Abstract
The pterional approach is a workhorse in neurosurgery, to the point where perfect knowledge of its execution is essential in neurosurgical daily practice. The pterional transsylvian corridor is used to treat aneurysms involving anterior circulation, basilar apex, the proximal segment of the superior cerebellar and posterior cerebral artery, arteriovenous malformations and cavernous hemangiomas of the basal forebrain, anterior and middle skull base tumors, gliomas of the frontal, parietal, and temporal opercula, insula, mediobasal temporal region, cerebral peduncles, interpeduncular fossa, and also orbital lesions. We herein overview the core technique and variations of the pterional approach aimed at broadening surgical freedom and decreasing the risk of approach-related complications. (www.actabiomedica.it)
Keywords: Intracranial Aneurysms, Neurovascular Surgery, Pterional Approach, Skull Base Tumors, Sylvian Fissure
Introduction
Introduced by Yasargil in the 1970s, the pterional or frontoternporosphenoidal approach is the most used approach in neurosurgery (1-3). Its critical point lies in the drilling of the lateral most part of the greater sphenoid wing to access the entire anterior and middle skull base.
The pterional approach has several vascular targets, namely the internal carotid artery (ICA) with its branches, middle cerebral artery (MCA), anterior communicating artery (ACoA), basilar tip, P1 segment of the posterior cerebral artery (PCA), and proximal segment of the superior cerebellar artery (SCA). Anterior and middle skull base lesions and intra-axial tumors of the frontal, temporal, and parietal opercula, uncus, insula, basal ganglia, lateral ventricle, and interpeduncular fossa may be reached through the pterional transsylvian route. The pterional approach may be also used to treat orbital tumors.
The present article reports the core technique and variations of the pterional approach. Hints and tips to maximize surgical freedom and avoid complications are also discussed.
Indications
The pterional approach allows access to the su-pratentorial basal cisterns and uppermost and median infratentorial cisterns. Accordingly, it is the approach of choice for most extra-axial lesions involving the anterior and middle skull base and a large proportion of intra-axial lesions affecting the basal forebrain and anterior midbrain.
Regarding the neurovascular pathology, the pterion-al approach is indicated in most anterior circulation an-eurysms and selected median and paramedian aneurysms involving the upper part of the posterior circulation.
Arteriovenous malformations and cavernous he-mangiomas involving the fronto-basal, anterior temporal, and opercular areas and the Sylvian fissure are approached by the pterional route in most cases.
Technique
Positioning
The patient is placed supine, with the head fixed to a Mayfield-Kees or Sugita skull clamp and elevated above the level of the heart to allow optimal venous outflow. In the classic description by Yasargil in 1976, an extension of approximately 20° is recommended. The malar eminence becomes the highest point of the patient’s head. A contralateral rotation of the head, ranging from 15° to 45°, based on the specific neurovascular target, is suggested (1-3) (Figure 1A).
Figure 1.
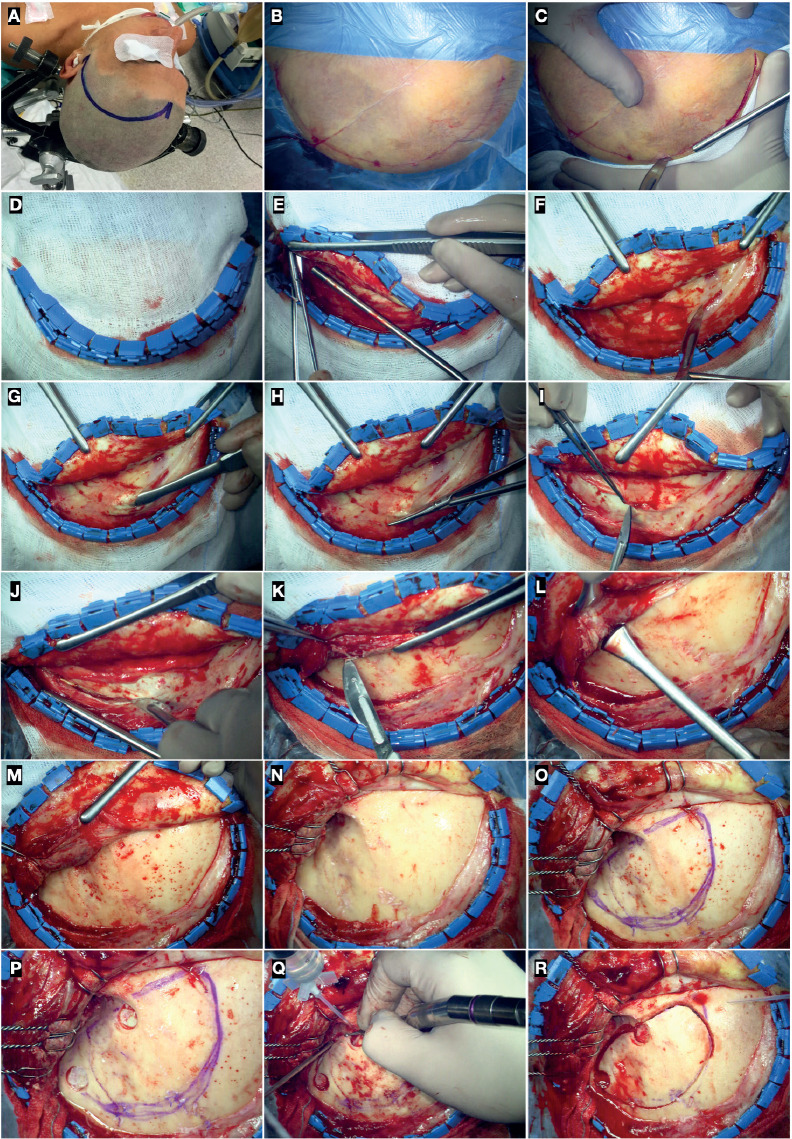
Surgical position (A), skin mark (B), and incision (C). (D) Hemostasis of the skin flap with Raney clips. (E-I) Preparation of the galea-pericranium flap; (J-N) Incision of the superficial temporal fascia, temporalis muscle, and deep temporal fascia (submuscular technique), and retrograde subperiosteal dissection of the deep temporalis fascia according to Oikawa’s technique. (O) Identification of the sutures of the pterional region and drawing of the bone flap. (P) The first and second burr-hole is placed just above the McCarty keyhole and above the posterior root of the zygoma, respectively. (Q) Partial drilling of the lateral third of the greater sphenoid wing before completing the craniotomy. (R) Frontotemporal bone flap.
Chaddad-Neto et al. reported a study in which they revised the concept of extension and rotation of the head for anterior circulation aneurysms (4). They demonstrated that excessive extension of the head causes a dramatic hindering of the optic-carotid complex by the orbital roof. The extension also deepens the anterior clinoid process and plane of the ICA, ultimately making anterior clinoidectomy and exposure of the ophthalmic artery more difficult. Conversely, a 15° rotation without extension makes the longest axis of the clinoid process parallel to the floor. Therefore, they recommended a neutral extension of the head with 15° of rotation for ophthalmic, posterior communicating artery (PCoA), and anterior choroidal artery (AChA) aneurysms. An extension of 15° and rotation of 10° is suggested for aneurysms involving the MCA bifurcation, ICA, and anterior and inferior projecting ACoA aneurysms (4).
Skin incision and soft tissue dissection
Local subcutaneous infiltration, along the skin incision mark, of lidocaine hydrochloride 1% or me-pivacaine chlorhydrate 2% plus adrenaline diluted in normal saline 0.9% (1:1) is recommended for antalgic purposes and to promote easier detachment of the skin from the subcutaneous layers.
The skin incision starts 1 cm in front of the tragus, anteriorly to the superficial temporal artery and auriculotemporal nerve. The incision curves upward, behind the hairline, to reach the midline. It should not involve the galea, which can be preserved and used as an au-tologous patch graft in case of duraplasty. The galea is subperiosteally dissected to prepare a double-layered galea-pericranium vascularized flap useful to repair the frontal sinus in case of accidental violation.
Hemostatic scalp clips, known as Raney clips, are placed along the edge of the incision to reflect the flap.
In the description by Yasargil, the skin flap is divided by the temporalis muscle and reflected forward.
To avoid damaging the frontal branch of the facial nerve, the superficial layer of the temporal fascia and the fat pad, within which the nerve courses, are separated from the deep layer (interfascial technique) (1, 2).
Two main variations have been reported for this step: the subfascial and submuscular techniques. The former involves the incision of the superficial and deep layers of the superficial temporal fascia, whereas the latter encompasses the incision of the deep temporal fascia, subperiosteal blunt dissection of the temporalis muscle, and forward reflection of the myocutaneous flap.
The submuscular technique is the most employed because faster and characterized by a lesser risk of iatrogenic damage to the frontal branch of the facial nerve. Regardless of the techniques, the temporalis muscle should be incised above the posterior root of the zygoma and subperiosteally detached in a retrograde superior to posterior and backward to forward direction. This technique has been described by Oi-kawa and colleagues (5). Electrocauterization must be avoided to preserve the anatomical integrity of the deep fascia along with the blood supply from the internal maxillary artery. Preservation of the deep fascia is the key to preventing temporalis muscle atrophy and functional and cosmetic complications (3, 5-7). A compulsive subperiosteal dissection of the muscle is also helpful in the recognition of skull sutures. The fronto-zygomatic suture and the superior aspect of the posterior root of the zygomatic process of the temporal bone should always be exposed (Figure 1).
Bony landmarks
The skull sutures at the level of the pterional region serve as landmarks for bone work.
The pterion is the joining point of the frontal, parietal, temporal, and sphenoid bone. Identification of the coronal, spheno-parietal, spheno-frontal, spheno-squamosal, and spheno-zygomatic sutures is of utmost importance to localize the lateral third of the greater sphenoid wing. The spheno-squamosal suture coincides with the anterior third of the temporoparietal suture, also known as squamosal. The highest point of the spheno-squamosal suture is located at the same level of the spheno-parietal and spheno-frontal sutures. It marks the uppermost point of the greater sphenoid wing and, indirectly, the superior orbital fissure (SOF).
The frontal process of the zygomatic bone anteriorly and the greater sphenoid wing posteriorly constitute the lateral wall of the orbit, of which the spheno-zygomatic suture labels the midportion. The fronto-zygomatic suture, located anterosuperior to the spheno-zygomatic one, is located at the level of the orbital roof. It also marks the limit between the lateral wall and roof. The supraorbital foramen and nerve should be identified early to mark the medial limit of the craniotomy (Figure 1O and Figures 2A and Figure 2C). Rarely, the air frontal sinus extends laterally to the supraorbital foramen. This point is important to avoid accidental violation of the frontal sinus.
Figure 2.
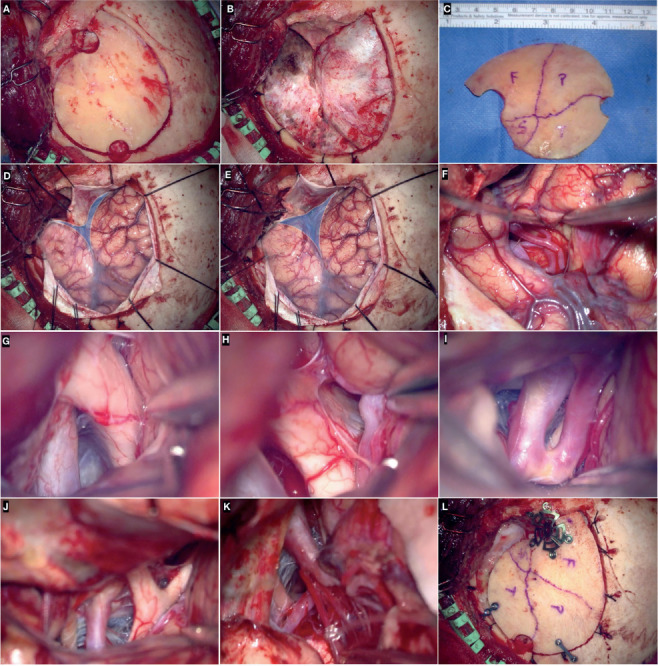
(A-C) left frontotemporal craniotomy and bone flap. Exposure of the sphenoidal part of the sylvian fissure (blue area) before (D) and after (E) drilling of the lateral third of the greater sphenoid wing. Splitting of the sylvian fissure (F) and intradural exposure of optico-carotid complex (G), lamina terminalis (H), anterior cerebral artery (I), Liliequist’s membrane (J), posterior communicating, and posterior cerebral artery (K). (L) Osteosynthesis of the bone flap with titanium low profile burr-hole covers, mini plates, and self-screwing unicortical screws.
Craniotomy
The MacCarty keyhole is placed 5 mm behind the connection between the fronto-zygomatic, sphenozygomatic, and fronto-sphenoidal sutures. The dura of the anterior fossa and periorbita, separated by the thin orbital roof, are thus exposed (8, 9). Nevertheless, in most cases, the exposure of the orbit and its contents is not necessary to treat the lesion for which the pterional approach has its indication. As a consequence, the first burr hole is generally placed just above the McCarty keyhole to access the anterior cranial fossa. The second burr hole should be placed at the level of the temporal squama, above the posterior root of the zygoma.
Two burr holes are enough in most cases. However, if the dura is firmly adherent to the bone, as in older patients with an internal frontal hyperostosis or chronic renal failure, a third burr hole can be positioned at the superior temporal line. Before crani-otomy, partial drilling of the lateral third of the greater sphenoid wing will facilitate the passage of the crani-otome and detachment of the bone flap. In doing so, the cancellous bone of the greater sphenoid wing and middle meningeal artery may bleed. The oozing can be managed with bone wax or bipolar coagulation.
The first and second burr holes are connected with the craniotome in a curvilinear fashion. In cases of hy-perpneumatization of the frontal sinus or where a huge subfrontal exposure is unnecessary, the lateral limit of craniotomy at the level of the frontal bone corresponds to the superior temporal line.
The bone flap is elevated with the aid of Penfield dissector no. 3, carefully completing the detachment of the underlying dura. In case the flap is still attached to the inner cortical surface of the greater sphenoid wing it can be easily fractured.
The key step of the pterional approach consists of wide drilling of the lateral part of the greater sphenoid wing until the SOF. This allows for a full and unobstructed view of the sphenoidal part of the Sylvian fissure which is the access door of the entire anterior and middle skull base. Drilling of the lateral part of the greater sphenoid wing avoids fixed brain retraction. The greater the drilling of this bony component, the lesser the need for spatulas. The meningo-orbital artery serves as a useful landmark to early identify the lateral third of the SOF between the lesser and greater sphenoid wing (10-12). Skeletonization and partial enlargement of the SOF are helpful in allowing the reflection of the dural flap. The thinning of the orbital roof with the drill is recommended to achieve a line of sight and working corridor as flat as possible to the anterior cranial fossa (Figures 2A-Figures 2C).
Dura opening
The dura may be opened in a curvilinear fashion around the SOF.
However, this cut is perpendicular to the Sylvian vein complex and middle meningeal artery, both of them being at risk of injury. To prevent this complication, a cut parallel to the posterior ramus of the Sylvian fissure can be alternatively performed, having so two dural leaflets. Tack-up sutures along the edge of the craniotomy and tenting stiches of the dural leaflets are important to keeping a clean surgical field (Figures 2d-e).
Intradural corridors
The pterional approach is related to the subfrontal, transsylvian, pretemporal, and anterior subtemporal working corridors. The transsylvian route to the posterior fossa is limited only by the Liliequist’s membrane, the opening of which allows access to the basilar bifurcation and anterior midbrain.
Three different deep windows may be utilized: the optic-carotid, carotid-oculomotor, and supra-carotid one. Splitting of the Sylvian fissure is the starting point for all of these corridors, which can be tailored on a case-by-case basis depending on the lesion to be treated. The combined subfrontal-transsylvian corridor is employed for the anterior circulation aneurysms, while the transsylvian deep windows, along with the pretemporal and subtemporal perspectives, are routes used for aneurysms of the basilar tip, P1 segment of the PCA, and proximal segment of the SCA. Anterior clinoidectomy is critical to expose the paraclinoid segment of the ICA (Figures 2F-K).
Closure
The dura is closed in a watertight fashion. In the case of duraplasty, the galea is widely preferred to any other heterologous or synthetic substitutes. The osteosynthesis of the bone flap is accomplished via low-profile titanium plates, burr-hole covers, and 4-mm or 5-mm self-tapping screws (Figure 2L).
The temporalis muscle is reapproximated with 2/0 Vicryl stiches. A separate suture of the superficial temporalis fascia is recommended to achieve better functional and cosmetic results. Neither interfascial nor submuscular technique requires a muscle cuff for reconstruction along the superior temporal line. Subcutaneous blood collections are avoided with the use of drainage. The skin suture is executed with 3/0 silk stiches to achieve a good aesthetic result (Figure 3).
Figure 3.
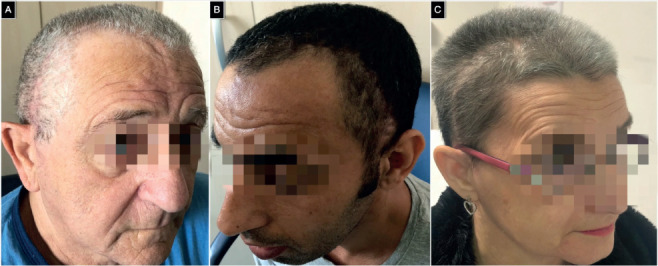
(A-C) Illustrative examples of good functional and cosmetic outcomes of three randomly selected patients of the patients’ cohort.
Tailoring the pterional approach
The pterional approach is characterized by tremendous versatility and it can be adapted to different lesions. The main aspects of tailoring are the size of the bone flap and the frontal versus the temporal extension of the craniotomy (13-23).
About aneurysms, a smaller craniotomy is generally adequate for unruptured paraclinoid aneurysms, where wide splitting of the Sylvian fissure is unnecessary, and small unruptured MCA bifurcation aneu-rysms. By contrast, larger bone flaps are required for ruptured and giant aneurysms and those complex of the ACoA. A wider subfrontal extension is useful if the subfrontal perspective is the prevalent working corridor, as for all the ACoA aneurysms. A greater temporal extension is critical for basilar apex and P1 PCA aneurysms. In these cases, the pretemporal corridor is the elective route to the target. In some basilar tip aneurysms, combined transsylvian-pretemporal working corridors are used in the so-called half-and-half approach (24, 25).
Complications avoidance
The pterional approach involves potential complications that can be functional or aesthetic in nature. Iatro-genic injury of the fronto-temporal branch of the facial nerve, temporalis muscle atrophy, masticatory imbalance, and hypo-anesthesia of the auricle and external acoustic meatus have a more concrete risk. The more frequent use of the submuscular technique has dramatically decreased the incidence of iatrogenic injury to the fronto-temporal branch of the facial nerve, which is associated with a poor aesthetic outcome mainly from the paralysis of the frontalis and corrugator supercilii muscles.
The implementation of a subperiosteal blunt and “cold” (avoiding electrocauterization) dissection of the deep temporal fascia is the key to preventing postoperative atrophy of the temporalis muscle with all related aesthetic and functional complications, masticatory imbalance for first.
The preservation of the auriculotemporal nerve during skin incision and soft tissue dissection prevents troublesome dysesthesia of the pinna.
Illustrative case
Multiple Bilateral Intracranial Aneurysms Treated in Single-Stage Surgery
58-year-old female suffering from an aneurysmal subarachnoid hemorrhage (GCS: 6; Hunt-Hess score: 4) underwent CT angiography that showed six anterior circulation aneurysms. An MCA bifurcation, PCoA, and AChA aneurysm were found on the left side, while a PCoA, Ml MCA, and MCA bifurcation aneurysm were on the right side. The patient was a candidate for a late treatment which was performed 2 months later in the light of a partial recovery. A left pterional transsylvian approach permitted to clip in a single-stage five of the six aneurysms. The suprachias-matic corridor was used to clip the contralateral, un-ruptured PCoA aneurysm. The right unruptured Ml MCA aneurysm was also clipped through the same corridor (Figure 4). The left MCA bifurcation aneu-rysm, which was giant and partially thrombosed, was surgically treated 2 months later through clip ligation. The patient furtherly recovered in the third month and the overall modified Ranking score was 3.
Figure 4.
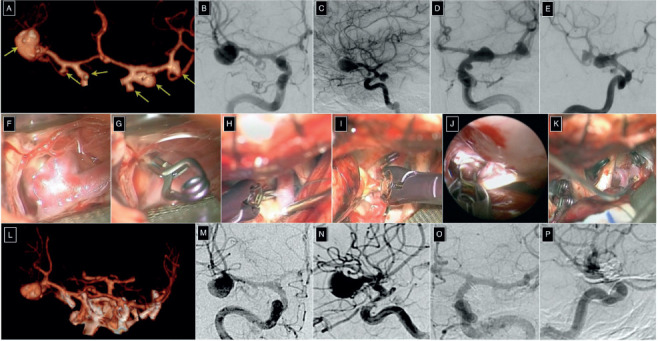
(A) Pre-operative 3D CT-angiography. Anterior (B) and lateral (C) projection digital subtraction angiography (DSA) of the right internal carotid artery (ICA). Anterior (D) and lateral (E) projection DSA of the left ICA. Clipping of the aneurysm of the left middle cerebral artery (MCA) bifurcation (F-G), left posterior communicating artery (PCoA) (H), left anterior choroidal artery (I), right PCoA ( J), and right M1 segment MCA (K) aneurysm. (L) Post-operative 3D CT-angiography. Anterior (M) and lateral (N) projection DSA of the right ICA. Anterior (O) and lateral (P) projection DSA of the left ICA.
Discussion
After >40 years from its original description by Yasargil (2), the pterional is still today the most used skull base approach in neurosurgery. It is routinely employed in the treatment of neurovascular pathologies, lesions of the anterior and middle skull base, and some intra-axial tumors (19, 20, 26, 27). Despite the constant refinements and advances about the techniques in neurosurgery (28-39), the pterional approach has maintained its primary role.
Several morphometric and dimensional studies have led to a better understanding of the technical aspects at the base of tailoring of the pterional approach in accordance with the lesion to be treated (40-46). They also have led to drawing the exact limits between the pterional approach and other anterolateral skull base approaches, classically considered as extensions of it. The reported illustrative case confirms the extreme versatility of the pterional approach.
Some technical tips of this workhorse approach deserve to be stressed.
The skin incision must be placed behind the hairline to achieve the best aesthetic outcome and must not be excessively anterior in those cases where full exposure of the posterior ramus of the Sylvian fissure is planned. This is because the more anterior the skin incision is, the more limited and anterior the size of the bone flap will be.
Another reason lies in the fact that the splitting of the Sylvian fissure should start at the tip of the pars tri-angularis of the frontal operculum, which may remain unexposed in case of an excessively anterior bone flap.
In performing the blunt subperiosteal dissection of the temporalis muscle according to the Oikawa technique, the best method to preserve the deep fascia is to detach the muscle from the back to the front but especially upward toward the superior temporal line which is the point where the muscle is more tenaciously attached (5, 7, 47-52). In doing so, sharp tips such as those of the Langenbeck elevator or even the tip of the knife plays better than blunt ones because the deep temporal fascia is extremely thin and may be damaged by coarse instruments. During the muscle detachment, a simple trick to be sure to have been adequately parallel to the skull base is to expose the midpoint of the spheno-zygomatic suture.
The key aspect of the pterional approach is drilling the most lateral aspect of the greater sphenoid wing along with the thinning of the orbital roof. This step allows for a working corridor that is fully parallel to the anterior skull base, thus avoiding the need for brain retraction. In comparison with the front-temporal approach, the drilling of the sphenoid wing allows for shortening the working distance and increasing the angular exposure of the target.
During the closure, the bone gap on the temporal side of the bone flap can be filled with hemostatics and fibrin glue sealant. Bone dust can also be used for this purpose having an intrinsic hemostatic power. To obtain a good aesthetic outcome, the use of silk or Ethilon 3/0 stiches is recommended.
Conclusion
The pterional approach is the backbone of all the anterolateral approaches to the skull base. Its tremendous versatility makes it appropriate for most neuro-vascular pathologies, lesions involving the anterior and middle skull base, as well as some intra-axial forebrain tumors.
Accurate planning, meticulous execution, and especially the extensive knowledge of the skull base anatomy are essential to exploit all advantages of this approach and decrease the risk of complications.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- Yaargil M, Yaşargil M. Interfascial pterional (frontotem-porosphenoidal) craniotomy: Georg Thieme Verlag. 1984:217–20 p. [Google Scholar]
- Yaşargil M.G. FJL, Ray M.W. 'The Operative Approach to Aneurysms of the Anterior Communicating Artery. In: al. KHe, editor. Advances and Technical Standards in Neurosurgery, vol 2. Vienna: Springer. 1975 [Google Scholar]
- Yasargil MG, Reichman MV, Kubik S. Preservation of the frontotemporal branch of the facial nerve using the inter-fascial temporalis flap for pterional craniotomy. Technical article. J Neurosurg. 1987;67(3):463–466. doi: 10.3171/jns.1987.67.3.0463. [DOI] [PubMed] [Google Scholar]
- Chaddad-Neto F, Doria-Netto HL, Campos-Filho JM, Ribas ES, Ribas GC, Oliveira E. Head positioning for anterior circulation aneurysms microsurgery. Arq Neuropsiqui-atr. 2014;72(11):832–840. doi: 10.1590/0004-282x20140156. [DOI] [PubMed] [Google Scholar]
- Oikawa S, Mizuno M, Muraoka S, Kobayashi S. Retrograde dissection of the temporalis muscle preventing muscle atrophy for pterional craniotomy. Technical note. J Neurosurg. 1996;84(2):297–299. doi: 10.3171/jns.1996.84.2.0297. [DOI] [PubMed] [Google Scholar]
- Zabramski JM, Kiris T, Sankhla SK, Cabiol J, Spetzler RF. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89(2):336–341. doi: 10.3171/jns.1998.89.2.0336. [DOI] [PubMed] [Google Scholar]
- Coscarella E, Vishteh AG, Spetzler RF, Seoane E. Zabram-ski JM. Subfascial and submuscular methods of temporal muscle dissection and their relationship to the frontal branch of the facial nerve. Technical note. J Neurosurg. 2000;92(5):877–880. doi: 10.3171/jns.2000.92.5.0877. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Tanriover N, Rhoton AL,, Jr, Yoshioka N, Fujii K. MacCarty keyhole and inferior orbital fissure in orbit-ozygomatic craniotomy. Neurosurgery. 2005;57(1 Sup-pl):152–159. doi: 10.1227/01.neu.0000163600.31460.d8. discussion -9. [DOI] [PubMed] [Google Scholar]
- Aziz KM, Froelich SC, Cohen PL, Sanan A, Keller JT, van Loveren HR. The one-piece orbitozygomatic approach: the MacCarty burr hole and the inferior orbital fissure as keys to technique and application. Acta Neurochir (Wien) 2002;144(1):15–24. doi: 10.1007/s701-002-8270-1. [DOI] [PubMed] [Google Scholar]
- Campero A, Baldoncini M, Villalonga JF, Saenz A. Orbit-omeningeal Band in Transcavernous Dissection and Anterior Clinoidectomy: 3-Dimensional Operative Video. Operative Neurosurgery. 2020;19(4) doi: 10.1093/ons/opaa037. E414-E. [DOI] [PubMed] [Google Scholar]
- Alejandro SA, Carrasco-Hernandez JP, da Costa MDS, Ferreira DS, Lima JVF, de Amorim BL, et al. Anterior Clinoidectomy: Intradural Step-by-Step En Bloc Removal Technique. World Neurosurg. 2021;146:217–231. doi: 10.1016/j.wneu.2020.11.002. [DOI] [PubMed] [Google Scholar]
- Baldoncini M, Luzzi S, Giotta Lucifero A, Flores-Justa A, Gonzalez-Lopez P, Campero A, et al. Optic Foraminotomy for Clipping of Superior Carotid-Ophthalmic Aneurysms. Frontiers in Surgery. 2021;8(639) doi: 10.3389/fsurg.2021.681115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meybodi AT, Lawton MT, Rubio RR, Yousef S, Benet A. Contralateral Approach to Middle Cerebral Artery Aneurysms: An Anatomical-Clinical Analysis to Improve Patient Selection. World Neurosurg. 2018;109:e274–e80. doi: 10.1016/j.wneu.2017.09.160. [DOI] [PubMed] [Google Scholar]
- Yu LH, Shang-Guan HC, Chen GR, Zheng SF, Lin YX, Lin ZY, et al. Monolateral Pterional Keyhole Approaches to Bilateral Cerebral Aneurysms: Anatomy and Clinical Application. World Neurosurg. 2017;108:572–580. doi: 10.1016/j.wneu.2017.09.048. [DOI] [PubMed] [Google Scholar]
- Serrano LE, Ayyad A, Archavlis E, Schwandt E, Nimer A, Ringel F, et al. A literature review concerning contralateral approaches to paraclinoid internal carotid artery aneurysms. Neurosurg Rev. 2019;42(4):877–884. doi: 10.1007/s10143-018-01063-3. [DOI] [PubMed] [Google Scholar]
- Nakao S, Kikuchi H, Takahashi N. Successful clipping of carotid-ophthalmic aneurysms through a contralateral pterional approach: Report of two cases. Journal of Neurosurgery. 1981;54(4):532–536. doi: 10.3171/jns.1981.54.4.0532. [DOI] [PubMed] [Google Scholar]
- Milenkovic Z, Gopic H, Antovic P, Jovicic V, Petrovic B. Contralateral pterional approach to a carotid-ophthalmic aneurysm ruptured at surgery: Case report. Journal of Neurosurgery. 1982;57(6):823–825. doi: 10.3171/jns.1982.57.6.0823. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Gragnaniello C, Giotta Lucifero A, Del Maestro M, Galzio R. Surgical Management of Giant Intracranial Aneurysms: Overall Results of a Large Series. World Neu-rosurg. 2020 doi: 10.1016/j.wneu.2020.08.004. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Gragnaniello C, Giotta Lucifero A, Del Maestro M, Galzio R. Microneurosurgical management of giant intracranial aneurysms: Datasets of a twenty-year experience. Data Brief. 2020;33:106537. doi: 10.1016/j.dib.2020.106537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Del Maestro M, Galzio R. Microneurosurgery for Paraclinoid Aneurysms in the Context of Flow Diverters. Acta Neurochir Suppl. 2021;132:47–53. doi: 10.1007/978-3-030-63453-7_7. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Gallieni M, Del Maestro M, Trovarelli D, Ricci A, Galzio R. Giant and Very Large Intracranial Aneurysms: Surgical Strategies and Special Issues. Acta Neurochir Suppl. 2018;129:25–31. doi: 10.1007/978-3-319-73739-3_4. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Giotta Lucifero A, Baldoncini M, Del Maestro M, Elbabaa SK, Galzio R. Paraclinoid Aneurysms: Outcome Analysis and Technical Remarks of a Microsurgical Series. Interdisciplinary Neurosurgery. 2021:101373. [Google Scholar]
- Luzzi S, Del Maestro M, Galzio R. Posterior Circulation Aneurysms: A Critical Appraisal of a Surgical Series in Endovascular Era. Acta Neurochir Suppl. 2021;132:39–45. doi: 10.1007/978-3-030-63453-7_6. [DOI] [PubMed] [Google Scholar]
- Zenonos GA, Chen SH, Sur S, Morcos JJ. Transcavern-ous Transclinoidal Half-and-Half Approach for Clipping of Twice-Recurrent Stent-Coiled Giant Basilar Tip Aneurysm: 3-Dimensional Surgical Video. World Neurosurg. 2019;127:330. doi: 10.1016/j.wneu.2019.04.045. [DOI] [PubMed] [Google Scholar]
- Lanzino G, Cannizzaro D, Villa SL, Meyer FB. Pretemporal (“Half-and-Half”) Approach for Posterior Circulation Aneurysms in a Patient With Internal Carotid Artery Occlusion. Oper Neurosurg (Hagerstown) 2018;14(4):457. doi: 10.1093/ons/opx153. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Gragnaniello C, Giotta Lucifero A, Del Maestro M, Galzio R. Surgical Management of Giant Intracranial Aneurysms: Overall Results of a Large Series. World Neu-rosurg. 2020;144:e119–e37. doi: 10.1016/j.wneu.2020.08.004. [DOI] [PubMed] [Google Scholar]
- Costa M, Baldoncini M, Tataryn ZL, Demichelis ME, Conde A, Purves C, et al. Microsurgical Clipping of Carotid-Ophthalmic Tandem Aneurysms: Case Report and Surgical Nuances. Medicina (Kaunas) 2021;57(7) doi: 10.3390/medicina57070731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Del Maestro M, Galzio R. Letter to the Editor. Preoperative embolization of brain arteriovenous malformations. J Neurosurg. 2019:1–2. doi: 10.3171/2019.6.JNS191541. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Del Maestro M, Elbabaa SK, Galzio R. Letter to the Editor Regarding “One and Done: Multimodal Treatment of Pediatric Cerebral Arteriovenous Malformations in a Single Anesthesia Event”. World Neurosurg. 2020;134:660. doi: 10.1016/j.wneu.2019.09.166. [DOI] [PubMed] [Google Scholar]
- Arnaout MM, Luzzi S, Galzio R, Aziz K. Supraorbital keyhole approach: Pure endoscopic and endoscope-assisted perspective. Clin Neurol Neurosurg. 2019;189:105623. doi: 10.1016/j.clineuro.2019.105623. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Del Maestro M, Elia A, Vincitorio F, Di Perna G, Zenga F, et al. Morphometric and Radiomorphometric Study of the Correlation Between the Foramen Magnum Region and the Anterior and Posterolateral Approaches to Ventral Intradural Lesions. Turk Neurosurg. 2019 doi: 10.5137/1019-5149.JTN.26052-19.2. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Zoia C, Rampini AD, Elia A, Del Maestro M, Carnevale S, et al. Lateral Transorbital Neuroendoscopic Approach for Intraconal Meningioma of the Orbital Apex: Technical Nuances and Literature Review. World Neuro-surg. 2019;131:10–7. doi: 10.1016/j.wneu.2019.07.152. [DOI] [PubMed] [Google Scholar]
- Zoia C, Bongetta D, Dorelli G, Luzzi S, Maestro MD. Gal-zio RJ. Transnasal endoscopic removal of a retrochiasmatic cavernoma: A case report and review of literature. Surg Neurol Int. 2019;10:76. doi: 10.25259/SNI-132-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Crovace AM, Del Maestro M, Giotta Lucifero A, Elbabaa SK, Cinque B, et al. The cell-based approach in neurosurgery: ongoing trends and future perspectives. Heliyon. 2019;5(11) doi: 10.1016/j.heliyon.2019.e02818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Giotta Lucifero A, Del Maestro M, Marfia G, Navone SE, Baldoncini M, et al. Anterolateral Approach for Retrostyloid Superior Parapharyngeal Space Schwannomas Involving the Jugular Foramen Area: A 20-Year Experience. World Neurosurg. 2019 doi: 10.1016/j.wneu.2019.09.006. [DOI] [PubMed] [Google Scholar]
- Millimaggi DF, Norcia VD, Luzzi S, Alfiero T, Galzio RJ, Ricci A. Minimally Invasive Transforaminal Lumbar Interbody Fusion with Percutaneous Bilateral Pedicle Screw Fixation for Lumbosacral Spine Degenerative Diseases. A Retrospective Database of 40 Consecutive Cases and Literature Review. Turk Neurosurg. 2018;28(3):454–461. doi: 10.5137/1019-5149.JTN.19479-16.0. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Gragnaniello C, Giotta Lucifero A, Marasco S, Elsawaf Y, Del Maestro M, et al. Anterolateral approach for subaxial vertebral artery decompression in the treatment of rotational occlusion syndrome: results of a personal series and technical note. Neurol Res. 2021;43(2):110–125. doi: 10.1080/01616412.2020.1831303. [DOI] [PubMed] [Google Scholar]
- Del Maestro M, Rampini AD, Mauramati S, Giotta Lucif-ero A, Bertino G, Occhini A, et al. Dye-Perfused Human Placenta for Vascular Microneurosurgery Training: Preparation Protocol and Validation Testing. World Neurosurg. 2020. [DOI] [PubMed]
- Luzzi S, Gragnaniello C, Marasco S, Lucifero AG, Del Maestro M, Bellantoni G, et al. Subaxial Vertebral Artery Rotational Occlusion Syndrome: An Overview of Clinical Aspects, Diagnostic Work-Up, and Surgical Management. Asian Spine J. 2020. [DOI] [PMC free article] [PubMed]
- Park J, Son W, Kwak Y, Ohk B. Pterional versus superciliary keyhole approach: direct comparison of approach-related complaints and satisfaction in the same patient. J Neuro-surg. 2018;130(1):220–226. doi: 10.3171/2017.8.JNS171167. [DOI] [PubMed] [Google Scholar]
- Yagmurlu K, Safavi-Abbasi S, Belykh E, Kalani MYS, Nakaji P, Rhoton AL,, Jr, et al. Quantitative anatomical analysis and clinical experience with mini-pterional and mini-or-bitozygomatic approaches for intracranial aneurysm surgery. J Neurosurg. 2017;127(3):646–659. doi: 10.3171/2016.6.JNS16306. [DOI] [PubMed] [Google Scholar]
- Mocco J, Komotar RJ, Raper DM, Kellner CP, Connolly ES, Solomon RA. The modified pterional keyhole craniotomy for open cerebrovascular surgery: a new workhorse? J Neurol Surg A Cent Eur Neurosurg. 2013;74(6):400–404. doi: 10.1055/s-0032-1333130. [DOI] [PubMed] [Google Scholar]
- Bir SC, Maiti T, Konar S, Nanda A. Comparison of the Surgical Outcome of Pterional and Frontotemporal-Orbit-ozygomatic Approaches and Determination of Predictors of Recurrence for Sphenoid Wing Meningiomas. World Neu-rosurg. 2017;99:308–319. doi: 10.1016/j.wneu.2016.10.057. [DOI] [PubMed] [Google Scholar]
- Giotta Lucifero A, Fernandez-Miranda JC, Nunez M, Bruno N, Tartaglia N, Ambrosi A, et al. The Modular Concept in Skull Base Surgery: Anatomical Basis of the Median, Paramedian and Lateral Corridors. Acta Biomed. 2021;92(S4):e2021411. doi: 10.23750/abm.v92iS4.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giotta Lucifero A, Baldoncini M, Bruno N, Tartaglia N, Ambrosi A, Marseglia GL, et al. Microsurgical Neurovascular Anatomy of the Brain: The Anterior Circulation (Part I) Acta Biomed. 2021;92(S4):e2021412. doi: 10.23750/abm.v92iS4.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giotta Lucifero A, Baldoncini M, Bruno N, Tartaglia N, Ambrosi A, Marseglia GL, et al. Microsurgical Neurovascular Anatomy of the Brain: The Posterior Circulation (Part II) Acta Biomed. 2021;92(S4):e2021413. doi: 10.23750/abm.v92iS4.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadri PA, Al-Mefty O. The anatomical basis for surgical preservation of temporal muscle. J Neurosurg. 2004;100(3):517–522. doi: 10.3171/jns.2004.100.3.0517. [DOI] [PubMed] [Google Scholar]
- al-Mefty O. Anand VK. Zygomatic approach to skull-base lesions. J Neurosurg. 1990;73(5):668–673. doi: 10.3171/jns.1990.73.5.0668. [DOI] [PubMed] [Google Scholar]
- Al-Mefty O. Skull base: zygomatic approach. Neurosurgery. 1986;19(4):674–675. doi: 10.1097/00006123-198610000-00034. [DOI] [PubMed] [Google Scholar]
- Chen CT, Robinson JB,, Jr, Rohrich RJ, Ansari M. The blood supply of the reverse temporalis muscle flap: anatomic study and clinical implications. Plast Reconstr Surg. 1999;103(4):1181–1188. doi: 10.1097/00006534-199904040-00012. [DOI] [PubMed] [Google Scholar]
- Cheung LK. The blood supply of the human temporalis muscle: a vascular corrosion cast study. J Anat. 1996;189((Pt 2)(Pt 2)):431–8. [PMC free article] [PubMed] [Google Scholar]
- Spetzler RF, Lee KS. Reconstruction of the temporalis muscle for the pterional craniotomy. Technical note. J Neuro-surg. 1990;73(4):636–7. doi: 10.3171/jns.1990.73.4.0636. [DOI] [PubMed] [Google Scholar]


