Abstract
Background
The risk of severe COVID-19 outcomes in people with immune-mediated inflammatory diseases and on immune-modifying drugs might not be fully mediated by comorbidities and might vary by factors such as ethnicity. We aimed to assess the risk of severe COVID-19 in adults with immune-mediated inflammatory diseases and in those on immune-modifying therapies.
Methods
We did a cohort study, using OpenSAFELY (an analytics platform for electronic health records) and TPP (a software provider for general practitioners), analysing routinely collected primary care data linked to hospital admission, death, and previously unavailable hospital prescription data. We included people aged 18 years or older on March 1, 2020, who were registered with TPP practices with at least 12 months of primary care records before March, 2020. We used Cox regression (adjusting for confounders and mediators) to estimate hazard ratios (HRs) comparing the risk of COVID-19-related death, critical care admission or death, and hospital admission (from March 1 to Sept 30, 2020) in people with immune-mediated inflammatory diseases compared with the general population, and in people with immune-mediated inflammatory diseases on targeted immune-modifying drugs (eg, biologics) compared with those on standard systemic treatment (eg, methotrexate).
Findings
We identified 17 672 065 adults; 1 163 438 adults (640 164 [55·0%] women and 523 274 [45·0%] men, and 827 457 [71·1%] of White ethnicity) had immune-mediated inflammatory diseases, and 16 508 627 people (8 215 020 [49·8%] women and 8 293 607 [50·2%] men, and 10 614 096 [64·3%] of White ethnicity) were included as the general population. Of 1 163 438 adults with immune-mediated inflammatory diseases, 19 119 (1·6%) received targeted immune-modifying therapy and 181 694 (15·6%) received standard systemic therapy. Compared with the general population, adults with immune-mediated inflammatory diseases had an increased risk of COVID-19-related death after adjusting for confounders (age, sex, deprivation, and smoking status; HR 1·23, 95% CI 1·20–1·27) and further adjusting for mediators (body-mass index [BMI], cardiovascular disease, diabetes, and current glucocorticoid use; 1·15, 1·11–1·18). Adults with immune-mediated inflammatory diseases also had an increased risk of COVID-19-related critical care admission or death (confounder-adjusted HR 1·24, 95% CI 1·21–1·28; mediator-adjusted 1·16, 1·12–1·19) and hospital admission (confounder-adjusted 1·32, 1·29–1·35; mediator-adjusted 1·20, 1·17–1·23). In post-hoc analyses, the risk of severe COVID-19 outcomes in people with immune-mediated inflammatory diseases was higher in non-White ethnic groups than in White ethnic groups (as it was in the general population). We saw no evidence of increased COVID-19-related death in adults on targeted, compared with those on standard systemic, therapy after adjusting for confounders (age, sex, deprivation, BMI, immune-mediated inflammatory diseases [bowel, joint, and skin], cardiovascular disease, cancer [excluding non-melanoma skin cancer], stroke, and diabetes (HR 1·03, 95% CI 0·80–1·33), and after additionally adjusting for current glucocorticoid use (1·01, 0·78–1·30). There was no evidence of increased COVID-19-related death in adults prescribed tumour necrosis factor inhibitors, interleukin (IL)-12/IL‑23 inhibitors, IL-17 inhibitors, IL-6 inhibitors, or Janus kinase inhibitors compared with those on standard systemic therapy. Rituximab was associated with increased COVID-19-related death (HR 1·68, 95% CI 1·11–2·56), with some attenuation after excluding people with haematological malignancies or organ transplants (1·54, 0·95–2·49).
Interpretation
COVID-19 deaths and hospital admissions were higher in people with immune-mediated inflammatory diseases. We saw no increased risk of adverse COVID-19 outcomes in those on most targeted immune-modifying drugs for immune-mediated inflammatory diseases compared with those on standard systemic therapy.
Funding
UK Medical Research Council, NIHR Biomedical Research Centre at King's College London and Guy's and St Thomas' NHS Foundation Trust, and Wellcome Trust.
Introduction
Although most people with COVID-19 have mild symptoms, estimates in unvaccinated individuals indicate that 15% develop pneumonia requiring hospital treatment and 5% progress to severe disease (ie, respiratory failure, septic shock, or multiple organ dysfunction).1 Previous research has shown that immune-mediated inflammatory diseases, including those affecting joints (rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis), the bowel (Crohn's disease and ulcerative colitis), and skin (psoriasis and hidradenitis suppurativa), are associated with an increased risk of severe COVID-19. However, most studies, except for one on rheumatoid arthritis,2 have found that this risk disappears after adjusting for comorbidities.3, 4 Most studies also show that use of targeted therapies does not confer risk of severe COVID-19, with the exception of rituximab or Janus kinase (JAK) inhibitors, with which some studies have reported worse outcomes.3, 5, 6, 7, 8, 9 The majority of these studies were from selected sources, such as disease-specific registries, rather than general population-based sources, and are hence subject to selection bias, small sample sizes, and absence of denominators.
We aimed to investigate risks of severe COVID-19 outcomes in people with immune-mediated inflammatory diseases and those on targeted immune-modifying therapies using English population-based electronic health record data linked to a new, unique national hospital prescribing dataset containing information on high-cost targeted immune-modifying therapies. The size of our study population and granularity of our data allowed us to perform post-hoc analyses stratified by ethnicity, which is an important risk factor for severe COVID-19.10, 11
Research in context.
Evidence before this study
We searched PubMed on Nov 2, 2021, using the terms “COVID-19”, “SARS-CoV-2” AND “rheumatoid arthritis”, “psoriatic arthritis”, “ankylosing spondylitis”, “Crohn's disease”, “ulcerative colitis”, “hidradenitis suppurativa” AND “psoriasis”, to identify primary research articles and systematic reviews, published in English, examining severe COVID-19 outcome risk in individuals with immune-mediated inflammatory diseases and those on immune-modifying therapy. Previous studies reported an increased risk of severe COVID-19 in people with immune-mediated inflammatory diseases that was largely mediated through comorbidities. Most published studies suggested that people on targeted therapies to treat immune-mediated inflammatory diseases were not at an increased risk of severe COVID-19 outcomes, with the exception of some studies reporting worse outcomes in those on rituximab or Janus kinase (JAK) inhibitors. Some therapies, such as tumour necrosis factor inhibitors, were found to be associated with a decreased risk of severe COVID-19 outcomes. The majority of studies focused on adverse outcomes in patients on systemic therapy for immune-mediated inflammatory diseases and used data from disease-specific registries, which can be subject to selection bias and lack denominator populations.
Added value of the study
In our large population-based study of more than 17 million individuals, including more than 1 million people with immune-mediated inflammatory diseases and about 200 000 receiving immune-modifying medications, people with immune-mediated inflammatory diseases had an increased risk of COVID-19-related death compared with the general population after adjusting for potential confounders and mediators. We also saw some evidence that patients with immune-mediated inflammatory diseases were more likely than the general population to have COVID-19-related critical care admission or death, and hospital admission. Non-White ethnic groups had a higher risk of severe COVID-19 than White ethnic groups. However, the increase in risk of severe COVID-19 associated with having an immune-mediated inflammatory disease was generally similar between ethnic groups. We saw no evidence of differences in severe COVID-19-related outcomes with most targeted immune-modifying therapies when compared with standard systemic therapy. However, rituximab was associated with an increased risk of COVID-19-related death, and critical care admission or death. There was also an increase in COVID-19-related hospital admissions in people prescribed rituximab or JAK inhibitors, compared with those on standard systemic therapy, although adjustment for confounding by unmeasured severity might explain at least part of this finding. This is the first study, to our knowledge, to use high-cost drug data on medicines supplied by hospitals at a national scale in England (to identify targeted therapies). The availability of these data fills an important gap in the medication record of patients with more specialist conditions treated by hospitals, creating an important opportunity to generate insights into these conditions and these medications
Implications of all the available evidence
Our study offers insights into future risk mitigation strategies and COVID-19 vaccination priorities for individuals with immune-mediated inflammatory diseases, as it highlights that patients with immune-mediated inflammatory diseases and those taking rituximab might be at risk of severe COVID-19 outcomes. Crucially, our study does not show a link between most targeted immune-modifying medications, compared with standard systemic therapy, and severe COVID-19 outcomes. However, the increased risk of adverse COVID-19 outcomes in people with immune-mediated inflammatory diseases and those treated with rituximab merits further study.
Methods
Study design and participants
We did a cohort study using OpenSAFELY, a new secure analytics platform for electronic health records that was created by our team for NHS England, and TPP, a general practitioner software provider. We used primary care records managed by TPP that are linked to the UK Office for National Statistics (ONS) death data, SARS-CoV-2 testing data, and a unique national hospital medication dataset (including high-cost drugs supplied by hospitals; appendix p 3).12 We accessed all data through OpenSAFELY. OpenSAFELY provides a secure software interface that allows analysis of pseudonymised primary care records in near real time within the electronic health record vendor's highly secure data centre, avoiding the need for data transfer off-site (minimising the re-identification risk). Pseudonymised datasets from other data providers are securely provided by the electronic health record vendor and linked to primary care data. The dataset analysed within OpenSAFELY was based on 24 million people currently registered at about 40% of general practitioner practices in England.
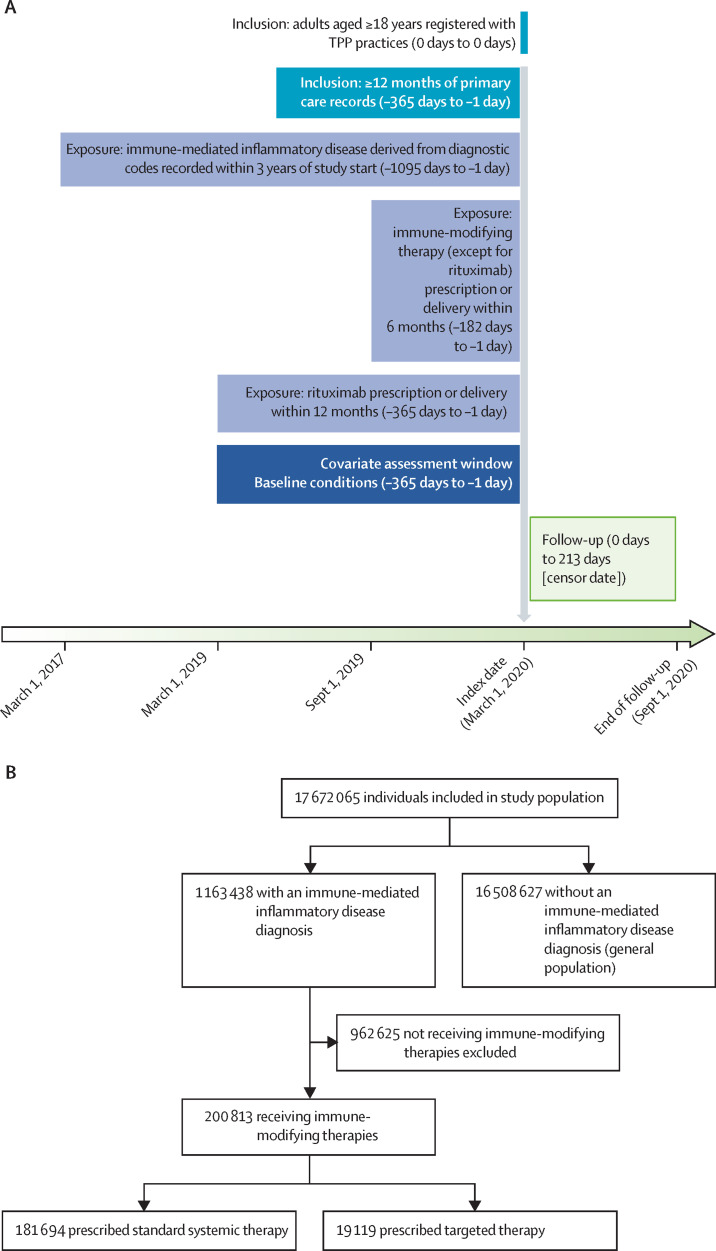
We included adults aged 18 years or older on March 1, 2020, who were registered with TPP practices with at least 12 months of primary care records before March, 2020 (figure 1A ). We followed up individuals from March 1, 2020 (UK SARS-CoV-2 outbreak start), to Sept 30, 2020 (study end), or until the specific outcome under analysis (ie, COVID-19-related death, critical care admission or death, or hospital admission).
Figure 1.
Study design and flow
We compiled diagnostic and therapeutic code lists (in machine-readable languages such as SNOMED-CT or UK National Health Service dictionary of medicines and devices) for all study variables (exposures, outcomes, and covariates). Detailed information on compilation and sources of code lists are freely available for inspection and re-use online. The study was approved by the Health Research Authority (Research Ethics Committee reference 20/LO/0651) and the London School of Hygiene and Tropical Medicine (London, UK) Ethics Board (reference 21863). All code used for data management and analyses, including all iterations of the prespecified study protocol archived with version control, is available online.
Exposures
Exposures were immune-mediated inflammatory diseases: inflammatory joint disease (rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis), inflammatory bowel disease (Crohn's disease, ulcerative colitis, or unclassified), and inflammatory skin disease (psoriasis or hidradenitis suppurativa); and prescription of systemic immune-modifying medication by general practitioners or supplied by hospitals through high-cost drug prescription procedures. We focused on these immune-mediated inflammatory diseases because they are similar in terms of disease mechanisms and therapies (eg, tumour necrosis factor [TNF] inhibitors).
We identified people with immune-mediated inflammatory diseases using diagnostic morbidity codes in primary care during the 3 years before March 1, 2020; people with multiple categories contributed to comparisons with the general population for all immune-mediated inflammatory disease categories for which they had records (eg, individuals with psoriatic arthritis and psoriasis, contributed to both joint and skin disease).
Immune-modifying medications were categorised as standard systemic therapy and targeted therapy. Standard systemic therapies included leflunomide, methotrexate, mycophenolate mofetil or mycophenolic acid, ciclosporin, sulphasalazine, mercaptopurine, thioguanine, and azathioprine. Targeted therapies comprised TNF inhibitors (etanercept, adalimumab, golimumab, certolizumab, and infliximab), interleukin (IL)-17 inhibitors (secukinumab, ixekizumab, and brodalumab), IL-12/IL-23 inhibitors (ustekinumab, guselkumab, risankizumab, and tildrakizumab), IL-6 inhibitors (tocilizumab and sarilumab), B-cell depletion therapy (rituximab), and JAK inhibitors (baricitinib and tofacitinib).13, 14, 15, 16, 17, 18 Individuals treated with both systemic therapy and targeted therapies were considered to be exposed to targeted therapies.
We identified standard systemic therapies using primary care prescribing data, and targeted immune-modifying medications using high-cost drugs invoices (appendix p 3). Drug exposure was defined by at least one prescription or delivery of medication to an individual before March 1, 2020 (date chosen because some medications were either specifically used or stopped owing to the pandemic). For each individual, we defined drug exposure on the basis of the closest drug recorded before the study start (March 1, 2020), allowing for a maximum of 6 months before the start of the study for all agents apart from rituximab, for which we permitted a 12-month exposure window (given the frequency of treatment and long duration of response).19, 20
Outcomes
Outcomes were COVID-19-related death, critical care admission or death, and hospital admission. We identified COVID-19-related deaths based on records of COVID-19-related International Classification of Diseases, revision 10, codes (U071, U072) anywhere on death certificates. We used COVID-19-related critical care admission (using data from the UK Intensive Care National Audit and Research Centre21) or death as a combined endpoint to reflect individuals with severe COVID-19 who died without being admitted to a critical care unit. We identified COVID-19-related hospital admission as a positive PCR test less than 28 days before admission and up to 5 days after admission to exclude nosocomial infection.
Statistical analysis
We selected potential confounders and mediators a priori based on clinical knowledge and previous evidence.10 In the relationship between immune-mediated inflammatory diseases and severe COVID-19 outcomes, we considered age (categorical variable), sex, deprivation (using quintiles of the Index of Multiple Deprivation),22, 23 and smoking status to be potential confounders; we considered body-mass index (BMI), cardiovascular disease, diabetes, and current glucocorticoid use to be potential mediators. In the relationship between immune-modifying therapy and severe COVID-19 outcomes, we considered age, sex, deprivation, smoking status, BMI, specific immune-mediated inflammatory disease (inflammatory joint, bowel, and skin disease), cardiovascular disease, cancer (excluding non-melanoma skin cancer), stroke, end-stage renal failure, chronic liver disease, chronic respiratory disease, and diabetes as potential confounders; we considered current glucocorticoid use as a potential mediator. Ethnicity (in five categories of White, South Asian, Black, mixed or other, and unknown) was used as a stratifying variable for subgroup analyses. A post-hoc analysis explored the effect of ethnicity on COVID-19 outcomes in each of the immune-mediated inflammatory disease subpopulations. Covariates were assessed within 12 months of study start as baseline conditions (definitions and figures representing assumed relationships between covariates, primary exposures, and outcomes are in the appendix pp 4, 27–28).
We described characteristics of the general population, people with immune-mediated inflammatory diseases, and those with immune-mediated inflammatory diseases prescribed immune-modifying therapy. We used Cox regression to estimate hazard ratios (HRs) with 95% CI comparing adults with immune-mediated inflammatory diseases with the general population, and people with immune-mediated inflammatory diseases on standard systemic drugs with those on targeted therapies. We adjusted models for confounding based on assumptions inherent in our conceptual frameworks (appendix pp 27–28). We tested Cox model assumptions using Schoenfeld residuals.
We repeated our main analyses in sensitivity analyses assessing robustness of our findings (appendix pp 5–7). We considered immune-mediated inflammatory disease severity and degree of shielding (ie, stay-at-home advice for vulnerable populations24) to be potential unmeasured confounders of associations between specific immune-modifying therapy and COVID-19 outcomes. We did a quantitative bias analysis using E values to assess how strongly associated unmeasured confounders would need to be with exposure and outcome to potentially fully explain observed non-null associations (ie, association adjusted for both measured covariates and the unmeasured confounder would be null).25
We used Python for data management, and Stata (version 16) or Python for analyses.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Of 17 672 065 people in the overall study population (figure 1B), 1 163 438 (6·6%) had an immune-mediated inflammatory disease diagnosis (table 1 ). Of these adults, 272 452 (23·4%) had inflammatory joint disease (183 485 [15·8%] with rheumatoid arthritis, 54 593 [4·7%] with psoriatic arthritis, and 35 138 [3·0%] with ankylosing spondylitis), 199 037 (17·1%) had an inflammatory bowel disease (69 788 [6·0%] with Crohn's disease, 100 617 [8·6%] with ulcerative colitis, and 32 093 [2·8%] with unclassified inflammatory bowel disease), and 769 816 (66·2%) had inflammatory skin disease (693 178 [59·6%] with psoriasis and 76 746 [6·6%] with hidradenitis suppurativa).
Table 1.
Descriptive characteristics of general population and people with immune-mediated inflammatory diseases
| General population (n=16 508 627) | Overall immune-mediated inflammatory diseases (n=1 163 438) | Inflammatory joint disease (n=272 452) | Inflammatory skin disease (n=769 816) | Inflammatory bowel disease (n=199 037) | |
|---|---|---|---|---|---|
| Age, years | |||||
| 18–39 | 5 808 217 (35·2%) | 252 718 (21·7%) | 25 238 (9·3%) | 191 634 (24·9%) | 46 099 (23·2%) |
| 40–49 | 2 727 833 (16·5%) | 183 130 (15·7%) | 32 366 (11·9%) | 130 758 (17·0%) | 32 057 (16·1%) |
| 50–59 | 2 882 387 (17·5%) | 232 525 (20·0%) | 56 192 (20·6%) | 155 223 (20·2%) | 39 513 (19·9%) |
| 60–69 | 2 235 982 (13·5%) | 209 384 (18·0%) | 62 359 (22·9%) | 129 432 (16·8%) | 34 853 (17·5%) |
| 70–79 | 1 797 487 (10·9%) | 186 613 (16·0%) | 62 200 (22·8%) | 107 331 (13·9%) | 31 215 (15·7%) |
| ≥80 | 1 056 721 (6·4%) | 99 068 (8·5%) | 34 097 (12·5%) | 55 438 (7·2%) | 15 300 (7·7%) |
| Sex | |||||
| Male | 8 293 607 (50·2%) | 523 274 (45·0%) | 107 104 (39·3%) | 356 220 (46·3%) | 96 054 (48·3%) |
| Female | 8 215 020 (49·8%) | 640 164 (55·0%) | 165 348 (60·7%) | 413 596 (53·7%) | 102 983 (51·7%) |
| Ethnicity* | |||||
| White | 10 614 096 (64·3%) | 827 457 (71·1%) | 195 851 (71·9%) | 547 080 (71·1%) | 141 986 (71·3%) |
| South Asian | 999 881 (6·1%) | 50 382 (4·3%) | 12 771 (4·7%) | 31 964 (4·2%) | 8685 (4·4%) |
| Black | 340 723 (2·1%) | 9960 (0·9%) | 2723 (1·0%) | 6071 (0·8%) | 1502 (0·8%) |
| Mixed or other | 494 119 (3·0%) | 16 797 (1·4%) | 3655 (1·3%) | 11 175 (1·5%) | 2736 (1·4%) |
| Missing | 4 059 808 (24·6%) | 258 842 (22·2%) | 57 452 (21·1%) | 173 526 (22·5%) | 44 128 (22·2%) |
| Body-mass index, kg/m2 | |||||
| Underweight (<18·5) | 314 887 (1·9%) | 21 231 (1·8%) | 5995 (2·2%) | 11 280 (1·5%) | 5158 (2·6%) |
| Normal (18·5–24·9) | 4 576 346 (27·7%) | 306 029 (26·3%) | 74 283 (27·3%) | 186 383 (24·2%) | 63 902 (32·1%) |
| Overweight (25·0–29·9) | 4 462 587 (27·0%) | 351 450 (30·2%) | 87 569 (32·1%) | 226 580 (29·4%) | 62 068 (31·2%) |
| Obese I (30·0–34·9) | 2 255 908 (13·7%) | 202 825 (17·4%) | 50 614 (18·6%) | 137 770 (17·9%) | 30 048 (15·1%) |
| Obese II (35·0–39·9) | 871 125 (5·3%) | 88 344 (7·6%) | 21 818 (8·0%) | 62 536 (8·1%) | 11 135 (5·6%) |
| Obese III (≥40·0) | 502 285 (3·0%) | 55 834 (4·8%) | 12 896 (4·7%) | 41 747 (5·4%) | 5744 (2·9%) |
| Missing | 3 525 489 (21·4%) | 137 725 (11·8%) | 19 277) (7·1%) | 103 520 (13·4%) | 20 982 (10·5%) |
| Index of Multiple Deprivation | |||||
| 1 (least deprived) | 3 337 475 (20·2%) | 242 175 (20·8%) | 57 464 (21·1%) | 156 444 (20·3%) | 44 874 (22·5%) |
| 2 | 3 280 436 (19·9%) | 235 706 (20·3%) | 56 059 (20·6%) | 152 956 (19·9%) | 42 621 (21·4%) |
| 3 | 3 294 811 (20·0%) | 233 866 (20·1%) | 56 398 (20·7%) | 152 627 (19·8%) | 40 775 (20·5%) |
| 4 | 3 330 769 (20·2%) | 228 552 (19·6%) | 53 089 (19·5%) | 152 678 (19·8%) | 37 674 (18·9%) |
| 5 (most deprived) | 3 129 886 (19·0%) | 213 903 (18·4%) | 47 616 (17·5%) | 148 866 (19·3%) | 31 274 (15·7%) |
| Missing | 135 250 (0·8%) | 9236 (0·8%) | 1826 (0·7%) | 6245 (0·8%) | 1819 (0·9%) |
| Smoking | |||||
| Never | 7 687 903 (46·6%) | 420 806 (36·2%) | 102 798 (37·7%) | 265 169 (34·4%) | 79 651 (40·0%) |
| Former | 5 310 393 (32·2%) | 509 886 (43·8%) | 128 484 (47·2%) | 327 811 (42·6%) | 91 893 (46·2%) |
| Current | 2 774 203 (16·8%) | 220 916 (19·0%) | 40 007 (14·7%) | 168 056 (22·8%) | 25 350 (12·7%) |
| Missing | 736 128 (4·5%) | 11 830 (1·0%) | 1163 (0·4%) | 8780 (1·1%) | 2143 (1·1%) |
| Comorbidities | |||||
| Diabetes | |||||
| HbA1c <58 mmol/mol (<7·5%) | 1 033 685 (6·3%) | 112 193 (9·6%) | 32 631 (12·0%) | 71 520 (9·3%) | 17 366 (8·7%) |
| HbA1c ≥58 mmol/mol (≥7·5%) | 456 388 (2·8%) | 48 951 (4·2%) | 13 058 (4·8%) | 32 388 (4·2%) | 7766 (3·9%) |
| Unknown HbA1c | 240 398 (1·5%) | 21 567 (1·9%) | 5741 (2·1%) | 14 071 (1·8%) | 3482 (1·7%) |
| Cardiovascular disease | 1 146 032 (6·9%) | 129 065 (11·1%) | 42 078 (15·4%) | 76 916 (10·0%) | 20 536 (10·3%) |
| Stroke | 372 332 (2·3%) | 40 523 (3·5%) | 12 872 (4·7%) | 24 075 (3·1%) | 6587 (3·3%) |
| Cancer | 962 622 (5·8%) | 94 832 (8·2%) | 27 779 (10·2%) | 56 751 (7·4%) | 17 150 (8·6%) |
| End-stage renal failure | 22 408 (0·1%) | 2190 (0·2%) | 580 (0·2%) | 1217 (0·2%) | 550 (0·3%) |
| Chronic respiratory disease | 666 384 (4·0%) | 94 350 (8·1%) | 33 690 (12·4%) | 53 614 (7·0%) | 14 725 (7·4%) |
| Chronic liver disease | 98 012 (0·6%) | 15 333 (1·3%) | 3877 (1·4%) | 9340 (1·2%) | 3758 (1·9%) |
| Glucocorticoid use | |||||
| One or more prescriptions in past 3 months† | 317 938 (1·9%) | 64 151 (5·5%) | 30 928 (11·4%) | 27 673 (3·6%) | 11 913 (6·0%) |
Data are n (%). People with diagnoses across subcategories contributed to multiple categories (eg, a person with psoriasis and psoriatic arthritis contributed to both skin and joint categories of immune-mediated inflammatory diseases). HbA1c=glycated haemoglobin.
Ethnicity was not adjusted for in the main analysis due to the high proportion of missing data, although we did adjust for ethnicity in a sensitivity analysis (appendix p 9).
Glucocorticoid use refers to individuals with one or more prescriptions for any dose of oral glucocorticoid in the 3 months before study start.
Compared with the general population, people with immune-mediated inflammatory diseases were older (≥70 years; 17·3% vs 24·5%), more likely to be female (49·8% vs 55·0%), White (64·3% vs 71·1%), and obese (BMI ≥30 kg/m2; 22·0% vs 29·8%), and with more comorbidities (table 1). There were differences between individuals with inflammatory joint, skin, and bowel diseases: for example, individuals with inflammatory joint disease were older than those with an inflammatory bowel disease or inflammatory skin disease (table 1).
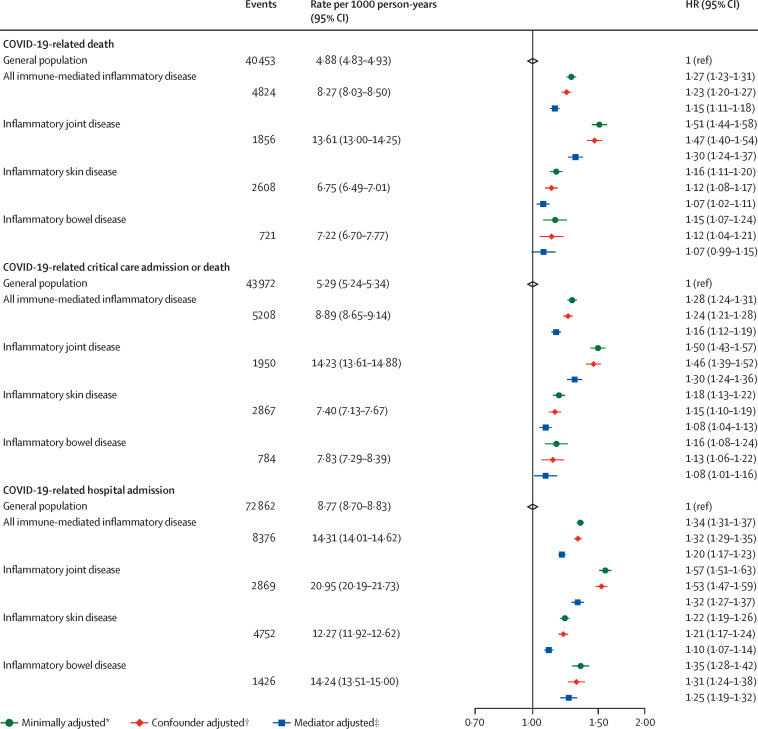
After adjusting for age and sex, people with immune-mediated inflammatory diseases had a greater risk of COVID-19-related death compared with the general population (HR 1·27, 95% CI 1·23–1·31). Evidence of association between immune-mediated inflammatory diseases and COVID-19-related death remained after additionally adjusting for the confounders deprivation, and smoking status (HR 1·23, 95% CI 1·20–1·27) and after further adjusting for the potential mediators BMI, cardiovascular disease, diabetes, and current glucocorticoid use (HR 1·15, 95% CI 1·11–1·18; figure 2 ; appendix p 8).
Figure 2.
COVID-19-related death, critical care admission or death, and hospital admission in people with immune-mediated inflammatory diseases versus the general population
The general population event counts shown are for the analyses comparing people with immune-mediated inflammatory diseases with the general population. HR=hazard ratio. *Adjusted for age and sex. †Adjusted (immune-mediated inflammatory disease population) for age, sex, deprivation, and smoking status. ‡Adjusted (immune-mediated inflammatory disease population): age, sex, deprivation, smoking status, body-mass index, cardiovascular disease, diabetes, and current glucocorticoid use.
After adjusting for age and sex, we saw increased COVID-19-related death in people with inflammatory joint (HR 1·51, 95% CI 1·44–1·58), bowel (1·15, 1·07–1·24), and skin (1·16, 1·11–1·20) diseases compared with the general population. After further adjusting for potential confounders, evidence for association between specific immune-mediated inflammatory disease types and COVID-19-related death persisted for all types of immune-mediated inflammatory diseases and was greatest for inflammatory joint disease (HR 1·47, 95% CI 1·40–1·54), with smaller effect estimates for inflammatory skin (1·12, 1·08–1·17) and bowel (1·12, 1·04–1·21) disease, and further attenuation after adjusting for potential mediators (figure 2; appendix p 8).
People with immune-mediated inflammatory diseases had greater risk of COVID-19-related critical care admission or death than the general population (HR 1·28, 95% CI 1·24–1·31), which persisted after adjusting for confounders (1·24, 1·21–1·28) and further adjusting for mediators (1·16, 1·12–1·19). Compared with the general population, there was evidence of increased COVID-19-related critical care admission or death in people with inflammatory joint, skin, and bowel diseases (figure 2; appendix p 8).
Compared with the general population, people with immune-mediated inflammatory diseases had greater risk of COVID-19-related hospital admission (HR 1·34, 95% CI 1·31–1·37), which remained after adjusting for potential confounders (1·32, 1·29–1·35) and mediators (1·20, 1·17–1·23). Risk of COVID-19-related hospital admission was increased in all immune-mediated inflammatory disease categories compared with the general population (figure 2; appendix p 8). Results from sensitivity analyses were broadly similar to the main analysis (appendix pp 9–10).
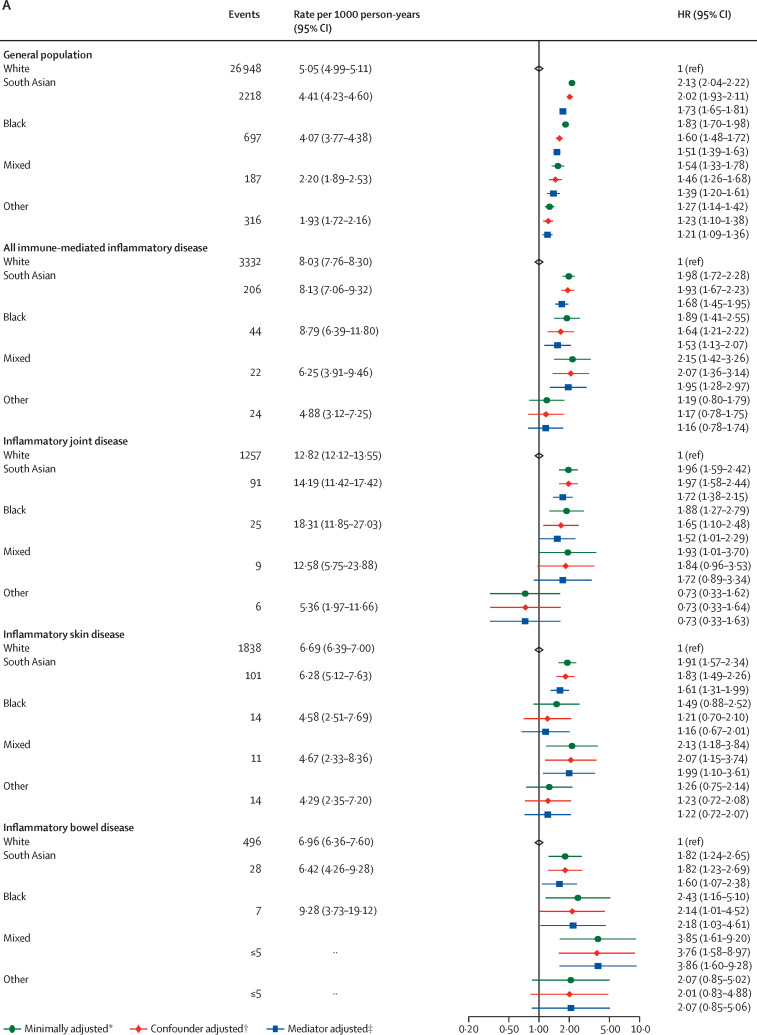
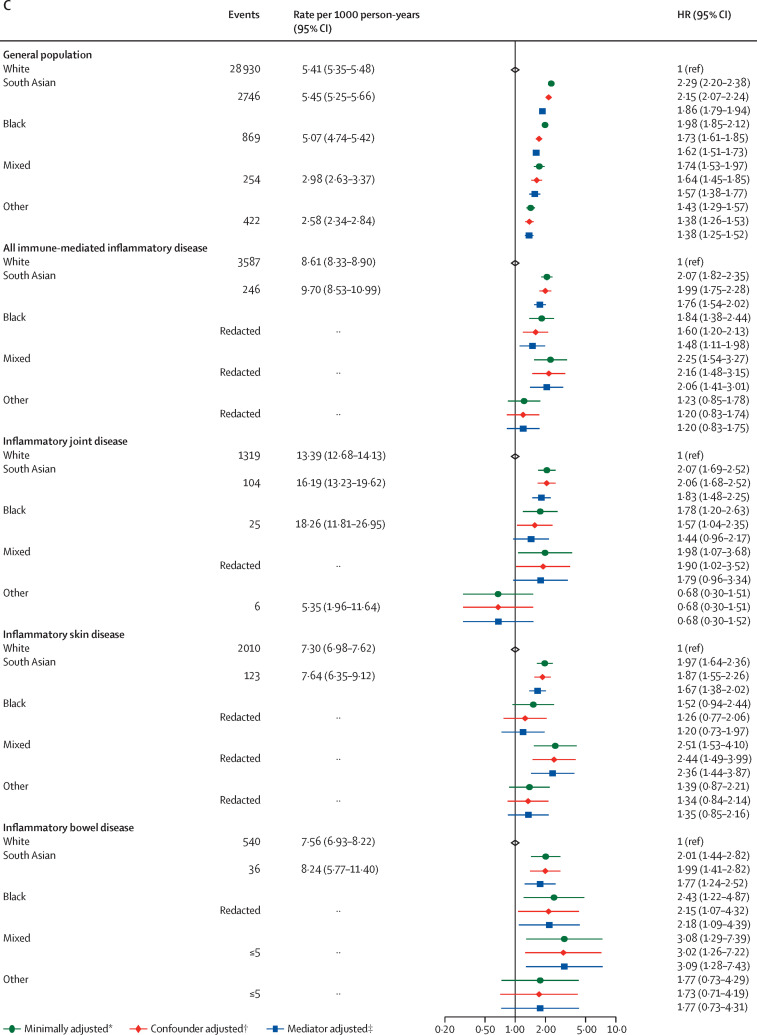
For age and sex distribution stratified by ethnicity see the appendix (p 29). 293 582 (35·5%) of 827 547 people in the White immune-mediated inflammatory disease population were younger than 50 years, versus 42 939 (55·7%) of 77 139 people in the non-White immune-mediated inflammatory disease population (p<0·0001). In analyses stratified by ethnicity and controlling for these age differences (appendix pp 30–33), we saw some attenuation of the estimates in people of South Asian ethnicity; for the other ethnic groups the numbers of events were small, leading to wide CIs. In the group with unknown ethnicity, we saw similar estimates to those for the White population. We also explored the effect of ethnicity itself on COVID-19 outcomes in each of the immune-mediated inflammatory disease subpopulations (figure 3 ). In each case, the effect of being in one of the non-White ethnic groups compared with the White population was similar to that observed in the general population.
Figure 3.
COVID-19-related death (A), critical care admission or death (B), and hospital admissions (C) in the general population and immune-mediated inflammatory disease subgroups comparing non-White with White ethnicities
Cells with counts less than or equal to five and cells that would potentially lead to a secondary risk of statistical disclosure have been redacted to protect anonymity. HR=hazard ratio. *Adjusted for age and sex. †Adjusted (immune-mediated inflammatory disease population) for age, sex, deprivation, and smoking status. ‡Adjusted (immune-mediated inflammatory disease population) for age, sex, deprivation, smoking status, body-mass index, cardiovascular disease, diabetes, and current glucocorticoid use.
200 813 (17·3%) of 1 163 438 adults with immune-mediated inflammatory diseases were prescribed either standard systemic therapy (181 694 [90·5%] of 200 813) or targeted immune-modifying therapy (19 119 [9·5%]; table 2 ; appendix p 11). Compared with people on standard systemic therapy, individuals receiving targeted therapy were younger and less likely to have comorbidities (eg, cardiovascular disease). The most commonly prescribed targeted therapies were TNF inhibitors, followed by rituximab, IL-12/IL-23 inhibitors, IL-17 inhibitors, JAK inhibitors, and IL-6 inhibitors (table 2).
Table 2.
Descriptive characteristics of immune-mediated inflammatory disease population on targeted and standard systemic immune-modifying therapy
| Standard systemic therapy*(n=181 694) | Any targeted immune-modifying therapy (n=19 119) | TNF inhibitor (n=13 524) | IL-12/IL-23 inhibitor (n=1379) | IL-17 inhibitor (n=1036) | JAK inhibitor (n=871) | Rituximab (n=1998) | IL-6 inhibitor (n=758) | |
|---|---|---|---|---|---|---|---|---|
| Immune-mediated inflammatory disease | ||||||||
| Joint disease | 98 830 (54·4%) | 12 929 (67·6%) | 8778 (64·9%) | 293 (21·2%) | 670 (64·7%) | 742 (85·2%) | 1998 (100·0%) | 758 (100·0%) |
| Skin disease | 31 695 (17·4%) | 5272 (27·6%) | 3392 (25·1%) | 893 (64·8%) | 838 (80·9%) | 96 (11·0%) | .. | .. |
| Bowel disease | 79 239 (43·6%) | 5094 (26·6%) | 4443 (32·9%) | 554 (40·2%) | 11 (1·1%) | 141 (16·2%) | .. | .. |
| Age, years | ||||||||
| 18–39 | 24 898 (13·7%) | 4276 (22·4%) | 3467 (25·6%) | 427 (31·0%) | 252 (24·3%) | 85 (9·8%) | 68 (3·4%) | 76 (10·0%) |
| 40–49 | 23 140 (12·7%) | 3301 (17·3%) | 2456 (18·2%) | 314 (22·8%) | 246 (23·7%) | 109 (12·5%) | 175 (8·8%) | 89 (11·7%) |
| 50–59 | 36 588 (20·1%) | 4405 (23·0%) | 3068 (22·7%) | 324 (23·5%) | 274 (26·4%) | 225 (25·8%) | 432 (21·6%) | 188 (24·8%) |
| 60–69 | 40 134 (22·1%) | 3826 (20·0%) | 2523 (18·7%) | 201 (14·6%) | 177 (17·1%) | 246 (28·2%) | 565 (28·3%) | 207 (27·3%) |
| 70–79 | 38 842 (21·4%) | 2616 (13·7%) | 1603 (11·9%) | 91 (6·6%) | 75 (7·2%) | 165 (18·9%) | 579 (29·0%) | 154 (20·3%) |
| ≥80 | 18 092 (10·0%) | 695 (3·6%) | 407 (3·0%) | 22 (1·6%) | 12 (1·2%) | 41 (4·7%) | 179 (9·0%) | 44 (5·8%) |
| Sex | ||||||||
| Male | 76 134 (41·9%) | 8341 (43·6%) | 6259 (46·3%) | 690 (50·0%) | 595 (57·4%) | 244 (28·0%) | 557 (27·9%) | 171 (22·6%) |
| Female | 105 560 (58·1%) | 10 778 (56·4%) | 7265 (53·7%) | 689 (50·0%) | 441 (42·6%) | 627 (72·0%) | 1 441 (72·1%) | 587 (77·4%) |
| Ethnicity† | ||||||||
| White | 130 217 (71·7%) | 13 353 (69·8%) | 9481 (70·1%) | 926 (67·2%) | 711 (68·6%) | 599 (68·8%) | 1406 (70·4%) | 535 (70·6%) |
| South Asian | 8451 (4·7%) | 1023 (5·4%) | 671 (5·0%) | 96 (7·0%) | 73 (7·0%) | 68 (7·8%) | 119 (6·0%) | 34 (4·5%) |
| Black | 1 361 (0·7%) | 179 (0·9%) | 123 (0·9%) | Redacted‡ | 8 (0·8%) | Redacted‡ | 25 (1·3%) | Redacted‡ |
| Mixed or other | 2183 (1·2%) | 277 (1·4 %) | 201 (1·5%) | Redacted§ | 22 (2·1%) | Redacted§ | 20 (1·0%) | Redacted§ |
| Missing | 39 482 (21·7%) | 4287 (22·4%) | 3048 (22·5%) | 335 (24·3%) | 222 (21·4%) | 176 (20·2%) | 428 (21·4%) | 169 (22·3%) |
| Body-mass index, kg/m2 | ||||||||
| Underweight (<18·5) | 3752 (2·1%) | 482 (2·5%) | 342 (2·5%) | 37 (2·7%) | 8 (0·8%) | 22 (2·5%) | 58 (2·9%) | 21 (2·8%) |
| Normal (18·5–24·9) | 52 050 (28·6%) | 5161 (27·0%) | 3761 (27·8%) | 318 (23·1%) | 168 (16·2%) | 252 (28·9%) | 560 (28·0%) | 210 (27·7%) |
| Overweight (25·0–29·9) | 59 223 (32·5%) | 5627 (29·4%) | 3989 (29·5%) | 340 (24·7%) | 299 (28·9%) | 254 (29·2%) | 646 (32·3%) | 216 (28·5%) |
| Obese I (30·0–34·9) | 32 671 (18·0%) | 3424 (17·9%) | 2334 (17·3%) | 265 (19·2%) | 227 (21·9%) | 163 (18·7%) | 388 (19·4%) | 136 (17·9%) |
| Obese II (35·0–39·9) | 13 370 (7·4%) | 1636 (8·6%) | 1071 (7·9%) | 150 (10·9%) | 132 (12·7%) | 82 (9·4%) | 172 (8·6%) | 70 (9·2%) |
| Obese III (≥40·0) | 7836 (4·3%) | 1011 (5·3%) | 650 (4·8%) | 115 (8·3%) | 88 (8·5%) | 44 (5·1%) | 89 (4·5%) | 55 (7·3%) |
| Missing | 12 792 (7·0%) | 1778 (9·3%) | 1377 (10·2) | 154 (11·2) | 114 (11·0%) | 54 (6·2%) | 85 (4·3%) | 50 (6·6%) |
| Index of Multiple Deprivation | ||||||||
| 1 (least deprived) | 39 830 (21·9%) | 4284 (22·4%) | 3104 (23·0%) | 254 (18·4%) | 240 (23·2%) | 187 (21·5%) | 401 (20·1%) | 189 (24·9%) |
| 2 | 38 618 (21·3%) | 4070 (21·3%) | 2904 (21·5%) | 281 (20·4%) | 193 (18·6%) | 218 (25·0%) | 427 (21·4%) | 150 (19·8%) |
| 3 | 37 626 (20·7%) | 3875 (20·3%) | 2724 (20·1%) | 288 (20·9%) | 210 (20·3%) | 156 (17·9%) | 443 (22·2%) | 149 (19·7%) |
| 4 | 34 698 (19·1%) | 3503 (18·3%) | 2473 (18·3%) | 272 (19·7%) | 187 (18·1%) | 146 (16·8%) | 370 (18·5%) | Redacted‡ |
| 5 (most deprived) | 29 508 (16·2%) | 3236 (16·9%) | 2209 (16·3%) | 274 (19·9%) | 195 (18·8%) | 155 (17·8%) | 345 (17·3%) | 144 (19·0%) |
| Missing | 1 414 (0·8%) | 151 (0·8%) | 110 (0·8%) | 10 (0·7%) | 11 (1·1%) | 9 (1%)) | 12 (0·6%) | Redacted§ |
| Smoking | ||||||||
| Never | 68 915 (37·9%) | 7156 (37·4%) | 5214 (38·6%) | 480 (34·8%) | Redacted§ | 311 (35·7%) | Redacted‡ | 276 (36·4%) |
| Former | 89 418 (49·2%) | 8437 (44·1%) | 5769 (42·7%) | 555 (40·2%) | Redacted§ | 439 (50·4%) | Redacted‡ | 355 (46·8%) |
| Current | 22 338 (12·3%) | 3300 (17·3%) | 2352 (17·4%) | 324 (23·5%) | Redacted§ | 117 (13·4%) | Redacted‡ | 120 (15·8%) |
| Missing | 1023 (0·6%) | (226) (1·2%) | 189 (1·4%) | 20 (1·5%) | Redacted§ | 131 (15·0%) | Redacted‡ | 7 (0·9%) |
| Comorbidities | ||||||||
| Diabetes | ||||||||
| HbA1c <58 mmol/mol (<7·5%) | 19 572 (10·8%) | 1654 (8·7%) | 1007 (7·4%) | 129 (9·4%) | 105 (10·1%) | 93 (10·7%) | 292 (14·6%) | 76 (10·0%) |
| HbA1c ≥58 mmol/mol (≥7·5%) | 7863 (4·3%) | 831 (4·3%) | 516 (3·8%) | 72 (5·2%) | 81 (7·8%) | 49 (5·6%) | 99 (5·0%) | 34 (4·5%) |
| Unknown HbA1c | 3343 (1·8%) | 390 (2·0%) | 245 (1·8%) | 37 (2·7%) | 24 (2·3%) | 22 (2·5%) | 53 (2·7%) | 15 (2·0%) |
| Cardiovascular disease | 24 056 (13·2%) | 1801 (9·4%) | 1074 (7·9%) | 109 (7·9%) | 96 (9·3%) | 111 (12·7%) | 345 (17·3%) | 94 (12·4%) |
| Stroke | 7204 (4·0%) | 480 (2·5%) | 273 (2·0%) | 36 (2·6%) | 21 (2·0%) | 35 (4·0%) | 92 (4·6%) | 36 (4·7%) |
| Cancer | 16 721 (9·2%) | 1143 (6·0%) | 487 (3·6%) | 48 (3·5%) | 66 (6·4%) | 59 (6·8%) | 458 (22·9%) | 50 (6·6%) |
| End-stage renal failure | 477 (0·3%) | 27 (0·1%) | 14 (0·1%) | Redacted‡ | Redacted‡ | Redacted‡ | 7 (0·4%) | Redacted‡ |
| Chronic respiratory disease | 19 549 (10·8%) | 1767 (9·2%) | 976 (7·2%) | 83 (6·0%) | 67 (6·5%) | 124 (14·2%) | 452 (22·6%) | 103 (13·6%) |
| Chronic liver disease | 3175 (1·7%) | 326 (1·7%) | 202 (1·5%) | 42 (3·0%) | 37 (3·6%) | 10 (1·1%) | 38 (1·9%) | 12 (1·6%) |
| Glucocorticoid use | ||||||||
| One or more prescription in past 3 months¶ | 20 254 (11·1%) | 2318 (12·1%) | 1292 (9·6%) | 92 (6·7%) | 69 (6·7%) | 223 (25·6%) | 537 (26·9%) | 197 (26·0%) |
Data are n (%). People with diagnoses across subcategories contributed to multiple categories (eg, someone with psoriasis and psoriatic arthritis, contributed to both skin and joint categories of immune-mediated inflammatory disease), therefore individuals may be included in more than one targeted immune-modifying treatment category. Individuals treated with both systemic therapy and targeted therapy were included in the targeted therapy cohort. HbA1c=glycated haemoglobin. IL=interleukin. JAK=Janus kinase. TNF=tumour necrosis factor.
Standard systemic therapies included leflunomide, methotrexate, mycophenolate mofetil or mycophenolic acid, ciclosporin, sulphasalazine, mercaptopurine, thioguanine, and azathioprine.
Ethnicity was not adjusted for in the main analysis due to the high proportion of missing data, although we did adjust for ethnicity in a sensitivity analysis (appendix p 9).
Cells that introduce a potential secondary statistical disclosure have been redacted to protect anonymity.
Cells with counts of less than or equal to five are redacted to protect anonymity.
Glucocorticoid use refers to individuals with one or more prescription for any dose of oral glucocorticoid in the 3 months before study start.
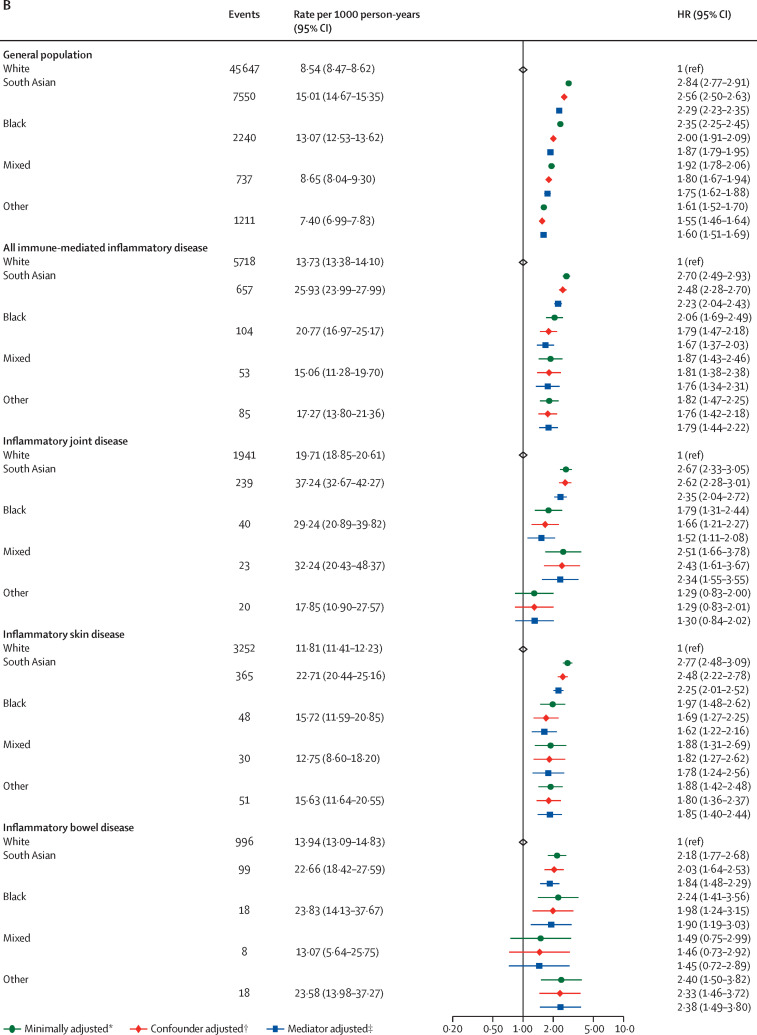
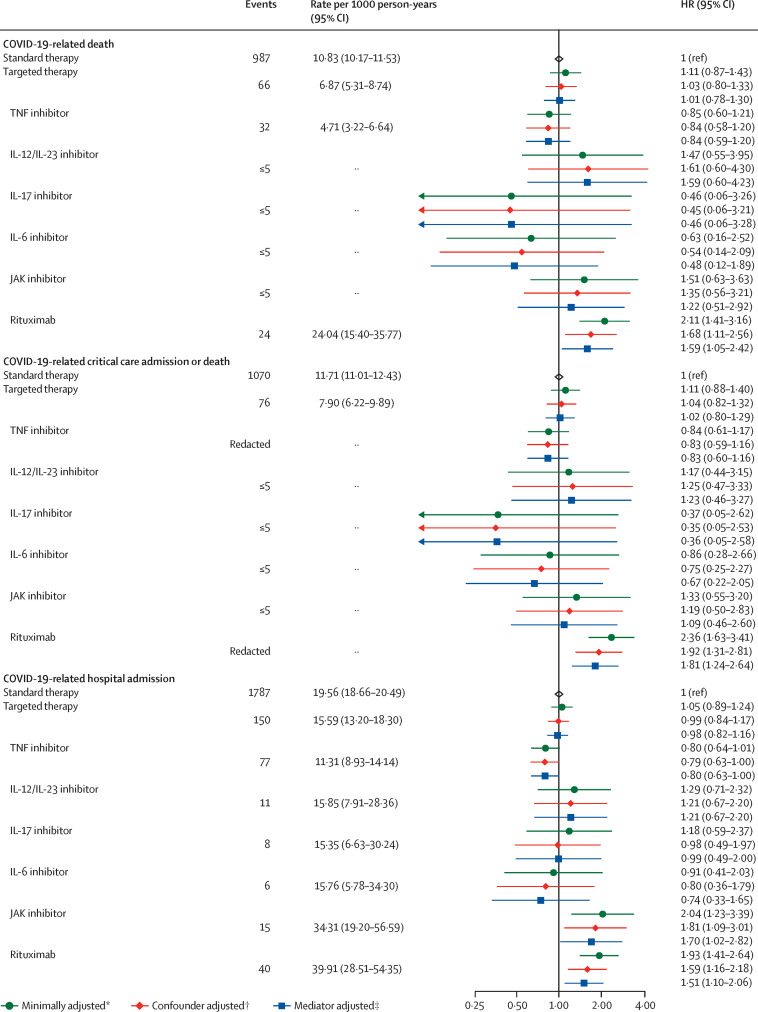
There was no difference in COVID-19-related death in people on targeted therapy compared with those on standard systemic therapy after adjusting for potential confounders (HR 1·03, 95% CI 0·80–1·33; adjusted for age, sex, deprivation, smoking status, BMI, immune-mediated inflammatory diseases [bowel, joint, skin], cardiovascular disease, cancer, stroke, end-stage renal failure, chronic liver disease, chronic respiratory disease, and diabetes) and mediators (1·01, 0·78–1·30; additionally adjusted for current glucocorticoid use; figure 4 ; appendix pp 12–13).
Figure 4.
COVID-19-related death, critical care admission or death, and hospital admission for targeted versus standard systemic immunosuppression
The general population event counts shown are for the analyses comparing patients with immune-mediated inflammatory diseases with the general population. Cells with counts less than or equal to five and cells that would potentially lead to a secondary risk of statistical disclosure have been redacted to protect anonymity. HR=hazard ratio. IL=interleukin. JAK=Janus kinase. TNF=tumour necrosis factor. *Adjusted for age and sex. †Adjusted for age, sex, deprivation, smoking status, body-mass index, specific immune-mediated inflammatory disease (joint, bowel, and skin), cardiovascular disease, cancer (excluding non-melanoma skin cancer), stroke, end-stage renal failure, chronic liver disease, chronic respiratory disease, and diabetes. ‡Adjusted for age, sex, deprivation, smoking status, body-mass index, specific immune-mediated inflammatory disease (joint, bowel, and skin), cardiovascular disease, cancer (excluding non-melanoma skin cancer), stroke, end-stage renal failure, chronic liver disease, chronic respiratory disease, diabetes, and current glucocorticoid use.
Compared with adults on standard systemic therapy, there was no observed increased risk of COVID-19 related death, COVID-19-related critical care admission or death, or COVID-19-related hospital admission, in individuals on TNF inhibitors, IL-12/IL-23 inhibitors, IL-17 inhibitors, JAK inhibitors, or IL-6 inhibitors, although CIs were wide in some groups (figure 4). Compared with people on standard systemic therapy, people receiving rituximab had an increased risk of COVID-19-related death (confounder-adjusted HR 1·68, 95% CI 1·11–2·56; based on 24 deaths in the rituximab group), and critical care admission or death (HR 1·92, 95% CI 1·31–2·81). We also observed an increased risk of COVID-19-related hospital admission in those receiving rituximab (HR 1·59, 95% CI 1·16–2·18; 40 events) and JAK inhibitors (1·81, 1·09–3·01; 15 events), compared with people on standard systemic therapy.
Excluding people with haematological cancers and organ transplants attenuated the effect estimate for rituximab (HR 1·54, 95% CI 0·95–2·49; 18 events). Otherwise, results from sensitivity analyses were similar to the main analysis (appendix pp 14–16, 19). In a quantitative bias analysis of individuals with immune-mediated inflammatory diseases taking rituximab or JAK inhibitors compared with those taking standard systemic therapy, we noted that an unmeasured confounder moderately associated with both exposure and outcome could potentially explain associations of rituximab and JAK inhibitors with adverse COVID-19 outcomes (appendix pp 22–25, 34–35).
Discussion
In this large population-based study using data from OpenSAFELY, we found that people with immune-mediated inflammatory diseases have a higher risk of COVID-19-related death, critical care admission or death, and hospital admissions than people without immune-mediated inflammatory diseases of the same age, sex, deprivation level, and smoking status. Adults with inflammatory joint disease had a greater increase in risk of all outcomes than those with inflammatory skin or bowel disease. We saw some very minor attenuation of estimates in people of South Asian ethnicity, but numbers of events were small in other ethnicities, precluding definitive conclusions.
We showed that compared with standard systemic immune-modifying therapies for immune-mediated inflammatory diseases, there was no increased risk of COVID-19-related death in people prescribed TNF, IL-12/IL-23, IL-17, IL-6, or JAK inhibitors. Rituximab was associated with an increased risk of death and critical care admission. However, this finding could be explained by residual confounding from factors such as frailty, a mechanistic link might be more plausible in the context of the wider evidence base.26, 27, 28
Our findings suggest that people with immune-mediated inflammatory diseases were at an increased risk of COVID-19-related death compared with people without immune-mediated inflammatory diseases of the same age, sex, deprivation, and smoking status. The mediator-adjusted effect estimates of our study also suggest that not all of the increased risk can be explained by mediation through comorbidities, as was found to be the case in most previous studies.3 Our finding that adults with immune-mediated inflammatory diseases were more likely to be admitted to hospital with COVID-19 than the general population is consistent with Canadian and Danish cohort studies29, 30 and reports of adverse COVID-19 outcomes for people with specific immune-mediated inflammatory diseases.31 However, factors leading to adverse COVID-19 outcomes are probably multifactorial, encompassing those associated with the likelihood of hospital admission, such as better access to care or a lower physician threshold for admission in patients on immune-modifying drugs, and factors associated with more severe symptoms. Explanations such as the presence of unmeasured confounders are also possible.
Our observation that adults on targeted therapies (except rituximab) do not have an increased risk of COVID-19 related death is consistent with data from international registries.3, 5, 6, 7 A recent meta-analysis using data from registries included 2766 individuals with autoimmune diseases and COVID-19 diagnoses reported higher rates of hospital admission and death in people prescribed combination standard systemic therapy and biologics or JAK inhibitors, but lower rates in those prescribed TNF inhibitor monotherapy.32 The Global Rheumatology Alliance reported no increase in COVID-19-related death with biological therapies compared with methotrexate monotherapy, but an increase in COVID-19-related death with JAK inhibitors and rituximab.6, 8, 26 The Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) reported no association in COVID-19-related death, critical care, or hospital admission in people on TNF inhibitors compared with those not prescribed TNF inhibitor therapy.5 A further pooled analysis from three international COVID-19 registries (including multiple immune-mediated inflammatory diseases) reported a reduction in severe COVID-19 outcomes among TNF inhibitor monotherapy users compared with those on other treatment regimens.9
Our rituximab findings are consistent with previous reports of increased mortality in people treated with B cell-depleting agents (eg, including for oncology indications).33 We were underpowered to assess effects of regular use of tocilizumab on COVID-19 outcomes, although trial data have shown benefit in patients admitted to hospital and critically ill patients.34, 35
Analyses of outcomes stratified by ethnicity within each immune-mediated inflammatory disease subpopulation showed that non-White groups were at a higher risk of death and hospital admission, consistent with a US-based study.36 An increase in risk was also seen in the non-immune-mediated inflammatory disease population in our analyses, with similar estimates to previously published studies; however, we did not see that the effect of immune-mediated inflammatory diseases was different in different ethnic groups.10, 11 Although non-White groups had similar or lower crude rates of severe COVID-19 compared with White groups in our analyses, this finding can be explained by the younger age distribution seen in these populations.
The key strengths of this study are the scale and completeness of underlying electronic health record data: all raw, single-event-level clinical events for all individuals at 40% of all general practitioner practices in England, including all tests, treatments, diagnoses, and clinical and demographic information linked to various sources of hospital data, including, for the first time, a comprehensive dataset of medications supplied by hospitals. We recognise some limitations. Information on high-cost drug prescriptions was not available after March, 2020. Therefore, we were not able to evaluate whether individuals stayed on their therapies throughout the study period. An ideal analysis would have evaluated medication just before COVID-19 diagnosis, and without this information, we must acknowledge potential for some misclassification bias, which could explain some of the null associations in our findings. Although English primary care records are longitudinal and comprehensive, certain confounders were not captured. Shielding, as recommended for groups of clinically vulnerable people by the Chief Medical Officer,24 might have reduced the risk of infection, thus likely biasing results towards the null. In mediator-adjusted models, we adjusted for concomitant use of oral glucocorticoids; however, this adjustment is likely to be imperfect, leading to residual confounding. We also considered cardiovascular disease and diabetes to be mediators in the relationship between immune-mediated inflammatory diseases and severe COVID-19 outcomes, but the timing of mediator assessment at index means that they could have predated the immune-mediated inflammatory disease diagnosis, and hence not be true mediators. Assessment of glucocorticoid exposure (and potentially immune-modifying drugs) is imperfect due to absent precise dose information, reducing dose regimens, low-cost medication administered in hospital alongside high-cost drugs, pandemic stockpiling, and patient-led discontinuation due to COVID-19-related concerns.
Finally, there is a possibility of misclassification of exposure status; this is highly unlikely for high-cost drug exposure because high-cost drug information is crucial for billing, but possible for standard systemic drugs resulting in underestimation of risks in the standard systemic group due to differential exclusion of patients whose first prescription was in hospital. We expect the effects of this misclassification to be minimal due to the short time window.
We have used one of the largest population-based datasets globally with linked data on immune-modifying drugs to describe COVID-19 risks for people with immune-mediated inflammatory diseases. We found that COVID-19 death and hospital admission were higher in people with immune-mediated inflammatory diseases; we saw no increased risk of adverse COVID-19 outcomes in adults on most targeted immune-modifying drugs for immune-mediated inflammatory diseases compared with standard systemics. The roll-out of a comprehensive vaccine programme alongside the development of other treatments for COVID-19 might mitigate some of the risks we describe. However, vaccine effectiveness in the immune-mediated inflammatory disease population on immunosuppressants has not been established37, 38, 39 and emergent evidence on the negative effect of immunosuppression on vaccine immunogenicity—notably, rituximab—suggests that some individuals will remain at greater risk of severe COVID-19 outcomes.
Our findings provide an evidence-base to inform policy on booster vaccination prioritisation and risk-mitigating behaviour advice, but must be interpreted in the context of UK public health policy on shielding. Findings will support health-care professionals engaging in shared decision making and communication of risk.
Data sharing
Access to the underlying identifiable and potentially re-identifiable pseudonymised electronic health record data is tightly governed by various legislative and regulatory frameworks, and restricted by best practice. The data in OpenSAFELY are drawn from general practice data across England where TPP is the data processor. TPP developers (CB, JC, JP, FH, and SH) initiate an automated process to create pseudonymised records in the core OpenSAFELY database, which are copies of key structured data tables in the identifiable records. These tables are linked onto key external data resources that have also been pseudonymised via SHA-512 one-way hashing of NHS numbers using a shared salt. DataLab developers and principal investigators (BG, LS, CEM, SB, AJW, KW, WH, HJC, DE, PI, SD, GH, BBC, RMS, ID, KBh, EW, and CTR) holding contracts with NHS England have access to the OpenSAFELY pseudonymised data tables as needed to develop the OpenSAFELY tools. These tools in turn enable researchers with OpenSAFELY Data Access Agreements to write and execute code for data management and data analysis without direct access to the underlying raw pseudonymised patient data and to review the outputs of this code. All code for the full data management pipeline—from raw data to completed results for this analysis—and for the OpenSAFELY platform as a whole is available for review online.
Declaration of interests
BG has received research funding from the Laura and John Arnold Foundation, the UK National Institute for Health Research (NIHR), the NIHR School of Primary Care Research, the NIHR Oxford Biomedical Research Centre, the Mohn-Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK (HDRUK), the Health Foundation, WHO, UK Research and Innovation (UKRI), Asthma UK, the British Lung Foundation, and the Longitudinal Health and Wellbeing strand of the National Core Studies programme; he also receives personal income from speaking and writing for lay audiences on the misuse of science and is a non-executive director of NHS Digital. CHS received departmental research funding from AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Leo, Pfizer, Novartis, Regeneron, SwedishOrphan Biovitrum, and Roche, and is an investigator within consortia that have industry partners. JG has received honoraria from AbbVie, Amgen, Celgene, Chugai, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Sobi, and UCB, and has research funding from Amgen, AstraZeneca, Gilead, Janssen, Medicago, Novovax, and Pfizer. MY has received honoraria from AbbVie and UCB. CWL has received honoraria from AbbVie, Bristol Myers Squibb, Celltrion, Ferring, Galapagos, Gilead, GlaxoSmithKline, Iterative Scopes, Janssen, Fresnius Kabi, Dr Falk, Vifor Pharma, Pfizer, Takeda, and Trellus Health. CHS and SML have received grants from the Horizon 2020 European Commission-funded consortium, which has industry partners involved in manufacture of treatments for immune-mediated inflammatory diseases (see the Biomap website for complete listing). EW has received payment from AstraZeneca for providing a training session, unrelated to the current manuscript. KEM has received consulting fees from Amgen. RM has received consulting fees from Amgen. LAT has received consulting fees from Bayer (payed to the institution), support for attending Medicines and Healthcare products Regulatory Agency meetings and is a member of two non-industry-funded trial advisory committees (unpaid). SN has received grants from Pfizer and honoraria for delivering educational presentations from Pfizer and Janssen. JB is funded by a studentship from GlaxoSmithKline. HIM was an occasional invited expert to the COVID-19 Vaccines Safety Surveillance Methodologies Expert Working Group, which has now come to a close. NAK has received departmental research funding from AbbVie, Biogen, Celgene, Celtrion, Galapagos, Merck Sharp & Dohme, Napp, Pfizer, Pharmacosmos, Roche, and Takeda; consulting fees from Amgen, Bristol Myers Squibb, Dr Falk, Janssen, Mylan, Pharmacosmos, Galapagos, Takeda, and Tillotts; honoraria from Allergan, Celltrion, Dr Falk, Ferring, Janssen, Pharmacosmos, Takeda, Tilllotts, and Galapagos; and support for meetings or travel from AbbVie, Dr Falk, and Janssen. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by the UK Medical Research Council (MRC; MR/V015737/1) and funded in part by the NIHR Biomedical Research Centre at King's College London and Guy's and St Thomas' NHS Foundation Trust and the Wellcome Trust (G205039/Z/16/Z). TPP and the North East Commissioning Support Unit North of England provided technical expertise and data infrastructure centre pro bono in the context of a national emergency. BG's work on better use of data in health care more broadly is currently funded in part by the NIHR Oxford Biomedical Research Centre, NIHR Applied Research Collaboration Oxford and Thames Valley, the Mohn-Westlake Foundation, NHS England, and the Health Foundation; all DataLab staff are supported by BG's grants on this work. LS reports grants from the Wellcome Trust, MRC, NIHR, UKRI, British Council, GlaxoSmithKline, British Heart Foundation, and Diabetes UK, outside this work. AS is employed by the London School of Hygiene & Tropical Medicine (LSHTM; UK) on a fellowship sponsored by GlaxoSmithKline. KBh holds a Sir Henry Dale fellowship jointly funded by the Wellcome Trust and the Royal Society. HIM is funded by the NIHR Health Protection Research Unit in Immunisation, a partnership between Public Health England and LSHTM. AYSW holds a fellowship from the British Heart Foundation. EW holds grants from the MRC. ID holds grants from NIHR and GlaxoSmithKline. RM holds a Sir Henry Wellcome Fellowship funded by the Wellcome Trust. HF holds a UKRI fellowship. RME is funded by HDR-UK and the MRC. SML was supported by a Wellcome Trust Senior Research Fellowship in Clinical Science (205039/Z/16/Z). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funders. SML was also supported by Health Data Research UK (grant number: LOND1), which is funded by the MRC, the UK Engineering and Physical Sciences Research Council, the UK Economic and Social Research Council, the Department of Health and Social Care (England), the Chief Scientist Office of the Scottish Government Health and Social Care Directorates, the Health and Social Care Research and Development Division (Welsh Government), the Public Health Agency (Northern Ireland), the British Heart Foundation, and the Wellcome Trust. SML is an investigator on the European Union Horizon 2020-funded BIOMAP Consortium. CHS acknowledges support for this research from the NIHR Biomedical Research Centre at King's College London and Guy's and St Thomas' NHS Foundation Trust and the Psoriasis Association. CWL is funded by a UKRI Future Leaders Fellowship. LAT is funded by UKRI, the NIHR, and the MRC. The views expressed are those of the authors and not necessarily those of the NIHR, ICNARC, NHS England, Public Health England, or the UK Department of Health and Social Care. We are very grateful for all the support received from the TPP Technical Operations team throughout this work, and for generous assistance from the information governance and database teams at NHS England and NHSX. Additionally, the North East Commissioning Support Unit provided support on behalf of all Commissioning Support Unit to aggregate the high-cost drugs data. This study uses electronic health records, data are provided by patients and collected by the NHS as part of their care and support. This publication is based on data derived from the Intensive Care National Audit & Research Centre (ICNARC) Case Mix Programme Database. The Case Mix Programme is the national, comparative audit of patient outcomes from adult critical care coordinated by ICNARC. We thank all the staff in the critical care units participating in the Case Mix Programme. For more information on the representativeness and quality of these data, please contact ICNARC. We are very grateful to Joe West from the University of Nottingham (UK) and Daniel Prieto-Alhambra from the University of Oxford (UK) for comments on an early version of the protocol.
Contributors
BG conceived the OpenSAFELY platform and the approach. LS and BG led the project overall and are guarantors. SML, CHS, NAK, CL, JG, KEM, BM, and SJWE conceptualised the study. SB led on software development. AM led on information governance. CJB, CEM, DH, RC, GH, TW, SCJB, PI, JC, DE, JP, and SH curated data. SML, CHS, NAK, CL, JG, KEM, BM, KBh, CTR, CJB, CEM, IJD, AJW, HIM, JC, HF, HJC, JT, RME, LAT, RME, AYSW and JP conceptualised disease categories and code lists. MY, JG, NAK, KBe, JB, and JM wrote the statistical analysis code. KEM, KBe, NAK, JG, SN, MY, and JB did data visualisation. EW, HJC, LS, and BG obtained ethical approvals. CJB, CEM, SCJB, SD, AG, LF, PI, AJW, JC, DE, WH, and FH contributed to software development. AS, SML, BMK, KEM, DH, CHS, KBe, JG, SN, MY, NAK, JM, LAT, RME, AYSW, RM, and SJWE reviewed and edited the manuscript. All authors were involved in design and conceptual development, and reviewed and approved the final manuscript. NAK, AR, and LF had full access to and validated the data in the study. All authors had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.England BR, Roul P, Yang Y, et al. Risk of COVID-19 in rheumatoid arthritis: a national Veterans Affairs matched cohort study in at-risk individuals. Arthritis Rheumatol. 2021;73:2179–2188. doi: 10.1002/art.41800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fagni F, Simon D, Tascilar K, et al. COVID-19 and immune-mediated inflammatory diseases: effect of disease and treatment on COVID-19 outcomes and vaccine responses. Lancet Rheumatol. 2021;3:e724–e736. doi: 10.1016/S2665-9913(21)00247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Silva KM, Jorge A, Cohen A, et al. COVID-19 outcomes in patients with systemic autoimmune rheumatic diseases compared to the general population: a US multicenter, comparative cohort study. Arthritis Rheumatol. 2021;73:914–920. doi: 10.1002/art.41619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491.e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahil SK, Dand N, Mason KJ, et al. Factors associated with adverse COVID-19 outcomes in patients with psoriasis—insights from a global registry-based study. J Allergy Clin Immunol. 2021;147:60–71. doi: 10.1016/j.jaci.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 Global Rheumatology Alliance physician registry. Ann Rheum Dis. 2021;80:1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izadi Z, Brenner EJ, Mahil SK, et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.29639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathur R, Rentsch CT, Morton CE, et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. 2021;397:1711–1724. doi: 10.1016/S0140-6736(21)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowan A, Bates C, Hulme W, et al. A comprehensive high cost drugs dataset from the NHS in England—an OpenSAFELY-TPP short data report. Wellcome Open Res. 2021;6:360. doi: 10.12688/wellcomeopenres.17360.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence Psoriasis: assessment and management. Clinical guideline [CG153] Oct 24, 2012. https://www.nice.org.uk/guidance/cg153/chapter/1-Recommendations [PubMed]
- 14.National Institute for Health and Care Excellence Rheumatoid arthritis in adults: management. NICE guideline [NG100] July 11, 2018. https://www.nice.org.uk/guidance/ng100/chapter/Recommendations [PubMed]
- 15.National Institute for Health and Care Excellence Spondyloarthritis in over 16s: diagnosis and management. NICE guideline [NG65] Feb 28, 2017. https://www.nice.org.uk/guidance/NG65/chapter/Recommendations [PubMed]
- 16.National Institute for Health and Care Excellence Crohn's disease: management. NICE guideline [NG129] May 3, 2019. https://www.nice.org.uk/guidance/ng129/chapter/Recommendations
- 17.National Institute for Health and Care Excellence Ulcerative colitis: management. NICE guideline [NG130] May 3, 2019. https://www.nice.org.uk/guidance/ng130/chapter/Recommendations [PubMed]
- 18.National Institute for Health and Care Excellence Adalimumab for treating moderate to severe hidradenitis suppurativa. Technology appraisal guidance [TA392] June 22, 2016. https://www.nice.org.uk/guidance/TA392/chapter/1-Recommendations
- 19.Buch MH, Smolen JS, Betteridge N, et al. Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:909–920. doi: 10.1136/ard.2010.144998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Assen S, Holvast A, Benne CA, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010;62:75–81. doi: 10.1002/art.25033. [DOI] [PubMed] [Google Scholar]
- 21.Richards-Belle A, Orzechowska I, Gould DW, et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020;46:2035–2047. doi: 10.1007/s00134-020-06267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministry of Housing. Communities and Local Government English indices of deprivation 2015. Sept 30, 2015. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015
- 23.Noble M, Wright G, Smith G, Dibben C. Measuring multiple deprivation at the small-area level. Environ Plann A. 2006;38:169–185. [Google Scholar]
- 24.UK Government Guidance on shielding and protecting people who are clinically extremely vulnerable from COVID-19. https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19
- 25.Ding P, VanderWeele TJ. Sensitivity analysis without assumptions. Epidemiology. 2016;27:368–377. doi: 10.1097/EDE.0000000000000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avouac J, Drumez E, Hachulla E, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol. 2021;3:e419–e426. doi: 10.1016/S2665-9913(21)00059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89:780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attauabi M, Seidelin JB, Felding OK, et al. Coronavirus disease 2019, immune-mediated inflammatory diseases and immunosuppressive therapies—a Danish population-based cohort study. J Autoimmun. 2021;118 doi: 10.1016/j.jaut.2021.102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eder L, Croxford R, Drucker A, et al. OP0285 COVID-19 hospitalizations, ICU admission, and death among patients with immune mediated inflammatory diseases (IMID)—a population-based study. Ann Rheum Dis. 2021;80(suppl 1):173. [Google Scholar]
- 31.Ludvigsson JF, Axelrad J, Halfvarson J, et al. Inflammatory bowel disease and risk of severe COVID-19: a nationwide population-based cohort study in Sweden. United European Gastroenterol J. 2021;9:177–192. doi: 10.1002/ueg2.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2021;80:384–391. doi: 10.1136/annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- 33.Lamure S, Dulery R, Delord M, et al. Abstract S09-02: high incidence of persistent COVID-19 among patients with lymphoma treated with B-cell depleting immunotherapy. Clin Cancer Res. 2021;27:S09. S02 (abstr). [Google Scholar]
- 34.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abani O, Abbas A, Abbas F, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gianfrancesco MA, Leykina LA, Izadi Z, et al. Association of race and ethnicity with COVID-19 outcomes in rheumatic disease: data from the COVID-19 Global Rheumatology Alliance Physician Registry. Arthritis Rheumatol. 2021;73:374–380. doi: 10.1002/art.41567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin S, Kennedy NA, Saifuddin A, et al. Antibody decay, T cell immunity and breakthrough infections following two SARS-CoV-2 vaccine doses in inflammatory bowel disease patients treated with infliximab and vedolizumab. Nat Commun. 2022;13 doi: 10.1038/s41467-022-28517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahil SK, Bechman K, Raharja A, et al. Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study. Lancet Rheumatol. 2022;4:e42–e52. doi: 10.1016/S2665-9913(21)00333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deepak P, Kim W, Paley MA, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to the underlying identifiable and potentially re-identifiable pseudonymised electronic health record data is tightly governed by various legislative and regulatory frameworks, and restricted by best practice. The data in OpenSAFELY are drawn from general practice data across England where TPP is the data processor. TPP developers (CB, JC, JP, FH, and SH) initiate an automated process to create pseudonymised records in the core OpenSAFELY database, which are copies of key structured data tables in the identifiable records. These tables are linked onto key external data resources that have also been pseudonymised via SHA-512 one-way hashing of NHS numbers using a shared salt. DataLab developers and principal investigators (BG, LS, CEM, SB, AJW, KW, WH, HJC, DE, PI, SD, GH, BBC, RMS, ID, KBh, EW, and CTR) holding contracts with NHS England have access to the OpenSAFELY pseudonymised data tables as needed to develop the OpenSAFELY tools. These tools in turn enable researchers with OpenSAFELY Data Access Agreements to write and execute code for data management and data analysis without direct access to the underlying raw pseudonymised patient data and to review the outputs of this code. All code for the full data management pipeline—from raw data to completed results for this analysis—and for the OpenSAFELY platform as a whole is available for review online.