Abstract
The genus Syzygium comprises 1200–1800 species that belong to the family of Myrtaceae. Moreover, plants that are belonged to this genus are being used in the traditional system of medicine in Asian countries, especially in China, India, and Bangladesh. The aim of this review is to describe the scientific works and to provide organized information on the available traditional uses, phytochemical constituents, and pharmacological activities of mostly available species of the genus Syzygium in Bangladesh. The information related to genus Syzygium was analytically composed from the scientific databases, including PubMed, Google Scholar, Science Direct, Web of Science, Wiley Online Library, Springer, Research Gate link, published books, and conference proceedings. Bioactive compounds such as flavanone derivatives, ellagic acid derivatives and other polyphenolics, and terpenoids are reported from several species of the genus Syzygium. However, many members of the species of the genus Syzygium need further comprehensive studies regarding phytochemical constituents and mechanism‐based pharmacological activities.
Keywords: Myrtaceae, pharmacological activities, phytochemical constituents, Syzygium, traditional use
The information related to genus Syzygium was analytically composed from the scientific databases, including PubMed, Google Scholar, Science Direct, Web of Science, Wiley Online Library, Springer, Research Gate link, published books, and conference proceedings. Bioactive compounds such as flavanone derivatives, ellagic acid derivatives and other polyphenolics, and terpenoids are reported from several species of the genus Syzygium. However, many members of the species of the genus Syzygium need further comprehensive studies regarding phytochemical constituents and mechanism‐based pharmacological activities.
1. INTRODUCTION
Plant is an essential source of medicine and plays a vital role in world health for its therapeutic or curative aids which have attained a commanding role in health system all over the world (Akkol, Tatlı, et al., 2021; Fernández et al., 2021; Hossain et al., 2020; Rahman et al., 2021). This comprises medicinal plants not only important for the treatment of diseases but also as potential material for maintaining good health and conditions. Better cultural acceptability, better compatibility and adaptability with the human body, and lesser side effects of plants made many countries in the world to depend on herbal medicine for their primary health care (Bari et al., 2021; CHOWDHURY et al., 2021; Hoque et al., 2021). For centuries, plants are being widely used for their natural resources isolated from various parts of a plant and have been used in the treatment of human diseases. Moreover, some of these plants produce edible fruits, while others simply produce dazzling flowers, which are so attractive and important economically as well. The knowledge of traditional medicine supports all other systems of medicine such as Ayurveda, Siddha, Unani, and even modern medicine. Plant‐based systems always play an essential role in health care, and their use by different cultures has been extensively documented. The number of plants used for treatment is estimated to be 20,000 named as medicinal plants. The studies on medicinal plants and active substances derived from these have increased the interest in plants for modern medicine in recent years (F. Hossain et al., 2021; S. Hossain et al., 2021; Sinan et al., 2021; Tallei et al., 2021).
The genus Syzygium is one of the genera of the myrtle family Myrtaceae comprising 1200–1800 species spread out over the world (B. Ahmad et al., 2016; Reza et al., 2021). The genus Syzygium (Myrtaceae) is named after a Greek word meaning “coupled,” an illusion to the paired branches and leaves (Nigam & Nigam, 2012). It has an extensive range that spread out from Africa and Madagascar, Asia throughout the Pacific (Tuiwawa et al., 2013), and highest levels of diversity ensue from Malaysia to Australia, where numerous species are very poorly known and countless species have not been portrayed taxonomically. These species are abundant components in the upper and medium strata of rainforests of the eastern Australia (Hyland, 1983). It is the biggest woody genus of the flowering plants in the world (B. Ahmad et al., 2016). Majority of the species of Syzygium genus vary from medium to large evergreen trees. Some of the species produce edible fruits (e.g., S. jambos, S. fibrosum), which are eaten freshly or commercially used to form them in jam and jellies (Table 1). The genus Syzygium has also a culinary use such as clove of some species, for example, S. aromaticum (B. Ahmad et al., 2016), whose unopened flower buds are used as a spice which is most important economically. Few species are used as flavoring agents for their attractive glossy foliage, while other species look ornamented. Many species of the genus Syzygium are known for their traditional use in various diseases. S. aromaticum's essential oil (CEO) is traditionally used in the treatment of burns and wounds, and as a pain reliever in dental care as well as treating tooth infections and toothache (Batiha et al., 2020). S. cordatum and S. guineese are used in abdominal pain, indigestion, and diarrhea (N. Dharani, 2016). S. cumini is used in diarrhea, dysentery, menorrhagia, asthma, and ulcers (Jadhav et al., 2009). S. jambos (L.) is traditionally used to treat hemorrhages, syphilis, leprosy, wounds, ulcers, and lung diseases (Reis et al., 2021). S. malaccense (L.) is used to treat mouth ulcers and irregular menstruation; S. samarangense flowers are used to treat diarrhea and fever; and S. suboriculare is used to treat coughs and colds, diarrhea, and dysentery (IE Cock & Cheesman, 2018). S. caryophyllatum, S. cumini, S. malaccense, and S. samarangens are used to treat diabetes mellitus (Ediriweera & Ratnasooriya, 2009).
TABLE 1.
A list of selected plants belonging to the Syzygium genus, including the plant parts with their traditional uses
| Plant Name | Part | Indications | References |
|---|---|---|---|
| Syzygium alternifolium (WT.) WALP | Leaf (juice), tender shoots (pulp) | Bacillary dysentery | (Yugandhar et al., 2015) |
| Fruits (powder) | Diarrhea and diabetes | (Yugandhar et al., 2015) | |
|
Syzygium anisatum (Vicfkery) Craven & Biffen |
Leaf (EO) | Antiseptic | (Bryant & Cock, 2016) |
|
Syzygium aqueum (Burm.f.) Alston |
Leaf | Antibiotic and childbirth pain | (T. Manaharan et al., 2013) |
|
Syzygium aromaticum (L) Merr. & Perry. |
Flower bud | Aromatic stomachic, anti‐inflammatory agent, deodorant, disinfectant | (Kasai et al., 2016) |
| Flower bud | Toothache, gum inflammation, coughs, colds, neuralgic pain, and rheumatism | (N. Dharani, 2016) | |
| Syzygium australe (H.L. Wendl. Ex Link) B. Hyland | _ | Fungal skin infections | (Noé et al., 2019) |
| Syzygium campanulatum Korth | _ | Stomach pain | (Abdul Hakeem Memon et al., 2020) |
| Syzygium calophyllifolium Walp. | Leaf | Skin diseases | (Chandran et al., 2016) |
| Fruit and bark | Aching tooth and inflammation | (Chandran et al., 2016) | |
|
Syzygium caryophyllatum (L.) Alston |
_ | Diabetes mellitus | (Ediriweera & Ratnasooriya, 2009) |
| Syzygium cordatum Hochst ex C Krauss | Leaf, root, and bark | Stomachaches, abdominal pains, indigestion, diarrhea, diabetes, and venereal diseases | (N. Dharani, 2016) |
| Leaf, root, bark and fruit | Gastrointestinal disorders, burns, sores, wounds, colds, cough, respiratory complaints, sexually transmitted infections (STIs), tuberculosis, fever, and malaria | (Maroyi, 2018) | |
| Syzygium cumini (L.) Skeels | Fruit | Cough, diabetes, dysentery, inflammation, ringworm, and gastrointestinal complaints | (N. Dharani, 2016) |
| Leaf | Diabetes, diarrhea, leucorrhea, and stomach pains | (N. Dharani, 2016) | |
| Stem bark | Bleeding gums, venereal ulcers, dysentery, and fresh wounds | (N. Dharani, 2016) | |
| Syzygium densiflorum Wall. ex Wt. & Arn. | Leaf and ripened fruit | Diabetes | (Krishnasamy et al., 2016) |
| Syzygium fruticosum (Roxb.) DC. | Leaf (juice) | Blood dysentery | (A. H. M. M. Rahman & Khanom, 2013) |
| ‐ | Stomachic, diabetes and bronchitis | (Chadni et al., 2014) | |
| Syzygium formosum (Wall.) Masam | Leaf | Allergy or skin rash | (Duyen Vu et al., 2019) |
| Syzygium grande (Wight) Walp. | _ | Diabetic‐related complications | (Huong et al., 2017) |
| Syzygium gratum (Wight) S.N. Mitra | _ | Dyspepsia, indigestion, peptic ulcer, diarrhea, bacterial infection, asthma, and cardiovascular diseases | (Senggunprai et al., 2010) |
| Syzygium guineense (Willd.) DC. | Root and stem bark | Stomachaches and infertility | (N. J. P. J. K. Dharani, 2016) |
| Leaf (decoction) | Intestinal parasites, stomachache, diarrhea and ophthalmia | (N. J. P. J. K. Dharani, 2016) | |
| Syzygium jambos L. (Alston) | _ | Hemorrhages, syphilis, leprosy, wounds, ulcers, and lung diseases | (Reis et al., 2021) |
| Syzygium lineatum (DC.) Merr. & L.M. Perry | _ | Cancer | (Castillo et al., 2018) |
| Syzygium luehmannii (F. Muell.) L.A.S. Johnson | _ | Fungal skin infections | (Noé et al., 2019) |
| Syzygium malaccense (L.)Merr. & L.M. Perry | Bark (decoction) | Mouth ulcers | (IE Cock & Cheesman, 2018) |
| Leaf | Irregular menstruation | (IE Cock & Cheesman, 2018) | |
| Syzygium myrtifolium Walp. | _ | Stomach aches; | (Kusriani et al., 2019) |
| Syzygium mundagam (Bourd.) Chitra | _ | Diabetes | (Chandran et al., 2017) |
|
Syzygium nervosum A. Cunn.ex DC. |
Leaf and flower bud | Abdominal pain, diarrhea, wounds, itchy sores and acne | (Pham et al., 2020) |
| Leaf and bark | Skin ulcers, scabies, and other skin diseases | (Pham et al., 2020) | |
| Leaf | Diarrhea, pimples, and breast inflammation | (Pham et al., 2020) | |
| Syzygium paniculatum Gaertn. | _ | Diabetes | (Konda et al., 2019) |
| Syzygium polyanthum (Wight) Walp. | Leaf |
diabetes mellitus, hypertension, gastritis, ulcers, diarrhea, skin diseases diabetes mellitus, hypertension, gastritis, ulcers, diarrhea, skin diseases Diabetes mellitus, hypertension, gastritis, ulcers, diarrhea, and skin diseases |
(Ismail & Ahmad, 2019) |
| Syzygium samarangense (Blume) Merr. and L.M. Perry | Flower | Diarrhea and fever | (IE Cock & Cheesman, 2018) |
| Syzygium suboriculare (Benth.) T.G. Hartley & L.M. Perry | _ | Cough, cold, diarrhea, and dysentery | (IE Cock & Cheesman, 2018) |
| Syzygium zeylanicum (L.) DC. | Leaf (extract) | Joint pain, headache, arthritis, and fever | (Anoop et al., 2015) |
| Stem bark | Diabetes mellitus | (Shilpa & Krishnakumar, 2015) |
Although many species of Syzygium genus are used as traditional medicine (Table 1), in this review, we tried to provide an overview on the phytochemical constituents and pharmacological activities of Syzygium species for the development of evidence‐based medicines. It is important to analyze the critique of these species in relation to current knowledge of bioactive compounds and biological activities, which may reduce the gaps between the traditional knowledge and evidence‐based research in future.
2. BOTANICAL DESCRIPTION
2.1. Nomenclature
Syzygium is an entirely old world genus. In past, many Syzygium species were originally described in Eugenia L. or Jambosa Adans. Taxonomic confusion in Eugenia and Syzygium resulted from the considerable overlap of macro‐ and micromorphological characters. Currently, it is clear that these genera are significantly different. Recent molecular evidence supports a scenario in which these two genera are in fact independent lineages (Widodo, 2011).
2.2. Phylogeny
Based upon evolutionary relationships as inferred from investigation of nuclear and plastid DNA sequence data, which is reinforced by morphological evidence presented an infrageneric classification of Syzygium. Six subgenera and seven sections were recognized. The six subgenera are Syzygium, Acmena, Sequestratum, Perikion, Anetholea, Wesa, and the seven sections are Gustavioides, Monimioides, Glenum, Waterhousea, Agaricoides, Acmena, Piliocalyx (Craven, Biffin, & Plants, 2010).
2.3. Morphology
Syzygium is mostly found in tropical or subtropical vegetation, ranging from lowland to montane rainforest, swamp, ultramafic forest, savannah to limestone forest, and also most common tree genera in the forest ecosystem. Some species arise in specified habitat such as along river or on ultramafic or limestone soil. Syzygium are morphologically categorized by a narrow leaf, short petiole, and flexible twig, and leaves are crowded at twig ends. Syzygium commonly blooms in masses in tropical rainforest. It is also important as a food resource for birds, insects, and mammals (Soh et al., 2017).
2.4. Geographical distribution
The genus is native to Bangladesh, India, Pakistan, Sri Lanka, Malaysia, the Philippines, Myanmar, China, and Thailand. It is considered as exotic in Australia, Algeria, Bahamas, Colombia, Ghana, Guatemala, Grenada, Guyana, Jamaica, Kenya, Mexico, Nepal, the Netherlands, Panama, South Africa, and United States of America (Nigam & Nigam, 2012).
3. METHODOLOGY
The present review article reports every aspect of the plant including its traditional uses, phytochemical constituents, and pharmacological activities from the genus Syzygium considering the literatures published prior to September 2020. All the available information on the genus Syzygium was conducted through searching variant scientific electronic databases, including PubMed, Google Scholar, Science Direct, Web of Science, Wiley Online Library, Springer, and Research Gate link, and additional information was conducted from other sources such as book and journals written in English. The literature searched is characterized under detail headings in individual section from the databases.
4. PHYTOCHEMICAL CONSTITUENTS
4.1. Flavonoids
Flavonoids are vital group of naturally occurring polyphenolic compounds having an antioxidant, anti‐inflammatory, antidiabetic, antiallergic activities, while some other flavonoid compounds exhibit potential antiviral activity (S. Ahmed et al., 2020; M. S. Islam et al., 2021; Karak & research, 2019). Methanol extract of S. aqueum leaves contained a number of 87 different compounds rich in flavonoids, for example, myricetin rhamnoside, myrigalone‐G pentoside, quercetin galloyl‐pentoside, cryptostrobin, in which myrigalone‐B and myrigalone‐G were the major flavonoid compounds (A. A. Ahmed et al., 2021; Sobeh, Mahmoud, et al., 2018, 2018). Six flavonoids (e.g., 4‐hydroxybenzaldehyde, myricetin‐3‐O‐rhamnoside, europetin‐3‐O‐rhamnoside, phloretin, myrigalone‐G, and myrigalone‐B) were isolated from the ethanol leaf extracts of S. aqueum. Among them, myricetin‐3‐O‐rhamnoside and europetin‐3‐O‐rhamnoside showed antihyperglycemic activity (Küpeli Akkol et al., 2020; Thamilvaani Manaharan et al., 2012). S. campanulatum n‐hexane and methanol leaf extract contained two flavonoid compounds (2S)‐7‐hydroxy‐5‐methoxy‐6,8‐dimethyl flavanone and (S)‐5,7‐dihydroxy‐6,8‐dimethyl‐flavanone assessed in HPLC method, showing strong antiproliferative activity against human colorectal carcinoma (HCT 116) cells (Hossen et al., 2021; Memon et al., 2015). S. corticosum chloroform leaf extract contained 19 compounds. Among them, two compounds were flavonoids (e.g., sideroxylin, 2,3‐dihydrosideroxylin) obtained through chromatographic separation (Ren et al., 2018). S. cumini contained very few number of flavonoid compounds in various parts such as leaf extract (ellagic acid; caffeic acid), bark extract (quercetin; kaemferol; Figure 1), seed extract (quercetin; rutin), and flower extract (kaemferol, dihydromyricetin) of the plants (Chhikara et al., 2018). Gallic acid methyl ester, a compound identified and characterized from S. fruticosum, exhibited strong antibacterial activity, cytotoxic activity, higher ferrous reducing antioxidant and DPPH free radical scavenging activities (Nasrin et al., 2018). Ethanolic extract of S. formosum leaves contained 28 compounds. Among these, 11 compounds were flavonoids (e.g., catechin, myricetin, quercetin, kaempferol pentoside, etc.), determined by HPLC method (Duyen Vu et al., 2019). S. samarangense methanol leaf extract contained 92 compounds determined by LC‐ESI‐MS/MS method where major compounds were (epi)‐catechin‐(epi)‐gallocatechin, (epi)‐gallocatechin gallate, (epi)‐catechin‐afzelechin, myricetin pentoside, myricetin rhamnoside, guaijaverin, and isorhamnetin rhamnoside (Sobeh et al., 2018). Four flavonoids (e.g. 2′‐hydroxy‐4′,6′‐dimethoxy‐3′‐methylchalcone, 2′,4′‐dihydroxy‐6′‐methoxy‐3′,5′‐dimethylchalcone, 2′,4′‐dihydroxy‐6′‐methoxy‐3′‐methylchalcone, and 7‐hydroxy‐5‐methoxy‐6,8‐dimethylflavanone) isolated from the hexane extract of S. samarangense showed dose‐dependent (10–1000 µg/ml) spasmolytic activity, indicating the usefulness of the plant in the treatment of diarrhea (Ghayur et al., 2006; Table 2).
FIGURE 1.
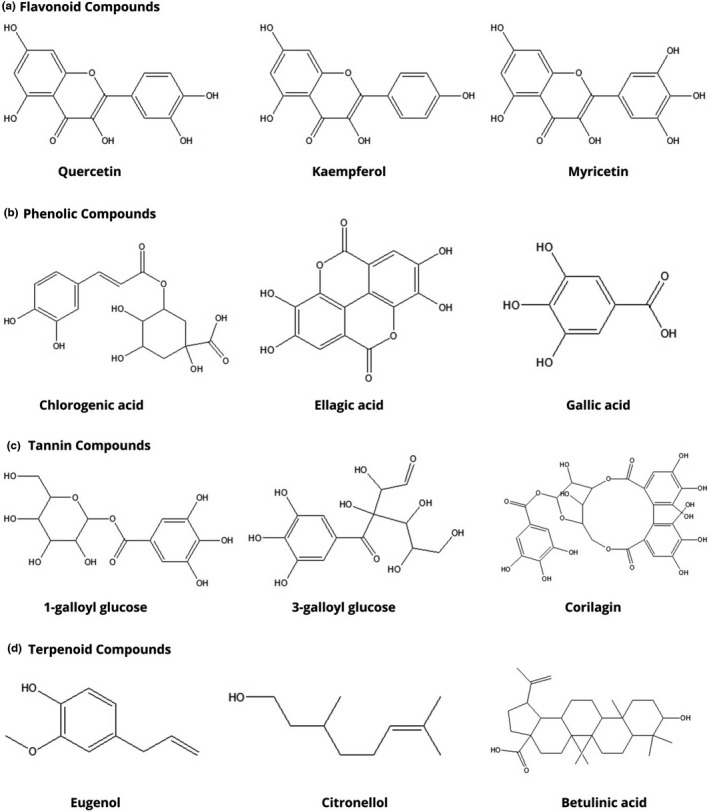
Different types of compounds isolated from Syzygium genus
TABLE 2.
A list of phytochemicals with their source of origin
| Sl. No. | Plant Name |
Extraction Solvent |
Plant Parts | Chemical Compounds | References |
|---|---|---|---|---|---|
| 01 |
Syzygium alternifolium (Wight) Walp. |
Methanol |
Stem Bark |
Octamethylcyclotetrasiloxane, hexamethylcyclotrisiloxane; 1,5‐Diphenyl−2H−1,2,4‐triazoline−3‐thione; cyclopentasiloxane; and decamethyl | (Yugandhar & Savithramma, 2017) |
| Methanol | Leaf | Diethoxydimethylsilane and acetaldehyde | (Yugandhar & Savithramma, 2017) | ||
| Methanol | Fruits | Diethoxydimethylsilane; flavone, 2',5,6,6'‐tetramethoxy‐; 4H−1‐Benzopyran−4‐one | (Yugandhar & Savithramma, 2017) | ||
| 02 |
Syzygium anisatum (Vicfkery) Craven & Biffen |
Aqueous | Leaf |
E‐anethole, methyl chavicol; Z‐ anethole; alpha‐Pinene; 1,8‐Cineole; alpha‐Farnesene; anisaldehyde |
(Brophy & Boland, 1991) |
| 03 |
Syzygium aqueum (Burm.f.) Alston |
Methanol | Leaf |
Myricetin rhamnoside; myrigalone‐G pentoside; quercetin galloyl‐pentoside; samarangenin A; (epi)‐gallocatechin gallate; digalloyl‐hexahydroxydiphenoyl (HHDP)‐hexoside; α‐selinene; β‐caryophyllene; and β‐selinene |
(M. Sobeh et al., 2016; Sobeh, Mahmoud, et al., 2018, 2018) |
| 04 | Syzygium aromaticum (L) Merr. & Perry. | _ | Leaf | Eugenol; β‐caryophyllene; 3‐hexen−1‐ol; and hexyl acetate; | (Kasai et al., 2016) |
| _ | Clove | Eugenol; eugenyl acetate; and β‐caryophyllene; | (Kasai et al., 2016) | ||
| Distilled water | Seed | Eugenol acetate; β‐carophyllene; eugenin; eugenol; methyl salicylate | (Ajiboye et al., 2016) | ||
| 05 |
Syzygium arnottianum Wall.ex Wight & Arn. |
Methanol | Leaf | 4‐Aminopyrimidine; Oxazole; Oct−3‐en−2‐yl ester Cyclopentanone | (Krishna & Mohan, 2012) |
| 06 | Syzygium australe (H.L. Wendl. Ex Link) B. Hyland |
Methanol & aqueous |
Leaf | 1‐Vinylheptanol; 2‐ethyl−1‐hexanol; 2‐heptyl−1,3‐dioxolane; 1‐methyloctyl butyrate; linalool; and 1‐terpineol | (Noé et al., 2019) |
| 07 |
Syzygium benthamianum (Duthie) Gamble |
Ethyl acetate | Leaf | 4‐(4‐Ethylcyclohexyl)−1‐pentyl‐Cyclohexene; Linoleic acid; 2,6,10,14,18‐Penta‐methyl−2,6,10,14,18‐eicosapentaene; 9,17‐Octadecadienal,(z)‐; Z,E−3,13‐Octadecadien−1‐ol; and 7‐Pentadecyne | (Kiruthiga et al., 2011) |
| Double distilled water | Leaf | Sitosteryl acetate; stigmastan−3,5,22‐trien; 2,6‐dimethyl−2‐octene; estra−1,3,5(10)‐trien−17.beta.‐ol; ergosta−4,7,22‐trien3.beta‐ol | (Deepika et al., 2013) | ||
| 08 | Syzygium campanulatum Korth | n‐Hexane methanol | Leaf | (2S)−7‐Hydroxy−5‐methoxy−6,8‐dimethyl flavanone; (S)−5,7‐dihydroxy−6,8‐dimethyl‐flavanone; (E)−2ʹ,4ʹ‐ dihydroxy−6ʹ‐methoxy−3ʹ,5ʹ‐dimethylchalcone; betulinic and ursolic acids | (A. H. Memon et al., 2015) |
| 09 | Syzygium calophyllifolium Walp. | Ethyl acetate | Leaf | Squalene; γ‐eudesmol; βvatirenene; 4‐methoxy‐naphthalene−1‐carboxylic acid, Eicosane, α‐gurjunene, 9‐Eicosyne, Germacrene D, β‐Elemene, (‐)‐Isoledene | (Vignesh et al., 2013) |
| Methanol | Fruit | 3‐Piperidinamine, 1‐ethyl‐; N‐[3‐[n‐aziridyl]propylidene]−3‐methylaminopropylamine; and 1,3‐Propanediamine, n′‐[3‐(dimethylamino)‐n–n‐dimethyl | (Sathyanarayanan et al., 2018) | ||
| 10 |
Syzygium caryophyllatum (L.) Alston |
Hydrodistillation ethanol |
Leaf(essential oil) | Phytol; α‐cadinol; globulol; humulene; and caryophyllene | (Khanh & Ban, 2020; Nadarajan & Pujari, 2014; Wathsara et al., 2020) |
| 11 | Syzygium cordatum Hochst ex C Krauss |
Hydrodistillation |
Leaf (EO) | Major compounds identified are 6,10,14‐trimethylpentadecane−2‐one; 2,3‐butanediol diacetate; n‐hexadeconic acid | (Chalannavar et al., 2011) |
| _ | Fruit | Major compounds identified are vanillic acid; caffeic acid; p‐coumaric acid; betulinic acid | (Maroyi, 2018) | ||
| _ | Bark | Major compounds identified are gallic acid; caffeic acid; arjunolic acid; epifriedelinol | (Maroyi, 2018) | ||
| 12 |
Syzygium corticosum (Lour.)Merr.& L.M. Perry |
Chloroform | Leaf | Major compounds identified are ursolic acid; fouquierol; melaleucic acid; 2,3‐dihydrosideroxylin | (Ren et al., 2018) |
| 13 |
Syzygium cumini (L.) Skeels |
_ | Leaf | Major compounds identified are myricetin; myricetin−4‐methyl ether 3‐O‐α‐rhamnopyranoside; ellagic acid; caffeic acid; nilocetin; acylated flavonol glycosides; Beta‐sitosterol | (Chhikara et al., 2018) |
| _ | Fruit | Major compounds identified are raffinose; gallic acid; cyanidingdiglycoside; petunidin; delphinidin−3‐gentiobioside; malvidin−3‐laminaribioside | (Sowjanya et al., 2013) | ||
| _ | Bark | Major compounds identified are quercetin; kaemferol; 3,3‐di‐O‐methyl ellagic acid; friedelin | (Chhikara et al., 2018) | ||
| _ | Seed | Major compounds identified are quercetin; rutin; ferulic acids; corilagin | (Chhikara et al., 2018) | ||
| _ | Flower | Major compounds identified are kaemferol; Oleanolic acid; eugenol; erategolic acid | (Chhikara et al., 2018) | ||
| _ | Root | Major compounds identified are Isohamnetin−3‐O‐rutinside and flavonoid glycosides | (Chhikara et al., 2018) | ||
| _ | Pulp | Major compounds identified are myricetin deoxyhexoside; gallic acid; citronellol | (Chhikara et al., 2018) | ||
| 14 | Syzygium densiflorum Wall. ex Wt. &Arn. |
Hydrodistillation |
Leaf (EO) | Major constituents are β‐maaliene; isoledene;α‐gurjunene; β‐elemene; and β‐vatirenene | (Saranya et al., 2012) |
| 15 |
Syzygium fruticosum DC. |
Ethyl acetate | Leaf | Gallic acid methyl ester | (Nasrin et al., 2018) |
| 16 | Syzygium formosum (Wall.) Masam | Ethanol | Leaf | Main constituents are gallic acid; protocatechuic acid; ursolic acid; and quercetin | (Duyen Vu et al., 2019) |
| 17 | Syzygium grande (Wight) Walp. | Hydrodistillation | Leaf (EO) | Main constituents are β‐caryophyllene; sabinene; (E)‐β‐ocimene;α‐copaene | (Huong et al., 2017; Samy et al., 2014; Sarvesan et al., 2015) |
| Hydrodistillation | Stem | Main constituents are β‐caryophyllene; sabinene; (E)‐β‐ocimene; δ‐Cadinene | (Huong et al., 2017) | ||
| 18 | Syzygium guineense (Willd.) DC. | n‐hexane | Leaf | Major compounds are tetratriacontane; 9‐octadecanoic acid; n‐hexadecenoic acid; and tetratriacontane | (Abok & Manulu, 2016) |
| 19 | Syzygium jambos L. (Alston) | Hydrodistillation | Leaf (EO) | Major compounds identified are (E)‐caryophyllene; n‐heneicosane; α‐humulene; thujopsan−2‐α‐ol | (Rezende et al., 2013) |
| _ | Stem bark | Major compounds identified are hexadecanoic acid; linoleic acid; and n‐butylidenephthalide | (LIN et al., 2013) | ||
| 20 | Syzygium lineatum (DC.) Merr. & L.M. Perry | Hydrodistillation | Leaf (EO) | Major compounds identified are β‐caryophyllene; α‐pinene; α‐selinene; and α‐humulene | (Khanh & Ban, 2020; Ruma, 2016) |
| 21 | Syzygium lanceolatum (Lam.) Wt. & Arn. | Hydrodistillation | Leaf (EO) | Major compounds identified are phenyl propanal; β‐caryophyllene; α‐humulene; and caryophyllene oxide. In another study, it was found that major compounds identified are 2,8‐Dimethyl−7‐methylene−1,8‐nonadien−3‐yne; germacrene D; elixene | (Benelli et al., 2018; Muthumperumal et al., 2016) |
| 22 | Syzygium luehmannii (F. Muell.) L.A.S. Johnson | Methanol & aqueous | Leaf | Major compounds identified are 2‐Ethyl−1‐hexanol; 2‐heptyl−1,3‐dioxolane; 1‐methyloctyl butyrate; Linalool; exo‐fenchol; 1‐terpineol; endo‐borneol; terpinen−4‐ol; and caryophyllene | (Noé et al., 2019) |
| 23 | Syzygium legatii Burtt Davy & Greenway | Acetone | Leaf | Major compounds identified are friedelan−3‐one; tetradecane; ethanedicarboxamide; dodecane | (Ibukun M Famuyide et al., 2020) |
| 24 | Syzygium malaccense (L.)Merr. & L.M. Perry | Hydrodistillation | Leaf | Major compounds identified are p‐cymene; (−)‐β‐caryophyllene; (−)‐β‐pinene and α‐terpineol | (Karioti et al., 2007) |
| 25 | Syzygium myrtifolium Walp. | Ethanol | Leaf | Major compounds identified are 1‐Octadecene; bis (2‐ethylhexyl) hexanedioic; and bis (2‐ethylhexyl) phthalate | (Novianti et al., 2019) |
| 26 | Syzygium polyanthum (Wight) Walp. | Aqueous | Leaf | Major compounds identified are 9‐octadecenoic; eicosanoic acid | (Widjajakusuma et al., 2019) |
| n‐hexane | Leaf | Major compounds identified are squalene; phytol; α‐pinene; and α‐tocopherol | (Abd Rahim et al., 2018) | ||
| Ethyl acetate | Leaf | Major compounds identified are squalene; phytol; β‐sitosterol | (Abd Rahim et al., 2018) | ||
| Methanol | Leaf | Major compounds identified are squalene; β‐sitosterol; pyrogallol; phytol | (Abd Rahim et al., 2018) | ||
| 27 | Syzygium paniculatum Gaertn. | Hydrodistillation (VO) | Fruit | Major compounds identified are α‐pinene; (Z)‐β‐ocimene; limonene; and α‐ terpineol | (Quijano‐Célis et al., 2013) |
| Hydrodistillation (VO) | Bark | Major compounds identified are α‐pinene; n‐hexadecanoic acid; limonene; and farnesol | |||
| 28 | Syzygium samarangense (Blume) Merr. and L.M. Perry | Methanol | Leaf | Major compounds identified were (epi)‐catechin‐(epi)‐gallocatechin; (epi)‐gallocatechin gallate; (epi)‐catechin‐afzelechin; myricetin pentoside | (Sobeh et al., 2018) |
| Hydrodistillation | Leaf (EO) | Major compounds identified were β‐selinene; α‐selinene; γ‐terpinene; β‐caryophyllene; and β‐gurjunene | (Reddy et al., 2011) | ||
| 29 |
Syzygium zeylanicum (L.) DC. |
Hydrodistillation | Leaf (EO) | Major compounds identified were α‐humulene and β‐elemene | (Govindarajan & Benelli, 2016) |
| _ | Seed | Major compounds identified were oleic acid; linoleic acid and palmitic acid | (Shilpa & Krishnakumar, 2015) | ||
| _ | Pulp | Major compounds identified were oleic acid; linoleic acid; and palmitic acid | (Shilpa & Krishnakumar, 2015) |
4.2. Phenols
Phenol is an aromatic hydrocarbon compound having antioxidant, antimicrobial, anti‐inflammation activities (Ağagündüz et al., 2021; Hossen et al., 2021; Minatel et al., 2017). S. alternifolium is rich in phenols in which nearly 40 types of different compounds were identified in methanol extracts of stem barks, leaves, and fruits through GC‐MS analysis. Among these, seven compounds were phenols (eg1‐butanol, 2‐furanmethanol, propol, methylpropylcarbinol, flavone, 2’,5,6,6’‐tetramethoxy‐; Yugandhar & Savithramma, 2017). Three polyphenols (e.g. gallic acid, myricitrin, and quercitrin; Figure 1), isolated from the methanol extract of S. antisepticum leaves, showed strong antioxidant activity (Mangmool et al., 2021). Methanol extract of S. aqueum leaves contained a few phenolic compounds (e.g. caffeic acid; Sobeh, Mahmoud, et al., 2018, 2018). Aqueous extract of S. aromaticum seeds contained a phenolic compound, eugenol acetate (Ajiboye et al., 2016). S. cordatum fruit extracts contained several phenolic compounds determined by HPLC/TLC. Among these, methanol fruit extract contained vanillic acid, caffeic acid, and p‐coumaric acid (Maroyi, 2018). S. cumini contained very few phenolic compounds in various parts of the plants such as leaf extract (e.g. ellagic acid; caffeic acid), fruit extract (e.g. gallic acid; Sowjanya et al., 2013), bark extract (e.g. 3,3‐di‐O‐methyl ellagic acid; 3,3,4‐tri‐O‐methyl ellagic acid), seed extract (e.g. ferulic acid), and flower extract (e.g. oleanolic acid; eugenol; Chhikara et al., 2018). S. formosum ethanolic leaf extract contained 28 compounds. Among these, four compounds were phenolic compounds (e.g., gallic acid; protocatechuic acid) determined by HPLC method (Duyen Vu et al., 2019). Gallic acid, isolated from methanol stem extract of S. litorale, exhibited strong antioxidant activity against 2,2‐diphenyl‐1‐picrylhydrazyl (DPPH; Tukiran et al., 2016). S. samarangense methanol leaf extract also contained gallic acid and p‐coumaroylquinic acid (Martínez et al., 2020; Sobeh et al., 2018).
4.3. Tannins
Tannins are polyphenolic biomolecules that have antioxidant, antimicrobial, antinutritional, anticancer, cardioprotective properties (Babar et al., 2019; Smeriglio et al., 2017). Methanol extract of S. aqueum leaves contained few number of tannin compounds (e.g. galloylquinic acid; Sobeh, Mahmoud, et al., 2018, 2018). S. cumini contained very few tannin compounds in various parts of the plants such as leaf extract (e.g. nilocetin) and seed extract (e.g. corilagin; Chhikara et al., 2018). S. samarangense methanol leaf extracts also contained galloylquinic acid and quinic acid (Khan et al., 2020; Sobeh et al., 2018).
4.4. Terpenoids
Terpenoids belong to the class of organic compounds which have a hepatoprotective, anti‐inflammatory, antimicrobial, analgesic, and immunomodulatory activities (Akkol, Çankaya, et al., 2021; Dzubak et al., 2006; Reza et al., 2018). n‐Hexane methanol extract of S. campanulatum leaves contained betulinic and ursolic acids assessed in HPLC method (Bristy et al., 2020; Memon et al., 2015; Rahman et al., 2020). Ethyl acetate extract of leaves of S. calophyllifolium contained higher proportions of sesquiterpenoids and triterpenoid compounds that showed effective antimicrobial activity. The extract also showed strong cytotoxicity and antioxidant activity. The major constituent was squalene assessed through GC‐MS analysis (Vignesh et al., 2013). S. cordatum fruit, bark, and wood extracts contained several triterpenoid compounds determined by TLC, IR, MS, CC. Among them, fruit extract contained betulinic acid. Bark and wood extract contained arjunolic acid, epifriedelinol, and friedelin (Maroyi, 2018). Chloroform extract of S. corticosum leaves contained 19 compounds. Among these, seven compounds were triterpenoids, and the major triterpenoid compounds were ursolic acid and melaleucic acid, obtained through chromatographic separation (Ren et al., 2018). S. cumini contained very few triterpenoid compounds in various parts of the plants such as leaf extract (e.g. acylated flavonol glycosides) and bark extract (e.g. friedelin; Chhikara et al., 2018). S. formosum ethanolic leaf extract contained 28 compounds. Among these, 13 compounds were triterpenoids (e.g., maslinic acid; ursolic acid) determined by HPLC method (Duyen Vu et al., 2019). Dichloromethane/methanol (1:1) extract of S. guineense was reported to contain 2, 3, 23‐trihydroxy methyl oleanate (Abera et al., 2018). S. legatii acetone leaf extract yielded 15 compounds with a triterpenoid compound, friedelan‐3‐one, determined by GC‐MS analysis (Famuyide et al., 2020).
4.5. Alkaloids
Alkaloids are naturally occurring organic compounds, which consist of at least one nitrogen atom and also have a wide range of pharmacological activities such as antibacterial, anticancer, analgesic, antihyperglycemic, and antimalarial (Tareq et al., 2020; Uddin et al., 2018). S. cumini seeds are reported to contain alkaloid, jambosine, and showed antidiabetic effect (Ayyanar & Subash‐Babu, 2012). There are various compounds isolated from different parts of Syzygium species such as S. cumini, S. polyanthum, and S. aromaticum (Abd Rahim et al., 2018; Hasanuzzaman et al., 2016). Methanol extract of S. cordatum fruit pulp and seed contains alkaloids (Sidney et al., 2015a).
4.6. Glycosides
Glycosides are the compounds in which a sugar is bound to another functional group via a glycosidic bond and many plants preserve chemicals as inactive form of glycosides (Ali Reza et al., 2021). It has several pharmacological activities, including antiarrhythmic and antihyperglycemic. 2,4,6‐Trihydroxy‐3‐methylacetophenone‐2‐O‐β‐d‐glucoside, a new acetophenone, was isolated from the flower buds of S. aromaticum (Ryu et al., 2016). S. cumini seeds contained glycoside jambolin or antimellin and showed antidiabetic effect by inhibiting the diastatic conversion of starch into sugar (Ayyanar & Subash‐Babu, 2012). There are few compounds found in different parts of S. species such S. cumini and S. polyanthum (Abd Rahim et al., 2018; Hasanuzzaman et al., 2016; Kusuma et al., 2011).
4.7. Others
There are so many classes of compounds such as alkaloid, fatty acid, saponin, anthocyanin, glycosides, etc. Gas chromatography coupled with mass spectrometry (GC‐MS) analysis of S. anisatum leaf essential oil showed that it contained large amounts of phenylpropene compounds (e.g. E‐anethole, methyl chavicol) and also contained monoterpenoids (e.g. 1,8‐Cineole; Brophy & Boland, 1991). Methanol extract of S. aqueum leaves contained 87 different compounds which were determined through high‐resolution LC‐ESI‐MS/MS analysis; among them, major proanthocyanins were samarangenin A; (epi)‐gallocatechin gallate; (epi)‐catechin‐(epi)‐gallocatechin‐(epi)‐gallocatechin gallate; and major ellagitannins compound was digalloyl‐hexahydroxydiphenoyl (HHDP)‐hexoside (Sobeh, Mahmoud, et al., 2018, 2018). GLC‐MS analysis of leaf essential oil contained large amounts of sesquiterpenes (e.g., α‐selinene; β‐caryophyllene; and β‐selinene; Sobeh et al., 2016). Extract of S. aromaticum clove leaves contained major constituents of phenylpropanoid compounds (e.g. eugenol), sesquiterpenes (e.g. β‐caryophyllene), and ester (e.g. 3‐hexen‐1‐ol and hexyl acetate); clove bud oils contained phenylpropanoid compounds (e.g. eugenol); sesquiterpenes (e.g. β‐caryophyllene), founded through statistical analysis using GC‐MS (Kasai et al., 2016). Methanol extract of S. arnottianum leaves contained 11 phytochemical compounds, among them some major compounds are oxazole; ketone (e.g. cyclopentanone); 1,2,4,5‐tetraethyl‐2‐thiopheneacetic acid; hexyl ester hydrazine; and 4,5‐dihydro‐2‐methyl‐dichloro acetic acid, analyzed through GC‐MS analysis (Krishna & Mohan, 2012). Both methanol and aqueous extracts of S. austral leaves contained major compounds such as 1‐vinylheptanol; 2‐ethyl‐1‐hexanol; 2‐heptyl‐1,3‐dioxolane; 1‐methyloctyl butyrate; and several terpenoids (e.g. linalool; exo‐fenchol; and 1‐terpineol), assessed through GC‐MS analysis (Noé et al., 2019). Ethyl acetate extract of S. benthamianum leaves contained total 24 compounds; among them, major constituents are 4‐(4‐ethylcyclohexyl)‐1‐pentyl‐cyclohexene; linoleic acid(fatty acid); 2,6,10,14,18‐penta‐methyl‐2,6,10,14,18‐eicosapentaene; 9,17‐octadecadienal,(z)‐; Z,E‐3,13‐octadecadien‐1‐ol; and 7‐pentadecyne, assessed through GC‐MS analysis (Kiruthiga et al., 2011). Further, GC‐MS analysis of leaves essential oil showed that it contained a total of 63 compounds; the major compounds obtained were sitosteryl acetate; stigmastan‐3,5,22‐trien; 2,6‐dimethyl‐2‐octene; estra‐1,3,5(10)‐trien‐17.beta.‐ol; ergosta‐4,7,22‐trien3.beta‐ol; and a number of other minor compounds (Deepika et al., 2013). S. campanulatum n‐hexane and methanol leaf extract contained chalcone (e.g. (E)‐2ʹ,4ʹ‐dihydroxy‐6ʹ‐methoxy‐3ʹ,5ʹ‐dimethylchalcone), assessed in HPLC method (A. H. Memon et al., 2015). Ethyl acetate extract of S. calophyllifolium leaves contained 60 compounds; among these, major compounds are γ‐eudesmol; β‐vatirenene; 4‐methoxy‐naphthalene‐1‐carboxylic acid; α‐gurjunene; eicosane; germacrene D, assessed through GC‐MS analysis (Vignesh et al., 2013). Methanol extract of fruits contained 12 compounds; among these, major compounds are 3‐piperidinamine; 1‐ethyl‐; N‐[3‐[n‐aziridyl]propylidene]‐3‐methylaminopropylamine; 1,3‐propanediamine; n′‐[3‐(dimethylamino)‐n–n‐dimethyl, determined through GC‐MS analysis (Sathyanarayanan et al., 2018). S. caryophyllatum leaves yielded essential oil upon hydrodistillation and identified 58 compounds through GC‐MS analysis; the major compounds are phytol; α‐cadinol; globulol; humulene; and caryophyllene (Wathsara et al., 2020). S. cordatum leaves yielded essential oil upon hydrodistillation and identified 60 compounds through GC‐FID (Gas chromatography analysis) and GC‐MS analysis; the major compounds identified are 6,10,14‐trimethylpentadecane‐2‐one; 2,3‐butanediol diacetate; n‐hexadeconic acid (Chalannavar et al., 2011). Mycaminose, a carbohydrate isolated from S. cumini seed extract, exhibited antidiabetic effects against STZ‐induced diabetic rats (A. Kumar et al., 2013). S. densiflorum essential oil compositions were identified from hexane extract, a total of 84 compounds were identified among which β‐maaliene; isoledene; α‐gurjunene; β‐elemene; and β‐vatirenene, analyzed by GC‐MS analysis (Saranya et al., 2012). S. grande leaves and stem yielded essential oil upon hydrodistillation and identified 22 and 43 compounds, respectively. The compounds were determined by GC‐MS analysis. Among all the compounds, the major constituents of leaves are β‐caryophyllene; sabinene; (E)‐β‐ocimene; α‐copaene, and the major constituents of stem are β‐caryophyllene; sabinene; (E)‐β‐ocimene; δ‐Cadinene. In both of them, majority of the compounds are sesquiterpene and monoterpene hydrocarbons (Huong et al., 2017). S. guineense n‐hexane leaf extract contained 12 compounds identified by GC‐MS analysis, and major compounds among them were tetratriacontane; 9‐octadecanoic acid; n‐hexadecenoic acid; and tetratriacontane. Organic acid followed by hydrocarbon are the major classes of the identified compounds (Abok & Manulu, 2016). S. jambos leaf essential oil yielded 62 compounds in GC‐MS analysis. Among them, major compounds identified are (E)‐caryophyllene; n‐heneicosane; α‐humulene; thujopsan‐2‐α‐ol (Rezende et al., 2013). Its stem bark essential oil yielded 22 compounds in GC‐MS analysis. Among them, major compounds identified are hexadecanoic acid; linoleic acid; and n‐butylidenephthalide (LIN et al., 2013). S. lineatum leaves yielded essential oil upon hydrodistillation and identified compounds through GC‐MS and gas chromatography flame ionization detector (GC‐FID) analysis. Sesquiterpenes were the major class of compounds. Among them, major compounds identified are β‐caryophyllene; α‐pinene; α‐selinene; and α‐humulene (Khanh & Ban, 2020). S. lanceolatum leaves yielded essential oil upon hydrodistillation, and 18 compounds in one study and 106 compounds in another study were identified through GC‐MS analysis. Alkenes were the major class of compounds, followed by sesquiterpenes. Among them, major compounds identified were phenyl propanal; β‐caryophyllene; α‐humulene; caryophyllene oxide; 2,8‐dimethyl‐7‐methylene‐1,8‐nonadien‐3‐yne; and germacrene D (Benelli et al., 2018; Muthumperumal et al., 2016). Both methanol and aqueous extracts of S. luehmannii leaves contained major compounds such as 2‐ethyl‐1‐hexanol; 2‐heptyl‐1,3‐dioxolane; 1‐methyloctyl butyrate; several terpenoids (e.g. Linalool; exo‐fenchol; 1‐terpineol; endo‐borneol; terpinen‐4‐ol; and caryophyllene), assessed through GC‐MS analysis (Noé et al., 2019). S. legatii acetone leaf extract yielded 15 compounds; among them, major compounds identified were friedelan‐3‐one; tetradecane; ethanedicarboxamide; dodecane, determined by GC‐MS analysis (Ibukun M Famuyide et al., 2020). S. malaccense leaves yielded essential oil upon hydrodistillation and identified 38 compounds through GC‐MS analysis. Monoterpenes (e.g. p‐cymene; (−)‐β‐pinene; α‐terpineol; (+)‐α‐pinene) were the major class of compounds, followed by sesquiterpene (e.g. (−)‐β‐caryophyllene; Karioti et al., 2007). S. myrtifolium ethanol leaf extracts yielded few compounds; among them, major compounds identified were 1‐octadecene; bis (2‐ethylhexyl) hexanedioic and bis (2‐ethylhexyl) phthalate, determined by GC‐MS analysis (Novianti et al., 2019). S. polyanthum aqueous leaf extract exhibited 12 compounds in GC‐MS analysis. Methyl esters were the major class of compounds. The major compounds are 9‐octadecenoic and eicosanoic acid (Widjajakusuma et al., 2019). S. paniculatum fruits yielded volatile oil upon hydrodistillation and identified 155 compounds through GC‐FID (Gas chromatography analysis) and GC‐MS analysis; the major compounds identified were α‐pinene; (Z)‐β‐ocimene; limonene; and α‐ terpineol, rich in terpenes (Quijano‐Célis et al., 2013). Moreover, several leaf extract of S. paniculatum are abundant in squalene; β‐sitosterol; phytol (Abd Rahim et al., 2018). The major compounds of bark were α‐pinene; n‐hexadecanoic acid; limonene; and farnesol (Okoh et al., 2019). S. samarangense leaves yielded essential oil upon hydrodistillation and identified 14 compounds through GC‐MS analysis; the major compounds identified were β‐selinene; α‐selinene; γ‐terpinene; β‐caryophyllene; and β‐gurjunene (Reddy & Jose, 2011). S. zeylanicum leaves yielded essential oil upon hydrodistillation and identified 18 compounds through GC‐MS analysis; the major compounds identified were α‐humulene and β‐elemene (Govindarajan & Benelli, 2016). Fatty acid composition of seeds and pulp yielded few compounds; among them, the major compounds were oleic acid; linoleic acid; and palmitic acid, same for both parts of the plant, determined by GC‐MS analysis (Shilpa & Krishnakumar, 2015).
5. PHARMACOLOGICAL ACTIVITIES
5.1. Antioxidant activities
Antioxidants are the elements which scavenge free radicals, improve protection level from oxidative damage, and also help in decreasing or inhibiting oxidative stress (OS; Figure 2). Many compounds isolated from plants are considered to be natural resources of antioxidants (Table 3). Consumption of several foods which are rich in flavonoid and phenolic compounds exhibits antioxidant effects that can be advantageous for health (Majumder et al., 2017). The ability to scavenge the oxygen free radicals was displayed by the leaf of S. anisatum (Konczak et al., 2010). S. aqueum leaf extract showed strong antioxidant properties in vitro and protected human keratinocytes (HaCaT cells) against UVA damage (Sobeh, Mahmoud, et al., 2018, 2018). Water and methanol extracts of S. aromaticum clove buds and leaves exhibited effective antioxidant activity (Kasai et al., 2016). Its essential clove oil showed high DPPH radical scavenging capacity and low hydroxyl radical inhibition (Radünz et al., 2019). Ethyl acetate leaf extract of S. benthamianum was found to act as potent free radical scavengers in comparison with BHT, a commercial antioxidant. Moreover, a concentration of 400 μg/ml of the extract showed significant inhibition of DPPH radical scavenging activity (Kiruthiga et al., 2011). Ethyl acetate extract of S. calophyllifolium leaves showed higher radical scavenging activity against DPPH free radical, and the activity of was concentration‐dependent manner (Vignesh et al., 2013). Its methanol extract of fruits also showed antioxidant activity (Sathyanarayanan et al., 2018). Ethyl acetate fraction of S. caryophyllatum leaves and n‐hexane fraction of S. caryophyllatum fruits exhibited significant antioxidant activity (Wathsara et al., 2020). Methanol extract of S. cordatum plant was found to be more effective in scavenging DPPH free radicals and exhibited antioxidant activity (Mzindle, 2017). S. cumini methanol leaf extract exhibited antioxidant activity using the DPPH free radical scavenging and ferric‐reducing antioxidant power (FRAP) assays (Ruan et al., 2008). Its seed powder with high carbohydrate diet supplementation prevented the rise of plasma OS markers (superoxide dismutase, catalase, and glutathione) and restored the anti‐oxidative enzymes activity (Ulla et al., 2017). Moreover, fruit extract of S. cumini showed antioxidant activity (Singh et al., 2016). Ethanol extract of S. densiflorum leaves showed significant antioxidant activity by decreasing super oxide dismutase (SOD) and TBARS levels at a dose of 200 mg/kg (MK et al., 2013). Ethanol extract of fruit also showed antioxidant activity by reducing blood glucose level (Krishnasamy et al., 2016). S. fruticosum chloroform fraction of methanol bark extract showed the highest free radical scavenging activity with IC50 value of 20.01 µg/ml (Chadni et al., 2014). Its methanol seed extract also exhibited significant antioxidant activity (S. Islam et al., 2013). Ethanol extract of S. formosum leaves showed better DPPH‐scavenging activities (Lee et al., 2006). S. grande leaves essential oil and leaf aqueous extract showed higher scavenging activity against hydrogen peroxide and in peritoneal macrophages of rat by dihydrofluorescein assay (Jothiramshekar et al., 2014; Kukongviriyapan et al., 2007). S. gratum aqueous and ethanol leaf extracts exhibited strong antioxidant and intracellular oxygen radical scavenging activities. Its aqueous leaf extract was further examined in C57BL/6J mice and showed antioxidant activity along with cytoprotective effect (Senggunprai et al., 2010). S. guineense ethanol leaf extract exhibited antioxidant activity against ferric nitriloacetate‐induced stress in the liver, heart, kidney, and brain tissues of Wistar rat homogenates by inhibiting the lipid peroxidation and restored the enzymatic and nonenzymatic activities (Nzufo et al., 2017). Ethanol leaf extract of S. jambos exhibited significant antioxidant activity with 50% inhibitory concentration (Bonfanti et al., 2013; H. Hossain et al., 2016; Sharma et al., 2013). Its fruit extract also exhibited antioxidant activity (Li et al., 2015). S. luzonense ethanol bark extract exhibited antioxidant activity (Walean et al., 2020). S. lanceolatum leaf essential oil exhibited strongest antioxidant activity with 69.97% inhibitory concentration, determined by the DPPH assay (Muthumperumal et al., 2016). S. malaccense methanol leaf extract exhibited strong antioxidant activity with 78.73% inhibitory concentration at a dose of 100μg /ml (Savitha et al., 2011). Its fruit extract also exhibited antioxidant activity (Nunes et al., 2016). S. mundagam methanol bark and leaf extracts exhibited antioxidant activity (Chandran et al., 2017). S. maire methanol fruit extract showed antioxidant activity (Gould et al., 2006). S. polyanthus methanol fruit extract exhibited antioxidant activity, determined by DPPH assay (Kusuma et al., 2011). S. paniculatum aqueous fruit extract exhibited antioxidant activity and decreased the levels of OS marker and protected the tissues (liver and kidney) against the cytotoxic action and OS‐induced diabetic rats (Konda et al., 2019; Vuong et al., 2014). S. samarangense methanol leaf extract showed antioxidant activity in Wistar rats by increasing the inhibition of GSH (reduced glutathione) and SOD levels and by decreasing the lipid peroxidation, determined by DPPH assay and reducing power assay (Majumder et al., 2017). S. zeylanicum methanol leaf extract exhibited strong antioxidant activity in DPPH assay (Nomi et al., 2012). Its fruit extract also exhibited antioxidant activity (Shilpa & Krishnakumar, 2015). Gallic acid methyl ester isolated and characterized from the ethyl acetate fraction of S. fruticosum leaves showed strong higher ferrous reducing antioxidant and DPPH free radical scavenging activities (Nasrin et al., 2018).
FIGURE 2.
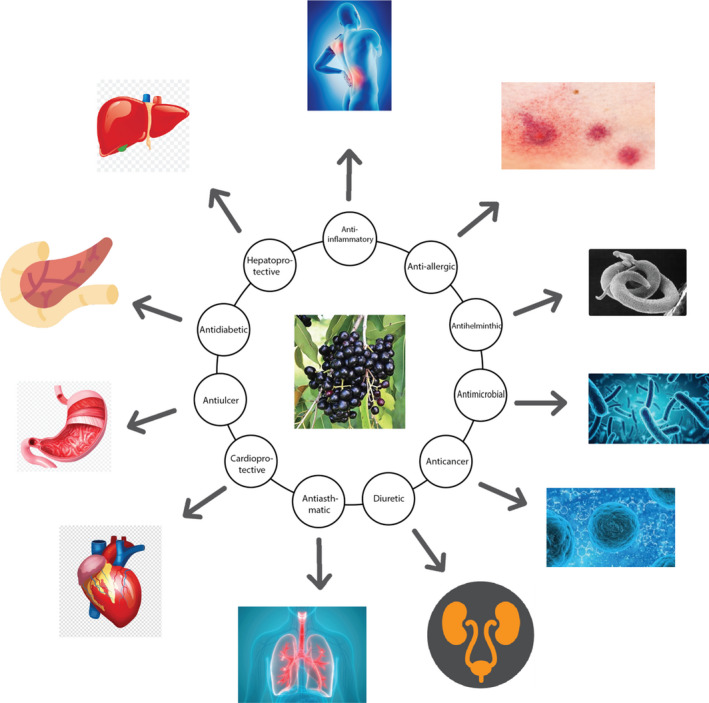
Graphical representation of pharmacological activities of different species of Syzygium genus
TABLE 3.
A list of plants belonging to the Syzygium genus including plant parts with their pharmacological activities
| Plant Name | Parts of Plant | Pharmacological activities | Reference |
|---|---|---|---|
| Syzygium alternifolium (WT.) WALP | Stem bark | Antimicrobial activity | (Yugandhar et al., 2015) |
| Leaf | Anticancer activity (in vitro) | (Komuraiah et al., 2014) | |
| Seeds | Antihyperglycemic and antihyperlipidemic activities | (Kasetti et al., 2010) | |
|
Syzygium anisatum (Vicfkery) Craven & Biffen |
Leaf | Antioxidant, antibacterial, anti‐inflammatory (in vitro), cytoprotective, and proapoptotic activities | (Bryant & Cock, 2016; Guo et al., 2014; Konczak et al., 2010; Sakulnarmrat et al., 2013) |
|
Syzygium aqueum (Burm.f.) Alston |
Leaf | Antioxidant, hepatoprotective, pain‐killing, anti‐inflammatory (in vitro), and antidiabetic activities | (T. Manaharan et al., 2013; Sobeh, Mahmoud, et al., 2018, 2018) |
| Syzygium aromaticum (L) Merr. & Perry. | Leaf | Antioxidant, antibacterial, and antibiofilm activities | (Kasai et al., 2016; Zhang et al., 2017) |
| Seeds | Antibacterial activity | (Ajiboye et al., 2016) | |
| Clove | Antifungal, antimicrobial, antioxidant, anticandidal, hepatoprotective (in vitro), larvicidal, ovicidal potentiality, and anticancer activities | (Hina et al., 2017; Hong et al., 2018; Nirmala et al., 2019; Park et al., 2007; Radünz et al., 2019) | |
| Flower bud | Antiulcer, antioxidant (in vitro), anti‐inflammatory, antituberculosis, antidiabetic, and anthelmintic activities | (Chniguir et al., 2019; Kasai et al., 2016; Kaur & Kaur, 2015; Patil et al., 2014; Santin et al., 2011; Tahir et al., 2016) | |
| Syzygium australe (H.L. Wendl. Ex Link) B. Hyland | Leaf | Antifungal activity (in vitro) | (Noé et al., 2019) |
| Fruit | Antibacterial and antiproliferative activities | (Jamieson et al., 2014; Sautron & Cock, 2014) | |
|
Syzygium benthamianum (Wight ex Duthie) Gamble |
Leaf | Antimicrobial, antioxidant, and anticancer activities | (Kiruthiga et al., 2011) |
| Syzygium campanulatum Korth | Leaf | Antiproliferative, antiangiogenesis, and antitumor activities | (Aisha et al., 2013; A. H. Memon et al., 2015) |
| Syzygium calophyllifolium Walp. | Bark | Antidiabetic, cytotoxic, analgesic, and anti‐inflammatory activities | (Chandran et al., 2016, 2018) |
| Leaf | Antibacterial, antifungal, antioxidant, and anticancer activities (in vitro) | (Vignesh et al., 2013) | |
| Fruit | Antioxidant and antibacterial activities (in vitro) | (Sathyanarayanan et al., 2018) | |
|
Syzygium caryophyllatum (L.) Alston |
Leaf | Antioxidant, antidiabetic (in vitro), antibacterial, antifungal, and anticancer activities | (Annadurai et al., 2012; Nadarajan & Pujari, 2014; Wathsara et al., 2020) |
| Fruit | Antioxidant and antidiabetic activities (in vitro) | (Wathsara et al., 2020) | |
| Root | Anti‐inflammatory activity (in vitro) | (Heendeniya et al., 2018) | |
| Syzygium cordatum Hochst ex C Krauss | Leaf | Antibacterial, antifungal, anti‐inflammatory activity; antidiarrheal, antidiabetic, antioxidant, antileishmanial, and antiplasmodial activities | (Bapela et al., 2017; I. E. Cock & van Vuuren, 2014; Deliwe & Amabeoku, 2013; Mulaudzi et al., 2012; Mzindle, 2017; Nondo et al., 2015) |
| Fruit | Antibacterial and antidiarrheal activities | (Maliehe et al., 2015; Sidney et al., 2015b) | |
| Seed | Antibacterial and antidiarrheal activities | (Maliehe et al., 2015; Sidney et al., 2015b) | |
| Bark | Antibacterial, antifungal, antimutagenic, and antiplasmodial activities | (I. E. Cock & van Vuuren, 2014; Nciki et al., 2016; Nondo et al., 2015; Verschaeve et al., 2004) | |
| Syzygium corticosum (Lour.)Merr.& L.M. Perry | Leaf | Anticancer activity (in vitro) | (Ren et al., 2018) |
| Syzygium cumini (L.) Skeels | Leaf | Antidiabetic, antioxidant, antinociceptive, anti‐leishmania, and antiallergic activities | (Brito et al., 2007; Quintans et al., 2014; Rodrigues et al., 2015; Ruan et al., 2008; Schoenfelder et al., 2010) |
| Fruit | Antioxidant (in vitro), antibacterial, and anticancer activities | (Afify et al., 2011; Singh et al., 2016) | |
| Bark | Antihelmintic activity | (Kavitha et al., 2011) | |
| Seed | Antibacterial, antihyperlipidemia, antioxidant, antidiabetic, and anti‐arthritis activities | (A. Kumar et al., 2013; E. Kumar et al., 2008; Ulla et al., 2017; Yadav et al., 2017) | |
| Syzygium densiflorum Wall. ex Wt. & Arn. | Leaf | Antidiabetic, antioxidant, antibacterial, and antifungal activities | (Eganathan et al., 2012; MK et al., 2013) |
| Fruit | Antidiabetic, antihyperlipidemic, and antioxidant activities | (Krishnasamy et al., 2016) | |
| Syzygium fruticosum (Roxb.) DC. | Leaf | Cytotoxic and thrombolytic activities | (Chadni et al., 2014) |
| Bark | Antibacterial and antioxidant activities | (Chadni et al., 2014) | |
| Seed | Antioxidant and anticancer activities | (S. Islam et al., 2013) | |
| Syzygium formosum (Wall.) Masam | Leaf | Antiallergic, anti‐inflammatory, and antioxidant activities | (Lee et al., 2006; Nguyen et al., 2018) |
| Syzygium francisii (F.M. Bailey) L.A.S. Johnson | Leaf | Antibacterial activity | (Ian Cock et al., 2013) |
| Syzygium forte (F. Muell.) B. Hyland | Leaf | Antibacterial activity | (Ian Cock et al., 2013) |
| Syzygium grande (Wight) Walp. | Leaf | Antibacterial and antioxidant activities | (Jothiramshekar et al., 2014; Sarvesan et al., 2015) |
| Bark | Antidiabetic activity | (Myint, 2017) | |
| Syzygium gratum (Wight) S.N. Mitra | Leaf | Antioxidant, cytoprotective, and anticancer activity | (Kukongviriyapan et al., 2007; Rocchetti et al., 2019; Senggunprai et al., 2010; Stewart et al., 2013) |
| Syzygium guineense (Willd.) DC. | Leaf | Anti‐inflammatory, analgesic, antibacterial, antimalarial, antidiarrheal, antidiabetic (in vitro), antioxidant, antihypertensive (in vivo), and vasodepressor (in vitro) activities | (Ayele et al., 2010; Djoukeng et al., 2005; Ezenyi & Igoli, 2018; Ezuruike et al., 2019; IOR et al., 2012; Nzufo et al., 2017; Tadesse & Wubneh, 2017) |
| Fruit | Cytotoxicity and antihelmintic activities | (Maregesi et al., 2016) | |
| Stem bark | Antituberculosis and antispasmodic activities | (Malele et al., 1997; Oladosu et al., 2017) | |
| Whole plant | Anticancer activity | (Koval et al., 2018) | |
| Syzygium jambos L. (Alston) | Leaf | Antidiabetic, antibacterial, anti‐inflammatory, antioxidant, hepatoprotective, antifungal (in vitro), antinociceptive, analgesic, antiulcerogenic, and anticancer activities | (Avila‐Peña et al., 2007; Bonfanti et al., 2013; Chua et al., 2019; Donatini et al., 2009; Gavillán‐Suárez et al., 2015; H. Hossain et al., 2016; M. R. Islam et al., 2012; Noé et al., 2019; Sharma et al., 2013) |
| Fruit | Antitumor, cytotoxic, and antioxidant activities | (Li et al., 2015; Tamiello et al., 2018) | |
| Stem bark | Antibacterial (in vitro), antidiabetic, and antileukemic activities | (Djipa et al., 2000; Hettiarachchi et al., 2004; Pardede et al., 2020) | |
| Syzygium johnsonii(F. Muell.) B. Hyland | Stem bark | Cytotoxicity and antibacterial activities | (Harris et al., 2011; Setzer et al., 2001) |
| Syzygium luzonense (Merr.)Merr. | Stem bark | Antibacterial, antioxidant, and antihyperglycemic | (Walean et al., 2020) |
| Syzygium lineatum(DC.)Merr.& L.M. Perry | Leaf | Cytotoxicity, anticancer, and antiproliferative activities | (Castillo et al., 2017; A; Castillo et al., 2018; Chua et al., 2019) |
| Syzygium lanceolatum (Lam.) Wt. & Arn. | Leaf | Larvicidal, antioxidant and antibacterial activities | (Benelli et al., 2018; Karuppusamy & Rajasekaran, 2009; Muthumperumal et al., 2016) |
| Syzygium luehmannii (F. Muell.) L.A.S. Johnson | Leaf | Antifungal activity (in vitro) | (Noé et al., 2019) |
| Fruit | Antifungal (in vitro), antibacterial, and antiproliferative activities | (Jamieson et al., 2014; Noé et al., 2019; Sautron & Cock, 2014) | |
| Syzygium legatii Burtt Davy & Greenway | Leaf | Antibacterial, antibiofilm, and anti‐quorum sensing activities | (I. Famuyide et al., 2019; I. M. Famuyide et al., 2019) |
| Syzygium malaccense (L.)Merr. & L.M. Perry | Leaf | Antifungal, antibacterial (in vitro), anti‐inflammatory, antioxidant, and cytotoxic (in vitro) activities | (Dunstan et al., 1997; Itam & Anna, 2020; Locher et al., 1995; Savitha et al., 2011) |
| Fruit | Antioxidant activity | (Nunes et al., 2016) | |
| Bark | Antiviral activity (in vitro) | (Locher et al., 1995) | |
| Syzygium myrtifolium Walp. | Leaf | Antidermatophytic, fungicidal, cytotoxic, antidiarrheal, and antispasmodic activities | (Abdul Hakeem Memon et al., 2020; Sit et al., 2018) |
| Syzygium mundagam (Bourd.) Chitra | Leaf | Antioxidant activity; | (Chandran et al., 2017) |
| Bark | Antidiabetic, antioxidant, antiproliferative, analgesic, and anti‐inflammatory activities | (Chandran et al., 2017a; Chandran et al., 2017b, 2017c, 2017d, 2020) | |
| Syzygium maire (A. Cunn) Sykes & Garn.‐Jones | Fruit | Antioxidant activity | (Gould et al., 2006) |
| Syzygium moorei F. Muell. | Leaf | Antibacterial and antihyperglycemic activities | (Ian Cock et al., 2013) |
| Syzygium paniculatum Gaertn. | Fruit | Antioxidant, anticancer (in vitro), antihyperglycemic, antihyperlipidemic, and antioxidant activities | (Konda et al., 2019; Vuong et al., 2014) |
| Leaf | Anticancer (Cytotoxic) activity; | (Rocchetti et al., 2019) | |
| Syzygium polyanthum (Wight) Walp. | Leaf | Antidiabetic, antibacterial, and anticancer activities | (Nordin et al., 2019; Ramli et al., 2017; Widjajakusuma et al., 2019; Widyawati et al., 2015) |
| Fruit | Antioxidant activity | (Kusuma et al., 2011) | |
| Syzygium puberulum Merr.& L.M. Perry | Leaf | Antibacterial activity | (Ian Cock et al., 2013) |
| Syzygium stocksii (Duthie) Gamble | Leaf | Antibacterial and antifungal activities | (Eganathan et al., 2012) |
| Syzygium samarangense (Blume) Merr. and L.M. Perry | Leaf | Antioxidant, hepatoprotective, antidiabetic, anti‐inflammatory, analgesic, CNS depressant, antibacterial, antidiarrheal, and anti‐obesity activities | (Adesegun et al., 2013; Ghayur et al., 2006; Kim et al., 2012; Majumder et al., 2017; Mollika et al., 2014; Qowiyyah et al.; Reddy et al., 2011; Resurreccion‐Magno et al., 2005; Sobeh et al., 2018) |
| Fruit | Anticancer, antihyperglycemic, anti‐inflammatory, and antiapoptotic activities | (Khamchan et al., 2018; Shen & Chang, 2013; Shen et al., 2012; Simirgiotis et al., 2008) | |
| Bark | Anthelmintic and anti‐acne activities | (Gayen et al., 2016; Sekar et al., 2017) | |
| Syzygium wilsonii (F. Muell.) B. Hyland | Leaf | Antibacterial activity | (Ian Cock et al., 2013) |
|
Syzygium zeylanicum (L.) DC. |
Leaf | Antioxidant, anti‐inflammatory, antibacterial, and larvicidal activities | (Anoop et al., 2015; Govindarajan & Benelli, 2016; Nomi et al., 2012; Shilpa et al., 2014) |
| Fruit | Antibacterial and antioxidant activities | (Shilpa & Krishnakumar, 2015) |
5.2. Anti‐inflammatory activity
Anti‐inflammatory is an action of a substance, which affects the CNS to block the pain signaling to the brain and helps to reduce inflammation and pain. Various compounds of several classes isolated from different species of the genus Syzygium exhibited anti‐inflammatory activity (Table 3). For instance, polyphenolic compounds help to reduce inflammation and also reduce pain. S. anisatum extract which is rich in polyphenols with the murine macrophage cells applied in various concentration inhibited the protein expression levels of iNOS and cyclooxygenase‐2 (COX‐2) in LPS‐activated RAW 264.7 cells and exhibited in vitro potential anti‐inflammatory activity (Guo et al., 2014). The polyphenol‐enriched leaf extract of S. aqueum exhibited promising anti‐inflammatory activities in vitro where it inhibited LOX, COX‐1, and COX‐2 with a higher COX‐2 selectivity than that of standards indomethacin and diclofenac and reduced the extent of lysis of erythrocytes upon incubation with hypotonic buffer solution (Sobeh, Mahmoud, et al., 2018, 2018). Aqueous extract of S. aromaticum flower buds inhibited human neutrophils myeloperoxidase and protected mice from LPS‐induced lung inflammation (Chniguir et al., 2019). S. calophyllifolium methanol bark extract at a dose of 200 mg/kg was found to be very effective against granuloma formation with an inhibition of 70.46% compared to standard drug indomethacin (57.81%), suggesting the efficiency of bark extract to inhibit the migration inflammatory cells and to prevent abnormal permeability of the blood capillaries and showed anti‐inflammatory activity (Chandran et al., 2018). S. caryophyllatum aqueous root extract exhibited concentration‐dependent anti‐inflammatory activity in vitro with an IC50 value of 6.229 μg/ml (Heendeniya et al., 2018). S. cordatum petroleum ether and dichloromethane extracts of leaves exhibited high inhibition activity toward both COX‐1 and COX‐2 (>70%; Mulaudzi et al., 2012). S. formosum ethanol leaf extract exhibited significant improvement in the inflammatory lesion in the small intestine and reduced the number of mast cells and eosinophils recruited to the lesion (Nguyen et al., 2018). S. guineense ethanol leaf extract exhibited significant (p < .05) anti‐inflammatory and analgesic effects on the writhing test at a concentration of 1000 mg/kg in rat models (IOR et al., 2012). S. jambos ethanol leaf extract exhibited anti‐inflammatory activity against the pathogenic Propionibacterium acnes through preventing the release of inflammatory cytokines IL‐8 and tumor necrosis factor‐alpha (TNF‐α) by suppressing them by 74%–99% (H. Hossain et al., 2016; Sharma et al., 2013). S. malaccense leaf extract exhibited anti‐inflammatory activity through inhibiting COX‐1 catalyzed prostaglandin biosynthesis (Dunstan et al., 1997). S. mundagam methanol bark extract exhibited anti‐inflammatory and analgesic activities by reducing inflammation and pain, respectively (Chandran et al., 2020). S. samarangense fruit extract exhibited anti‐inflammatory activity through preventing the intracellular inflammatory signal, reestablishing the PI3K‐Akt/PKB insulin signaling pathway and also increasing glucose uptake in TNF‐α treated FL83B mouse (Shen et al., 2012). Methanol leaf extract also exhibited anti‐inflammatory activity through significant (p < .05) inhibition of carrageenan‐induced paw edema (Mollika et al., 2014). S. zeylanicum ethyl acetate leaf extract exhibited anti‐inflammatory activity through inhibition of cyclooxygenase, 5‐lipoxygenase, and also protein denaturation (Anoop, Bindu, & Review, 2015).
5.3. Antibacterial activities
Antibacterial agents are the substances used principally against pathogenic bacteria to kill or inhibit them to protect cells. Aqueous extract of S. alternifolium stem bark showed antimicrobial properties (Yugandhar et al., 2015). S. anisatum methanol and aqueous leaf extract significantly inhibited both gram‐positive and gram‐negative bacteria (Bryant & Cock, 2016). S. aromaticum leaf essential oil eugenol exhibited antibacterial activity (90.84%) against P. gingivalis concentration of 31.25 μM (Zhang et al., 2017). S. aromaticum seed extract showed antibacterial activity with minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values of 0.06 and 0.10 mg/ml, respectively. Time kill susceptibility at MBC value showed significant decrease in the optical density and colony‐forming unit (CFU) of Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus (Ajiboye et al., 2016). S. aromaticum essential oil showed in vitro inhibitory and bactericidal effects against Staph. aureus (Radünz et al., 2019). Methanol extract of S. austral fruit exhibited greater antibacterial activity in disk diffusion assay method (Sautron & Cock, 2014). Ethyl acetate extract of S. benthamianum leaves showed antibacterial activity by inhibiting the growth of Staph. aureus at the MIC of 500 μg/ml, and other microbial species used in this study showed MIC at 250 μg/ml (Kiruthiga et al., 2011). Ethyl acetate extract of S. calophyllifolium leaves showed maximum zone of inhibition against E. Faecalis and expressed its antibacterial activity (Vignesh et al., 2013). Its methanol extract showed antibacterial activity against E. coli and others at a concentration of 100 mg/ml (Sathyanarayanan et al., 2018). Isolated compounds from S. caryophyllatum leaf essential oil exhibited potent antibacterial activity against both gram‐positive and gram‐negative bacteria carried out by disk diffusion method against six pathogenic bacteria (e.g. Bacillus cereus; Nadarajan & Pujari, 2014). Aqueous extract of S. cordatum leaves exhibited good antibacterial activity, determined by using the microdilution bioassay in 96‐well plate (Mulaudzi et al., 2012). Its methanol extract of fruits and seeds also showed antibacterial activity. The fruit pulp extract exhibited the lowest MIC value of 3.13 mg/ml against some gram‐positive and gram‐negative bacteria, while the seed extract showed the lowest MIC (Maliehe et al., 2015; Sidney et al., 2015b). The aqueous and dichloromethane–methanol extracts of barks exhibited antibacterial activity by producing inhibition effect against several bacterial pathogens, determined by the microtiter plate dilution assay (Nciki et al., 2016). S. cumini methanol seed extract exhibited antibacterial activity against the B. subtilis by agar well diffusion assay. The data showed that B. subtilis is susceptible to methanol seed extract with a zone of inhibition of 20.03 mm (Yadav et al., 2017). Its fruit extract also showed antibacterial activity (Singh et al., 2016). Ethyl acetate extract of S. densiflorum leaves exhibited antibacterial activity against six bacterial strains (e.g. Pseudomonas aeroginosa), determined by disk diffusion method (Eganathan et al., 2012). Chloroform and aqueous fractions of methanol bark extract of S. fruticosum showed mild antibacterial activity against both gram‐positive (e.g. B. cereus) and gram‐negative bacteria (e.g. E. coli) with zone of inhibition ranging from 7 to 14 mm as compared to standard ciprofloxacin (zone of inhibition of 50 mm), determined by disk diffusion method (Chadni et al., 2014). S. francisii, S. forte, S. moorei, S. puberulum, and S. wilsonii leaf methanol extracts inhibited the growth of several gram‐positive and gram‐negative bacterial strains and exhibited antibacterial activity. Moreover, leaf extract of these species showed lower toxicity (LC50 > 1000 μg/ml)) except S. forte (Ian Cock et al., 2013). S. grande leaf essential oil exhibited the antibacterial activity and produced a maximum zone of inhibition against B. subtilis and a minimum zone of inhibition against E. coli (Sarvesan et al., 2015). S. guineense leaf extract showed antibacterial activity against gram‐positive and gram‐negative bacteria, determined by disk diffusion method (Djoukeng et al., 2005). S. jambos ethanol leaf extract exhibited antibacterial activity by inhibiting the growth of Propionibacterium acnes with an MIC value of 31.3 and 7.9 μg/ml (Sharma et al., 2013). Acetone and aqueous bark extracts also exhibited antibacterial activity against several bacterial strains, determined by the agar dilution method (Djipa et al., 2000). S. johnsonii ethanol and chloroform bark extracts exhibited antibacterial activity against both gram‐positive bacteria (e.g. B. cereus), with MIC value 624 and 1250 ppm, respectively, and gram‐negative bacteria (e.g. P. aeruginosa), with MIC value 156 and 624 ppm, respectively, determined using the microbroth dilution technique (Harris et al., 2011; Setzer et al., 2001). S. luzonense ethanol bark extract exhibited antibacterial activity against both gram‐positive bacteria (e.g., Staph. aureus) and gram‐negative bacteria (e.g. E. coli), determined by Kirby–Bauer method (Walean et al., 2020). S. lanceolatum leaf essential oil exhibited antibacterial activity against six bacterial strains, namely, B. cereus, B. licheniformis, Staph. aureus, Staph. hominis, A. viridian and E. coli (Muthumperumal et al., 2016). S. luehmannii methanol fruit extracts exhibited greater antibacterial activity in disk diffusion assay (Sautron & Cock, 2014). S. legatii acetone leaf extract showed strong antibacterial activity against both gram‐positive (e.g., B. cereus) and gram‐negative bacteria (e.g., E. coli) with zone of significant inhibition (I. M. Famuyide et al., 2019). S. malaccense aqueous leaf extract exhibited antibacterial activity against only gram‐positive bacteria (e.g., S. pyogenes, Staph. aureus) with significant zone of inhibition (Locher et al., 1995). S. polyanthus methanol leaf extract exhibited antibacterial activity against foodborne pathogen (e.g., Listeria monocytogenes, P. aeruginosa) with significant zone of inhibition (Widjajakusuma et al., 2019). Ethyl acetate extract of S. stocksii leaves exhibited antibacterial activity against six bacterial strains (e.g. P. aeruginosa), determined by disk diffusion method (Eganathan et al., 2012). S. samarangense leaves essential oil extract exhibited strong antibacterial activity against both gram‐positive (e.g. B. cereus) and gram‐negative bacteria (e.g. E. coli) with significant zone of inhibition, determined by agar well diffusion method (Reddy et al., 2011). Methanol and aqueous extracts of S. zeylanicum bark and leaves exhibited antibacterial activity, and this activity was independent of gram reaction (Shilpa et al., 2014). Its fruits extract also exhibited antibacterial activity (Shilpa & Krishnakumar, 2015).
5.4. Antifungal activity
S. aromaticum oil (clove oil) showed strong antifungal activity against Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum gypseum, and Microsporum canis, and Eugenol was the most effective antifungal constituent of clove oil (Park et al., 2007). S. australe (leaf), S. jambos (leaf), S. luehmannii (leaf and fruit) methanol extracts showed potent activity against fungal growth through inhibiting the growth of human dermatophytes (Noé et al., 2019). Ethyl acetate extract of S. benthamianum leaves showed antifungal activity by inhibiting the growth of Proteus vulgaris at the MIC of 100 μg/ml (Kiruthiga et al., 2011). Ethyl acetate extract of S. calophyllifolium leaves showed maximum zone of inhibition against T. mentagrophytes and expressed its antifungal activity (Vignesh et al., 2013). Methyl acetate extract of S. caryophyllatu leaves exhibited antifungal activity against three fungal strains (e.g. Alternaria alternata), assessed in disk diffusion method (Annadurai et al., 2012). Dichloromethane, ethanol, and water extracts of S. cordatum leaves exhibited the best antifungal activity with MIC values of 0.20, 0.39, and 0.78 mg/ml, respectively (Mulaudzi et al., 2012). The aqueous and dichloromethane–methanol extracts of bark exhibited antifungal activity producing inhibition effect against several bacterial pathogens, determined by using the microtiter plate dilution assay (Nciki et al., 2016). Ethyl acetate extract of S. densiflorum leaves exhibited antifungal activity against three fungal species (Aspergillus niger), determined by disk diffusion method (Eganathan et al., 2012). S. malaccense methanol leaf extract exhibited selective antifungal activity against Microsporum canis, Trichophyton rubrum and Epidermophyton floccosum through inhibiting their growth (Locher et al., 1995). Ethyl acetate extract of S. stocksii leaves exhibit antifungal activity against three fungal species (A. niger), determined by disk diffusion method (Eganathan et al., 2012; Table 3).
5.5. Anticancer activity
Anticancer substances are those substances which exhibited its cytotoxic effect against different cancer cell lines (Table 3). In vitro anticancer activity of leaf hexane and methanol extracts and its isolated two compounds (eucalyptin and epibetulinic acid) of S. alternifolium was showed significant activity (IC50 values 8.177 and 2.687 µg/ml) when compared with others human cancer cell lines (MCF‐7) and human prostate cancer cell lines (DU‐145). S. aromaticum bud essential oil extract was evaluated to determine the cytotoxicity using MTT assay, colony formation assay, and Annexin V‐FITC assay against the thyroid cancer cell line (HTh‐7) and found that the extract showed significant anticancer activity (Nirmala et al., 2019). Methanol and aqueous extracts of S. austral fruits were potent inhibitors of cell proliferation against CaCo2 and HeLa cancer cells, determined by an MTS‐based cell proliferation assay (Jamieson et al., 2014). Ethyl acetate extract of S. benthamianum leaves showed higher activity on Hep2 cells by inhibiting the cell growth, determined by MTT assay (Kiruthiga et al., 2011). n‐Hexane methanol extract of S. campanulatum leaves showed antiproliferative activity on human colon cancer (HCT 116) cell line (Memon et al., 2015). Ethyl acetate extract of S. calophyllifolium leaves showed anticancer activity; the extract has higher cytotoxic activity against Hep2 cell lines (Vignesh et al., 2013). Its methanol extract of bark showed antiproliferative and cell death‐inducing ability analyzed by using MCF‐7 breast cancer cell (Chandran et al., 2018). Ethyl acetate extract of S. caryophyllatum leaves exhibited maximum cell inhibition at higher concentration on cell viability of Hep2 cell lines determined by MTT assay (Annadurai et al., 2012). Ursolic acid and (+)‐2,3‐dihydrosideroxylin isolated from the leaves of S. corticosum were evaluated for their cytotoxicity against the HT‐29 human colon cancer cell line, and it was reported that both the compounds produced cytotoxic effect against the cancer cell line (Ren et al., 2018). S. cumini ethanol fruit extract showed anticancer property through exhibiting a significant dose‐dependent inhibitory effect on cancer cell lines or on AML (acute myeloid leukemia, immature monocytes) cell line (Afify et al., 2011). S. fruticosum methanol seed extract showed anticancer property through exhibiting a significant dose‐dependent inhibitory effect on Ehrlich's Ascite cell (EAC)‐induced Swiss albino mice (S. Islam et al., 2013). S. gratum leaf extract produced cytotoxicological effects on gastric and breast cancer cell lines (e.g., Kato‐III, NUGC‐4, MCF‐7, MDA‐MB‐231), determined by MTT assay (Rocchetti et al., 2019; Stewart et al., 2013). S. guineense methanol plant extract showed anticancer activity against triple‐negative breast cancer and colon cancer cells through inhibiting Wnt‐signaling and proliferation of Wnt‐dependent tumors (Koval et al., 2018). S. jambos exhibited anticancer activity (Chua et al., 2019). Ethanol and chloroform bark extracts of S. johnsonii showed cytotoxicity against several cancer cell lines such as HepG2 and MDA‐MB‐231(Harris et al., 2011; Setzer et al., 2001). S. lineatum leaf extract exhibited antiproliferative effects on HUVEC (human umbilical vein endothelial cells) and cytotoxic effect on Hela (cervical cancer cell line), determined by MTT assay (Castillo et al., 2017). Methanol and aqueous extracts of S. luehmannii fruits were potent inhibitors of cell proliferation against CaCo2 and HeLa cancer cells, determined by an MTS‐based cell proliferation assay (Jamieson et al., 2014). Methanol extract of S. malaccense fruit exhibited anticancer activity against MCF‐7 and MDA‐MB‐231 (Itam & Anna, 2020). S. mundagam methanol bark extract exhibited anticancer activity inducing toxicity in MCF7 breast cancer cells (Chandran et al., 2020). S. polyanthum hydro‐methanol leaf extract exhibited anticancer activity against 4T1 and MCF‐7 mammary carcinoma cells (Nordin et al., 2019). S. paniculatum fruit extract exhibited anticancer activity by reducing cell viability in both MiaPaCa‐2 and ASPC‐1 pancreatic cancer cells (Vuong et al., 2014). Its leaf extract also exhibited cytotoxic effects against MCF‐7 breast adenocarcinoma and MDA‐MB‐231 breast cancer cell lines (Rocchetti et al., 2019). Three compounds (2`,4`‐dihydroxy‐3`,5`‐dimethyl‐6`‐methoxychalcone; 2`,4`‐dihydroxy‐3`‐methyl‐6`‐methoxychalcone (stercurensin); and 2`,4`‐dihydroxy‐6`‐methoxychalcone (cardamonin)) isolated from the methanol extracts of the pulp and seeds of the fruits of S. samarangense exhibited cytotoxic effect against human colon cancer cell line (SW‐480; Simirgiotis et al., 2008).
5.6. Antidiabetic activity
The substances which are used to treat diabetes mellitus through altering the blood glucose level in blood are called antidiabetic or hypoglycemic or antihyperglycemic agents. Many compounds of several classes isolated from different species of the genus exhibited antidiabetic activity. For instance, flavanone 5‐O‐methyl‐4′‐desmethoxymatteucinol 2 exhibited antihyperglycemic effects through altering the blood glucose level (Resurreccion‐Magno et al., 2005). Aqueous extract of S. alternifolium seeds at a dose of 50 mg/kg exhibited antihyperglycemic activity and produced maximum fall of 83% in the blood glucose level in diabetic rat (Kasetti et al., 2010). Bioactive compounds (e.g., 4‐hydroxybenzaldehyde, myricetin‐3‐O‐rhamnoside) of leaf extract of S. aqueum effectively increased adipogenesis, stimulated glucose uptake, and also increased adiponectin secretion and showed antidiabetic potentiality (T. Manaharan et al., 2013). S. aromaticum essential oil from bud extract exhibited a stronger antidiabetic activity with 95.30% inhibition of α‐amylase (Tahir et al., 2016). Methanol extract of S. calophyllifolium barks reduced the blood glucose level and exhibited antidiabetic effect in streptozotocin‐nicotinamide (STZ‐NA)‐induced diabetic rats (Chandran et al., 2016). Hexane fraction of S. caryophyllatum fruits exhibited significantly high antiamylase activity with IC50 value of 2.27 ± 1.81 μg/ml and also exhibited antidiabetic effects (Wathsara et al., 2020). Aqueous extract of S. cordatum leaves exhibited strong antidiabetic effects on streptozotocin (STZ)‐induced diabetic rats through lowering the blood glucose levels (Deliwe & Amabeoku, 2013). Ethyl acetate and methanol extracts of S. cumini seed exhibited the antidiabetic activity against STZ‐induced diabetic rats. Both extracts produced significant (p < .05) reduction in blood glucose level (A. Kumar et al., 2013). Its ethanol leaf extract exhibited the antidiabetic activity against alloxan‐induced diabetic rats by reducing blood glucose level (Schoenfelder et al., 2010). S. densifloru methanol leaf extract exhibited significant reduction in the elevated blood glucose level where the percentage of activity at a concentration of 200 mg/kg b.w was higher than the standard drug metformin (MK et al., 2013). Ethanol fruit extract also exhibited antidiabetic activity in STZ‐ and nicotinamide (NA)‐induced diabetic rats (Krishnasamy et al., 2016). S. grande bark extract showed antidiabetic properties (Myint, 2017). S. guineense aqueous leaf extract exhibited in vitro antidiabetic activity by effecting on glutathione levels within HepG2 cells and inhibiting P‐glycoprotein efflux (Ezuruike et al., 2019). S. jambos aqueous leaf extract exhibited antidiabetic activity by reducing blood glucose level in diabetes genetic mouse models (db/db; Gavillán‐Suárez et al., 2015). Aqueous bark extract also exhibited antidiabetic activity by reducing blood glucose level at a high dose determined by using normoglycemic (in fasted and nonfasted states) and STZ‐induced diabetic rats (Hettiarachchi et al., 2004). S. luzonense ethanol bark extract exhibited antihyperglycemic activity on alloxan‐induced rats by reducing the blood sugar with an optimal dose of 300 mg/kg b.w (Walean et al., 2020). S. mundagam methanol bark extract exhibited antidiabetic activity by reducing blood glucose level in STZ‐NA‐induced diabetic rats (Chandran et al., 2017). S. paniculatum methanol and aqueous fruit extract exhibited antidiabetic activity by reducing blood glucose level in STZ‐induced diabetic rats (Konda et al., 2019). S. polyanthum aqueous leaf extract exhibited antidiabetic activity through reducing blood glucose level in alloxan‐induced diabetic rats (Widjajakusuma et al., 2019). Its methanol leaf extract also exhibited antihyperglycemic effects on STZ‐induced diabetic rats (Widyawati et al., 2015). Vescalagin, a compound isolated from S. samarangense fruit, exhibited antidiabetic activity against high‐fructose diet (HFD)‐induced diabetic Wistar rats by lowering the plasma insulin and C‐peptide levels (Shen & Chang, 2013). In another study, a compound isolated from leaves of S. samarangense exhibited antihyperglycemic effects on alloxan‐induced diabetic rats (Resurreccion‐Magno et al., 2005).
5.7. Antidiarrheal activity
Antidiarrheal agents are fiber‐forming substances which are used to treat or relieve the symptoms of diarrhea (S. Ahmad et al., 2020; Ansari et al., 2017). S. cordatum leaf aqueous extract reduced the number of diarrheal episodes, decreased the stool mass, and delayed the onset of castor oil‐induced diarrhea in mice (Deliwe & Amabeoku, 2013). Its methanol extract of fruit pulp and seed extract exhibited the antidiarrheal activity by reducing the number of wet stools, total stools, and onset time in castor oil‐induced rats (Sidney et al., 2015b). S. guineense ethanol leaf extract exhibited antidiarrheal activity in mice significantly (p < .05) by inhibiting the intrinsic small intestinal propulsion and itopride‐induced propulsive activity (Ezenyi & Igoli, 2018). Isolated compounds from S. myrtifolium ethanol leaf extract exhibited antidiarrheal and antispasmodic potentiality for selected therapeutic effect (Memon et al., 2020). Hexane extract of S. samarangense leaves exhibited spasmolytic activity by relaxing the high K+‐induced contractions and also decreased the Ca++ dose–response in a dose‐dependent manner (Ghayur et al., 2006; Table 3).
5.8. Hepatoprotective activity
The ability of a substance to prevent damage or injury of liver is called antihepatotoxicity or hepatoprotective activity. For example, CCl4 causes hepatotoxic effect through various appliances, and the antihepatotoxic substances such as flavonoids (e.g. myricetin) as natural resource of plants counteract that effects by reducing the injury level by different facts (Sobeh et al., 2018). S. aqueum leaf extract showed hepatoprotective activity by reducing the elevated levels of ALT, AST, total bilirubin (TB), total cholesterol (TC), and triglycerides (TG) in rats with acute CCl4‐induced intoxication. In addition to reducing the high MDA level, the extract noticeably restored GSH and SOD to the normal control levels in liver tissue homogenate and counteracted the deleterious histopathologic changes in liver after CCl4 injection (Sobeh, Mahmoud, et al., 2018, 2018). S. aromaticum clove oil extract showed in vitro hepatoprotective potential against CCl4‐induced hepatotoxicity using rat liver slice culture (LSC) model (Hina et al., 2017). S. cumini seed powder with HCHF (high carbohydrate high fat) food supplementation reduced the high‐fat diet‐induced fatty liver or hepatic steatosis in rats. It is also noted that its seed powder prevented the rise of plasma TC and TG levels (Ulla et al., 2017). Methanol extract of S. densifloru fruits showed antihyperlipidemic activity (Krishnasamy et al., 2016). S. jambos ethanol leaf extract exhibited hepatoprotective activity in a rat model of CCl4‐induced liver damage (Islam et al., 2012). S. samarangense methanol leaf extract showed hepatoprotective activity by reducing liver injury using CCl4‐inducedrats (Sobeh et al., 2018).
5.9. Others
The addition of polyphenolic‐rich extracts from the leaves of S. anisatum to the culture media exerted supreme cytotoxic effect through reducing cell viability of the following cancer cell lines: HT‐29, AGS, BL13, and HepG2, in a dose‐dependent manner (Sakulnarmrat et al., 2013). S. aromaticum leaves contain eugenol that inhibited biofilm formation and reduced preformed biofilm of P. gingivalis at different concentrations (Zhang et al., 2017). Its bud methanol extract and hydrodistillate showed larvicidal and ovicidal potentiality against third‐instar larvae and eggs of B. procera (Hong et al., 2018). Its essential oils displayed antiulcer activities in the rat models of indomethacin and ethanol‐induced ulcer (Santin et al., 2011). Also bud extracts exhibited antituberculosis activity, the proportion of inhibition for M. tuberculosis H37Rv, was found to be dose dependent (Kaur & Kaur, 2015). Its ethanol bud extract also showed anthelmintic activity (Patil et al., 2014). n‐Hexane extract of S. campanulatum leaves suppressed expression of VEGF in endothelial cells. It inhibited angiogenesis and tumor growth in nude mice and showed antiangiogenesis effect and antitumor activity (Aisha et al., 2013). S. cordatum bark extracts significantly lowered the effect of the mutagen mitomycin C (MMC) and showed antimutagenic effects (Verschaeve et al., 2004). Its leaf extracts exhibited significant leishmanicidal activity with acceptable SI values (SI ≥10) to determine their potential lethality or safe therapeutic application against rat skeletal myoblast L6 cell (Bapela et al., 2017). S. cordatum also showed antiplasmodial activity through inhibiting the growth of the chloroquine‐resistant Dd2 malaria parasite strains (Nondo et al., 2015). S. cumini ethanol leaf extracts exhibited antinociceptive effect through showing marked inhibition (p < .01 or p < .001) of glutamate‐induced orofacial nociception (38.8, 51.7, and 54.7%) when compared with the control group (Quintans et al., 2014). Its aqueous leaf extract showed antiallergic properties inhibiting the paw edema induced by C48/80, a potent mast cell degranulator, to an extent comparable to the effect of promethazine, a classical antihistaminic used to relieve symptoms of allergic reactions (Brito et al., 2007). Its methanol and aqueous bark extract exhibited anthelmintic effect using Pheretima posthuma as the animal models, and the effect of the extract is comparable to that of standard drug, Albendazole (Kavitha et al., 2011). Its ethanol extract of seeds at the dose of 250mg/kg and 500 mg/kg inhibited the Freund's complete adjuvant (FCA) induced arthritis in rats (E. Kumar et al., 2008). Carbon tetrachloride soluble fraction of S. fruticosum leaf extract showed significant lethality having the LC50 value 0.65µg/ml. It also exhibited thrombolytic activity (Chadni et al., 2014). Ethanol extract of S. formosum leaves showed antiallergic activity through inhibiting the allergic symptoms to a significant extent in a dose‐dependent manner, examined with a mouse model of chicken ovalbumin (cOVA)‐induced food allergy (Nguyen et al., 2018). S. guineense leaf extract exhibited antimalarial activity in mice through the suppression of parasite (e.g., malaria parasite) at doses of 600 and 400 mg/kg (Tadesse & Wubneh, 2017). Its hydro‐alcoholic leaf extract exhibited in vivo antihypertensive activity in a rat model by reducing blood pressure and also showed in vitro vasodepressor activity by relaxation of aorta precontracted with KCl (Ayele et al., 2010). Its chloroform stem bark extract exhibited antituberculosis activity assessed by using the Mycobacterium Growth Indicator Tube (MGIT) method (Oladosu et al., 2017). Its ethanol fruit extract exhibited cytotoxicity and antihelmintic activity against Artemia salina and Pherithema posthuma pathogen, respectively (Maregesi et al., 2016). Methanol stem bark extract inhibited intrinsic contractions of rabbit and also produced sustained hypotension in anaesthetized rats and reduce systolic, diastolic blood pressure exhibiting antispasmodic activity (Malele et al., 1997). S. jambos hydro‐alcoholic leaf extract exhibited significant antinociceptive and analgesic activity in a rat model (Avila‐Peña et al., 2007). Its hydro‐ethanol leaf extract exhibited antiulcerogenic activity in a rat model reducing gastric injury induced by HCl/ethanol (Donatini et al., 2009). Its chloroform‐methanol fruit extract exhibited antitumor activity in Ehrlich tumor‐bearing mice reducing the tumor growth (Tamiello et al., 2018). Its fruit extract exhibited cytotoxic effects on melanoma cells by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide assay (Li et al., 2015). Methanol bark extract exhibited antileukemic activity with the survived cell percentage (HL‐60 cells) of 12.7% among tested sample (Pardede et al., 2020). S. lanceolatum leaf essential oil exhibited larvicidal effect against anopheline and culicine species (Benelli et al., 2018). S. legatii acetone leaf extract showed antibiofilm activity through reducing biofilm formation (I. M. Famuyide et al., 2019). Its acetone leaf extract also showed anti‐quorum sensing activity determined by inhibition of quorum sensing (QS)‐controlled violacein pigment production in Chromobacterium violaceum (I. Famuyide et al., 2019). S. malaccense aqueous bark extract exhibited antiviral activity against Herpes Simplex Virus‐1 and 2 and inhibited the growth of these viruses (Locher et al., 1995). Its leaven‐hexane, ethyl acetate, and methanol extract exhibited cytotoxicity properties on brine shrimp (Itam & Anna, 2020). Methanol and water extracts of S. myrtifolium leaves exhibited antidermatophytic activity against Trichophyton rubrum and T. interdigitale dermatophytes. These extraction also exhibited fungicidal activity and cytotoxic activity against several isolates of T. tonsurans determined by spread plate method and epithelial (Vero) cell line of monkey, respectively (Sit et al., 2018). S. samarangense methanol leaf extract exhibited analgesic and CNS depressant activities, determined by writhing test and reduction of locomotor and exploratory activities in the open field and hole cross tests, respectively (Mollika et al., 2014). Its fruit extracts exhibited antiapoptotic properties against STZ‐induced pancreatic ß‐cell damage in diabetic rats. Ethanol bark extracts exhibited significant anthelmintic activity against five worms (Gayen et al., 2016). Its fruit extracts exhibited anti‐acne activity (Goni et al., 2021; Sekar et al., 2017). Leaf extracts of n‐hexane and ethyl acetate fraction exhibited anti‐obesity effect on Wistar rat through inhibiting body weight. S. zeylanicum leaf essential oil exhibited potent larvicidal effect against larvae of mosquitos (e.g. Aedes albopictus, Anopheles subpictus; Govindarajan & Benelli, 2016).
6. CONCLUSION
In this review article, we have tried to present the organized information on traditional uses, phytochemical constituents, and mechanism‐based pharmacological activities of plants belonging to the genus Syzygium. The collected data from various literatures turned evident that the plants of the genus Syzygium had prominent traditional uses along with potential pharmacological properties. The reviewed investigation revealed many different pharmacological activities obtained from several organic extracts, essential oils, and compounds isolated from Syzygium species. Some of the activities such as antioxidant, anti‐inflammatory, antibacterial, anticancer, hepatoprotective, antidiarrheal activities, etc. were exhibited as pharmacological activities. Many of them also applied on the animal model for better result along with in vitro screening. The in vivo data complied with the data obtained from the in vitro studies. However, only few species of the genus were studied for their phytochemical constituents that could arbitrate pharmacological activities and still many are unidentified and multiple gaps in the knowledge exist for the lack of isolated compounds. Many of the implemented pharmacological studies were limited to the in vitro screening, many of the pharmacological studies were not correlated with traditional uses, and also many of the animal model‐based investigations were done without mentioning their detailed mechanisms of action. This review article linked the phytochemical constituents to pharmacological activities and provided perception of the biological potential of the genus Syzygium. Study of pharmacological activities delivered supportive evidence for therapeutic effect of this genus. However, many members of the genus Syzygium need more inclusive studies regarding phytochemical constituents and mechanism‐based pharmacological activities. Also, in vitro and in vivo animal studies are necessary to ascertain the safety, clarification of the effective doses, and the mechanisms of action before future clinical studies.
CONFLICT OF INTEREST
All the authors have read and approved the manuscript for this journal. They do not have any conflict of interest.
AUTHOR CONTRIBUTIONS
A. B. M Neshar Uddin: investigated the study and provided resources. Farhad Hossain: performed data curation, investigated the study, contributed to software, and wrote the original draft. A. S. M. Ali Reza: conceived and designed the review. Mst Samima Nasrin: interpreted the data and drafted the manuscript. A. H. M. Khurshid Alam: corrected the manuscript and interpreted the data.
ACKNOWLEDGMENTS
The authors are grateful to the Department of Pharmacy, International Islamic University Chittagong, Bangladesh for giving their supports to perform this study.
Uddin, A. B. M. N. , Hossain, F. , Reza, A. S. M. A. , Nasrin, M. S. , & Alam, A. H. M. K. (2022). Traditional uses, pharmacological activities, and phytochemical constituents of the genus Syzygium: A review. Food Science & Nutrition, 10, 1789–1819. 10.1002/fsn3.2797
REFERENCES
- Abd Rahim, E. N. A. , Ismail, A. , Omar, M. N. , Rahmat, U. N. , & Ahmad, W. A. N. W. J. P. J. (2018). GC‐MS analysis of phytochemical compounds in Syzygium polyanthum leaves extracted using ultrasound‐assisted method. Pharmacognosy Journal, 10(1). [Google Scholar]
- Abera, B. , Adane, L. , & Mamo, F. J. J. P. P. (2018). Phytochemical investigation the root extract of Syzygium guineense and isolation of 2, 3, 23‐trihydroxy methyl oleanate. Journal of Pharmacognosy and Phytochemistry, 7(2), 3104–3111. [Google Scholar]
- Abok, J. , & Manulu, C. J. C. S. I. J. (2016). TLC analysis and GC‐MS profiling of Hexane extract of Syzygium guineense Leaf. American Chemical Science Journal, 16(3), 1–6. 10.9734/ACSJ/2016/27372 [DOI] [Google Scholar]
- Adesegun, S. A. , Samuel, O. F. , Anthony, B. O. , Folasade, B. O. , & Mary, S. K. J. (2013). Essential Oil of Syzygium samarangense; A Potent antimicrobial and inhibitor of partially purified and characterized extracellular protease of Escherichia coli , British Journal of Pharmacology and Toxicology, 4(6), 215–221. [Google Scholar]
- Afify, A.‐E.‐M.‐M. , Fayed, S. A. , Shalaby, E. A. , El‐Shemy, H. (2011). Syzygium cumini (pomposia) active principles exhibit potent anticancer and antioxidant activities. African Journal of Pharmacy and Pharmacology, 5(7), 948–956. [Google Scholar]
- Ağagündüz, D. , Çelik, M. N. , Dazıroğlu, M. E. Ç. , & Capasso, R. (2021). Emergent drug and nutrition interactions in COVID‐19: A comprehensive narrative review. Nutrients, 13(5), 1550. 10.3390/nu13051550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, B. , Baider, C. , Bernardini, B. , Biffin, E. , Brambach, F. , Burslem, D. , Byng, J. W. , Christenhusz, M. , Florens, F. V. , Lucas, E. , & Ray, A. (2016). Syzygium (Myrtaceae): Monographing a taxonomic giant via 22 coordinated regional revisions: PeerJ Preprints.
- Ahmad, S. , Nasrin, M. S. , Reza, A. A. , Chakrabarty, N. , Hoque, M. A. , Islam, S. , Hafez Kabir, M. S. , Tareq, S. M. , Alam, A. K. , Haque, M. A. , & Arman, M. S. I. (2020). Curculigo recurvata WT Aiton exhibits anti‐nociceptive and anti‐diarrheal effects in Albino mice and an in silico model. Animal Models and Experimental Medicine, 3(2), 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, A. A. , Rahman, M. A. , Hossen, M. A. , Reza, A. A. , Islam, M. S. , Rashid, M. M. , Rafi, M. K. J. , Siddiqui, M. T. A. , Al‐Noman, A. , & Uddin, M. N. (2021). Epiphytic Acampe ochracea orchid relieves paracetamol‐induced hepatotoxicity by inhibiting oxidative stress and upregulating antioxidant genes in in vivo and virtual screening. Biomedicine & Pharmacotherapy, 143, 112215.– 10.1016/j.biopha.2021.112215 [DOI] [PubMed] [Google Scholar]
- Ahmed, S. , Khan, H. , Aschner, M. , Mirzae, H. , Küpeli Akkol, E. , & Capasso, R. (2020). Anticancer potential of furanocoumarins: Mechanistic and therapeutic aspects. International Journal of Molecular Sciences, 21(16), 5622.– 10.3390/ijms21165622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisha, A. F. A. , Ismail, Z. , Abu‐Salah, K. M. , Siddiqui, J. M. , Ghafar, G. , & Abdul Majid, A. M. S. (2013). Syzygium campanulatum korth methanolic extract inhibits angiogenesis and tumor growth in nude mice. BMC Complementary and Alternative Medicine, 13(1), 168. 10.1186/1472-6882-13-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajiboye, T. O. , Mohammed, A. O. , Bello, S. A. , Yusuf, I. I. , Ibitoye, O. B. , Muritala, H. F. , & Onajobi, I. B. (2016). Antibacterial activity of Syzygium aromaticum seed: Studies on oxidative stress biomarkers and membrane permeability. Microbial Pathogenesis, 95, 208–215. 10.1016/j.micpath.2016.03.011 [DOI] [PubMed] [Google Scholar]
- Akkol, E. K. , Çankaya, I. T. , Karatoprak, G. Ş. , Carpar, E. , Sobarzo‐Sánchez, E. , & Capasso, R. (2021). Natural compounds as medical strategies in the prevention and treatment of psychiatric disorders seen in neurological diseases. Frontiers in Pharmacology, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkol, E. K. , Tatlı, I. I. , Karatoprak, G. Ş. , Ağar, O. T. , Yücel, Ç. , Sobarzo‐Sánchez, E. , & Capasso, R. (2021). Is Emodin with Anticancer Effects Completely Innocent? Two Sides of the Coin. Cancers, 13(11), 2733. 10.3390/cancers13112733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Reza, A. S. M. , Nasrin, M. S. , Hossen, M. A. , Rahman, M. A. , Jantan, I. , Haque, M. A. , & Sobarzo‐Sánchez, E. (2021). Mechanistic insight into immunomodulatory effects of food‐functioned plant secondary metabolites. Critical Reviews in Food Science and Nutrition, 1–31. 10.1080/10408398.2021.2021138 [DOI] [PubMed] [Google Scholar]
- Annadurai, G. , Masilla, B. R. P. , Jothiramshekar, S. , Palanisami, E. , Puthiyapurayil, S. , Parida, A. K. (2012). Antimicrobial, antioxidant, anticancer activities of Syzygium caryophyllatum (L.) Alston. International Journal of Green Pharmacy, 6(4), 285–288. [Google Scholar]
- Anoop, M. , & Bindu, A. R. (2015). In‐vitro anti‐inflammatory activity studies on Syzygium zeylanicum (L) DC leaves. International Journal of Pharma Research & Review, 4(8), 18–27. [Google Scholar]
- Ansari, P. , Uddin, M. J. , Rahman, M. M. , Abdullah‐Al‐Mamun, M. , Islam, M. R. , Ali, M. H. , & Reza, A. A. (2017). Anti‐inflammatory, anti‐diarrheal, thrombolytic and cytotoxic activities of an ornamental medicinal plant: Persicaria orientalis. Journal of Basic and Clinical Physiology and Pharmacology, 28(1), 51–58. 10.1515/jbcpp-2016-0023 [DOI] [PubMed] [Google Scholar]
- Avila‐Peña, D. , Peña, N. , Quintero, L. , & Suárez‐Roca, H. (2007). Antinociceptive activity of Syzygium jambos leaves extract on rats. Journal of Ethnopharmacology, 112(2), 380–385. 10.1016/j.jep.2007.03.027 [DOI] [PubMed] [Google Scholar]
- Ayele, Y. , Urga, K. , & Engidawork, E. (2010). Evaluation of in vivo antihypertensive and in vitro vasodepressor activities of the leaf extract of Syzygium guineense (Willd) D.C. Phytotherapy Research. Phytotherapy Research, 24(10), 1457–1462. 10.1002/ptr.3141 [DOI] [PubMed] [Google Scholar]
- Ayyanar, M. ,& Subash‐Babu, P. (2012). Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pacific Journal of Tropical Biomedicine, 2(3), 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babar, Z. , Jaswir, I. , Tareq, A. , Ali Reza, A. M. , Azizi, W. , Hafidz, M. , & Uddin, M. R. (2019). In vivo anxiolytic and in vitro anti‐inflammatory activities of water‐soluble extract (WSE) of Nigella sativa (L.) seeds. Natural Product Research, 35(16), 2793–2798. [DOI] [PubMed] [Google Scholar]
- Bapela, M. J. , Kaiser, M. , & Meyer, J. J. M. (2017). Antileishmanial activity of selected South African plant species. South African Journal of Botany, 108, 342–345. 10.1016/j.sajb.2016.08.014 [DOI] [Google Scholar]
- Bari, M. S. , Khandokar, L. , Haque, E. , Romano, B. , Capasso, R. , Seidel, V. , Haque, M. A. , & Rashid, M. A. (2021). Ethnomedicinal uses, phytochemistry, and biological activity of plants of the genus Gynura. Journal of Ethnopharmacology, 271, 113834. [DOI] [PubMed] [Google Scholar]
- Batiha, G.‐E.‐S. , Alkazmi, L. M. , Wasef, L. G. , Beshbishy, A. M. , Nadwa, E. H. , & Rashwan, E. K. (2020). Syzygium aromaticum L. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules, 10(2), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli, G. , Rajeswary, M. , & Govindarajan, M. (2018). Towards green oviposition deterrents? Effectiveness of Syzygium lanceolatum (Myrtaceae) essential oil against six mosquito vectors and impact on four aquatic biological control agents. Environmental Science and Pollution Research, 25(11), 10218–10227. 10.1007/s11356-016-8146-3 [DOI] [PubMed] [Google Scholar]
- Bonfanti, G. , Bitencourt, P. R. , Bona, K. S. , Silva, P. S. , Jantsch, L. B. , Pigatto, A. S. , Boligon, A. , Athayde, M. , Gonçalves, T. , & Moretto, M. (2013). Syzygium jambos and Solanum guaraniticum show similar antioxidant properties but induce different enzymatic activities in the brain of rats. Molecules, 18(8), 9179–9194. 10.3390/molecules18089179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristy, T. A. , Barua, N. , Montakim Tareq, A. , Sakib, S. A. , Etu, S. T. , Chowdhury, K. H. , Jyoti, M. A. , Aziz, M. A. I. , Reza, A. S. M. A. , Caiazzo, E. , Romano, B. , Tareq, S. M. , Emran, T. B. , & Capasso, R. (2020). Deciphering the Pharmacological Properties of Methanol Extract of Psychotria calocarpa Leaves by In Vivo, In Vitro and In Silico Approaches. Pharmaceuticals, 13(8), 183. 10.3390/ph13080183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito, F. A. , Lima, L. A. , Ramos, M. , Nakamura, M. J. , Cavalher‐Machado, S. C. , Siani, A. C. , Henriques, M. , & Sampaio, A. (2007). Pharmacological study of anti‐allergic activity of Syzygium cumini (L.) Skeels. Brazilian Journal of Medical and Biological Research, 40(1), 105–115. 10.1590/S0100-879X2007000100014 [DOI] [PubMed] [Google Scholar]
- Brophy, J. J. , & Boland, D. J. (1991). The leaf essential oil of two chemotypes of Backhousia anisata vickery. Flavour and Fragrance Journal, 6(3), 187–188. 10.1002/ffj.2730060305 [DOI] [Google Scholar]
- Bryant, K. , & Cock, I. E. J. P. C. (2016). Growth inhibitory properties of Backhousia myrtifolia Hook. & Harv. and Syzygium anisatum (Vickery) Craven & Biffen extracts against a panel of pathogenic bacteria. Pharmacognosy Communications, 6(4), 194. [Google Scholar]
- Castillo, A. , Ibana, F. , & Macabeo, A. (2017). Cytotoxicity and α‐glucosidase Inhibitory Potentials of the Leaf Extract and Phenolic Derivatives of Syzygium lineatum .
- Castillo, A. , Ibana, F. , & Macabeo, A. (2018). Alkenylated Phenolic Natural Products Validate the Claimed Anti‐Cancer Property of Syzygiumlineatum (“Lubeg”) .
- Chadni, S. H. , Al Hasan, A. , & Azam, A. Z. J. B. P. J. (2014). Antimicrobial, Cytotoxic, Thrombolytic and Antioxidant Activities of Syzygium fruticosum (Roxb.) DC. Bangladesh Pharmaceutical Journal, 17(1), 51–54. [Google Scholar]
- Chalannavar, R. K. , Baijnath, H. , Odhav, B. J. (2011). Chemical constituents of the essential oil from Syzygium cordatum. African Journal of Biotechnology, 10(14), 2741–2745. [Google Scholar]
- Chandran, R. , Abrahamse, H. , & Parimelazhagan, T. (2018). Cytotoxic, analgesic and anti‐inflammatory properties of Syzygium calophyllifolium bark. Biomedicine & Pharmacotherapy, 103, 1079–1085. 10.1016/j.biopha.2018.04.067 [DOI] [PubMed] [Google Scholar]
- Chandran, R. , Abrahamse, H. , Parimelazhagan, T. , & Durai, G. J. B. (2017). Syzygium mundagam bark methanol extract restores skin to normal in diabetic wounded rats. Biomedicine & Pharmacotherapy, 94, 781–786. 10.1016/j.biopha.2017.07.114 [DOI] [PubMed] [Google Scholar]
- Chandran, R. , George, B. P. , & Abrahamse, H. J. M. (2020). Anti‐proliferative, analgesic and anti‐inflammatory properties of Syzygium mundagam bark methanol extract. Molecules, 25(12), 2900– 10.3390/molecules25122900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran, R. , George, B. P. , Abrahamse, H. , & Parimelazhagan, T. (2017). Therapeutic effects of Syzygium mundagam bark methanol extract on type‐2 diabetic complications in rats. Biomedicine & Pharmacotherapy, 95, 167–174. 10.1016/j.biopha.2017.08.061 [DOI] [PubMed] [Google Scholar]
- Chandran, R. , George, B. P. , Abrahamse, H. , Parimelazhagan, T. Therapeutic effects of Syzygium mundagam bark methanol extract on type‐2 diabetic complications in rats. Biomedicine & Pharmacotherapy, 95, 167–174. 10.1016/j.biopha.2017.08.061 [DOI] [PubMed] [Google Scholar]
- Chandran, R. , Parimelazhagan, T. , George, B. P. (2017). Antihyperglycemic activity of the bark methanolic extract of Syzygium mundagam in diabetic rats. Alexandria Journal of Medicine, 53(4), 317–324. [Google Scholar]
- Chandran, R. , Parimelazhagan, T. , Shanmugam, S. , & Thankarajan, S. (2016). Antidiabetic activity of Syzygium calophyllifolium in Streptozotocin‐Nicotinamide induced Type‐2 diabetic rats. Biomedicine & Pharmacotherapy, 82, 547–554. 10.1016/j.biopha.2016.05.036 [DOI] [PubMed] [Google Scholar]
- Chhikara, N. , Kaur, R. , Jaglan, S. , Sharma, P. , Gat, Y. , & Panghal, A. (2018). Bioactive compounds and pharmacological and food applications of Syzygium cumini ‐ a review. Food & Function, 9(12), 6096–6115. 10.1039/c8fo00654g [DOI] [PubMed] [Google Scholar]
- Chniguir, A. , Zioud, F. , Marzaioli, V. , El‐Benna, J. , & Bachoual, R. (2019). Syzygium aromaticum aqueous extract inhibits human neutrophils myeloperoxidase and protects mice from LPS‐induced lung inflammation. Pharmaceutical Biology, 57(1), 55–63. 10.1080/13880209.2018.1557697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, K. H. , Chowdhur, R. , Hasan, M. , Uddin, M. J. , Hasan, Z. , Nasrin, S. , & Reza, A. (2021). Xylia xylocarpa (Roxb.) Taub. Leaves Ameliorates Inflammation and Pain in Experimental Mice and Computer‐Aided Model. Walailak . Journal of Science & Technology, 18(15). [Google Scholar]
- Chua, L. K. , Lim, C. L. , Ling, A. P. K. , Chye, S. M. , & Koh, R. Y. (2019). Anticancer Potential of Syzygium Species: A Review. Plant Foods for Human Nutrition, 74(1), 18–27. 10.1007/s11130-018-0704-z [DOI] [PubMed] [Google Scholar]
- Cock, I. , & Cheesman, M. (2018). Plants of the genus Syzygium (Myrtaceae): A review on ethnobotany, medicinal properties and phytochemistry. In Bioactive Compounds of Medicinal Plants: Properties and Potential for Human Health (pp. 35–84). [Google Scholar]
- Cock, I. , Chikowe, G. , & Mpala, L. (2013). Antibacterial activity of selected Australian Syzygium species. Pharmacognosy Communications, 3, 77–83. [Google Scholar]
- Cock, I. E. , & van Vuuren, S. F. (2014). Anti‐Proteus activity of some South African medicinal plants: Their potential for the prevention of rheumatoid arthritis. Inflammopharmacology, 22(1), 23–36. 10.1007/s10787-013-0179-3 [DOI] [PubMed] [Google Scholar]
- Craven, L. A. , Biffin, E. J. (2010). An infrageneric classification of Syzygium (Myrtaceae). Blumea‐Biodiversity, Evolution and Biogeography of Plants, 55(1), 94–99. [Google Scholar]
- Deepika, N. , Eganathan, P. , Sujanapal, P. , & Parida, A. (2013). Chemical Composition of Syzygium benthamianum (Wt. ex Duthie) gamble essential oil ‐ an endemic and vulnerable tree species. Journal of Essential Oil Bearing Plants, 16(2), 289–293. 10.1080/0972060X.2013.793976 [DOI] [Google Scholar]
- Deliwe, M. , & Amabeoku, G. (2013). Evaluation of the antidiarrhoeal and antidiabetic activities of the leaf aqueous extract of Syzygium cordatum hoscht. ex C. Krauss (Mytraceae) in rodents.
- Dharani, N. (2016). A review of traditional uses and phytochemical constituents of indigenous Syzygium species in east Africa. Pharmaceutical Journal of Kenya, 22(4), 123–127. [Google Scholar]
- Djipa, C. D. , Delmée, M. , & Quetin‐Leclercq, J. (2000). Antimicrobial activity of bark extracts of Syzygium jambos (L.) Alston (Myrtaceae). Journal of Ethnopharmacology, 71(1), 307–313. 10.1016/S0378-8741(99)00186-5 [DOI] [PubMed] [Google Scholar]
- Djoukeng, J. D. , Abou‐Mansour, E. , Tabacchi, R. , Tapondjou, A. L. , Bouda, H. , & Lontsi, D. (2005). Antibacterial triterpenes from Syzygium guineense (Myrtaceae). Journal of Ethnopharmacology, 101(1–3), 283–286. 10.1016/j.jep.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Donatini, R. S. , Ishikawa, T. , Barros, S. , & Bacchi, E. M. J. (2009). Antiulcerogenic and antioxidant activities of leaf extract of Syzygium jambos (L.). Alston (Myrtaceae). Revista Brasileira De Farmacognosia, 19(1A & 1B), 89–94. [Google Scholar]
- Dunstan, C. A. , Noreen, Y. , Serrano, G. , Cox, P. A. , Perera, P. , & Bohlin, L. (1997). Evaluation of some Samoan and Peruvian medicinal plants by prostaglandin biosynthesis and rat ear oedema assays. Journal of Ethnopharmacology, 57(1), 35–56. 10.1016/s0378-8741(97)00043-3 [DOI] [PubMed] [Google Scholar]
- Duyen Vu, T. P. , Quan Khong, T. , Nguyet Nguyen, T. M. , Kim, Y. H. , & Kang, J. S. (2019). Phytochemical profile of Syzygium formosum (Wall.) Masam leaves using HPLC‐PDA‐MS/MS and a simple HPLC‐ELSD method for quality control. Journal of Pharmaceutical and Biomedical Analysis, 168, 1–12. 10.1016/j.jpba.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Dzubak, P. , Hajduch, M. , Vydra, D. , Hustova, A. , Kvasnica, M. , Biedermann, D. , Markova, L. , Urban, M. , & Sarek, J. (2006). Pharmacological activities of natural triterpenoids and their therapeutic implications. Natural Product Reports, 23(3), 394–411. [DOI] [PubMed] [Google Scholar]
- Ediriweera, E. , & Ratnasooriya, W. (2009). A review on herbs used in treatment of diabetes mellitus by Sri Lankan ayurvedic and traditional physicians. Ayu, 30(4), 373–391. [Google Scholar]
- Eganathan, P. , Saranya, J. , Sujanapal, P. , & Parida, A. (2012). Antimicrobial Activity of Syzygium stocksii (Duthie) Gamble and Syzygium densiflorum Wall. ex Wt. & Arn. leaves. Journal of Biologically Active Products from Nature, 2(6), 360–364. 10.1080/22311866.2012.10719144 [DOI] [Google Scholar]
- Ezenyi, I. C. , & Igoli, J. O. (2018). Antidiarrhoeal properties of Syzygium guineense leaf extract and identification of chemical constituents in its active column fractions. Journal of Complementary and Integrative Medicine, 16(2), 10.1515/jcim-2016-0074 [DOI] [PubMed] [Google Scholar]
- Ezuruike, U. F. , Chieli, E. , & Prieto, J. M. (2019). In Vitro Modulation of Glibenclamide Transport by P‐glycoprotein Inhibitory Antidiabetic African Plant Extracts. Planta Medica, 85(11–12), 987–996. 10.1055/a-0948-9072 [DOI] [PubMed] [Google Scholar]
- Famuyide, I. M. , Aro, A. O. , Fasina, F. O. , Eloff, J. N. , & McGaw, L. J. (2019). Antibacterial and antibiofilm activity of acetone leaf extracts of nine under‐investigated south African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complementary and Alternative Medicine, 19(1), 141. 10.1186/s12906-019-2547-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famuyide, I. , Fasina, F. , Eloff, J. , & McGaw, L. J. (2019). In vitro biological activities of some South African Syzygium and Eugenia (Myrtaceae) species with potential as phytogenic feed additives. Planta Medica, 85(18), PV‐05. [Google Scholar]
- Famuyide, I. M. , Fasina, F. O. , Eloff, J. , & McGaw, L. J. (2020). The ultrastructural damage caused by Eugenia zeyheri and Syzygium legatii acetone leaf extracts on pathogenic Escherichia coli. BMC Veterinary Research, 16(1), 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández, J. , Silván, B. , Entrialgo‐Cadierno, R. , Villar, C. J. , Capasso, R. , Uranga, J. A. , Lombó, F. , & Abalo, R. (2021). Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomedicine & Pharmacotherapy, 143, 112241. 10.1016/j.biopha.2021.112241 [DOI] [PubMed] [Google Scholar]
- Gavillán‐Suárez, J. , Aguilar‐Perez, A. , Rivera‐Ortiz, N. , Rodríguez‐Tirado, K. , Figueroa‐Cuilan, W. , Morales‐Santiago, L. , Cubano, L. A. , & Martínez‐Montemayor, M. M. (2015). Chemical profile and in vivo hypoglycemic effects of Syzygium jambos, Costus speciosus and Tapeinochilos ananassae plant extracts used as diabetes adjuvants in Puerto Rico. BMC Complementary and Alternative Medicine, 15, 244. 10.1186/s12906-015-0772-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayen, P. R. , Al Hossain, A. M. , Saifuzzaman, M. , Faroque, A. (2016). Anthelmintic activity of ethanolic extract of Syzygium samarangense (Blume) Merril & Perry. Dhaka University Journal of Pharmaceutical Sciences, 15(1), 109–111. [Google Scholar]
- Ghayur, M. , Gilani, A. , Khan, A. , Amor, E. , Villaseñor, I. , & Choudhary, M. J. (2006). Presence of calcium antagonist activity explains the use of Syzygium samarangense in diarrhoea. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 20(1), 49–52. [DOI] [PubMed] [Google Scholar]
- Goni, O. , Khan, M. F. , Rahman, M. M. , Hasan, M. Z. , Kader, F. B. , Sazzad, N. , Sakib, M. A. , Romano, B. , Haque, M. A. , & Capasso, R. (2021). Pharmacological insights on the antidepressant, anxiolytic and aphrodisiac potentials of Aglaonema hookerianum Schott. Journal of Ethnopharmacology, 268, 113664. 10.1016/j.jep.2020.113664 [DOI] [PubMed] [Google Scholar]
- Gould, K. S. , Thodey, K. , Philpott, M. , & Ferguson, L. R. (2006). Antioxidant activities of extracts from traditional Maori food plants. New Zealand Journal of Botany, 44(1), 1–4. 10.1080/0028825X.2006.9513001 [DOI] [Google Scholar]
- Govindarajan, M. , & Benelli, G. (2016). α‐Humulene and β‐elemene from Syzygium zeylanicum (Myrtaceae) essential oil: Highly effective and eco‐friendly larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus (Diptera: Culicidae). Parasitology Research, 115(7), 2771–2778. 10.1007/s00436-016-5025-2 [DOI] [PubMed] [Google Scholar]
- Guo, Y. , Sakulnarmrat, K. , & Konczak, I. (2014). Anti‐inflammatory potential of native Australian herbs polyphenols. Toxicology Reports, 1, 385–390. 10.1016/j.toxrep.2014.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S. M. , McFeeters, H. , Ogungbe, I. V. , Cruz‐Vera, L. R. , Setzer, W. N. , Jackes, B. R. , & McFeeters, R. L. (2011). Peptidyl‐tRNA hydrolase screening combined with molecular docking reveals the antibiotic potential of Syzygium johnsonii bark extract. Natural Products Communications, 6(10), 1421–1424. [PubMed] [Google Scholar]
- Hasanuzzaman, M. , Islam, W. , & Islam, M. J. (2016). Phytochemical screening of Syzygium cumini (L.) extracts in different solvents. Journal of Bio‐Science, 24, 11–18. [Google Scholar]
- Heendeniya, S. , Ratnasooriya, W. D. & Pathirana, R. N. (2018). In vitro investigation of anti‐inflammatory activity and evaluation of phytochemical profile of Syzygium caryophyllatum. Journal of Pharmacognosy and Phytotherapy, 7(1), 1759–1763. [Google Scholar]
- Hettiarachchi, H. , Jayakody, J. , & Ratnasooriya, W. J. (2004). Antidiabetic activity of aqueous bark extract of Syzygium jambos. Australian Journal of Medical Herbalism , 16(2), 56. [Google Scholar]
- Hina, S. , Rehman, K. , Shahid, M. , & Jahan, N. (2017). In vitro antioxidant, hepatoprotective potential and chemical profiling of Syzygium aromaticum using HPLC and GC‐MS. Pakistan Journal of Pharmaceutical Sciences, 30(3(Suppl.)), 1031–1039. [PubMed] [Google Scholar]
- Hong, T. K. , Perumalsamy, H. , Jang, K. H. , Na, E. S. , & Ahn, Y. J. (2018). Ovicidal and larvicidal activity and possible mode of action of phenylpropanoids and ketone identified in Syzygium aromaticum bud against Bradysia procera. Pesticide Biochemistry and Physiology, 145, 29–38. 10.1016/j.pestbp.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Hoque, M. A. , Ahmad, S. , Chakrabarty, N. , Khan, M. F. , Kabir, M. S. H. , Brishti, A. , Raihan, M. O. , Alam, A. H. M. K. , Haque, M. A. , Nasrin, M. S. , Haque, M. A. , & Reza, A. S. M. A. (2021). Antioxidative role of palm grass rhizome ameliorates anxiety and depression in experimental rodents and computer‐aided model. Heliyon, 7(10), e08199. 10.1016/j.heliyon.2021.e08199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain, F. , Mostofa, M. G. , & Alam, A. K. (2021). Traditional uses and pharmacological activities of the genus leea and its phytochemicals: A review. Heliyon, 7(2), e06222. 10.1016/j.heliyon.2021.e06222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain, H. , Rahman, S. E. , Akbar, P. N. , Khan, T. A. , Rahman, M. M. , & Jahan, I. A. (2016). HPLC profiling, antioxidant and in vivo anti‐inflammatory activity of the ethanol extract of Syzygium jambos available in Bangladesh. BMC Research Notes, 9, 191. 10.1186/s13104-016-2000-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain, K. H. , Rahman, M. A. , Taher, M. , Tangpong, J. , Hajjar, D. , Alelwani, W. , & Reza, A. A. (2020). Hot Methanol Extract of Leea Macrophylla (Roxb.) manages chemical‐induced inflammation in Rodent Model. Journal of King Saud University‐Science. [Google Scholar]
- Hossain, S. , Urbi, Z. , Karuniawati, H. , Mohiuddin, R. B. , Moh Qrimida, A. , Allzrag, A. M. M. , & Capasso, R. (2021). Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy. Life, 11(4), 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen, M. A. , Ali Reza, A. , Amin, M. B. , Nasrin, M. S. , Khan, T. A. , Rajib, M. H. R. , & Haque, M. A. (2021). Bioactive metabolites of Blumea lacera attenuate anxiety and depression in rodents and computer‐aided model. Food Science & Nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen, M. A. , Reza, A. A. , Ahmed, A. A. , Islam, M. K. , Jahan, I. , Hossain, R. , Khan, M. F. , Maruf, M. R. A. , Haque, M. A. , & Rahman, M. A. (2021). Pretreatment of Blumea lacera leaves ameliorate acute ulcer and oxidative stress in ethanol‐induced Long‐Evan rat: A combined experimental and chemico‐biological interaction. Biomedicine & Pharmacotherapy, 135, 111211. 10.1016/j.biopha.2020.111211 [DOI] [PubMed] [Google Scholar]
- Huong, L. T. , Hung, N. V. , Chac, L. D. , Dai, D. N. , & Ogunwande, I. A. (2017). Essential Oils from Syzygium grande (Wight) Walp. and Syzygium sterrophyllum Merr. et Perry. Journal of Essential Oil Bearing Plants, 20(6), 1620–1626. 10.1080/0972060X.2017.1409658 [DOI] [Google Scholar]
- Hyland, B. P. M. J. (1983). A revision of Syzygium and allied genera (Myrtaceae) Australia. Australian Journal of Botany Supplementary Series, 13(9), 1–164. [Google Scholar]
- Ior, I. , Otimenyin, I. , & Umar, M. (2012). Anti‐inflammatory and analgesic activities of the ethanolic extract of the leaf of Syzygium guineense in rats and mice. IOSR Journal of Pharmacy (IOSRPHR), 2(4), 33–36. 10.9790/3013-24303336 [DOI] [Google Scholar]
- Islam, M. R. , Parvin, M. S. , & Islam, M. E. (2012). Antioxidant and hepatoprotective activity of an ethanol extract of Syzygium jambos (L.) leaves. Drug Discoveries & Therapeutics, 6(4), 205–211. 10.5582/ddt.2012.v6.4.205 [DOI] [PubMed] [Google Scholar]
- Islam, M. S. , Rashid, M. M. , Ahmed, A. A. , Reza, A. A. , Rahman, M. A. , & Choudhury, T. R. (2021). The food ingredients of different extracts of Lasia spinosa (L.) Thwaites can turn it into a potential medicinal food. NFS Journal, 25, 56–69. 10.1016/j.nfs.2021.11.002 [DOI] [Google Scholar]
- Islam, S. , Nasrin, S. , Khan, M. A. , Hossain, A. S. , Islam, F. , Khandokhar, P. , & Medicine, A. (2013). Evaluation of antioxidant and anticancer properties of the seed extracts of Syzygium fruticosum Roxb. growing in Rajshahi. Bangladesh, 13(1), 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail, A. , & Ahmad, W. A. N. W. (2019). Syzygium polyanthum (Wight) Walp: A Potential Phytomedicine. Pharmacognosy Journal, 11(2), 429–438. 10.5530/pj.2019.11.67 [DOI] [Google Scholar]
- Itam, A. , & Anna, L. (2020). Antioxidant activities, cytotoxic properties and total phenolic content of Syzygium malaccense (L.) Merr. & L.M. Perry leaves extracts: A West Sumatera Indonesian plant. Pak J Pharm Sci, 33(1), 175–181. [PubMed] [Google Scholar]
- Jadhav, V. , Kamble, S. , & Kadam, V. (2009). Herbal medicine: Syzygium cumini: A review. Journal of Pharmacy Research, 2(8), 1212–1219. [Google Scholar]
- Jamieson, N. , Sirdaarta, J. , & Cock, I. J. P. C. (2014). The anti‐proliferative properties of Australian plants with high antioxidant capacities against cancer cell lines. Pharmacognosy Communications, 4(4), 71–82. [Google Scholar]
- Jothiramshekar, S. , Eganathan, P. , & Puthiyapurayil, S. (2014). Antioxidant Activity of the Leaf Essential Oil of Syzygium calophyllifolium, Syzygium makul, Syzygium grande and Eugenia cotinifolia ssp. codyensis. Journal of Biologically Active Products from Nature, 4(1), 12–18. 10.1080/22311866.2014.886960 [DOI] [Google Scholar]
- Karak, P. J. (2019). Biological activities of flavonoids: An overview. International Journal of Pharmaceutical Sciences and Research, 10(4), 1567–1574. [Google Scholar]
- Karioti, A. , Skaltsa, H. , & Gbolade, A. A. (2007). Analysis of the leaf Oil of Syzygium malaccense Merr. et Perry from Nigeria. Journal of Essential Oil Research, 19(4), 313–315. 10.1080/10412905.2007.9699290 [DOI] [Google Scholar]
- Karuppusamy, S. , Rajasekaran, K. J. (2009). High throughput antibacterial screening of plant extracts by resazurin redox with special reference to medicinal plants of Western Ghats. Global Journal of Pharmacology, 3(2), 63–68. [Google Scholar]
- Kasai, H. , Shirao, M. , & Ikegami‐Kawai, M. (2016). Analysis of volatile compounds of clove (syzygium aromaticum) buds as influenced by growth phase and investigation of antioxidant activity of clove extracts. Flavour and Fragrance Journal 31(2), 178–184. 10.1002/ffj.3299 [DOI] [Google Scholar]
- Kasetti, R. B. , Rajasekhar, M. D. , Kondeti, V. K. , Fatima, S. S. , Kumar, E. G. , Swapna, S. , Ramesh, B. , & Rao, C. A. (2010). Antihyperglycemic and antihyperlipidemic activities of methanol:Water (4:1) fraction isolated from aqueous extract of Syzygium alternifolium seeds in streptozotocin induced diabetic rats. Food and Chemical Toxicology, 48(4), 1078–1084. 10.1016/j.fct.2010.01.029 [DOI] [PubMed] [Google Scholar]
- Kaur, R. , & Kaur, H. (2015). Antitubercular activity and phytochemical screening of selected medicinal plants. Oriental Journal of Chemistry, 31(1), 597–600. 10.13005/ojc/310176 [DOI] [Google Scholar]
- Kavitha, K. , Murali, M. , & Jayachandra, K. J. (2011). Priliminary Phytochemical Screening, Anthelmintic Activity of Methanolic and Aqueous Extract of Syzygium Cumini Linn. Bark (Myrtaceae). Journal of Pharmaceutical Sciences and Research, 3(9), 1460. [Google Scholar]
- Khamchan, A. , Paseephol, T. , Hanchang, W. J. (2018). Protective effect of wax apple (Syzygium samarangense (Blume) Merr. & LM Perry) against streptozotocin‐induced pancreatic ß‐cell damage in diabetic rats. Biomedicine & Pharmacotherapy, 108, 634–645. [DOI] [PubMed] [Google Scholar]
- Khan, M. F. , Kader, F. B. , Arman, M. , Ahmed, S. , Lyzu, C. , Sakib, S. A. , Tanzil, S. M. , Zim, A. F. M. I. U. , Imran, M. A. S. , Venneri, T. , Romano, B. , Haque, M. A. , & Capasso, R. (2020). Pharmacological insights and prediction of lead bioactive isolates of Dita bark through experimental and computer‐aided mechanism. Biomedicine & Pharmacotherapy, 131, 110774. 10.1016/j.biopha.2020.110774 [DOI] [PubMed] [Google Scholar]
- Khanh, T. H. , & Ban, P. H. (2020). Analysis of Essential Oils from Leaf of Syzygium hancei Merr. & Perry, Syzygium caryophyllatum (L.) Alston and Syzygium lineatum (DC.). Merr. & Perry from Vietnam. Journal of Essential Oil Bearing Plants, 23(3), 548–558. 10.1080/0972060X.2020.1790429 [DOI] [Google Scholar]
- Kim, Y.‐J. , Kim, H.‐C. , Ko, H. , Amor, E. C. , Lee, J. W. , & Yang, H. O. (2012). Inhibitory effects of aurentiacin from Syzygium samarangense on lipopolysaccharide‐induced inflammatory response in mouse macrophages. Food and Chemical Toxicology, 50(3), 1027–1035. 10.1016/j.fct.2011.11.050 [DOI] [PubMed] [Google Scholar]
- Kiruthiga, K. , Saranya, J. , Eganathan, P. , Sujanapal, P. , & Parida, A. (2011). Chemical Composition, Antimicrobial, Antioxidant and Anticancer Activity of Leaves of Syzygium benthamianum (Wight ex Duthie) Gamble. Journal of Biologically Active Products from Nature, 1(4), 273–278. 10.1080/22311866.2011.10719094 [DOI] [Google Scholar]
- Komuraiah, B. , Chinde, S. , Kumar, A. N. , Srinivas, K. , Venu, C. , Kumar, J. K. , Sastry, K. P. , & Grover, P. (2014). Isolation of phytochemicals from anticancer active extracts of Syzygium alternifolium Walp. Leaf. Pharmacognosy Journal, 6(4), 83–85. 10.5530/pj.2014.4.13 [DOI] [Google Scholar]
- Konczak, I. , Zabaras, D. , Dunstan, M. , & Aguas, P. (2010). Antioxidant capacity and phenolic compounds in commercially grown native Australian herbs and spices. Food Chemistry, 122(1), 260–266. 10.1016/j.foodchem.2010.03.004 [DOI] [Google Scholar]
- Konda, P. Y. , Dasari, S. , Konanki, S. , & Nagarajan, P. (2019). In vivo antihyperglycemic, antihyperlipidemic, antioxidative stress and antioxidant potential activities of Syzygium paniculatum gaertn streptozotocin‐induced diabetic rats. Heliyon, 5(3), e01373. 10.1016/j.heliyon.2019.e01373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval, A. , Pieme, C. A. , Queiroz, E. F. , Ragusa, S. , Ahmed, K. , Blagodatski, A. , Wolfender, J.‐L. , Petrova, T. V. , & Katanaev, V. L. (2018). Tannins from Syzygium guineense suppress Wnt signaling and proliferation of Wnt‐dependent tumors through a direct effect on secreted Wnts. Cancer Letters, 435, 110–120. 10.1016/j.canlet.2018.08.003 [DOI] [PubMed] [Google Scholar]
- Krishna, M. , & Mohan, M. J. (2012). Evalution of Phytoconstituents of Syzygium arnottianum Leaves. International Journal of Pharmacognosy and Phytochemical Research 9, 1380–1385. [Google Scholar]
- Krishnasamy, G. , Muthusamy, K. , Chellappan, D. R. , & Subbiah, N. (2016). Antidiabetic, antihyperlipidaemic, and antioxidant activity of Syzygium densiflorum fruits in streptozotocin and nicotinamide‐induced diabetic rats. Pharmaceutical Biology, 54(9), 1716–1726. 10.3109/13880209.2015.1125932 [DOI] [PubMed] [Google Scholar]
- Kukongviriyapan, U. , Luangaram, S. , Leekhaosoong, K. , Kukongviriyapan, V. , & Preeprame, S. (2007). Antioxidant and vascular protective activities of Cratoxylum formosum, Syzygium gratum and Limnophila aromatica. Biological and Pharmaceutical Bulletin, 30(4), 661–666. 10.1248/bpb.30.661 [DOI] [PubMed] [Google Scholar]
- Kumar, A. , Ilavarasan, R. , Deecaraman, M. , Aravindan, P. , Padmanabhan, N. , Krishan, M. J. (2013). Anti‐diabetic activity of Syzygium cumini and its isolated compound against streptozotocin‐induced diabetic rats. Journal of Medicinal Plants Research 2(9), 246–249. [Google Scholar]
- Kumar, A. , Ilavarasan, R. , Jayach, T. , Deecaraman, M. , Aravindan, P. , Padmanabhan, N. , Krishan, M. J. (2013). Anti‐diabetic activity of Syzygium cumini and its isolated compound against streptozotocin‐induced diabetic rats. Journal of Medicinal Plants Research, 2(9), 246–249. [Google Scholar]
- Kumar, E. , Mastan, S. , Reddy, K. R. , Reddy, G. A. , Raghunandan, N. , & Chaitanya, G. J. (2008). Anti‐arthritic property of the methanolic extract of Syzygium cumini seeds. International Journal of Integrative Biology, 4(1), 55–61. [Google Scholar]
- Küpeli Akkol, E. , Genç, Y. , Karpuz, B. , Sobarzo‐Sánchez, E. , & Capasso, R. (2020). Coumarins and coumarin‐related compounds in pharmacotherapy of cancer. Cancers, 12(7), 1959. 10.3390/cancers12071959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusriani, R. H. , Rosandhy, S. M. , & Elfahmi, E. (2019). Luteolin, a flavonoid from Syzygium myrtifolium Walp, Current Research on Biosciences and Biotechnology, 1(1), 31–33. 10.5614/crbb.2019.1.1/FKAN4064 [DOI] [Google Scholar]
- Kusuma, I. W. , Kuspradini, H. , Arung, E. T. , Aryani, F. , Min, Y. H. , Kim, J. S. , & Kim, Y. U. (2011). Biological activity and phytochemical analysis of three Indonesian medicinal plants, Murraya koenigii, Syzygium polyanthum and Zingiber purpurea. Journal of Acupuncture and Meridian Studies, 4(1), 75–79. 10.1016/s2005-2901(11)60010-1 [DOI] [PubMed] [Google Scholar]
- Lee, M.‐H. , Jiang, C.‐B. , Juan, S.‐H. , Lin, R.‐D. , & Hou, W.‐C. (2006). Antioxidant and heme oxygenase‐1 (HO‐1)‐induced effects of selected Taiwanese plants. Fitoterapia, 77(2), 109–115. 10.1016/j.fitote.2005.11.012 [DOI] [PubMed] [Google Scholar]
- Li, G. Q. , Zhang, Y. B. , Wu, P. , Chen, N. H. , Wu, Z. N. , Yang, L. , Qiu, R. X. , Wang, G. C. , & Li, Y. L. (2015). New Phloroglucinol Derivatives from the Fruit Tree Syzygium jambos and their cytotoxic and antioxidant activities. Journal of Agriculture and Food Chemistry, 63(47), 10257–10262. 10.1021/acs.jafc.5b04293 [DOI] [PubMed] [Google Scholar]
- Lin, D.‐D. , Liu, J.‐W. , Li, W.‐G. , Cheng, J.‐L. , & Chen, W.‐W.‐J. (2013). Analysis of Supercritical CO2 Fluid Extraction from the Stem of Syzygium jambos by GC‐MS. China Pharmacy, 2013(31), 32. [Google Scholar]
- Locher, C. P. , Burch, M. T. , Mower, H. F. , Berestecky, J. , Davis, H. , Van Poel, B. , Lasure, A. , Berghe, D. A. V. , & Vlietinck, A. J. (1995). Anti‐microbial activity and anti‐complement activity of extracts obtained from selected Hawaiian medicinal plants. Journal of Ethnopharmacology, 49(1), 23–32. 10.1016/0378-8741(95)01299-0 [DOI] [PubMed] [Google Scholar]
- Majumder, R. , Alam, M. B. , Chowdhury, S. T. , Bajpai, V. K. , & Shukla, S. (2017). Quantitative measurement of bioactive compounds from leaves of Syzygium samarangense with antioxidant efficacy. Journal of the National Science Foundation of Sri Lanka, 45(2), 169– 10.4038/jnsfsr.v45i2.8182 [DOI] [Google Scholar]
- Malele, R. S. , Moshi, M. J. , Mwangi, J. W. , Achola, K. J. , & Munenge, R. W. (1997). Pharmacological properties of extracts from the stem bark of Syzygium guineense on the ileum and heart of laboratory rodents. African Journal of Health Sciences, 4(1), 43–45. [PubMed] [Google Scholar]
- Maliehe, S. , Shandu, S. , Basson, K. J. (2015). Evaluation of the antibacterial activity of Syzygium cordatum fruit‐pulp and seed extracts against bacterial strains implicated in gastrointestinal tract infections. African Journal of Biotechnology, 14(16), 1387–1392. [Google Scholar]
- Manaharan, T. , Appleton, D. , Cheng, H. M. , Palanisamy, U. D. (2012). Flavonoids isolated from Syzygium aqueum leaf extract as potential antihyperglycaemic agents. Food Chemistry, 132(4), 1802–1807. 10.1016/j.foodchem.2011.11.147 [DOI] [Google Scholar]
- Manaharan, T. , Ming, C. H. , & Palanisamy, U. D. (2013). Syzygium aqueum leaf extract and its bioactive compounds enhances pre‐adipocyte differentiation and 2‐NBDG uptake in 3T3‐L1 cells. Food Chemistry, 136(2), 354–363. 10.1016/j.foodchem.2012.08.056 [DOI] [PubMed] [Google Scholar]
- Mangmool, S. , Kunpukpong, I. , Kitphati, W. , & Anantachoke, N. (2021). Antioxidant and Anticholinesterase Activities of Extracts and Phytochemicals of Syzygium antisepticum Leaves. Molecules, 26(11), 3295. 10.3390/molecules26113295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maregesi, S. , Kagashe, G. , Messo, C. W. , Mugaya, L. (2016). Determination of Mineral content, Cytotoxicity and Anthelmintic activity of Syzygium guineense Fruits. Journal of Medical and Pharmaceutical Sciences, 2(54), 95–99. [Google Scholar]
- Maroyi, A. (2018). Syzygium cordatum hochst. ex Krauss: An overview of its ethnobotany, phytochemistry and pharmacological properties. Molecules, 23(5), 10.3390/molecules23051084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez, V. , Iriondo De‐Hond, A. , Borrelli, F. , Capasso, R. , Del Castillo, M. D. , & Abalo, R. (2020). Cannabidiol and other non‐psychoactive cannabinoids for prevention and treatment of gastrointestinal disorders: Useful nutraceuticals? International Journal of Molecular Sciences, 21(9), 3067. 10.3390/ijms21093067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon, A. H. , Ismail, Z. , Al‐Suede, F. S. , Aisha, A. F. , Hamil, M. S. , Saeed, M. A. , Laghari, M. , & Majid, A. (2015). Isolation, characterization, crystal structure elucidation of two flavanones and simultaneous RP‐HPLC determination of five major compounds from Syzygium campanulatum Korth. Molecules, 20(8), 14212–14233. 10.3390/molecules200814212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon, A. H. , Tan, M. H. , Khan, M. S. S. , Hamil, M. S. R. , Saeed, M. A. A. , Ismail, Z. , Asmawi, M. Z. , Majid, A. M. S. A. , & Singh, G. K. C. (2020). Toxicological, Antidiarrhoeal and Antispasmodic Activities of Syzygium myrtifolium. Revista Brasileira De Farmacognosia, 30(3), 397–405. 10.1007/s43450-020-00054-0 [DOI] [Google Scholar]
- Minatel, I. O. , Borges, C. V. , Ferreira, M. I. , Gomez, H. A. G. , Chen, C.‐Y.‐O. , Lima, G. P. P. (2017). Phenolic compounds: Functional properties, impact of processing and bioavailability, Phenolic Compd. Biol. Act, 8, 1–24. [Google Scholar]
- MK, M. M. R. , Agilandeswari, D. , & Dhanabal, S. (2013). Pharmacognostical, antidiabetic and antioxidant studies on Syzygium densiflorum leaves, Contemporary Investigations and Observations in Pharmacy, 2(2), 43–51. [Google Scholar]
- Mollika, S. , Islam, N. , Parvin, N. , Kabir, A. , Sayem, M. , & Luthfunnesa, S. R. J. (2014). Evaluation of analgesic, anti‐Inflammatory and CNS activities of the methanolic extract of Syzygium samarangense leave. Global Journal of Pharmacology, 8(1), 39–s46. [Google Scholar]
- Mulaudzi, R. B. , Ndhlala, A. R. , Kulkarni, M. G. , & Van Staden, J. (2012). Pharmacological properties and protein binding capacity of phenolic extracts of some Venda medicinal plants used against cough and fever. Journal of Ethnopharmacology, 143(1), 185–193. 10.1016/j.jep.2012.06.022 [DOI] [PubMed] [Google Scholar]
- Muthumperumal, C. , Stalin, N. , Das, A. , Swamy, P. (2016). Chemical profiling of leaf essential oil, Antioxidant potential and Antibacterial activity of Syzygium lanceolatum (Lam.) Wt. & Arn (Myrtaceae) Free Radicals and Antioxidants, Saudi Journal of Medical and Pharmaceutical Sciences. 6(1), 13–22. [Google Scholar]
- Myint, P. J. I. J. C. A. M. (2017). Anti‐diabetic potential of some myanmar traditional medicinal plants. International Journal of Complementary & Alternative Medicine, 8(2), 00252. 10.15406/ijcam.2017.08.00252 [DOI] [Google Scholar]
- Mzindle, N. B. (2017). Anti‐inflammatory, anti‐oxidant and wound‐healing properties of selected South Africa medicinal plants .
- Nadarajan, S. , & Pujari, S. S. (2014). Leaf Essential Oil Composition and Biochemical Activity of an Endangered Medicinal Tree Syzygium caryophyllatum (L.) Alston, (Wild black plum). Journal of Essential Oil Bearing Plants, 17(3), 371–379. 10.1080/0972060X.2014.895198 [DOI] [Google Scholar]
- Nasrin, M. , Mostofa, M. G. , Harun‐Or‐Rashid, M. , Islam, M. S. , & Khurshid, A. (2018). Antioxidant, free radical scavenging, antibacterial and cytotoxic compound from the leaves of Syzygium fruticosum. International Journal of Pharmaceutical Sciences and Research, 4, 69–73. [Google Scholar]
- Nciki, S. , Vuuren, S. , van Eyk, A. , & de Wet, H. (2016). Plants used to treat skin diseases in northern Maputaland, South Africa: Antimicrobial activity and in vitro permeability studies. Pharmaceutical Biology, 54(11), 2420–2436. 10.3109/13880209.2016.1158287 [DOI] [PubMed] [Google Scholar]
- Nguyen, T. M. N. , Lomunova, M. , Vu, T. P. D. , Le, B. V. , Kim, Y. H. , Kang, J. S. , & Hwang, I. (2018). Anti‐allergic effects of the ethanol extract of Syzygium formosum (Wall.) Masam leaves and its immunoregulatory mechanisms. Journal of Ethnopharmacology, 211, 171–179. 10.1016/j.jep.2017.09.026 [DOI] [PubMed] [Google Scholar]
- Nigam, V. & Nigam, R. J. C. R. I. P. S. (2012). Distribution and medicinal properties of Syzygium species. 73–80.
- Nirmala, M. J. , Durai, L. , Gopakumar, V. , & Nagarajan, R. (2019). Anticancer and antibacterial effects of a clove bud essential oil‐based nanoscale emulsion system. International Journal of Nanomedicine, 14, 6439–6450. 10.2147/ijn.S211047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noé, W. , Murhekar, S. , White, A. , Davis, C. , & Cock, I. E. (2019). Inhibition of the growth of human dermatophytic pathogens by selected australian and asian plants traditionally used to treat fungal infections. Journal De Mycologie Médicale, 29(4), 331–344. 10.1016/j.mycmed.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Nomi, Y. , Shimizu, S. , Sone, Y. , Tuyet, M. T. , Gia, T. P. , Kamiyama, M. , Shibamoto, T. , Shindo, K. , & Otsuka, Y. (2012). Isolation and antioxidant activity of zeylaniin A, a new macrocyclic ellagitannin from Syzygium zeylanicum leaves. Journal of Agriculture and Food Chemistry, 60(41), 10263–10269. 10.1021/jf302977n [DOI] [PubMed] [Google Scholar]
- Nondo, R. S. , Zofou, D. , Moshi, M. J. , Erasto, P. , Wanji, S. , Ngemenya, M. N. , Titanji, V. P. , Kidukuli, A. W. , & Masimba, P. J. (2015). Ethnobotanical survey and in vitro antiplasmodial activity of medicinal plants used to treat malaria in Kagera and Lindi regions, Tanzania. Journal of Medicinal Plants Research, 9(6), 179–192. [Google Scholar]
- Nordin, M. L. , Othman, A. A. , Kadir, A. A. , Shaari, R. , Osman, A. Y. , & Mohamed, M. (2019). Antibacterial and cytotoxic activities of the Syzygium polyanthum leaf extract from Malaysia. Veterinary World, 12(2), 236–242. 10.14202/vetworld.2019.236-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novianti, T. , Saleh, C. , & Erwin, E. J. J. K. M. (2019). Identifikasi senyawa metabolit sekunder ekstrak n‐heksana daun berwarna merah dari Syzygium myrtifolium Walp. Jurnal Kimia Mulawarman, 17(1), 11–15. [Google Scholar]
- Nunes, P. C. , Aquino Jde, S. , Rockenbach, I. I. , & Stamford, T. L. (2016). Physico‐Chemical Characterization, Bioactive Compounds and Antioxidant Activity of Malay Apple [Syzygium malaccense (L.) Merr. & L.M. Perry]. PLoS One, 11(6), e0158134. doi: 10.1371/journal.pone.0158134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzufo, F. T. , Pieme, C. A. , Njimou, J. R. , Nya, P. C. , Moukette, B. M. , Marco, B. , Angelo, C. , & Yonkeu, N. J. (2017). Organo‐protective and antioxidant properties of leaf extracts of Syzygium guineense var macrocarpum against ferric nitriloacetate‐induced stress of Wistar rats. Journal of Complementary and Integrative Medicine, 14(1), 10.1515/jcim-2015-0086 [DOI] [PubMed] [Google Scholar]
- Okoh, S. O. , Okoh, O. O. , & Okoh, A. I. (2019). Seasonal variation of volatile oil composition and antioxidant property of aerial parts of Syzygium paniculatum Gaertn. grown in the Eastern Cape, South Africa. Natural Product Research, 33(15), 2276–2280. 10.1080/14786419.2018.1497032 [DOI] [PubMed] [Google Scholar]
- Oladosu, I. A. , Lawson, L. , Aiyelaagbe, O. O. , Emenyonu, N. , & Afieroho, O. E. (2017). Anti‐tuberculosis lupane‐type isoprenoids from Syzygium guineense Wild DC. (Myrtaceae) stem bark. Future Journal of Pharmaceutical Sciences, 3(2), 148–152. 10.1016/j.fjps.2017.05.002 [DOI] [Google Scholar]
- Pardede, A. , Wardhani, R. A. A. K. , & Frasisca, E. J. E. (2020). Antileukemic Activity of Methanol Extract From Stem of Baccaurea macrocarpa, Syzygium jambos, Bouea macrophylla Griff., and Diospyros discolor Willd. EduChemia (Jurnal Kimia Dan Pendidikan), 5(2), 111–118. [Google Scholar]
- Park, M. J. , Gwak, K. S. , Yang, I. , Choi, W. S. , Jo, H. J. , Chang, J. W. , Jeung, E. B. , & Choi, I. G. (2007). Antifungal activities of the essential oils in Syzygium aromaticum (L.) Merr. Et Perry and Leptospermum petersonii Bailey and their constituents against various dermatophytes. Journal of Microbiology, 45(5), 460–465. [PubMed] [Google Scholar]
- Patil, R. , Kadam, J. , Chavan, J. , & Salunkhe, A. J. G. T. (2014). Anthelmintic activity of ethanolic bud extract of syzygium aromaticum against pheretima posthuma. Goldenesearch Thoughts, 3(7), 2. [Google Scholar]
- Pham, G. N. , Nguyen, T. T. T. , Nguyen‐Ngoc, H.‐J.‐E.‐B.‐C. , & Medicine, A. (2020). Ethnopharmacology, Phytochemistry, and Pharmacology of Syzygium nervosum . Evidence‐Based Complementary and Alternative Medicine, 2020, 1–14. 10.1155/2020/8263670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qowiyyah, A. , Ihsan, S. , Hamdani, S. , & Syifa, L. Anti‐obesity activity of extract under various fractions of jambu air samarang (Syzygium Samarangense) leaves on wistar female Rats.
- Quijano‐Célis, C. E. , Echeverri‐Gil, D. , Ruiz, Y. , & Pino, J. A. J. N. P. C. (2013). Volatiles from Syzygium paniculatum fruit. Natural Product Communications, 8(1), 1934578X1300800131. [PubMed] [Google Scholar]
- Quintans, J. S. , Brito, R. G. , Aquino, P. G. , França, P. H. , Siqueira‐Lima, P. S. , Santana, A. E. , & Quintans‐Júnior, L. J. (2014). Antinociceptive activity of Syzygium cumini leaves ethanol extract on orofacial nociception protocols in rodents. Pharmaceutical Biology, 52(6), 762–766. 10.3109/13880209.2013.870582 [DOI] [PubMed] [Google Scholar]
- Radünz, M. , da Trindade, M. L. M. , Camargo, T. M. , Radünz, A. L. , Borges, C. D. , Gandra, E. A. , & Helbig, E. (2019). Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chemistry, 276, 180–186. 10.1016/j.foodchem.2018.09.173 [DOI] [PubMed] [Google Scholar]
- Rahman, A. H. M. M. , & Khanom, A. (2013). A Taxonomic and Ethno‐Medicinal Study of Species from Moraceae (Mulberry) Family in Bangladesh Flora. Research in Plant Sciences, 1(3), 53–57. [Google Scholar]
- Rahman, J. , Tareq, A. M. , Hossain, M. , Sakib, S. A. , Islam, M. N. , Ali, M. , Uddin, A. B. M. N. , Hoque, M. , Nasrin, M. S. , Emran, T. B. , Capasso, R. , Reza, A. S. M. A. , & Simal‐Gandara, J. (2020). Biological eValuation, DFT calculations and molecular docking studies on the antidepressant and cytotoxicity activities of cycas pectinata buch.‐Ham. Compounds. Pharmaceuticals, 13(9), 232. 10.3390/ph13090232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, M. , Uddin, M. , Reza, A. , Tareq, A. M. , Emran, T. B. , & Simal‐Gandara, J. (2021). Ethnomedicinal Value of Antidiabetic Plants in Bangladesh: A Comprehensive Review. Plants, 10(4), 729. 10.3390/plants10040729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramli, S. , Radu, S. , Shaari, K. , & Rukayadi, Y. (2017). Antibacterial Activity of Ethanolic Extract of Syzygium polyanthum L. (Salam) Leaves against Foodborne Pathogens and Application as Food Sanitizer. BioMed Research International, 2017, 9024246. 10.1155/2017/9024246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, L. J. , & Jose, B. (2011). Chemical composition and antibacterial activity of the volatile oil from the leaf of Syzygium samarangense (Blume). Merr. & LM Perry. Asian Journal of Biochemical and Pharmaceutical Research, 1(3), 263–269. [Google Scholar]
- Reis, A. S. , de Sousa Silva, L. , Martins, C. F. , & de Paula, J. R. (2021). Analysis of the volatile oils from three species of the gender Syzygium. Research, Society and Development, 10(7), e13510716375–e13510716375. 10.33448/rsd-v10i7.16375 [DOI] [Google Scholar]
- Ren, Y. , Anaya‐Eugenio, G. D. , Czarnecki, A. A. , Ninh, T. N. , Yuan, C. , Chai, H. B. , Soejarto, D. D. , Burdette, J. E. , de Blanco, E. J. C. , & Kinghorn, A. D. (2018). Cytotoxic and NF‐κB and mitochondrial transmembrane potential inhibitory pentacyclic triterpenoids from Syzygium corticosum and their semi‐synthetic derivatives. Bioorganic & Medicinal Chemistry, 26(15), 4452–4460. 10.1016/j.bmc.2018.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resurreccion‐Magno, M. H. C. , Villaseñor, I. M. , Harada, N. , & Monde, K. (2005). Antihyperglycaemic flavonoids from Syzygium samarangense (Blume) Merr. and Perry. Phytotherapy Research, 19(3), 246–251. 10.1002/ptr.1658 [DOI] [PubMed] [Google Scholar]
- Reza, A. A. , Haque, M. A. , Sarker, J. , Nasrin, M. S. , Rahman, M. M. , Tareq, A. M. , Khan, Z. , Rashid, M. , Sadik, M. G. , Tsukahara, T. , & Alam, A. K. (2021). Antiproliferative and antioxidant potentials of bioactive edible vegetable fraction of Achyranthes ferruginea Roxb. in cancer cell line. Food Science & Nutrition, 9(7), 3777–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza, A. A. , Hossain, M. S. , Akhter, S. , Rahman, M. R. , Nasrin, M. S. , Uddin, M. J. , Sadik, G. , & Khurshid Alam, A. H. M. (2018). In vitro antioxidant and cholinesterase inhibitory activities of Elatostema papillosum leaves and correlation with their phytochemical profiles: A study relevant to the treatment of Alzheimer’s disease. BMC Complementary and Alternative Medicine, 18(1), 123. 10.1186/s12906-018-2182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende, W. P. , Borges, L. L. , Alves, N. M. , Ferri, P. H. , & Paula, J. R. (2013). Chemical variability in the essential oils from leaves of Syzygium jambos. Revista Brasileira De Farmacognosia, 23(3), 433–440. 10.1590/S0102-695X2013005000035 [DOI] [Google Scholar]
- Rocchetti, G. , Lucini, L. , Ahmed, S. R. , & Saber, F. R. (2019). In vitro cytotoxic activity of six Syzygium leaf extracts as related to their phenolic profiles: An untargeted UHPLC‐QTOF‐MS approach. Food Research International, 126, 108715. 10.1016/j.foodres.2019.108715 [DOI] [PubMed] [Google Scholar]
- Rodrigues, K. A. , Amorim, L. V. , Dias, C. N. , Moraes, D. F. , Carneiro, S. M. , & Carvalho, F. A. (2015). Syzygium cumini (L.) Skeels essential oil and its major constituent α‐pinene exhibit anti‐Leishmania activity through immunomodulation in vitro. Journal of Ethnopharmacology, 160, 32–40. 10.1016/j.jep.2014.11.024 [DOI] [PubMed] [Google Scholar]
- Ruan, Z. P. , Zhang, L. L. , & Lin, Y. M. J. M. (2008). Evaluation of the antioxidant activity of Syzygium cumini leaves. Molecules, 13(10), 2545–2556. 10.3390/molecules13102545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruma, O. C. J. A. J. N. A. S. (2016). Phytochemical screening of selected indigenous edible plants from the towns of Isabela, Philippines. Asian Journal of Natural & Applied Sciences, 5, 36–45. [Google Scholar]
- Ryu, B. , Kim, H. M. , Woo, J.‐H. , Choi, J.‐H. , & Jang, D. S. J. F. (2016). A new acetophenone glycoside from the flower buds of Syzygium aromaticum (cloves). Fitoterapia, 115, 46–51. 10.1016/j.fitote.2016.09.021 [DOI] [PubMed] [Google Scholar]
- Sakulnarmrat, K. , Fenech, M. , Thomas, P. , & Konczak, I. (2013). Cytoprotective and pro‐apoptotic activities of native Australian herbs polyphenolic‐rich extracts. Food Chemistry, 136(1), 9–17. 10.1016/j.foodchem.2012.07.089 [DOI] [PubMed] [Google Scholar]
- Samy, M. N. , Sugimoto, S. , Matsunami, K. , Otsuka, H. , & Kamel, M. S. (2014). One new flavonoid xyloside and one new natural triterpene rhamnoside from the leaves of Syzygium grande. Phytochemistry Letters, 10, 86–90. 10.1016/j.phytol.2014.08.009 [DOI] [Google Scholar]
- Santin, J. R. , Lemos, M. , Klein‐Júnior, L. C. , Machado, I. D. , Costa, P. , de Oliveira, A. P. , Tilia, C. , de Souza, J. P. , de Sousa, J. P. B. , Bastos, J. K. , & de Andrade, S. F. (2011). Gastroprotective activity of essential oil of the Syzygium aromaticum and its major component eugenol in different animal models. Naunyn‐Schmiedeberg's Archives of Pharmacology, 383(2), 149–158. 10.1007/s00210-010-0582-x [DOI] [PubMed] [Google Scholar]
- Saranya, J. , Eganathan, P. , Sujanapal, P. , & Parida, A. (2012). Chemical Composition of Leaf Essential Oil of Syzygium densiflorum Wall. ex Wt. & Arn.‐ A vulnerable tree species. Journal of Essential Oil Bearing Plants, 15(2), 283–287. 10.1080/0972060X.2012.10644048 [DOI] [Google Scholar]
- Sarvesan, R. , Eganathan, P. , Saranya, J. , & Sujanapal, P. (2015). Chemical composition and antimicrobial activity of leaf essential oil of syzygium grande (Wight) Walp. Journal of Essential Oil Bearing Plants, 18(3), 642–646. 10.1080/0972060X.2014.958572 [DOI] [Google Scholar]
- Sathyanarayanan, S. , Chandran, R. , Thankarajan, S. , Abrahamse, H. , & Thangaraj, P. (2018). Phytochemical composition, antioxidant and anti‐bacterial activity of Syzygium calophyllifolium Walp. fruit. Journal of Food Science and Technology, 55(1), 341–350. 10.1007/s13197-017-2944-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautron, C. , & Cock, I. J. P. C. (2014). Antimicrobial activity and toxicity of Syzygium australe and Syzygium leuhmannii fruit extracts. Pharmacognosy Communication, 4(1), 53–60. 10.5530/pc.2014.1.8 [DOI] [Google Scholar]
- Savitha, R. C. , Padmavathy, S. , & Sundhararajan, A. (2011). Invitro antioxidant activities on leaf extracts of Syzygium malaccense (L.) merr and perry. Ancient Science of Life, 30(4), 110. [PMC free article] [PubMed] [Google Scholar]
- Schoenfelder, T. , Warmlin, C. Z. , Manfredini, M. S. , Pavei, L. L. , Réus, J. V. , Tristão, T. C. , Fernandes, M. S. , & Costa‐Campos, L. (2010). Hypoglycemic and hypolipidemic effect of leaves from Syzygium cumini (L.) Skeels, Myrtaceae. in diabetic rats. Revista Brasileira De Farmacognosia, 20(2), 222–227. [Google Scholar]
- Sekar, M. , Halim, F. (2017). Formulation and evaluation of natural anti‐acne cream containing Syzygium samarangense fruits extract. Annual Research & Review in Biology, 17(3), 1–7. 10.9734/ARRB/2017/36467 [DOI] [Google Scholar]
- Senggunprai, L. , Kukongviriyapan, V. , Prawan, A. , & Kukongviriyapan, U. (2010). Consumption of Syzygium gratum promotes the antioxidant defense system in mice. Plant Foods for Human Nutrition, 65(4), 403–409. 10.1007/s11130-010-0200-6 [DOI] [PubMed] [Google Scholar]
- Setzer, M. C. , Setzer, W. N. , Jackes, B. R. , Gentry, G. A. , & Moriarity, D. M. (2001). The medicinal value of tropical rainforest plants from paluma, north Queensland. Australia. Pharmaceutical Biology, 39(1), 67–78. 10.1076/phbi.39.1.67.5944 [DOI] [Google Scholar]
- Sharma, R. , Kishore, N. , Hussein, A. , & Lall, N. (2013). Antibacterial and anti‐inflammatory effects of Syzygium jambos L. (Alston) and isolated compounds on acne vulgaris. BMC Complementary and Alternative Medicine, 13, 292. 10.1186/1472-6882-13-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, S.‐C. , & Chang, W.‐C. (2013). Hypotriglyceridemic and hypoglycemic effects of vescalagin from Pink wax apple [Syzygium samarangense (Blume) Merrill and Perry cv. Pink] in high‐fructose diet‐induced diabetic rats. Food Chemistry, 136(2), 858–863. 10.1016/j.foodchem.2012.08.037 [DOI] [PubMed] [Google Scholar]
- Shen, S.‐C. , Chang, W.‐C. , & Chang, C.‐L.‐J.‐I. (2012). Fraction from wax apple [Syzygium samarangense (Blume) Merrill and Perry] fruit extract ameliorates insulin resistance via modulating insulin signaling and inflammation pathway in tumor necrosis factor α‐treated FL83B mouse hepatocytes. International Journal of Molecular Sciences, 13(7), 8562–8577. 10.3390/ijms13078562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilpa, K. J. , & Krishnakumar, G. (2015). Nutritional, fermentation and pharmacological studies of Syzygium caryophyllatum (L.) Alston and Syzygium zeylanicum (L.) DC fruits. Cogent Food & Agriculture, 1(1), 2–13. 10.1080/23311932.2015.1018694 [DOI] [Google Scholar]
- Shilpa, K. J. , Krishnakumar, G. , & Sooryaprakash, S. (2014). Phytochemical composition, antioxidant, and antibacterial activities of two Syzygium spp. Journal of Herbs, Spices & Medicinal Plants, 20(1), 45–54. 10.1080/10496475.2013.821432 [DOI] [Google Scholar]
- Sidney, M. T. , Siyabonga, S. J. , & Kotze, B. A. (2015). The antibacterial and antidiarreal activities of the crude methanolic Syzygium cordatum [S. Ncik, 48 (UZ)] fruit pulp and seed extracts. Journal of Medicinal Plants Research, 9(33), 884–891. 10.5897/JMPR2015.5789 [DOI] [Google Scholar]
- Sidney, M. T. , Siyabonga, S. J. , & Kotze, B. A. J. J. O. M. P. R. (2015b). The antibacterial and antidiarreal activities of the crude methanolic Syzygium cordatum [S. Ncik, 48 (UZ)] fruit pulp and seed extracts. Journal of Medicinal Plants Research, 9(33), 884‐891. 10.5897/JMPR2015.5789 [DOI]
- Simirgiotis, M. J. , Adachi, S. , To, S. , Yang, H. , Reynertson, K. A. , Basile, M. J. , Gil, R. R. , Weinstein, I. B. , & Kennelly, E. J. (2008). Cytotoxic chalcones and antioxidants from the fruits of Syzygium samarangense (Wax Jambu). Food Chemistry, 107(2), 813–819. 10.1016/j.foodchem.2007.08.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinan, K. I. , Akpulat, U. , Aldahish, A. A. , Celik Altunoglu, Y. , Baloğlu, M. C. , Zheleva‐Dimitrova, D. , Gevrenova, R. , Lobine, D. , Mahomoodally, M. F. , Etienne, O. K. , Zengin, G. , Mahmud, S. , & Capasso, R. (2021). LC‐MS/HRMS analysis, anti‐cancer, anti‐enzymatic and anti‐oxidant effects of boerhavia diffusa extracts: A potential raw material for functional applications. Antioxidants, 10(12), 2003. 10.3390/antiox10122003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, J. P. , Kaur, A. , Singh, N. , Nim, L. , Shevkani, K. , Kaur, H. , & Arora, D. S. (2016). In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT ‐ Food Science and Technology, 65, 1025–1030. 10.1016/j.lwt.2015.09.038 [DOI] [Google Scholar]
- Sit, N. W. , Chan, Y. S. , Lai, S. C. , Lim, L. N. , Looi, G. T. , Tay, P. L. , Tee, Y. T. , Woon, Y. Y. , Khoo, K. S. , & Ong, H. C. (2018). In vitro antidermatophytic activity and cytotoxicity of extracts derived from medicinal plants and marine algae. Journal De Mycologie Médicale, 28(3), 561–567. 10.1016/j.mycmed.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Smeriglio, A. , Barreca, D. , Bellocco, E. , & Trombetta, D. (2017). Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. British Journal of Pharmacology, 174(11), 1244–1262. 10.1111/bph.13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeh, M. , Braun, M. S. , Krstin, S. , Youssef, F. S. , Ashour, M. L. , & Wink, M. (2016). Chemical Profiling of the Essential Oils of Syzygium aqueum, Syzygium samarangense and Eugenia uniflora and Their Discrimination Using Chemometric Analysis. Chemistry & Biodiversity, 13(11), 1537–1550. 10.1002/cbdv.201600089 [DOI] [PubMed] [Google Scholar]
- Sobeh, M. , Mahmoud, M. F. , Petruk, G. , Rezq, S. , Ashour, M. L. , Youssef, F. S. , El‐Shazly, A. M. , Monti, D. M. , Abdel‐Naim, A. B. , & Wink, M. (2018). Syzygium aqueum: A Polyphenol‐ Rich Leaf Extract Exhibits Antioxidant, Hepatoprotective. Pain‐Killing and Anti‐inflammatory Activities in Animal Models, 9, 566. 10.3389/fphar.2018.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeh, M. , Youssef, F. S. , Esmat, A. , Petruk, G. , El‐Khatib, A. H. , Monti, D. M. , Ashour, M. L. , & Wink, M. (2018). High resolution UPLC‐MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4‐induced hepatic damage. Food and Chemical Toxicology, 113, 145–153. 10.1016/j.fct.2018.01.031 [DOI] [PubMed] [Google Scholar]
- Soh, W. , Nair, K. J. T. G. S. , Cumini, S. , & Species, O. U. (2017). Taxonomy of Syzygium, 1–5.
- Sowjanya, K. , Swathi, J. , Narendra, K. , & Satya, A. K. J. I. J. N. P. S. (2013). A review on phytochemical constituents and bioassay of Syzygium cumini . International Journal of Natural Product Science, 3(2), 1–11. [Google Scholar]
- Stewart, P. , Boonsiri, P. , Puthong, S. , & Rojpibulstit, P. (2013). Antioxidant activity and ultrastructural changes in gastric cancer cell lines induced by Northeastern Thai edible folk plant extracts. BMC Complementary and Alternative Medicine, 13, 60. 10.1186/1472-6882-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse, S. A. , & Wubneh, Z. B. (2017). Antimalarial activity of Syzygium guineense during early and established Plasmodium infection in rodent models. BMC Complementary and Alternative Medicine, 17(1), 21. 10.1186/s12906-016-1538-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir, H. U. , Sarfraz, R. A. , Ashraf, A. , & Adil, S. (2016). Chemical composition and antidiabetic activity of essential oils obtained from two spices (Syzygium aromaticum and Cuminum cyminum). International Journal of Food Properties, 19(10), 2156–2164. 10.1080/10942912.2015.1110166 [DOI] [Google Scholar]
- Tallei, T. E. , Niode, N. J. , Idroes, R. , Zidan, B. , Mitra, S. , Celik, I. , Nainu, F. , Ağagündüz, D. , Emran, T. B. , & Capasso, R. (2021). A Comprehensive review of the potential use of green tea polyphenols in the management of COVID‐19. Evidence‐Based Complementary and Alternative Medicine, 2021, 1–13. 10.1155/2021/7170736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiello, C. S. , Adami, E. R. , de Oliveira, N. M. T. , Acco, A. , Iacomini, M. , & Cordeiro, L. M. C. (2018). Structural features of polysaccharides from edible jambo (Syzygium jambos) fruits and antitumor activity of extracted pectins. International Journal of Biological Macromolecules, 118(Pt B), 1414–1421. 10.1016/j.ijbiomac.2018.06.164 [DOI] [PubMed] [Google Scholar]
- Tareq, A. M. , Farhad, S. , Uddin, A. N. , Hoque, M. , Nasrin, M. S. , Uddin, M. M. R. , Hasan, M. , Sultana, A. , Munira, M. S. , Lyzu, C. , Moazzem Hossen, S. M. , Ali Reza, A. , & Emran, T. B. (2020). Chemical profiles, pharmacological properties, and in silico studies provide new insights on Cycas pectinata. Heliyon, 6(6), e04061. 10.1016/j.heliyon.2020.e04061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuiwawa, S. , Craven, L. A. , Sam, C. , & Crisp, M. D. (2013). The genus Syzygium (Myrtaceae) in Vanuatu. Blumea‐Biodiversity, Evolution and Biogeography of Plants, 58(1), 53–67. [Google Scholar]
- Tukiran, T. , Mahmudah, F. , Hidayati, N. , & Shimizu, K. J. M. (2016). Gallic acid: A phenolic acid and its antioxidant activity from stem bark of chloroform extracts of syzygium litorale (blume) amshoff (myrtaceae). Molekul, 11(2), 180–189. 10.20884/1.jm.2016.11.2.215 [DOI] [Google Scholar]
- Uddin, M. , Ali Reza, A. , Abdullah‐Al‐Mamun, M. , Kabir, M. S. , Nasrin, M. , Akhter, S. , Arman, M. S. I. , & Rahman, M. A. (2018). Antinociceptive and Anxiolytic and Sedative Effects of Methanol Extract of Anisomeles indica: An Experimental Assessment in Mice and Computer Aided Models. Frontiers in Pharmacology, 9, 246. 10.3389/fphar.2018.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulla, A. , Alam, M. A. , Sikder, B. , Sumi, F. A. , Rahman, M. M. , Habib, Z. F. , Mohammed, M. K. , Subhan, N. , Hossain, H. , & Reza, H. M. (2017). Supplementation of Syzygium cumini seed powder prevented obesity, glucose intolerance, hyperlipidemia and oxidative stress in high carbohydrate high fat diet induced obese rats. BMC Complementary and Alternative Medicine, 17(1), 289. 10.1186/s12906-017-1799-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschaeve, L. , Kestens, V. , Taylor, J. L. , Elgorashi, E. E. , Maes, A. , Van Puyvelde, L. , & Van Staden, J. (2004). Investigation of the antimutagenic effects of selected South African medicinal plant extracts. Toxicology in Vitro, 18(1), 29–35. 10.1016/s0887-2333(03)00131-0 [DOI] [PubMed] [Google Scholar]
- Vignesh, R. , Puhazhselvan, P. , Sangeethkumar, M. , Saranya, J. , Eganathan, P. , & Sujanapal, P. (2013). GC‐MS analysis, antimicrobial, scavenging ability and cytotoxic activity of leaves of Syzygium calophyllifolium walp. Journal of Biologically Active Products from Nature, 3(2), 121–129. 10.1080/22311866.2013.817741 [DOI] [Google Scholar]
- Vuong, Q. V. , Hirun, S. , Chuen, T. L. K. , Goldsmith, C. D. , Bowyer, M. C. , Chalmers, A. C. , Phillips, P. A. , & Scarlett, C. J. (2014). Physicochemical composition, antioxidant and anti‐proliferative capacity of a lilly pilly (Syzygium paniculatum) extract. Journal of Herbal Medicine, 4(3), 134–140. 10.1016/j.hermed.2014.04.003 [DOI] [Google Scholar]
- Walean, M. , Melpin, R. , Rondonuwu, M. , Pinontoan, K. , Maliangkay, H. , & Astriani, M. (2020). Phytochemical screening and biological activities of pakoba (Syzygium luzonense) stem bark ethanol extract. Biodiversitas Journal of Biological Diversity, 21, 2377–2382. 10.13057/biodiv/d210606 [DOI] [Google Scholar]
- Wathsara, H. P. T. , Weeratunge, H. D. , Mubarak, M. N. A. , Godakumbura, P. I. , & Ranasinghe, P. (2020). In Vitro Antioxidant and Antidiabetic Potentials of Syzygium caryophyllatum L Alston. Evidence‐Based Complementary and Alternative Medicine, 2020, 9529042. 10.1155/2020/9529042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjajakusuma, E. C. , Jonosewojo, A. , Hendriati, L. , Wijaya, S. , Ferawati, Surjadhana, A. , Sastrowardoyo, W. , Monita, N. , Muna, N. M. , Fajarwati, R. P. , Ervina, M. , Esar, S. Y. , Soegianto, L. , Lang, T. , & Heriyanti, C. (2019). Phytochemical screening and preliminary clinical trials of the aqueous extract mixture of Andrographis paniculata (Burm. f.) Wall. ex Nees and Syzygium polyanthum (Wight.) Walp leaves in metformin treated patients with type 2 diabetes. Phytomedicine, 55, 137–147. 10.1016/j.phymed.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Widodo, P. (2011). New nomenclature in Syzygium (Myrtaceae) from Indonesia and Its Vicinities. Reinwardtia, 13, 235–240. [Google Scholar]
- Widyawati, T. , Yusoff, N. A. , Asmawi, M. Z. , & Ahmad, M. (2015). Antihyperglycemic effect of methanol extract of Syzygium polyanthum (Wight.) leaf in streptozotocin‐induced diabetic rats. Nutrients, 7(9), 7764–7780. 10.3390/nu7095365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, A. K. , Saraswat, S. , Sirohi, P. , Rani, M. , Srivastava, S. , Singh, M. P. , & Singh, N. K. (2017). Antimicrobial action of methanolic seed extracts of Syzygium cumini Linn. on Bacillus subtilis. AMB Express, 7(1), 196. 10.1186/s13568-017-0500-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugandhar, P. , Haribabu, R. , & Savithramma, N. (2015). Synthesis, characterization and antimicrobial properties of green‐synthesised silver nanoparticles from stem bark extract of Syzygium alternifolium (Wt.) Walp. 3 Biotech, 5(6), 1031–1039. 10.1007/s13205-015-0307-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugandhar, P. , & Savithramma, N. (2017). Spectroscopic and chromatographic exploration of different phytochemical and mineral contents from Syzygium alternifolim (Wt.) Walp. an endemic, endangered medicinal tree taxon. Journal of Applied Pharmaceutical Science, 7, 73–085. 10.7324/JAPS.2017.70110 [DOI] [Google Scholar]
- Zhang, Y. , Wang, Y. , Zhu, X. , Cao, P. , Wei, S. , & Lu, Y. (2017). Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microbial Pathogenesis, 113, 396–402. 10.1016/j.micpath.2017.10.054 [DOI] [PubMed] [Google Scholar]


