Abstract
Background:
There is a growing population of adolescent and young adult (AYA, age 15–39 years) acute leukemia survivors in whom long-term mortality outcomes are largely unknown.
Methods:
The current study utilized the Surveillance, Epidemiology, and End Results (SEER) registry to assess long-term outcomes of AYA acute leukemia 5-year survivors. The impact of diagnosis age, sex, race/ethnicity, socioeconomic status, and decade of diagnosis on long-term survival were assessed utilizing an accelerated failure time model.
Results:
1,938 AYA acute lymphoblastic leukemia (ALL) and 2,350 AYA acute myeloid leukemia (AML) survivors diagnosed between 1980 and 2009 were included with a median follow-up of 12.3 and 12.7 years, respectively. Ten-year survival for ALL and AML survivors was 87% and 89%, respectively, and 99% for the general population. Survival for AYA leukemia survivors remained below that of the age-adjusted general population at up to 30 years of follow-up. Primary cancer mortality was the most common cause of death in early survivorship with non-cancer causes of death becoming more prevalent in later decades of follow-up. Male AML survivors had significantly worse survival than females (Survival Time Ratio: 0.61, 95% Confidence Interval: 0.45–0.82).
Conclusions:
AYA leukemia survivors have higher mortality rates than the general population that persist for decades after diagnosis.
Impact:
While there have been improvements in late-mortality, long-term survival for AYA leukemia survivors remains below that of the general population. Studies investigating risk factors for mortality and disparities in late-effects among long-term AYA leukemia survivors are needed.
Keywords: Adolescent and Young Adult Oncology, AYA, Acute Lymphoblastic Leukemia, Acute Myeloid Leukemia, ALL, AML, Survival, Disparities
INTRODUCTION
The incidence of cancers among adolescent and young adults (AYA; ages 15–39 years) has increased by approximately 30% since the 1970s,(1) with approximately 90,000 new diagnoses per year in the United States.(2) This has been accompanied by improvement in 5-year survival rates to >80% across all AYA cancer types,(3, 4) leading to an increasing population of survivors. Compared with the general population, AYA cancer survivors have higher mortality rates that persist decades into survivorship.(5, 6)
Leukemias, specifically acute lymphocytic leukemia (ALL) and acute myeloid leukemia (AML), are two of the more common AYA cancer diagnoses, with combined incidence rates of 3.1, 2.9, and 4.1 per 100,000 among those aged 15–19, 20–29, and 30–39 years, respectively.(3) Five-year survival for both ALL and AML decreases with older age at diagnosis, from 74% and 66% in those diagnosed at age 15–19 years to 52% and 59% in those diagnosed at 20–39 years, and 51% and 57% for those diagnosed at 30–39 years, respectively.(2) Compared with younger children diagnosed with ALL, AYAs with ALL are at increased risk of treatment-related toxicity,(7) and among AYA cancer diagnoses, a leukemia diagnosis carries a higher 20-year mortality burden than most other common AYA cancer types.(8) Additionally, the majority of AYA patients with AML are recommended to undergo hematopoietic cell transplantation (HCT) in first remission, which itself carries a high risk of late effects,(9) making investigation of long-term survival patterns fundamental in the AYA leukemia survivor population.
While recent studies have reported improving overall survival for AYA leukemia patients,(8) demographic factors impact survival in the AYA population. Specifically, 5-year survival rates among AYA cancer survivors varies by sex, race/ethnicity and socioeconomic status (SES). Male, non-Hispanic Black (hereafter, Black), Hispanic patients, and those living in rural counties and areas with lower SES often experience worse survival compared with female, non-Hispanic White (hereafter, White) patients and those living in metro counties and areas of higher SES.(10–16) In AYA leukemia patients, racial disparities in survival have been demonstrated at up to 10-years of follow-up,(17, 18) however, more data are needed to assess whether these and other disparities persist further into survivorship. The current study used the Surveillance, Epidemiology, and End Results (SEER) database, a United States population-based registry, to characterize long-term mortality patterns among 5-year survivors of AYA ALL and AML, as well as determine the impact of race, SES, rurality, diagnosis age, and sex on long-term survival in this population. While previous studies among AYA leukemia patients have assessed survival from time of diagnosis, the current study is unique in its inclusion of only 5-year survivors, thus focusing on disparities specific to the survivorship period.
MATERIALS and METHODS
Data on each diagnosis were extracted from SEER for the years 1975–2011. Information was identified for 2,313 patients with ALL and 2,842 patients with AML who were alive 5 years after diagnosis. Of those, 375 ALL survivors and 492 AML survivors were excluded due to missing race/ethnicity code or classified as American Indian (due to low numbers), missing county level statistics, missing Rural-Urban Continuum code, or were diagnosed prior to 1980 or after 2009 (Supplemental Figures 1 and 2). As long-term survival by decade of diagnosis was assessed, patients were excluded if they were diagnosed prior to 1980 or after 2009 as data spanning the entire decade of diagnosis was not available for the 1970s or 2010s. In the SEER dataset, race/ethnicity information is based on medical records. When SEER race/ethnicity data has been compared with self-report data, agreement was reported as excellent for race and moderate to substantial on Hispanic ethnicity.(19)
A county-level socioeconomic deprivation index (SES index) was derived as previously defined by Truong et al.(20) County-level variables used to derive the SES index included poverty (P) per the percentage with income below the 200th percentile of the poverty line, low educational attainment (E) per the percentage with less than a high school education obtained, household crowding (C) per the percentage with greater than one person per room, unemployment (U) per the percentage unemployed, levels of immigration (I) per the percentage of foreign-born, and language isolation (L) per the percentage of language isolation. The variables P, E, C, U, I, L were standardized within the ALL and AML populations as the difference from the mean divided by the standard deviation. The county SES index was calculated as (((P + E + C + U)/4) + ((I + L)/2))/2. As this is a socioeconomic deprivation index, higher values indicate greater deprivation.
Continuous variables were summarized by decade and overall as mean, standard deviation, median, minimum, and maximum. Discrete variables were summarized by decade and overall as count, percentage, mortality count, and mortality percentage. Unadjusted Kaplan-Meier curves were used to summarize the relationships between survival and age group at diagnosis, sex, race/ethnicity, and SES tertile.(21)
The age-adjusted survival curve for the US general population was derived from the 2014 US life tables obtained from the National Vital Statistics Report,(22) per a hypothetical table cohort of 100,000. The cumulative survival probability for each age was estimated using the product-limit method, based upon the change in number of individuals surviving to a specific age, adjusted with reference to age 30 (the mean of the mean ages of AYA ALL and AML 5 years following diagnosis). Pairwise differences among the US National cohort, ALL, and AML were assessed by the log-rank test. Cause of death was obtained from the SEER database and categorized into two groups, death by acute leukemia and death by other. In AYA ALL and AML survivors, death by acute leukemia was defined as cause of death reported as ALL, AML, aleukemic leukemia, subleukemic leukemia, leukemia not otherwise specified, other acute leukemia, or other myeloid/monocytic leukemia. Death by other causes included all other causes of death as reported in SEER. Cumulative incidence plots were used to illustrate the competing risks of incidence of death by acute leukemia versus death by other than acute leukemia among AYA 5-year survivors separately by ALL and AML.
Separately by ALL and AML, a time-to-event model was used to model the time (following 5 years survival) to death with relation to diagnosis decade (1980s, 1990s, 2000s) and covariates including age at diagnosis (numeric years), rurality (numeric integer between 0 and 8), race and origin (Non-Hispanic White, Hispanic (All Races), Non-Hispanic Black, Non-Hispanic Asian or Pacific Islander), sex (female versus male), and SES index. A Cox proportional hazards model was considered, but due to violations of the proportionality of hazards assumption, an accelerated failure time (AFT) model was selected instead.(23) Weibull, exponential, Gaussian, logistic, log-normal, and log-logistic distributions were evaluated. The log-normal distribution was chosen as optimal for ALL, and the Weibull distribution was chosen as optimal for AML, due to lower Akaike Information Criterion (AIC) together with a good fit of a Kaplan-Meier plot of model residuals to the model distribution. Differences in survival among discrete variable levels were assessed by Tukey-adjusted contrasts.
Statistical analyses were performed using R statistical software (R Core Team, 2020, version 3.6.3). In all statistical tests, two-sided alpha=.05. Survival modeling was performed using the “survival” package.(24, 25) Cumulative incidence plots were produced using the “cmprsk” package.(26) Assessment of differences among discrete variable levels in the accelerated failure time model were estimated using the “emmeans” package;(27) this includes adjusted means weighted proportionally to covariate marginal frequencies.
RESULTS
Patient Characteristics
The characteristics of 1,938 AYA ALL 5-year survivors and 2,350 AYA AML 5-year survivors are shown in Table 1. Of the ALL survivors, 6% were Black, 29% were Hispanic, 7% were non-Hispanic Asian, or Pacific Islander (hereafter Asian or Pacific Islander), and 58% were White. Among AML survivors, 9% were Black, 22% were Hispanic, 10% were Asian or Pacific Islander, and 59% were White. The median age of diagnosis was 23 years (range 15–39) for ALL and 28 years (range 15–39) for AML. Median follow-up times (from 5-year survival) were 7.3 and 7.7 years for ALL and AML, respectively, with a common range of 0.1 to 31.9 years. The county-level SES indices, as well as the county-level variables that informed the indices, are summarized in Table 1, with index measurements ranging from −1.6 (lowest) to 2.5 (highest) and −1.5 to 2.5 for ALL and AML survivors, respectively, and respective medians of −0.13 and −0.10.
Table 1.
Survivor Characteristics
| Characteristic | ALL Survivors, No. (%) | AML Survivors, No. (%) |
|---|---|---|
| Sex | ||
| Female | 724 (37.4) | 1217 (51.8) |
| Male | 1214 (62.6) | 1133 (48.2) |
| Age at Diagnosis | ||
| Mean ± STD | 22.6±7.3 | 28.3±7.2 |
| Median(range) | 20.0 (15.0–39.0) | 29.0 (15.0–39.0) |
| Follow-up Time | ||
| Mean ± STD | 9.1±7.0 | 9.0±6.5 |
| Median(range) | 7.3 (0.1 – 31.9) | 7.7 (0.1–31.9) |
| Race/Ethnicity | ||
| Non-Hispanic White | 1129 (58.3) | 1393 (59.3) |
| Non-Hispanic Black | 111 (5.7) | 219 (9.3) |
| Hispanic (All Races) | 557 (28.7) | 507 (21.6) |
| Non-Hispanic Asian or Pacific | 141 (7.3) | 231 (9.8) |
| Islander | ||
| Decade of Diagnosis | ||
| 1980s | 199 (10.3) | 206 (8.8) |
| 1990s | 397 (20.5) | 538 (22.9) |
| 2000s | 1342 (69.2) | 1606 (68.3) |
| Rurality Index, Mean ± STD | 0.7±1.4 | 0.7±1.4 |
| County % <200% Poverty | ||
| Mean ± STD | 31.6±9.0 | 31.6±9.1 |
| Median(range) | 30.9 (10.3–61.9) | 30.7 (10.3–71.9) |
| County % HS Education | ||
| Mean ± STD | 14.0±6.1 | 13.7±5.9 |
| Median(range) | 12.5 (2.1–32.4) | 12.5 (3.2–32.4) |
| County % Housed >1 per Room | ||
| Mean ± STD | 5.4±3.7 | 5.1±3.6 |
| Median(range) | 3.7 (0.0–13.0) | 3.5 (0.0–13.0) |
| County % Unemployed | ||
| Mean ± STD | 7.0±2.0 | 7.0±2.1 |
| Median(range) | 6.9 (1.3–16.0) | 6.9 (2.1–16.1) |
| County % Foreign Born | ||
| Mean ± STD | 19.7±11.5 | 19.2±11.4 |
| Median(range) | 20.7 (0.1–43.0) | 20.0 (0.3–43.0) |
| County % Language Isolated | ||
| Mean ± STD | 6.7±4.4 | 6.4±4.2 |
| Median(range) | 6.3 (0.0–21.9) | 6.2 (0.0–21.9) |
| SES Index | ||
| Mean ± STD | 0.0±0.8 | 0.0±0.8 |
| Median(range) | −0.1 (−1.6–2.5) | −0.1 (−1.5–2.5) |
Overall Survival and Cause of Death
Kaplan-Meier plots of overall survival of 5-year AYA ALL and AML survivors compared with the age-adjusted expected survival of the general population through 30 years of follow-up are shown in Figure 1A. There was no evidence of difference in survival between ALL and AML survivors (p=0.3), however, each had significantly lower survival with comparison to the US general population (p<0.0001 in each comparison).
Figure 1.
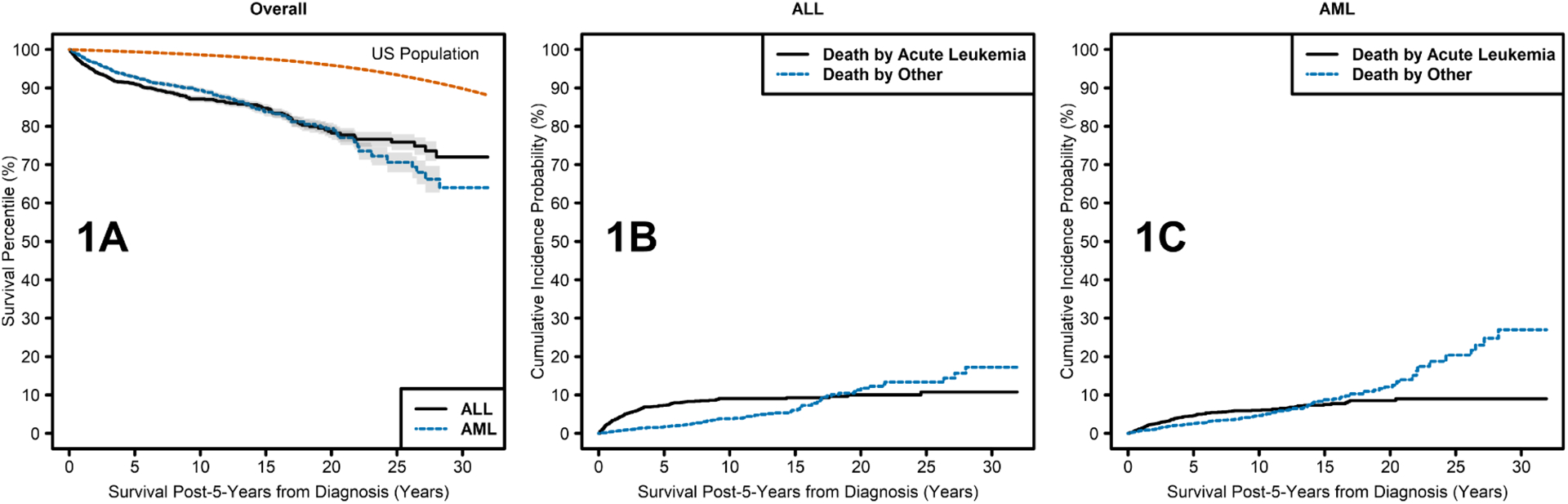
Kaplan Meier curves showing: A) survival over time among 5-year survivors of AYA ALL and AML compared with the age-adjusted expected survival of the general population; B) death by acute leukemia and death by other causes over time in 5-year survivors of AYA ALL; C) death by acute leukemia and death by other causes over time in 5-year survivors of AYA AML.
For ALL, 10-year survival for those diagnosed in the 1980s, 1990s, and 2000s was 83%, 88%, and 88%, respectively. Ten-year survival for AML was 82%, 90%, and 90% for those diagnosed in the 1980s, 1990s, and 2000s, respectively. Causes of death are shown in Supplemental Table 1. In the earlier survivorship years the most common cause of death for both ALL and AML survivors was acute leukemia, however, deaths due to acute leukemia plateaued approximately 10 years post diagnosis for patients with both ALL and AML. Deaths due to other causes continued to climb throughout the survivorship period (Figures 1B and 1C). Death due to subsequent malignancies and cardiac disease were the most common causes of death due to other causes for both ALL and AML survivors.
Survival by Age at Diagnosis and Sex
In the covariate-adjusted models, age at diagnosis was significantly associated with differential long-term survival (p<0.0001 for both ALL and AML), with each additional year at diagnosis associated with a 6% and 5% decrease in long-term survival for ALL and AML survivors, respectively (Table 2). These trends of declining survival with increasing age are evident in the unadjusted Kaplan-Meier plots of Figures 2A and 2B, where age is categorized into discrete ranges (15–19, 20–29, and 30–39 years).
Table 2.
Differences in Survival by Age, and Rurality and SES Index. These are estimated from the accelerated failure time survival model which included the covariates diagnosis decade, age at diagnosis, race and origin, sex, rurality, and SES index. Age at diagnosis and rurality p-values are unadjusted due to an absence of multiple comparisons.
| Continuous Variable | Survival Time Ratio (95% CI) | p-value |
|---|---|---|
| ALL Survivors | ||
| Age at Diagnosis | 0.94 (0.92–0.97) | <0.0001 |
| Rurality | 0.96 (0.82–1.12) | 0.61 |
| SES Index | 0.80 (0.58–1.11) | 0.17 |
| AML Survivors | ||
| Age at Diagnosis | 0.95 (0.93–0.97) | <0.0001 |
| Rurality | 1.06 (0.94–1.18) | 0.36 |
| SES Index | 1.09 (0.86–1.37) | 0.49 |
Figure 2.
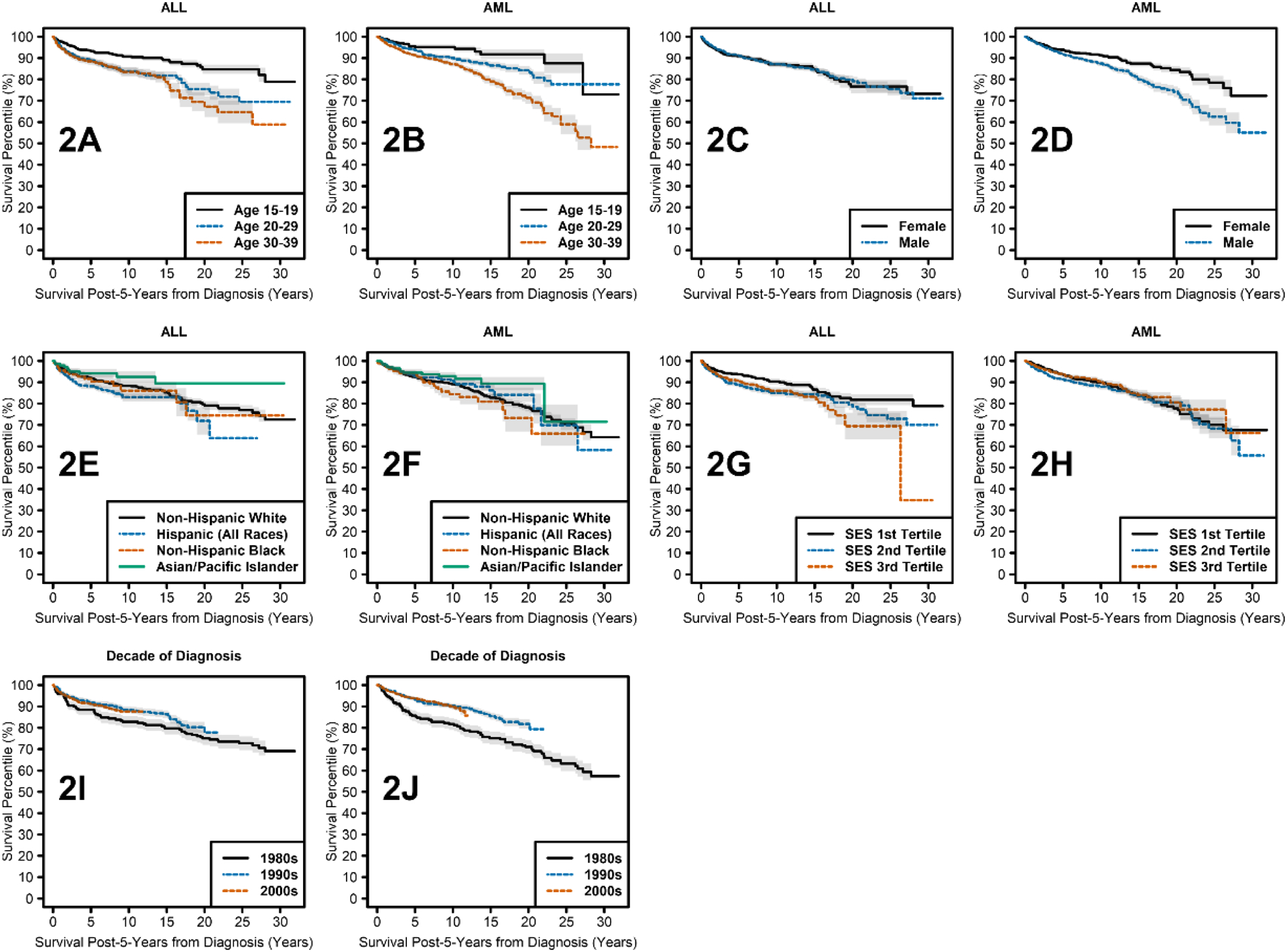
Kaplan Meier curves showing survival over time by: A) age at diagnosis in 5-year survivors of AYA ALL; B) age at diagnosis in 5-year survivors of AYA AML; C) sex in 5-year survivors of AYA ALL; D) sex in 5-year survivors of AYA AML; E) race ethnicity in 5-year survivors of AYA ALL; F) race/ethnicity in 5-year survivors of AYA AML; G) SES by tertile in 5-year survivors of AYA ALL; H) SES by tertile in 5-year survivors of AYA AML; I) decade of diagnosis in 5-year survivors of AYA ALL; J) decade of diagnosis in 5-year survivors of AYA AML.
Survival time ratios (STR) report the percent duration of survival for a group of interest compared with the referent group. Male AYA AML survivors had significantly worse survival than female survivors (p<0.0001), with males surviving only 61% as long as females (STR: 0.61, 95% Confidence Interval (CI): 0.45–0.82, Table 3, Figure 2D). However, no evidence of a survival difference due to sex was found for ALL (STR: 0.96, 95% CI: 0.62–1.49, Table 3, Figure 2C).
Table 3.
Differences in Survival by Sex and Decade of Diagnosis. These are estimated from the accelerated failure time survival model which included the covariates diagnosis decade, age at diagnosis, race and origin, sex, rurality, and SES index. Tukey adjustment was not applicable to comparison between sexes, since there was only one comparison.
| Survival Time Ratio (95% CI) | Unadjusted p-value | Tukey Adjusted p-value | |
|---|---|---|---|
| Sex Comparison | |||
| ALL Survivors | |||
| Male - Female | 0.96 (0.62–1.49) | 0.87 | Not Applicable |
| AML Survivors | |||
| Male-Female | 0.61 (0.45–0.82) | 0.0009 | Not Applicable |
| Decade Comparison | |||
| ALL Survivors | |||
| 1990s – 1980s | 2.62 (1.29–5.31) | 0.008 | 0.021 |
| 2000s – 1980s | 2.37 (1.24–4.55) | 0.009 | 0.025 |
| 2000s – 1990s | 0.91 (0.54–1.52) | 0.71 | 0.93 |
| AML Survivors | |||
| 1990s – 1980s | 2.18 (1.44–3.29) | 0.0002 | 0.0007 |
| 2000s – 1980s | 2.17 (1.43–3.30) | 0.0003 | 0.0008 |
| 2000s – 1990s | 1.0 (0.70–1.43) | 0.99 | 1.00 |
Survival by Race/Ethnicity
Asian or Pacific Islander ALL survivors had higher survival time than Hispanic ALL survivors (unadjusted p=0.009, Tukey p=0.047, STR: 3.77, 95% CI: 1.39–10.28, Table 4). Additionally, Hispanic ALL survivors had a trend towards lower survival time than White ALL survivors (unadjusted p=0.036, Tukey p=0.15, STR: 0.56, 95% CI: 0.32–0.96, Table 4, Figure 2E). Other differences in long-term survival between race/ethnicity groups assessed lacked evidence of significance (Table 4, Figures 2E and 2F).
Table 4.
Differences in Survival by Race and Ethnicity. These are estimated from the accelerated failure time survival model which included the covariates diagnosis decade, age at diagnosis, race and origin, sex, rurality, and SES index, with interactions of diagnosis decade with sex, race and origin, and SES index.
| Race/Ethnicity Comparison | Survival Time Ratio (95% CI) | Unadjusted p-value | Tukey Adjusted p-value |
|---|---|---|---|
| ALL Survivors | |||
| Non-Hispanic Black – Non-Hispanic White | 0.82 (0.32–2.08) | 0.68 | 0.98 |
| Hispanic (All Races) – Non-Hispanic White | 0.56 (0.32–0.96) | 0.036 | 0.15 |
| Non-Hispanic Black – Hispanic (All Races) | 1.47 (0.55–3.94) | 0.44 | 0.87 |
| Non-Hispanic Asian or Pacific Islander – Non-Hispanic White | 2.10 (0.79–5.56) | 0.1 | 0.44 |
| Non-Hispanic Asian or Pacific Islander – Hispanic (All Races) | 3.77 (1.39–10.28) | 0.009 | 0.047 |
| Non-Hispanic Asian or Pacific Islander – Non-Hispanic Black | 2.56 (0.71–9.19) | 0.15 | 0.47 |
| AML Survivors | |||
| Non-Hispanic Black – Non-Hispanic White | 0.70 (0.43–1.13) | 0.14 | 0.45 |
| Hispanic (All Races) – Non-Hispanic White | 0.92 (0.6–1.43) | 0.72 | 0.98 |
| Non-Hispanic Black – Hispanic (All Races) | 0.75 (0.42–1.34) | 0.33 | 0.77 |
| Non-Hispanic Asian or Pacific Islander – Non-Hispanic White | 1.49 (0.81–2.77) | 0.20 | 0.58 |
| Non-Hispanic Asian or Pacific Islander – Hispanic (All Races) | 1.62 (0.82–3.19) | 0.16 | 0.50 |
| Non-Hispanic Asian or Pacific Islander – Non-Hispanic Black | 2.15 (1.04–4.45) | 0.039 | 0.17 |
Survival by SES and Rurality
The county SES index took into account county-level education level, poverty, unemployment, household crowding, and immigration status. Kaplan-Meier curves showing survival by SES index categorized into discrete tertiles ranging from highest SES (tertile 1), middle SES (tertile 2), and lowest SES (tertile 3) are shown for ALL (Figure 2G) and AML survivors (Figure 2H). Socioeconomic status did not significantly impact long-term survival in our population of 5-year AYA ALL and AML survivors (Table 2). Likewise, rurality also did not significantly impact long-term survival in our population of 5-year AYA ALL and AML survivors (Table 2).
Survival by Decade of Diagnosis
Long-term survival of both AYA ALL and AML survivors was significantly associated with decade of diagnosis (Table 3, Figures 2I and 2J). Long-term survival times for those diagnosed in the 1990s were more than twice that of patients diagnosed in the 1980s for ALL (unadjusted p=0.008, Tukey p=0.021, STR: 2.62, 95% CI: 1.29–5.31) and AML (unadjusted p=0.0002, adjusted p=0.0007, STR: 2.18, 95% CI: 1.44–3.29). Similarly, survival times more than doubled for those diagnosed in the 2000s compared with those in the 1980s for ALL (unadjusted p=0.009, Tukey p=0.025, STR: 2.37, 95% CI: 1.24–4.55) and AML (unadjusted p=0.0003, Tukey p=0.0008, STR: 2.17, 95% CI: 1.43–3.30). However, there were no significant long-term survival differences for those diagnosed in the 2000s compared with the 1990s for either ALL or AML.
DISCUSSION
Overall, we found that 5-year survivors of AYA leukemias have worse survival compared with the age-adjusted expected survival of the overall population at up to 30 years of follow-up. Primary disease remained the most common cause of death in the early survivorship period, however the incidence of death from other causes increased over time, overtaking primary disease as the most common cause of death at about 15 years of follow-up. Importantly, there have been improvements in long-term survival for 5-year AYA leukemia survivors over time, with those diagnosed in the 1990s and 2000s, demonstrating more than twice the survival time compared with those diagnosed in the 1980s. In addition to overall inferior survival compared with the general population, persistent ethnic disparities were found. Older age at diagnosis also negatively impacted long-term survival, as did male sex among AML survivors.
Increased long-term mortality rates in 5-year survivors of childhood leukemias compared with age-adjusted expected rates of the general population have previously been demonstrated, (28) and long-term survival rates of AYA leukemia survivors in the current study appear to be lower than those of childhood leukemia survivors. Specifically, data from the Childhood Cancer Survivor Study indicate an overall survival rate of about 94% at 20 years of follow-up for 5-year survivors of childhood leukemia, which is higher than observed in AYAs.(29, 30) While AYAs are older at diagnosis and thus have a higher burden of age-related comorbidities over time, an increased risk for treatment-related toxicity in AYAs compared with pediatric patients could also be contributing to these differences, leading to increased risk for a multitude of late effects, including second cancers, cardiac, pulmonary, and endocrine diseases, all of which increase morbidity.(7, 31, 32)
Additionally, we found that acute leukemia remains the most common cause of death in 5-year AYA leukemia survivors until about 20 years of follow-up. In the childhood cancer survivor primary disease progression/recurrence remains the most common cause of death until about 30 years of follow-up.(28) Common late causes of death in AYA cancer survivors include secondary malignancies, cardiovascular disease, and infectious diseases.(33, 34) This further points to the need to focus on early identification and treatment of co-morbidities and late effects, as well as upfront risk modification in the AYA population.
Previous studies assessing racial/ethnic disparities in 5-year survival among AYA leukemia patients have had differing findings. Among AYA ALL patients, the majority of studies to date have found that White patients have improved survival compared with Black patients.(11, 17) However, a recent large population-based analysis showed similar survival between White and Black AYA ALL patients with the exception of male ALL patients.(14) Hispanic AYA ALL patients have consistently been found to have worse survival outcomes compared with White patients.(11, 14, 17) Hispanic ALL patients are more likely to have high-risk genetic alterations that portend worse prognosis, such as Philadelphia chromosome-positive ALL and Philadelphia chromosome-like ALL.(35–37) In addition to increasing the risk of early mortality, treatment regimens for these subtypes are more likely to include high-intensity chemotherapy and HCT, thus increasing risks of late effects.(38, 39) While adjusted analyses do not show evidence of a difference in long-term survival, the current analysis suggests that disparities among Hispanic AYA ALL survivors compared with White survivors persist well beyond 5 years post-diagnosis. Adjustment for SES may be contributing to these findings not reaching statistical significance and it is also possible that our cohort size limited the ability to detect differences in survival between Hispanic and White AYA ALL survivors in the second and third decade of survivorship. As SEER does not contain patient-level SES data, it is difficult to fully evaluate the extent to which SES status impacts racial/ethnic survival disparities among long-term AYA leukemia survivors. Future studies are needed to clarify this interaction and work towards reducing both racial/ethnic and SES disparities in long-term survival.
Analyses of disparities in 5-year mortality of AYA AML patients have had less consistent results. Similar to findings in AYA ALL, survival for Black patients has been found to be both worse and similar compared with White patients.(11, 14, 17, 18) However, when comparing between Hispanic patients and White patients, studies have predominantly found similar survival between the two groups,(11, 14) though both increased and decreased survival for Hispanic patients have also been reported.(17, 18) Data reporting differences in survival for Asian or Pacific Islander AYA leukemia patients are sparse.
SES impacts multiple aspects of cancer care. Lower SES status has been reported to impact survival in the AYA cancer population,(11, 12, 40, 41) however, assessing AYA leukemia specifically, SES has been found to inconsistently impact cancer-specific survival, with one analysis only finding an impact among White patients.(42) In the current study, there was no evidence of SES independently impacting long-term survival among AYA leukemia survivors. A limitation that comes with utilizing SEER is that this database collects SES data on a county population level, rather than an individual level, which makes interpretation of SES data in this population complicated, particularly in the case of the current analysis as data is collected at the time of diagnosis and our focus is on long-term survivorship. Lower SES, compared with higher SES, impacts access to care, including decreased use of subspecialty services and survivorship care as well as an increasing the likelihood of diagnosis delays thus likely contributing to disparities in both short- and long-term mortality.(43–46) While we did not find an association between SES and long-term survival in AYA leukemia patients, this should be a focus of future studies.
Sex-based disparities in long-term mortality were seen for AML survivors, however none was found for ALL. The absence of disparities for ALL by sex was somewhat unexpected, as females have longer lifespans than males in the general population,(47) and in a previous analysis, our group found dramatic sex-based disparities in long-term survival among 5-year AYA lymphoma survivors.(48) One potential reason for this finding is that anthracyclines, commonly used to treat AYA leukemia and known to carry a risk of cardiotoxicity, differentially impact males and females in late effects profile, with females more likely to develop cardiac morbidity and mortality,(49) though these agents are also used to treat lymphoma patients. In childhood ALL patients, females experience significantly more acute treatment related toxicities and treatment-related deaths than males.(50) More data are needed on differences in short- and long-term treatment toxicities by sex in the AYA population.
Older age at diagnosis was associated with inferior long-term survival. While the natural course of age-related comorbidities occurring earlier into survivorship for older patients likely contributed to this finding, AYA leukemia patients diagnosed at older ages also have cancer- and treatment-specific factors placing them at higher risk for long-term survival deficits. Increasing age is associated with an increase in more unfavorable cytogenetics in both AYA ALL and AML patients, as well as higher likelihood of HCT and increased risk of treatment related mortality.(51, 52) Additionally, participation in clinical trials has been found to reduce risk of relapse for AYAs with leukemia, however trial enrollment decreases with increasing age in this population.(51, 53, 54)
Similar to findings in the childhood cancer population,(55) we found that long-term survival for AYA leukemia survivors improved in more recent decades of diagnosis. Importantly, treatment advances leading to increased cure rates and improvements in supportive care, specifically for patients receiving stem cell transplants, have improved survival for AYA leukemia patients diagnosed in more recent decades.(56) For example, reduced-intensity conditioning therapy was introduced in the 1990s, leading to a decrease in treatment toxicities which could reduce risk of late-effect related long-term mortality.(57, 58) Also in the 1990s, treatment with lower doses of anthracyclines were shown to have similar 5-year survival and lower risk of cardiac dysfunction following therapy.(59) There is a strong association between anthracycline dose and risk of late-cardiotoxicity,(60) thus the lower dose treatments likely contributed to improved long-term survival for those AYA leukemia patients treated in the 1990s and 2000s. Early ALL treatment included cranial radiation for most patients, however, starting in the 1980s with the introduction of triple intrathecal therapy, the use of prophylactic cranial radiation declined and with continued advances in systemic therapy the need for prophylactic cranial radiation was eliminated by the late 2000s.(61) This change likely contributed to decreased risk secondary malignancies, endocrinopathies, and frailty among survivors treated in more recent decades.(62) Finally, tyrosine kinase inhibitors first used in the early 2000s, resulting in improvement in survival for PH+ ALL, and although data are limited, does not appear to have significantly increased the long-term toxicity profile associated with early mortality.(63) The growing use of immunotherapy has improved short term survival in relapsed/refractory and high-risk cases, although knowledge of treatment-related late-effects are largely unknown.(64) As treatment regimens continue to evolve with particular focus to reduce toxicity in the treatment of high-risk genetic subtypes,(65) improvements are likely to continue, though whether these efforts result in decreasing racial/ethnic disparities in long-term survival need to be assessed.
SEER is considered as a definitive source of U.S. population-level cancer data, however, there are limitations with this dataset. Data on specific treatment regimens are not available, thus the extent to which treatment variables impacted disparities in long-term survival is unknown. SEER does contain cause of death data, however, due to coding it is difficult to discern whether death from acute leukemia represents primary disease mortality or secondary cancer. As such, we grouped all forms of acute leukemias together to broadly capture primary disease. It is likely that some of these cases represented secondary cancers, specifically patients with ALL whose cause of death in SEER is reported as AML. For all AYA cancer survivors, the cumulative incidence of secondary cancers at 30 years of follow-up is 13.9% and for AYA ALL and AML survivors, secondary leukemias represent less than 3% of total secondary malignancies.(66) The ability to capture rural-urban disparities in long-term survival of AYA leukemia survivors may also be limited as rural populations are underrepresented in the SEER dataset. As previously discussed, SES data from SEER is captured on a population level. While census tract SES data have previously been shown to more accurately reflect individual mortality than county-level data,(67) census tract data is unavailable in SEER prior to the year 2000. Therefore, we utilized county-level data to create a county-level SES deprivation index. As SES data was collected at time of diagnosis and not tracked over time, this also limits conclusions that can be drawn. Strengths of the current study include a population-based cohort with large sample size and length of follow-up time for two diagnoses that are classified as rare disease in AYAs. Despite the large sample size, there are relatively lower numbers of racial/ethnic minorities represented, which may limit the ability to detect disparities in long-term survival between these populations.
The findings in the current study have demonstrated that 5-year survivors of AYA leukemia have survival outcomes inferior to the age-adjusted expected survival rates of the general population, with race/ethnicity, age at diagnosis, and decade of diagnosis impacting survival at up to three decades of follow-up for AYA leukemia survivors, as well as sex among AML survivors. While long-term survival has improved in recent decades, disparities remain. In this study, the median ages at diagnosis were 23 years for ALL and 28 years for ALL, thus survivors have the potential to live another 50+ years from diagnosis. Adherence to preventive care guidelines and aggressive risk factor reduction should be emphasized in the care of long-term AYA leukemia survivors. While identification of vulnerable populations of AYA leukemia survivors is an important step towards targeted interventions, further understanding of factors placing survivors at risk for late morbidity and mortality is needed to develop surveillance strategies and reduce risk factors.
Supplementary Material
Funding:
This work was supported by the National Cancer Institute at the National Institutes of Health grant numbers P30 CA016672 (MR) and R38-HL143612 (AB) and research support from the Archer Foundation and LyondellBasell (MR, JAL).
Footnotes
Conflict of Interest Disclosures: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Scott AR, Stoltzfus KC, Tchelebi LT, Trifiletti DM, Lehrer EJ, Rao P, et al. Trends in Cancer Incidence in US Adolescents and Young Adults, 1973–2015. JAMA Netw Open. 2020;3(12):e2027738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70(6):443–59. [DOI] [PubMed] [Google Scholar]
- 3.Close AG, Dreyzin A, Miller KD, Seynnaeve BKN, Rapkin LB. Adolescent and young adult oncology-past, present, and future. CA Cancer J Clin. 2019;69(6):485–96. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Moke DJ, Tsai KY, Hwang A, Freyer DR, Hamilton AS, et al. A Reappraisal of Sex-Specific Cancer Survival Trends Among Adolescents and Young Adults in the United States. J Natl Cancer Inst. 2019;111(5):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkman AM, Livingston JA, Merriman K, Hildebrandt M, Wang J, Dibaj S, et al. Long-term survival among 5-year survivors of adolescent and young adult cancer. Cancer. 2020;126(16):3708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson C, Smitherman AB, Nichols HB. Conditional relative survival among long-term survivors of adolescent and young adult cancers. Cancer. 2018;124(14):3037–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Damania RC, Talati R, O’Riordan MA, Matloub YH, Ahuja SP. Increased Toxicity Among Adolescents and Young Adults Compared with Children Hospitalized with Acute Lymphoblastic Leukemia at Children’s Hospitals in the United States. J Adolesc Young Adult Oncol. 2021. [DOI] [PubMed] [Google Scholar]
- 8.Anderson C, Nichols HB. Trends in late mortality among adolescent and young adult (AYA) cancer survivors. J Natl Cancer Inst. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CJ, Kim S, Tecca HR, Bo-Subait S, Phelan R, Brazauskas R, et al. Late effects after ablative allogeneic stem cell transplantation for adolescent and young adult acute myeloid leukemia. Blood Adv. 2020;4(6):983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avila JC, Livingston JA, Rodriguez AM, Kirchhoff AC, Kuo YF, Kaul S. Disparities in Adolescent and Young Adult Sarcoma Survival: Analyses of the Texas Cancer Registry and the National SEER Data. J Adolesc Young Adult Oncol. 2018;7(6):681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moke DJ, Tsai K, Hamilton AS, Hwang A, Liu L, Freyer DR, et al. Emerging Cancer Survival Trends, Disparities, and Priorities in Adolescents and Young Adults: A California Cancer Registry-Based Study. JNCI Cancer Spectr. 2019;3(2):pkz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keegan TH, Clarke CA, Chang ET, Shema SJ, Glaser SL. Disparities in survival after Hodgkin lymphoma: a population-based study. Cancer Causes Control. 2009;20(10):1881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puthenpura V, Canavan ME, Poynter JN, Roth M, Pashankar FD, Jones BA, et al. Racial/ethnic, socioeconomic, and geographic survival disparities in adolescents and young adults with primary central nervous system tumors. Pediatr Blood Cancer. 2021;68(7):e28970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy CC, Lupo PJ, Roth ME, Winick NJ, Pruitt SL. Disparities in cancer survival among adolescents and young adults: a population-based study of 88,000 patients. J Natl Cancer Inst. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hossain MJ, Xie L. Sex disparity in childhood and young adult acute myeloid leukemia (AML) survival: Evidence from US population data. Cancer Epidemiol. 2015;39(6):892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KJ, Wang X, Barnes JM, Delavar A. Associations between geographic residence and US adolescent and young adult cancer stage and survival. Cancer. 2021;127(19):3640–50. [DOI] [PubMed] [Google Scholar]
- 17.Kahn JM, Keegan TH, Tao L, Abrahao R, Bleyer A, Viny AD. Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer. 2016;122(17):2723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durani U, Go RS. Racial and ethnic disparities in the survival of adolescents and young adults with acute myeloid leukemia: a retrospective study using the US National Cancer Data Base. Leuk Lymphoma. 2017;58(5):1184–9. [DOI] [PubMed] [Google Scholar]
- 19.Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18(2):177–87. [DOI] [PubMed] [Google Scholar]
- 20.Truong B, Green AL, Friedrich P, Ribeiro KB, Rodriguez-Galindo C. Ethnic, Racial, and Socioeconomic Disparities in Retinoblastoma. JAMA Pediatr. 2015;169(12):1096–104. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan E, Meier P. Nonparametric estimation from incomplete observation. Journal of the American Statistical Association. 1958;53(282):457–81. [Google Scholar]
- 22.Arias E, Heron M, Xu J. United States Life Tables, 2014. Natl Vital Stat Rep. 2017;66(4):1–64. [PubMed] [Google Scholar]
- 23.Cox D Regression Models and Life-Tables. Journal of the Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- 24.Therneau T, Grambsh P. Modeling Survival Data: Extending the Cox Model. New York: SPringer; 2000. [Google Scholar]
- 25.Therneau TM. A Package for Survival Analysis in R. R package version 3.2–3 2020 [Available from: https://CRAN.R-project.org/package=survival>.
- 26.Gray B cmprsk: Subdistribution Analysis of Competing Risks. R package version 2.2–10. 2020. [Google Scholar]
- 27.Lenth R emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.6 2020 [Available from: https://CRAN.R-project.org/package=emmeans.
- 28.Armstrong GT, Liu Q, Yasui Y, Neglia JP, Leisenring W, Robison LL, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulrooney DA, Dover DC, Li S, Yasui Y, Ness KK, Mertens AC, et al. Twenty years of follow-up among survivors of childhood and young adult acute myeloid leukemia: a report from the Childhood Cancer Survivor Study. Cancer. 2008;112(9):2071–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon SB, Chen Y, Yasui Y, Pui CH, Hunger SP, Silverman LB, et al. Reduced Morbidity and Mortality in Survivors of Childhood Acute Lymphoblastic Leukemia: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2020;38(29):3418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muffly L, Maguire FB, Li Q, Kennedy V, Keegan TH. Late Effects in Survivors of Adolescent and Young Adult Acute Lymphoblastic Leukemia. JNCI Cancer Spectr. 2020;4(4):pkaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abrahao R, Huynh JC, Benjamin DJ, Li QW, Winestone LE, Muffly L, et al. Chronic medical conditions and late effects after acute myeloid leukaemia in adolescents and young adults: a population-based study. Int J Epidemiol. 2021;50(2):663–74. [DOI] [PubMed] [Google Scholar]
- 33.Anderson C, Lund JL, Weaver MA, Wood WA, Olshan AF, Nichols HB. Disparities in Mortality from Noncancer Causes among Adolescents and Young Adults with Cancer. Cancer Epidemiol Biomarkers Prev. 2019;28(9):1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armenian SH, Xu L, Cannavale KL, Wong FL, Bhatia S, Chao C. Cause-specific mortality in survivors of adolescent and young adult cancer. Cancer. 2020;126(10):2305–16. [DOI] [PubMed] [Google Scholar]
- 35.Raca G, Abdel-Azim H, Yue F, Broach J, Payne JL, Reeves ME, et al. Increased Incidence of IKZF1 deletions and IGH-CRLF2 translocations in B-ALL of Hispanic/Latino children-a novel health disparity. Leukemia. 2021;35(8):2399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvey RC, Mullighan CG, Chen IM, Wharton W, Mikhail FM, Carroll AJ, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tasian SK, Hurtz C, Wertheim GB, Bailey NG, Lim MS, Harvey RC, et al. High incidence of Philadelphia chromosome-like acute lymphoblastic leukemia in older adults with B-ALL. Leukemia. 2017;31(4):981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koo HH. Philadelphia chromosome-positive acute lymphoblastic leukemia in childhood. Korean J Pediatr. 2011;54(3):106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bleckmann K, Schrappe M. Advances in therapy for Philadelphia-positive acute lymphoblastic leukaemia of childhood and adolescence. Br J Haematol. 2016;172(6):855–69. [DOI] [PubMed] [Google Scholar]
- 40.DeRouen MC, Parsons HM, Kent EE, Pollock BH, Keegan THM. Sociodemographic disparities in survival for adolescents and young adults with cancer differ by health insurance status. Cancer Causes Control. 2017;28(8):841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lara J, Brunson A, Keegan TH, Malogolowkin M, Pan CX, Yap S, et al. Determinants of Survival for Adolescents and Young Adults with Urothelial Bladder Cancer: Results from the California Cancer Registry. J Urol. 2016;196(5):1378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent EE, Sender LS, Largent JA, Anton-Culver H. Leukemia survival in children, adolescents, and young adults: influence of socioeconomic status and other demographic factors. Cancer Causes Control. 2009;20(8):1409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pisu M, Kenzik KM, Oster RA, Drentea P, Ashing KT, Fouad M, et al. Economic hardship of minority and non-minority cancer survivors 1 year after diagnosis: another long-term effect of cancer? Cancer. 2015;121(8):1257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dreyer MS, Nattinger AB, McGinley EL, Pezzin LE. Socioeconomic status and breast cancer treatment. Breast Cancer Res Treat. 2018;167(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desmond RA, Jackson BE, Waterbor JW. Disparities in Cancer Survivorship Indicators in the Deep South Based on BRFSS Data: Recommendations for Survivorship Care Plans. South Med J. 2017;110(3):181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garner EF, Maizlin II, Dellinger MB, Gow KW, Goldfarb M, Goldin AB, et al. Effects of socioeconomic status on children with well-differentiated thyroid cancer. Surgery. 2017;162(3):662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collaborators GBDM. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1684–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berkman AM, Andersen CR, Puthenpura V, Livingston JA, Ahmed S, Cuglievan B, et al. Impact of race, ethnicity and socioeconomic status over time on the long-term survival of adolescent and young adult Hodgkin lymphoma survivors. Cancer Epidemiol Biomarkers Prev. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meiners B, Shenoy C, Zordoky BN. Clinical and preclinical evidence of sex-related differences in anthracycline-induced cardiotoxicity. Biol Sex Differ. 2018;9(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meeske KA, Ji L, Freyer DR, Gaynon P, Ruccione K, Butturini A, et al. Comparative Toxicity by Sex Among Children Treated for Acute Lymphoblastic Leukemia: A Report From the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62(12):2140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Creutzig U, Kutny MA, Barr R, Schlenk RF, Ribeiro RC. Acute myelogenous leukemia in adolescents and young adults. Pediatr Blood Cancer. 2018;65(9):e27089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boissel N, Baruchel A. Acute lymphoblastic leukemia in adolescent and young adults: treat as adults or as children? Blood. 2018;132(4):351–61. [DOI] [PubMed] [Google Scholar]
- 53.Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29(30):4045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fern LA, Bleyer A. Dynamics and Challenges of Clinical Trials in Adolescents and Young Adults With Cancer. Cancer J. 2018;24(6):307–14. [DOI] [PubMed] [Google Scholar]
- 55.Yeh JM, Ward ZJ, Chaudhry A, Liu Q, Yasui Y, Armstrong GT, et al. Life Expectancy of Adult Survivors of Childhood Cancer Over 3 Decades. JAMA Oncol. 2020;6(3):350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ribera JM, Ribera J, Genesca E. Treatment of adolescent and young adults with acute lymphoblastic leukemia. Mediterr J Hematol Infect Dis. 2014;6(1):e2014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martino R, Caballero MD, Perez-Simon JA, Canals C, Solano C, Urbano-Ispizua A, et al. Evidence for a graft-versus-leukemia effect after allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning in acute myelogenous leukemia and myelodysplastic syndromes. Blood. 2002;100(6):2243–5. [DOI] [PubMed] [Google Scholar]
- 58.Bachanova V, Verneris MR, DeFor T, Brunstein CG, Weisdorf DJ. Prolonged survival in adults with acute lymphoblastic leukemia after reduced-intensity conditioning with cord blood or sibling donor transplantation. Blood. 2009;113(13):2902–5. [DOI] [PubMed] [Google Scholar]
- 59.Sorensen K, Levitt G, Bull C, Chessells J, Sullivan I. Anthracycline dose in childhood acute lymphoblastic leukemia: issues of early survival versus late cardiotoxicity. J Clin Oncol. 1997;15(1):61–8. [DOI] [PubMed] [Google Scholar]
- 60.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125(1):47–58. [DOI] [PubMed] [Google Scholar]
- 61.Pui CH, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol. 2013;50(3):185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kizilocak H, Okcu F. Late Effects of Therapy in Childhood Acute Lymphoblastic Leukemia Survivors. Turk J Haematol. 2019;36(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larson S, Stock W. Progress in the treatment of adults with acute lymphoblastic leukemia. Curr Opin Hematol. 2008;15(4):400–7. [DOI] [PubMed] [Google Scholar]
- 64.Rank CU, Schmiegelow K. Optimal approach to the treatment of young adults with acute lymphoblastic leukemia in 2020. Semin Hematol. 2020;57(3):102–14. [DOI] [PubMed] [Google Scholar]
- 65.Prescott K, Jacobs M, Stock W, Wynne J. New Approaches to Treating Challenging Subtypes of ALL in AYA Patients. Curr Hematol Malig Rep. 2020;15(6):424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee JS, DuBois SG, Coccia PF, Bleyer A, Olin RL, Goldsby RE. Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer. 2016;122(1):116–23. [DOI] [PubMed] [Google Scholar]
- 67.Moss JL, Johnson NJ, Yu M, Altekruse SF, Cronin KA. Comparisons of individual- and area-level socioeconomic status as proxies for individual-level measures: evidence from the Mortality Disparities in American Communities study. Popul Health Metr. 2021;19(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


