Institute, U01-HL49298
Contributor Information
Howard N. Hodis, Harry J. Bauer and Dorothy Bauer Rawlins Professor of Cardiology, Professor of Medicine and Preventive Medicine, Professor of Molecular Pharmacology and Toxicology, Director, Atherosclerosis Research Unit, Keck School of Medicine, University of Southern California, 2250 Alcazar Street, CSC 132, Los Angeles, CA 90033.
Wendy J Mack, Professor of Preventive Medicine, Keck School of Medicine, University of Southern California, 2001 North Soto Street, SSB 202Y, Los Angeles, CA 90033.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2.Mehta LS, Beckie TM, DeVon HA, et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 2016;13:916–947. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med 1976;85:447–452. [DOI] [PubMed] [Google Scholar]
- 5.Hodis HN, Mack WJ, Lobo RA, et al. , for the Estrogen in the Prevention of Atherosclerosis Trial Research Group. Estrogen in the prevention of atherosclerosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001;135:939–953. [DOI] [PubMed] [Google Scholar]
- 6.Hodis HN, Mack WJ, Azen SP, et al. , for the WELL-HART Research Group. Hormone therapy and the progression of coronary artery atherosclerosis in postmenopausal women. N Engl J Med 2003;349:535–545. [DOI] [PubMed] [Google Scholar]
- 7.Blankenhorn DH, Hodis HN. Duff Memorial Lecture: arterial imaging and atherosclerosis reversal. Arterioscl Thromb 1994;14:177–192. [DOI] [PubMed] [Google Scholar]
- 8.Holm P, Andersen HL, Andersen MR, Erhardtsen E, Stender S. The direct antiatherogenic effect of estrogen is present, absent, or reversed, depending on state of the arterial endothelium; a time course study in cholesterol-clamped rabbits. Circulation 1999;100:1727–1733. [DOI] [PubMed] [Google Scholar]
- 9.Tarhouni K, Guihot AL, Vessieres E, et al. Determinants of flow-mediated outward remodeling in female rodents: respective roles of age, estrogens, and timing. Arterioscler Thromb Vasc Biol 2014;34:1281–1289. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld ME, Kauser K, Martin-McNulty B, et al. Estrogen inhibits the initiation of fatty streaks throughout the vasculature but does not inhibit intra-plaque hemorrhage and the progression of established lesions in apolipoprotein E deficient mice. Atherosclerosis 2002;164:251–259. [DOI] [PubMed] [Google Scholar]
- 11.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol 1997;17:217–221. [DOI] [PubMed] [Google Scholar]
- 12.Clarkson TB, Anthony MS, Jerome CP. Lack of effect of raloxifene on coronary artery atherosclerosis of postmenopausal monkeys. J Clin Endocrinol Metab 1988:83;721–726. [DOI] [PubMed] [Google Scholar]
- 13.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab 2001:86;41–47. [DOI] [PubMed] [Google Scholar]
- 14.Williams JK, Anthony MS, Honore EK, et al. Regression of atherosclerosis in female monkeys. Arterioscler Thromb Vasc Biol 1995;15:827–836. [DOI] [PubMed] [Google Scholar]
- 15.Geary RL, Adams MR, Benjamin ME, Williams JK. Conjugated equine estrogens inhibit progression of atherosclerosis but have no effect on intimal hyperplasia or arterial remodeling induced by balloon catheter injury in monkeys. J Am Coll Cardiol 1998;31:1158–1164. [DOI] [PubMed] [Google Scholar]
- 16.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy menopausal women: principal results from the Women’s Health Initiative randomized controlled trial. J Am Med Assoc 2002;288:321–323. [DOI] [PubMed] [Google Scholar]
- 17.Hodis HN, Mack WJ. Randomized controlled trials and the effects of postmenopausal hormone therapy on cardiovascular disease: facts, hypotheses and clinical perspective. In: Treatment of the Postmenopausal Woman (Lobo RA [editor]). 3rd edition. Elsevier Academic Press, Philadelphia, PA, pp 529–564, 2007. [Google Scholar]
- 18.Grodstein F, Stampfer M. The epidemiology of coronary heart disease and estrogen replacement in postmenopausal women. Prog Cardiol Dis 1995;38:199–210. [DOI] [PubMed] [Google Scholar]
- 19.Grodstein F, Stampfer M. Estrogen for women at varying risk of coronary disease. Maturitas 1998;30:19–26. [DOI] [PubMed] [Google Scholar]
- 20.The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. J Am Med Assoc 2004;291:1701–1712. [DOI] [PubMed] [Google Scholar]
- 21.Hodis HN, Mack WJ. Hormone replacement therapy and the association with coronary heart disease and overall mortality: clinical application of the timing hypothesis. J Steroid Biochem Mol Biol 2014;142:68–75. [DOI] [PubMed] [Google Scholar]
- 22.Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement treatment on cardiovascular events in recently postmenopausal women: randomized trial. Br Med J 2012;345:e6409. [DOI] [PubMed] [Google Scholar]
- 23.Hodis HN, Mack WJ, Shoupe D, et al. , for the Early versus Late Intervention Trial with Estradiol Research Group. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause 2015:22:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salpeter SR, Walsh JME, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med 2004;19:791–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salpeter SR, Walsh JME, Greyber E, Salpeter EE. Coronary heart disease events associated with hormone therapy in younger and older women: a meta-analysis. J Gen Intern Med 2006;21:363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in postmenopausal women. Cochrane Database of Systemic Reviews 2015, Issue 3:CD002229. DOI: 10.1002/14651858.CD002229.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodis HN, Mack WJ, Henderson VW, et al. , for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 2016;374:1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manson J, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. J Am Med Assoc 2017;318:927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women. Am J Med 2009;12:1016–1022. [DOI] [PubMed] [Google Scholar]
- 30.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. Estrogen therapy and risk of cognitive decline: results from the Women’s Estrogen for Stroke Trial (WEST). Am J Obstet Gynecol 2005;192:387–393. [DOI] [PubMed] [Google Scholar]
- 31.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. J Am Med Assoc 2013;310:1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodis HN, Mack WJ. The timing hypothesis and hormone replacement therapy: a paradigm shift in the primary prevention of coronary heart disease in women. Part 2: comparative risks. J Am Geriatr Soc 2013;61:1011–1018. [DOI] [PubMed] [Google Scholar]
- 33.Walsh JME, Pignone M. Drug treatment of hyperlipidemia in women. J Am Med Assoc 2004;291:2243–2252. [DOI] [PubMed] [Google Scholar]
- 34.Petretta M, Costanzo P, Perrone-Filardi P, et al. Impact of gender in primary prevention of coronary heart disease with statin therapy: a meta-analysis. Int J Cardiol 2010;138:25–31. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno K, Nakaya N, Ohashi Y, et al. Usefulness of pravastatin in primary prevention of cardiovascular events in women: analysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA Study). Circulation 2008;117:494–502. [DOI] [PubMed] [Google Scholar]
- 36.Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. Br Med J 2009;338:b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mora S, Glynn RJ, Hsia J, et al. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity c-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010;121:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. New Engl J Med 2016;374:2021–2031. [DOI] [PubMed] [Google Scholar]
- 39.Berger JS, Roncaglioni MC, Avanzini F, et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. J Am Med Assoc 2006;295:306–313. [DOI] [PubMed] [Google Scholar]
- 40.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. New Engl J Med 2005;352:1293–1304. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa H, Nakayama M, Morimoto T, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. J Am Med Assoc 2008;300:2134–2141. [DOI] [PubMed] [Google Scholar]
- 42.Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 2018;392:1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. New Engl J Med 2018;379:1529–1539. [DOI] [PubMed] [Google Scholar]
- 44.McNeil JJ, Woods RL, Nelson MR, et al. Effect of aspirin on disability-free survival in the healthy elderly. New Engl J Med 2018;379:1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. New Engl J Med 2018;379:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. New Engl J Med 2018;379:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wing LMH, Reid CM, Ryan P, et al. A comparison of outcomes with angiotensin-converting-enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med 2003;348:583–592. [DOI] [PubMed] [Google Scholar]
- 48.Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin-converting-enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol 2003;41:1529–1538. [DOI] [PubMed] [Google Scholar]
- 49.Council for International Organizations of Medical Science (CIOMS). Benefit-Risk Balance for Marketed Drugs: Evaluating Safety Signals. Report of CIOMS Working Group IV. Geneva, Switzerland: CIOMS; 1998. Available at: www.cioms.ch/publications/g4-benefit-risk.pdf. Accessed November 15, 2021. [Google Scholar]
- 50.Hodis HM, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007;14:944–957. [DOI] [PubMed] [Google Scholar]
- 51.Hodis HN, Sarrel PM. Menopausal hormone therapy and breast cancer: what is the evidence from randomized trials? Climacteric 2018;21:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sacks FM, Pfeffer MA, Moye LA, et al. , for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001–1009. [DOI] [PubMed] [Google Scholar]
- 53.Shepherd J, Blauw GJ, Murphy MB, et al. , on behalf of the PROSPER Study Group. Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER): a randomized controlled trial. Lancet 2002;360: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 54.Strandberg TE, Pyorala K, Cook TJ, et al. , for the 4S Group. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet 2004;364:771–777. [DOI] [PubMed] [Google Scholar]
- 55.Clearfield M, Downs JR, Weis S, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): efficacy and tolerability of long-term treatment with lovastatin in women. J Womens Health Gend Based Med 2001;10:971–981. [DOI] [PubMed] [Google Scholar]
- 56.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 57.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 2002;288:2998–3007. [DOI] [PubMed] [Google Scholar]
- 58.Collins R, Armitage J, Parish S, Sleight P, Peto R, for the Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004;363:757–767. [DOI] [PubMed] [Google Scholar]
- 59.Dale KM, Coleman CI, Henyan NN, et al. Statins and cancer risk: a meta-analysis. J Am Med Assoc 2006;295:74–80. [DOI] [PubMed] [Google Scholar]
- 60.Bonovas S, Filioussi K, Tsavaris N, et al. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol 2005;23:8606–8612. [DOI] [PubMed] [Google Scholar]
- 61.Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 62.Li CI, Daling JR, Tang MTC, Haugen KL, Porter PL, Malone KE. Use of hypertensive medications and breast cancer among women aged 55 to 74 years. J Am Med Assoc Intern Med 2013;173:1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.U.S. Food and Drug Administration. FDA Drug Safety Communication: Important Safety Label Changes to Cholesterol Lowering Statin Drugs [on-line]. Available at https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-important-safety-label-changes-cholesterol-lowering-statin-drugs. Accessed November 15, 2021.
- 64.Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care 2009;32:1924–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomized statin trials. Lancet 2010;375:735–742. [DOI] [PubMed] [Google Scholar]
- 66.Preiss D, Seshasai SRK, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. J Am Med Assoc 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 67.Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med 2012;172:144–152. [DOI] [PubMed] [Google Scholar]
- 68.Bonds DE, Lasser N, Qi L, et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomized trial. Diabetologia 2006;49:459–468. [DOI] [PubMed] [Google Scholar]
- 69.Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative hormone trial. Diabetologia 2004;47:1175–1187. [DOI] [PubMed] [Google Scholar]
- 70.Kanaya AM, Herrington D, Vettinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. Ann Intern Med 2003;138:1–9. [DOI] [PubMed] [Google Scholar]
- 71.Salpeter SR, Walsh JME, Ormiston TM, et al. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab 2006;8:538–564. [DOI] [PubMed] [Google Scholar]
- 72.Slopien R, Wender-Ozegowska E, Rogowicz-Frontczak A, et al. Menopause and diabetes: EMAS clinical guide. Maturitas 2018;117:6–10. [DOI] [PubMed] [Google Scholar]
- 73.Narayan KMV, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk of diabetes mellitus in the United States. J Am Med Assoc 2003;290:1884–1890. [DOI] [PubMed] [Google Scholar]
- 74.Kannel WB. Lipid, diabetes, and coronary heart disease: insights from the Framingham study. Am Heart J 1985;110:1100–1107. [DOI] [PubMed] [Google Scholar]
- 75.Park SK, Harlow SD, Zheng H, et al. Association between changes in oestradiol and follicle-stimulating hormone levels during the menopausal transition and risk of diabetes. Diabet Med 2017;34:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pereira RI, Casey BA, Swibas TA, et al. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab 2015;100:4456–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salpeter SR, Buckley NS, Liu H, et al. The cost-effectiveness of hormone therapy in younger and older postmenopausal women. Am J Med 2009;122:42–52. [DOI] [PubMed] [Google Scholar]
- 78.Donneyong MM, Chang TJ, Roth JA, et al. The women’s health initiative estrogen-alone trial had differential disease and medical expenditure consequences across age groups. Menopause 2020;27:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sarrel P Adding up the healthcare costs when estrogen therapy is avoided after hysterectomy. Menopause 2020;27:625–627. [DOI] [PubMed] [Google Scholar]
- 80.Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause 2015;22:260–266. [DOI] [PubMed] [Google Scholar]
- 81.Lobo RA, Pickar JH, Stevenson JC, Hodis HN, Mack WJ. Back to the future: hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis 2016:254:282–290. [DOI] [PubMed] [Google Scholar]
- 82.Nelson HD, Vesco KK, Haney E, et al. Nonhumoral therapies for menopausal hot flashes: systematic review and meta-analysis. J Am Med Assoc 2006;295:2057–2071. [DOI] [PubMed] [Google Scholar]
- 83.Zhu L, Jiang X, Sun y, Shu W Effect of hormone therapy on the risk of bone fractures: a systematic review and meta-analysis of randomized controlled trials. Menopause 2016;23:461–470. [DOI] [PubMed] [Google Scholar]