Abstract
Background
Priming COVID-19 vaccine schedules have been deployed at variable intervals globally, which might influence immune persistence and the relative importance of third-dose booster programmes. Here, we report exploratory analyses from the Com-COV trial, assessing the effect of 4-week versus 12-week priming intervals on reactogenicity and the persistence of immune response up to 6 months after homologous and heterologous priming schedules using the vaccines BNT162b2 (tozinameran, Pfizer/BioNTech) and ChAdOx1 nCoV-19 (AstraZeneca).
Methods
Com-COV was a participant-masked, randomised immunogenicity trial. For these exploratory analyses, we used the trial's general cohort, in which adults aged 50 years or older were randomly assigned to four homologous and four heterologous vaccine schedules using BNT162b2 and ChAdOx1 nCoV-19 with 4-week or 12-week priming intervals (eight groups in total). Immunogenicity analyses were done on the intention-to-treat (ITT) population, comprising participants with no evidence of SARS-CoV-2 infection at baseline or for the trial duration, to assess the effect of priming interval on humoral and cellular immune response 28 days and 6 months post-second dose, in addition to the effects on reactogenicity and safety. The Com-COV trial is registered with the ISRCTN registry, 69254139 (EudraCT 2020–005085–33).
Findings
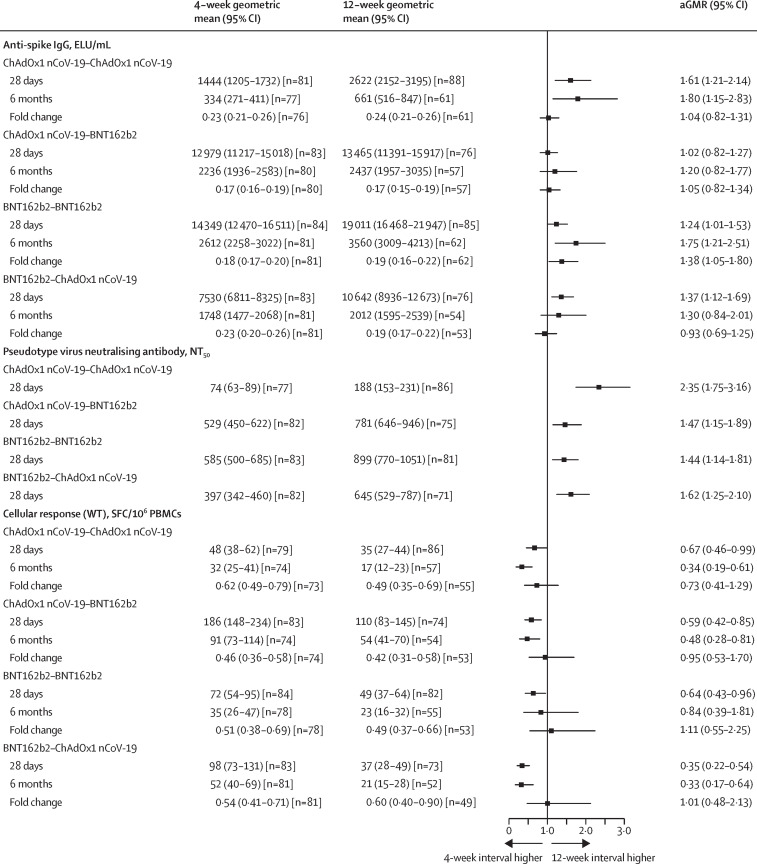
Between Feb 11 and 26, 2021, 730 participants were randomly assigned in the general cohort, with 77–89 per group in the ITT analysis. At 28 days and 6 months post-second dose, the geometric mean concentration of anti-SARS-CoV-2 spike IgG was significantly higher in the 12-week interval groups than in the 4-week groups for homologous schedules. In heterologous schedule groups, we observed a significant difference between intervals only for the BNT162b2–ChAdOx1 nCoV-19 group at 28 days. Pseudotyped virus neutralisation titres were significantly higher in all 12-week interval groups versus 4-week groups, 28 days post-second dose, with geometric mean ratios of 1·4 (95% CI 1·1–1·8) for homologous BNT162b2, 1·5 (1·2–1·9) for ChAdOx1 nCoV-19–BNT162b2, 1·6 (1·3–2·1) for BNT162b2–ChAdOx1 nCoV-19, and 2·4 (1·7–3·2) for homologous ChAdOx1 nCoV-19. At 6 months post-second dose, anti-spike IgG geometric mean concentrations fell to 0·17–0·24 of the 28-day post-second dose value across all eight study groups, with only homologous BNT162b2 showing a slightly slower decay for the 12-week versus 4-week interval in the adjusted analysis. The rank order of schedules by humoral response was unaffected by interval, with homologous BNT162b2 remaining the most immunogenic by antibody response. T-cell responses were reduced in all 12-week priming intervals compared with their 4-week counterparts. 12-week schedules for homologous BNT162b2 and ChAdOx1 nCoV-19–BNT162b2 were up to 80% less reactogenic than 4-week schedules.
Interpretation
These data support flexibility in priming interval in all studied COVID-19 vaccine schedules. Longer priming intervals might result in lower reactogenicity in schedules with BNT162b2 as a second dose and higher humoral immunogenicity in homologous schedules, but overall lower T-cell responses across all schedules. Future vaccines using these novel platforms might benefit from schedules with long intervals.
Funding
UK Vaccine Taskforce and National Institute for Health and Care Research.
Research in context.
Evidence before this study
Previous Com-COV results have shown good humoral and cellular immune responses to all homologous and heterologous schedules involving ChAdOx1 nCoV-19 and BNT162b2 when given at a 28-day priming interval. However, the effect of priming interval on immunogenicity is not fully understood. The original ChAdOx1 nCoV-19 efficacy trial showed an increase in immunogenicity and efficacy with a longer priming interval, and non-randomised data from the PITCH study suggest that prolonging priming interval in homologous BNT162b2 schedules modestly increases the humoral response and might have an effect on the profile of the cellular response, with the proportion of CD4-positive, IL-2 producing T cells increasing with a longer priming. However, it is not clear whether this change in profile is due to the difference in interval or the time elapsed since the first vaccine dose. We searched PubMed for research articles published between database inception and March 1, 2022, using the search terms (COVID) AND (Vaccin*) AND ((Heterologous) OR (Interval)) NOT (BCG) with no language restrictions. Besides our previously published reactogenicity and immunogenicity results, the search yielded five cohort studies that found variable increases in binding or neutralising antibody titres ranging from 1·5 to 9 times greater in longer interval groups than in shorter ones. Additionally, another cohort study found no difference in antibody response between intervals for inactivated vaccines. All these studies were non-randomised with differences in their baseline populations and had significant variability in the priming intervals received by their participants, as well as in the timing of antibody concentrations measurement. Aside from the PITCH consortium, all these studies had very low numbers of participants. None of these studies evaluated vaccine schedules other than those including only mRNA vaccines. Three statistical modelling studies assessed the overall benefit of prolonging priming interval without considering differences in immune response, with one suggesting that delaying the second dose to approximately 12 weeks would have a positive effect on death and hospitalisation in the context of increasing numbers of SARS-CoV-2 cases.
Added value of this study
We report the results on immunogenicity, reactogenicity, and safety of the first participant-masked randomised clinical trial using two vaccines approved by WHO for emergency use, ChAdOx1 nCoV-19 and BNT162b2, when administered at a 12-week interval in heterologous and homologous vaccine schedules (ChAdOx1 nCoV-19–ChAdOx1 nCoV-19, ChAdOx1 nCoV-19–BNT162b2, BNT162b2–BNT162b2, and BNT162b2–ChAdOx1 nCoV-19), which more closely mirrors the real-world vaccine rollout across many different countries. We also showed the effect of prolonging priming interval on reactogenicity, peak immune response, and decay rate of these schedules. The maximal humoral responses in the schedules with a 12-week interval were all at least as great as those in the equivalent 4-week schedules, and the decay rate of humoral response was reduced in the homologous BNT162b2 schedule with a longer interval. Reactogenicity at the second dose was greatly reduced in schedules with a 12-week interval and BNT162b2 as a second dose. This lends support to the decision by many national immunisation programmes to add flexibility to the priming interval and informs future vaccine development for non-SARS-CoV-2 pathogens. No safety concerns were raised.
Implications of all the available evidence
Delivery of COVID-19 vaccines to large proportions of the world has large logistical implications, especially in low-income and middle-income countries, where health-care and public health infrastructures might not be as robust. The results from this study support flexibility in use both of heterologous priming schedules and prolongation of priming interval, which might help to mitigate some of these logistical challenges. There is also evidence that, where feasible, longer interval schedules might be preferable to increase the magnitude of humoral response and reduce the rate of humoral decay, which might ultimately correlate with better levels of protection against COVID-19 over time. These data will inform the development of future vaccines and vaccine schedules against non-SARS-CoV-2 pathogens.
Introduction
Com-COV was a participant-masked randomised study investigating the safety, reactogenicity, and immunogenicity of heterologous and homologous primary COVID-19 immunisation schedules using BNT162b2 (tozinameran, Pfizer/BioNTech) and ChAdOx1 nCoV-19 (AstraZeneca). Previously reported data from 4-week interval schedules showed greater reactogenicity in heterologous versus homologous schedules.1, 2 At 28 days after the second dose, anti-SARS-CoV-2 IgG concentrations were highest in the homologous BNT162b2 and heterologous ChAdOx1 nCoV-19–BNT162b2 schedules, whereas measured T-cell responses at 14 days and 28 days post-second dose were highest in the ChAdOx1 nCoV-19–BNT162b2 schedule.
On the basis of the primary findings of Com-COV and other studies,1, 2, 3, 4 WHO has issued guidance on the use of heterologous COVID-19 vaccination,5 and many national primary immunisation campaigns have deployed schedules with combinations of viral vector and mRNA vaccines.6, 7, 8 Pressures on vaccine supply and logistical difficulties in global vaccine distribution9 have resulted in many national vaccine programmes, including in the UK,10 extending the priming interval beyond initial manufacturer recommendations.11 For the homologous ChAdOx1 nCoV-19 vaccination, this extension was supported by non-randomised, post-hoc trial analyses suggesting improved immunogenicity and efficacy,12 which contributed to the WHO recommendation to prolong the homologous ChAdOx1 nCoV-19 interval from 4 weeks to 8–12 weeks.13 Subsequently, observational data from the UK Health Security Agency suggest that a prolonged priming interval increases both humoral immunogenicity and vaccine effectiveness for homologous BNT162b2 but does so less clearly for homologous ChAdOx1 nCoV-19, although analyses were confounded by substantial differences in baseline populations.14
To our knowledge, no randomised data has been published to date on the effect of short versus long intervals for primary immunisation on reactogenicity and initial immunogenicity of these vaccines, whether given in homologous or heterologous schedules. Similarly, there are no randomised data on the long-term maintenance of immunity against both ancestral and variant SARS-CoV-2 strains, which is particularly relevant in the context of many countries choosing to deploy third-dose booster immunisations due to concerns regarding waning vaccine effectiveness and in countries planning their primary immunisation programmes.
Accordingly, we present secondary analyses from Com-COV, examining the effect of dose interval on reactogenicity, peak immune response, and waning of immune response, as these data are key to guiding decisions of national immunisation programmes for priming intervals, as well as whether, and when, to deliver booster programmes. Additionally, we assessed the effect of prophylactic paracetamol on reactogenicity and immunogenicity given previous concerns about increased reactogenicity after 4-week heterologous versus homologous schedules.
Methods
Com-COV trial design and outcomes
The Com-COV trial (ISRCTN 69254139; protocol available online and in the appendix pp 54–111) has been previously reported.2 Briefly, two COVID-19 vaccines, ChAdOx1 nCoV-19 and BNT162b2, were used; in the general cohort, 730 participants were randomly assigned to one of four permutations of priming schedules (homologous ChAdOx1 nCoV-19, ChAdOx1 nCoV-19–BNT162b2, homologous BNT162b2, and BNT162b2–ChAdOx1 nCoV-19) at two priming intervals (4 weeks and 12 weeks). An additional immunology cohort was comprised of 100 participants who were separately randomly assigned to 4-week groups, with extra early study visits to characterise the initial cellular response. Randomisation and masking are described in the appendix (p 38). The trial was approved by the South-Central Berkshire Research Ethics Committee (21/SC/0022), the University of Oxford, the Medicines and Healthcare Products Regulatory Agency and the National Health Service (NHS) Research Ethics Service (UK Human Research Authority). An independent data safety monitoring board reviewed safety data, and local trial-site physicians provided oversight of all adverse events in real time.
Given concerns regarding increased reactogenicity with heterologous 4-week vaccine schedules, the study protocol was amended to include a voluntary paracetamol sub-study for participants receiving their second dose at a 12-week interval, at the point of their second dose. Consenting participants were randomly assigned to receive advice either to take paracetamol soon after immunisation, and three further doses over the following 24 h regardless of symptoms (prophylactic paracetamol) or to take paracetamol if they became symptomatic (reactive paracetamol). Electronic diaries of participants were monitored with three additional yes-no questions regarding the impact of symptoms caused by vaccination on daily activities.
Eligible participants were adults aged 50 years or older who were naive to COVID-19 vaccines, had no or well controlled mild-moderate comorbidities, and had no history of laboratory-confirmed SARS-CoV-2 infection. Full inclusion and exclusion criteria are provided in the study protocol. Adverse events were collected as per the study protocol.
Anti-spike IgG, T-cell ELISpot, and pseudotyped virus neutralising antibody titres assessed 28 days post-second dose in participants vaccinated at a 4-week interval have already been reported.2 The key outcomes reported here are the effects of priming interval on these immunological outcomes and later timepoints.
Of note, the evaluation of immune persistence was affected by the rapid rollout of the national third-dose booster campaign. Although final blood tests were brought forward to accommodate this, the final outcome was that only approximately half of participants on a 4-week interval schedule had their final visit, and thus we did not use data from this final visit in the final interval comparison analysis. These comparisons were therefore done on the 5-month timepoint post-second dose for the 4-week participants and the 6-month timepoint for the 12-week participants, although the timing of this visit was more variable. The median visit time post-second dose were 5·1 months for the 4-week group and 6·0 months for the 12-week group (hereafter, we use 6 months to refer to the timepoints in the 4-week and 12-week groups).
The assays used in Com-COV have been previously described.2, 15, 16, 17 In brief, serum samples were analysed at Nexelis (Laval, Canada) to determine SARS-CoV-2 anti-spike IgG concentrations by ELISA and 50% neutralising antibody titre (NT50) for a SARS-CoV-2 pseudotype virus neutralisation assay, by use of a vesicular stomatitis virus backbone adapted to bear the spike protein of an ancestral SARS-CoV-2 strain.18 The conversion factors to international standard units can be found in the appendix (p 38). Serum samples were analysed at the UK Health Security Agency (Porton Down, UK), by ECLIA (Cobas platform, Roche Diagnostics) to determine anti-SARS-CoV-2 nucleocapsid IgG status. Interferon-γ-secreting T cells specific to whole spike protein epitopes, based on the ancestral Wuhan-Hu-1 sequence (YP_009724390.1), were detected on fresh samples with use of a modified T-SPOT-Discovery test done at Oxford Immunotec (Abingdon, UK). The assay was repeated on frozen samples for the Wuhan-Hu-1 sequence and the beta (B.1.351) and delta (B.1.617·2) variants.19 We report T-cell frequencies as spot forming cells per 250 000 peripheral blood mononuclear cells (PBMCs) with a lower limit of detection of one in 250 000 PBMCs, multiplying these results by four to express frequencies per 106 PBMCs. Microneutralisation assays to determine 50% focus reduction neutralisation titres (FRNT50) for live SARS-CoV-2 virus lineages (SARS-CoV-2/human/AUS/VIC01/2020 [Victoria] strain and beta, delta, and omicron [B.1.1.529] variants) were done at the University of Oxford.
Participants who tested positive for SARS-CoV-2 during the trial (symptomatic or asymptomatic) were reviewed at an additional safety visit.
Statistical analysis
The sample size calculation has been described previously.2 In this study, we focused on an exploratory analysis of the original trial, evaluating the effect of interval on different vaccine schedules, and thus no formal sample size power calculation was done for this analysis. All analyses were done on an intention-to-treat (ITT) basis. The immunogenicity analysis population consisted of participants with no evidence of SARS-CoV-2 infection, defined as self-reported SARS-CoV-2 infection or anti-nucleocapsid IgG of 1·0 or higher, up until 6 months post-second dose. For the 28 days post-second dose timepoint, we calculated the geometric mean ratio (GMR) with 95% CIs as the antilogarithm of the difference between the mean of the log10-transformed titre in the 12-week interval group with the 4-week group as reference, after adjusting for study site as a randomisation stratification variable and paracetamol usage in the first 24 h post-second dose in the linear regression model (as only 12-week groups were randomly assigned to the paracetamol sub-study). Since the timing of the 6-month post-second dose visit was slightly different between 4-week and 12-week interval groups, we further adjusted the day post-second dose in the linear regression model to estimate the GMR for the 6-month post-second dose timepoint. Sensitivity analyses included participants in the immunology cohort. The interactions between schedules (heterologous or homologous) and intervals (4 weeks or 12 weeks) were further explored by the same linear regression model after further adjusting for age, sex, and ethnicity. Subgroup analyses were done by age, sex, and baseline comorbidities. We report p values for interaction between subgroups and interval using the Wald test, and we set the two-sided significance level for interaction at 0·0014 using Bonferroni correction (36 interaction tests). Due to the rollout of the national third-dose booster vaccine programme, we expected the proportion of withdrawals in the 12-week interval group to be higher than the 4-week interval group at the visit done 6 months post second dose. Accordingly, the immunogenicity at 28 days and 3 months post second dose were compared between participants with missing versus available data at 6 months to explore any potential bias caused by the missing data.
The analysis population for safety and reactogenicity included all participants who had received at least one dose of study vaccine. To describe the presence or absence of each solicited adverse event, logistic regression models were fitted, adjusting for study site and any paracetamol use in the first 24 h to evaluate the effect of interval on reactogenicity. In the paracetamol sub-study among 12-week interval participants, the analysis population comprised all participants with available endpoint data who consented to the sub-study. The analyses were done on an ITT basis, and the comparisons for reactogenicity and immunogenicity between prophylactic and reactive paracetamol use groups were reported following the statistical analysis described, with the prophylactic paracetamol group as the reference group.
All statistical analyses were done with R, version 4.1.1, SAS, version 9.4, and Stata 17. The Com-COV trial is registered with the ISRCTN registry, 69254139 (EudraCT 2020–005085–33).
Role of the funding source
The funders had no role in data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit for publication.
Results
Between Feb 11 and 26, 2021, 975 participants were screened at eight study sites across England, of whom 830 were randomly assigned in the study. Of these, 100 participants were enrolled into the immunology cohort and were randomly assigned to one of the 4-week interval groups (appendix p 3). The remaining 730 participants enrolled into the general cohort were randomly assigned to one of eight groups (combination of four priming schedules and two priming intervals). The mean age of participants in the general cohort was 58·2 years (SD 4·7); 323 (44%) of 730 participants were women and 407 (56%) men, while 165 (23%) were from non-White ethnic backgrounds. Baseline characteristics were well balanced across all groups, except for comorbidity frequency. After excluding 36 participants who were seropositive for SARS-CoV-2 at baseline and another 30 participants with evidence of COVID-19 infection before 6 months post-second dose within the trial, the immunogenicity analysis to compare 4-week and 12-week interval groups included 664 participants (appendix p 17; table ).
Table.
Baseline demographics and characteristics by study group in the analysis population of the general cohort
|
4-week interval study groups |
12-week interval study groups |
Overall (n=664) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ChAd–ChAd (n=83) | ChAd–BNT (n=83) | BNT–BNT (n=84) | BNT–ChAd (n=83) | ChAd–ChAd (n=89) | ChAd–BNT (n=77) | BNT–BNT (n=87) | BNT–ChAd (n=78) | ||
| Age, years | |||||||||
| Mean | 58·4 (4·8) | 58·3 (4·8) | 58·3 (5·0) | 57·5 (4·6) | 57·6 (5·1) | 58·8 (4·4) | 58·2 (4·6) | 58·2 (4·4) | 58·2 (4·7) |
| Median | 57·6 (54·3–62·4) | 58·1 (54·1–62·4) | 57·6 (54·2–62·4) | 56·7 (53·8–60·5) | 57·7 (53·1–61·2) | 58·5 (55·5–61·0) | 57·2 (54·9–61·8) | 58·4 (54·6–60·6) | 57·7 (54·3–61·6) |
| Aged 50–59 years | 50 (60%) | 50 (60%) | 55 (65%) | 61 (73%) | 63 (71%) | 49 (64%) | 55 (63%) | 50 (64%) | 433 (65%) |
| Aged ≥60 years | 33 (40%) | 33 (40%) | 29 (35%) | 22 (27%) | 26 (29%) | 28 (36%) | 32 (37%) | 28 (36%) | 231 (35%) |
| Gender | |||||||||
| Men | 49 (59%) | 47 (57%) | 42 (50%) | 45 (54%) | 47 (53%) | 44 (57%) | 47 (54%) | 49 (63%) | 370 (56%) |
| Women | 34 (41%) | 36 (43%) | 42 (50%) | 38 (46%) | 42 (47%) | 33 (43%) | 40 (46%) | 29 (37%) | 294 (44%) |
| Ethnicity | |||||||||
| White | 66 (80%) | 62 (75%) | 68 (81%) | 60 (72%) | 68 (76%) | 60 (78%) | 67 (77%) | 62 (79%) | 513 (77%) |
| Black | 1 (1%) | 1 (1%) | 0 | 2 (2%) | 3 (3%) | 1 (1%) | 2 (2%) | 1 (1%) | 11 (2%) |
| Asian | 12 (14%) | 13 (16%) | 6 (7%) | 9 (11%) | 8 (9%) | 10 (13%) | 9 (10%) | 9 (12%) | 76 (11%) |
| Mixed | 4 (5%) | 5 (6%) | 8 (10%) | 9 (11%) | 7 (8%) | 5 (6%) | 6 (7%) | 4 (5%) | 48 (7%) |
| Other | 0 | 2 (2%) | 2 (2%) | 3 (4%) | 3 (3%) | 1 (1%) | 3 (3%) | 2 (3%) | 16 (2%) |
| Comorbidities | |||||||||
| Cardiovascular | 19 (23%) | 16 (19%) | 16 (19%) | 20 (24%) | 17 (19%) | 21 (27%) | 16 (18%) | 18 (23%) | 143 (22%) |
| Respiratory | 13 (16%) | 10 (12%) | 9 (11%) | 11 (13%) | 7 (8%) | 8 (10%) | 12 (14%) | 9 (12%) | 79 (12%) |
| Diabetes | 7 (8%) | 8 (10%) | 0 | 2 (2%) | 2 (2%) | 1 (1%) | 1 (1%) | 4 (5%) | 25 (4%) |
| Timing of 6-month visit, days* | |||||||||
| Mean | 153 (5) | 154 (7) | 153 (6) | 154 (5) | 176 (19) | 178 (19) | 177 (15) | 176 (17) | 164 (17) |
| Median | 154 (139–171) | 154 (142–198) | 154 (120–174) | 154 (141–168) | 180 (138–225) | 179 (141–239) | 181 (140–208) | 176 (138–223) | 154 (120–239) |
Data are n (%), mean (SD), or median (IQR).
Days since second dose.
Homologous schedules had significantly increased anti-spike binding IgG titres with a 12-week schedule compared with a 4-week schedule at both 28 days and 6 months post-second dose. With heterologous schedules, we observed a significant increase between intervals only at 28 days for BNT162b2–ChAdOx1 nCoV-19, with no significant differences observed between intervals for ChAdOx1 nCoV-19–BNT162b2 at either timepoint (Figure 1, Figure 2 ; appendix p 18). All schedules showed a significant increase in pseudotyped virus neutralisation titres against the Victoria strain at 28 days post-second dose with the 12-week interval, with GMRs of 1·4 (95% CI 1·1–1·8) for homologous BNT162b2, 1·5 (1·2–1·9) for ChAdOx1 nCoV-19–BNT162b2, 1·6 (1·3–2·1) for BNT162b2–ChAdOx1 nCoV-19, and 2·4 (1·7–3·2) for homologous ChAdOx1 nCoV-19. (figure 1). Overall, regardless of interval, the rank order of schedules by humoral response (homologous BNT162b2, ChAdOx1 nCoV-19–BNT162b2, BNT162b2–ChAdOx1 nCoV-19, and homologous ChAdOx1 nCoV-19) did not change. However, the magnitude of difference between both sets of homologous and heterologous schedules was reduced in 12-week schedules (appendix p 4). The decay rates of anti-spike IgG between 28 days and 6 months post-second dose were similar between the 4-week and 12-week interval groups, except that the 12-week interval showed a slightly slower decay rate compared with that of the 4-week interval for homologous BNT162b2 in the adjusted analysis. We observed similar concentrations of anti-spike IgG at 28 days and 3 months post-second dose between participants in the 12-week interval groups who had 6-month post-second dose anti-spike IgG data missing and those who did not (appendix p 5).
Figure 1.
Immune responses between 4-week and 12-week intervals at 28 days and 6 months post-second dose in the general cohort
Data presented are the geometric means and 95% CIs. Fold changes were calculated by dividing the immune response at 6 months post-second dose by that at 28 days post-second dose. GMRs between schedules with 4-week and 12-week intervals were adjusted for study site and paracetamol usage in the first 24 h post-vaccination (yes or no) for the 28-day data; 6-month visit time (in days) was further adjusted for the 6-month data and fold change. The vertical line is the line of no difference between 4-week and 12-week interval groups. aGMR=adjusted geometric mean ratio. ELU=ELISA units. GMR=geometric mean ratio. NT50=50% neutralisation titre. PBMCs=peripheral blood mononuclear cells. SFC=spot-forming cell. WT=wild type.
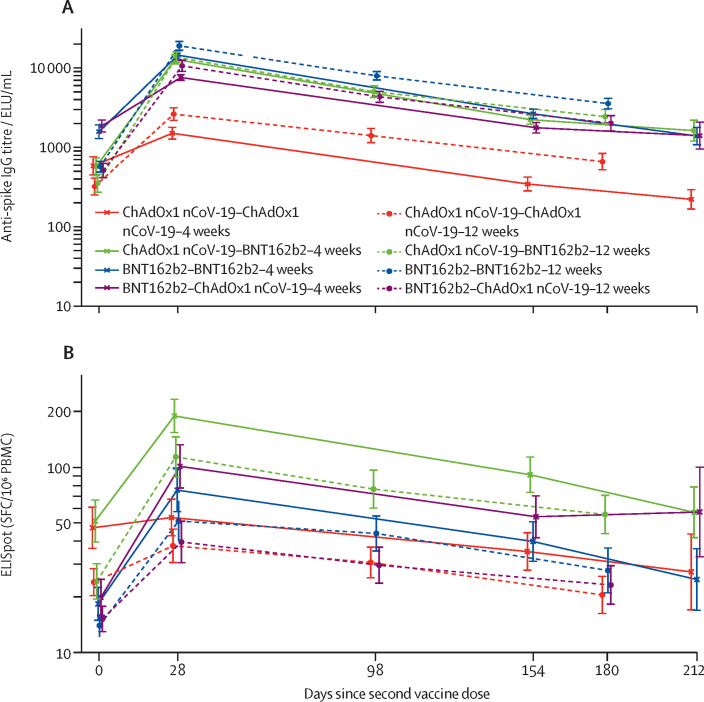
Figure 2.
Kinetics of immune response over time with all schedules normalised by time of second dose in the seronegative general cohort
Figure shows results for anti-spike IgG titres (A) and T-cell ELISpot counts (B). Day 0 refers to time of second dose. Datapoints are geometric mean concentrations, with whiskers showing the 95% CIs. Numbers of participants per timepoint are shown in the appendix (p 16). ELU=exponential linear unit. PBMCs=peripheral blood mononuclear cells. SFC=spot-forming cell.
By contrast, T-cell ELISpot counts were all approximately a third lower 28 days post-second dose in the 12-week interval groups than in the 4-week interval groups with homologous ChAdOx1 nCoV-19, homologous BNT162b2, and ChAdOx1 nCoV-19–BNT162b2 schedules (although not all were significant), with a greater reduction of two thirds seen with the BNT162b2–ChAdOx1 nCoV-19 schedule (GMR 0·35, 95% CI 0·22–0·54). This difference was maintained at 6 months post second dose (figure 1). Regardless of interval, ChAdOx1 nCoV-19–BNT162b2 generated the highest cellular immune response; however, the other three schedules converged at longer timepoints when the longer 12-week interval was used. ELISpot counts after the second dose were similar between intervals when measured from time after first dose for homologous BNT162b2, ChAdOx1 nCoV-19–BNT162b2, and homologous ChAdOx1 nCoV-19 (appendix p 18).
We observed consistent results in sensitivity analyses (appendix p 19) and in the analysis by subgroup for the effect of interval on humoral and cellular immunogenicity (appendix pp 20–23).
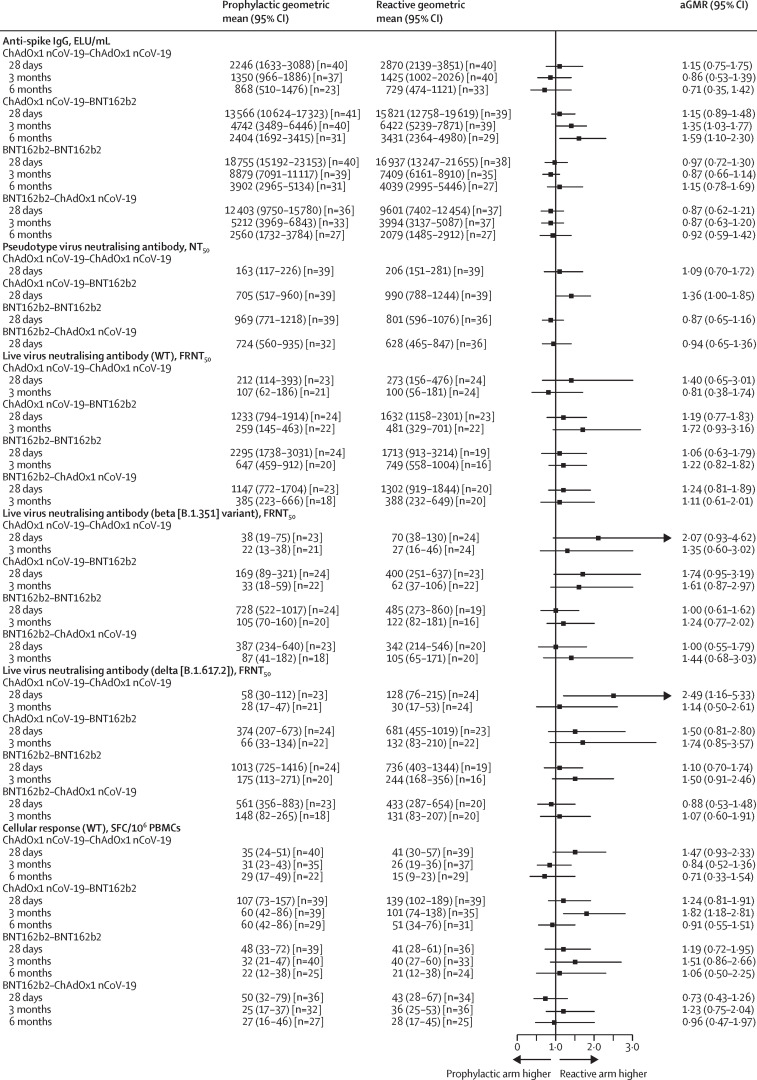
Serum samples from participants in the 12-week groups showed apparent reductions in neutralising titres against beta, delta, and omicron variants compared with the Victoria strain at 28 days and 3 months post-second dose (appendix p 24). We observed no differences in the reduction of neutralising titres against variants of concern between the four schedule groups, and hence no differences in the rank order of schedules. No differences were observed in T-cell ELISpot count (for beta and delta variants alone) elicited across variants (appendix pp 6, 25). The paracetamol sub-study (figure 3 , appendix pp 7, 26) did not show a significant advantage for reactive paracetamol over prophylactic paracetamol in terms of the magnitude of humoral immune response.
Figure 3.
Immune responses between prophylactic and reactive paracetamol use at 28 days, 3 months, and 6 months post-second dose in the 12-week interval groups
Data shown are geometric means (95% CI) in the intention-to-treat population. GMRs and two-sided 95% CIs were adjusted for study site, age, gender, and pre-boost immunogenicity (anti-spike IgG for humoral responses and ELISpot for cellular response). Numbers of participants per timepoint are shown in the appendix (p 16). aGMR=adjusted geometric mean ratio. ELU=ELISA units. FRNT50=50% focal reduction neutralisation titre. GMR=geometric mean ratio. NT50=50% neutralisation titre. PBMCs=peripheral blood mononuclear cells. SFC=spot-forming cell. WT=wild type.
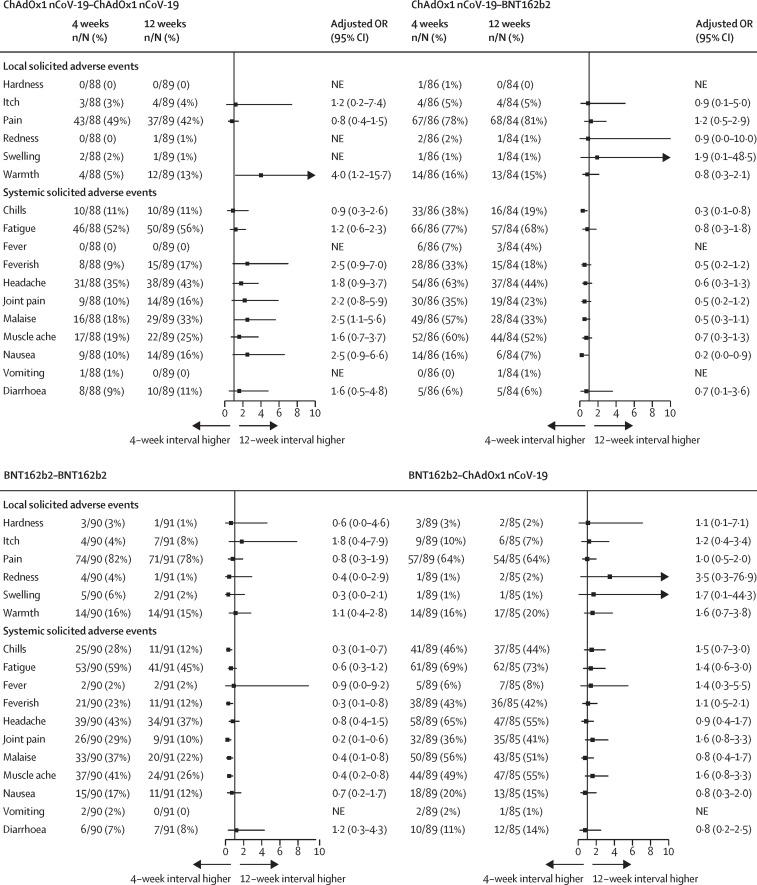
Extending the vaccination interval from 4 to 12 weeks greatly reduced the frequency of nearly all systemic solicited adverse events in participants receiving BNT162b2 as a second dose, regardless of prime vaccine, by up to 80%. Homologous ChAdOx1 nCoV-19 second-dose reactogenicity was increased in the 12-week interval but, given the low level of reactogenicity in the 4-week interval, this absolute increase was small. The frequency of systemic solicited adverse events in participants receiving BNT162b2–ChAdOx1 nCoV-19 was not affected, nor was the frequency or severity of local symptoms in any vaccine schedule (figure 4 ). Therefore, the 12-week heterologous schedules were still associated with greater systemic reactogenicity than homologous schedules, but the magnitude of the difference was generally lower than in 4-week schedules (appendix pp 27–29) and was still limited to the first 48 h (appendix pp 30–31).
Figure 4.
Forest plots of solicited adverse events in days 0–7 post-second dose by vaccine schedule comparing 12-week interval groups to 4-week interval groups in the general cohort
Models adjusted for paracetamol recommendation (reactive or prophylactic use). Models with no adjusted OR were non-estimable due to few events in that study group. The vertical line is the line of no difference between 4-week and 12-week interval groups. NE=not estimable. OR=odds ratio.
With the paracetamol sub-study, done in participants on 12-week intervals, we observed no difference in local symptoms between any study schedule for those advised for prophylactic versus reactive paracetamol. Headache was less frequently reported in all prophylactic paracetamol groups than in reactive groups. The adherence of paracetamol usage in the prophylactic group ranged from 88% to 98%, whereas the rates of reactive paracetamol usage in the first 24 h varied from 29% to 73% across schedules, mirroring their respective reactogenicity (appendix p 8). BNT162b2–ChAdOx1 nCoV-19 was the most reactogenic schedule and had the largest reduction in frequency of systemic symptoms with prophylactic paracetamol (appendix pp 32–33). Observations were similar when comparing participants on 4-week intervals to either all participants on 12-week intervals or only those randomly assigned to reactive paracetamol.
Advice for prophylactic or reactive paracetamol overall was not shown to affect activities of daily living, ability to attend work, or the seeking of medical attention. Absolute numbers of participants who were affected in this manner were low across all groups (appendix pp 34–37).
Between Feb 11 and Oct 22, 2021, there were 1004 adverse events reported in 462 participants (appendix p 9), proportionally split across groups. Descriptions of all non-serious adverse events of grade 3 or higher are presented in the appendix (pp 10–12). There were five adverse events of special interest (excluding events related to SARS-CoV-2 and COVID-19; appendix p 13) and 11 serious adverse events across all groups (appendix p 14). One of these was deemed possibly related to immunisation (IgA nephropathy–minimal change disease overlap, possibly precipitated by COVID-19 infection soon after first dose of BNT162b2). As of the writing of this Article, this participant was under further follow-up regarding an ongoing fall in renal function. 40 participants tested positive for SARS-CoV-2 (all but four cases occurred at least 2 weeks post second dose). Combining over both intervals, these cases were distributed by group as the following: homologous ChAdOx1 nCoV-19 (11 cases), ChAdOx1 nCoV-19–BNT162b2 (nine cases), homologous BNT162b2 (nine cases), and BNT162b2–ChAdOx1 nCoV-19 (seven cases; appendix p 15). No participants were hospitalised.
Discussion
To our knowledge, we report here the first randomised data elucidating the effect of time interval and prophylactic paracetamol use for homologous and heterologous priming schedules using ChAdOx1 nCoV-19 and BNT162b2. Longer intervals enhanced the maximal humoral neutralising immune response for all schedules 28 days post-second dose. Significant increases in binding antibody response were present only for homologous ChAdOx1 nCoV-19, homologous BNT162b2, and BNT162b2–ChAdOx1 nCoV-19 at the same timepoint. The difference was maintained at the 6-month timepoint only in homologous schedules. Reduced cellular responses were observed for all schedules with a 12-week interval. Additionally, a prolonged interval had a slightly reduced rate of antibody decay in homologous BNT162b2. Finally, longer intervals greatly reduced second-dose systemic reactogenicity in schedules with BNT162b2 as the second dose. No safety concerns were reported.
The rank order of schedules of maximal or later humoral responses was not affected by interval, with the most immunogenic schedule remaining homologous BNT162b2, meaning that the vaccines that were used as part of the schedule had a greater effect on immunogenicity than changing the interval between doses. The larger proportional increase in humoral response in a 12-week interval for homologous ChAdOx1 nCoV-19 compared with a 4-week interval might well translate into increased efficacy, as suggested by non-randomised data from a randomised control trial.12 Contrastingly, although an observational study of immunogenicity and national effectiveness14 suggested that both of these increased with longer priming intervals for homologous BNT162b2, the effect was not as evident for homologous ChAdOx1 nCoV-19. However, this study was not randomised, with significant differences between baseline vaccinated populations. This limitation also applies to the UK National COVID survey, which showed a contrasting finding of no effect of interval on effectiveness for homologous BNT162b2.20
Despite some significant results within the paracetamol sub-study, the small advantages noted in some assay readouts are probably due to chance as a result of the large number of comparisons, with prophylactic paracetamol unlikely to have a significant effect on immunogenicity, although further work is required to clarify this.
T-cell ELISpot counts were lower 28 days post-second dose for participants with a 12-week interval than for those with a 4-week interval. The biological significance of this reduction is unclear because in the UK programme, which predominantly used longer priming intervals, protection against severe disease has been maintained for longer than protection against symptomatic infection,21 but these results are consistent with non-randomised studies.14, 17 For the commonly used schedules (homologous ChAdOx1 nCoV-19, ChAdOx1 nCoV-19–BNT162b2, and homologous BNT162b2), the T-cell ELISpot level achieved after a 12-week interval coincided with the waning level of T-cell response after a 4-week interval and followed the same waning trajectory, suggesting that the time from prime vaccination might play an important role in the T-cell response to the second antigenic exposure, and this will require further exploratory immunological work to elucidate.
Schedules with a BNT162b2 second dose had lower reactogenicity with a longer interval, whereas homologous ChAdOx1 nCoV-19, which had a low level of second dose reactogenicity, showed a small increase. High rates of reactogenicity seem always to accompany the first exposure to the ChAdOx1 nCoV-19 vaccine, regardless of whether it is the first or second vaccine dose. No clear pattern between reactogenicity and immunogenicity was observed between schedules.
Within the paracetamol sub-study, the clearest reduction in reactogenicity with prophylactic paracetamol was in the most reactogenic group (BNT162b2–ChAdOx1 nCoV-19), with lower baseline reactogenicity and small group sizes probably contributing to the absence of a clear effect in other schedules. Liberal use of paracetamol in the reactive groups probably further reduced measurable differences. This sub-study also potentially confounded the reactogenicity interval comparison, as it would have increased overall paracetamol usage in 12-week intervals; however, a comparison of participants in a 4-week interval with those in a 12-week interval randomly assigned to advice for reactive paracetamol showed similar patterns, and the comparisons between intervals were adjusted for paracetamol use. With these caveats, advice for prophylactic paracetamol might be considered for deployment routinely by national immunisation programmes, and it would be worth assessing in the context of booster doses, as there was no substantial effect of advice for prophylactic paracetamol on immunogenicity shown.
Our study has some limitations. The sample size of the Com-COV trial was calculated to assess non-inferiority of the primary endpoints and was thus not powered to detect significant differences among the additional assays done to evaluate immunological activity against variants. T-cell ELISpot assays on variants of concern were done on frozen samples by necessity; this might have adversely affected the sensitivity of the assay, and thus its ability to detect differences between variants of concern. Due to sample size and design, as well as low SARS-CoV-2 positivity across all groups, our data do not allow comment on vaccine effectiveness. However, a Swedish nationwide cohort study22 suggests that schedules of ChAdOx1 nCoV-19 followed by an mRNA vaccine might be more effective than homologous ChAdOx1 nCoV-19 schedules. The applicability of these findings to a younger cohort is unclear; however, previous efficacy trials suggest that the immunogenic differences after two doses between older and younger adults were minimal, and the similarity with results from the PITCH study suggests that our results might have broader external validity.17
In conclusion, our study suggests that the choice of vaccines used in a COVID-19 priming schedule had a greater effect on immunogenicity than the dose interval. Nonetheless, both heterologous and homologous schedules using ChAdOx1 nCoV-19 and BNT162b2 induced robust immune responses with a 12-week interval similar or greater than those elicited by the schedules with 4-week intervals, allowing for greater flexibility in vaccine deployment, both in terms of which vaccines are included in a schedule and the interval between doses. For homologous BNT162b2, a longer interval had a slightly slower rate of decay than its respective shorter interval counterpart. This might be relevant when considering how the waning of immunity might affect the decision for, and timing of, booster immunisation programmes. This might well indicate a difference in immunological development by challenging the immune response with a second antigenic exposure at a different point in the immune maturation process after priming and requires further investigation to elucidate.
National immunisation programmes will consider many factors when deciding how to deliver vaccine doses, including vaccine availability, risk of disruption to vaccine supply, health-care infrastructure, current and projected rates of COVID-19 transmission, and public health messaging. When comparing shorter to longer intervals, policy makers are faced with a choice of higher antibody levels in the vaccinated population sooner rather than later, which might be preferable in high-transmission settings, when rapid deployment of vaccines is essential and logistically possible and the supply is abundant. Alternatively, lower antibody levels initially, but at higher levels subsequently and for longer, might be achievable in lower-transmission settings and are potentially better suited for regions with reduced vaccine supply rates and constrained logistics. Importantly, Com-COV provides a robust evidence base on which to base these decisions. These unique data might also be used to optimise immunogenicity and minimise reactogenicity for future deployment of these novel platforms against non-COVID-19 pathogens.
Data sharing
The study protocol is provided in the appendix. Individual participant data will be made available when the trial is complete, upon requests directed to the corresponding author; after approval of a proposal, data can be shared through a secure online platform.
Declaration of interests
MDS acts on behalf of the University of Oxford as an investigator on studies funded or sponsored by vaccine manufacturers AstraZeneca, GlaxoSmithKline, Pfizer, Novavax, Janssen, Medimmune, and MCM vaccines, receiving no personal financial payment for this work. JSN-V-T was seconded to the Department of Health and Social Care (DHSC), England. AMC and DMF are investigators on studies funded by Pfizer and Unilever, receiving no personal financial payment for this work. AF is a member of the Joint Committee on Vaccination and Immunisation and Chair of the WHO European Technical Advisory Group of Experts on immunisation; he is an investigator, provides consultative advice, or both on clinical trials and studies of COVID-19 vaccines produced by AstraZeneca, Janssen, Valneva, Pfizer, and Sanofi and of other vaccines from these and other manufacturers GlaxoSmithKline, VPI, Takeda, and Bionet Asia, receiving no personal remuneration or benefits for any of this work. SNF acts on behalf of University Hospital Southampton NHS Foundation Trust as an investigator, provider of consultative advice, or both on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers Janssen, Pfizer, AstraZeneca, GlaxoSmithKline, Novavax, Seqirus, Sanofi, Medimmune, Merck, and Valneva vaccines and antimicrobials, receiving no personal financial payment for this work. PTH acts on behalf of St George's University of London as an investigator on clinical trials of COVID-19 vaccines funded or sponsored by vaccine manufacturers Janssen, Pfizer, AstraZeneca, Novavax, and Valneva, receiving no personal financial payment for this work. CAG acts on behalf of University Hospitals Birmingham NHS Foundation Trust as an investigator on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers Janssen, Pfizer, AstraZeneca, Novavax, CureVac, Moderna, and Valneva vaccines, receiving no personal financial payment for this work. VL acts on behalf of University College London Hospitals NHS Foundation Trust as an investigator on clinical trials of COVID-19 vaccines funded or sponsored by vaccine manufacturers Pfizer, AstraZeneca, and Valneva, receiving no personal financial payment for this work. TL is named as an inventor on a patent application covering this SARS-CoV-2 vaccine (GB2003670.3) and is an occasional consultant to Vaccitech, unrelated to this work. Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1 nCoV-19.
Acknowledgments
Acknowledgments
The study is funded by the UK Government through the National Institute for Health and Care Research (NIHR) and the Vaccine Task Force (VTF). This research was supported by the NIHR Oxford Biomedical Research Centre and delivered through the NIHR funded National Immunisation Schedule Evaluation Consortium (NISEC). MDS and SNF are NIHR Senior Investigators. The views expressed are those of the authors and not necessarily those of the NIHR, VTF, or the Department of Health and Social Care. We express our gratitude for the contribution of all the trial participants and the invaluable advice of the international Data Safety Monitoring Board. We additionally acknowledge the broader support from the various teams within the University of Oxford including the Department of Paediatrics, Clinical Trials Research Governance, and Research Contracts and Public Affairs Directorate.
Contributors
MDS and JSN-V-T conceived the trial, and MDS is the chief investigator. MDS, ASVS, RHS, and XL contributed to the protocol and design of the study. ASVS, ELP, and RHS led the implementation of the study. XL, RHS, and MG did the statistical analysis and have verified the underlying data. ASVS, RHS, MG, XL, and MDS drafted the report. All other authors contributed to the implementation and data collection. All authors reviewed and approved the final report.
Contributor Information
Com-COV Study Group:
Alasdair P.S. Munro, Jazz Bartholomew, Laura Presland, Sarah Horswill, Sarah Warren, Sophie Varkonyi-Clifford, Stephen Saich, Kirsty Adams, Marivic Ricamara, Nicola Turner, Nicole Y. Yee Ting, Sarah Whittley, Tommy Rampling, Amisha Desai, Claire H. Brown, Ehsaan Qureshi, Karishma Gokani, Kush Naker, Johanna K. Kellett Wright, Rachel L. Williams, Tawassal Riaz, Florentina D. Penciu, Amy Carson, Claudio Di Maso, Gracie Mead, Elizabeth G. Howe, Iason Vichos, Mujtaba Ghulam Farooq, Rabiullah Noristani, Xin L. Yao, Neil J. Oldfield, Daniel Hammersley, Sue Belton, Simon Royal, Alberto San Francisco Ramos, Cecilia Hultin, Eva P. Galiza, Rebecca Crook, Marcin Bula, Fred Fyles, Hassan Burhan, Flora Maelin, Elen Hughes, and Emmanuel Okenyi
Supplementary Material
References
- 1.Shaw RH, Stuart A, Greenland M, Liu X, Nguyen Van-Tam JS, Snape MD. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397:2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, Shaw RH, Stuart AS, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hillus D, Schwarz T, Tober-Lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;9:1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Interim recommendations for heterologous COVID-19 vaccine schedules. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-heterologous-schedules
- 6.Danish Health Authority Denmark continues its vaccine rollout without the COVID-19 vaccine from AstraZeneca. 2021. https://www.sst.dk/en/english/news/2021/denmark-continues-its-vaccine-rollout-without-the-covid-19-vaccine-from-astrazeneca#:~:text=The%20Danish%20Health%20Authority%20has,assessment%20regarding%20the%20AstraZeneca%20vaccine
- 7.French Health Authority COVID-19: quelle stratégie vaccinale pour les moins de 55 ans ayant déjà reçu une dose d'AstraZeneca? 2021. https://www.has-sante.fr/jcms/p_3260335/en/covid-19-quelle-strategie-vaccinale-pour-les-moins-de-55-ans-ayant-deja-recu-une-dose-d-astrazeneca
- 8.Public Health Agency of Sweden Information on the use of the AstraZeneca vaccine in the vaccination of people 65 and older. 2021. https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/communicable-disease-control/covid-19/vaccination-against-covid-19/
- 9.Ghebreyesus TA. WHO Director-General's opening remarks at the media briefing on COVID-19—4 August 2021. 2021. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-4-august-2021
- 10.UK Department of Health and Social Care Optimising the COVID-19 vaccination programme for maximum short-term impact. 2021. https://www.gov.uk/government/publications/prioritising-the-first-covid-19-vaccine-dose-jcvi-statement/optimising-the-covid-19-vaccination-programme-for-maximum-short-term-impact
- 11.Medicines & Healthcare products Regulatory Agency Summary of product characteristics for COVID-19 vaccine Pfizer/BioNTech. 2021. https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19/summary-of-product-characteristics-for-covid-19-vaccine-pfizerbiontech
- 12.Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO The Oxford/AstraZeneca COVID-19 vaccine: what you need to know. 2021. https://www.who.int/news-room/feature-stories/detail/the-oxford-astrazeneca-covid-19-vaccine-what-you-need-to-know
- 14.Amirthalingam G, Bernal JL, Andrews NJ, et al. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nat Commun. 2021;12 doi: 10.1038/s41467-021-27410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.v Stuart AS, Shaw RH, Liu X, Greenland M, Aley PK, Andrews NJ, et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2021;399:36–49. doi: 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Payne RP, Longet S, Austin JA, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184:5699. doi: 10.1016/j.cell.2021.10.011. 714.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bewley KR, Coombes NS, Gagnon L, et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat Protoc. 2021;16:1–33. doi: 10.1038/s41596-021-00536-y. [DOI] [PubMed] [Google Scholar]
- 19.Oxford Immunotec T-Cell Xtend. 2017. http://www.oxfordimmunotec.com/international/products-services/t-cell-xtend/
- 20.Pouwels KB, Pritchard E, Matthews PC, et al. Effect of delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27:2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UK Health Security Agency SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 31. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1042367/technical_briefing-31-10-december-2021.pdf
- 22.Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic COVID-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur. 2021;11 doi: 10.1016/j.lanepe.2021.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol is provided in the appendix. Individual participant data will be made available when the trial is complete, upon requests directed to the corresponding author; after approval of a proposal, data can be shared through a secure online platform.