ABSTRACT
STUDY QUESTION
What are the patient-reported outcomes (PROs) and patient-reported experiences (PREs) in home-based monitoring compared to those in hospital-based monitoring of ovulation for scheduling frozen–thawed embryo transfer (FET)?
SUMMARY ANSWER
Women undergoing either home-based or hospital-based monitoring experience an increase in anxiety/sadness symptoms over time, but women undergoing home-based monitoring felt more empowered during the treatment and classified the monitoring as more discreet compared to hospital-based monitoring.
WHAT IS KNOWN ALREADY
FET is at the heart of modern IVF. The two types of FET cycles that are mainly are used are artificial cycle FET, using artificial preparation of the endometrium with exogenous progesterone and oestrogen, and natural cycle FET (NC-FET). During a natural cycle FET, women visit the hospital repeatedly and receive an ovulation trigger to time FET (i.e. modified NC-FET or hospital-based monitoring). The previously published Antarctica randomised controlled trial (NTR 1586) showed that modified NC-FET is more cost-effective compared to artificial cycle FET. From the women’s point of view a more natural approach using home-based monitoring of ovulation with LH urine tests to time FET may be desired (true NC-FET or home-based monitoring). Currently, the multicentre Antarctica-2 randomised controlled trial (RCT) is comparing the cost-effectiveness of home-based monitoring of ovulation with that of hospital-based monitoring of ovulation. The Antarctica-2 RCT enables us to study PROs, defined as the view of participating women of their healthcare status, and PREs, defined as the perception of the received care of participating women, in both FET strategies.
STUDY DESIGN, SIZE, DURATION
PROs and PREs were assessed alongside the Antarctica-2 RCT. PROs were assessed using the validated EuroQol-5D-5L questionnaire. Currently, there are no guidelines for assessing PREs in this population. Therefore, members of the Dutch Patient Organisation for Couples with Fertility Problems (FREYA) filled out an online survey and selected the following PREs to assess (i) anxiety about missing ovulation, (ii) perceived level of partner participation, (iii) level of discretion, (iv) feeling of empowerment and (v) satisfaction with treatment.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Women participating in the RCT also participated in PRO and PRE assessment. We assessed PROs and PREs at three time points: (i) before randomisation, (ii) at the time of the FET and (iii) at the time of the pregnancy test. A sample size of 200 participants was needed to find a difference of 0.3 with a standard deviation in both groups of 0.7, an alpha of 5%, power of 80% and a drop-out rate of 10%. We performed mixed model analysis for between-group comparison of treatment and time effects.
MAIN RESULTS AND ROLE OF CHANCE
A total of 260 women were randomised. Of these, 132 women were treated with home-based monitoring and 128 women were treated with hospital-based monitoring. Data before randomisation were available for 232 women (home-based monitoring n = 116, hospital-based monitoring n = 116). For the PROs, we found a significant increase in anxiety/sadness symptoms over time (P < 0.001) in both groups. We found no treatment effect of home-based versus hospital-based monitoring for the PROs (P = 0.8). Concerning the PRES, we found that women felt more empowered during home-based monitoring (P = 0.001) and classified the home-based monitoring as more discreet (P = 0.000) compared to the hospital-based monitoring.
LIMITATIONS, REASONS FOR CAUTION
The results are applicable only to women undergoing NC-FET and not to women undergoing artificial cycle FET.
WIDER IMPLICATIONS OF THE FINDINGS
Apart from clinical outcomes, PROs and PREs are also of importance in clinical decision-making and to support tailoring treatment even more specifically to the wishes of patients. Measurement of PROs and PREs should therefore be incorporated in future clinical research.
STUDY FUNDING/COMPETING INTEREST(S)
The Antarctica-2 RCT is supported by a grant of the Netherlands Organisation for Health Research and Development (ZonMw 843002807). J.B. receives unconditional educational grants from Merck Serono and Ferring and is a member of the medical advisory board of Ferring. C.L. reports that his department receives unrestricted research grants from Ferring, Merck and Guerbet. E.G. receives personal fees from Titus Health Care outside submitted work. The remaining authors have no conflicts of interest.
TRIAL REGISTRATION NUMBER
Trial NL6414 (NTR6590)
TRIAL REGISTER DATE
23 July 2017
DATE OF FIRST PATIENT’S ENROLMENT
10 April 2018
Introduction
Frozen–thawed embryo transfer (FET) is at the heart of modern IVF and has been enabled by ongoing improvements in laboratory techniques for freezing and thawing of embryos and FET cycle procedures (Wong et al., 2014). The number of FET cycles has increased substantially over the past decade (De Geyter et al., 2018; ESHRE, 18 February 2018; Pereira et al., 2019).
For FET to be effective, the endometrium needs to be synchronised with the developmental stage or age of the embryo to allow implantation. Two types of methods are mainly used to achieve this, firstly embryo transfer in the natural cycle (NC-FET) with repeated ultrasound monitoring of the dominant follicle followed by hCG triggering for ovulation (modified NC-FET). The second method is embryo transfer in an artificial cycle, in which the endometrium is artificially prepared by using exogenous oestrogen and the timing of the thaw and transfer is initiated by start of exogenous progesterone (Glujovsky et al., 2010). In 2016, the multicentre non-inferiority Antarctica randomised controlled trial (RCT) performed in the Netherlands showed that modified NC-FET is preferred over artificial cycle FET based on cost-effectiveness (Groenewoud et al., 2016). As an effect, we have already observed in the Netherlands that the majority of IVF centres perform FET in a modified natural cycle (2017 unpublished data based on a national survey on clinical protocols in all 13 IVF centres in the Netherlands).
During a modified NC-FET cycle, an average of three hospital visits are needed for ultrasound monitoring (unpublished data Antarctica RCT, Netherlands trial register Trial NL1515 (NTR1586)). From the woman’s perspective a more natural approach and less interference with private and working life may be desired (Gerris and De Sutter, 2010). Home-based ultrasound monitoring of follicle growth in fresh IVF cycles indeed improved patient-reported outcomes and experiences such as contentedness, empowerment, discretion and partner participation (Gerris et al., 2014). Therefore, home-based monitoring might also be the preferred treatment for women in FET cycles. An alternative to the previously described NC-FET, which implies hospital-based monitoring, is home-based monitoring in which natural ovulation is monitored using urinary LH tests (also known as true NC-FET). One could question why hospital-based monitoring is the norm given that urinary LH tests are widely performed by women themselves to monitor their ovulation. This approach reduces direct costs of repeated ultrasound visits and medication and indirect costs of transportation to the clinic and productivity loss. Because of these advantages, home-based monitoring is increasingly applied in the Netherlands, albeit in the absence of evidence supporting its cost-effectiveness. Therefore, to compare the (cost-) effectiveness of home-based monitoring with that of hospital-based monitoring of the ovulation, we designed the currently ongoing Antarctica-2 RCT.
Apart from clinical outcomes, patient-reported outcomes (PROs) measuring the women’s views of their healthcare status and patient-reported experiences (PREs) measuring the women’s perceptions of their experience whilst receiving care are also of importance in clinical shared decision-making and to be able to tailor treatments even more specifically to the wishes of women (Doyle et al., 2013; Lavallee et al., 2016; Ahern et al., 2017; Kingsley and Patel, 2017). In the field of FET, PROs and PREs have not been studied before. Our aim was to investigate whether women have a better experience after home-based monitoring as compared to hospital-based monitoring. We hypothesise that home-based monitoring will provide a better experience, less interference with private and working life, more partner participation, more perceived discretion and a higher level of empowerment. In the current study we therefore investigated the PROs and PRES of women during a natural FET cycle regarding home or hospital monitoring of ovulation to time FET alongside the ongoing Antarctica-2 RCT.
Materials and Methods
Participants
We included women who were about to be scheduled for FET in the Netherlands as part of the Antarctica-2 RCT (trial registration Trial NL6414 (NTR6590)). The Antarctica-2 RCT is an ongoing national multicentre randomised trial that compares home-based monitoring with hospital-based monitoring of ovulation to study the effect on ongoing pregnancies, within the Dutch Consortium for Healthcare Evaluation and Research in Obstetrics and Gynaecology.
We included women between the age of 18 and 45 with ovulatory cycles and who were Dutch or English speaking. We excluded women with anovulatory cycles, women who were ovulatory with ovulation induction and women with a contra-indication for pregnancy. Six centres randomising women for the Antarctica-2 trial between April 2018 and March 2019 invited women to participate in the present study. This study was reviewed and approved by the Medical Ethics Committee of AMC Amsterdam (number 2018_004, MEC AMC, Code 018, https://www.ccmo.nl/metcs/publicaties/publicaties/2018/11/20/erkende-metcs-met-code). All participants provided written informed consent for the study.
Randomisation and masking
Eligible women who gave informed consent were randomly allocated to home-based monitoring (true NC-FET; experimental arm) or hospital-based monitoring (modified NC-FET; standard intervention or control arm) with a 1:1 allocation using a web-based data system. The ongoing Antarctica-2 RCT is an open-label study as masking women or their healthcare providers to the assigned intervention is not possible.
Assessments
The handling of personal data was performed according to the standards of General Data Protection Regulation 2018. We obtained demographic and clinical information from the medical record file. To assess PROs and PREs, we sent digital questionnaires through our web-based data system (Castor EDC, CIWIT B.V.). Women who did not open or complete the questionnaire received one email as a reminder.
Patient-reported outcomes (PROs)
PROs were assessed before randomisation (T0), after completion of ovulation monitoring but before FET (T1) and before the pregnancy test (T2). PROs were assessed using the five-level EQ-5D (EQ-5D-5L), a validated self-reported questionnaire to measure health-related quality of life (Herdman et al., 2011; Doyle et al., 2013; Lavallee et al., 2016; Kingsley and Patel, 2017). This six-item questionnaire is used to measure the generic quality of life. In EQ-5D-5L, women rank five dimensions of quality of life on a five-point Likert scale: mobility, self-care, usual activities, pain/discomfort and anxiety/depression, in which a score of 1 indicates no problems and a score of 5 indicates extreme problems. EQ-5D-5L also contains a visual analogue scale (VAS), in which respondents grade their perceived health status on a scale ranging from 0 (worst possible health status) to 100 (best possible health status). The EQ-5D-5L questionnaire is shown in Supplementary Figure S1.
Selection of patient-reported experience measurement to assess patient-reported experiences (PREs)
PREs were assessed after completion of ovulation monitoring but before FET (T1) and before the pregnancy test (T2). We selected potential patient-reported experience measurements from the literature (Gerris and De Sutter, 2010; Gerris et al., 2014) and asked FREYA, the Dutch Patient Organisation for Couples with Fertility Problems, to prioritise these patient-reported experience measurements by an online survey among their members. The call to participate in this survey was distributed through social media member groups of FREYA in February 2017, and 197 women responded. The risk of missing ovulation and the level of partner participation (weighted means of 3.81 and 3.45 respectively: scale 1 to 4) were valued as high (Table I). Feeling of empowerment, interference with professional life, interference with social life and transportation costs were valued moderately high (mean weights of 2.88, 2.89, 2.87 and 2.40: scale 1 to 4) (Table I). The level of discretion of the treatment and satisfaction with the treatment were not specifically assessed in the FREYA questionnaire but were selected based on the literature (Gerris and De Sutter, 2010; Gerris et al., 2014).
Table I. Outcomes of FREYA questionnaire for selection of patient-reported experience measurements for assessment of PREs in the Antarctica-2 RCT.
| PRE items | Weighted average * |
|---|---|
| Risk to miss ovulation | 3.81 |
| Partner participation | 3.45 |
| Feeling of empowerment | 2.88 |
| Interference with social life | 2.87 |
| Interference with professional life | 2.89 |
| Transportation costs | 2.40 |
FREYA, Dutch Patient Organisation for Couples with Fertility Problems; PREs, patient-reported experiences; RCT, randomised controlled trial.
*Scale ranging from 1 (not important at all) to 4 (very important).
The final set of selected patient-reported experience measurements consists of five questions (scale 1 to 5). Four questions concern potential perceived anxiety on missing ovulation and satisfaction with the ovulation detection method and one question concerned the perceived level of partner participation: (i) Did you experience anxiety/sadness about missing your ovulation window? (ii) Did you experience empowerment during the treatment (ovulation method)? (iii) Was the treatment (ovulation method) discreet? (iv) How satisfied were you with the treatment (ovulation method)? (v) Was your partner involved in the treatment (ovulation method)? The PRE questionnaire is shown in Supplementary Figure S2.
Statistical analysis
A sample size of 200 women was needed to find a difference of 0.3 with a standard deviation in both groups of 0.7 at an alpha of 5%, power of 80% and a drop-out rate of 10%. The analysis was done according to the intention-to-treat principle. For PRO and PRE assessment, we used a subgroup of the participants of the Antarctica-2 trial. This trial is still ongoing for assessment of cost-effectiveness.
For PROs, the EQ-5D-5L descriptive system and EQ-VAS were analysed separately according to standards of EuroQol (EuroQol.org, User Guide, 2015). The results of the EQ-5D-5L descriptive system were reported as mean (SD) of the total score on the EQ-5D-5L descriptive system per group per time point and also as problems/no problems per group per dimension at the three different time points. The results of the EQ-VAS score were reported as mean (SD) and median (−25th, −75th) per group per time point. Linear mixed models were used to perform between-group comparison of treatment and time effects. These models included fixed effects for groups and different time points (before randomisation, at the time of FET and at the time of the pregnancy test). For each model, we obtained P-values for the overall group-by-time interaction, differences among group means at the different time points and pairwise comparisons between the home-based monitoring and hospital-based monitoring treatment groups.
For PREs, analyses were performed per item. Therefore, each PRE question was analysed separately. The results of the PREs were reported as mean (SD) per group per time point. Linear mixed models were used to perform between-group comparison of treatment and time effects. These models included fixed effects for groups and different time points (before randomisation, before FET and before pregnancy test). For each model, we obtained P-values for the overall group-by-time interaction, differences among group means at the different time points and pairwise comparisons between the home-based monitoring and hospital-based monitoring treatment groups.
Results
Baseline characteristics
A total of 334 women were invited, of whom 260 women agreed to participate and were randomised. Of these, 132 women were allocated to home-based monitoring and 128 women were allocated to hospital-based monitoring. Of the 260 women recruited, the response rate was 89.2% (232/260). Five women in the home-based monitoring group dropped out: one because she became pregnant spontaneously, two because of preference for hospital-based monitoring, one withdrew from the study because of a logistic reason and one for an unknown reason. In the hospital-based monitoring group three women dropped out: one because of logistic reasons and two because of need for an artificial cycle (Fig. 1).
Figure 1. CONSORT flow diagram (2010).
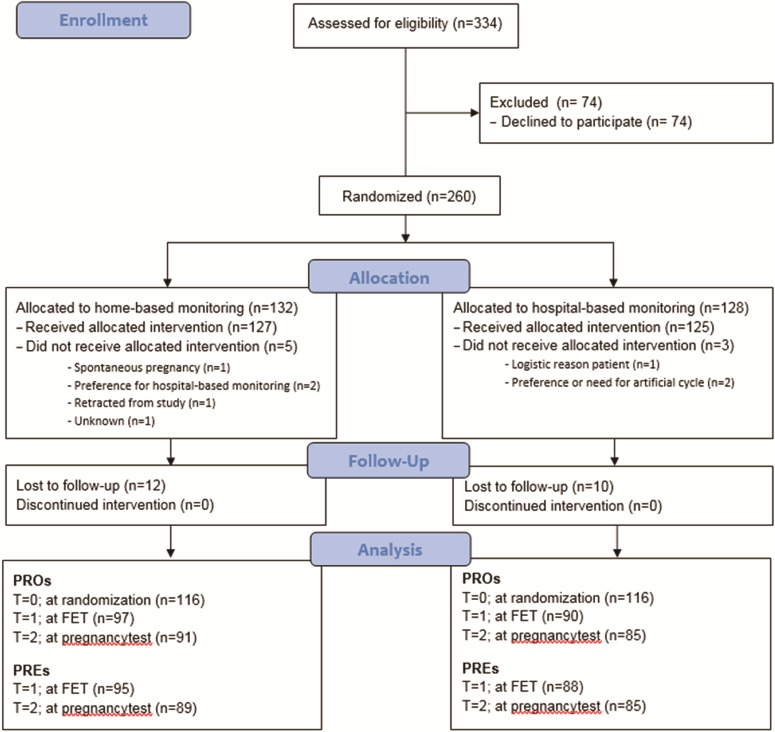
Table II describes the characteristics of 260 analysed women (132 home-based monitoring, 128 hospital-based monitoring). Both groups were comparable with respect to age, smoking status, BMI, fertility status and duration of subfertility in months (Table II).
Table II. Baseline characteristics of 260 women participating in the PRO and PRE assessment of the Antarctica-2 RCT (in number (%) or mean (SD)).
| Characteristics | Home-based monitoring | Hospital-based monitoring |
|---|---|---|
| Total | n = 132 | n = 128 |
| Female age in years, mean (SD) | 35.12 (4.69) | 35.65 (3.94) |
| Current smoker | n = 11 (8.3%) | n = 11 (8.6%) |
| BMI, mean (SD) | 24.16 (4.58) | 23.85 (3.89) |
| Fertility status | ||
| Primary | n = 55 (41.7%) | n = 46 (35.9%) |
| Secondary | n = 74 (56.1%) | n = 82 (64.1%) |
| Number of parity > 16 weeks | ||
| 0 | n = 12 | n = 21 |
| 1 | n = 47 | n = 47 |
| ≥2 | n = 15 | n = 10 |
| Duration of subfertility in months, mean (SD) | 32.85 (44.64) | 35.60 (50.82) |
| Diagnoses | ||
| Unexplained or mild male subfertility (pre-wash TMSC 3 to 10 million) | n = 25 (18.9%) | n = 32 (25.0%) |
| Male subfertility | n = 79 (59.9%) | n = 60 (46.8%) |
| Tubal factor | n = 6 (4.5%) | n = 7 (5.5%) |
| Endometriosis | n = 3 (2.3%) | n = 6 (4.6%) |
| PGD | n = 9 (6.8%) | n = 9 (7.0%) |
| Other | n = 16 (12.2%) | n = 18 (14.1%) |
| Initial treatment | ||
| IVF | n = 29 (22.3%) | n = 50 (39.1%) |
| IVF-ICSI | n = 101 (77.7%) | n = 78 (60.9%) |
PROs, patient-reported outcomes; TMSC, total motile sperm count.
In the home-based monitoring group, data at T1 were collected after completion of the ovulation monitoring for all the women, although 35% of the women completed the questionnaire after FET. In the hospital-based monitoring group, data at T1 were collected after completion of the ovulation monitoring for all the women and 26% of the women completed the questionnaire after FET. This was not significantly different between groups (P = 0.40).
In the home-based monitoring group, data at T2 were collected for 96% of the women before the scheduled pregnancy test and for 4% of the women after the scheduled pregnancy test. In the hospital-based monitoring group, data at T2 were collected for 98% of the women before the scheduled pregnancy test and for 2% of the women after the scheduled pregnancy test.
PROs: EQ-5D-5L descriptive system
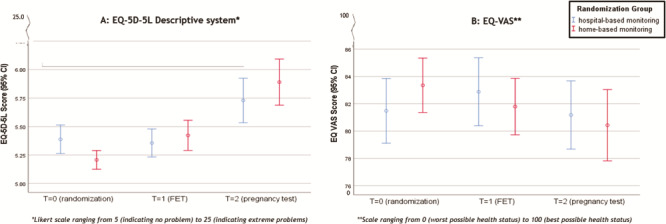
For the EQ-5D-5L descriptive system, we found no treatment effect of home-based monitoring versus hospital-based monitoring (P = 0.8) (Table III, Fig. 2A). We did find a significant time effect on the EQ-5D-5L descriptive system in both groups for the dimension anxiety/sadness, indicating an increase in anxiety/sadness symptoms over time (P < 0.001) in both groups. The mixed model analysis indicated an interaction effect on the EQ-5D-5L descriptive system between treatment groups and stage of the treatment (prior to randomisation, at the time of FET and pregnancy test) (P = 0.034) (Table III). This means that the level of increase in anxiety/sadness symptoms differs between home-based monitoring and hospital-based monitoring. The means (SD) on the EQ-5D-5L descriptive system for both groups are presented in Table III. In the home-based monitoring group, the mean score on the EQ-5D-5L descriptive system was 0.21 higher at T = 1 compared to that at T = 0. In the hospital-based monitoring group, the mean score on the EQ-5D-5L descriptive system was 0.03 lower at T = 1 compared to that at T = 0. In the home-based monitoring group, the mean score on the EQ-5D-5L descriptive system was 0.48 higher at T = 2 compared to that at T = 1. In the hospital-based monitoring group, the mean score on the EQ-5D-5L descriptive system was 0.37 higher at T = 2 compared to that at T = 1 (Table III, Fig. 2A).
Table III. Outcomes of the assessed PROs using the EQ-5D-5L descriptive system. 1 .
| EQ-5D descriptive system * | T0 (baseline) | T1 (FET) | T2 (pregnancy test) | Treatment effect | Time effect | Interaction |
|---|---|---|---|---|---|---|
| Mean (SD) | ||||||
| Home-based monitoring | n = 116; 5.21 (0.45) | n = 97; 5.42 (0.66) | n = 91; 5.90 (0.97) | P = 0.8 | P < 0.001 | P = 0.034 |
| Hospital-based monitoring | n = 116; 5.39 (0.68) | n = 90; 5.36 (0.59) | n = 85; 5.73 (0.90) |
1Data for the two randomisation groups of the Antarctica-2 RCT before randomisation (T = 0), after completion of ovulation monitoring but before frozen–thawed embryo transfer (FET) (T = 1) and before pregnancy test (T = 2). In the home-based monitoring group 19% (22/116) of the women reported problems at T = 0 compared to 29% (34/116) of the women in the hospital-based monitoring group. At T = 1, 33% (32/97) of the women in the home-based monitoring group reported problems compared to 30% (27/90) of the women in the hospital-based monitoring group. In the home-based monitoring group 56% (51/91) of the women reported problems at T = 2 compared to 49% (42/85) of the women in the hospital-based monitoring group. At all three time points problems were only reported on the dimension ‘anxiety/depression’; women did not report problems on any of the other four dimensions.
*Likert scale ranging from 5 (indicating no problem) to 25 (indicating extreme problems).
Figure 2. PROs using EQ-5D-5L. (A) Patient-reported outcomes (PROs) using the EQ-5D-5L descriptive system. (B) PROs using EQ-VAS. VAS, visual analogue scale.
PROs: EQ-VAS
Based on the mixed model analysis, we did not find any treatment effect of home-based monitoring versus hospital-based monitoring on the EQ-VAS score (P = 0.99). Our analysis did not show a time effect (P = 0.34) nor an interaction effect (P = 0.36) on the VAS score (Table IV, Fig. 2B).
Table IV. Outcomes of the assessed PROs using EQ-VAS.
| EQ-VAS * | T0 (baseline) | T1 (FET) | T2 (pregnancy test) | Treatment effect | Time effect | Interaction |
|---|---|---|---|---|---|---|
| Mean (SD) | ||||||
| Home-based monitoring | n = 116; 83.35 (10.83) | n = 97; 81.79 (10.27) | n = 91; 80.43 (12.54) | P = 0.99 | P = 0.34 | P = 0.36 |
| Hospital-based monitoring | n = 116; 81.48 (12.89) | n = 90; 82.88 (11.88) | n = 84; 81.18 (11.51) | |||
| Median (−25th, −75th) | ||||||
| Home-based monitoring | n = 116; 82.50 (80.00–90.00) | n = 97; 80.00 (75.00–90.00) | n = 91; 80.00 (73.00–90.00) | |||
| Hospital-based monitoring | n = 116; 80.50 (75.00–90.00) | n = 90; 84.50 (75.00–92.00) | n = 84; 81.00 (76.00–89.8) |
Data for the two randomisation groups of the Antarctica-2 RCT before randomisation (T = 0), after completion of ovulation monitoring but before FET (T = 1) and before pregnancy test (T = 2).
VAS, visual analogue scale.
*Scale ranging from 0 (worst possible health status) to 100 (best possible health status).
PREs: five dimensions
PREs were examined using the five different dimensions as defined in the Materials and Methods section.
Based on the mixed model analysis, we found a significant treatment effect on the PREs: empowerment during the treatment (P = 0.001) and discretion of the method (P = 0.000) in favour of the home-based monitoring (Table V, Fig. 3A and B). We did not find any significant treatment effect of home-based monitoring versus hospital-based monitoring on the other three of the five PREs: anxiety/sadness about missing the ovulation window (P = 0.66), satisfaction with the treatment (P = 0.17) and partner participation during the treatment (P = 0.07) (Table V, Fig. 4A–C).
Table V. Outcomes of the assessed PREs.
|
PRE items
Mean (SD) |
T1 (FET) | T2 (pregnancy test) | Treatment effect | Time effect | Interaction |
|---|---|---|---|---|---|
| Anxiety/sadness about missing ovulation* | |||||
| Home-based monitoring | n = 95; 1.73 (0.85) | n = 89; 1.97 (0.96) | P = 0.66 | P = 0.047 | P = 0.594 |
| Hospital-based monitoring | n = 88; 1.60 (0.82) | n = 85; 1.74 (0.93) | |||
| Empowerment during treatment** | |||||
| Home-based monitoring | n = 95; 3.23 (0.92) | n = 89; 3.29 (0.92) | P = 0.001 | P = 0.41 | P = 0.83 |
| Hospital-based monitoring | n = 88; 2.90 (0.90) | n = 85; 3.00 (0.95) | |||
| Discretion of method** | |||||
| Home-based monitoring | n = 94; 4.13 (0.79) | n = 88; 4.18 (0.78) | P = 0.000 | P = 0.66 | P = 0.30 |
| Hospital-based monitoring | n = 86; 3.84 (0.85) | n = 85; 3.71 (0.90) | |||
| Satisfaction with method** | |||||
| Home-based monitoring | n = 94; 3.83 (1.05) | n = 88; 3.95 (0.97) | P = 0.17 | P = 0.97 | P = 0.187 |
| Hospital-based monitoring | n = 86; 3.83 (0.75) | n = 85; 3.69 (0.82) | |||
| Partner? | |||||
| Home-based monitoring | Yes n = 89, No n = 5 | Yes n = 86, No n = 3 | NA | NA | NA |
| Hospital-based monitoring | Yes n = 81, No n = 6 | Yes n = 79, No n = 6 | |||
| Partner involvement during treatment** | |||||
| Home-based monitoring | n = 89; 3.99 (0.96) | n = 85; 4.01 (0.85) | P = 0.069 | P = 0.71 | P = 0.485 |
| Hospital-based monitoring | n = 80; 4.24 (0.83) | n = 78; 4.21 (0.93) |
Data for the two randomisation groups of the Antarctica-2 RCT after completion of ovulation monitoring but before FET (T = 1) and before pregnancy test (T = 2).
*Scores scale: 1 = indicating no problem; 2 = indicating slight problems; 3 = indicating moderate problems; 4 = indicating severe problems; 5 = indicating extreme problems. **Scores scale: 1 = no empowerment/discretion/satisfaction/partner involvement at all; 2 = no empowerment/discretion/satisfaction/partner involvement; 3 = neutral; 4 = experienced empowerment/discretion/satisfaction/partner involvement; 5 = experienced very much empowerment/discretion/satisfaction/partner involvement.
Figure 3. PREs with significant difference between home-based monitoring and hospital-based monitoring. (A) feeling of empowerment during treatment. (B) perceived level of discretion during treatment. PREs, patient-reported experiences.
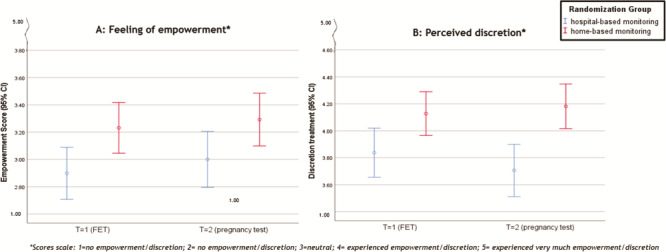
Figure 4. PREs without significant difference between home-based monitoring and hospital-based monitoring. (A) anxiety/sadness about missing ovulation. (B) satisfaction with treatment. (C) perceived level of partner participation during treatment.
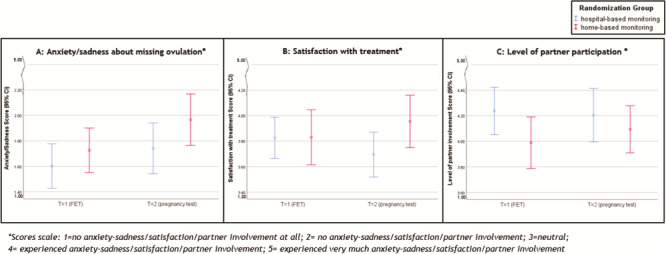
We found a very small time effect of home-based monitoring versus hospital-based monitoring on the anxiety/sadness about missing the ovulation window, meaning that both groups felt increasingly anxious/sad over time (P = 0.047). On the other four PREs, we did not find any time effect (Table V).
Our analysis did not show an interaction effect of home-based monitoring versus hospital-based monitoring on any of the five PREs (Table V).
Discussion
Compared to hospital-based monitoring, home-based monitoring did not affect PROs but improved PREs on two dimensions: more perceived discretion and feeling of empowerment during the treatment. Home-based monitoring did not result in higher levels of anxiety compared to hospital-based monitoring, albeit both treatments were associated with a minor increase in anxiety/sadness symptoms over time.
We hypothesised that home-based monitoring resulted in less interference with private and working life, more partner participation, a feeling of discretion and a feeling of empowerment as compared to hospital-based monitoring. Satisfaction with either treatment was not different in our study. We further hypothesised that assistance of the partner with performing urinary LH tests at home might increase the level of perceived partner participation. The reported level after home-based monitoring compared to that after hospital-based monitoring was not statistically significant. Remarkably, the patient organisation FREYA members valued partner participation as an important PRE. Possibly no good answer to this question resulted from not having a strict definition of what should have been exactly considered as participation of the partner. This could also be explained by the partner accompanying the patient to the hospital-based monitoring. Future focus groups may further investigate this.
In comparison with results of the study on home-based monitoring during IVF-stimulation (the ‘fresh cycle’), the outcomes of our study differ slightly. Women during home-based monitoring of IVF-stimulation experienced an increased level of satisfaction with the treatment and an increased level of partner participation. These PREs were not significantly better for the home-based monitoring group in our study. The increased level of perceived discretion and feeling of empowerment during home-based monitoring in FET cycles is in concordance with another study on home-based monitoring during IVF-stimulation in a fresh embryo transfer (Gerris and De Sutter, 2010; Gerris et al., 2014). Based on this, it seems that a more natural and home-based approach is preferred.
Before the start of this study, from the patient organisation FREYA members came, as a potential disadvantage, that home-based monitoring might result in a higher level of perceived anxiety due to the potential risk of missing the ovulation. Our study did not confirm this higher anxiety. Both treatments were associated with an increase in anxiety/sadness symptoms over time. Other studies on anxiety during fertility treatments found similar results. Most women undergoing fertility care experience increased emotional distress over time including anxiety and depressive symptoms (Thiering et al., 1993; Csemiczky et al., 2000; Gameiro et al., 2015). This may partly be explained by the fact that unfortunately, one-third of women end fertility treatment without achieving pregnancy (Pinborg et al., 2009) and experience difficulties in adjusting to unmet parenthood goals (Gameiro et al., 2014). However, even when a pregnancy is achieved, IVF mothers perceive more anxiety symptoms during the course of the pregnancy compared to women with a spontaneous pregnancy (Hammarberg et al., 2008; Garcia-Blanco et al., 2018). In line with the results of our study, a recently performed study shows that the stress and anxiety level in women undergoing fresh or FET cycles increased significantly in the period between pre-embryo transfer and the pregnancy test (Cheung et al., 2019).
A major strength of this study is its randomised design and its pre-defined sample size. To our knowledge, this study is the first to report on PROs and PREs in FET cycles. Considering the increasing number of FET cycles worldwide, it is of great importance to gain more knowledge about PROs and PREs of these treatments in order to be able to make a shared decision about the treatment and to tailor FET cycles even more specifically to the preference of individual women (De Geyter et al., 2018; ESHRE, 18 February 2018; Pereira et al., 2019).
Knowledge about PROs and PREs is also important given that most women undergoing IVF treatment (including FET) experience increased emotional distress over time including anxiety and depressive symptoms (Thiering et al., 1993; Csemiczky et al., 2000; Gameiro et al., 2015; Cheung et al., 2019). It is even suggested that higher levels of depressive symptoms or anxiety during fertility treatment might be related to lower pregnancy rates (Thiering et al., 1993; Csemiczky et al., 2000; Gameiro et al., 2015). In the ESHRE guideline, it is advised that fertility staff should be aware that patients’ emotional stress peaks at the embryo transfer and the waiting period before the pregnancy test (Gameiro et al., 2015). In our study we found an increase in depression/anxiety over time between FET and the pregnancy test in both groups, which is comparable to the data presented in the this ESHRE guideline. Therefore, we believe that the first step in psychosocial care is awareness among healthcare providers of the psychosocial burden women and partners experience during a FET treatment cycle and offering further psychosocial counselling as indicated. During our study, we did not investigate the effect of specific interventions for psychosocial support. Still, according to the ESHRE guideline, it is appropriate to offer patients access to specialised psychosocial care.
Several uncertainties should be acknowledged. PROs are assessed often in patients and partners undergoing reproductive techniques. However, the validated questionnaires that are generally used in other fields of medicine to assess PROs, such as the EQ-5D-5L, may not be perfectly applicable to this young and (usually) physically fit population. In order to evaluate the PROs in this healthy population, questions concerning level of mobility, self-care, usual activities and pain/discomfort may not be sufficiently sensitive; therefore, development of validated patient-reported outcome measurements specific for FET cycles in fertility care is desired. Second, this study lacks data on partners of women undergoing natural cycle FET. Third, data at T1 were collected for the majority of the participants before FET and therefore the majority of the women did not know the quality of the embryo when they completed the questionnaire. A minority of the women might have known the quality of the embryo, but given the randomised design, this knowledge was comparable between groups (P = 0.40). Data at T2 were collected in 97% of the women in both arms before the scheduled pregnancy test, rendering it unlikely that the timing of the T1 and T2 data collection has influenced the outcomes and corresponding conclusions.
Concerning implications for future research, development of validated patient-reported outcome measurements specifically for women and partners in different types of fertility care, like FET, is desired (Dancet et al., 2010). The specialised Fertility Quality of Life (FertiQol) tool mainly focuses on quality of life in men and women who have just been diagnosed with fertility problems (Boivin et al., 2011a,b). Although the FertiQol tool is useful to assess PROs in our population, we considered this full questionnaire too time consuming for the participants together with the PRE questionnaire. We are aware that EQ-5D-5L is not sufficient to assess anxiety and depression separately. We therefore assessed anxiety separately in the PRE questionnaire. We did not study depression; depression was, however, not prioritised by the patient organisation FREYA as assessed by a questionnaire among their members.
To evaluate PREs in fertility care and to be able to compare outcomes of PREs between studies, we need to pre-define and validate patient-reported experience measurements. Assessing PREs in our study population is a new strategy for measurement of patient experience and satisfaction and has therefore not yet been validated. Further research and future validation of patient-reported experience measurements is warranted, to be able to establish a standardised measuring instrument. Furthermore, it would be of interest to investigate whether quality of life and patient satisfaction in an artificial cycle FET are comparable to those observed in our study, as this type of cycle is still being used in different countries.
The answer to our research question is women have a better experience (more perceived feeling of empowerment and discretion) after home-based monitoring compared with that after hospital-based monitoring. This study suggests that there is no effect of FET monitoring strategy on quality of life and indicates there might be a positive evaluation of care in favour of home-based monitoring. The outcome of the PREs is only based on a non-validated set of items, which were prioritised by the Dutch patient organisation FREYA. Before a final recommendation regarding home-based or hospital-based monitoring can be made, we need to await the completion of the Antarctica-2 RCT, which will generate data on pregnancy rates and cost-effectiveness.
Acknowledgements
We would like to thank EuroQol for allowing us permission to use EQ-5D-5L for the assessment of PROs. We also would like to thank the Dutch Patient Organization for Couples with Fertility Problems (FREYA) for their input and their contribution to the development of the PRE assessment.
Supplementary data
Supplementary data are available at Human Reproduction online.
Authors’ roles
T.Z.: study design, data collection, data analysis and manuscript preparation. J.B.: study design, data collection, supervision and manuscript preparation. M.G.: study design, supervision and manuscript preparation. J.V.: data collection and manuscript preparation. E.K.: data collection and manuscript preparation. C.B.: data collection and manuscript preparation. E.G.: data collection and manuscript preparation. M.W.: study design, data analysis and manuscript preparation. F.M.: study design, data collection, supervision and manuscript preparation.
Funding
Netherlands Organisation for Health Research and Development (ZonMw 843002807).
Conflict of interest
J.B. receives unconditional educational grants from Merck Serono and Ferring and is a member of the medical advisory board of Ferring. C.L. reports that his department receives unrestricted research grants from Ferring, Merck and Guerbet. E.G. receives personal fees from Titus Health Care outside submitted work. The remaining authors have no conflicts of interest to declare.
References
- ESHRE ESoHRaE. ART fact sheet. 18 February 2018.
- Ahern S, Ruseckaite R, Ackerman IN. Collecting patient-reported outcome measures. Intern Med J 2017;47:1454–1457. [DOI] [PubMed] [Google Scholar]
- Boivin J, Takefman J, Braverman A. The Fertility Quality of Life (FertiQoL) tool: development and general psychometric properties. Hum Reprod 2011a;26:2084–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin J, Takefman J, Braverman A. The Fertility Quality of Life (FertiQoL) tool: development and general psychometric properties. Fertil Steril 2011b;96:409–415e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C, Saravelos SH, Chan T, Sahota DS, Wang CC, Chung PW, Li TC. A prospective observational study on the stress levels at the time of embryo transfer and pregnancy testing following in vitro fertilisation treatment: a comparison between women with different treatment outcomes. BJOG 2019;126:271–279. [DOI] [PubMed] [Google Scholar]
- Csemiczky G, Landgren BM, Collins A. The influence of stress and state anxiety on the outcome of IVF-treatment: psychological and endocrinological assessment of Swedish women entering IVF-treatment. Acta Obstet Gynecol Scand 2000;79:113–118. [DOI] [PubMed] [Google Scholar]
- Dancet EA, Nelen WL, Sermeus W, De Leeuw L, Kremer JA, D'Hooghe TM. The patients’ perspective on fertility care: a systematic review. Hum Reprod Update 2010;16:467–487. [DOI] [PubMed] [Google Scholar]
- De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, Scaravelli G, Smeenk J, Vidakovic S, Goossens Vet al. ART in Europe, 2014: results generated from European registries by ESHRE: the European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod 2018;33:1586–1601. [DOI] [PubMed] [Google Scholar]
- Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EuroQol.org . User Guide 2015https://euroqol.org/wp-content/uploads/2016/09/EQ-5D-5L_UserGuide_2015.pdf.
- Gameiro S, Boivin J, Dancet E, Klerk C, Emery M, Lewis-Jones C, Thorn P, Van den Broeck U, Venetis C, Verhaak CMet al. ESHRE guideline: routine psychosocial care in infertility and medically assisted reproduction-a guide for fertility staff. Hum Reprod 2015;30:2476–2485. [DOI] [PubMed] [Google Scholar]
- Gameiro S, Belt-Dusebout AW, Bleiker E, Braat D, Leeuwen FE, Verhaak CM. Do children make you happier? Sustained child-wish and mental health in women 11-17 years after fertility treatment. Hum Reprod 2014;29:2238–2246. [DOI] [PubMed] [Google Scholar]
- Garcia-Blanco A, Diago V, Hervas D, Ghosn F, Vento M, Chafer-Pericas C. Anxiety and depressive symptoms, and stress biomarkers in pregnant women after in vitro fertilization: a prospective cohort study. Hum Reprod 2018;33:1237–1246. [DOI] [PubMed] [Google Scholar]
- Gerris J, De Sutter P. Self-operated endovaginal telemonitoring (SOET): a step towards more patient-centred ART? Hum Reprod 2010;25:562–568. [DOI] [PubMed] [Google Scholar]
- Gerris J, Delvigne A, Dhont N, Vandekerckhove F, Madoc B, Buyle M, Neyskens J, Deschepper E, De Bacquer D, Pil Let al. Self-operated endovaginal telemonitoring versus traditional monitoring of ovarian stimulation in assisted reproduction: an RCT. Hum Reprod 2014;29:1941–1948. [DOI] [PubMed] [Google Scholar]
- Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev 2010;CD006359. [DOI] [PubMed] [Google Scholar]
- Groenewoud ER, Cohlen BJ, Al-Oraiby A, Brinkhuis EA, Broekmans FJ, Bruin JP, Dool G, Fleisher K, Friederich J, Goddijn Met al. A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer. Hum Reprod 2016;31:1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarberg K, Fisher JR, Wynter KH. Psychological and social aspects of pregnancy, childbirth and early parenting after assisted conception: a systematic review. Hum Reprod Update 2008;14:395–414. [DOI] [PubMed] [Google Scholar]
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley C, Patel S. Patient-reported outcome measures and patient-reported experience measures. BJA Educ 2017;17:137–144. [Google Scholar]
- Lavallee DC, Chenok KE, Love RM, Petersen C, Holve E, Segal CD, Franklin PD. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Aff (Millwood) 2016;35:575–582. [DOI] [PubMed] [Google Scholar]
- Pereira N, Petrini AC, Hancock KL, Rosenwaks Z. Fresh or frozen embryo transfer in in vitro fertilization: an update. Clin Obstet Gynecol 2019;62:293–299. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Hougaard CO, Nyboe Andersen A, Molbo D, Schmidt L. Prospective longitudinal cohort study on cumulative 5-year delivery and adoption rates among 1338 couples initiating infertility treatment. Hum Reprod 2009;24:991–999. [DOI] [PubMed] [Google Scholar]
- Thiering P, Beaurepaire J, Jones M, Saunders D, Tennant C. Mood state as a predictor of treatment outcome after in vitro fertilization/embryo transfer technology (IVF/ET). J Psychosom Res 1993;37:481–491. [DOI] [PubMed] [Google Scholar]
- Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril 2014;102:19–26. [DOI] [PubMed] [Google Scholar]


