Abstract
Aging represents the single greatest risk factor for chronic diseases, including osteoporosis, a skeletal fragility syndrome that increases fracture risk. Optimizing bone strength throughout life reduces fracture risk. Factors critical for bone strength include nutrition, physical activity, and vitamin D status, whereas unhealthy lifestyles, illnesses, and certain medications (eg, glucocorticoids) are detrimental. Hormonal status is another important determinant of skeletal health, with sex steroid concentrations, particularly estrogen, having major effects on bone remodeling. Aging exacerbates bone loss in both sexes and results in imbalanced bone resorption relative to formation and is associated with increased marrow adiposity, osteoblast/osteocyte apoptosis, and accumulation of senescent cells. The mechanisms underlying skeletal aging are as diverse as the factors that determine the strength (and thus fragility) of bone. This review updates our current understanding of the epidemiology, pathophysiology, and treatment of osteoporosis and provides an overview of the underlying hallmark mechanisms that drive skeletal aging.
BACKGROUND
In our recent evolutionary past, population growth and human life expectancy have increased dramatically in developed countries,1 and these trends are projected to continue.2 With longer lifespans less shaped by natural selection, however, the period of disease-free life (ie, healthspan) has not kept pace, creating challenges humans remain poorly equipped to handle including an enormous burden of late-life morbidity due to age-related diseases and chronic morbidities that often co-exist in the elderly. These include cancers, cardiovascular disease, renal and neurodegenerative diseases, metabolic dysfunction, frailty, pulmonary fibrosis, osteoarthritis, and osteoporosis, among numerous others.3
One of the most common diseases associated with aging is osteoporosis (meaning “porous bone”), a skeletal fragility disorder characterized by reduced bone strength. Bone strength reflects both bone mineral density (BMD) and bone quality, with reductions in bone strength contributing to age-related morbidity via increasing susceptibility to fragility fractures. Despite a range of therapeutic options that are safe, effective and approved for the prevention or treatment of osteoporosis, including oral or intravenous bisphosphonates, estrogen, raloxifene, denosumab, teriparatide/abaloparatide and romosozumab, the majority of individuals with osteoporosis remain untreated. In fact, the proportion of persons receiving appropriate osteoporosis therapy following a hip fracture has actually decreased in recent years.4 This disconnect stems in part from concerns for rare side effects associated with osteoporosis-specific drugs such as atypical femoral fractures and osteonecrosis of the jaw. Sadly, this reluctance to initiate and/or adhere to osteoporosis therapies has contributed to a rising prevalence of osteoporosis and an increased fracture burden among the elderly.
Bone is a unique tissue that serves paradoxical functions across the lifespan. For example, it must be light enough to permit movement yet strong to resist trauma. In the elderly, the most common form of major trauma results from the impact associated with falling. When an applied force exceeds a bone’s strength, structural failure in the form of a fracture will occur. Thus, optimizing bone strength reduces fracture risk. Although partly dependent on the amount of bone acquired during development and growth, skeletal strength is a function of its mass, material (both matrix and mineral), and both macro- and microarchitecture (eg, trabecular connectively, cortical porosity). As reviewed in more detail below, numerous factors contribute to maximal bone strength across the lifespan including genetics, sex steroid levels (particularly estrogen) in both women and men, nutrition, physical activity and vitamin D status, whereas other factors such as unhealthy lifestyles, illnesses, and certain medications (eg, glucocorticoids) can be detrimental to bone strength. As an example of the pivotal role for estrogen in maintenance of skeletal health, the rapid decline in estrogen levels that herald menopausal onset are paralleled by accelerated and progressive bone loss. Coincident with such hormonal changes, aging itself further exacerbates bone loss in both sexes, and the underlying mechanisms mediating the pathogenesis of skeletal aging are as diverse as the factors that determine the strength (and thus fragility) of bone.
Another issue of growing concern in the elderly is polypharmacy, which has been shown to be an independent risk factor for hip fractures.5 Notably, most drug discovery efforts for osteoporosis and other aging-associated diseases have historically followed the mantra of treating each disease as a separate entity (ie, one disease, one drug), leading to a greater risk for adverse drug interactions in elderly populations. However, an approach focused on developing interventions to delay or treat osteoporosis as well as other aging-associated diseases as a group has recently gained momentum.6 In addition to efforts to optimize physical activity and nutrition, an approach to therapeutically targeting basic mechanisms of aging may be feasible, as recent pre-clinical models have shown healthspan extension in studies of aged animals.7 Fundamental to addressing these issues is additional understanding of the pathogenesis of skeletal aging. Herein, we provide an update on the current understanding of the epidemiology, optimal clinical assessment, pathophysiology, and treatments for osteoporosis, as well as an overview of the underlying hallmark mechanisms that drive the aging process across tissues, including bone.
Epidemiology of osteoporosis and fractures
Among adults aged 50 years and older in the United States (U.S.), BMD measurements obtained at the femoral neck and lumbar spine in the National Health and Nutrition Examination Survey (NHANES) 2005-2010 U.S. Census population counts suggest that there are an estimated 10.2 million (10.3%) Americans with osteoporosis, and that 54% (53.6 million) of U.S. adults aged 50 years and older have osteopenia.8 Similar patterns have been observed across various racial and ethnic groups as well as in other developed countries.9 Consistent with the incidence and prevalence of the disease, osteoporosis-related fractures are more common in women than men, and it is generally estimated that 1 in 3 women and 1 in 5 men aged 50 years will suffer an osteoporotic fracture in their remaining lifetime.9–12 With life expectancy continuing to increase, these demographic trends are only expected to rise. Therefore, identifying at-risk persons through proper clinical assessments and treating those individuals at greatest risk for fragility fractures is increasingly important.
Clinical assessment of skeletal aging
Assessing musculoskeletal health in older adults involves the careful evaluation of multiple layers of clinical, radiographic and laboratory testing to elucidate the intricate interplay between bone, muscle, and fat. Indeed, the evaluation of age-related bone loss is generally best performed alongside evaluations of frailty and sarcopenia, as well as the assessments of potential physical or cognitive dysfunction, and additional social determinants of health.
Assessment of skeletal fragility
The ultimate indicator of skeletal fragility is the incidence of a fragility fracture. A fragility fracture is defined as any fracture that occurs following a fall from standing height or less. Sites of injury that predict the incidence of subsequent fractures include fractures at the hip, spine, or distal forearm. Vertebral fractures that are clinically “silent” and therefore discovered incidentally on radiologic imaging, are similarly considered to reflect underlying bone fragility.
In addition to age, numerous factors can contribute to bone loss and increase the risk of fractures. These include race and ethnicity, lifestyle factors (smoking, alcohol, etc.), endocrine disorders (hyperparathyroidism, hypercortisolism, etc.), genetic disorders (cystic fibrosis, etc.), and medications (glucocorticoids, anticonvulsants, etc.).13
Surrogate markers and clinical tools have been developed to identify patients at risk for fracture and who would potentially benefit from intervention. Validated tools that identify common risk factors are available and can provide risk estimates for hip and major osteoporotic fractures. Commonly utilized tools include the fracture risk assessment tool (FRAX®) (https://www.sheffield.ac.uk/FRAX/) and the Garvan Institute bone fracture risk calculator (https://www.garvan.org.au/promotions/bone-fracture-risk/calculator/index.php). While FRAX® is currently most widely used in clinical practice, the Garvan tool may be have additional value for evaluating fracture risk in patients with recurrent falls and factures.14
Using low dose X-ray beams, dual energy X-ray absorptiometry (DXA) provides an estimate of bone mineral content and areal BMD. Low BMD has been associated with progressively increased risk of fragility fractures. In postmenopausal women and men 50 years or older, BMD is commonly expressed in terms of a T-score, which represents the standard deviation of an individual’s BMD from the young adult mean BMD. The World Health Organization defines a T-score ≥ −1.0 as normal, a T-score between −1.0 and −2.5 as osteopenia, and a T-score ≤ −2.5 as osteoporosis. DXA can also provide comprehensive vertebral morphometric measurements that indicate the presence of a vertebral compression deformity or fracture.
The National Osteoporosis Foundation (NOF) recommends measurement of BMD in: (i) women age 65 and older and men age 70 and older; (ii) postmenopausal women and men above age 50 with additional clinical risk factors; and (iii) postmenopausal women and men age 50 and older who have fractured in adulthood.13
Laboratory testing also aids in the assessment of skeletal fragility given that as many as a third of postmenopausal women and 50-80% of men with osteoporosis may have a previously unrecognized metabolic bone disease.15,16 Testing should be individualized but may include serum electrolytes (such as calcium and phosphorus), vitamin D levels, and kidney function. Serum and urine markers of bone turnover [eg, procollagen type 1 N-terminal propeptide (P1NP) and C-telopeptide of type I collagen (CTx)] may provide insight into the extent of a patient’s bone formation and resorption, respectively.
Assessment of falls, frailty, and sarcopenia
Broadly defined, sarcopenia is the progressive loss of muscle mass and function. It is closely associated with frailty and an increased risk of falls and fractures as well as other poor health outcomes including mortality. In order to diagnose sarcopenia, measurements of muscle mass, muscle strength and physical performance are essential.
A number of tools are available in the research setting but have limited clinical availability; cost incurred by some of these tools further limits their widespread use.17 BMI and body circumference are not considered reliable to evaluate for sarcopenia. Total body DXA can provide estimates of lean muscle mass. Gait speed or Timed Up and Go (TUG) are easy tools to replicate in the clinical setting and can provide valuable physical performance assessment.18,19 In the TUG test, the patient is asked to stand up from a sitting position and walk for 10 feet (3 meters) while being timed. Grip strength has also been shown to have significant clinical relevance but requires a calibrated dynamometer and consistent measurement environment.20,21
Commonly used fracture risk assessment tools (eg, the FRAX® calculator) do not fully account for frailty or sarcopenia and may thus underestimate fracture risk in older adults.22 The Fracture Risk Assessment in Long-term Care (FRAiL) calculator is a recently developed tool that relies on a host of clinical factors, including physical performance and muscle function, to predict the two-year risk for hip fractures in adults residing in nursing homes.23
Fall risk is typically evaluated by inquiring about a history of falls, particularly within the past 12 months, as recent falls are associated with an increased risk of subsequent falls.24 Additional details about cognitive dysfunction, sensory deficits, and polypharmacy have all been associated with a higher risk of falls and hip fractures.25–29
Changes in BMD, skeletal microarchitecture, material properties, and strength with aging
There has been considerable progress over the past few decades in our understanding of the patterns of changes in BMD and additional measurable components of bone strength. As noted above, bone health is most frequently assessed in clinical practice by DXA, which permits assessments of areal BMD. Changes in BMD throughout the lifespan from growth to aging are shown for women and men in Figure 1. Because of the widespread availability of DXA, the majority of emphasis has focused on bone mass and BMD, although given significant limitations of DXA,30 more recent research has relied on quantitative computed tomography (QCT), which has advantages for measuring volumetric BMD and bone structure as well as for separation of trabecular (more metabolically active) versus cortical (structurally relevant) compartments. Longitudinal studies using these tools have demonstrated lifetime losses of trabecular bone of ~45% in men and ~55% in women, with cortical bone losses of ~18% in men and ~25% in women.31 Bone loss is accelerated in women around the time of menopause, with losses of ~20-30% trabecular (spine) and ~5-10% cortical (distal radius) bone over the 6-10 year peri-menopausal transition.31,32 In both sexes, continuous bone loss occurs with aging unless pharmacologic intervention is undertaken.
Figure 1.
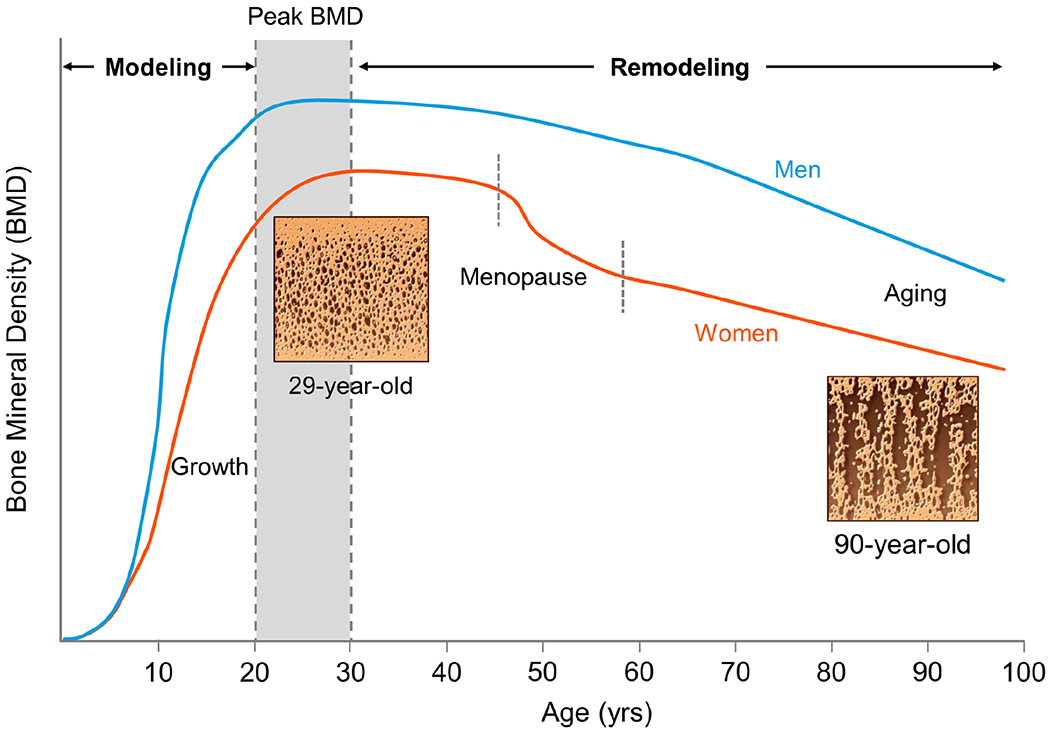
Skeletal changes in BMD throughout the female and male lifespan, including representative micrographs of cadaveric bone from a 29-year-old and 90-year-old women showing the progressive loss of bone with aging. BMD, bone mineral density; yrs, years.
Bone mass and BMD, however, are clearly not the only determinants of fracture risk. As an example, in the setting of equivalent femoral neck BMD T-scores as determined by DXA, older individuals are typically at significantly higher risk for fracture when compared to younger persons.33 In addition to aging, increased fracture risk independent of BMD has also been established in specific populations, including patients treated with glucocorticoids34 and those with type 2 diabetes mellitus (T2DM).35 These observations thus highlight the importance of assessing aspects of bone quality, including the material properties and microarchitecture of bone that have been shown to be altered in, for example, patients with T2DM.36,37 However, there is currently a paucity of data on longitudinal changes in these parameters that occur with aging. Nevertheless, with the recent availability of high-resolution peripheral QCT (HRpQCT) to measure bone microarchitecture in vivo, BMD-independent effects of aging have been shown. In one study of young versus older individuals with DXA-matched areal BMD, similar trabecular bone microarchitectural parameters were seen at the distal radius, but cortical porosity was significantly higher in the older subjects.38 Thus, aspects of bone quality are likely to at least partly explain the BMD-independent higher fracture risk in elderly populations.
Changes in bone remodeling with aging
Throughout life, the skeleton is a highly metabolically active organ that undergoes continuous bone remodeling with removal of old and damaged bone by osteoclasts followed by self-renewal and repair by osteoblasts which lay down new bone matrix. The actions of osteoclasts and osteoblasts are both spatially and temporally coordinated, with this coordination at least in part overseen by osteocytes as well as by an array of both local and systemic factors released by various cell types to ultimately sculpt the unique composition and architecture of the skeleton. At the cellular level as shown in Figure 2, remodeling occurs via teams of short-lived cells comprising bone multicellular units (BMUs) that constitute three consecutive phases: i) resorption, when osteoclasts digest old or damaged bone; ii) reversal – when mononuclear cells invade the space; and iii) formation – when osteoblasts are recruited to the site of resorption to fill in new bone until the excavated cavity is completely replaced.39 On a microscopic level, these remodeling cycles occur continuously throughout the skeleton, adjusting skeletal mass, size, and shape to meet mechanical demands, respond to stress or injury, and to repair the continuous accumulation of microdamage that occurs with time. Collectively, these functions result from the complex interplay of cells in the bone microenvironment. However, around midlife in women, and later in men, this normally balanced bone remodeling process becomes unbalanced – ie, increased resorption occurs along with insufficient formation, ultimately resulting in a net loss of bone.
Figure 2.
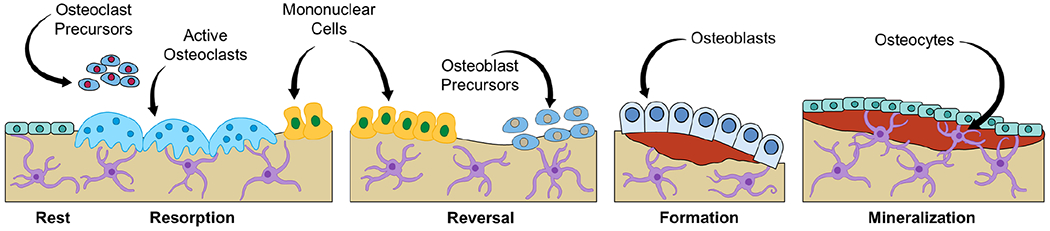
The bone remodeling cycle of resorption, reversal, and formation. Osteoclast and osteoblast precursors are recruited from the marrow to become active osteoclasts and osteoblasts on bone surfaces where they arrange themselves into temporary structures termed BMUs that execute bone remodeling in coordination with the actions of matrix-embedded osteocytes. BMUs, basics multicellular units.
Old Bone
With aging, this fundamental remodeling imbalance drives bone loss and structural decay in both sexes. If this state of negative bone balance remains uncorrected (eg, via pharmacological intervention), bone loss will continue from trabecular, endocortical, and intracortical surfaces, eventually resulting in an aged osteoporotic skeleton.40 Characteristics of osteoporotic bone include loss of trabecular connectivity, thinning or complete removal of trabeculae, endocortical bone loss resulting in cortical thinning, as well as increased remodeling within Haversian canals resulting in increased cortical porosity.41 Much of this bone loss reflects aging-associated deficits in osteoblast-mediated bone formation. For example, mean wall trabecular thickness, a surrogate measure of the work done by osteoblasts, decreases substantially with aging in both women and men.42 It should be noted, however, that although there is a fairly steady decline in circulating biochemical markers of bone formation in men with aging, higher circulating bone formation markers following menopausal onset are generally seen in women, reflecting higher bone turnover due to the coordinated coupling that occurs between osteoclasts which remove bone and osteoblasts which work to replace bone within these excavated spaces.43,44 Despite this increased bone turnover, however, because bone resorption in postmenopausal women remains higher relative to formation at a cellular level, a negative bone balance ensues. Thus, aging is associated with defective bone formation in both sexes.
The adult skeleton comprises ~20% trabecular and ~80% cortical bone. Given that loss of trabecular bone generally occurs both earlier and more rapidly than cortical bone, the proportion of bone loss that is trabecular effectively decelerates with aging, leading to an inherent effective acceleration of cortical bone loss that ultimately dominates with advancing age.45,46 The aging-associated cortical bone loss may contribute to the greater prevalence of fractures, including a higher proportion of non-vertebral fractures, among the elderly.9–12 Furthermore, bone loss at specific skeletal locations (eg, at the femoral neck and distal forearm) likely increases the risk for fracture at those sites relative to others. Collectively, loss of both trabecular and cortical bone with aging contributes to both reduced bone quality and bone strength, ultimately placing older individuals at higher risk for fractures.
Bone marrow adiposity
The marrow cavity within bone is the only location in humans where bone and fat coexist in adjacent proximity to one another.47 Notably, whereas osteoblast numbers decline with advancing age and ultimately lead to reduced bone formation,42 aging is also associated with bone marrow adipose tissue (BMAT) accumulation.48 Within the marrow cavity, adipocytes accumulate along the endosteal surface and surrounding regions of the appendicular skeleton with both aging and osteoporosis, but also in other settings including anorexia nervosa, diabetes, caloric restriction, and skeletal unloading.49–52 In addition, exposure to irradiation or chemotherapy can result in a profound, rapid accumulation of BMAT both locally and at skeletal sites distant to where the initial exposure occurred.53,54 Therefore, the physiological and pathophysiological roles of BMAT are diverse and context-specific, and remain incompletely understood.
Despite such unknowns, it is now well-recognized that BMAT accumulates within the marrow cavity at both appendicular and axial osteoporotic skeletal sites with advancing age and that this inverse relationship of decreased bone mass and increased marrow adipose is a hallmark feature of skeletal aging. Studies in both animals and humans have begun to shed light on how and why this paradox may exist. For example, because both osteoblasts and adipocytes originate from the same pool of pluripotent mesenchymal stem cell (MSC) progenitors,55 their ultimate lineage fate may be altered by one or more fundamental mechanisms of aging (as reviewed below). Indeed, it is possible that the marked decrease in the number of osteoblasts found on bone surfaces in old age is due to an age-related change in lineage allocation toward one which favors the differentiation of MSCs into adipocytes.47 However, it remains unclear how and precisely from where bone marrow adipocytes arise. Thus, a simple dichotomous tradeoff between fat and bone is unlikely to be the only explanation for reduced bone formation with aging. An alternative potential mechanism is that dwindling MSC progenitor pools and/or insufficient activation or defective differentiation of these progenitors may underlie this observation. Despite numerous remaining questions, therapeutic interventions directed at modulating the bone marrow niche as well as specific cell populations within it may yet prove beneficial for slowing or perhaps even reversing the age-related defects in bone formation.
Osteoblast and osteocyte apoptosis and age-related changes in the osteocyte canicular network
Throughout life, all normal nucleated cells experience various internal and external stressful stimuli (eg, DNA damage, oxidative stress, oncogenic insults, etc.) and in response their default fate is apoptosis, although a cell can undergo alternative fates, such as cellular senescence (as reviewed below and in more detail elsewhere56). However, when the signals to die overpower the various mechanisms of survival, programed cell death (ie, apoptosis) ensues. Because bone is a tissue that must constantly self-renew, apoptosis is necessary for the regeneration of new cells and to initiate bone remodeling. For example, the number of osteoblasts on bone surfaces and their lifespan, which is only ~12 days in mice and ~150 days in humans,57,58 is regulated by a multitude of factors in the bone microenvironment that fine tune both their birth from MSCs and their death by apoptosis.59 Indeed, while a subset of osteoblasts will differentiate into either lining cells or osteocytes, the majority (~60-90%) undergo apoptosis.60,61 While aging contributes to increased osteoblast apoptosis and reduced osteoblast numbers,57,58 osteocytes by contrast are long-lived cells that survive under normal circumstances essentially until their local environment is remodeled; however, when they eventually do undergo apoptosis, their empty lacunae hold the remnants of their degraded DNA. Osteocyte apoptosis results in the recruitment of osteoclasts to the vicinity to thereby initiate remodeling and is exacerbated with, for example, glucocorticoid excess,62 unloading63, and aging.64,65 Under these conditions osteocyte apoptosis contributes to the disruption of the osteocyte lacuno-canalicular system, including loss of osteocyte connectivity as well as deficient pericellular fluid flow, and results in deficient bone quality.64,65 By contrast, normal physiological strains imparted from mechanical loading are important toward the generation of survival signals (eg, nitric oxide [NO], prostaglandins [PGE2], and WNTs) that prevent osteocyte apoptosis.66–69 In addition, autophagy is an essential intracellular recycling pathway whereby, for example, during periods of caloric restriction (ie, fasting), misfolded proteins and damaged organelles are escorted for lysosomal degradation to thereby maintain cell survival, which suggests that impaired osteocyte autophagy that occurs with glucocorticoid excess, skeletal aging, or obesity-associated inflammation may exacerbate osteocyte apoptosis.70–72 Therefore, adequate exercise and healthy dietary habits represent important lifestyle choices to maintain the integrity of the mechanosensory osteocyte canicular network and thus bone health in the elderly.
Fundamental aging mechanisms that contribute to the pathogenesis of skeletal aging
Rather than living in a disease-free state or suffering from only a single age-related disease, the elderly typically experience multi-morbidity. It is increasingly recognized that this is at least in part due to a myriad of fundamental biological aging mechanisms that drive many (if not all) chronic diseases of aging.7 Importantly, when these biological underpinnings are triggered in an age-dependent manner, chronic diseases tend to accumulate in a groupwise fashion. Therefore, an improved knowledge of the underlying aging processes shared across tissues and systems is fundamental for any future efforts to therapeutically target these mechanisms in order to delay the appearance and/or progression of age-related diseases in unison. Accordingly, a major current goal within the field of Geroscience is to develop interventions that slow aging in order to improve quality of life via extension of years of health while simultaneously compressing years spent with multi-morbidity.
In a seminal review,7 nine fundamental mechanisms of aging that cause functional decline were identified. Importantly, all are both shared across various tissues and also common to mammalian organisms. These hallmarks of aging include: genomic instability, epigenetic alterations, telomere attrition, loss of proteostasis, cellular senescence, mitochondrial dysfunction, dysregulated nutrient sensing, stem cell exhaustion, and altered intercellular communication.7 Each of these aging hallmarks meets the following criteria: i) manifests during aging; ii) experimental aggravation accelerates aging; and iii) experimental amelioration slows aging and extends healthspan.7 It should be noted that these hallmark mechanisms of aging are linked in that either aggravation or amelioration of one hallmark could in theory accelerate or alleviate other hallmarks, respectively. Importantly, there is now substantial evidence, for example in old mice, demonstrating that therapeutically targeting fundamental aging mechanisms can collectively delay the onset of or alleviate the progression of numerous age-related diseases.
With regard to bone, research on the fundamental biology of the aging skeleton has accelerated rapidly over the past few decades. An interesting outcome of this surge is the now ample evidence that each of the identified hallmarks of aging is present within bone, that each can drive skeletal aging, and that each fundamental mechanism is manifest in old bone to varying degrees.6 As is the case with aging in other tissues, primary hallmarks of bone aging include genomic instability, telomere attrition, epigenetic alterations, and loss of proteostasis (eg, autophagy), each of which universally acts as a stressor to induce damage to a variety of cell types within the bone microenvironment.6 In particular, long-lived cells such as osteocytes in bone appear to be uniquely susceptible to these age-associated insults.73,74 In contrast, the antagonistic hallmarks of aging (ie, dysregulated nutrient sensing, mitochondria dysfunction, and cellular senescence) evolved to limit cellular damage. While they are effective in doing so initially, with advancing age they become exacerbated and in turn deleterious.7 As an example, cells in the bone microenvironment that incur damage in the form of primary aging hallmarks may become senescent as a compensatory mechanism to prevent malignant transformation.75 However, as senescent cells accumulate in the aged bone microenvironment, which has been demonstrated in old mice and humans,76 these damaged cells themselves may further contribute to skeletal aging via their release of senescence-associated factors (eg, inflammatory cytokines, chemokines, etc.) which act as pro-resorptive factors or potentially contribute to alter the lineage allocation of MSC progenitors into BMAT.73 Both are examples of the integrative hallmarks of aging – ie, altered intercellular communication and stem cell exhaustion, that are signatures of skeletal aging.6 Therefore, safely interfering with fundamental mechanisms of aging may represent new therapeutic strategies for age-related chronic diseases, including for example targeting cellular senescence in old age to prevent osteoporosis,77 and extending healthspan.78
In summary, aging exacerbates bone loss in both sexes and results in imbalanced bone resorption relative to formation and is associated with increased marrow adiposity, osteoblast/osteocyte apoptosis, and accumulation of senescent cells (Figure 3).
Figure 3.
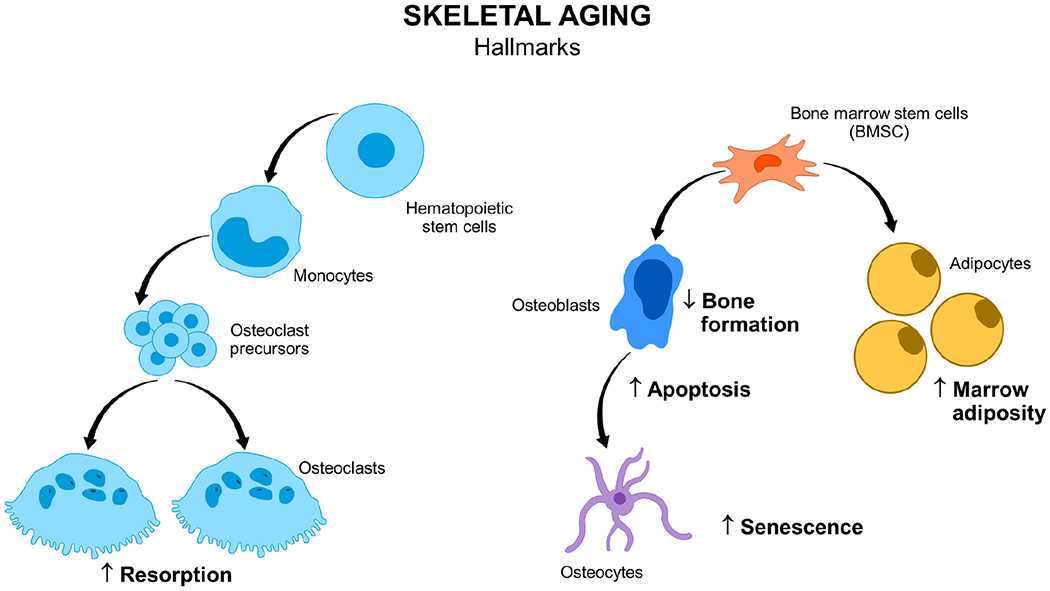
Hallmarks of skeletal aging in old bone. BMSC, bone marrow stem cell.
Targeting fundamental aging mechanisms in humans
Because of the mounting evidence from preclinical studies that several age-related diseases can be alleviated by therapeutically targeting fundamental mechanisms of aging,7 potential interventions to test this approach in humans are gaining momentum. For example, because senescent cells accumulate with age in various tissues and at anatomical sites of disease,56 one logical strategy has been to utilize drugs that selectively kill senescent cells (ie, “senolytics”), which are in various stages of clinical development.79 Indeed, given that pharmacological elimination of senescent cells using first generation senolytics prevents aspects of skeletal aging with apparent advantages over anti-resorptive therapy in old mice,73,77,78 and also improves physical function and resilience,80 the aim of an ongoing randomized controlled trial (ClinicalTrials.gov NCT04313634) in older women with a high cellular senescence burden is to translate these novel findings in mice to humans. Thus, depending on the success of similar ongoing and future trials, drugs that target fundamental aging mechanisms may one day complement currently approved osteoporosis-specific therapies to simultaneously prevent or delay multiple age-related diseases.
Management of skeletal fragility
Efforts to prevent the age-related increase in fractures are aimed at bone loss as well as addressing underlying chronic illnesses that may contribute to skeletal fragility and sarcopenia. Importantly, the “osteoporosis prescription” should be individualized to each patient’s goals and take into account comorbidities and psychosocial factors. In addition, identifying access to community resources such as geriatric-friendly fitness centers, transportation, and affordable home modification strategies are likely to contribute significantly to the success of the management plan.81
Physical activity
Exercise programs designed to strengthen core muscles and improve balance have consistently been shown to reduce the risk of falls.82,83 In addition, resistance training, including weight-bearing and medium-to-high impact exercise, for 12-18 months can modestly improve BMD.83–86 High intensity progressive training, including in a supervised multimodal program, can similarly lead to improvements in BMD and physical performance.87–89 In older adults, exercise programs should be personalized to be safe, sustainable, and reproducible.90,91 For instance, low intensity exercise such as walking, sitting, and standing exercises can be progressively increased as tolerated by patients (Table 1).
Table 1:
Mayo Clinic Osteoporosis Exercise Chart
| Low Intensity Exercises |
|---|
| 1. Walking and standing posture: |
| a. Walking purpose: To strengthen legs and heart and improve balance. |
| b. Standing posture purpose: To learn to stand properly which will improve posture |
| 2. Wall arch: to stretch shoulders and calves and tone the back and abdomen |
| 3. Chin tuck: to help straighten head and shoulders |
| 4. Chest stretch: to stretch chest and improved back posture |
| 5. Upper back extension: to stretch chest, strengthen upper back muscles and improve back posture |
| 6. Pelvic tilt: to strengthen lower back and abdominal muscles |
| 7. Back and shoulder stretch: to stretch upper back and shoulders |
| Moderate Intensity Exercises |
| 8. Back posture exercise: To flatten upper back, stretch chest and improved posture |
| 9. Sitting stretch: To stretch calf and thigh muscles and improve muscle tone of legs |
| 10. Calf stretch: To stretch back of thighs and calf muscles, improved posture and stretch heel cords |
| 11. Upper back lift: To strengthen back muscles |
| 12. Abdomen strengthening: To strengthen abdomen |
| 13. Shoulders strengthening: To help strengthen shoulder and back muscles |
| 14. Spine and hip exercise: To strength in arms spine and hips and improve muscle tone |
Type, intensity, and frequency of the exercises are individualized by the treating physician or physical therapist.
Modified from Mayo Clinic Osteoporosis Exercise Chart, Mehrsheed Sinaki, Stephen Hodgson, patient education booklet MIC200054. Used with permission of Mayo Foundation for Medical Education and Research.
Yoga has gained significant enthusiasm in the past number of years, particularly in older adults. Moderate duration yoga exercises may in fact improve balance and prevent falls.92,93 Sudden rotational movements or excessive flexion of the spine, however, can predispose to vertebral compression fractures and should be avoided.94 There is also moderate quality evidence that Tai chi improves balance, reduces falls, and benefits overall bone health, particularly in older adults and women with osteoarthritis.95
Calcium and Vitamin D
Both calcium and vitamin D play central roles in maintenance of the musculoskeletal system. Hypovitaminosis D in older men and women is associated with decreased muscle strength and an increased risk of hip fractures.96,97 Vitamin D replacement improves both BMD and muscle function,98 and, in institutionalized older adults, may also reduce the risk for falls.99–101
In ambulatory healthy older women residing in nursing homes, treatment with 800 IU of vitamin D and 1200 mg of elemental calcium was associated with a significant reduction in hip and nonvertebral fractures compared to placebo.102 A pooled analysis of trials looking at vitamin D supplementation and osteoporotic fractures showed similar results in older men and women independent from the type of dwelling.103 On the other hand, in a randomized controlled trial of older men and women with history of falls, various doses of vitamin D failed to reduce fall risk, although all groups had improvement in overall physical performance.104,105 In the absence of malabsorptive conditions, daily vitamin D supplementation is preferred over larger intermittent doses (such as monthly or annually) as the latter were associated with an increased risk of falls in older adults.104–107
It is recommended that all adults aged 70 years and older receive 1200 mg of calcium per day and 800 IU of vitamin D per day from all sources, including diet. Dietary sources of calcium are varied and include dairy products and nuts, whereas vitamin D is limited to oily fish and fortified juices. Excessive doses of either calcium or vitamin D can be associated with adverse events, but daily calcium intake of up to 2000-2500 mg and daily vitamin D intake of up to 4000 IU are considered safe.101,108
Pharmacotherapy
Pharmacologic interventions to prevent age-related bone loss and reduce the risk of fractures include estrogen, raloxifene, four bisphosphonates (ie, alendronate, ibandronate, risedronate, zoledronate), denosumab (RANKL-neutralizing monoclonal antibodies), teriparatide (parathyroid hormone [PTH] 1-34), abaloparatide (PTHrP analog), and romosozumab (sclerostin-neutralizing monoclonal antibodies). The skeletal benefits and overall risks associated with these drugs in patients with osteoporosis have been reviewed recently109 and particularly in older adults.110
Estrogen has been shown to improve BMD and reduce overall fracture risk, although its use is limited to the early postmenopausal years given the associated risk of cardiovascular events and breast cancer in older women.111 Bisphosphonates, whether in oral or intravenous form, are the most commonly prescribed osteoporosis pharmacotherapy; they provide 40-70% reduction in risk of both vertebral and hip fractures. Although generally well tolerated, their prolonged use may be associated with extremely rare, yet serious side effects including atypical femoral fractures and osteonecrosis of the jaw.112,113 Denosumab is a more potent antiresorptive agent that provides a similar fracture risk reduction to bisphosphonates but a more robust increase in BMD.114 Denosumab may be associated with hypocalcemia, particularly in patients with advanced kidney disease, as well as an increased risk of mild upper respiratory or superficial skin infections.115 Delayed dosing or discontinuation of denosumab, particularly after long-term use, is associated with a rapid rebound bone loss and an increased risk of vertebral fractures.116
Osteoanabolic agents (ie, teriparatide and abaloparatide) stimulate bone formation and provide a greater increase in BMD than anti-resorptive agents, which translates into a 30-50% reduction in nonvertebral fractures and 60-80% in vertebral fractures.117,118 Although abaloparatide is stable at room temperature for up to 30 days, teriparatide requires refrigeration at temperatures between 36°F and 46°F (2°C to 8°C). These are both self-administered as daily injections and their use is largely limited to patients with a high fracture risk because of greater costs and possible higher rates of discontinuation among older adults.119,120 Post-marketing surveillance data showed that teriparatide did not increase the risk of adult osteosarcoma;121 therefore, in November of 2020, the U.S. Food and Drug Administration (FDA) no longer requires a black box warning to that effect.
Romosozumab, a dual anti-resorptive and osteoanabolic agent, provides ~70% reduction in vertebral fractures over one year but a nonsignificant reduction in nonvertebral fractures.122 In women, an increase in adjudicated serious cardiovascular events was observed with romosozumab when compared to alendronate, but not when compared to placebo.123 A similar increase was seen in men receiving romosozumab as compared to placebo.124 Aggregate data from six romosozumab trials showed a slight increase (relative risk=1.39, 95% CI 1.01-1.90) in composite four-point major adverse cardiovascular events (myocardial infarction, stroke, heart failure, and atrial fibrillation), although the risk was not significant when each event was considered separately.125 It is thus recommended to avoid romosozumab in patients with a high cardiovascular risk, specifically those with a history of a cardiovascular event in the preceding year.
Non-skeletal benefits of osteoporosis therapy
Pre-clinical and clinical studies have also examined additional non-skeletal effects of a number of these drugs. This may influence the choice of therapy, particularly in frail older adults. In addition, consideration should be made for patients’ neurobehavioral and social environments when choosing the most appropriate drug.
Zoledronate infusion every 18 months has been shown to reduce the overall incidence of cancers, particularly breast cancer in women with osteopenia.126 A 3.3% absolute risk reduction in mortality was also seen in the HORIZON Recurrent Fracture Trial, with similar mortality benefits being observed in other trials of zoledronate.127,128 However, a single zoledronate infusion did not improve secondary functional outcomes in institutionalized frail women 65 years or older.129
Non-skeletal effects of denosumab have similarly been investigated, particularly in patients with osteosarcopenia. In postmenopausal women, denosumab was associated with a significant increase in lean mass and grip strength after three years.130 In community dwelling older adults, denosumab significantly improved balance measures and decreased the fear of falls as compared to zoledronate; measures of physical function (ie, gait speed and TUG test) improved significantly to comparable degrees with both agents.131 Denosumab also reduced fall incidence in a pooled analysis of five trials, with a greater reduction observed in those less than 75 years of age.132
CONCLUSION
As the population ages to longer lifespans less shaped by natural selection, healthspan will not keep pace, creating challenges humans remain poorly equipped to handle including an enormous burden of late-life morbidity due to age-related diseases and chronic morbidities that often co-exist in the elderly. The skeleton is not exempt as aging exacerbates bone loss in both sexes and results in imbalanced bone resorption relative to formation and is associated with increased marrow adiposity, osteoblast/osteocyte apoptosis, and accumulation of senescent cells. While available pharmacological interventions are safe, effective, and important in preventing skeletal again, new approaches focused on developing interventions to delay or treat osteoporosis as well as other aging-associated diseases as a group have gained momentum.
Supplementary Material
Grant Support:
Supported by NIH grants P01 AG062413 (SK, JNF), R21 AG065868 (JNF, SK), and R01 DK128552 (JNF).
Abbreviations
- BMD
bone mineral density
- U.S.
United States
- NHANES
National Health and Nutrition Examination Survey
- FRAX®
Fracture Risk Assessment Tool
- DXA
dual energy X-ray absorptiometry
- NOF
National Osteoporosis Foundation
- P1NP
procollagen type 1 N-terminal propeptide
- CTx
C-telopeptide of type I collagen
- TUG
Timed Up and Go
- FRAiL
Fracture Risk Assessment in Long-term Care
- QCT
quantitative computed tomography
- T2DM
type 2 diabetes mellitus
- HRpQCT
high-resolution peripheral quantitative computed tomography
- BMUs
bone multicellular units
- BMAT
bone marrow adipose tissue
- MSC
mesenchymal stem cell
- PTH
parathyroid hormone
- FDA
Food and Drug Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Author Statement: Jad G. Sfeir: Conceptualization, Writing- Original Draft Preparation; Matthew T. Drake: Writing- Reviewing and Editing; Sundeep Khosla: Writing- Reviewing and Editing; and Joshua N. Farr: Conceptualization, Writing- Original Draft Preparation; Writing- Reviewing and Editing; Visualization; and Supervision.
Potential Competing Interests: The authors report no competing interests.
Disclosure Summary: The authors have nothing to disclose.
REFERENCES:
- 1.Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296(5570):1029–1031. [DOI] [PubMed] [Google Scholar]
- 2.Kontis V, Bennett JE, Mathers CD, et al. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389(10076):1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22(17):R741–752. [DOI] [PubMed] [Google Scholar]
- 4.Kim BT, Mosekilde L, Duan Y, et al. The structural and hormonal basis of sex differences in peak appendicular bone strength in rats. J Bone Miner Res. 2003;18:150–155. [DOI] [PubMed] [Google Scholar]
- 5.Lai SW, Liao KF, Liao CC, et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine (Baltimore). 2010;89(5):295–299. [DOI] [PubMed] [Google Scholar]
- 6.Farr JN, Almeida M. The Spectrum of Fundamental Basic Science Discoveries Contributing to Organismal Aging. J Bone Miner Res. 2018;33(9):1568–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis EM, van der Velde R, Moon RJ, et al. Epidemiology of fractures in the United Kingdom 1988-2012: Variation with age, sex, geography, ethnicity and socioeconomic status. Bone. 2016;87:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanis JA, Johnell O, Oden A, et al. Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int. 2000;11:669–674. [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ, Chrischilles EA, Cooper C, Lane AW, Riggs BL. How many women have osteoporosis. J Bone Miner Res. 1992;7:1005–1010. [DOI] [PubMed] [Google Scholar]
- 12.Melton LJ III, Atkinson EJ, O’Connor MK, O’Fallon WM, Riggs BL. Bone density and fracture risk in men. J Bone Miner Res. 1998;13(12):1915–1923. [DOI] [PubMed] [Google Scholar]
- 13.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Bergh JP, van Geel TA, Lems WF, Geusens PP. Assessment of individual fracture risk: FRAX and beyond. Curr Osteoporos Rep. 2010;8(3):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bours SP, van den Bergh JP, van Geel TA, Geusens PP. Secondary osteoporosis and metabolic bone disease in patients 50 years and older with osteoporosis or with a recent clinical fracture: a clinical perspective. Curr Opin Rheumatol. 2014;26(4):430–439. [DOI] [PubMed] [Google Scholar]
- 16.Ebeling PR, Nguyen HH, Aleksova J, et al. Secondary Osteoporosis. Endocr Rev. 2021. [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. Jama. 2011. ;305(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bischoff HA, Stahelin HB, Monsch AU, et al. Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing. 2003;32(3):315–320. [DOI] [PubMed] [Google Scholar]
- 20.Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69(5):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9(12):e113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry SD, Zullo AR, Lee Y, et al. Fracture Risk Assessment in Long-term Care (FRAiL): Development and Validation of a Prediction Model. J Gerontol A Biol Sci Med Sci. 2018;73(6):763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiely DK, Kiel DP, Burrows AB, Lipsitz LA. Identifying nursing home residents at risk for falling. J Am Geriatr Soc. 1998;46(5):551–555. [DOI] [PubMed] [Google Scholar]
- 25.Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age Ageing. 2012;41(3):299–308. [DOI] [PubMed] [Google Scholar]
- 26.Lawlor DA, Patel R, Ebrahim S. Association between falls in elderly women and chronic diseases and drug use: cross sectional study. Bmj. 2003;327(7417):712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ensrud KE, Blackwell TL, Mangione CM, et al. Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc. 2002;50(10):1629–1637. [DOI] [PubMed] [Google Scholar]
- 28.Wu Q, Bencaz AF, Hentz JG, Crowell MD. Selective serotonin reuptake inhibitor treatment and risk of fractures: a meta-analysis of cohort and case-control studies. Osteoporos Int. 2012;23(1):365–375. [DOI] [PubMed] [Google Scholar]
- 29.Rabenda V, Nicolet D, Beaudart C, Bruyere O, Reginster JY. Relationship between use of antidepressants and risk of fractures: a meta-analysis. Osteoporos Int. 2013;24(1):121–137. [DOI] [PubMed] [Google Scholar]
- 30.Seeman E An exercise in geometry. J Bone Miner Res. 2002;17:373–380. [DOI] [PubMed] [Google Scholar]
- 31.Riggs BL, Melton LJ III, Robb RA, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. [DOI] [PubMed] [Google Scholar]
- 32.Riggs BL, Melton LJI, Robb RA, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanis JA, Johnell O, Oden A, et al. Risk of hip fracture according to the world health organization criteria for osteopenia and osteoporosis. Bone. 2000;27:585–590. [DOI] [PubMed] [Google Scholar]
- 34.Van Staa TP, Laan RF, Barton IP, et al. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48(11):3224–3229. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz AV, Vittinghoff E, Bauer DC, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. Jama. 2011;305(21):2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farr JN, Drake MT, Amin S, et al. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29(4):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burghardt AJ, Issever AS, Schwartz AV, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(11):5045–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicks KM, Amin S, Atkinson EJ, et al. Relationship of age to bone microstructure independent of areal bone mineral density. J Bone Miner Res. 2012;27(3):637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hattner R, Epker BN, Frost HM. Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature. 1965;206(983):489–490. [DOI] [PubMed] [Google Scholar]
- 40.Seeman E Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci. 2013;68(10):1218–1225. [DOI] [PubMed] [Google Scholar]
- 41.Parfitt AM. Skeletal heterogeneity and the purposes of bone remodeling: implications for the understanding of osteoporosis. Osteoporosis. 1996;12:315–329. [Google Scholar]
- 42.Lips P, Courpron P, Meunier PJ. Mean wall thickness of trabecular bone packets in the human iliac crest: changes with age. Calcif Tissue Res. 1978;26:13–17. [DOI] [PubMed] [Google Scholar]
- 43.Eriksen EF. Normal and pathological remodeling of human trabecular bone: three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr Rev. 1986;7:379–408. [DOI] [PubMed] [Google Scholar]
- 44.Khosla S Pathogenesis of age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68(10):1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ensrud KE, Palermo L, Black DM, et al. Hip and calcaneal bone loss increase with advancing age: longitudinal results from the Study of Osteoporotic Fractures. J Bone Miner Res. 1995;10:1778–1787. [DOI] [PubMed] [Google Scholar]
- 46.Zebaze RMD, Ghasem-Zadeh A, Bohte A, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. [DOI] [PubMed] [Google Scholar]
- 47.Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015;3(2):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz AV, Sigurdsson S, Hue TF, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98(6):2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bredella MA, Fazeli PK, Miller KK, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ecklund K, Vajapeyam S, Feldman HA, et al. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25(2):298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baum T, Yap SP, Karampinos DC, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging. 2012;35(1):117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trudel G, Payne M, Madler B, et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol (1985). 2009;107(2):540–548. [DOI] [PubMed] [Google Scholar]
- 53.Wright LE, Buijs JT, Kim HS, et al. Single-Limb Irradiation Induces Local and Systemic Bone Loss in a Murine Model. J Bone Miner Res. 2015;30(7):1268–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandra A, Lagnado AB, Farr JN, et al. Targeted Reduction of Senescent Cell Burden Alleviates Focal Radiotherapy-Related Bone Loss. J Bone Miner Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. J Clin Invest. 1998;102:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parfitt AM, Han ZH, Palnitkar S, et al. Effects of ethnicity and age or menopause on osteoblast function, bone mineralization, and osteoid accumulation in iliac bone. J Bone Miner Res. 1997;12:1864–1873. [DOI] [PubMed] [Google Scholar]
- 59.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. [DOI] [PubMed] [Google Scholar]
- 60.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. [DOI] [PubMed] [Google Scholar]
- 61.Jilka RL, Weinstein RS, Parfitt AM, Manolaga SC. Quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J Bone Miner Res. 2007;22:1492–1501. [DOI] [PubMed] [Google Scholar]
- 62.O’Brien CA, Jia D, Plotkin LI, et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145(4):1835–1841. [DOI] [PubMed] [Google Scholar]
- 63.Aguirre JI, Plotkin LI, Stewart SA, et al. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21:605–615. [DOI] [PubMed] [Google Scholar]
- 64.Tiede-Lewis LM, Xie Y, Hulbert MA, et al. Degeneration of the osteocyte network in the C57BL/6 mouse model of aging. Aging (Albany NY). 2017;9(10):2190–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schurman CA, Verbruggen SW, Alliston T. Disrupted osteocyte connectivity and pericellular fluid flow in bone with aging and defective TGF-beta signaling. Proc Natl Acad Sci U S A. 2021. ;118(25). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts--correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217(2):640–648. [DOI] [PubMed] [Google Scholar]
- 67.Ajubi NE, Klein-Nulend J, Nijweide PJ, et al. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes--a cytoskeleton-dependent process. Biochem Biophys Res Commun. 1996;225(1):62–68. [DOI] [PubMed] [Google Scholar]
- 68.Joeng KS, Lee YC, Lim J, et al. Osteocyte-specific WNT1 regulates osteoblast function during bone homeostasis. J Clin Invest. 2017;127(7):2678–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noble BS, Peet N, Stevens HY, et al. Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol. 2003;284(4):C934–943. [DOI] [PubMed] [Google Scholar]
- 70.Yao W, Dai W, Jiang JX, Lane NE. Glucocorticoids and osteocyte autophagy. Bone. 2013;54(2):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Onal M, Piemontese M, Xiong J, et al. Suppression of autophagy in osteocytes mimics skeletal aging. J Biol Chem. 2013;288(24):17432–17440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ju L, Han J, Zhang X, et al. Obesity-associated inflammation triggers an autophagy-lysosomal response in adipocytes and causes degradation of perilipin 1. Cell Death Dis. 2019;10(2):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farr JN, Khosla S. Cellular senescence in bone. Bone. 2019;121:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farr JN, Kaur J, Doolittle ML, Khosla S. Osteocyte Cellular Senescence. Curr Osteoporos Rep. 2020;18(5):559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130(2):223–233. [DOI] [PubMed] [Google Scholar]
- 76.Farr JN, Fraser DG, Wang H, et al. Identification of Senescent Cells in the Bone Microenvironment. J Bone Miner Res. 2016;31(11):1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farr JN, Xu M, Weivoda MM, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(9):1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaur J, Farr JN. Cellular senescence in age-related disorders. Transl Res. 2020;226:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robbins PD, Jurk D, Khosla S, et al. Senolytic Drugs: Reducing Senescent Cell Viability to Extend Health Span. Annu Rev Pharmacol Toxicol. 2021. ;61:779–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coll PP, Phu S, Hajjar SH, et al. The prevention of osteoporosis and sarcopenia in older adults. J Am Geriatr Soc. 2021;69(5):1388–1398. [DOI] [PubMed] [Google Scholar]
- 82.Guirguis-Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL. Interventions to Prevent Falls in Older Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama. 2018;319(16):1705–1716. [DOI] [PubMed] [Google Scholar]
- 83.Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Troy KL, Mancuso ME, Butler TA, Johnson JE. Exercise Early and Often: Effects of Physical Activity and Exercise on Women’s Bone Health. Int J Environ Res Public Health. 2018;15(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2011;(11):CD004963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;(7):CD000333. [DOI] [PubMed] [Google Scholar]
- 87.Daly RM, Gianoudis J, Kersh ME, et al. Effects of a 12-Month Supervised, Community-Based, Multimodal Exercise Program Followed by a 6-Month Research-to-Practice Transition on Bone Mineral Density, Trabecular Microarchitecture, and Physical Function in Older Adults: A Randomized Controlled Trial. J Bone Miner Res. 2020;35(3):419–429. [DOI] [PubMed] [Google Scholar]
- 88.Gianoudis J, Bailey CA, Ebeling PR, et al. Effects of a targeted multimodal exercise program incorporating high-speed power training on falls and fracture risk factors in older adults: a community-based randomized controlled trial. J Bone Miner Res. 2014;29(1):182–191. [DOI] [PubMed] [Google Scholar]
- 89.Watson SL, Weeks BK, Weis LJ, et al. High-Intensity Resistance and Impact Training Improves Bone Mineral Density and Physical Function in Postmenopausal Women With Osteopenia and Osteoporosis: The LIFTMOR Randomized Controlled Trial. J Bone Miner Res. 2018;33(2):211–220. [DOI] [PubMed] [Google Scholar]
- 90.Dent E, Morley JE, Cruz-Jentoft AJ, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J Nutr Health Aging. 2018;22(10):1148–1161. [DOI] [PubMed] [Google Scholar]
- 91.WHO Guidelines Approved by the Guidelines Review Committee, in Global Recommendations on Physical Activity for Health. 2010, World Health Organization: Geneva. [PubMed] [Google Scholar]
- 92.Schmid AA, Van Puymbroeck M, Koceja DM. Effect of a 12-week yoga intervention on fear of falling and balance in older adults: a pilot study. Arch Phys Med Rehabil. 2010;91 (4):576–583. [DOI] [PubMed] [Google Scholar]
- 93.Youkhana S, Dean CM, Wolff M, Sherrington C, Tiedemann A. Yoga-based exercise improves balance and mobility in people aged 60 and over: a systematic review and meta-analysis. Age Ageing. 2016;45(1):21–29. [DOI] [PubMed] [Google Scholar]
- 94.Sfeir JG, Drake MT, Sonawane VJ, Sinaki M. Vertebral compression fractures associated with yoga: a case series. Eur J Phys Rehabil Med. 2018;54(6):947–951. [DOI] [PubMed] [Google Scholar]
- 95.Zou L, Wang C, Chen K, et al. The Effect of Taichi Practice on Attenuating Bone Mineral Density Loss: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int J Environ Res Public Health. 2017;14(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Visser M, Deeg DJ, Lips P, Longitudinal Aging Study A. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–5772. [DOI] [PubMed] [Google Scholar]
- 97.Cauley JA, Lacroix AZ, Wu L, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149(4):242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao JG, Zeng XT, Wang J, Liu L. Association Between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. Jama. 2017;318(24):2466–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of Vitamin D on falls: a meta-analysis. Jama. 2004;291(16):1999–2006. [DOI] [PubMed] [Google Scholar]
- 100.Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med. 2015;175(5):703–711. [DOI] [PubMed] [Google Scholar]
- 101.Reid IR. Osteoporosis: evidence for vitamin D and calcium in older people. Drug Ther Bull. 2020;58(8):122–125. [DOI] [PubMed] [Google Scholar]
- 102.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327(23):1637–1642. [DOI] [PubMed] [Google Scholar]
- 103.Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40–49. [DOI] [PubMed] [Google Scholar]
- 104.Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly High-Dose Vitamin D Treatment for the Prevention of Functional Decline: A Randomized Clinical Trial. JAMA Intern Med. 2016;176(2):175–183. [DOI] [PubMed] [Google Scholar]
- 105.Waterhouse M, Sanguineti E, Baxter C, et al. Vitamin D supplementation and risk of falling: outcomes from the randomized, placebo-controlled D-Health Trial. J Cachexia Sarcopenia Muscle. 2021;12(6):1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ginde AA, Blatchford P, Breese K, et al. High-Dose Monthly Vitamin D for Prevention of Acute Respiratory Infection in Older Long-Term Care Residents: A Randomized Clinical Trial. J Am Geriatr Soc. 2017;65(3):496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pekkarinen T, Valimaki VV, Aarum S, et al. The same annual dose of 292000 IU of vitamin D (cholecalciferol) on either daily or four monthly basis for elderly women: 1-year comparative study of the effects on serum 25(OH)D concentrations and renal function. Clin Endocrinol (Oxf). 2010;72(4):455–461. [DOI] [PubMed] [Google Scholar]
- 108.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5(11):898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sfeir JG, Pignolo RJ. Pharmacologic Interventions for Fracture Risk Reduction in the Oldest Old: What Is the Evidence? JBMR Plus. 2021;5(10):e10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. Jama. 2003;290(13):1729–1738. [DOI] [PubMed] [Google Scholar]
- 112.Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29(1):1–23. [DOI] [PubMed] [Google Scholar]
- 113.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–1491. [DOI] [PubMed] [Google Scholar]
- 114.Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–523. [DOI] [PubMed] [Google Scholar]
- 115.McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354(8):821–831. [DOI] [PubMed] [Google Scholar]
- 116.Zanchetta MB, Boailchuk J, Massari F, et al. Significant bone loss after stopping long-term denosumab treatment: a post FREEDOM study. Osteoporos Int. 2018;29(1):41–47. [DOI] [PubMed] [Google Scholar]
- 117.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. [DOI] [PubMed] [Google Scholar]
- 118.Miller PD, Hattersley G, Riis BJ, et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. Jama. 2016;316(7):722–733. [DOI] [PubMed] [Google Scholar]
- 119.Niimi R, Kono T, Nishihara A, et al. Usefulness of daily teriparatide treatment in elderly patients over 80 years of age. Osteoporos Int. 2016;27(5):1869–1874. [DOI] [PubMed] [Google Scholar]
- 120.McClung MR, Harvey NC, Fitzpatrick LA, et al. Effects of abaloparatide on bone mineral density and risk of fracture in postmenopausal women aged 80 years or older with osteoporosis. Menopause. 2018;25(7):767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gilsenan A, Midkiff K, Harris D, et al. Teriparatide Did Not Increase Adult Osteosarcoma Incidence in a 15-Year US Postmarketing Surveillance Study. J Bone Miner Res. 2021. ;36(2):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med. 2016;375(16):1532–1543. [DOI] [PubMed] [Google Scholar]
- 123.Saag KG, Petersen J, Brandi ML, et al. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med. 2017;377(15):1417–1427. [DOI] [PubMed] [Google Scholar]
- 124.Lewiecki EM, Blicharski T, Goemaere S, et al. A Phase III Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men With Osteoporosis. J Clin Endocrinol Metab. 2018;103(9):3183–3193. [DOI] [PubMed] [Google Scholar]
- 125.Lv F, Cai X, Yang W, et al. Denosumab or romosozumab therapy and risk of cardiovascular events in patients with primary osteoporosis: Systematic review and meta- analysis. Bone. 2020. ;130:115121. [DOI] [PubMed] [Google Scholar]
- 126.Reid IR, Horne AM, Mihov B, et al. Effects of Zoledronate on Cancer, Cardiac Events, and Mortality in Osteopenic Older Women. J Bone Miner Res. 2020;35(1):20–27. [DOI] [PubMed] [Google Scholar]
- 127.Boonen S, Black DM, Colon-Emeric CS, et al. Efficacy and safety of a once-yearly intravenous zoledronic acid 5 mg for fracture prevention in elderly postmenopausal women with osteoporosis aged 75 and older. J Am Geriatr Soc. 2010;58(2):292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Greenspan SL, Perera S, Ferchak MA, Nace DA, Resnick NM. Efficacy and safety of single-dose zoledronic acid for osteoporosis in frail elderly women: a randomized clinical trial. JAMA Intern Med. 2015;175(6):913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest. 2019;129(8):3214–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Phu S, Bani Hassan E, Vogrin S, Kirk B, Duque G. Effect of Denosumab on Falls, Muscle Strength, and Function in Community-Dwelling Older Adults. J Am Geriatr Soc. 2019;67(12):2660–2661. [DOI] [PubMed] [Google Scholar]
- 132.Chotiyarnwong P, McCloskey E, Eastell R, et al. A Pooled Analysis of Fall Incidence From Placebo-Controlled Trials of Denosumab. J Bone Miner Res. 2020;35(6):1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


