Abstract
Background:
Primary HHV7 infection is almost ubiquitous, and it can present as exanthema subitem. Little is known on the clinical relevance of HHV7 neuroinvasion in immunocompetent children.
Methods:
We describe 12 patients (median age 9.45 years, 50% males) with acute encephalopathy and active HHV7 infection. In all patients, HHV7-DNA was detected on cerebrospinal fluid (CSF) by RT-PCR.
Results:
7/12 patients had meningoencephalitis (two with ADEM and one with MOG antibody-associated CIS); 5/12 showed acute neuropsychiatric symptoms. EEG showed anomalies exclusively in patients with meningoencephalitis. Six patients had RMN anomalies. CSF HHV7 copies ranged between 20 and 3,500 copies/mL (median 66 copies/mL) and mean HHV7 CSF/blood ratio was 0.75. Outcome was favorable in all children, although 3/12 had minor neurobehavioral sequelae. Mean follow-up period of 5.2 months.
Conclusion:
HHV7 can determine neuroinvasion in immunocompetent children, leading to acute encephalopathy. Blood-brain barrier damage and high CSF/ blood viral copies ratio correlated with a more severe presentation. We speculate on the importance of immune-mediated mechanisms in provoking clinical features. (www.actabiomedica.it)
Keywords: ADEM, Encephalitis, HHV7, Meningoencephalitis, PANS, Pediatric
Introduction
The human herpes virus 7 (HHV-7) is a b-herpes virus. It was first discovered by June et al. in 1990. HHV-7 is genetically, epidemiologically and clinically highly related to the more investigated and widely described human herpes virus 6 (HHV-6) (1). HHV-7 infects CD4+ T-lymphocytes and, less frequently, CD8+ and immature T-cells (2). Similarly to other Herpes viruses, HHV-7 establishes a life-long infection in its human hosts after the primary infection, switching between latent and lytic phases (3).
HHV-7 is ubiquitous, and the primary infection mostly occurs during early childhood, peaking between 18 and 36 months of life, which is slightly later than HHV-6. By the age of 5 years, more than 90% of the general population has been infected by HHV-7 (4, 5). Alike HHV-6, HHV-7 infection has different clinical presentations in children, such as exanthema subitum, fever without rash, febrile seizures and febrile status epilepticus (6-8).
Little is known on how HHV-7 can enter the blood brain barrier (BBE) and cause invasion of the central nervous system (CNS) (9). Recently, single case reports and case series of HHV-7 related encephalitis or encephalopathy have been described, in both immunocompetent and immunocompromised children and adults (10). Clinical presentation of CNS involvement seems highly heterogeneous, to be distinguished from other neurological diseases, and includes febrile seizures, encephalitis, meningoencephalitis, facial palsy, vestibular neuritis, severe headache, somnolence, fatigue, nausea, vomiting, photosensitivity, ataxia, and coma (10-18).
In this single-center retrospective study, we evaluated HHV-7-related CNS disorders and analyzed its clinical manifestations and outcome in children and adolescents.
Materials and Method
All cerebrospinal fluid (CSF) samples collected from January 1st 2012 to December 31th 2019 for a suspect of CNS infection were tested for HHV-7 DNA by real-time polymerase chain reaction (RT-PCR)15. Other neurotropic viruses including HHV-6, human herpes simplex (HSV), varicella zoster virus (VZV), enterovirus (EV) human cytomegalovirus (CMV) and Epstein-Barr virus (EBV) were analyzed (19-21).
Study inclusion criteria for HHV-7 related CNS infection were: 1) pediatric age (>28 days and <18 years); 2) acute/subacute onset of neurological symptoms; 3) evidence of HHV-7 DNA in the CSF; 4) full clinical and demographic data available. Patients with known immunodeficiency or with a final differential diagnosis were excluded from the study.
Clinical data were retrospectively collected using the hospital charts and anonymously analyzed.
The following variables were recorded: age, sex, comorbidities, personal and family history, signs and symptoms at onset, clinical and neurological examination, blood and CSF chemistry, extended microbiological investigations (including evidence of co-infections), electroencephalographic (EEG) abnormalities, evidence of CNS lesions on computed tomography (CT) and/or magnetic resonance imaging (MRI), therapy and outcome. Disability at disease onset (worse clinical score) and follow up was determined according to the modified Rankin Scale for children (mRS) (22, 23). Central nervous system demyelinating disorders were classified according to diagnostic criteria of IPMSSG (24).
Extended investigations including isoelettrofocusing and search for neuronal cell auto-antibodies were carried out by the Neuroimmunology Laboratory of IRCCS Mondino Foundation (Pavia, Italy). The patients’ serum and CSF were tested with an in-house screening immunohistochemistry technique on lightly-fixed rat brain tissue (25, 26) and with a CBA for MOG, AQP4, NMDAR, LGI1, CASPR2, AMPAR1/2, GABABR Abs (Euroimmun, Lubeck, Germany).
Written informed consent was obtained from all parents or legal tutors of the patients.
Results
A total of 456 pediatric inpatients had their CSF tested for HHV-7 in the seven years study period. HHV-7 DNA was detected in 16/456 (3.5%) patients. Four patients were excluded from our study: two with an alternative final diagnosis (one with WNV encephalitis and one with Listeria monocytogenes menin-goencephalitis), and two others because of lack of clinical data. Twelve patients were finally included: six females and six males (Figure 1).
Figure 1.
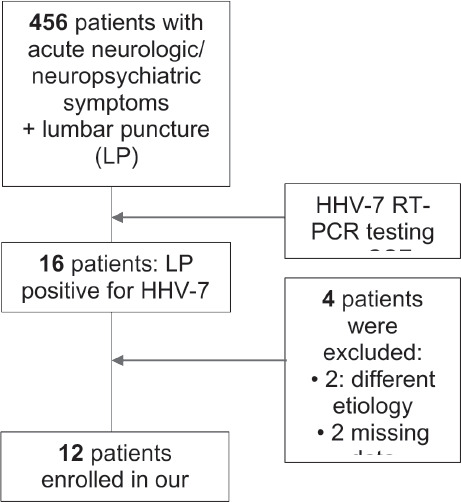
Study flow-chart
Median age was 9.5 years (range 1-16 years). Demographic, clinical and laboratory features are summarized in Table 1.
Table 1.
Study population
| patient | gender/age | HHV7 (copies/mL) | CSF/serum HHV-7 copies | CSF -WBC | CSF -CSF– polyclonal bands | CSF -CSF– polyclonal oligoclonal | anti-MOG | BBB damage | diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F; 9.4 y | 72 | 0.18 | 36 | Yes | none | none | none | meningoencephalitis (ADEM) |
| 2 | F; 1.7 y | 1080 | 0.13 | 5 | yes | none | none | none | encephalitis |
| 3 | F; 11 y | 1500 | 0.8 | 90 | none | yes | none | none | meningoencephalitis |
| 4 | M; 16.2 y | 3500 | 4.87 | / | / | / | / | meningoencephalitis | |
| 5 | M; 9.1 y | 40 | 0.4 | 26 | none | none | yes | meningoencephalitis (AHEM) | |
| 6 | F; 12.6 y | 100 | / | neg | yes | none | none | none | meningoencephalitis with III cranial nerve palsy |
| 7 | M; 8.2 | 100 | 0.058 | 60 | none | none | yes | none | meningoencephalitis (CIS anti-MOG +) |
| 8 | F; 7 y | 40 | 0.23 | neg | / | / | / | / | neuropsychiatric symptoms |
| 9 | M; 14.4 y | 20 | 0.0014 | neg | / | / | / | / | neuropsychiatric symptoms (PANS) |
| 10 | M; 13.6 y | 60 | / | neg | yes | none | none | none | neuropsychiatric symptoms (PANS) |
| 11 | F; 2 y | 33 | 0.825 | neg | yes | none | none | none | neuropsychiatric symptoms |
| 12 | M; 9.5 y | 20 | 0.04 | neg | / | / | / | / | neuropsychiatric symptoms (PANS) |
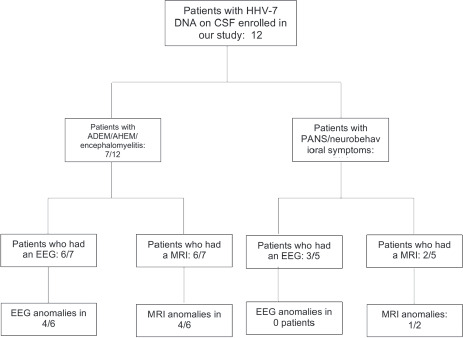
All patients were healthy before the acute event, except for one patient with a prior diagnosis of Gilles de la Tourette syndrome, and one with learning disability. Five/12 (41.7%) had fever, and none of our patients had typical features of exanthema subitum. All of them showed acute or subacute onset of CNS symptoms: 7/12 with meningoencephalitic symptoms and 5/12 with prominent neuropsychiatric manifestations (Figure 2).
Figure 2.
Clinical manifestations of acute HHV7 CNS infection
Of the seven patients with meningoencephalitis, two had acute disseminated encephalomyelitis (ADEM), and one had acute hemorrhagic encephalomyelitis (AHEM). All these seven patients had acute encephalopathy (defined as altered consciousness that persisted for longer than 24h, including lethargy, irritability, or a change in personality and behaviour), and acute (multi)focal neurologic symptoms (i.e. cranial nerves palsy, dysarthria, loss of sphincter control, nystagmus). Four/7 (57%) had fever, and 4/7 showed meningeal signs (including at least two of the following: rigor nucalis, headache, photophobia/phonophobia, or vomiting). Only a 19-months old patient with encephalitis experienced recurrent febrile seizures over 24 hours. Finally, one patient had acute unilateral recurrent oculomotor nerve palsy, as the sole focal neurological manifestation.
Among the five patients with predominant neuropsychiatric manifestations, four were ultimately classified as PANS (Pediatric Acute-onset Neuropsychiatric Syndrome) (27). They acutely displayed tics and OCD (obsessive-compulsive disorder). In one of these three we observed also echolalia, coprolalia, soliloquy, self-harming and hetero-aggressive behavior.
CSF analysis was performed in all 12 patients. In all cases it was crystalline, although 5/12 showed pleocytosis (mean cell count 43.4 cells/mL – range 5 – 90 cells/mL), and one hyperproteinorrachia (181 mg/ dL). We detected blood-brain barrier (BBB) damage (i.e. increased CSF/serum transfer of albumin) in the patient with AHEM, and oligoclonal bands (OcB) in another patient with meningoencephalitis. All CSF and sera were tested for autoimmune encephalitides, only one patient presenting with ADEM was tested positive for anti-MOG antibodies.
HHV-7 DNA was detected in all CSF and in 10/12 whole blood (WB) samples (two not tested). Other neurotropic viral infections were excluded, and no coinfection was detected on CSF. In just one case (a patient with ADEM that developed anti-MOG antibodies), circulating copies of HHV-6 DNA were detected on WB, but not in the CSF. Median HHV-7 DNA copies was 66/mL in the CSF (range: 20c/ mL – 3,500/mL) and 660/mL in WB (range 40/mL – 14490/mL). Mean number of HHV-7 DNA copies was 547/mL in the CSF and 2,838/mL in WB. The mean CSF/WB HHV-7 DNA copies ratio was 0.75. Mean CSF/WB ratio was—higher in patients with meningoencephalitis compared to those with neuropsychiatric symptoms (1.073 vs 0.2741, p<.05) (Table 2).
Table 2.
CSF and blood analysis in the two diagnostic sub-groups
| ADEM/meningoencephalitis (Median values) | PANS/neuropsychiatric symptoms (Median values) | |
|---|---|---|
| HHV7 -CSF (cpp/mL) | 913.14 | 34.6 |
| HHV7 -WB (cpp/mL) | 2188.3 | 3812.5 |
| LCR -WBC (cell/mmc) | 31.29 | 0.4 |
| LCR – protein (mg/dL) | 51.4 | 19.2 |
| LCR -glucose (mg/dL) | 59.4 | 57.8 |
EEG was performed on 9/12 patients. EEG abnormalities were seen in 7/9 patients, all within the meningoencephalitis group, showing diffuse or focal slowing of the background activity.
MRI was performed in 8/12 patients and evidenced inflammatory lesions with T2-hyperintensity in five. Four of these five belong to the meningoencephalitis group and three out of four showed demyelinating lesions, believed to be immune-mediated.
All patients received multiple drugs, especially antivirals (acyclovir), antibiotics (mostly, amoxicillin/ clavulanic acid, ceftriaxone and azithromycin) and anti-inflammatories (ibuprofen, paracetamol, steroids). Besides, anti-epileptic drugs or hypnotic-sedative drugs were associated depending on the clinical manifestations. Antibiotics (predominantly amoxicillin, ceftriaxone and azithromycin) were used in 7/12 cases, while acyclovir was only given as empirical treatment to patients with meningoencephalitis, in 5/12 cases, and hypnotic-sedative drugs (i.e. benzodiazepines) to patients with acute psychosis and psycho-motor agitation, in 3/12 cases (Table 3).
Table 3.
Therapy of acute HHV7-related CNS infection
| Antiviral (Acyclovir) | Antibiotics | Anti-Inflammatories | Aed | Hypnotic-Sedative | Paracetamol | |
|---|---|---|---|---|---|---|
| n° 1 | yes | yes: ceftriaxone, ampicillina | yes | no | no | yes |
| n° 2 | yes | yes: amoxicillina | no | no | no | no |
| n° 3 | yes | yes: ceftriaxone | yes | no | no | yes |
| n° 4 | no | no | yes | no | no | yes |
| n° 5 | yes | yes: ceftriaxone, azitromicina | yes | yes | no | no |
| n° 6 | no | no | no | no | no | no |
| n° 7 | no | no | yes | no | no | no |
| n° 8 | yes | yes: amoxiclav | yes | no | yes | no |
| n° 9 | no | yes: amoxiclav e penicillina | yes | no | no | no |
| n° 10 | no | yes: amoxiclav e azitromicina | no | no | no | no |
| n° 11 | no | no | no | no | yes | yes |
| n° 12 | no | no | no | no | yes | no |
| total | 5 | 7 | 7 | 1 | 3 | 4 |
Outcome at short and medium term was favorable in all cases. Worse mRS at diagnosis was 1 in 6/12 patients, 2 in 3/12 and 3 in 3/12. Only the patient with AHEM scored 5 and required admission to the Intensive Care Unit.
Mean follow up was 3.6 months (range 0-15 months). At last visit, 9/12 patients had a mRS of 0, while 3/12 scored 1 for residual cognitive and behavioral deficits, including two patients with PANS and the one with AHEM.
Discussion
Among the Herpes virus family, many members are known to have a high neurotropism and cause central and peripheral neurological symptoms (28, 29). Whether this is mainly caused by a direct cytopathic effect, or by the inflammatory reaction of the activated immune system, remains largely unknown (7, 30). However, alike other neurotropic viruses, the CNS involvement of HHV-7 has been poorly and only very recently investigated (8, 31-34).
In 1996, Torigoe and colleagues first described HHV-7-associated neurological symptoms as febrile seizures, hemiplegia, and loss of consciousness (7). Almost all Torigoe’s patients presented with the typical rash (exanthema subitum), which is a clue for first infection. Several years later, the causative role of HHV-7 in febrile status epilepticus was investigated in a large international study, called the FEBSTAT study, which stated that HHV-7, together with HHV-6B, account for one third of febrile status epilepticus (8). Lately, Schwartz and colleagues conducted the largest retrospective study on 57 patients with HHV-7 associated CNS symptoms, identifying cases of acute encephalomyelitis, meningitis, and ADEM, mostly as a result of delayed HHV-7 primary infection (30).
In our study, we exclusively considered pediatric patients (<18 years) with definite acute/subacute neurological symptoms and evidence of HHV-7 DNA in CSF by RT-PCR detection. This technique does not allow to differentiate primary infection from viral reactivation, or re-infection. In fact, historically, differentiation among these entities has been carried out with serological studies. However, the scarce sensitivity and specificity of these tests, due to cross-reactivity with other Herpesviridae such as cytomegalovirus and HHV-6, hinders any solid interpretation of HHV-7 serology (7, 12, 30).
The detection rate of HHV-7 was 2.6% among all suspect meningitis/encephalitis in our Centre. The median age of our patients was 9.5 years, without gender predominance. These results are in line with the study by Schwartz and colleagues, in which the median age was 10 years (30), with a slight male preponderance and a global detection rate of 1.9%. To note, immuno-compromised patients were excluded from our study to avoid possible confounding factors: in his study, Schwartz also included three immunocompromised patients (5.3% of the study population), but the causative role of HHV-7 in their CNS manifestations was excluded in all of them. This might suggest that immunocompromised patients might develop a higher tolerance to HHV-7, and that the CNS manifestations are rather caused by an abnormal immune reaction to the virus, rather than by direct viral invasion of the CNS.
All patients in our study presented with acute encephalopathy according to the Granerod criteria but clinical presentation could be further classified in two distinct patterns: 58% presented acute encephalomyelitis with or without meningeal signs, while 42% showed acute neuropsychiatric symptoms (35). To the best of our knowledge, this is the first report of a possible association between HHV7 infection and acute psychiatric manifestations, that meet diagnostic criteria for PANS. The two groups were largely similar in terms of demographic features.
As Ward et al. stated in their work, we observed that HHV-7 infection is usually associated with febrile seizures in children younger than 2 years of age (12). The relative lack of febrile seizures in our cohort is probably due to a selection bias, since only children and adolescents with suspicion of CNS infection/inflammation had their CSF tested. The higher age and absence of exanthema subitum may suggest either a late primary infection or a re-infection. Moreover, in any of our case there was an alternative cause, according to our inclusion criteria: HHV-7 was the only virus detected in CSF and there was no co-infection. This differs from Schwartz’s study, in which an alternative etiology was identified for half of the children who had HHV-7 in their CSF. Both studies used the same detection test (RT-PCR) and the population was demographically similar, then, which is the reason of this difference is still not clear. In addition, except for one patient, children in Schwartz’s work suffered from a minor learning disability as a long-term sequelae of HHV-7 encephalitis. Besides our patients had a full and good recovery. Uniquely, patients with acute neuropsychiatric symptoms still suffered from slightly form of neurobehavioral disorder during the follow-up.
Shwartz’s study stated that, in order to prove HHV-7 infection, HHV-7 DNA detection needs to be associated to be confirmed serologically, instead we did not consider serum avidity tests (30). Interpretaion of serological assays is complicated due to an high cross-reactivity of beta-herpesvirures and CMV (36, 37). Conversely, we took into account even autoantibodies, essential to be investigated considering that a case of post-HSV NMDAR encephalitis was reported (38, 39).
Concerning CSF testing for viral DNA, it must be underlined that just a few published researches have performed it until now. Besides Schwartz’s works, Fay described a clinical case with a brain stem hemorrhagic encephalitis, which closely resembles our AHEM (30, 40, 41). Considering these two last cases, both patients were immunocompetent, healthy before the acute event and HHV-7 was the only potential etiological agent on CSF. Again, as for other neurological fields, polypharmacotherapy was implemented in both (42-46). This has been realized with steroids, plasma exchange, IVIg; the prognosis was favorable in both cases.
It seems likely that the older the patient, the more severe HHV-7 related encephalopathy will be the potential for severe disease with delayed primary infection is analogous to that seen with other herpesviruses, i.e. VZV and EBV (30). During early childhood, febrile seizures are largely prevalent, whereas neurologic events are usually more serious when the patient grows up. This clinical feature was firstly highlighted by Chan (11). The reason why delayed HHV-7 primary infection is often more aggressive is due to a more aggressive inflammatory response produced by a more mature immune system, as it happens for other exanthematous diseases (e.g. varicella, rubella, measles).
Our work is the first to show how HHV-7 can be responsible for acute, or subacute, psychotic events. Four of them were diagnosed for PANS: what is atypical and new is that the immune-mediated response which elicits the major symptoms of this syndrome, e.g. OCD and tics, is simultaneous with the viral infection and HHV-7 DNA copies detection in CSF.
According to a 2012 publication, diagnostic criteria for PANS include abrupt and dramatic onset of obsessive-compulsive disorder or severely restricted food intake (< 48h), concurrent neuropsychiatric symptoms, with severe and acute onset and exclusion of a known neurologic or medical disorder, such as Tourette syndrome (47). PANS are suspected to be postinfectious in origin, although no single microbe has been associated with the onset of typical neuropsychiatric symptoms (27). HHV-7 has never been reported before, whereas a few instances of other Herpes viruses (Herpes simplex and Varicella Zoster virus) related to PANS onset or flares have been documented (27). An autoimmune etiology has been proposed, according to Kirvan’s study: in fact, behavioral and movement disorders are believed to have antibody responses where mimicry and signal transduction are responsible for neuropsychiatric anomalies (48).
Laboratory analysis confirmed the suspected encephalopathy in all cases. The detection on HHV-7 DNA in CSF confirmed how this virus is capable of passing BBB and causing a neuro-invasion. Relating HHV-7 DNA copies on CSF and whole blood, we observed that the CSF/blood ratio is higher in more severely ill patients, similarly to what already observed in HHV6 encephalitis by our group (49).
Unfortunately, due to the fact that this is a retrospective study we could not analyze the lumbar puncture results on follow-up, therefore we do not have any data regarding the absence of HHV-7 copies concurrently with the symptoms regression.
Lastly, the detection of OcB on CSF by isoelettrofocusing exam is secondary to an intrathecal synthesis: this data is in line with the hypothesis that HHV-7 is able to replicate inside CNS and to trigger an immune response.
There is a lack of evidence about the best treatment strategies for HHV-7-related encephalopathy. In this study, all patients underwent empirical polypharmacotherapy including acyclovir and antibiotics, although no evidence is available regarding the therapeutic effect of antiviral therapy on HHV7-related CNS infection. Of note, however, we must highlight that neurological prognosis was favorable in all cases.
Conclusions
HHV-7 is capable of neuro-invasion and, once inside CNS, can replicate and trigger an immune and inflammatory response with a related encephalopathy.
Neuro-invasion has been confirmed by HHV-7 DNA copies in CSF, CSF anomalies (e.g. pleocytosis, hyperproteinorrachia), EEG pattern slow abnormalities and CNS lesions assessed by MRI.
The older the pediatric patients are, the more severe the neurologic clinical presentation HHV-7 infection will be, e.g. febrile seizures during early childhood and encephalomyelitis and ADEM/AHEM during adolescence and pre-adolescence.
Furthermore, clinical severity seems to be related to the viral replication rate inside CNS: the greater the number of DNA copies, the worse the clinical presentation is. The same was assessed taking into account CSF/WB HHV-7 DNA copies ratio: the bigger the ratio, the worse the clinical events will be.
Prognosis is favorable, even if some patients had severe presentation in the beginning. Our patients were all healthy and immunocompetent: thus, we speculate that cytokine patterns could be involved in pathogenesis. Nevertheless, we considered two pathogenetic hypothesis: a direct viral invasion from blood vessels to CNS, through BBB disruptions, during a delayed first infection; or, a viral reactivation, due to the fact HHV-7 persist, latent, inside CNS cells.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- Frenkel N, Schirmer EC, Wyatt LS, Katsafanas G, Roffman E, Danovich RM, et al. Isolation of a new herpesvirus from human CD4+ T cells. Proc Natl Acad Sci U S A. 1990;87(2):748–752. doi: 10.1073/pnas.87.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berneman ZN, Ablashi DV, Li G, Eger-Fletcher M, Reitz MS,, Jr, Hung CL, et al. Human herpesvirus 7 is a T-lym-photropic virus and is related to, but significantly different from, human herpesvirus 6 and human cytomegalovirus. Proc Natl Acad Sci U S A. 1992;89(21):10552–6. doi: 10.1073/pnas.89.21.10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneth J. Ryane. Sherris medical microbiology : an introduction to infectious diseases: Third edition. Norwalk, Conn. : Appleton & Lange. [1994] ©1994; 1994.
- Ward KN. The natural history and laboratory diagnosis of human herpesviruses-6 and -7 infections in the immunocompetent. J Clin Virol. 2005;32(3):183–193. doi: 10.1016/j.jcv.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Huang LM, Lee CY, Liu MY, Lee PI. Primary infections of human herpesvirus-7 and herpesvirus-6: a comparative, longitudinal study up to 6 years of age. Acta Paediatr. 1997;86(6):604–608. doi: 10.1111/j.1651-2227.1997.tb08942.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Kondo T, Torigoe S, Okada S, Mukai T, Yamanishi K. Human herpesvirus 7: another causal agent for roseola (exanthem subitum) J Pediatr. 1994;125(1):1–5. doi: 10.1016/s0022-3476(94)70113-x. [DOI] [PubMed] [Google Scholar]
- Torigoe S, Koide W, Yamada M, Miyashiro E, Tanaka-Taya K, Yamanishi K. Human herpesvirus 7 infection associated with central nervous system manifestations. J Pediatr. 1996;129(2):301–305. doi: 10.1016/s0022-3476(96)70259-7. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Shinnar S, Hesdorffer DC, Nordli DR, Ha-midullah A, Benn EK, et al. Human herpesvirus 6 and 7 in febrile status epilepticus: the FEBSTAT study. Epilepsia. 2012;53(9):1481–1488. doi: 10.1111/j.1528-1167.2012.03542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapenko S, Roga S, Skuja S, Rasa S, Cistjakovs M, Svirskis S, et al. Detection frequency of human herpesviruses-6A, -6B, and -7 genomic sequences in central nervous system DNA samples from post-mortem individuals with unspecified encephalopathy. J Neurovirol. 2016;22(4):488–497. doi: 10.1007/s13365-015-0417-0. [DOI] [PubMed] [Google Scholar]
- Ongradi J, Ablashi DV, Yoshikawa T, Stercz B, Ogata M. Roseolovirus-associated encephalitis in immunocompetent and immunocompromised individuals. J Neurovirol. 2017;23(1):1–19. doi: 10.1007/s13365-016-0473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PK, Chik KW, To KF, Li CK, Shing MM, Ng KC, et al. Case report: human herpesvirus 7 associated fatal encephalitis in a peripheral blood stem cell transplant recipient. J Med Virol. 2002;66(4):493–496. doi: 10.1002/jmv.2171. [DOI] [PubMed] [Google Scholar]
- Ward KN, Andrews NJ, Verity CM, Miller E, Ross EM. Human herpesviruses-6 and -7 each cause significant neurological morbidity in Britain and Ireland. Arch Dis Child. 2005;90(6):619–623. doi: 10.1136/adc.2004.062216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl-Koppe A, Blay M, Jager G, Weiss M. Human herpes virus type 7 DNA in the cerebrospinal fluid of children with central nervous system diseases. Eur J Pediatr. 2001;160(6):351–358. doi: 10.1007/s004310100732. [DOI] [PubMed] [Google Scholar]
- Venancio P, Brito MJ, Pereira G. Vieira JP Anti-N-me-thyl-D-aspartate receptor encephalitis with positive serum antithyroid antibodies, IgM antibodies against mycoplasma pneumoniae and human herpesvirus 7 PCR in the CSF. Pediatr Infect Dis J. 2014;33(8):882–883. doi: 10.1097/INF.0000000000000408. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Del Maestro M, Elbabaa SK, Galzio R. Letter to the Editor Regarding “One and Done: Multimodal Treatment of Pediatric Cerebral Arteriovenous Malformations in a Single Anesthesia Event”. World Neurosurg. 2020. pp. 134–660. [DOI] [PubMed]
- Bellantoni G, Guerrini F, Del Maestro M, Galzio R, Luzzi S. Simple schwannomatosis or an incomplete Coffin-Siris? Report of a particular case. eNeurologicalSci. 2019;14:31–3. doi: 10.1016/j.ensci.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciappetta P, D’Urso P I, Luzzi S, Ingravallo G, Cimmino A, Resta L. Cystic dilation of the ventriculus terminalis in adults. J Neurosurg Spine. 2008;8(1):92–9. doi: 10.3171/SPI-08/01/092. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Giotta Lucifero A, Brambilla I, Semeria Man-telli S, Mosconi M, Foiadelli T, et al. Targeting the medulloblastoma: a molecular-based approach. Acta Biomed. 2020;91(7-s):79–100. doi: 10.23750/abm.v91i7-S.9958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzinger F, Ebner K, Lion T. Detection and monitoring of virus infections by real-time PCR. Mol Aspects Med. 2006;27(2-3):254–298. doi: 10.1016/j.mam.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldanti F, Gatti M, Furione M, Paolucci S, Tinelli C, Comoli P, et al. Kinetics of Epstein-Barr virus DNA load in different blood compartments of pediatric recipients of T-cell-depleted HLA-haploidentical stem cell transplantation. J Clin Microbiol. 2008;46(11):3672–3677. doi: 10.1128/JCM.00913-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furione M, Rognoni V, Cabano E, Baldanti F. Kinetics of human cytomegalovirus (HCMV) DNAemia in transplanted patients expressed in international units as determined with the Abbott RealTime CMV assay and an inhouse assay. J Clin Virol. 2012;55(4):317–322. doi: 10.1016/j.jcv.2012.08.017. [DOI] [PubMed] [Google Scholar]
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- Bigi S, Fischer U, Wehrli E, Mattle HP, Boltshauser E, Bur-ki S, et al. Acute ischemic stroke in children versus young adults. Ann Neurol. 2011;70(2):245–254. doi: 10.1002/ana.22427. [DOI] [PubMed] [Google Scholar]
- Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Graus F. Antibody-Mediated Encephalitis. N Engl J Med. 2018;378(9):840–851. doi: 10.1056/NEJMra1708712. [DOI] [PubMed] [Google Scholar]
- Gastaldi M, Scaranzin S, Jarius S, Wildeman B, Zard-ini E, Mallucci G, et al. Cell-based assays for the detection of MOG antibodies: a comparative study. J Neurol. 2020;267(12):3555–3564. doi: 10.1007/s00415-020-10024-0. [DOI] [PubMed] [Google Scholar]
- Chang K, Frankovich J, Cooperstock M, Cunningham MW, Latimer ME, Murphy TK, et al. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol. 2015;25(1):3–13. doi: 10.1089/cap.2014.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Ihira M, Suzuki K, Suga S, Matsubara T, Furukawa S, et al. Invasion by human herpesvirus 6 and human herpesvirus 7 of the central nervous system in patients with neurological signs and symptoms. Arch Dis Child. 2000;83(2):170–171. doi: 10.1136/adc.83.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral I, Sainz de la Maza S, Rodriguez M, Kawiorski MM, Lopez-Martmez MJ, Galan JC. Molecular detection of human herpesvirus 7 DNA in cerebrospinal fluid from adult patients with neurological disorders. J Neurovirol. 2018;24(3):333–338. doi: 10.1007/s13365-018-0618-4. [DOI] [PubMed] [Google Scholar]
- Schwartz KL, Richardson SE, Ward KN, Donaldson C, MacGregor D, Banwell B, et al. Delayed primary HHV-7 infection and neurologic disease. Pediatrics. 2014;133(6):e1541–7. doi: 10.1542/peds.2013-3344. [DOI] [PubMed] [Google Scholar]
- Foiadelli T, Savasta S, Battistone A, Kota M, Passera C, Fiore S, et al. Nucleotide variation in Sabin type 3 poliovirus from an Albanian infant with agammaglobulinemia and vaccine associated poliomyelitis. BMC Infect Dis. 2016;16:277. doi: 10.1186/s12879-016-1587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manti S, Licari A, Montagna L, Votto M, Leonardi S, Brambilla I, et al. SARS-CoV-2 infection in pediatric population. Acta Biomed. 2020;91(11-s):e2020003. doi: 10.23750/abm.v91i11-S.10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savasta S, Rovida F, Foiadelli T, Campana AM, Percivalle E, Marseglia GL, et al. West-Nile virus encephalitis in an immunocompetent pediatric patient: successful recovery. Ital J Pediatr. 2018;44(1):140. doi: 10.1186/s13052-018-0574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini A, Corsi M, Santangelo A, Riva A, Peroni D, Foia-delli T, et al. Challenges and management of neurological and psychiatric manifestations in SARS-CoV-2 (COVID-19) patients. Neurol Sci. 2020;41(9):2353–2366. doi: 10.1007/s10072-020-04544-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- Foa-Tomasi L, Avitabile E, Ke L, Campadelli-Fiume G. Polyvalent and monoclonal antibodies identify major immunogenic proteins specific for human herpesvirus 7-in-fected cells and have weak cross-reactivity with human herpesvirus 6. J Gen Virol. 1994;75(Pt 10):2719–2727. doi: 10.1099/0022-1317-75-10-2719. [DOI] [PubMed] [Google Scholar]
- Sutherland S, Christofinis G, O’Grady J, Williams R. A serological investigation of human herpesvirus 6 infections in liver transplant recipients and the detection of cross-reacting antibodies to cytomegalovirus. J Med Virol. 1991;33(3):172–176. doi: 10.1002/jmv.1890330306. [DOI] [PubMed] [Google Scholar]
- Morris NA, Kaplan TB, Linnoila J, Cho T. HSV encephalitis-induced anti-NMDAR encephalitis in a 67-year-old woman: report of a case and review of the literature. J Neu-rovirol. 2016;22(1):33–7. doi: 10.1007/s13365-015-0364-9. [DOI] [PubMed] [Google Scholar]
- Nosadini M, Granata T, Matricardi S, Freri E, Ragona F, Papetti L, et al. Relapse risk factors in anti-N-methyl-D-aspartate receptor encephalitis. Dev Med Child Neurol. 2019;61(9):1101–1107. doi: 10.1111/dmcn.14267. [DOI] [PubMed] [Google Scholar]
- Hall CB, Long CE, Schnabel KC, Caserta MT, McIntyre KM, Costanzo MA, et al. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med. 1994;331(7):432–438. doi: 10.1056/NEJM199408183310703. [DOI] [PubMed] [Google Scholar]
- Fay AJ, Noetzel MJ, Mar SS. Pediatric Hemorrhagic Brainstem Encephalitis Associated With HHV-7 Infection. Pediatr Neurol. 2015;53(6):523–526. doi: 10.1016/j.pediatrneurol.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Crovace AM, Del Maestro M, Giotta Lucifero A, Elbabaa SK, Cinque B, et al. The cell-based approach in neurosurgery: ongoing trends and future perspectives. Heliyon. 2019;5(11):e02818. doi: 10.1016/j.heliyon.2019.e02818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giotta Lucifero A, Luzzi S, Brambilla I, Trabatti C, Mosconi M, Savasta S, et al. Innovative therapies for malignant brain tumors: the road to a tailored cure. Acta Biomed. 2020;91(7-s):5–17. doi: 10.23750/abm.v91i7-S.9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Giotta Lucifero A, Brambilla I, Magistrali M, Mosconi M, Savasta S, et al. Adoptive immunotherapies in neuro-oncology: classification, recent advances, and translational challenges. Acta Biomed. 2020;91(7-s):18–31. doi: 10.23750/abm.v91i7-S.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foiadelli T, Naso M, Licari A, Orsini A, Magistrali M, Trabatti C, et al. Advanced pharmacological therapies for neurofibromatosis type 1-related tumors. Acta Biomed. 2020;91(7-s):101–114. doi: 10.23750/abm.v91i7-S.9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giotta Lucifero A, Luzzi S, Brambilla I, Schena L, Mosconi M, Foiadelli T, et al. Potential roads for reaching the summit: an overview on target therapies for high-grade gliomas. Acta Biomed. 2020;91(7-s):61–78. doi: 10.23750/abm.v91i7-S.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur C, Bitnun A, Kronenberg S, Laxer RM, Levy DM, Logan WJ, et al. PANDAS/PANS in childhood: Controversies and evidence. Paediatr Child Health. 2019;24(2):85–91. doi: 10.1093/pch/pxy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006;179(1-2):173–179. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Berzero G, Campanini G, Vegezzi E, Paoletti M, Pichiec-chio A, Simoncelli AM, et al. Human Herpesvirus 6 Encephalitis in Immunocompetent and Immunocompromised Hosts. Neurol Neuroimmunol Neuroinflamm. 2021;8(2) doi: 10.1212/NXI.0000000000000942. [DOI] [PMC free article] [PubMed] [Google Scholar]


