Abstract
Feline sporotrichosis is a major clinical problem among cats in Brazil and is also a neglected, but important, public health issue, due to its zoonotic potential. The nasal clinical form of the disease is particularly challenging, having treatment refractoriness and clinical signs relapse as common features. This case series study aimed to preliminarily describe the effects of the azolic antifungal drug, clotrimazole, as a topical 1% solution spray, together with per os itraconazole on inducing disease remission, as well as treatment tolerability and safety. Medical records of the Feline Medicine Service from the Universidade Federal Rural do Rio de Janeiro were reviewed, and 7 feline patients met the inclusion criteria (confirmatory diagnostic reached, available follow-up records, and use of intranasal clotrimazole 1% solution –1 spray per nostril every 24 hours– as adjunctive therapy to itraconazole – 100 mg/cat per os every 24 hours). Among these, 4 had a history of treatment refractoriness done until then. Follow-up records included clinical evaluation, along with complementary tests and owner reports on tolerability and occurrence of adverse reactions. All patients have undergone clinical remission within 60 days. Tolerability were satisfactory, and adverse reactions were only found on complementary tests (hepatic enzyme elevation), without clinical repercussion. The intranasal use of 1% clotrimazole solution has shown as a promising adjunctive therapy to itraconazole for feline nasal sporotrichosis, even in previous refractory cases.
Keywords: azolic antifungal, subcutaneous mycosis, Sporothrix brasiliensis, nasal granuloma
Resumo
A esporotricose felina é um dos principais problemas clínicos entre os gatos no Brasil. É também um problema de saúde pública negligenciado, mas importante, devido ao seu potencial zoonótico. A forma clínica nasal da doença é particularmente desafiadora, sendo a refratariedade ao tratamento e a recidiva dos sinais clínicos características comuns. Este estudo de série de casos teve como objetivo descrever preliminarmente o efeito da infusão intranasal do antifúngico azólicos clotrimazol, na forma de solução "spray" a 1%, com itraconazol dado via oral, na indução da remissão da doença em pacientes felinos, bem como a tolerabilidade e segurança do tratamento. Os registros médicos do Serviço de Medicina Felina da Universidade Federal Rural do Rio de Janeiro foram revisados, e 7 pacientes felinos atenderam aos critérios de inclusão (diagnóstico confirmatório alcançado, prontuários de acompanhamento disponíveis e uso de solução intranasal de clotrimazol 1% –1 borrifada por narina a cada 24 horas– como terapia adjuvante ao itraconazol –100 mg / gato por via oral a cada 24 horas). Destes, 4 tinham história de refratariedade ao tratamento realizado até então. Os registros de acompanhamento incluíram avaliação clínica, realização de testes complementares e relatórios dos responsáveis pelos animais acerca da tolerabilidade e ocorrência de efeitos adversos. Todos os pacientes apresentaram remissão clínica em até 60 dias. A tolerabilidade e a segurança foram satisfatórias, sendo encontrados efeitos adversos apenas em exames complementares (elevação das enzimas hepáticas), sem repercussão clínica. O uso tópico de solução de clotrimazol a 1% mostrou-se uma promissora terapia adjuvante ao itraconazol dado por via oral para o tratamento da esporotricose nasal felina, mesmo em casos previamente refratários.
Palavras-chave: antifúngico azólico, micose subcutânea Sporothrix brasiliensis, granuloma nasal
Introduction
Sporotrichosis is a subcutaneous zoonotic fungal disease that is caused by Sporothrix spp. and has become endemic in Brazil during the last decades (Gremião et al., 2021). Epidemiologically, cats play an important role in this disease cycle since their lesions present a high burden of yeast cells (Souza et al., 2018). Additionally, infected cats may easily transmitte the fungi by scratches and bite wounds to other cats because of their territorial and predatory behavior, and to humans due to cat-human bond (Montenegro et al., 2014).
In the southeast region, more specifically in the state of Rio de Janeiro, sporotrichosis has reached hyperendemic proportions, with Sporothrix brasiliensis as the most prevalent species (Rodrigues et al., 2020). Many studies have described this species’ features, which is now known for its impressive adaptation for parasitism, virulence in both feline and human patients, and widespread resistance to azoles the first-line drugs used for this infection (Nakasu et al., 2021). In the feline patient, the treatment for nasal sporotrichosis is particularly challenging, with many cases being refractory to standard therapies (i.e., per os itraconazole ± potassium iodide) (Nakasu et al., 2021). The reason is not fully understood; however, according to Malik et al. (2004), this region in the cat is relatively poorly vascularized, which may prevent systemic drugs from reaching it in appropriate concentrations. Additionally, evidence showed that this fungus can invade bone and cartilage tissues and infect osteoclasts in this region, which further complicates the complete healing (Gremião et al., 2015).
Clotrimazole is an azolic antifungal that is highly effective against S. brasiliensis isolates in vitro (Gagini et al., 2017). In vivo, it has been used topically by intravesical infusion in a cat with cystitis and candiduria secondary to diabetes mellitus (Toll et al., 2003) and by intranasal infusion in two cats with nasal aspergillosis (Furrow & Groman, 2009), both with good efficacy. Therefore, this case series aimed to describe a series of cases where the the azolic antifungal drug clotrimazole were used as a intranasal solution spray at 1% together with per os itraconazole for treating the nasal form of feline sporotrichosis.
Materials and methods
This case series is based on the medical records of cats presented to the Feline Medicine Service at the Veterinary Hospital from the Universidade Federal Rural do Rio de Janeiro-UFRRJ (Seropédica-Rio de Janeiro) between 2018 and 2021 with nasal sporotrichosis (lesions extending from the nasal bridge to the intern mucosa). Included cases were those with a confirmatory diagnosis that was reached by fungal culture (± other laboratory tests such as cytology and serology); the veterinarian in charge prescribed an intranasal infusion of clotrimazole spray (1% clotrimazole in sodium chloride solution; Grupo TudoDVet Farmácia Veterinária ®; one spray in each nostril at approximately 1 mL per nostril, SID) (Figure 1) as adjunctive therapy to per os itraconazole (100 mg/cat PO SID); with follow-up records of a minimum of 30 days from the beginning of drug usage; and with posttreatment follow-up evaluation times. In all cases, written consent was obtained from the owner.
Figure 1. Feline sporotrichosis patient (case 7) receiving intranasal administration of 1% clotrimazole spray through its nostril. The infusion was performed by introducing the applicator tip through each one of the nostrils and pulling the valve one time per nostril (volume infused equivalent to 1 mL/nostril).
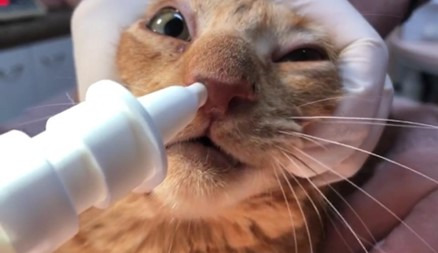
The clotrimazole spray intranasal infusion was performed by introducing the applicator tip through the nostril and pulling the valve one time per nostril, as shown in Figure 1.
The collected data included gender, age, neutering and retroviral status, body weight, lifestyle, signalment, diagnostic methods used to confirm infection, previous treatments (including drug(s) utilized and duration), and complementary tests. Cases were considered refractory if the previous treatment had not induced any clinical improvement for >3 months. Follow-up data were also collected, including reported adverse reactions by owners, clinical evaluation, and complimentary exam findings. Patients were considered in clinical remission if clinical signs, such as nasal bridge bulging, stertor, sneezing, edema, and ulceration, were significantly atenuated within 60 days.
Results
A total of 7 cases met the inclusion criteria, of which 4 cases (cases 1, 3, 6, and 7) had a history of treatment refractoriness used until then. Patient data regarding age, gender, weight, signalment, lifestyle, retroviral, and castration status, as well as the performed diagnostic tests, adverse reactions, and treatment outcomes are shown in Table 1.
Table 1. Data regarding age, gender, weight, previous treatments, lifestyle, neutered and retroviral status, diagnostic methods, adverse reactions, and outcome of each feline sporotrichosis case.
| Case | Age | Gender | Weight | Previous treatments | Lifestyle | Neuter status | Retroviral status | Signalment | Diagnostic method(s) | Adverse reactions | Treatment time | Follow-up time after treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 yo | M | 4.28 kg | Itraconazole (15–50 mg) for 12 months | Outdoor | Neutered | FeLV+ | An ulcerated bulge on the nasal bridge, and airway flow obstruction | Cytology, culture, histopathology | None | 3 months | 32 days | Total clinical remission |
| 2 | 7 yo | M | 7.90 kg | Itraconazole (100 mg) for 1 month | Indoor | Neutered | Negative | Nasal bridge bulging | Cytology, culture | None | 3 months | 78 days | Total clinical remission |
| 3 | 4 yo | F | 3.28 kg | Itraconazole (25–100 mg) for 18 months, potassium iodide (10 mg). and fluconazole (50 mg) for 2 months, and tropical ketoconazole for 1 month | Indoor | Spayed | Negative | An ulcerated bulge on the nasal bridge, sneezing, and airway flow obstruction | Cytology, culture | ↑ ALT | 2 months | 44 days | Total clinical remission |
| 4 | 1 yo | M | 1.55 kg | Itraconazole (100 mg) for 1 month | Indoor | Neutered | Negative | Nasal bridge bulging and respiratory stertor | Serology, culture | ↑ ALP and ALT, salivation | 6 months | 30 days | Clinical remission with residual nasal bulging |
| 5 | 5 yo | F | 3.88 kg | Itraconazole (100 mg) for 1 month | Indoor | Spayed | Negative | Sneezing and airway flow obstruction | Serology, culture | None | 5 months | 87 days | Clinical remission with residual nasal bulging |
| 6 | 7 yo | M | 4.470 kg | Itraconazole (100 mg) and potassium iodide (15 mg) for 5 months | Outdoor/ indoor | Neutered | FIV+ | An ulcerated bulge on the nasal bridge airway, and flow obstruction | Culture | ↑ ALT | 2 months | 93 days | Total clinical remission |
| 7 | 6 yo | F | 3.67 kg | Itraconazole and potassium iodide for 48 months (no dosages available) | Indoor | Neutered | Negative | An ulcerated bulge on the nasal bridge, airway flow obstruction, and breathing difficulty | Serology, culture | None | 3 months | 16 days | Clinical remission with residual nasal bulging |
yo: years old; F: female; M: male; FeLV+: positive for Feline Leukemia Virus infection; FIV+: positive for Feline Immunodeficiency Virus infection; ALT: alanine-aminotransferases; ALP: alkaline phosphatase.
This case series patients’ group included 4 males and 3 females. All animals were castrated and kept strictly indoors except for cats 1 and 6. The mean age and body weight were 5.14 years old and 4.14 kg, respectively. Diagnostic methods used to confirm infection included fungal isolation for all seven cases, quantitative serology for anti-SsCBF IgG antibodies on cases 4, 5, and 7 (titers of 1:1600, 1:1600, and 1:12800, respectively; cutoff titer of 1:400) and cytology on cases 1, 2, and 3. The mean previous treatment duration time was 12.2 months (1–48 months). All the cats have received per os itraconazole (25–100 mg/cat), and potassium iodide (5–50 mg/kg) was also given in cases 3, 4, 6, and 7. Other therapies included fluconazole (case 3), famciclovir, human recombinant-interferon, enrofloxacin, N-acetylcysteine (case 5), amoxicillin (case 2), and topical ketoconazole cream (case 4).
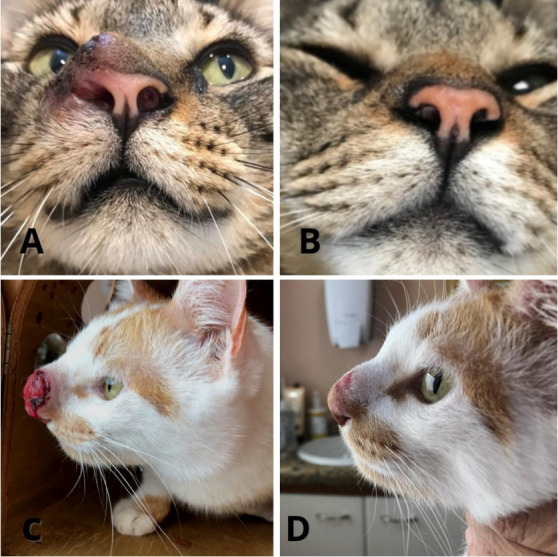
Treatment efficacy was evaluated by clinical remission of lesions and signalment, such as nasal bridge bulging, ulceration, edema, airway flow obstruction, sneezing, respiratory stertor, and nasal discharge. Treatment outcomes are presented in Table 1. The mean treatment duration was 3.4 months (2–6 months). In most patients, clinical sign remission occurred within 60 days, whereas in only 30 days in case 3 (Figure 2).
Figure 2. Clinical evolution observed in feline sporotrichosis patients with the use of clotrimazol 1% spray intranasally and oral itraconazole. (A) Pretreatment aspect of case 1’s lesion. Note for edema, ulceration, hyperemia, and nasal flow obstruction by the proliferative lesion in the left nostril. (B) Same patient after 60 days of therapy. Note for resolution of hyperemia, proliferation, ulceration, and nasal flow obstruction. (C) Pretreatment aspect of case 7’s lesion. Intense proliferation, hyperemia, edema, and ulceration on the nasal bridge are observed. This patient also had severe stertor and airflow obstruction. (D) Patient 7 after 60 days of therapy. A dramatic reduction in ulceration, proliferation, and airway obstruction are perceived. Residual nasal bulging is still present.
Except for salivation following accidental oral administration of clotrimazole, no other adverse reactions were reported by owners. The complementary tests revealed that 3 patients (cases 3, 4, and 6) showed mild to moderate alanine-aminotransferase elevation (ALT: 94–324 U/L; reference range: 5–60 U/L) and 2 (cases 4 and 6) showed mild alkaline phosphatase elevations (ALP: 101–116 UI/L; reference range: >90 UI/L) at some treatment points. However, the clinical signs of hepatic injury (e.g., vomiting, weight loss, and inappetence) were absent according to these owners. Other biochemical parameters (such as creatinine, blood urea nitrogen, and albumin) were normal in all cats, as well as the complete blood count (CBC).
All but one owner reported no difficulty with therapy administration. The owner who found infusing the drug difficult reported struggles with patient restraints, and on some occasions, the drug was infused orally instead because of the cat’s reluctance. With this, salivation was observed. The mean follow-up time after treatment discontinuation was 54 days (range: 16–93 days), during which no relapses occurred.
Discussion
Sporotrichosis is a major health problem in Brazil’s feline population and has zoonotic implications, which also configures it into a public health issue. Gremião et al. (2021) stated that early and successful treatment of feline sick patients is the key to breaking this agent cycle and preventing new infections. However, refractoriness is a common finding in cats with nasal sporotrichosis, which makes this specific clinical form very frustrating for both veterinarians and owners. Previous studies have demonstrated the efficacy of alternative therapies in these refractory cases, such as the use of cryosurgery and intralesional amphotericin B (Gremião et al., 2009; Souza et al., 2016). However, these options can be expensive due to the geographic distribution of sporotrichosis in our country, where most patients come from poor families, therefore prohibitive for many owners (Gutierrez-Galhardo et al., 2015; Sanchotene et al., 2015). Furthermore, some owners will not consent to submit their cats to these procedures because of their inherent invasiveness. However, as revealed in this case series, compounded 1% clotrimazole spray is a cheaper and non-invasive alternative that may be added in cases with little effect from the standard therapy.
Similarly to itraconazole, this drugs’ mechanism of action consist in the sterol 14a-demethylase enzyme (CYP51) inhibition, interefering, thus, with the ergosterol (main component of the fungus plasmatic membrane) synthesis (Ferreira et al., 2020). Gagini et al. (2017) revealed that clotrimazole is highly efficient against S. brasiliensis isolates in vitro, either alone or associated with itraconazole; however, in vivo studies were lacking until the present. Other studies have already reported the successful use of intranasally delivered clotrimazole for fungal rhinitis in cats (Barrs et al., 2012; Furrow & Groman, 2009; Tomsa et al., 2003). The etiological agent in those was Aspergillus sp., with differences regarding therapeutic protocols (i.e., patients were anesthetized and clotrimazole was infused through a catheter inserted intranasally); however, they revealed that this drug may be well-tolerated and safe for this route in feline patients.
Nevertheless, the employed clotrimazole solution in at least one of those previous reports contained isopropanol and propylene glycol, which potentially cause pharyngeal edema and inflammation. Tomsa et al. (2003) reported local irritation after clotrimazole infusion in one cat and, therefore, recommended the placement of an enteral feeding tube to prevent it from stopping eating. However, this was not reported in patients described herein, possibly, due to the composition of the used clotrimazole solution in these patients, which only contains clotrimazole in sodium chloride solution, according to the manufacturer. Additionally, differences were found in the drug administration and volume between our patients and the ones from these studies, wherein drug delivery was accomplished by infusion of 8–10 mL of clotrimazole solution per nostril in the latter, whereas only 1 spray pressing (approximately 1 mL/nostril) inside the applied cat nostril in our study.
At the follow-up appointments, generally performed every 30 days after treatment initiation, blood samples for CBC and serum biochemistry were collected to assess the therapy tolerability. Some cats showed mild elevations in hepatic enzymes, such as ALT and ALP. Possibly, such alterations were due to oral itraconazole administration, which is known to cause hepatic injury (Gremião et al., 2021; Pereira et al., 2010; Reis et al., 2016). However, they were not accompanied by clinical signs, such as inappetence, vomiting, and weight loss, as expected in hepatotoxicity. In all these cases, hepatic enzymes were decreased following the institution of hepaprotective therapy (silymarin at 90 mg/cat PO once a day). Nonetheless, more studies are needed to verify the issue of hepatotoxicity in topical clotrimazole therapy.
Our case series had median treatment duration of 12.2 months before presentation. This is considerably longer compared to studies that evaluate other alternative therapies to refractory sporotrichosis, such as intralesional amphotericin B or cryosurgery (8 and 3.59 months, respectively) (Gremião et al., 2009; Souza et al., 2016). However, the effectiveness was similar. This finding is encouraging, as this could mean that 1% clotrimazole spray may be efficient even in long-lasting refractory cases, which comprise a great portion of feline patients with nasal sporotrichosis. The authors believe that this is most likely because the topical application of the drug produces high concentrations within nasal tissues, thus increasing its ability to mitigate the infection, similar to Sharman and Mansfield (2012) in their review concerning topical therapies for intranasal mycosis in dogs.
Noteworthy, a significant limitation of this therapy is that the owner must handle the patient daily to intranasally infuse the drug. This may represent a zoonotic risk, which is even more significant if the person in charge of drug administration is immunocompromised (e.g., human immunodeficiency virus-positive and oncologic patients) (Chakrabarti et al., 2015). Additionally, some cats may not tolerate restraint during drug application, and owners might find it difficult to adapt the therapy. In this study, only one owner reported struggling with drug administration, and were accidentally administered orally on some occasions, eliciting salivation. This probably reflects the bitter taste of the drug since such an event did not happen when it was properly infused. All the other owners described ease at administration and good acceptance by their cats.
However, careful handling, and probably, the help of a second person is advisable both to ease the drug infusion procedure and reduce the likelihood of zoonotic transmission, even though the latter is considered low, as yeast cells burden is drastically decreased once systemic and topical treatments are adopted (Gremião et al., 2021). The authors recommend discussing the risks with owners before treatment prescription.
The in vivo utilization of clotrimazol for treating feline sporotrichosis due to S. brasiliensis has never been described before. In this case series, its intranasal administration daily togheter with oral itraconazole has shown as a valuable therapeutic option to induce the disease’s clinical remission. In order to further evaluate the efficacy of this therapy, as well as its safety, adverse reactions and tolerability, prospective controlled studies are warranted.
In conclusion, the use of 1% clotrimazole solution for intranasal infusion together with oral itraconazole has determined the clinical remission of feline nasal sporotrichosis in the patients described and may be viewed as a promising therapy modality to be considered in cases where the traditional oral therapy alone shows little effect on nasal sporotrichosis treatment.
Acknowledgements
The authors thank the drug’s manufacturer “TudoDVet” for accepting to formulate the drug and also for providing all information regarding the process.
Footnotes
How to cite: Santi, J. P., Santos, C. R. G. R., Santos, A. S., & Souza, H. J. M. (2022). Intranasal clotrimazole spray 1% associated with oral itraconazole for nasal feline sporotrichosis: a case series. Brazilian Journal of Veterinary Medicine, 44, e004821. https://doi.org/10.29374/2527-2179.bjvm004821
Ethics statement: All procediments were consented by the animals’ owners.
Financial support: JPS – Received scholarship from MEC (Ministério da Educação). CRGRS – Received scholarship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). ASS and HJMS – None.
The study was carried out at the Hospital Veterinário de Pequenos Animais – HVPA, from the Universidade Federal Rural do Rio de Janeiro, Campus Seropédica, RJ, Brasil.
Availability of complementary results: Please, contact the correspondent author for complementary information on this subject.k
References
- Barrs V. R., Halliday C., Martin P., Wilson B., Krockenberger M., Gunew M., Bennett S., Koehlmeyer E., Thompson A., Fliegner R., Hocking A., Sleiman S., O’Brien C., Beatty J. A. Sinonasal and sino-orbital aspergillosis in 23 cats: Aetiology, clinicopathological features and treatment outcomes. Veterinary Journal. 2012;191(1):58–64. doi: 10.1016/j.tvjl.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A., Bonifaz A., Gutierrez-Galhardo M. C., Mochizuki T., Li S. Global epidemiology of sporotrichosis. Medical Mycology. 2015;53(1):3–14. doi: 10.1093/mmy/myu062. [DOI] [PubMed] [Google Scholar]
- Ferreira P., Noronha L., Teixeira R., Vieira Í., Borba-Santos L., Viçosa A., Moraes M., Calil-Elias S., Freitas Z., Silva F., Rozental S., Futuro D., Ferreira V. Investigation of a microemulsion containing clotrimazole and itraconazole for transdermal delivery for the treatment of sporotrichosis. Journal of Pharmaceutical Sciences. 2020;109(2):1026–1034. doi: 10.1016/j.xphs.2019.10.009. [DOI] [PubMed] [Google Scholar]
- Furrow E., Groman R. P. Intranasal infusion of clotrimazole for the treatment of nasal aspergillosis in two cats. Journal of the American Veterinary Medical Association. 2009;235(10):1188–1193. doi: 10.2460/javma.235.10.1188. [DOI] [PubMed] [Google Scholar]
- Gagini T., Borba-Santos L. P. B., Rodrigues A. M., Camargo Z. P., Rozental S. Clotrimazole is highly effective in vitro against feline Sporothrix brasiliensis isolates. Journal of Medical Microbiology. 2017;66(11):1573–1580. doi: 10.1099/jmm.0.000608. [DOI] [PubMed] [Google Scholar]
- Gremião I. D. F., Rocha E. M. F., Montenegro H., Carneiro A. J. B., Xavier M. O., Farias M. R., Monti F., Mansho W., Pereira R. H. M. A., Pereira S. A., Lopes-Bezerra L. M. Guideline for the management of feline sporotrichosis caused by Sporothrix brasiliensis and literature revision. Brazilian Journal of Microbiology. 2021;52(1):107–124. doi: 10.1007/s42770-020-00365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremião I. D. F., Schubach T. M. P., Pereira S. A., Rodrigues A. M., Chaves A. R., Barros M. B. Intralesional amphotericin B in a cat with refractory localised sporotrichosis. Journal of Feline Medicine and Surgery. 2009;11(8):720–723. doi: 10.1016/j.jfms.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremião I. D., Menezes R. C., Schubach T. M., Figueiredo A. B., Cavalcanti M. C., Pereira S. A. Feline sporotrichosis: Epidemiological and clinical aspects. Medical Mycology. 2015;53(1):15–21. doi: 10.1093/mmy/myu061. [DOI] [PubMed] [Google Scholar]
- Gutierre-Galhardo M. C., Freitas D. F S., Valle A. C. F., Almeida-Paes R., Almeida M. M. E., Zancopé-Oliveira R. M. Epidemiological aspects of sporotrichosis epidemic in Brazil. Epidemiological aspects of sporotrichosis epidemic in Brazil. Current Fungal Infection Reports. 2015;9(4):238–245. doi: 10.1007/s12281-015-0237-y. [DOI] [Google Scholar]
- Malik R., Vogelnest L., O’Brien C. R., White J., Hawke C., Wigney D. I., Martin P., Norris J. M. Infections and some other conditions affecting the skin and subcutis of the naso-ocular region of cats: Clinical experience (1987–2003) Journal of Feline Medicine and Surgery. 2004;6(6):383–390. doi: 10.1016/j.jfms.2004.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro H., Rodrigues A. M., Dias M. A. G., da Silva E. A., Bernardi F., de Camargo Z. P. Feline sporotrichosis due to Sporothrix brasiliensis: An emerging animal infection in São Paulo, Brazil. BMC Veterinary Research. 2014;10(1):269. doi: 10.1186/s12917-014-0269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasu C. C. T., Waller S. B., Ripoll M. K., Ferreira M. R. A., Conceição F. C., Gomes A. R., Osório L. G., de Faria R. P., Cleff M. B. Feline sporotrichosis: A case series of itraconazole-resistant Sporothrix brasiliensis infection. Brazilian Journal of Microbiology. 2021;52(1):163–171. doi: 10.1007/s42770-020-00290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira S. A., Passos S. R. L., Silva J. N., Gremião I. D. F., Figueiredo F. B., Teixeira J. L., Monteiro P. C. F., Schubach T. M. P. Response to azolic antifungal agents for treating feline sporotrichosis. The Veterinary Record. 2010;166(10):290–294. doi: 10.1136/vr.166.10.290. [DOI] [PubMed] [Google Scholar]
- Reis É. G., Schubach T. M. P., Pereira S. A., Silva J. N., Carvalho B. W., Quintana M., Gremião I. D. Association of itraconazole and potassium iodide in the treatment of feline sporotrichosis: A prospective study. Medical Mycology. 2016;54(7):684–690. doi: 10.1093/mmy/myw027. [DOI] [PubMed] [Google Scholar]
- Rodrigues A. M., Della Terra P. P., Gremião I. D., Pereira S. A., Orofino-Costa R., de Camargo Z. P. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020;185(5):813–842. doi: 10.1007/s11046-020-00425-0. [DOI] [PubMed] [Google Scholar]
- Sanchotene K. O., Madrid I. M., Klafke G. B., Bergamashi M., Terra P. P. D., Rodrigues A. M., De Camargo Z. P., Xavier M. O. Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses. 2015;58(11):652–658. doi: 10.1111/myc.12414. [DOI] [PubMed] [Google Scholar]
- Sharman M. J., Mansfield C. S. Sinonasal aspergillosis in dogs: A review. The Journal of Small Animal Practice. 2012;53(8):434–444. doi: 10.1111/j.1748-5827.2012.01245.x. [DOI] [PubMed] [Google Scholar]
- Souza C. P., Lucas R., Ramadinha R. H., Pires T. B. Cryosurgery in association with itraconazole for the treatment of feline sporotrichosis. Journal of Feline Medicine and Surgery. 2016;18(2):137–143. doi: 10.1177/1098612X15575777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza E. W., Borba C. M., Pereira S. A., Gremião I. D. F., Langohr I. M., Oliveira M. M. E., Oliveira R. V. C., Cunha C. R., Zancopé-Oliveira R. M., Miranda L. H. M., Menezes R. C. Clinical features, fungal load, coinfections, histological skin changes, and itraconazole treatment response of cats with sporotrichosis caused by Sporothrix brasiliensis. Scientific Reports. 2018;8(1):9074. doi: 10.1038/s41598-018-27447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll J., Ashe C. M., Trepanier L. A. Intravesicular administration of clotrimazole for treatment of candiduria in a cat with diabetes mellitus. Journal of the American Veterinary Medical Association. 2003;223(8):1156–1158, 1129. doi: 10.2460/javma.2003.223.1156. [DOI] [PubMed] [Google Scholar]
- Tomsa K., Glaus T. M., Zimmer C., Greene C. E. Fungal rhinitis and sinusitis in three cats. Journal of the American Veterinary Medical Association. 2003;222(10):1380–1384, 1365. doi: 10.2460/javma.2003.222.1380. [DOI] [PubMed] [Google Scholar]


