Abstract
Introduction:
Treatment of cranial neurovascular pathology requires a detailed understanding of the brain, head, and neck vasculature. This study aims at a comprehensive overview of the microsurgical anatomy of the anterior cerebral circulation.
Methods:
Five formalin-fixed adult cadaveric heads were used. Common carotid arteries, vertebral arteries, and internal jugular veins were injected with colored latex (red for arteries and blue for veins). The heads were dissected under a surgical microscope with magnifications ranging between 3× to 40× focusing on the anterior circulation. A synoptic approach was used to describe in detail the segments, branches, perforating arteries, veins, and vascular territories of the cerebral arteries and veins.
Results:
The anterior arterial circulation of the brain is provided by the internal carotid artery (ICA), anterior cerebral artery (ACA), middle cerebral artery (MCA), anterior communicating artery (ACoA), and perforating arteries. Perforating arteries of the anterior circulation arise from the ICA, ACA, MCA, ACoA, and posterior communicating artery (PCoA). The distal segments and collateral branches of the ICA, ACA, and MCA give the arterial supply to the largest part of the forebrain, whereas perforating arteries of the anterior circulation are related to the striatum, thalamus, and basal ganglia. The ACoA is the core functional anastomosis between the left and right ICA systems. The external carotid artery provides the vascular supply to the region of the face, head, and neck, and most of the meninges. The internal jugular venous system is composed of the internal and external jugular veins, which constitutes the outflow of the cerebral and facial venous system, respectively.
Conclusion:
Thorough knowledge of the topographic, cisternal, and functional anatomy of the anterior circulation of the brain is critical for surgery of the supratentorial lesions. (www.actabiomedica.it)
Keywords: Anterior Cerebral Artery, Anterior Communicating Artery, External Carotid Artery, Internal Carotid Artery, Middle Cerebral Artery, Perforating Arteries
Introduction
Thorough knowledge of the vascular anatomy of the brain, head, and neck is essential for the safe and successful management of all vascular pathologies affecting the central nervous system. Among these, particularly aneurysms, arteriovenous malformations, and cranial dural arteriovenous fistulas are nowadays of interest for both neurosurgeons and interventional neuroradiologists in the context of a multidisciplinary approach (1-6). Most of these pathologies predominantly involve the supratentorial region of the brain and, accordingly, in-depth mastery of the microsurgical anatomy of the anterior circulation is the key for the correct topographic localization of the lesion on CT, MRI, and catheter-based angiography, as well as the planning of surgery. Moreover, the intraoperative identification of the different segments and branches of the internal carotid artery (ICA), anterior cerebral artery (ACA), middle cerebral artery (MCA), and anterior communicating artery (ACoA) is based upon the knowledge of specific parenchymal landmarks within the different topographic regions.
The present study consists of a detailed overview of the microneurosurgical anatomy of the anterior cerebral circulation as it relates to the management of the neurovascular pathologies affecting the intracranial supratentorial region.
Methods
Five adult cadaveric heads were used for the study. Immediately after death, both the common carotid arteries, vertebral arteries, and internal jugular veins were isolated in the neck, cannulated with latex tubes, and flushed with tap water to remove blood clots and debris. The heads were then formalin-fixed and stored for three weeks. Afterward, the specimens were injected with a 100 mL syringe containing a mixture of colored latex, thinner solution, and catalyst. Three heads were employed to study the arterial system, for which red latex was used. The remaining two heads were injected with blue latex and used for the study of the venous anatomy. The heads were stored in a plastic bag for 48 hours to achieve the hardening of the latex and then dissected under a surgical microscope (OPMI pico, Carl Zeiss, Oberkochen, Germany) with magnifications ranging between 3x to 40x depending on the type of vessel. Dissections were focused on the anterior circulation and multiple digital pictures were acquired during each step of dissection. The course of arterial and venous segments, collateral branches, and vascular territories were studied in detail, and data were summarized in synoptic tables.
Results
Internal Carotid Artery
The ICA arises in the neck from the common carotid artery at the level of Farabeuf's triangle, which is delineated by the internal jugular vein (IJV ) posteriorly, the thyrolinguofacial venous trunk anteriorly, and the hypoglossal nerve superiorly. Classically, the ICA is noted to originate at the level of the superior border of the thyroid cartilage, but in fact, the site of origin varies largely (7). The ICA ascends toward the external orifice of the carotid canal, initially superficial to the external carotid artery (ECA), then turning medially and passing posterior to the ECA. Before entering the skull base, the ICA passes through the parapharyngeal space which is divided into pre-and post-styloid compartments and bordered laterally by the posterior belly of the digastric muscle (8, 9). The cervical ICA has no branches. Inside the temporal bone, the ICA has three well-defined segments, namely, the posterior vertical, horizontal, and anterior vertical segments. The posterior genu lies between the posterior vertical and horizontal segments, whereas the anterior genu is located between the horizontal and anterior vertical segments. The posterior vertical segment gives rise to the caroticotympanic artery. The horizontal segment is cushioned by a venous plexus and the distal segment runs inferior to the third trigeminal division lying within Meckel's cave. This area corresponds to the foramen lacerum, which is formed by the union between the petrous apex, the lateral aspect of the dorsum sellae, and the sphenoid body. Above the foramen lacerum, the petrolingual ligament, lying between the petrous apex and the lingual process of the sphenoid bone, envelopes the anterior genu, fixes the ICA to the carotid sulcus on the lateral aspect of the body of the sphenoid bone and marks the caudal limit of the carotid sulcus itself. The carotid sulcus, where the ICA runs superiorly, is located within the cavernous sinus; this is where the cavernous ICA segment begins. The cavernous ICA makes a vertical posterior bend, then has a horizontal forward segment and a horizontal anterior bend, the uppermost part of which passes medial to the anterior clinoid process, and pierces the roof of the cavernous sinus (10). The so-called clinoid segment of the ICA is comprised in between the proximal and distal dural rings that define the limit of the carotid collar (11, 12). The clinoid segment is bounded medially by the carotid sulcus, anteriorly by the optic strut, and medially by the anterior clinoid process. The distal dural ring is tightly adherent to the anterior and lateral aspect of the clinoid segment, but not the medial and posterior aspect. This posteromedial area around the distal clinoid segment is known as the carotid cave; aneurysms of the carotid cave hemorrhage into the subarachnoid space (11-13). Not infrequently, the superior hypophyseal artery arises from the carotid cave. The cavernous segment gives rise to the meningohypophyseal artery, the inferolateral trunk, and the capsular arteries. Above the level of the anterior clinoid process, the supraclinoid ICA ascends superiorly, posteriorly, and laterally toward the lateral aspect of the optic chiasm, then up to the anterior perforated substance where it bifurcates into the anterior and middle cerebral arteries (Figure 1,2).
Figure 1.
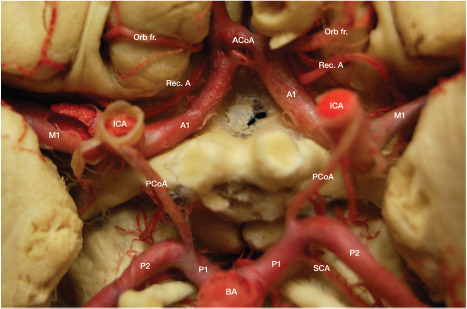
Circle of Willis.
Figure 2.
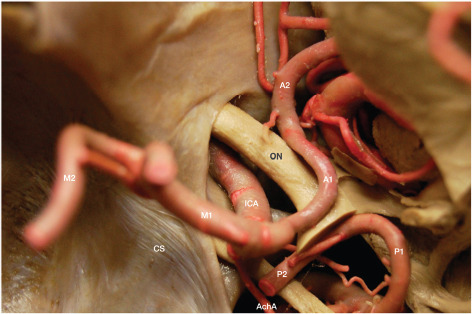
Supraclinoid segment of the internal carotid artery.
From proximal to distal, the supraclinoid ICA gives rise to the ophthalmic artery (OphA), posterior communicating artery (PCoA), and anterior choroidal artery (AChA), which mark the ophthalmic, posterior communicating, and choroidal segments of the ICA. The ophthalmic is the longest segment, whereas the posterior communicating is the shortest one (10). The OphA emerges from the ventral aspect of the supraclinoid ICA just inferior to the optic nerve and the anterior clinoid. An anterior clinoidectomy is generally necessary to expose the OphA. The PCoA and AChA arise from the back wall of the ICA, along with the superior hypophyseal perforating arteries arising specifically from the back wall of the ophthalmic segment. The largest of these arteries is referred to as the superior hypophyseal artery (10, 14). The PCoA arises from the posteromedial aspect of the back wall of the ICA; its diameter may be as large as that of the posterior cerebral artery in cases of persistent fetal PCoA. The AChA also arises from the posteromedial aspect of the ICA and courses posteriorly toward the lateral aspect of the lateral geniculate body. The first AChA segment, coursing parallel to the optic tract within the crural cistern, is known as the cisternal segment, which is delineated anterolaterally by the uncus and posteromedially by the cerebral peduncle. The lateral geniculate body marks the transition from the cisternal to the plexal segment of the AChA, which turns abruptly from medial to lateral to reach the inferior choroidal point (15-20). Along its course, the AChA gives rise to important branches to the optic tract, uncus, cerebral peduncle, temporal horn, lateral geniculate body, hippocampus, dentate gyrus and fornix, and anterior perforated substance (16, 17, 19). Within the temporal horn of the lateral ventricle, the plexal segment of the AChA widely anastomoses with the lateral posterior choroidal artery to form a dense arterial plexus supplying the choroid plexus.
Over the years, several ICA segment classifications have been proposed; some of these have considerable importance from a surgical standpoint (21-25). The extremely detailed seven-segment classification by Bouthillier and colleagues probably conforms best to the needs of most surgeons (23). Table 1 summarizes the main ICA segment classification schemes and highlights the differences between them (Table 1).
Table 1.
Internal Carotid Artery Segment Classifications
| Authors | Segment Nomenclature | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cervical | Petrous | Lacerum | Paraclival | Para sellar | Cavernous | Clinoid | Supraclinoid | |||
| Vertical Cavernous | Horizontal Cavernous | Ophthalmic | Communicating | |||||||
| Distal Anatomie Border | ||||||||||
| External orifice of the carotid canal | Foramen laceram | Petrolingual ligament | Superior edge of the petroclival fissure | Proximal durai ring | Primitive maxillary artery | Trigeminal artery | Distal durai ring | PCoA | ICA bifurcation | |
| Perlmutter et al. 1976 (21) | Cervical | Petrous | Cavernous | Supraclinoid | ||||||
| Lasjaunias et al. 1984 (22) | Cervical | Petrous | Vertical Cavernous | Horizontal Cavernous | Clinoid | Cisternal | ||||
| Boutliillier et al. 1996 (23) | CI | C2 | C3 | C4 | C5 | C6 | C7 | |||
| Ziyal et al. 2005 (24) | Cervical | Petrous | Cavernous | Clinoid | Cisternal | |||||
| Labib et al. 2014 (25) | Parapharyngeal | Petrous | Paraclival | Paraseli ar | Paraclinoid | Intradural | ||||
Table 2 describes the collateral branches arising from the different ICA segments according to the Bouthillier classification (23) (Table 2).
Table 2.
Collateral Branches of the ICA segments and Vascular Supply
| ICA segment | Collateral Branches | Vascular Supply | ||
|---|---|---|---|---|
| CI Cervical | ||||
| C2 Petrous | Caroticotympanic artery | Tympanic cavity | ||
| C3 Lacerum | Vidian artery (45% of cases) | Upper part of the pharynx and the auditory tube | ||
| C4 Cavernous | Meningohypophyseal trunk | Medial tentorial artery (Bernasconi-Cassinari) | CN III and IV, roof of cavernous sinus, medial third of tentorium, and posterior attachment of the faix cerebri | |
| Lateral tentorial artery | Lateral third of tentorium | |||
| Dorsal meningeal artery | CN VI into Dorello's canal, dura of dorsum sellae | |||
| Inferior hypophyseal artery | Pituitary gland | |||
| Medial clival artery | Dura over posterior clinoid, dorsum sellae, and medial wall of cavernous sinus | |||
| Inferolateral trunk | Superior division | CNs III and IV, roof of cavernous sinus, medial third of tentorium, and posterior attachment of the faix cerebri | ||
| Anterior division | CNs III, IV, VI and cavernous sinus dura around superior orbital fissure, V2, dura around foramen rotundum | |||
| Posterior division | VI, V3, CN VII and dura around gasserian ganglion | |||
| Capsular arteries | Dura of sellar floor | |||
| CS Clinoid | ||||
| C6 Ophthalmic | Ophthalmic artery | Central retinal artery | Inner retinal layers | |
| Lacrimal artery | Lacrimal gland, eyelids and conjunctiva | |||
| Posterior ciliary arteries | Posterior uveal tract | |||
| Muscular branches | Extraocular muscles | |||
| Supraorbital artery | Muscles and skin of the forehead | |||
| Anterior Ethmoidal artery | Anterior and middle ethmoidal sinuses; dura of the anterior cranial fossa | |||
| Posterior Ethmoidal artery | Posterior ethmoidal sinuses; dura of the anterior cranial fossa | |||
| Medial palpebral arteries | Eyelid | |||
| Superior hypophyseal arteries (n. 4 on average). The largest of the branches is often referred to as the superior hypophyseal artery (14, 19, 20) | Infundibulum of the pituitary gland, optic nerve, chiasm, floor of the third ventricle | |||
| C7 Communicating | Posterior Communicating artery | Perforating arteries (n. 4-16, inconstant) (10, 27) Premammfflary artery (anterior thalamoperforating artery) | Contribution to the posterior limb of the internal capsule | |
| Anterior Choroidal artery (15-19) | Choroid plexus of the lateral ventricle and third ventricle; optic chiasm and optic tract; posterior limb of the internal capsule; lateral geniculate body; globus pallidus; tail of the caudate nucleus; hippocampus; amygdala;substantia nigra; red nucleus; eras cerebri | |||
CN: cranial nerve; ICA: internal carotid artery
Anterior Cerebral Artery
The ACA is the smallest branch of the ICA bifurcation. Like the MCA, the ACA originates below the anterior perforated substance then courses medially to pass above the chiasm or optic nerve and below the lateral olfactory stria to reach the basal aspect of the interhemispheric fissure where it joins the ACoA. From this point, the ACA turns abruptly upward within the interhemispheric fissure until the subcallosal area where it courses inside the callosal sulcus following the profile of the rostrum, genu, body, and splenium of the corpus callosum until it joins the distal posterior cerebral artery (PCA); the ACA and PCA are widely anastomosed above the splenium of the corpus callosum. The average ACA length and diameter at the origin are 12.7 mm and 2.5 mm, respectively (10, 21). However, the two ACAs are seldom equal in length and diameter. In the case of hypoplastic ACA, the ACoA is of larger diameter to compensate. The ACA is classically divided into five segments, from A1 to A5. The ACoA marks the limit between the A1 and A2 segments. If an A1 segment is shorter than the contralateral one, an unavoidable twist of the ACoA occurs on the axial plane, this being the main reason why the ACoA is often better seen on oblique angiographic projections. Altogether, the A2 to A5 segments are also referred to as the pericallosal artery because of the intimate relationship existing between the distal ACA and the corpus callosum (Figure 3,44).
Figure 3.
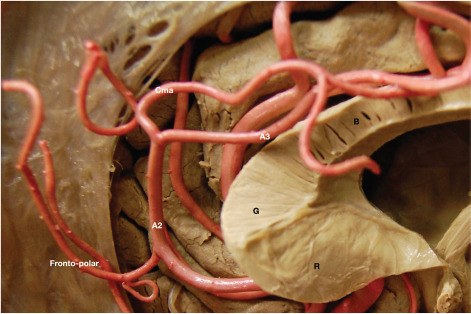
Anterior cerebral and callosomarginal arteries.
Figure 4.
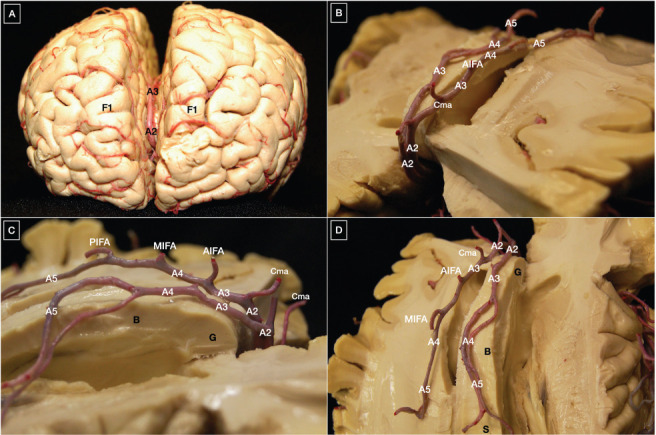
(A) Course of the anterior cerebral artery along the genu of the corpus callosum; (B-D) pericallosal artery.
Except for its posterior portion, the pericallosal artery is located below the free margin of the falx, this aspect being paramount for orientation during surgery of pericallosal artery aneurysms. Within the interhemispheric fissure, the left pericallosal artery courses above the right one in most cases (26). During its complex course, the ACA gives rise to a series of medial lenticulostriate arteries, the most medial one of which is also known as the recurrent artery of Heubner (median striatal artery) (21, 27), as well as eight cortical branches through which, apart from the caudate nucleus and the anterior limb of the internal capsule, the ACA vascularizes the medial part of the orbital gyri, gyrus rectus, olfactory bulb and tract, superior frontal gyrus, and the superior parts of the precentral, central, and postcentral gyri (10, 21, 26). The strip of lateral cortex vascularized by the ACA is wider anteriorly and narrower progressively posteriorly. Not infrequently, the ACA ends in the callosomarginal artery, the largest branch from the A3 segment running inside the cingulate sulcus, which is related to the cingulate gyrus (26). This pattern is responsible for the angiographic "bullnose" appearance of the ACA (28). Table 3 presents a detailed description of the ACA segments, along with their limits, collateral branches, and vascular supply (Table 3).
Table 3.
Segments, Collateral and Terminal Branches, and Vascular Supply of the Anterior Cerebral Artery
| Anterior Cerebral artery segment | Distal Anatomical Border | Collateral and Terminal Branches | Vascular Supply | |||
|---|---|---|---|---|---|---|
| Proximal (precommunicating) (10) | Al (precommunicating/ horizontal) | Anterior Communicating artery | Medial lenticulostriate arteries (n. 8 on average (27)) | Caudate nucleus; anterior limb of the internal capsule | ||
| Distal (postcommunicating) (10) | A2 (infracallosal) | Callosomarginal artery | Recurrent artery of Heubner* (21, 27) | Internal capsule | ||
| Orbitofrontal artery | Inferomedial surface of the frontal lobe; gyrus rectus | |||||
| Frontopolar artery | Frontal pole | |||||
| A3 (pericallosal) | Paracentral artery | Calloso marginal artery | Medial frontal arteries MIFA PIFA | AI FA | Anterior third of the mesial aspect of the superior frontal gyrus | |
| Middle third of the mesial aspect of the superior frontal gyrus | ||||||
| Posterior third of the mesial aspect of the superior frontal gyrus | ||||||
| A4 (pericallosal) | Inferior parietal artery | Paracentral artery | Cingulate branches | Paracentral lobule | ||
| A5 (postcallosal) | Anastomosis with posterior cerebral artery branches | Superior parietal artery | Superior half of the precuneus | |||
| Inferior parietal artery | Superior half of the precuneus | |||||
Middle Cerebral Artery
Like the ACA, the MCA arises from the ICA below the anterior perforated substance and courses laterally behind the sphenoid ridge to reach the limen insula where it turns abruptly upward making a genu that is very well defined on an anterior-posterior angiographic view (Figure 5).
Figure 5.
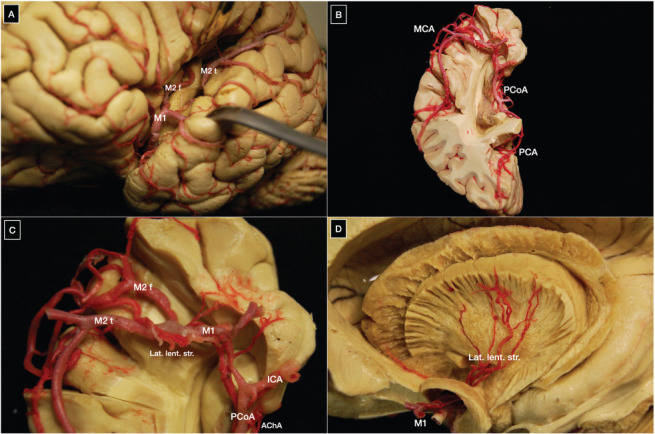
(A-C) Origin and course of the middle cerebral artery within the Sylvian fissure; (D) Lateral lenticulostriate perforating arteries arising from the Ml segment of the middle cerebral artery supply the internal capsule and basal ganglia. A1, A2, A3, A4, and A5: segments of the anterior cerebral artery; ACoA: anterior communicating artery; AIFA: anterior inferior frontal artery; Ant. perf.: anterior perforated substance; R, G, B, S: rostrum, genu, body and splenium of the corpus callosum; BA: basilar artery; Cma: callosomarginal artery; Dist. ACA: distal anterior cerebral artery; F1: superior frontal gyrus; Fronto-polar: fronto-polar artery; ICA: internal carotid artery; MIFA: middle inferior frontal artery; Orb fr: orbitofrontal artery; P1, P2: segments of the posterior cerebral artery; PCoA: posterior communicating artery; PIFA: posterior inferior frontal artery; Rec. A: recurrent artery of Heubner; MCA: middle cerebral artery; M2 f: M2 frontal trunk; M2 t: M2 temporal trunk; PCA: posterior cerebral artery; SCA: superior cerebellar artery.
The MCA has a bifurcation (78%), trifurcation (12%), or rarely, a quadrifurcation (10%) point located proximal to the genu of the M1 segment in more than 90% of cases (3, 22). Bifurcation further divides the M1 segment into pre-bifurcation and post-bifurcation segments, the latter of which has a superior and inferior trunk. Arteries arising from the pre-bifurcation segment other than perforating arteries are generally cortical and called early branches. Regarding the size of the post-bifurcation segment trunks, equal size, inferior trunk dominant, and superior trunk dominant have been reported in 18%, 32%, and 28% of hemispheres, respectively (3, 22). Nearly half of MCAs send early branches directed to the temporal lobe in more than 90% of cases (22). Uncal and temporopolar arteries, and the anterior temporal artery, which is an important landmark for orientation during surgery of MCA aneurysms, are examples of early cortical branches (23). Ascending along the insular cortex, the MCA trunks reach the circular sulcus of the insula and the operculum of the frontal, temporal, and parietal lobes before supplying a wide cortical area consisting of most of the lateral surface and some of the basal surface of the hemisphere. Classically, the cortex supplied by the MCA is divided into 12 areas: orbitofrontal, prefrontal, precentral, central, anterior parietal, posterior parietal, angular, temporo-occipital, posterior temporal, middle temporal, anterior temporal, and temporopolar (3, 22). This scheme follows a clockwise order around the Sylvian fissure starting at 8 o'clock. Each cortical area is generally supplied by a single stem artery. The posterior temporal stem artery supplying the angular gyrus is called the angular artery; this particular artery has a diameter of 1.4 mm on average, the critical size required for long-term bypass patency, and is widely used as the recipient vessel in most cerebral vascular bypasses to the MCA (24). Table 3 describes the segments of the MCA as well as their limits, collateral branches, and vascular supply (Table 4).
Table 4.
Segments, Collateral and Terminal Branches, and Vascular Supply of the Middle Cerebral Artery
| Middle Cerebral artery segment | Distal Anatomical Border | Collateral and Terminal Branches | Vascular Supply | ||
|---|---|---|---|---|---|
| Ml (sphenoidal) * | Limen insula | Superior surface Insular arteries (n. 1-6 in 55% (31)) | Lateral lenticulostriate arteries (n. 10 on average (27, 30)) | Caudate, putamen, globus pallidus, superior half of the internal capsule, and corona radiata | |
| Limen insulae | |||||
| Inferior surface Temporopolar arteries Anterior temporal artery | Uncal arteries | Uncal region | |||
| Polar and anterolateral portions of the temporal lobe | |||||
| Anterior third of the superior, middle, and inferior temporal gyri | |||||
| M2 (insular) * | Circular sulcus of the insula | Superior and inferior trunk** | Superior branches | Insular arteries (n. >10 90% (31)) | Insular cortex, extreme capsule |
| Anterior branches | Orbitofrontal artery (Origin from Ml is rare. In most cases, it arises from the A2 segment of the anterior cerebral artery) | Inferomedial surface of the frontal lobe, gyrus rectus | |||
| Operculofrontal artery | Frontal opercular area | ||||
| Central sulcus artery | Superior part of the precentrai gyrus and the inferior one-half of the postcentral gyrus | ||||
| Posterior branches | Posterior parietal artery | Posterior part of the superior and inferior parietal lobules; supramarginal gyrus | |||
| Angular artery | Angular gyrus, supramarginal gyrus, posterior superior temporal gyrus, parietooccipital sulcus | ||||
| Posterior temporal artery | Middle and posterior third of the superior, middle, and inferior temporal gyri | ||||
| M3 (opercular) | Cortical surface of the sylvian fissure | Insular arteries (n. 1-2 in 25% (31)) | Superior or inferior periinsular sulcus | ||
| M4 (cortical) | Terminal territories on the lateral convexity | Cortical branches | MCA territory: 12 areas, namely, orbitofrontal, prefrontal, precentrai, central, anterior parietal, posterior parietal, angular, temporo-occipital, posterior temporal, middle temporal, anterior temporal, and temporopolar (10, 30) | ||
*The middle cerebral artery bifurcation into superior and inferior trunks may located be at Ml, M2, or the M1-M2 junction. A trifurcation, with superior, middle, and inferior trunks, is present in approximately 30% of cases, whereas a quadrifurcation is rare; ** Superior and inferior trunks are seldom symmetrical and equal in size; MCA: middle cerebral artery.
Anterior Communicating Artery
The ACoA establishes the vascular connection between the left and right ICA systems in the midline; crossing blood flow is perfectly balanced in cases of equally sized A1 segments. On par with the ICAs, PCoAs, and A1 segments of the ACA, the ACoA contributes to forming the anterior part of the Circle of Willis (Figure 1). Mean ACoA diameter and length are 1.5 mm and 4 mm, respectively (21). Larger ACoAs are generally associated with significant diameter differences between left and right A1s. The ACoA gives rise to three important groups of perforating arteries: the subcallosal, hypothalamic, and chiasmatic (29). Within the anterior circulation, the ACoA is among the sites having a high frequency of anatomic variation. Serizawa and colleagues reported the main types and relative frequency of these variations as follows: plexiform (33%), dimple (33%), fenestration (21%), duplication (18%), string (18%), fusion (12%), median artery of the corpus callosum (6%), and azygous anterior cerebral artery (3%) (29). Despite its small average length and diameter, the ACoA has considerable importance from a functional standpoint in pathologic conditions because it allows blood flow to the contralateral hemisphere in cases of ICA occlusion. The balloon test occlusion routinely performed in clinical practice for ICA aneurysms and various occlusive ICA pathologies is aimed to assess the functional role of the ACoA, which may vary case-by-case. The origin of the ACoA is a common site of aneurysms.
Perforating Arteries
Perforating arteries of the anterior circulation supply the striatum, thalamus, and basal ganglia. They also serve as feeders in deep-seated arteriovenous malformations. During clipping or endovascular embolization of aneurysms, preservation of the perforating arteries is fundamental. Perforating arteries related to the anterior circulation arise from the ICA, PCoA, ACA, MCA, and ACoA. The lateral lenticulostriate arteries arise in all cases from the entire pre-bifurcation M1 segment of the MCA and also from the post-bifurcation segment in half of the cases (10, 30). In more than 70% of cases, the lenticulostriate arteries are located less than 5 mm from the bifurcation. The M2 segment rarely has perforating arteries (27, 31) (Figure 5).
The earlier the bifurcation, the greater the number of post-bifurcation branches, and vice versa. No branches arise from the post-bifurcation segment if the bifurcation point is located more than 2.5 cm from the origin of the MCA (10, 30, 31). This data is important for the assessment of the risk of perforating artery occlusion during surgery for MCA bifurcation aneurysms. Table 5 summarizes the main groups and the vascular supply of the perforating arteries of the anterior circulation, according to their origin from the parent artery (Table 5).
Table 5.
Perforating Arteries of the Anterior Circulation
| Parent Vessel | Perforating Arteries | Vascular Supply | ||
|---|---|---|---|---|
| ICA | С 6 Ophthalmic | Superior hypophyseal arteries (n. 4 an average). The largest of the branches is often referred to as the superior hypophyseal artery (14,19,20) | Infundibulum of the pituitary gland, optic nerve, chiasm, floor of the third ventricle | |
| C7 Communicating | PCoA | Perforating arteries (n. 4-16, inconstant) (10) Premammillary artery (anterior thalamoperforating artery) | Contribution to the posterior limb of the internal capsule | |
| Choroidal branches (n. 4, range 1-9) (10, 27) (from the choroidal C4 segment according to the Perlmutter classification (21)) | Anterior perforated substance | |||
| AChA (n. 9 branches on average) (15-19) | Posterior limb of the internal capsule; lateral geniculate body; globus pallidus; tail of the caudate nucleus; hippocampus; amygdala; substantia nigra; red nucleus; crus cerebri | |||
| ACA | Al (precommunicating or horizontal) | Medial lenticulostriate arteries (n. 8 on average (27)) | Caudate nucleus; anterior limb of the internal capsule | |
| A2 (infracallosal) | Recurrent artery of Heubner* (21, 27) | Internal capsule | ||
| MCA | Ml (sphenoidal) | Lateral lenticulostriate arteries (n. 10 on average (27, 30)) | Caudate, putamen, globus pallidus, superior half of the internal capsule, and corona radiata | |
| ACoA | Subcallosal (29) | Subcallosal area, septal nuclei | ||
| Hypothalamic (29) | Hypothalamus | |||
| Chiasmatic (29) | Optic chiasm | |||
ICA: internal carotid artery; АСА: anterior cerebral artery; MCA: middle cerebral artery; ACoA: anterior communicating artery; АСҺА: anterior choroidal artery.
External Carotid Artery
The ECA is a branch of the common carotid artery. It provides the vascular supply for much of the face, head, and neck and most of the meninges (32-36). It has a role in several vascular pathological conditions, including arteriovenous fistulas and arteriovenous malformations. Most cerebral vascular bypasses are extracranial to intracranial and involve branches from the ECA. Table 6 reports the main branches of the ECA and their vascular supply (Table 6).
Table 6.
Collateral and Terminal Branches, and Vascular Supply of the External Carotid Artery
| Collateral and Terminal Branches | Vascular Supply | ||
|---|---|---|---|
| Superior thyroid artery | Infrahyoid branch | Muscles attached onto the hyoid bone | |
| Sternocleidomastoid branch | Sternocleidomastoid muscle | ||
| Superior laryngeal artery | Muscles, mucous membrane, and glands of the larynx | ||
| Cricothyroid artery | Larynx | ||
| Ascending pharyngeal artery (32-35) | Pharyngeal trunk | Superior branches | Pharyngeal submucosal spaces |
| Middle branches | |||
| Inferior pharyngeal branches | |||
| Neuromeningeal trunk | Hypoglossal branch | Meninges of the posterior fossa; CN XII | |
| Jugular branch | Meninges of the posterior fossa and jugular foramen; CN IX, X, and XI | ||
| Inferior tympanic branch | Caroticotympanic branch of the internal carotid artery; CN XI | ||
| Musculospinal artery | CN XI; superior sympathetic ganglion | ||
| Lingual artery | Deep lingual artery | Genioglossus muscle | |
| Sublingual artery | |||
| Facial artery | Cervical | Ascending palatine artery | Mimic muscles |
| Tonsillar branch | |||
| Submental artery | |||
| Glandular branches | |||
| Facial | Inferior labial artery | ||
| Superior labial artery | |||
| Lateral nasal branch | |||
| Angular artery | |||
| Occipital artery (35, 36) | Muscular branches | Digastric, stylohyoid, splenius, and longus capitis muscles | |
| Sternocleidomastoid branch | Sternocleidomastoid muscles | ||
| Auricular branch | Skin of the back of the ear; mastoid air cells | ||
| Meningeal branch | Meninges of the posterior fossa | ||
| Descending branches | Trapezius muscle | ||
| Posterior auricular artery (35) | Stylomastoid artery | Tympanic cavity, the tympanic antrum and mastoid cells, and the semicircular canals; CN VII | |
| Maxillary artery* | Mandibular segment | Deep auricular artery | Tympanic membrane |
| Anterior tympanic artery | Middle ear | ||
| Middle meningeal artery | Meninges of the middle fossa | ||
| Inferior alveolar artery | Pulp of the teeth; chin; mylohyoid muscle | ||
| Accessory meningeal artery | Meninges of the middle fossa | ||
| Pterygoid segment | Masseteric artery | Masseter muscle | |
| Pterygoid branches | Lateral and medial pterygoid muscle | ||
| Deep temporal arteries | Temporalis muscle; periosteum of the temporal fossa | ||
| Buccal artery | Cheek; buccinator muscle | ||
| Pterygomaxillary segment | Sphenopalatine artery | Mucosa of the nasal cavity | |
| Descending palatine artery | Mucosa of hard and soft palate | ||
| Infraorbital artery | Inferior rectus and inferior oblique muscles; Mucosa of the maxillary sinus | ||
| Posterior superior alveolar artery | Molar and premolar teeth; mucosa of the alveolar process of the maxilla | ||
| Artery of the pterygoid canal | Upper part of the pharynx; auditory tube | ||
| Pharyngeal branch | Middle and lower pharynx | ||
| Alveolar arteries | Mucosa of the alveolar process of the maxilla | ||
| Superficial temporal artery* | Frontal branch | Pericranium, skin and muscles of the forehead | |
| Parietal branch | Superficial layer of the temporalis fascia; skin of the parietal region | ||
CN: cran ial nerve; *Maxillary and superficial temporal are considered terminal branches of the external carotid artery.
Veins
The complex venous system of the head and neck is a tributary of the internal and external jugular veins (37). The IJV system involves the cerebral and facial systems; the former is further composed of superficial and deep venous systems that drain into the dural sinuses. Table 7 describes the general arrangement of the head and neck venous system (Table 7).
Table 7.
General Arrangement of the Head and Neck Venous System
| External Jugular Vein System | ||||||||||||||||||||||||
| Superficial temporal (anterior auricular) | Retromandibular | External jugular vein | ||||||||||||||||||||||
| Maxillary (pterygoid plexus) | ||||||||||||||||||||||||
| Posterior auricular | ||||||||||||||||||||||||
| Transverse cervical | ||||||||||||||||||||||||
| Suprascapular | ||||||||||||||||||||||||
| Anterior jugular | ||||||||||||||||||||||||
| Internal Jugular Vein System | ||||||||||||||||||||||||
| Cerebral system | Dural sinuses | Superior ophthalmic vein | Cavernous sinus (Anterior and posterior cavernous sinuses) | Superior petrosal sinus | Basilar plexus | Breschet's veins (Extradural neural axis compartment) (37) | ||||||||||||||||||
| Inferior ophthalmic vein | ||||||||||||||||||||||||
| Superficial Venous system | Superficial Middle cerebral vein (Superficial Sylvian vein) | Sphenoparietal sinus | Inferior petrosal sinus | |||||||||||||||||||||
| Deep middle cerebral vein (deep Sylvian vein) | ||||||||||||||||||||||||
| Superior cerebral veins | Superior sagittal sinus | Torcular Herophili | Transverse sinus | Sigmoid sinus | Jugular bulb | Internal jugular vein | ||||||||||||||||||
| Middle cerebral veins | ||||||||||||||||||||||||
| Trolard vein (superior anastomotic) | ||||||||||||||||||||||||
| Labbé vein (inferior anastomotic) | ||||||||||||||||||||||||
| Inferior cerebral veins | ||||||||||||||||||||||||
| Deep Venous system | Superior thalamostriate vein | Internal cerebral vein | Vein of Galen | Straight sinus | ||||||||||||||||||||
| Septal vein | ||||||||||||||||||||||||
| Basal vein of Rosenthal | ||||||||||||||||||||||||
| Inferior sagittal sinus | ||||||||||||||||||||||||
| Occipital sinus | Marginal sinus | |||||||||||||||||||||||
| External Jugular Vein System | ||||||||||||||||||||||||
| Facial system | Deep Facial vein | Facial vein | Internal jugular vein | |||||||||||||||||||||
| Inferior Labial vein | ||||||||||||||||||||||||
| Superior Labial vein | ||||||||||||||||||||||||
| Angular vein | ||||||||||||||||||||||||
| Supraorbital vein | ||||||||||||||||||||||||
| Frontal vein | ||||||||||||||||||||||||
| Middle thyroid vein | ||||||||||||||||||||||||
| Superior thyroid vein | ||||||||||||||||||||||||
| Pharyngeal veins | ||||||||||||||||||||||||
| Lingual veins | ||||||||||||||||||||||||
Discussion
The present anatomic study focused on the normal vascular anatomy of the anterior cerebral circulation, the mastery of which is pivotal in dealing with neurovascular pathology affecting the intracranial supratentorial compartment.
From the surgical standpoint, the in-depth understanding of the anterior circulation involves two main aspects, namely the course of the ICA, ACA, ACoA, MCA, and their branches within the supratentorial subarachnoid cisterns, and the knowledge of the possible anatomic variations of the anterior portion of the Circle of Willis.
Anatomic Variations
The physiologic patterning of the vasculature of the developing brain and spinal cord has been reported to be affected mainly by motor pathways and motor neurons (38-43). The vasculogenesis underling to the anatomic variations of the intracranial vessels has been assumed to be attributable to the same molecules affecting the neoangiogenesis of brain tumors and traumatic brain and spinal cord injuries, namely the Vascular Endothelial Growth Factor (VEGF) and semaphorin 3A (Sema3A) (44-47). Several deviations from the normal arterial blood supply of the brain have been reported. Segmental agenesis of the ICA may potentially affect each of its embryological segments, specifically the cervical, petrous, vertical cavernous, horizontal cavernous, clinoid, and cisternal one (22). Lasjaunias and Santoyo-Vazquez reported that, in the case of segmental agenesis, the ICA blood flow is typically rerouted toward the distal agenetic segment (22). Aberrant ICA originates from the union of the inferior tympanic branch of the ascending pharyngeal artery with the caroticotympanic artery as a consequence of the agenesis of the first cervical segment (22, 48-50). Aberrant ICA is more usual on the right side and in women (90%) (51). The persistent stapedial artery is a very rare but possible finding (0.02–0.01%) (52), as well as the complete agenesis (congenital absence) of the ICA (53-56). The evidence of a narrowed or absent carotid canal at the skull base is paramount in the differential diagnosis between congenital and acquired, pathological, absence of ICA as in tumors, dissection, or fibromuscular dysplasia (55). Hypoplasia of the ICA has an incidence of 0.079% (54, 55). Kinking and looping of the cervical ICA have been reported in 15% of angiograms (54). Further possible variants include duplication, fenestration, high or low branching (from Th2 to C1 level) from the common carotid artery, origin directly from the aorta, and persistent primitive olfactory artery and dorsal ophthalmic artery (54, 57-61). Some of the variations related to the ICA may selectively affect its intracavernous branches (62). Because of the hemodynamic imbalance or genetic landscape associated with the anomaly, anatomic variants of the ICA are associated with aneurysms in 24-34% of cases (63). Anatomic variations of the A1 segment of the ACA comprehend asymmetry (80%), aplasia (10%), hypoplasia (2%), fenestration (0.058%4%) (64-66), and infraoptic course, this last extremely rare embryological variant consisting of an ipsilateral or bilateral carotid-anterior cerebral artery anastomosis (67-71). The asymmetry of the A1 segment deriving from the hypoplasia of one side reduces the chance for arterial cross-flow from the contralateral side in the case of ICA, ACA, of MCA occlusion, and also affects the side selection for surgery of ACoA aneurysms (72, 73). Hypoplasia of A1 has a role as a risk factor in the formation of ACoA aneurysms (74), and may even affect their morphology (75). Variations of the A2 segment involve the existence of a common trunk (azy-gos-ACA) (0.3–2%) (76-78), accessory/triplicated A2ACA (2–13%) (64, 65), fenestration (79), and bihemispheric ACA where one A2 gives branches to both hemispheres (57, 64, 77). Azygos-ACA is frequently associated with saccular and non-saccular aneurysms (76, 80). Hypoplasia/aplasia of the ACoA has been reported to have an incidence of 5% (64), whereas the incidence of ACoA fenestration is larger by far (40%) (64, 79, 81). Loukas and colleagues reported that the recurrent artery of Heubner origin at the level of confluence of ACA and ACoA in 62.3%, and from A1 or proximal A2 segment in 14.3% and 23.3% of cases, respectively (82). The so-called ACoA complex, formed by distal A1 segment, ACoA, and proximal A2 segment, may be rotated on an axial plane (twisted ACoA complex) or also vertical plane (tilted ACoA complex), this aspect having great relevance in ACoA aneurysms surgery (83-86). From the phylogenetical standpoint, ACA is the continuation of the ICA, while MCA, which develops later than ACoA, is considered a side-branch of the ICA (87). Anatomic variations of the MCA include differences in the course of the M1 segment in the horizontal and vertical plane, branching variations, variations of the MCA division, doubled MCA, accessory MCA, and fenestrations (57, 88-91). Yasargil reported that in the horizontal plane the M1 segment may be straight diagonal, temporal convex, orbital convex, and S-shaped, whereas on vertical one it may course straight diagonal (45%), posterior (10%), or anterior (40%). The M1 segment can also rarely make a double anterior loupe (5%) (88, 89). About the branching pattern, the most frequent findings are the origin of a single common trunk from M1 consisting of temporal arteries (temporal early bifurcation, 10%), orbital and frontal arteries (frontal early bifurcation 18%), or both (early pseudobifurcation 2%) (88, 89). Accessory MCA has an incidence of 0.5%, and early pseudobifurcation and accessory MCA may be rarely encountered (0.1%) (88, 89). Variations of MCA divisions entail no bifurcation (2%), equal bifurcation superior and inferior trunks (50%), early divisions of superior and inferior trunks (pseudotetrabifurcation 8%), and origin of the middle trunk from the proximal temporal trunk (10%), frontal trunk (15%), or distal temporal trunk (15%) (88, 89). Duplicated MCAs have been reported to arise from the distal ICA above the origin of the AChA and before theMCA. Conversely, accessory MCA takes off from the A1 segment of the MCA and its course is parallel to the MCA (90, 91). Altogether these variations have an incidence of about 3% in post-mortem examinations (92). Ukino et al. found only 6 fenestrated MCAs out of 2000 MRIs (0.3%) (93). It should be highlighted that, differently from the embryological remnants of the central nervous system, which are of ectodermal derivation, those involving the neurovascular system imply aberrant differentiation of the mesoderm(87, 94, 95).
Cisternal Anatomy of the Anterior Circulation
Inoue and colleagues identified and accurately described 9 cisterns and 11 inner arachnoid membranes within the supratentorial space, thus completing the systematic classification reported by Yasargil (96-98). The supratentorial cisterns comprehend the 1) Sylvian, 2) carotid, 3) chiasmatic, 4) lamina terminalis, 5) pericallosal, 6) olfactory, 7) crural, 8) ambient, and 9) interpeduncular cistern. The interpeduncular cistern is considered as a "transitional" cistern between the supra-and infratentorial spaces. For practical purposes, we prefer to discuss the microsurgical anatomy of the interpeduncular cistern and the Liliequist's membrane in the chapter about the infratentorial compartment. The inner arachnoid membranes are the 1) proximal sylvian, 2) medial carotid, 3) lateral carotid, 4) lateral and 5) medial lamina terminalis, 6) olfactory, 7) intracrural, 8) lateral, 9) intermediate, and 10) medial intrasylvian (96, 99). The proximal Sylvian membrane attaches to the lateral orbital gyrus and the uncus and separates the Sylvian and carotid cisterns. The carotid and chiasmatic cisterns are separated by the medial carotid membrane, which attaches to the superior aspect of the oculomotor nerve and basically corresponds to the arachnoid web of the opticocarotid triangle (36-42). The lateral carotid membrane extends from the optic to the oculomotor nerve and forms the lateral boundary of the carotid cistern (96, 99). Lateral and medial lamina terminalis membranes are attached on the lateral and medial parts of the gyrus rectus, respectively, and the optic chiasm. The lateral lamina terminalis membrane separates the lamina terminalis and the carotid cisterns, whereas the medial lamina terminalis membrane separates the lamina terminalis and the pericallosal cisterns. The olfactory membrane joins the posterior orbital gyri posteriorly and the gyrus rectus anteriorly forming the posteroinferior margin of the olfactory cistern. Intracrural membrane is comprised between the uncus to the cerebral peduncle and divides the crural cistern into superior and inferior compartments. The AChA and PCA courses above and below the intracrural membrane, respectively. Lateral, intermediate, and medial intrasylvian membranes divide the Sylvian cisterns attaching to the MCA at various levels. The Sylvian cistern, delimited by the opercular part of the frontal, temporal, and parietal lobe, has three distinct segments, namely the proximal (pre-insular), middle (insular), and posterior (retro-insular) one (89). The pre-insular one is the deepest segment. It consists of the Sylvian vallecula, between the ICA bifurcation and the limen insula. The Sylvian vallecula is the segment where the M1 segment of the MCA, lateral lenticulostriate arteries, and deep Sylvian vein run. Yasargil reported that the length and width of the vallecula range between 30 and 50 mm, and 5-6 mm, respectively (89). The lateral most aspect of the Sylvian vallecula, which is related to the limen insula, marks the limits between the M1 and M2 segments of the MCA (10, 89). The chiasmatic arteries of the ACoA originate within the lamina terminal cistern and provide for the arterial supply of the upper part of the chiasm in the chiasmatic cistern. The A2 segment of the ACA and subcallosal perforating arteries of the ACoA lie within the subcallosal cistern (100). The cavernous ICA has no relationship with the subarachnoid space. Conversely, it is a potential site of spontaneous and traumatic carotid-cavernous fistulas (101-104). The clinoid, ophthalmic, and communicating segment of the ICA, the superior hypophyseal arteries, and the perforating arteries from the back wall of the ICA course within the carotid cistern. PCoA and AChA run in the lateral upper part of the interpeduncular cistern and crural cistern, respectively. ICA bifurcation, perforatings from the ICA terminus, and anterior perforated substance lie in the olfactory cistern. The A1 segment of the ACA, ACoA, recurrent artery of Heubner, and medial lenticulostriate arteries are related to the lamina terminalis cistern, while the M1 segment of the MCA and lateral lenticulostriates to the and Sylvian cistern.
Cisternal Approach to the Supratentorial Region
The anterior circulation of the brain is related to the subarachnoid cisterns of the supratentorial region and the cisternal approach to the supratentorial lesions consists of a compartmental opening of one or more of these cisterns (88, 97, 98, 100, 105-107). Any lesion may grow epiarachnoidally or also within one or more cisterns but, in both cases, specific cisternal corridors are necessary to achieve the lesion. In several situations, it is necessary to go through more than a single cistern to reach the target (97, 98, 108, 109). During aneurysm surgery, the compartmental approach to the supratentorial subarachnoid cisterns allows to expose the different segments of the anterior portion of the Circle of Willis and, through some of these subarachnoid corridors, even part of the posterior circulation (88, 107). The pterional approach permits the access to all of the supratentorial cisterns, being considered for this reason the workhorse of anterior and middle skull base surgery. The cisternal approach is required also for those aneurysms of the more proximal segments of the ICA, as the clinoid one (110).
In the last few years, the results of the initial experience about the use of the endoscope in intracranial aneurysms surgery have been reported (111, 112). However, the practicability of this approach for aneurysms is still distant from that of skull base lesions (113, 114).
Conclusion
The internal and external carotid arteries provide arterial vascularization of the anterior circulation of the head and neck. They are widely anastomosed at different sites. The ACoA is the main physiologic functional anastomosis between the left and right ICA systems.
The anterior cerebral circulation involves a large number of perforating arteries arising from the ICA, PCoA, AChA, ACA, ACoA, and MCA that provide vascular supply to the striatum, thalamus, and basal ganglia.
The ECA supplies the soft tissues of the face, head, and neck, and also the meninges.
The venous outflow of the head is primarily a tributary of the IJV and is composed of superficial and deep venous systems, both draining into the dural sinuses.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Luzzi S, Del Maestro M, Galzio R. Letter to the Editor. Preoperative embolization of brain arteriovenous malformations. J Neurosurg. 2019:1–2. doi: 10.3171/2019.6.JNS191541. [DOI] [PubMed] [Google Scholar]
- Ricci A, Di Vitantonio H, De Paulis D, Del Maestro M, Raysi SD, Murrone D, et al. Cortical aneurysms of the middle cerebral artery: A review of the literature. Surg Neurol Int. 2017;8:117. doi: 10.4103/sni.sni_50_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Del Maestro M, Elbabaa SK, Galzio R. Letter to the Editor Regarding “One and Done: Multimodal Treatment of Pediatric Cerebral Arteriovenous Malformations in a Single Anesthesia Event”. World Neurosurg. 2020;134:660. doi: 10.1016/j.wneu.2019.09.166. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Gragnaniello C, Giotta Lucifero A, Del Maestro M, Galzio R. Surgical Management of Giant Intracranial Aneurysms: Overall Results of a Large Series. World Neu-rosurg. 2020;144:e119–e37. doi: 10.1016/j.wneu.2020.08.004. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Gragnaniello C, Giotta Lucifero A, Del Maestro M, Galzio R. Microneurosurgical management of giant intracranial aneurysms: Datasets of a twenty-year experience. Data Brief. 2020;33:106537. doi: 10.1016/j.dib.2020.106537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maestro M, Rampini AD, Mauramati S, Giotta Lucifero A, Bertino G, Occhini A, et al. Dye-Perfused Human Placenta for Vascular Microneurosurgery Training: Preparation Protocol and Validation Testing. World Neurosurg. 2020 doi: 10.1016/j.wneu.2020.11.034. [DOI] [PubMed] [Google Scholar]
- Al-Rafiah A. AA EL-H, Aal IH, Zaki AI. Anatomical study of the carotid bifurcation and origin variations of the ascending pharyngeal and superior thyroid arteries. Folia Morphol (Warsz). 2011;70(1):47–55. [PubMed] [Google Scholar]
- Luzzi S, Giotta Lucifero A, Del Maestro M, Marfia G, Navone SE, Baldoncini M, et al. Anterolateral Approach for Retrostyloid Superior Parapharyngeal Space Schwannomas Involving the Jugular Foramen Area: A 20-Year Experience. World Neurosurg. 2019 doi: 10.1016/j.wneu.2019.09.006. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Gragnaniello C, Giotta Lucifero A, Marasco S, Elsawaf Y, Del Maestro M, et al. Anterolateral approach for subaxial vertebral artery decompression in the treatment of rotational occlusion syndrome: results of a personal series and technical note. Neurol Res. 2021;43(2):110–125. doi: 10.1080/01616412.2020.1831303. [DOI] [PubMed] [Google Scholar]
- Rhoton AL., Jr. The supratentorial arteries. Neurosurgery. 2002;51(4 Suppl S53):120. [PubMed] [Google Scholar]
- Rhoton AL., Jr. The cavernous sinus, the cavernous venous plexus, and the carotid collar. Neurosurgery. 2002;51(4 Suppl):S375–410. [PubMed] [Google Scholar]
- Seoane E, Rhoton AL,, Jr, de Oliveira E. Microsurgical anatomy of the dural collar (carotid collar) and rings around the clinoid segment of the internal carotid artery. Neurosurgery. 1998;42(4):869–884. doi: 10.1097/00006123-199804000-00108. discussion 84-6. [DOI] [PubMed] [Google Scholar]
- Joo W, Funaki T, Yoshioka F, Rhoton AL. Jr. Microsurgi-cal anatomy of the carotid cave. Neurosurgery. 2012;70(2 Suppl Operative):300–311. doi: 10.1227/NEU.0b013e3182431767. discussion 11-2. [DOI] [PubMed] [Google Scholar]
- Gibo H, Lenkey C, Rhoton AL. Jr. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981;55(4):560–574. doi: 10.3171/jns.1981.55.4.0560. [DOI] [PubMed] [Google Scholar]
- Frigeri T, Rhoton A, Paglioli E, Azambuja N. Cortical projection of the inferior choroidal point as a reliable landmark to place the corticectomy and reach the temporal horn through a middle temporal gyrus approach. Arq Neurop-siquiatr. 2014;72(10):777–781. doi: 10.1590/0004-282x20140165. [DOI] [PubMed] [Google Scholar]
- Rhoton AL,, Jr, Fujii K, Fradd B. Microsurgical anatomy of the anterior choroidal artery. Surg Neurol. 1979;12(2):171–187. [PubMed] [Google Scholar]
- Tubbs RS, Miller JH, Cohen-Gadol AA, Spencer DD. Intraoperative anatomic landmarks for resection of the amygdala during medial temporal lobe surgery. Neurosurgery. 2010;66(5):974–977. doi: 10.1227/01.NEU.0000368105.64548.71. [DOI] [PubMed] [Google Scholar]
- Wen HT, Rhoton AL,, Jr, de Oliveira E, Cardoso AC, Tedeschi H, Baccanelli M, et al. Microsurgical anatomy of the temporal lobe: part 1: mesial temporal lobe anatomy and its vascular relationships as applied to amygdalohippocampectomy. Neurosurgery. 1999;45(3):549–591. doi: 10.1097/00006123-199909000-00028. discussion 91-2. [DOI] [PubMed] [Google Scholar]
- Fujii K, Lenkey C, Rhoton AL. Jr. Microsurgical anatomy of the choroidal arteries: lateral and third ventricles. J Neu-rosurg. 1980;52(2):165–188. doi: 10.3171/jns.1980.52.2.0165. [DOI] [PubMed] [Google Scholar]
- Tubbs RS, Hansasuta A, Loukas M, Louis RG,, Jr, Shoja MM, Salter EG, et al. Branches of the petrous and cavernous segments of the internal carotid artery. Clin Anat. 2007;20(6):596–601. doi: 10.1002/ca.20434. [DOI] [PubMed] [Google Scholar]
- Perlmutter D, Rhoton AL. Jr. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J Neurosurg. 1976;45(3):259–272. doi: 10.3171/jns.1976.45.3.0259. [DOI] [PubMed] [Google Scholar]
- Lasjaunias P, Santoyo-Vazquez A. Segmental agenesis of the internal carotid artery: angiographic aspects with embryological discussion. Anat Clin. 1984;6(2):133–141. doi: 10.1007/BF01773165. [DOI] [PubMed] [Google Scholar]
- Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38(3):425–432. doi: 10.1097/00006123-199603000-00001. discussion 32-3. [DOI] [PubMed] [Google Scholar]
- Ziyal IM, Ozgen T, Sekhar LN, Ozcan OE, Cekirge S. Proposed classification of segments of the internal carotid artery: anatomical study with angiographical interpretation. Neurol Med Chir (Tokyo) 2005;45(4):184–190. doi: 10.2176/nmc.45.184. discussion 90-1. [DOI] [PubMed] [Google Scholar]
- Labib MA, Prevedello DM, Carrau R, Kerr EE, Naudy C, Abou Al-Shaar H, et al. A road map to the internal carotid artery in expanded endoscopic endonasal approaches to the ventral cranial base. Neurosurgery. 2014;10(Suppl 3):44871. doi: 10.1227/NEU.0000000000000362. discussion 71. [DOI] [PubMed] [Google Scholar]
- Perlmutter D, Rhoton AL. Jr. Microsurgical anatomy of the distal anterior cerebral artery. J Neurosurg. 1978;49(2):204–228. doi: 10.3171/jns.1978.49.2.0204. [DOI] [PubMed] [Google Scholar]
- Rosner SS, Rhoton AL,, Jr, Ono M, Barry M. Microsurgi-cal anatomy of the anterior perforating arteries. J Neuro-surg. 1984;61(3):468–485. doi: 10.3171/jns.1984.61.3.0468. [DOI] [PubMed] [Google Scholar]
- Osborn AG. Diagnostic cerebral angiography Lippincott Williams & Wilkins. 1999 [Google Scholar]
- Serizawa T, Saeki N, Yamaura A. Microsurgical anatomy and clinical significance of the anterior communicating artery and its perforating branches. Neurosurgery. 1997;40(6):1211–1216. doi: 10.1097/00006123-199706000-00019. discussion 6-8. [DOI] [PubMed] [Google Scholar]
- Gibo H, Carver CC, Rhoton AL,, Jr, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981;54(2):151–169. doi: 10.3171/jns.1981.54.2.0151. [DOI] [PubMed] [Google Scholar]
- Ture U, Yasargil MG, Al-Mefty O, Yasargil DC. Arteries of the insula. J Neurosurg. 2000;92(4):676–687. doi: 10.3171/jns.2000.92.4.0676. [DOI] [PubMed] [Google Scholar]
- Lasjaunias P, Moret J. The ascending pharyngeal artery: normal and pathological radioanatomy. Neuroradiology. 1976;11(2):77–82. doi: 10.1007/BF00345017. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey L, Daniels DL, Ulmer JL, Mark LP, Smith MM, Strottmann JM, et al. The ascending pharyngeal artery: branches, anastomoses, and clinical significance. AJNR Am J Neuroradiol. 2002;23(7):1246–1256. [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti DD, Reis CV, Hanel R, Safavi-Abbasi S, Deshmukh P, Spetzler RF, et al. The ascending pharyngeal artery and its relevance for neurosurgical and endovascular procedures. Neurosurgery. 2009;65(6 Suppl):114–120. doi: 10.1227/01.NEU.0000339172.78949.5B. discussion 20. [DOI] [PubMed] [Google Scholar]
- Martins C, Yasuda A, Campero A, Ulm AJ, Tanriover N, Rhoton A. Jr. Microsurgical anatomy of the dural arteries. Neurosurgery. 2005;56(2 Suppl):211–251. doi: 10.1227/01.neu.0000144823.94402.3d. discussion -51. [DOI] [PubMed] [Google Scholar]
- Lasjaunias P, Theron J, Moret J. The occipital artery. Anat-omy--normal arteriographic aspects--embryological significance. Neuroradiology. 1978;15(1):31–7. doi: 10.1007/BF00327443. [DOI] [PubMed] [Google Scholar]
- Parkinson D. Extradural neural axis compartment. J Neu-rosurg. 2000;92(4):585–588. doi: 10.3171/jns.2000.92.4.0585. [DOI] [PubMed] [Google Scholar]
- Ohtaka-Maruyama C, Okado H. Molecular Pathways Underlying Projection Neuron Production and Migration during Cerebral Cortical Development. Front Neurosci. 2015;9:447. doi: 10.3389/fnins.2015.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Rarick KR, Ramchandran R. Established New and Emerging Concepts in Brain Vascular Development. Front Physiol. 2021;12(636736) doi: 10.3389/fphys.2021.636736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S. Molecular pathways controlling the sequential steps of cortical projection neuron migration. Adv Exp Med Biol. 2014;800:1–24. doi: 10.1007/978-94-007-7687-6_1. [DOI] [PubMed] [Google Scholar]
- Himmels P, Paredes I, Adler H, Karakatsani A, Luck R, Marti HH, et al. Motor neurons control blood vessel patterning in the developing spinal cord. Nat Commun. 2017;8:14583. doi: 10.1038/ncomms14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes I, Himmels P, Ruiz de Almodovar C. Neurovascular Communication during CNS Development. Dev Cell. 2018;45(1):10–32. doi: 10.1016/j.devcel.2018.01.023. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274(5290):1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Bellantoni G, Guerrini F, Del Maestro M, Galzio R, Luzzi S. Simple schwannomatosis or an incomplete Coffin-Siris? Report of a particular case. eNeurologicalSci. 2019;14:31–3. doi: 10.1016/j.ensci.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Crovace AM, Del Maestro M, Giotta Lucifero A, Elbabaa SK, Cinque B, et al. The cell-based approach in neurosurgery: ongoing trends and future perspectives. Heliyon. 2019;5(11) doi: 10.1016/j.heliyon.2019.e02818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giotta Lucifero A, Luzzi S, Brambilla I, Trabatti C, Mosconi M, Savasta S, et al. Innovative therapies for malignant brain tumors: the road to a tailored cure. Acta Biomed. 2020;91(7-s):5–17. doi: 10.23750/abm.v91i7-S.9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo P, Lombardi F, Augello FR, Giusti I, Luzzi S, Dolo V, et al. NOS2 inhibitor 1400W Induces Autophagic Flux and Influences Extracellular Vesicle Profile in Human Glioblastoma U87MG Cell Line. Int J Mol Sci. 2019;20(12) doi: 10.3390/ijms20123010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvaget E, Paris J, Kici S, Kania R, Guichard JP, Chapot R, et al. Aberrant internal carotid artery in the temporal bone: imaging findings and management. Arch Otolaryngol Head Neck Surg. 2006;132(1):86–91. doi: 10.1001/archotol.132.1.86. [DOI] [PubMed] [Google Scholar]
- Roll JD, Urban MA, Larson TC,, 3rd, Gailloud P, Jacob P, Harnsberger HR. Bilateral aberrant internal carotid arteries with bilateral persistent stapedial arteries and bilateral duplicated internal carotid arteries. AJNR Am J Neuroradiol. 2003;24(4):762–765. [PMC free article] [PubMed] [Google Scholar]
- Meder JF, Blustajn J, Trystram D, Godon-Hardy S, Devaux B, Zuber M, et al. Radiologic anatomy of segmental agenesis of the internal carotid artery. Surg Radiol Anat. 1997;19(6):385–394. doi: 10.1007/BF01628506. [DOI] [PubMed] [Google Scholar]
- Caldemeyer KS, Carrico JB, Mathews VP. The radiology and embryology of anomalous arteries of the head and neck. AJR Am J Roentgenol. 1998;170(1):197–203. doi: 10.2214/ajr.170.1.9423632. [DOI] [PubMed] [Google Scholar]
- Silbergleit R, Quint DJ, Mehta BA, Patel SC, Metes JJ, Noujaim SE. The persistent stapedial artery. AJNR Am J Neuroradiol. 2000;21(3):572–577. [PMC free article] [PubMed] [Google Scholar]
- Nicoletti G, Sanguigni S, Bruno F, Tardi S, Malferrari G. Hypoplasia of the internal carotid artery: collateral circulation and ultrasonographic findings. A case report. J Ultrasound. 2009;12(1):41–4. doi: 10.1016/j.jus.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan MR, Deveikis JP. Handbook of Cerebrovascular Disease and Neurointerventional Technique: Springer International Publishing. 2018 [Google Scholar]
- Ta§ar M. Yeti^er S, Ta§ar A, Ugurel S, Gonul E, Saglam M. Congenital absence or hypoplasia of the carotid artery: radioclinical issues. Am J Otolaryngol. 2004;25(5):339–349. doi: 10.1016/j.amjoto.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Lee JH, Oh CW, Lee SH, Han DH. Aplasia of the internal carotid artery. Acta Neurochir (Wien) 2003;145(2):117–25. doi: 10.1007/s00701-002-1046-y. discussion 25. [DOI] [PubMed] [Google Scholar]
- Makowicz G, Poniatowska R, Lusawa M. Variants of cerebral arteries — anterior circulation. Pol J Radiol. 2013;78(3):42–7. doi: 10.12659/PJR.889403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharatha A, Aviv RI, White J, Fox AJ, Symons SP. Intracranial arterial fenestrations: frequency on CT angiography and association with other vascular lesions. Surg Radiol Anat. 2008;30(5):397–401. doi: 10.1007/s00276-008-0340-7. [DOI] [PubMed] [Google Scholar]
- van Rooij SB, van Rooij WJ, Sluzewski M, Sprengers ME. Fenestrations of intracranial arteries detected with 3D rotational angiography. AJNR Am J Neuroradiol. 2009;30(7):1347–1350. doi: 10.3174/ajnr.A1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino A, Sawada A, Takase Y, Kudo S. MR angiography of anomalous branches of the internal carotid artery. AJR Am J Roentgenol. 2003;181(5):1409–1414. doi: 10.2214/ajr.181.5.1811409. [DOI] [PubMed] [Google Scholar]
- TANAKA M. Persistent primitive dorsal ophthalmic artery associated with paraclinoid internal carotid artery aneurysm. Journal of Neuroendovascular Therapy. 2009;3(1):39–41. [Google Scholar]
- Willinsky R, Lasjaunias P, Berenstein A. Intracav-ernous branches of the internal carotid artery (ICA). Comprehensive review of their variations. Surg Radiol Anat. 1987;9(3):201–215. doi: 10.1007/BF02109631. [DOI] [PubMed] [Google Scholar]
- Lath N, Taneja M. Bilateral congenital hypoplasia of the internal carotid arteries. J HK Coll Radiol. 2008;11:129–31. [Google Scholar]
- Dimmick SJ, Faulder KC. Normal variants of the cerebral circulation at multidetector CT angiography. Radiographics. 2009;29(4):1027–1043. doi: 10.1148/rg.294085730. [DOI] [PubMed] [Google Scholar]
- Uchino A, Nomiyama K, Takase Y, Kudo S. Anterior cerebral artery variations detected by MR angiography. Neuroradiology. 2006;48(9):647–652. doi: 10.1007/s00234-006-0110-3. [DOI] [PubMed] [Google Scholar]
- Zhao HW, Fu J, Lu ZL, Lu HJ. Fenestration of the anterior cerebral artery detected by magnetic resonance angiography. Chin Med J (Engl) 2009;122(10):1139–1142. [PubMed] [Google Scholar]
- Given CA,, 2nd, Morris PP. Recognition and importance of an infraoptic anterior cerebral artery: case report. AJNR Am J Neuroradiol. 2002;23(3):452–454. [PMC free article] [PubMed] [Google Scholar]
- Kochar PS, Soin P, Elfatairy K. Infraoptic anterior cerebral artery or carotid-anterior cerebral artery anastomosis: A very rare embryological variation. Case series and review of literature. Clin Imaging. 2021;79:8–11. doi: 10.1016/j.clinimag.2021.03.014. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Uchino A, Saito N, Ishida J, Suzuki T. Carotid-anterior cerebral artery (ACA) anastomosis associated with azygos ACA and ophthalmic artery arising from the middle meningeal artery: a case report. Surg Radiol Anat. 2020;42(2):211–214. doi: 10.1007/s00276-019-02353-1. [DOI] [PubMed] [Google Scholar]
- Wong ST, Yuen SC, Fok KF, Yam KY, Fong D. Infraoptic anterior cerebral artery: review, report of two cases and an anatomical classification. Acta Neurochir (Wien) 2008;150(10):1087–1096. doi: 10.1007/s00701-008-0016-4. [DOI] [PubMed] [Google Scholar]
- Uchino A, Saito N, Tanahashi N. Bilateral carotid-anterior cerebral artery anastomoses associated with bilateral ophthalmic arteries arising from the anastomotic arteries diagnosed by magnetic resonance angiography: a case report. Surg Radiol Anat. 2018;40(6):721–725. doi: 10.1007/s00276-017-1953-5. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Fujisawa H, Ishihara H, Yoneda H, Kato S, Ogawa A. Side selection of pterional approach for anterior communicating artery aneurysms--surgical anatomy and strategy. Acta Neurochir (Wien) 2008;150(1):31–9. doi: 10.1007/s00701-007-1466-9. discussion 9. [DOI] [PubMed] [Google Scholar]
- Hyun S-J, Hong S-C, Kim J-S. Side selection of the pterional approach for superiorly projecting anterior communicating artery aneurysms. Journal of Clinical Neuroscience. 2010;17(5):592–596. doi: 10.1016/j.jocn.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhang F, Wang H, Yu Y. Contribution of the Hemodynamics of A1 Dysplasia or Hypoplasia to Anterior Communicating Artery Aneurysms: A 3-Dimensional Numerical Simulation Study. Journal of Computer Assisted Tomography. 2012;36(4):421–426. doi: 10.1097/RCT.0b013e3182574dea. [DOI] [PubMed] [Google Scholar]
- Rinaldo L, McCutcheon BA, Murphy ME, Bydon M, Rabinstein AA, Lanzino G. Relationship of A1 segment hypoplasia to anterior communicating artery aneurysm morphology and risk factors for aneurysm formation. Journal of Neurosurgery JNS. 2017;127(1):89–95. doi: 10.3171/2016.7.JNS16736. [DOI] [PubMed] [Google Scholar]
- Auguste KI, Ware ML, Lawton MT. Nonsaccular aneurysms of the azygos anterior cerebral artery. Neurosurg Focus. 2004;17(5):E12. doi: 10.3171/foc.2004.17.5.12. [DOI] [PubMed] [Google Scholar]
- LeMay M, Gooding CA. The clinical significance of the azygos anterior cerebral artery (A.C.A.) Am J Roentgenol Radium Ther Nucl Med. 1966;98(3):602–610. doi: 10.2214/ajr.98.3.602. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Waga S, Kojima T, Shimosaka S. Aneurysm of distal anterior cerebral artery associated with azygos anterior cerebral artery. Acta Neurochir (Wien) 1981;59(1-2):65–9. doi: 10.1007/BF01411192. [DOI] [PubMed] [Google Scholar]
- Bozek P, Pilch-Kowalczyk J, Kluczewska E. Zymon-Zagorska A. Detection of cerebral artery fenestrations by computed tomography angiography. Neurol Neurochir Pol. 2012;46(3):239–244. doi: 10.5114/ninp.2012.29132. [DOI] [PubMed] [Google Scholar]
- Huh JS, Park SK, Shin JJ, Kim TH. Saccular aneurysm of the azygos anterior cerebral artery: three case reports. J Korean Neurosurg Soc. 2007;42(4):342–345. doi: 10.3340/jkns.2007.42.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gast AN, van Rooij WJ, Sluzewski M. Fenestrations of the anterior communicating artery: incidence on 3D angiography and relationship to aneurysms. AJNR Am J Neuroradiol. 2008;29(2):296–298. doi: 10.3174/ajnr.A0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas M, Louis RG,, Jr, Childs RS. Anatomical examination of the recurrent artery of Heubner. Clin Anat. 2006;19(1):25–31. doi: 10.1002/ca.20229. [DOI] [PubMed] [Google Scholar]
- Dehdashti AR, Chiluwal AK, Regli L. The Implication of Anterior Communicating Complex Rotation and 3-Dimensional Computerized Tomography Angiography Findings in Surgical Approach to Anterior Communicating Artery Aneurysms. World Neurosurg. 2016;91:34–42. doi: 10.1016/j.wneu.2016.03.051. [DOI] [PubMed] [Google Scholar]
- Swiqtnicki W, Radomiak-Zaluska A, Heleniak M, Komunski P. Factors determining the best surgical exposure and safe clip positioning in surgical treatment of anterior communicating artery (AComA) aneurysms — particular significance of AComA complex rotation in the axial plane. Pol Przegl Chir. 2019;91(6):6–10. doi: 10.5604/01.3001.0013.5285. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Darder JM, Feliu R, Pesudo JV, Borras JM, Gomez R, Daz C, et al. Surgical management of anterior communicating artery aneurysms based on computed tomographic angiography with three-dimensional reconstruction and without preoperative angiography. Neurocirugia (Astur) 2002;13(6):446–454. [PubMed] [Google Scholar]
- Chen L, Agrawal A, Kato Y, Karagiozov KL, Kumar MV, Sano H, et al. Role of aneurysm projection in “A2” fork orientation for determining the side of surgical approach. Acta Neurochir (Wien) 2009;151(8):925–933. doi: 10.1007/s00701-009-0407-1. discussion 33. [DOI] [PubMed] [Google Scholar]
- Raybaud C. Normal and abnormal embryology and development of the intracranial vascular system. Neurosurg Clin N Am. 2010;21(3):399–426. doi: 10.1016/j.nec.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Yasargil MG. Microneurosurgery: Clinical considerations, surgery of the intracranial aneurysms and results: Georg Thieme. 1984 [Google Scholar]
- Yasargil MG, Krayenbuhl N, Roth P, Hsu SP. Yasargil DC. The selective amygdalohippocampectomy for intractable temporal limbic seizures. J Neurosurg. 2010;112(1):168–185. doi: 10.3171/2008.12.JNS081112. [DOI] [PubMed] [Google Scholar]
- Komiyama M, Nakajima H, Nishikawa M, Yasui T. Middle cerebral artery variations: duplicated and accessory arteries. AJNR Am J Neuroradiol. 1998;19(1):45–9. [PMC free article] [PubMed] [Google Scholar]
- Teal JS, Rumbaugh CL, Bergeron RT, Segall HD. Anomalies of the middle cerebral artery: accessory artery, duplication, and early bifurcation. Am J Roentgenol Radium Ther Nucl Med. 1973;118(3):567–575. doi: 10.2214/ajr.118.3.567. [DOI] [PubMed] [Google Scholar]
- Jain KK. SOME OBSERVATIONS ON THE ANATOMY OF THE MIDDLE CEREBRAL ARTERY. Can J Surg. 1964;7:134–139. [PubMed] [Google Scholar]
- Uchino A, Takase Y, Nomiyama K, Egashira R, Kudo S. Fenestration of the middle cerebral artery detected by MR angiography. Magn Reson Med Sci. 2006;5(1):51–5. doi: 10.2463/mrms.5.51. [DOI] [PubMed] [Google Scholar]
- de Lahunta A, Glass EN, Kent M. Embryonic Development of the Central Nervous System. Vet Clin North Am Small Anim Pract. 2016;46(2):193–216. doi: 10.1016/j.cvsm.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Ciappetta P, D’Urso P I, Luzzi S, Ingravallo G, Cimmino A, Resta L. Cystic dilation of the ventriculus terminalis in adults. J Neurosurg Spine. 2008;8(1):92–9. doi: 10.3171/SPI-08/01/092. [DOI] [PubMed] [Google Scholar]
- Inoue K, Seker A, Osawa S, Alencastro LF, Matsushima T, Rhoton AL. Jr. Microsurgical and endoscopic anatomy of the supratentorial arachnoidal membranes and cisterns. Neurosurgery. 2009;65(4):644–664. doi: 10.1227/01.NEU.0000351774.81674.32. discussion 65. [DOI] [PubMed] [Google Scholar]
- Yasargil MG. Microneurosurgery Volume I: Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain, Diagnostic Studies, General Operative Techniques and Pathological Considerations of the Intracranial Aneurysms: Thieme. 1984 [Google Scholar]
- Yasargil MG. Microneurosurgery Volume II: Clinical Considerations, Surgery of the Intracranial Aneurysms and Results: Thieme. 1984 [Google Scholar]
- Wackenheim A, Braun JP, Babin E, Megret M. The carotid cistern. Neuroradiology. 1973;5(2):82–4. doi: 10.1007/BF00337488. [DOI] [PubMed] [Google Scholar]
- Yasargil MG, Kasdaglis K, Jain KK, Weber HP. Anatomical observations of the subarachnoid cisterns of the brain during surgery. J Neurosurg. 1976;44(3):298–302. doi: 10.3171/jns.1976.44.3.0298. [DOI] [PubMed] [Google Scholar]
- Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62(2):248–256. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- Fattahi TT, Brandt MT, Jenkins WS, Steinberg B. Traumatic Carotid-Cavernous Fistula: Pathophysiology and Treatment. Journal of Craniofacial Surgery. 2003;14(2):240–246. doi: 10.1097/00001665-200303000-00020. [DOI] [PubMed] [Google Scholar]
- Debrun G, Lacour P, Vinuela F, Fox A, Drake CG, Caron JP. Treatment of 54 traumatic carotid-cavernous fistulas. Journal of Neurosurgery. 1981;55(5):678–692. doi: 10.3171/jns.1981.55.5.0678. [DOI] [PubMed] [Google Scholar]
- Liang W, Xiaofeng Y, Weiguo L, Wusi Q, Gang S, Xuesh-eng Z. Traumatic Carotid Cavernous Fistula Accompanying Basilar Skull Fracture: a Study on the Incidence ofTraumatic Carotid Cavernous Fistula in the Patients With Basilar Skull Fracture and the Prognostic Analysis About Traumatic Carotid Cavernous Fistula. Journal of Trauma and Acute Care Surgery. 2007;63(5):1014–1020. doi: 10.1097/TA.0b013e318154c9fb. [DOI] [PubMed] [Google Scholar]
- Ya§argil M.G. FJL, Ray M.W. The Operative Approach to Aneurysms of the Anterior Communicating Artery. In: al. KHe, editor. Advances and Technical Standards in Neurosurgery vol 2. Vienna: Springer. 1975 [Google Scholar]
- Yasargil MG. Internal carotid aneurysms, distal medial wall aneurysms and aneurysms of superior wall of internal carotid artery. Microneurosurgery. 1984;2:58–89. [Google Scholar]
- Yasargil MG, Fox JL. The microsurgical approach to intracranial aneurysms. Surg Neurol. 1975;3(1):7–14. [PubMed] [Google Scholar]
- Ya§argil MG. Microneurosurgery: Thieme. 1984 [Google Scholar]
- Ya§argil MG, Abernathey CD. Microneurosurgery of CNS Tumors: Thieme. 1996 [Google Scholar]
- Luzzi S, Del Maestro M, Galzio R. Microneurosurgery for Paraclinoid Aneurysms in the Context of Flow Diverters. Acta Neurochir Suppl. 2021;132:47–53. doi: 10.1007/978-3-030-63453-7_7. [DOI] [PubMed] [Google Scholar]
- Gardner PA, Vaz-Guimaraes F, Jankowitz B, Koutour-ousiou M, Fernandez-Miranda JC, Wang EW, et al. Endoscopic Endonasal Clipping of Intracranial Aneurysms: Surgical Technique and Results. World Neurosurg. 2015;84(5):1380–1393. doi: 10.1016/j.wneu.2015.06.032. [DOI] [PubMed] [Google Scholar]
- Szentirmai O, Hong Y, Mascarenhas L, Salek AA, Stieg PE, Anand VK, et al. Endoscopic endonasal clip ligation of cerebral aneurysms: an anatomical feasibility study and future directions. J Neurosurg. 2016;124(2):463–468. doi: 10.3171/2015.1.JNS142650. [DOI] [PubMed] [Google Scholar]
- Arnaout MM, Luzzi S, Galzio R, Aziz K. Supraorbital keyhole approach: Pure endoscopic and endoscope-assisted perspective. Clin Neurol Neurosurg. 2019;189:105623. doi: 10.1016/j.clineuro.2019.105623. [DOI] [PubMed] [Google Scholar]
- Zoia C, Bongetta D, Dorelli G, Luzzi S, Maestro MD, Galzio RJ. Transnasal endoscopic removal of a retrochiasmatic cavernoma: A case report and review of literature. Surg Neurol Int. 2019;10:76. doi: 10.25259/SNI-132-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


