Abstract
Purpose of the review:
The coronavirus disease 2019 (COVID) pandemic has resulted in significant mortality and morbidity globally. Patients who survive infection may develop continuing disease collectively known as the post-acute sequelae of SARS CoV-2 infection (PASC), which includes neurologic symptoms especially fatigue and cognitive impairment. The pathogenic mechanisms driving PASC are unknown although a post-infectious process, persistent infection, or lasting pathophysiological changes that occur during acute infection are all suspected to contribute.
Recent findings:
Here we review the current evidence underlying potential pathogenic mechanisms of the neurological complications of PASC with particular emphasis on the evidence for post-infectious immune processes and viral persistence.
Summary:
Immune dysregulation favoring persistent inflammation, including neuroinflammation and enhanced autoimmunity, are present in patients with COVID and likely contribute to the development of PASC. Limited evidence of viral persistence exists but may explain the ongoing inflammatory processes and affinity maturation observed in some patients recovering from COVID infections. No specific studies to date have tied persistent infection to PASC. CNS trauma, in particular hypoxic changes in the CNS, and psychiatric complications occur with greater frequency in patients with COVID and may contribute to the development of PASC. Future research is needed to fully understand the pathophysiological mechanisms driving PASC.
Keywords: SARS-CoV-2, post-acute sequelae of SARS CoV-2 infection, long-COVID, neurological impairments, autoimmune
Introduction
The coronavirus disease 2019 (COVID) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over five million deaths and 353 million infections [1]. Approximately 30% of patients develop continuing disease that impairs quality of life, collectively known as post-acute sequelae of SARS CoV-2 infection (PASC) or “long-haul COVID” [2]. PASC occurs even in patients with mild infections and is comprised of a spectrum of symptoms, including neurological manifestations such as fatigue and cognitive impairments which have resulted in disability and are creating a long-term health burden from this pandemic [3–5]. PASC is an important ongoing consequence of SARS-CoV-2, yet its cause(s) remains undefined. The spectrum of neurological symptoms associated with PASC may be due to a post-infectious process, a persistent infection, or from lasting pathophysiological changes that are initiated during acute infection. The goal of this review is to present the current evidence underlying these potential disease drivers with particular emphasis on the evidence for post-infectious processes and SARS-CoV-2 viral persistence.
It is important to acknowledge that patients with PASC are heterogeneous in their clinical presentation [4, 6–8] . PASC is likely not a single disease entity driven by a unifying disease process, but instead it is an umbrella term for a spectrum of complications that are associated with SARS-CoV-2. It is also important to acknowledge that there is much about COVID that remains unknown. This review therefore has many limitations, the most important being that the COVID pandemic has not ended and that our understanding of the virus and its physiologic impacts continues to evolve. As this manuscript is being prepared, the Omicron surge has begun, and we do not yet know if this variant will have similar phenotypes or rates of neurologic complications associated with it.
Neurologic complications associated with SARS-CoV-2
Persistent neurologic symptoms are the most frequently reported PASC complications. In the patient-led study that initially described long-haul symptoms, fatigue was overwhelmingly the most frequent complaint. Fatigue, body aches, difficulty concentrating, headaches, difficulty sleeping, anxiety, memory problems, and dizziness were nine of the top ten most frequently endorsed complaints [9]. These findings have been now replicated in multiple studies within the first month after COVID recovery [8, 10, 11] and a recent study shows that fatigue and cognitive dysfunction often continues to persist after six months [4].
The symptom complex in PASC is consistent with those previously described as the “postviral fatigue syndrome”. As initially described by Behan and Behan in 1984 [12], the postviral fatigue syndrome is an aversive constellation of symptoms where the “principle symptom is severe muscle fatiguability, but there may be a range of secondary symptoms, such as the aching of muscles, disequilibrium, and psychiatric manifestations.” Estimates of the incidence of postviral fatigue syndrome after viral meningitis, Epstein-Barr virus, and Ross River virus infections range between 10-12% [13, 14]. These persistent issues have also been reported as sequelae of other coronaviruses, including SARS and MERS [15].
There are other meaningful neurological complications from PASC. Peripheral neuropathies have been reported as sequela of COVID [16] and may be the consequence of COVID immune-mediated neuronal or vascular injury, medication toxicity, or compression injury [17]. Anosmia, parosmia, ageusia, and parageusia are also commonly reported PASC complications. It is currently thought that these neurological complications are caused by injury [18] of neuronal supportive cells and dysinnervation during healing.
Potential pathogenic mechanisms of PASC
As described, PASC includes neurologic complications unique from the acute disease phase. These symptoms may be due to immune mediated post-infectious processes, direct or indirect damage driven by chronic viral infection, or from stress, damage, and failed healing of injury sustained from the acute infection (Figure 1). Evidence exists for all these mechanisms to contribute to the development of PASC.
Figure 1. Potential pathogenesis of neurologic complications observed in patients with PASC.
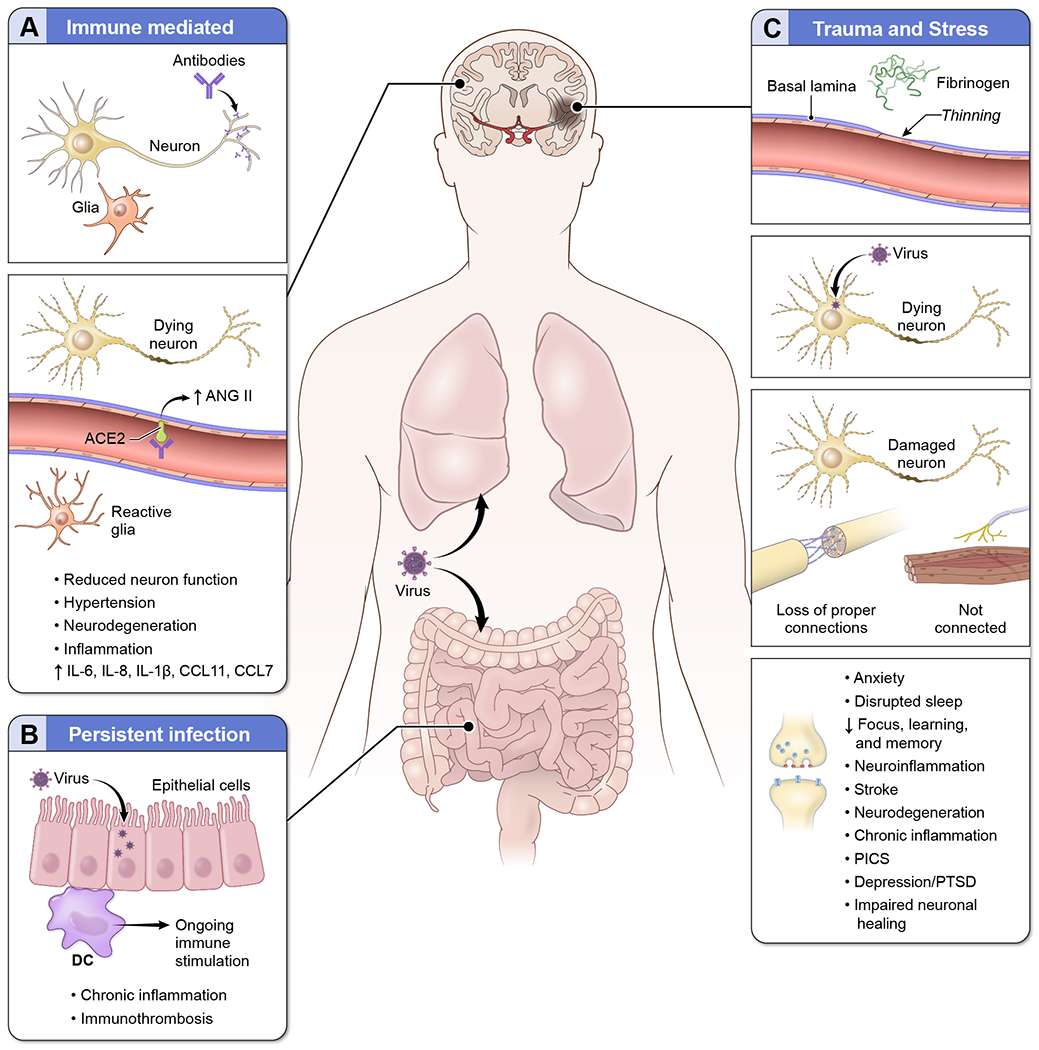
Multiple pathogenic mechanisms likely contribute to the development of neurologic complications in patients with PASC including (A) immune mediated processes. Antibodies that specifically target neuronal proteins have been detected in patients with COVID. These antibodies can impair neurologic function, induce neurodegeneration, and trigger proinflammatory processes within the CNS. Antibodies that recognize ACE2 on endothelial cells can inhibit enzyme function leading to increased levels of ANG II in the CNS, which in turn can drive neurodegeneration and neuroinflammation. Elevated neuroinflammation, including reactive glial cells and increased proinflammatory cytokines, has been observed in patients with SARS-CoV-2 infection even in the absence of virus in the brain. (B) Persistent infection may also contribute to the development of PASC. In some patients, virus appears to persist in the lung and GI tract. The virus in these compartments can cause ongoing immune stimulation and persistent inflammation. Systemic inflammation is associated with cognitive impairments including executive function and memory. Chronic inflammation is also associated with the development of thrombi which can increase the risk of stroke and drive further inflammation. (C) Trauma and stress may also contribute to the development of PASC. Stroke and hemorrhage into the CNS occur in patients with COVID resulting in CNS tissue destruction and neuronal loss. Microvascular damage and associated hypoxia induced changes can also result in neurodegeneration and promote neuroinflammation. Direct infection of the CNS has been reported, including in neurons, which can also contribute to neurodegeneration. In neurons that have been damaged, repair processes may also be impaired resulting in loss of proper connections or in neurons that do not fully re-connect to innervated tissues. Lack of proper healing may also contribute to the development of neurological symptoms in PACS. Depression, anxiety, post-traumatic stress disorder, and PICS can also directly contribute to suppression synaptic receptors and CNS function resulting in impairments in focus, learning and memory.
Immune mediated post-infectious processes
Recent work has revealed that immune dysregulation persists in patients who develop PASC as compared to patients who fully recover [19**, 20*]. Patients with PASC could be distinguished with a collection of proinflammatory biomarkers [19] and alterations in transcription in immune cells were detected in patients with PASC at 24 weeks post-infection [20]. Notably, increased gene transcription of cell cycle and translation were increased in patients with PASC and there was a robust decrease in platelet gene expression [20]. Importantly, there were no differences detected at earlier times points, suggesting that patients with PASC have ongoing gene expression alterations as compared to patients that recover. Alterations in neuroinflammation are also detected in patients with PASC. Within the CNS SARS-CoV-2 drives microglial activation, myelin loss, and increases CSF proinflammatory cytokines even in the absence of CNS infection [21**]. CCL11, which has been found to be correlated with impaired neurogenesis [22], was elevated in patients with PASC as compared to patients who fully recovered from infection. As both systemic and neuro-inflammation are associated with impaired cognition [23], these processes may be contributing to the neurologic complications observed in patients with PASC (Figure 1A).
SARS-CoV-2 also appears to increase the risk of development of autoimmunity. Multiple studies have demonstrated that patients with COVID develop autoantibodies at elevated levels as compared to uninfected controls [24–26]. Some of the immune specificities detected in SARS-CoV-2 patients are known autoantigens including La [27], Ro [28], ANA [24–26], and phospholipids [29, 30]. Although frank autoimmune encephalitis associated with COVID appears infrequent [31], antibodies associated with these conditions including NMDAR [32–34], CASPR2 [35], MOG [36, 37], and GAD65 [38] have been described in patients with COVID and neurological disease, many which responded to immunotherapy. A recent report of NMDAR encephalitis occurring in a patient post-COVID vaccination [39*] emphasizes that this process is immune mediated. In addition to anti-neuronal antibodies, antibodies that alter vasculature and thrombotic processes could both directly and indirectly impact the CNS and contribute to the development of PASC. Antibodies that inhibit the function of ACE2, the receptor for SARS-CoV-2, have been described in patients with COVID and are associated with elevated levels of ANG II [40]. In addition to increasing blood pressure, ANG II can drive inflammation and neurodegeneration (reviewed in [41]). Antibodies to neutrophil extracellular traps (NETs) have been detected in patients with COVID [42, 43]. These antibodies appear to induce thrombosis by triggering the formation and impairing the clearance of NETs. Excessive presence of NETs is associated with the formation of blood clots (reviewed in [44]) which may increase the risk of stroke and neurological injury (see “Trauma and stress” section below) in patients with COVID [45]. However, to date, no studies have specifically looked at anti-neuronal, NETs, or ACE2 immune specificities in patients with PASC.
The few studies that have specifically examined autoantibodies in patients with PASC are suggestive that post-infectious autoimmune processes persist. In one cohort of 95 patients with PASC, 80% of the patients had autoantibodies 110 days after infection with SARS-CoV-2 and 40% of the patients had antibodies to two or more immune specificities [46*]. Another study of 31 patients, composed primarily of patients with neurological symptoms, found antibodies to β2 adrenoceptor, muscarinic M2 receptor, angiotensin II AT1 receptor, and angiotensin 1-7 MAS receptor [47*]. Antibodies to the β2 adrenoceptor and muscarinic receptors have been noted in subsets of patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) or Postural Orthostatic Tachycardia Syndrome (POTS) [48, 49]. The overlap of symptoms with ME/CFS and POTS, especially fatigue, suggests that infection-initiated immune-mediated dysregulation of nervous system receptors may have a causal role in a subset of patients with PASC.
Persistent virus
Another hypothesis is that PASC is due to persistent infection in a subset of patients, which in addition to direct viral damage, could drive chronic inflammation and immune-mediated tissue damage (Figure 1B). While most patients clear infectious virus within nine days, and viral RNA ceases to be detectable between 14-17 days [50, 51], emerging evidence suggests that subsets of patients could have persistent infection. Extended duration of viral RNA detectable in the respiratory tract and feces of several months has been described [50, 52–54], as have intermittent PCR negative and positive tests, including in patients with PASC [55–57]. However, without sequential viral sequencing it is unclear if this represents reinfection or reemergence of the original viral infection. Studies with such sequencing suggest both reinfection and chronic infection are possible.
In a large cohort of patients (n=133,266), two people were identified as having persistent infections with high titer virus that was sequenced and shown to be the same virus as the original infection at day 80 and 62 post original PCR [58]. In a group of 38 patients with long-term infection, defined as a positive PCR test for greater than four weeks, viral RNA was detectable in oral swabs and sputum samples that were analyzed weekly for six weeks [59]. In two of the sputum samples analyzed, infectious virus was cultured, representing viable virus 102 and 73 days after initial infection. Whole genome sequencing of the cultured virus demonstrated genomic alignment to virus circulating at the time of initial infection and repeated sequencing showed minimal or no genetic alterations, strongly suggesting persistent infection as compared to reinfection [59]. Yet in another study, sequencing showed different lineage of virus from two people identified as having a second PCR positive test 108 and 126 days after infection, suggesting reinfection [55]. Collectively, sequential sequencing data suggests that although infrequent, both reinfection and persistent infection of replication competent virus can occur. Post-mortem studies also suggest that some patients may have chronic infections. Case reports and small studies have found that the virus can persist in the lung months after infection, despite clearance from other clinical samples such as the sputum or nasal secretions [60, 61**]. Importantly, in addition to virus being present in the lung, three patients that died between day 19 and 43 of disease had detectable spike protein in subsets of neurons in the CNS, indicating that neuronal infection with SARS-CoV-2 can occur [61]. However, the frequency in which virus invades or persists in the brain remains unclear, as is the direct contribution of CNS infection on the development of PASC [62].
Studies evaluating ongoing immune response evolution also suggest that SARS-CoV-2 can persist. In one study, immune responses from 87 patients were examined one and six-months after infection. While no patients were PCR positive at the six-month time point, there was ongoing IgA affinity maturation [63*]. As this process requires antigen the authors collected biopsies from the GI tract of 14 individuals, five of which showed viral antigen by immunofluorescence, confirmed to be SARS-CoV-2 by sequencing [63]. This suggests that virus can persist in some body compartments and continue to drive immune responses. Similar antiviral IgA kinetics were observed in a study that monitored antibody titers for 14 months in 278 people infected with SARS-CoV-2 with mild disease [64]. While virus was not detected by PCR, IgA levels continued to rise steadily in a subset of patients, suggesting ongoing immune stimulation [64]. In another study of 203 post-symptomatic patients, the majority of which had mild illness, 5% of patients were PCR positive greater than 90 days post infection and a subset of these patients had high viral loads, similar to those observed during acute infections [65*, 66]. Further characterization of the immune response in these patients revealed increased levels of viral specific CD8+ T cells that recognized more epitopes as compared to patients without a PCR positive test at 90 days [65]. Collectively these studies suggest that in a subset of patients recovering from viral infection there is ongoing viral replication that results in continued immune system stimulation. However, no studies to date have examined extended viral persistence and continued immune evolution on the development of PASC.
While there is not a consensus on persistent SARS-CoV-2 infections, it is important to note that the formation of cell-free replication competent virions is not a requirement for viruses to drive CNS pathologies or establish chronic CNS infections. For example, measles and other viruses (reviewed in [67]) can persist in the CNS and spread via cell-to-cell contact by various mechanisms. Importantly, neutralizing antibodies are not able to reduce viral transmission and there is a reduction in the ability of virus to be detected in fluids such as the CSF as cell free viral particles are not released [68–71]. While SARS-CoV-2 does appear to infect the CNS of a subset of patients, we do not currently know if SARS-CoV-2 establishes a reservoir in the CNS. Although case reports of viral RNA detected in the CSF of patients with PASC have been published [72], no systematic evaluation of CSF samples from a large set of patients has thus far shed light on this issue.
Trauma and stress
The neurologic complications observed in patients with PASC could also arise from permanent damage and neuronal reorganization sustained by the CNS during acute infection (Figure 1C). One of the major risks to the CNS from SARS-CoV-2 is ischemic stroke, which is greatly elevated in patients with COVID as compared to other viral infections [73]. While the risk of thrombosis appears to increase with COVID disease severity (reviewed in [74]), a quarter of the patients in a retrospective study presenting to the emergency room for stroke were found to have COVID [73], suggesting that stroke can occur even in patients not requiring hospitalization for their SARS-CoV-2 infection. An additional study examining asymptomatic male patients who were younger than 50 years of age further confirmed that there is an increased risk of stroke after COVID, with the mean time to stroke event being 54 days post infection [75]. Further, a post-mortem series demonstrated microvascular damage in the CNS of patients including non-hospitalized COVID patients that died suddenly [62*]. Here the authors demonstrated that abnormalities on MRI represented thinning of the basal lamina of the endothelial layer with corresponding fibrinogen leakage into the CNS [62]. While no patients in this series had PASC, persistent endothelial dysfunction has been linked to PASC [76] and this series suggests that endothelial dysfunction occurs even in those not requiring hospitalization. Other post-mortem series have also described hypoxic changes and vascular damage in the CNS [77] and these concur with MRI studies demonstrating that patients with neurologic symptoms have imaging results consistent with infarcts, hemorrhage, or global hypoxic ischemic encephalopathy [78].
It is notable that cognitive deficits [79] and chronic headaches [80] are both common complications in patients recovering from mild ischemic stroke and those with PASC. Vascular damage leading to permanent CNS tissue damage can occur in the wake of even mild ischemic events. Beyond the direct tissue damage, neural repair after stroke involves multiple integrated processes with changes in both structure and function. There is a substantial amount of personal variation in this recovery that belies variation in genetics, environment, and behavior [81]. It seems likely that the vasculopathy of COVID contributes to the development of PASC symptoms in some patients.
Another contributor to PASC may be stress and psychological trauma. It has been demonstrated that post-intensive care syndrome (PICS), which include neurological complications such as post-traumatic stress disorder, depression, neuromuscular weakness, chronic inflammation, and cognitive impairments, occurs frequently in patients with COVID and that COVID is associated with elevated rates of depression even in mild cases not requiring hospitalization [82, 83]. Further, patients with COVID with new onset depression or adjustment disorders show elevated levels of systemic inflammation [84]. As patients with stress-related disorders, such as PICS, are at an increased risk of developing autoimmune diseases [85], it is important to consider that the outlined potential pathogenic mechanisms are not independent from each other but may overlap and fuel other mechanisms driving the neurological manifestations of PASC. Stress, ischemic events, and chronic infections are all proinflammatory and can drive immune mediated damage to the CNS. In turn, chronic inflammation is a risk factor for thrombotic events such as stroke [86] and can contribute to neurocognitive impairments and neurodegeneration [87, 88].
Conclusions
PASC is an important complication of SARS-CoV-2 infection and currently we are not able to predict who will develop long-term complications, if they will persist or resolve, or if PASC is preventable. A barrier to these advancements is that patients with PASC may have very different disease processes despite similarities in clinical phenotype. In this review we suggest that the neurologic manifestations of PASC are driven by multiple mechanisms including immune-mediated processes, chronic viral infection, and trauma and stress. It is important for future research to take these processes into consideration and to group patients, by deeply phenotyping them, into biotypes based on pathogenic processes as the prognosis and therapeutic avenues for patients with PASC will likely differ among these groups. The phenotyping should include neurological and psychological evaluations to understand the confluence of these processes on the development and persistence of PASC. Further, the development of biomarkers for chronic inflammation, including neuroinflammation, may identify patients that would benefit from immune suppressive therapies. Additional studies are needed to determine if chronic infection with SARS-CoV-2 occurs and if so, where the relevant reservoirs of virus reside and how these potential reservoirs might be eliminated.
Key points.
Neurologic complications are common features of PASC.
Multiple disease mechanisms including chronic infection, post-infectious immune processes, hypoxic CNS injury, and stress may contribute to PASC development.
PASC is heterogeneous in cause and will require clinicians and researchers to consider these potential mechanisms as patients are evaluated to avoid misclassification and treatment errors.
Grouping patients with PASC into biotypes based on pathogenesis may help elucidate therapeutic avenues.
Acknowledgements:
The authors would like to acknowledge Alan Hoofring for assistance in preparation of Figure 1.
Financial support and sponsorship:
Supported by intramural funds from NINDS (NS003157).
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, et al. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw Open. 2021;4(2):e210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hampshire A, Trender W, Chamberlain SR, Jolly AE, Grant JE, Patrick F, et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. 2021;39:101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heightman M, Prashar J, Hillman TE, Marks M, Livingston R, Ridsdale HA, et al. Post-COVID-19 assessment in a specialist clinical service: a 12-month, single-centre, prospective study in 1325 individuals. BMJ Open Respir Res. 2021;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasserie T, Hittle M, Goodman SN. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients With COVID-19: A Systematic Review. JAMA Netw Open. 2021;4(5):e2111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–64. [DOI] [PubMed] [Google Scholar]
- 8.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert NJ, Survivor Corps. COVID-19 “Long Hauler” Symptoms Survey Report. Indiana University School of Medicine; 2020. [Google Scholar]
- 10.Fair Health Inc. A Detailed Study of Patients with Long-Haul COVID. 2021.
- 11.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behan PO, Behan WM, Bell EJ. The postviral fatigue syndrome--an analysis of the findings in 50 cases. J Infect. 1985;10(3):211–22. [DOI] [PubMed] [Google Scholar]
- 13.Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333(7568):575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotopf M, Noah N, Wessely S. Chronic fatigue and minor psychiatric morbidity after viral meningitis: a controlled study. J Neurol Neurosurg Psychiatry. 1996;60(5):504–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med. 2020;52(5):jrm00063. [DOI] [PubMed] [Google Scholar]
- 16.Attal N, Martinez V, Bouhassira D. Potential for increased prevalence of neuropathic pain after the COVID-19 pandemic. Pain Rep. 2021;6(1):e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finsterer J, Scorza FA, Scorza CA, Fiorini AC. Peripheral neuropathy in COVID-19 is due to immune-mechanisms, pre-existing risk factors, anti-viral drugs, or bedding in the Intensive Care Unit. Arq Neuropsiquiatr. 2021;79(10):924–8. [DOI] [PubMed] [Google Scholar]
- 18.Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6(31). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022. [DOI] [PubMed] [Google Scholar]; ** This study demonstrates that patients with PACS have ongoing immune persistent immune activation eight months after infection with SARS-CoV-2 as compared to individuals that fully recovered from SARS-CoV-2 infection, other cornavirus infections, or unifected controls. The authors demonstrate both innate and adaptive immune activation and defined a set of biomarkers that could identify patients with PACS. Inflammatory biomarkers in PACS may identify patients who would benefit from early immune intervention.
- 20.Ryan FJ, Hope CM, Masavuli MG, Lynn MA, Mekonnen ZA, Yeow AEL, et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022;20(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Analysis of immune responses 12, 16, and 24 weeks after SARS-CoV-2 infection shows that patients with PACS had alterations in transcriptional profiles as compared to patients that fully recovered.
- 21.Fernandez-Castaneda A, Lu P, Geraghty AC, Song E, Lee MH, Wood J, et al. Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. bioRxiv. 2022. [Google Scholar]; ** This non-peer reviewed pre-print demonstrates multiple lines of evidence of neuroinflammation during SARS-CoV-2 infection even in the absense of CNS infection. Murine models and human post-mortem studies revealed white matter microglial cell activation, increased CSF inflammatory cytokine concentrations, loss of myelin, and impaired hippocampal neurogenesis. CCL11 was elevated in the CSF in both the murine model and in patients with PACS.
- 22.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker KA, Gottesman RF, Wu A, Knopman DS, Gross AL, Mosley TH Jr., et al. Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. Neurology. 2019;92(11):e1256–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emmenegger M, Kumar SS, Emmenegger V, Malinauskas T, Buettner T, Rose L, et al. Anti-prothrombin autoantibodies enriched after infection with SARS-CoV-2 and influenced by strength of antibody response against SARS-CoV-2 proteins. PLoS Pathog. 2021;17(12):e1010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang SE, Feng A, Meng W, Apostolidis SA, Mack E, Artandi M, et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun. 2021;12(1):5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacchi MC, Tamiazzo S, Stobbione P, Agatea L, De Gaspari P, Stecca A, et al. SARS-CoV-2 infection as a trigger of autoimmune response. Clin Transl Sci. 2021;14(3):898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell. 2020;183(4):982–95 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujii H, Tsuji T, Yuba T, Tanaka S, Suga Y, Matsuyama A, et al. High levels of anti-SSA/Ro antibodies in COVID-19 patients with severe respiratory failure: a case-based review : High levels of anti-SSA/Ro antibodies in COVID-19. Clin Rheumatol. 2020;39(11):3171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valencia Sanchez C, Theel E, Binnicker M, Toledano M, McKeon A. Autoimmune Encephalitis After SARS-CoV-2 Infection: Case Frequency, Findings, and Outcomes. Neurology. 2021;97(23):e2262–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allahyari F, Hosseinzadeh R, Nejad JH, Heiat M, Ranjbar R. A case report of simultaneous autoimmune and COVID-19 encephalitis. J Neurovirol. 2021;27(3):504–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burr T, Barton C, Doll E, Lakhotia A, Sweeney M. N-Methyl-d-Aspartate Receptor Encephalitis Associated With COVID-19 Infection in a Toddler. Pediatr Neurol. 2021;114:75–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guilmot A, Maldonado Slootjes S, Sellimi A, Bronchain M, Hanseeuw B, Belkhir L, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. 2021;268(3):751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durovic E, Bien C, Bien CG, Isenmann S. MOG antibody-associated encephalitis secondary to Covid-19: case report. BMC Neurol. 2021;21(1):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dias da Costa M, Leal Rato M, Cruz D, Valadas A, Antunes AP, Albuquerque L. Longitudinally extensive transverse myelitis with anti-myelin oligodendrocyte glycoprotein antibodies following SARS-CoV-2 infection. J Neuroimmunol. 2021;361:577739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valadez-Calderon J, Ordinola Navarro A, Rodriguez-Chavez E, Vera-Lastra O. Co-expression of anti-NMDAR and anti-GAD65 antibodies. A case of autoimmune encephalitis in a post-COVID-19 patient. Neurologia. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flannery P, Yang I, Keyvani M, Sakoulas G. Acute Psychosis Due to Anti-N-Methyl D-Aspartate Receptor Encephalitis Following COVID-19 Vaccination: A Case Report. Front Neurol. 2021;12:764197. [DOI] [PMC free article] [PubMed] [Google Scholar]; * First case report of anti-NMDAR encephalitis following SARS-CoV-2 immunization. The patient presented with anxiety and delusions and progressed to psychosis and catatonia. Immunomodulatory therapy was successful in treating this patient.
- 40.Arthur JM, Forrest JC, Boehme KW, Kennedy JL, Owens S, Herzog C, et al. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLoS One. 2021;16(9):e0257016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuo Y, Estes SK, Ali RA, Gandhi AA, Yalavarthi S, Shi H, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12(570). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo Y, Yalavarthi S, Navaz SA, Hoy CK, Harbaugh A, Gockman K, et al. Autoantibodies stabilize neutrophil extracellular traps in COVID-19. JCI Insight. 2021;6(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gasecka A, Borovac JA, Guerreiro RA, Giustozzi M, Parker W, Caldeira D, et al. Thrombotic Complications in Patients with COVID-19: Pathophysiological Mechanisms, Diagnosis, and Treatment. Cardiovasc Drugs Ther. 2021;35(2):215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerrero JI, Barragan LA, Martinez JD, Montoya JP, Pena A, Sobrino FE, et al. Central and peripheral nervous system involvement by COVID-19: a systematic review of the pathophysiology, clinical manifestations, neuropathology, neuroimaging, electrophysiology, and cerebrospinal fluid findings. BMC Infect Dis. 2021;21(1):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodruff MC, Walker TA, Truong AD, Dixit AN, Han JE, Ramonell RP, et al. Evidence of Persisting Autoreactivity in Post-Acute Sequelae of SARS-CoV-2 Infection. medRxiv. 2021:2021.09.21.21263845. [Google Scholar]; * This non-peer reviewed pre-print manuscript describes the screening for autoantibodies in 95 patients with PACS. 80% of patients with PACS had detectable autoantibodies and 40% of patients with PACS had antibodies to two or more distinct immune specifities. ANA was the most common immune specificity detected, however several other immue specificites were described including anti-dsDNA, RNA polumerase III, and phospholipids. Importantly, these autoimmune responses persisted up to 14 months post-SARS-CoV-2 infection.
- 47. Wallukat G, Hohberger B, Wenzel K, Furst J, Schulze-Rothe S, Wallukat A, et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun. 2021;4:100100. * This study describes the detection of antibodies that impair or activate G-protein coupled receptors from the serum of patients with PACS.
- 48.Loebel M, Grabowski P, Heidecke H, Bauer S, Hanitsch LG, Wittke K, et al. Antibodies to beta adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain Behav Immun. 2016;52:32–9. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Yu X, Liles C, Khan M, Vanderlinde-Wood M, Galloway A, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3(1):e000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Kampen JJA, van de Vijver D, Fraaij PLA, Haagmans BL, Lamers MM, Okba N, et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun. 2021;12(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li N, Wang X, Lv T. Prolonged SARS-CoV-2 RNA shedding: Not a rare phenomenon. J Med Virol. 2020;92(11):2286–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing YH, Ni W, Wu Q, Li WJ, Li GJ, Wang WD, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020;53(3):473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu CLH, Raval M, Schnall JA, Kwong JC, Holmes NE. Duration of Respiratory and Gastrointestinal Viral Shedding in Children With SARS-CoV-2: A Systematic Review and Synthesis of Data. Pediatr Infect Dis J. 2020;39(9):e249–e56. [DOI] [PubMed] [Google Scholar]
- 55.Hicks JT, Das S, Matanock A, Griego-Fisher A, Sosin D. Characteristics of Persons with Secondary Detection of SARS-CoV-2 >/=90 days After First Detection - New Mexico, 2020. J Infect Dis. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Armstrong JN, Campbell L, Rabatsky-Her T, Leung V, Parikh S. Repeat positive SARS-CoV-2 RNA testing in nursing home residents during the initial 9 months of the COVID-19 pandemic: an observational retrospective analysis. Lancet Reg Health Am. 2021;3:100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdallah H, Porterfield F, Fajgenbaum D. Symptomatic relapse and long-term sequelae of COVID-19 in a previously healthy 30-year-old man. BMJ Case Rep. 2020;13(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abu-Raddad LJ, Chemaitelly H, Malek JA, Ahmed AA, Mohamoud YA, Younuskunju S, et al. Two prolonged viremic SARS-CoV-2 infections with conserved viral genome for two months. Infect Genet Evol. 2021;88:104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Q, Zheng XS, Shen XR, Si HR, Wang X, Wang Q, et al. Prolonged shedding of severe acute respiratory syndrome coronavirus 2 in patients with COVID-19. Emerg Microbes Infect. 2020;9(1):2571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Y, Xia Z, Liang W, Li J, Liu L, Huang D, et al. SARS-CoV-2 persisted in lung tissue despite disappearance in other clinical samples. Clin Microbiol Infect. 2020;26(10):1424–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021;218(3). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study demonstrates that SARS-CoV-2 can unfect the CNS using orthogonal approaches including human brain organoids, a murine model, and human post-mortem studies. SARS-CoV-2 was detected in cortical neurons in human brains post-mortem.
- 62.Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, et al. Microvascular Injury in the Brains of Patients with Covid-19. N Engl J Med. 2021;384(5):481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This post-mortem series describes the pathology and demonstrates endothelial wall thinning and protein leakage into the CNS of patients who died suddenly of mild SARS-CoV-2 infection. No evidence of viral infection in the CNS was found in any patient.
- 63.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study describes the ongoing affinity maturation of the IgA compartment in patients recovering from SARS-CoV-2 infection one and 6 months post-infection. Biopsies from asymptomatic individuals four months after infection demonstrated SARS-CoV-2 protein and nucleic acid in the GI tract of seven of 14 individuals. This study suggests that chronic infection is possible in a subset of patients and that the GI may be a location for this viral persistence. Further, this study suggests that alterations in the IgA subset may indicate ongoing viral presence.
- 64.Burgi JJ, Rosslein M, Hornung H, Jentsch J, Boller VL, Dollenmaier G, et al. Divergent humoral responses in mild to moderate SARS-CoV-2 infection over time - indication of persistence of the virus? J Infect. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vibholm LK, Nielsen SSF, Pahus MH, Frattari GS, Olesen R, Andersen R, et al. SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine. 2021;64:103230. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This cohort study of 203 post-symptomatic SARS-CoV-2 infected patients demonstrated that after 90 days 5% of the patients remained PCR positive despite similar antibody titers as patients who were PCR negative. Additionally, the group that remained PCR positive showed enhanced CD8+ T cell responses, suggesting ongoing immune stimulation from the virus. This study suggests that viral persistence occurs in a small subset of patients and may contribute to ongoing inflammation.
- 66.Yu F, Yan L, Wang N, Yang S, Wang L, Tang Y, et al. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin Infect Dis. 2020;71(15):793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nath A, Johnson TP. Mechanisms of viral persistence in the brain and therapeutic approaches. FEBS J. 2021. [DOI] [PubMed] [Google Scholar]
- 68.O’Hara BA, Morris-Love J, Gee GV, Haley SA, Atwood WJ. JC Virus infected choroid plexus epithelial cells produce extracellular vesicles that infect glial cells independently of the virus attachment receptor. PLoS Pathog. 2020;16(3):e1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lannes N, Garcia-Nicolas O, Demoulins T, Summerfield A, Filgueira L. CX3CR1-CX3CL1-dependent cell-to-cell Japanese encephalitis virus transmission by human microglial cells. Sci Rep. 2019;9(1):4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bello-Morales R, Praena B, de la Nuez C, Rejas MT, Guerra M, Galan-Ganga M, et al. Role of Microvesicles in the Spread of Herpes Simplex Virus 1 in Oligodendrocytic Cells. J Virol. 2018;92(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson TP, Larman HB, Lee MH, Whitehead SS, Kowalak J, Toro C, et al. Chronic Dengue Virus Panencephalitis in a Patient with Progressive Dementia with Extrapyramidal Features. Ann Neurol. 2019;86(5):695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Viszlayova D, Sojka M, Dobrodenkova S, Szabo S, Bilec O, Turzova M, et al. SARS-CoV-2 RNA in the Cerebrospinal Fluid of a Patient with Long COVID. Ther Adv Infect Dis. 2021;8:20499361211048572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, et al. Risk of Ischemic Stroke in Patients with Covid-19 versus Patients with Influenza. medRxiv. 2020. [Google Scholar]
- 74.Jenner WJ, Gorog DA. Incidence of thrombotic complications in COVID-19 : On behalf of ICODE: The International COVID-19 Thrombosis Biomarkers Colloquium. J Thromb Thrombolysis. 2021;52(4):999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tu TM, Seet CYH, Koh JS, Tham CH, Chiew HJ, De Leon JA, et al. Acute Ischemic Stroke During the Convalescent Phase of Asymptomatic COVID-2019 Infection in Men. JAMA Netw Open. 2021;4(4):e217498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Charfeddine S, Ibn Hadj Amor H, Jdidi J, Torjmen S, Kraiem S, Hammami R, et al. Long COVID 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights From TUN-EndCOV Study. Front Cardiovasc Med. 2021;8:745758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, et al. Neuropathological Features of Covid-19. N Engl J Med. 2020;383(10):989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alonazi B, Farghaly AM, Mostafa MA, Al-Watban JA, Zindani SA, Altaimi F, et al. Brain MRI in SARS-CoV-2 pneumonia patients with newly developed neurological manifestations suggestive of brain involvement. Sci Rep. 2021;11(1):20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marsh EB, Brodbeck C, Llinas RH, Mallick D, Kulasingham JP, Simon JZ, et al. Poststroke acute dysexecutive syndrome, a disorder resulting from minor stroke due to disruption of network dynamics. Proc Natl Acad Sci U S A. 2020;117(52):33578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harriott AM, Karakaya F, Ayata C. Headache after ischemic stroke: A systematic review and meta-analysis. Neurology. 2020;94(1):e75–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Regenhardt RW, Takase H, Lo EH, Lin DJ. Translating concepts of neural repair after stroke: Structural and functional targets for recovery. Restor Neurol Neurosci. 2020;38(1):67–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rousseau AF, Minguet P, Colson C, Kellens I, Chaabane S, Delanaye P, et al. Post-intensive care syndrome after a critical COVID-19: cohort study from a Belgian follow-up clinic. Ann Intensive Care. 2021;11(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.da Graca B, Bennett MM, Powers MB, Gottlieb RL, Waddimba AC, Warren AM. Psychological differences in adults with and without a COVID-19 diagnosis. J Ment Health. 2022:1–8. [DOI] [PubMed] [Google Scholar]
- 84.Iglesias-Gonzalez M, Boigues M, Sanagustin D, Giralt-Lopez M, Cuevas-Esteban J, Martinez-Caceres E, et al. Association of serum interleukin-6 and C-reactive protein with depressive and adjustment disorders in COVID-19 inpatients. Brain Behav Immun Health. 2022;19:100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song H, Fang F, Tomasson G, Arnberg FK, Mataix-Cols D, Fernandez de la Cruz L, et al. Association of Stress-Related Disorders With Subsequent Autoimmune Disease. JAMA. 2018;319(23):2388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–31. [DOI] [PubMed] [Google Scholar]
- 87.Chitnis T, Weiner HL. CNS inflammation and neurodegeneration. J Clin Invest. 2017;127(10):3577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gorelick PB. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci. 2010;1207:155–62. [DOI] [PubMed] [Google Scholar]


