Abstract
Treatment of neonates with persistent pulmonary hypertension of newborn includes optimization of ventilatory support, use of pulmonary vasodilators, and/or inotropic support. If refractory to this management, some may require extracorporeal membrane oxygenation. We describe a case series of 10 neonates with refractory persistent pulmonary hypertension of newborn treated with vasopressin in a single tertiary center. Mean initiation time of vasopressin was at 30 h of life with a dose ranging from 10 to 85 milliunits/kg/h. Oxygenation index decreased after 12 h of vasopressin exposure (25 to 11) and mean arterial pressure improved after 1 h (45 to 58 mm Hg). Extracorporeal membrane oxygenation was averted in 50% of the cases with transient hyponatremia as the only notable side effect. Although our findings are exploratory and further research is needed to establish safety and efficacy, our experience suggests that vasopressin may have rescue properties in the management of refractory persistent pulmonary hypertension of newborn.
Keywords: Persistent pulmonary hypertension of newborn, extracorporeal membrane oxygenation, Extracorporeal Life Support Organization, oxygenation index
Introduction
Persistent pulmonary hypertension of the newborn (PPHN) is a result of delayed transition from fetal to neonatal circulation. It is characterized by elevated right ventricular (RV) pressure, right to left shunt at the ductal level and/or foramen ovale, and hypoxemia. The incidence is 1.9 per 1000 live births, and mortality ranges between 4% and 33%. 1 Treatment strategies include optimization of ventilatory support, use of inhaled or intravenous pulmonary vasodilators, and/or inotropic support. Neonates who are refractory to medical management require extracorporeal membrane oxygenation (ECMO) as rescue modality. Medical advances such as inhaled nitric oxide (iNO), high-frequency oscillatory ventilation, and surfactant led to a decrease in neonatal respiratory ECMO from 1992 to 2000.2,3 However, from 2000 onwards for the last two decades, ECMO use has plateaued, indicating the need for new approaches in managing PPHN-related respiratory failure. 4
Vasopressin has been suggested for neonatal PPHN management as it is thought to be selective systemic vasoconstrictors. 5 Arginine vasopressin increases systemic vascular resistance (SVR) and mean arterial blood pressure (MAP) with minimal chronotropic effects by acting on V1 receptors. 6 In an animal model, its action on pulmonary endothelial V1 receptors induces nitric oxide release, which is critical in decreasing pulmonary vascular resistance (PVR). 7 However, hesitance on use of vasopressin centers around the potential to cause electrolyte disturbances such as hyponatremia and/or reduce splanchnic circulation, thereby increasing the risk for necrotizing enterocolitis. 8 Vasopressin has been successfully used to treat septic shock in premature neonates, children, and adults. 9 - 11 However, data on use of vasopressin in neonates with PPHN are limited. We describe our center’s experience with the use of vasopressin in a group of neonates with refractory PPHN, the effect on hemodynamic parameters, and short-term outcomes.
Case
This is a retrospective case series of 10 neonates with severe PPHN treated with vasopressin in a tertiary academic neonatal intensive care unit from January 2017 to December 2020. The group had a male predominance (70%) with a mean gestational age and birthweight of 38 weeks and 3200 g, respectively (Table 1). Meconium aspiration was the leading cause of PPHN (50%). All patients received iNO and were on a median of two vasopressors/inotropes before initiation of vasopressin. Five out of ten patients needed ECMO. Indication of ECMO in this cohort was meconium aspiration syndrome, hypoxic respiratory failure, and sepsis.
Table 1.
Characteristics of patients receiving vasopressin for PPHN.
| Subject | Birth weight (g) | Sex | Gestational age (weeks) | Diagnosis | Need for therapeutic hypothermia | Need for ECMO | Age at initiation (h) | Initial vasopressin dose (milliunits/kg/h) | Maximum vasopressin dose (milliunits/kg/h) | Duration of vasopressin (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3663 | M | 34.3 | Naphthalene exposure | N/A | N | 47 | 40 | 60 | 212 |
| 2 | 2710 | M | 39 | MAS | N/A | Y (VA) | 17 | 40 | 80 | 5 |
| 3 | 3730 | M | 39 | HIE with pulmonary hemorrhage | Y | N | 21 | 60 | 80 | 157 |
| 4 | 2590 | M | 40.1 | HIE with pneumothorax | Y | Y (VA) | 43 | 40 | 80 | 12 |
| 5 | 3200 | M | 41.1 | MAS | N/A | N | 25 | 60 | 80 | 54 |
| 6 | 2321 | F | 40 | MAS with pneumothorax | N/A | N | 37 | 20 | 20 | 41 |
| 7 | 3145 | F | 39.3 | HIE with MAS | Y | N | 20 | 30 | 80 | 14 |
| 8 | 3940 | M | 38.5 | HIE, COVID-19 exposure | Y | Y (VA) | 28 | 60 | 80 | 7 |
| 9 | 3510 | M | 35.1 | HIE and LV dysfunction | Y | Y (VV) | 30 | 10 | 80 | 20 |
| 10 | 3134 | F | 41 | MAS/HIE/sepsis | Y | Y (VA) | 35 | 20 | 85 | 9.5 |
COVID-19: Coronavirus disease-19; ECMO: extracorporeal membrane oxygenation; F: female; HIE: hypoxic ischemic encephalopathy; LV: left ventricle; M: male; MAS: meconium aspiration syndrome; N: no; N/A: not applicable; PPHN: persistent pulmonary hypertension of newborn; VA: veno-arterial; VV: veno-venous; Y: yes.
Six patients (60%) received therapeutic hypothermia for moderate to severe hypoxic ischemic encephalopathy (HIE). Frequency of vasopressors/inotropes used included dopamine, 9 dobutamine, 8 epinephrine, 4 and milrinone. 2 The mean initiation time of vasopressin was at 30 ± 10 h of life with dose ranging from 10 to 85 milliunits/kg/h and mean duration of 53 ± 72 h (Table 1). The median time between initiation of vasopressin and ECMO in those five patients was 7 h.
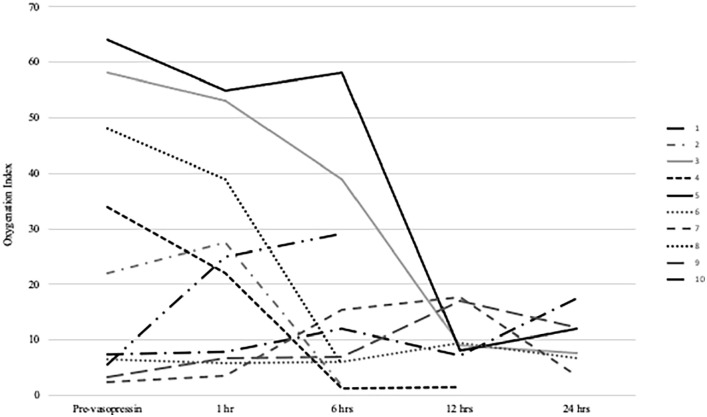
Oxygenation index (OI) was noted to decrease following vasopressin initiation, most notable after 12 h of exposure (mean 25.10 ± 24.14 to 11.36 ± 4.67, Table 2). 12 One patient had rebound in OI associated with rewarming phase of therapeutic hypothermia (Figure 1). MAP improved at 1 h of vasopressin exposure (mean 44.50 ± 7.80 to 57.90 ± 13.27 mm Hg). There was a decrease in mean inotropic score (IS) at 12 h (24.70 ± 12.94 to 10.5 ± 10.25). Mean vasoactive-inotropic score (VIS) decreased gradually, lowest at 24 h (25.50 ± 12.78 to 16.57 ± 8.45, Table 2). 13 Five patients (50%) averted ECMO, and all had vasopressin therapy for >12 h. Of those who required ECMO, only one out of five received vasopressin for >12 h.
Table 2.
Effects of vasopressin on clinical and hemodynamic variables.
| Variable | Pre-vasopressin | 1 h | 6 h | 12 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|---|---|---|
| Oxygenation index | 25.10 ± 24.14 | 24.52 ± 19.29 | 23.78 ± 19.29 | 11.36 ± 4.67 | 9.52 ± 5.37 | 19.40 ± 23.79 | 14 ± 12.12 |
| Mean arterial blood pressure (mm Hg) | 44.50 ± 7.80 | 57.90 ± 13.27 | 60.85 ± 9.20 | 62.33 ± 16.66 | 63.80 ± 14.49 | 63.25 ± 10.21 | 71.33 ± 24.11 |
| Inotropic score (mean, SD) | 24.70 ± 12.94 | 21.52 ± 10.20 | 14.85 ± 10.83 | 10.5 ± 10.25 | 10.6 ± 6.3 | 13.75 ± 5.31 | 20.33 ± 17.89 |
| Vasoactive-inotropic score (mean, SD) | 25.20 ± 12.78 | 30.81 ± 12.36 | 24.90 ± 13.91 | 18.18 ± 12.08 | 16.57 ± 8.45 | 19.62 ± 11.55 | 26.16 ± 23.00 |
| Heart rate (per min) | 145.90 ± 30.15 | 145.30 ± 21.13 | 130.42 ± 12.80 | 133.33 ± 10.30 | 130.80 ± 13.95 | 132.25 ± 11.29 | 141.67 ± 13.79 |
| Urine output (mL/kg/h) | 2.66 ± 1.70 | 4.93 ± 5.9 | 2.95 ± 1.62 | 2.90 ± 1.85 | 2.65 ± 1.58 | 3.03 ± 0.75 | 4.65 ± 1.62 |
| Serum sodium (mmol/L) | 135.90 ± 5.02 | 133 ± 1.87 | 133 ± 5.76 | 130.33 ± 3.50 | 126.20 ± 4.49 | 129 ± 5.22 | 134.33 ± 9.71 |
| Serum lactate (mmol/L) | 3.56 ± 1.87 | 3.85 ± 1.20 | 4.65 ± 3.16 | 3.60 ± 2.89 | 2.72 ± 1.97 | 2.10 ± 0.56 | 2.43 ± 0.49 |
SD: standard deviation.
Figure 1.
Oxygenation index trends per subject.
Despite a down-trending heart rate from mean of 146 beats per minute (bpm) to 130 bpm after 6 h of vasopressin, there was a concurrent worsening lactic acidosis with peak of 4.65 ± 3.16 at 6 h (Table 2). This was followed by a gradual decrease at 48 h with mean lactate of 2.1 ± 0.56. There was no notable change in urine output. Transient hyponatremia was appreciated after 12 h of vasopressin (135.90 ± 1.87 to 130.33 ± 3.50 mmol/L) with nadir identified at 24 h (126.20 ± 4.49 mmol/L) and subsequent improvement by 72 h (134.22 ± 9.71 mmol/L). There were no cases of necrotizing enterocolitis. Three (30%) required a gastrostomy tube and nine (90%) were discharged in room air with one (10%) requiring low flow oxygen via nasal cannula. All neonates survived to discharge.
Discussion
The ideal approach to treating PPHN should target a decrease in PVR, while simultaneously increasing SVR and MAP. This combined approach results in left to right shunting, improved oxygenation, and organ perfusion. Inhaled nitric oxide selectively reduces PVR; however, there are data suggesting approximately 30% of patients with PPHN are non-responders. 14 Sildenafil and milrinone reduce PVR via their actions as phosphodiesterase 5 and 3 inhibitors, respectively. However, their effects on SVR can induce systemic hypotension and decreased end organ perfusion. Commonly used inotropes like dopamine and epinephrine can be used to deliberately achieve supra-systemic blood pressures and reverse the right to left shunt. Unfortunately, their non-specific vasoconstriction can also worsen underlying PPHN.
Vasopressin in principle is ideal for treating PPHN. Vasopressin decreases PVR, improving RV function while concomitantly increasing SVR, thereby sustaining perfusion. 8 Although vasopressin has been successfully used in preterm infants for septic shock,9,15 its use in neonatal PPHN is limited to case reports. Scheurer et al. 16 described successful use in a late preterm and term infant with post-operative pulmonary hypertension on multiple inotropes following cardiac surgery. One hour after initiation of vasopressin, a marked improvement in MAP allowed for discontinuation of epinephrine. However, both patients developed hyponatremia and vasopressin was discontinued.
Mohamed et al. 17 described the use of vasopressin in 10 term neonates with refractory PPHN on multiple pressors. They used vasopressin as a rescue therapy with a dosing range of 6–72 milliunits/kg/h. All patients had significant OI reduction at 6 h, and MAP improved at 24 h. The lowest sodium reported was 127 mmol/L and no patients had necrotizing enterocolitis. Acker et al. 18 described the use of vasopressin in 13 patients with congenital diaphragmatic hernia who all met ECMO criteria. After vasopressin use for 12 h, with a dose ranging from 6 to 120 milliunits/kg/h, the MAP improved, and six patients did not require ECMO. They reported hyponatremia (mean 117 mmol/L) in all patients who received vasopressin for greater than 24 h.
Our cohort had more than a 50% reduction in mean OI after 12 h of vasopressin exposure with a dose ranging from 10 to 85 milliunits/kg/h and a mean duration of 53 h. Like Scheurer et al., we saw an improvement in MAP after 1 h of initiation, permitting the reduction of inotropic support as reflected by lower mean IS at 12 h. While all patients in our series met ECMO criteria prior to vasopressin initiation, five (50%) avoided ECMO. Our findings of transient hyponatremia, with nadir at 24 h, are comparable to other studies warranting close monitoring of electrolytes.16,17 No case of necrotizing enterocolitis was identified in our case series. Limitations of the study are its retrospective design, case series, unknown confounders, and 4 years of data collection.
Conclusion
Rescue therapy with vasopressin stabilized hemodynamics in a subset of neonates with refractory PPHN. Although our findings are exploratory and further research is needed to establish safety and efficacy, our experience suggests that vasopressin may have rescue properties in the management of refractory PPHN. The near immediate improvement in MAP coupled with maximum OI effect at 12 h suggests at least 12 h of exposure would be beneficial to avoid need for ECMO. Further study is needed to determine the optimal timing of vasopressin initiation and duration in this unique population.
Acknowledgments
The authors are indebted to the ECMO team for their efforts, our patients, and their families.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This case series was reviewed and approved by Drexel University IRB with an HIPAA Waiver of Authorization, ID: 1911007503.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Waiver of consent provided for this study by Institutional Ethics Committee Number 100.
ORCID iD: Swosti Joshi  https://orcid.org/0000-0003-4028-7213
https://orcid.org/0000-0003-4028-7213
References
- 1. Walsh-Sukys MC, Tyson JE, Wright LL, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics 2000; 105(1Pt. 1): 14–20. [DOI] [PubMed] [Google Scholar]
- 2. Hintz SR, Suttner DM, Sheehan AM, et al. Decreased use of neonatal extracorporeal membrane oxygenation (ECMO): how new treatment modalities have affected ECMO utilization. Pediatrics 2000; 106(6): 1339–1343. [DOI] [PubMed] [Google Scholar]
- 3. Redaelli S, Magliocca A, Malhotra R, et al. Nitric oxide: clinical applications in critically ill patients. Nitric Oxide 2022; 121: 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ELSO International registry report. Annual respiratory neonatal runs, January 2016. https://www.pedsurglibrary.com/apsa/view/Pediatric-Surgery-NaT/829025/all/Extracorporeal_Life_Support
- 5. Lakshminrusimha S, Keszler M. Persistent pulmonary hypertension of the newborn. Neoreviews 2015; 16(12): e680–e692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thibonnier M, Coles P, Thibonnier A, et al. The basic and clinical pharmacology of nonpeptide vasopressin receptor antagonists. Annu Rev Pharmacol Toxicol 2001; 41: 175–202. [DOI] [PubMed] [Google Scholar]
- 7. Evora PR, Pearson PJ, Schaff HV. Arginine vasopressin induces endothelium-dependent vasodilatation of the pulmonary artery. Chest 1993; 103(4): 1241–1245. [DOI] [PubMed] [Google Scholar]
- 8. Klinzing S, Simon M, Reinhart K, et al. Moderate-dose vasopressin therapy may impair gastric mucosal perfusion in severe sepsis: a pilot study. Anesthesiology 2011; 114: 1396–1402. [DOI] [PubMed] [Google Scholar]
- 9. Meyer S, Loffler G, Polcher T, et al. Vasopressin in catecholamine-resistant septic and cardiogenic shock in very-low-birthweight infants. Acta Paediatr 2006; 95(10): 1309–1312. [DOI] [PubMed] [Google Scholar]
- 10. Malay MB, Ashton RC, Jr, Landry DW, et al. Low-dose vasopressin in the treatment of vasodilatory septic shock. J Trauma 1999; 47: 699–703. [DOI] [PubMed] [Google Scholar]
- 11. Vail EA, Gershengorn HB, Hua M, et al. Epidemiology of vasopressin use for adults with septic shock. Ann Am Thorac Soc 2016; 13(10): 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muniraman HK, Song AY, Ramanathan R, et al. Evaluation of oxygen saturation index compared with oxygenation index in neonates with hypoxemic respiratory failure. JAMA Netw Open 2019; 2: e19179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McIntosh AM, Tong S, Deakyne SJ, et al. Validation of the vasoactive-inotropic score in pediatric sepsis. Pediatr Crit Care Med 2017; 18(8): 750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldman AP, Tasker RC, Haworth SG, et al. Four patterns of response to inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 1996; 98(4Pt. 1): 706–713. [PubMed] [Google Scholar]
- 15. Bidegain M, Greenberg R, Simmons C, et al. Vasopressin for refractory hypotension in extremely low birth weight infants. J Pediatr 2010; 157(3): 502–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scheurer MA, Bradley SM, Atz AM, et al. Vasopressin to attenuate pulmonary hypertension and improve systemic blood pressure after correction of obstructed total anomalous pulmonary venous return. J ThoracCardiovasc Surg 2005; 129: 464–466. [DOI] [PubMed] [Google Scholar]
- 17. Mohamed A, Nasef N, Shah V, et al. Vasopressin as a rescue therapy for refractory pulmonary hypertension in neonates. Pediatric Critical Care Medicine 2014; 15(2): 148–154. [DOI] [PubMed] [Google Scholar]
- 18. Acker SN, Kinsella JP, Abman SH, et al. Vasopressin improves hemodynamic status in infants with congenital diaphragmatic hernia. J Pediatr 2014; 165(1): 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]