Abstract
Statistical models were used to predict the effects of tryptone, glucose, yeast extract (TGY) and Mn on biomass formation of the highly radioresistant bacterium Deinococcus radiodurans. Results suggested that glucose had marginal effect on biomass buildup, but Mn was a significant factor for biomass formation. Mn also facilitated glucose interactions with other nutrient components. These predictions were verified by in vivo and in vitro experiments. TGY-grown cells metabolized glucose solely by the pentose phosphate pathway (PPP). Although only a fraction of glucose from the medium was transported into the cells, glucose was incorporated into the DNA efficiently after cells were exposed to UV light. The presence of glucose also enhanced the radioresistance of the culture. Mn could induce an Embden-Meyerhof-Parnas (EMP) pathway in D. radiodurans. The EMP pathway and the PPP of the Mn-treated cells oxidized glucose simultaneously at a 6:1 ratio. Although glucose was hydrolyzed rapidly by the Mn-treated cells, most glucose was released as CO2. Mn-treated cultures retained less glucose per cell than cells grown without Mn, and still less glucose was incorporated into the DNA after cells were exposed to UV light. Mn-treated cells were also more sensitive to UV light. Results suggested that metabolites of glucose generated from the PPP enhanced the survival of D. radiodurans. Induction of the EMP pathway by Mn may deplete metabolites for DNA repair and may induce oxidative stress for the cell, leading to reduction of radioresistance.
The genus Deinococcus is composed of a group of bacteria characterized by their extreme resistance to ionizing radiation (1, 7, 9, 17–19). Most experts agree that Deinococcus radiodurans has a very effective system to repair its DNA, but the molecular basis of this effective system is not clear (14, 17, 18). It appears that D. radiodurans achieves its resistance to radiation by changing the operating conditions of many “ordinary” enzymes (3). Most researchers use the tryptone-glucose-yeast extract (TGY) medium to grow Deinococcus (American Type Culture Collection catalogue. 1996. American Type Culture Collection, Manassas, Va.) (19), even though these organisms are generally considered inactive to sugars (21). Chou and Tan (5) showed that the addition of Mn to a stationary-phase culture of D. radiodurans could induce new rounds of cell division. They termed this mode of growth Mn-induced cell division (MnCD) and suggested that MnCD is a form of starvation growth (23). The authors also showed that the addition of Mn to the culture also induced higher superoxide dismutase (SOD) and catalase activity in the culture. Interestingly, D. radiodurans also became more sensitive to radiation when Mn was included in the culture. Mn is required in many metabolic enzymes. In D. radiodurans, this element serves as a cofactor of SOD (12). The binding of Mn to the chromosomes of the intact cells may contribute to its resistance to radiation (14). Evans and Moseley (9) suggested that Mn served as a cofactor for the DNA repair endonuclease UV endonuclease β. Therefore, the presence of Mn should increase, rather than decrease, the radioresistance of the cells. It is more likely that the high concentrations needed for MnCD induction were involved in a different physiological response in the cell.
Microbiologists often assume that when nutrients are exhausted, or when waste products have accumulated to a toxic level, a culture will stop growing and enter the stationary phase. The initial objective of this study was to determine how MnCD could occur. Results showed that MnCD was complex but that MnCD was not likely due to starvation growth. In addition to MnCD, Mn also could induce a functional but futile glycolytic pathway that subsequently reduced the radioresistance of the cell.
MATERIALS AND METHODS
Organism and growth conditions.
D. radiodurans IR was a gift from S.-T. Tan, National Tsing Hua University, Taiwan, Republic of China (5, 26–28). Cells (25 ml) were grown at 30°C in a 250-ml sidearm flask and were incubated in an incubator shaker at 200 rpm. TGY medium was composed of 0.5% Difco tryptone, 0.1% glucose, and 0.3% yeast extract. TGY+Mn medium was TGY medium enriched with 0.1 mM MnCl2. TGY+Ba medium was TGY medium enriched with 0.1 mM BaCl2. TY medium was the same as TGY medium, but without added glucose. Unless otherwise specified, cells were pregrown in the test medium twice before inoculation. The size of inoculum was 0.05%. MnCD was induced by addition of MnCl2 (final concentration, 10 μM) to a 27-h culture of TGY-grown D. radiodurans culture.
At intervals, samples (0.2 ml) were removed from the cultures to determine the cell density and the amount of glucose in the medium. Cell number was determined by direct cell counting in a Petroff-Hausser bacterial counting chamber. Growth was also monitored photometrically using a Klett colorimeter with a green filter and by dry weight analysis. These methods of biomass measurement resulted in similar growth curves.
Chemical analysis.
Glucose concentration in the medium was determined by a glucose kit 510-DA from Sigma Chemical Co. (St. Louis, Mo.). Alternatively, glucose in the medium was separated by thin-layer chromatography (TLC) and was stained with silver nitrate as described by Wong (30). Protein concentration was quantified by the Coomassie blue assay (Bio-Rad, Richmond, Calif.) using bovine serum albumin as a standard.
Statistical models.
The effects of nutrients on total biomass formation were analyzed by a resolution V, four-way factorial design with 24 combinations of tryptone, yeast extract, glucose, and Mn prepared in triplicate. These combinations included high and low levels of glucose (0%, 0.05%, and 0.2%), yeast extract (0.1% and 0.4%), tryptone (0.1% and 0.5%), and manganese (0 and 100 μM). D. radiodurans growing on TGY medium (24 h) was used as an inoculum. After 40 h of incubation, the cell density of the culture in each tube was recorded.
The effects of nutrients on biomass production before and after MnCD were analyzed by a resolution V, three-way factorial design. The experiment consisted of 18 combinations of tryptone (0%, 0.1%, and 0.5%), yeast extract (0%, 0.06%, and 0.3%), and glucose (0% and 0.1%) prepared in triplicate. D. radiodurans growing on TGY medium (24 h) was used as an inoculum. After the culture reached stationary phase (27 h), the cell density of each culture was recorded as the initial biomass. Mn (0.1 mM final concentration) was then added to each culture to induce MnCD growth. After another 20 h of incubation, the cell density of the culture in each tube was recorded as the final biomass. The biomass of MnCD cells was the difference between the final biomass and the initial biomass.
The biomass variances between each set of samples were analyzed by the SAS program (6, 8) performed on a mainframe VAX computer. Results were expressed as distribution factors (F values), which were >0 and were defined as F (α, 1, γ2) = t2(α/2, γ/2) where t = Student's t test distribution; α = significance level; γ = degrees of freedom. P values of less than 0.001 were considered significant.
Radiorespirometry.
The metabolism of glucose in D. radiodurans was analyzed by trapping the 14CO2 released from the position-labeled [14C]glucose. Specifically, exponential-phase cultures of TGY- and TGY+Mn-grown cells (about 8.4 × 108 cells/ml) were washed and transferred into 15 ml of their corresponding medium in a 50-ml presterilized flask. The flask was immediately sealed with a rubber stopper with an inlet capillary tube and an outlet tube. The inlet capillary tube was cotton plugged at one end and was submerged into the sample medium at the other end. The outlet tube was connected to a train of three CO2 traps, each containing 25 ml of 1 M KOH. The last trap was connected to a vacuum pump, which generated a regulated negative pressure to allow a gentle air flow through the inlet tube to the cell sample and to the trapping tubes. The efficiency of trapping CO2 was better than 87%. The sample was aerated continuously by a magnetic stir bar. After 5 h of acclimation at room temperature, the experiment was initiated by the addition of 0.5 μCi of d-glucose-1-14C (specific activity = 54.3 mCi/mmol [Sigma]), or 0.5 μCi of d-glucose-3,4-14C (specific activity = 53.6 mCi/mmol [NEN, Boston, Mass.]), to the cell suspension. Duplicates of 100 μl of KOH were withdrawn from each trap every 20 min for up to 6 h. The KOH samples were mixed with 1 ml of Ecolit scintillation cocktail (ICN, Irvine, Calif.) for 14C determination by using a Beckman Model LS7000 scintillation counter. The remaining samples in the flask were checked for contamination at the end of the experiment. No contamination was detected. Results expressed in count-per-minute interval were calculated by using the present sample's count-per-minute value minus the count-per-minute value of the previous sample.
Effects of other metals.
Divalent metal chlorides, including MnCl2, ZnCl2, MgCl2, CaCl2, BaCl2, CuCl2, NiCl2, CoCl2, and FeCl2, were tested for their ability to induce cell growth and glucose consumption. Cultures were grown in 13-by-150-mm test tubes in triplicate. Each tube contained 1 ml of half-strength TY medium enriched with 5 mM glucose and 1 μl of d-glucose-UL-14C (specific activity = 3.7 mCi/mmole [Sigma]). Chloride salts were filter sterilized and added to the medium to the final concentration of 100 μM. A half-strength medium without added metal was used as a control. A culture that was pregrown on TGY medium was used as an inoculum. Culture tubes were placed in a test tube rack and were tilted at a 40° angle to maximize aeration. After 40 h, half of each sample was used to determine the cell density. The remaining cell sample was washed twice with 50 mM Tris-HCl (pH 7.5), and the cell pellets were suspended in 1 ml of Ecolit scintillation cocktail to determine the amount of radioisotope accumulated in the cells. Results were analyzed by one-way analysis of variance using SigmaStat software program (Jandel, Sausalito, Calif.). The significance level was set at 95%.
Resistance to UV irradiation.
Mid-log phase cultures of D. radiodurans growing on TY, TY+Mn, TGY, TGY+Ba, and TGY+Mn media were washed twice with distilled water. Cells were resuspended in phosphate buffer (50 mM, pH 7.0) to an optical density at 600 nm (OD600) of 0.6. Fifteen-milliliter samples of each cell suspension were transferred to sterile petri dishes and were exposed to UV irradiation (254 nm) at 3 Jm−2s−1 for various periods. The irradiated cells were serially diluted and plated on plate count agar (Difco). Colonies that appeared on the plates were counted 72 h after inoculation.
Incorporation of 14C from d-glucose into the bacterial DNA.
D. radiodurans was grown on 10 ml of TGY and TGY+Mn medium to mid-log phase. Two microcuries of d-glucose-UL-14C was added to each of the cultures. After 2 h of acclimation, each culture was divided into two halves. One half of each culture was transferred into a small petri dish (50-mm diameter). The petri dish (with the cover off) was placed under a UV light (254 nm) in an illumination box for UV treatment. The intensity of the UV irradiation was about 0.1 Jm−2s−1. The illumination box was placed on a rotor mixer to facilitate mixing of the bacteria. Samples were irradiated continuously for 5 h. The other half of the culture was treated similarly but without UV irradiation. After 5 h, 100 μl were withdrawn from each sample to determine the cell density and the amount of 14C present in the cells. The rest of the cells were adjusted to an OD600 of 0.6 and were used for DNA extraction. DNA from each sample was extracted by an established procedure (16, 20). The ratios of OD260 to OD280 of all extracted DNA samples were between 1.8 and 1.9. The extracted DNA was mixed with 5 ml of Ecolit scintillation cocktail for radioisotope counting.
Enzyme assay.
The cytoplasmic fraction was used to determine the activities if the pentose phosphate pathway (PPP) and the Embden-Meyerhof-Parnas (EMP) pathway. Glucose-6-phosphate dehydrogenase (G6PDH) was determined by monitoring the reduction of NADP at 340 nm. The reaction mixture contained 1 mM glucose-6-phosphate and 0.5 mM NADP in a total of 1 ml of 50 mM Tris-HCl buffer (pH 8.2). Reaction was initiated by the addition of the cell-free extracts (about 0.4 mg/ml) of TY-, TY+Mn-, TGY-, or TGY+Mn-grown cells or similarly by the addition of prepared cell-free extracts of Escherichia coli grown on TGY medium. One unit reduces 1 μmol of NADP per min at 25°C. The d-fructose 1,6-bisphosphate aldolase (ALD) activity was determined by a modification of the method of Jagannathan et al. (11) in which 3-phosphoglyceraldehyde reacts with hydrazine to form a hydrazone which absorbs at 240 nm. One unit is described as a change in absorbance of 1.00 per min at 25°C and pH 7.5. The reaction mixture contained 100 μl of cytoplasmic extract (about 0.2 mg/ml), 2 mM hydrazine, and 2.4 mM fructose 1,6-biphosphate in 50 mM Tris-HCl buffer (pH 7.5). Enzyme reaction was initiated by the addition of fructose 1,6-bisphosphate. Reaction mixture without cell-free extract was used as a reference blank.
RESULTS
We repeated the growth experiments described by Chou and Tan (5) and confirmed their results. Initial experiments showed that MnCD could still occur in double-strength and half-strength TGY media. The cell yields of D. radiodurans growing on these media were proportional to the concentrations of TGY. This suggests that the culture of D. radiodurans reached its stationary phase due to nutrient depletion, not due to accumulation of toxic materials. However, we also noticed that the cell yields of MnCD varied significantly. Further studies showed that the earlier Mn was added to the cultures, the greater was the MnCD response. Under our growth conditions, cultures older than 120 h never exhibited the MnCD response. Cell yield was highest when D. radiodurans was grown on TGY+Mn medium. However, cultures growing on TGY+Mn medium did not exhibit the MnCD response.
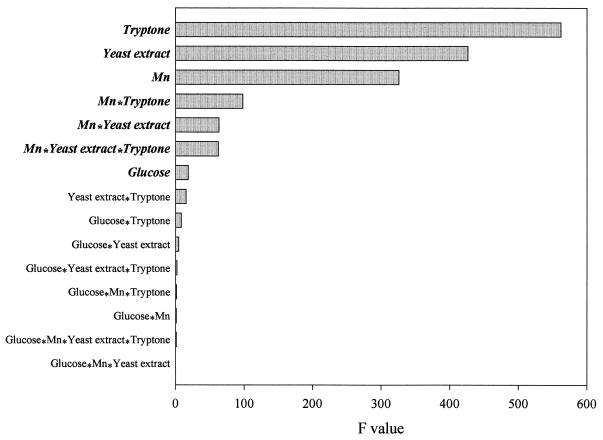
A two-level factorial design experiment was used to analyze the nutritional requirements and the interactions between these nutrient components on the biomass yield. Results (Fig. 1) showed that biomass formation in D. radiodurans was largely supported by tryptone and yeast extract. This supported the notion that this organism is proteolytic (21). Interestingly, Mn, an inorganic metal, was a significant factor in promoting biomass formation. The role of this metal in biomass formation was due to the strong interactions between this metal and tryptone, yeast extract, and tryptone-plus-yeast extract. Glucose also contributed to biomass formation. Nevertheless, the effect of glucose on biomass formation was relatively small. Results also revealed that there was no significant interaction between glucose and other nutrient components in promoting biomass formation.
FIG. 1.
A two-level factorial design experiment showing the effects of components in the TGY medium on the biomass formation in D. radiodurans. Parameters shown in bold and italic are those that had significant effects (P > 0.001) on biomass formation.
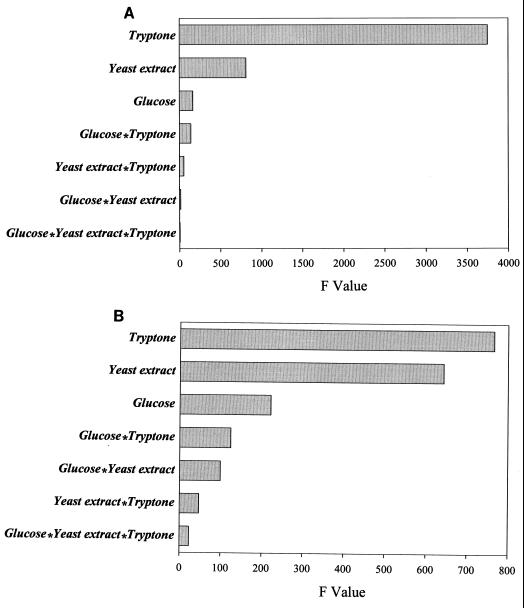
It should be noted that the statistical model presented in Fig. 1 does not identify events that occurred before or after Mn addition. Nutrient requirements for the MnCD-grown cell were obviously different from that of normal TGY-grown cells. To identify these differences, a multilevel factorial design experiment was used. Results are shown in Fig. 2. Before Mn addition, the source of nutrient that supported biomass formation was mainly from tryptone with minor contribution from yeast extract. The contribution of glucose to the biomass was only marginal (Fig. 2A). The biomass formed from MnCD cells was still protein based, with tryptone and yeast extract as major nutrients. However, the role of glucose in biomass formation became more significant (Fig. 2B). In addition, interactions between glucose and other nutrients have increased significantly. MnCD did not occur in media without tryptone. Cultures grown without glucose still exhibited MnCD response, albeit the response was smaller than those with glucose.
FIG. 2.
A multilevel factorial design experiment showing the effects of nutrient components on biomass formation before (A) and after (B) Mn-induced cell division. Only parameters that had a significant effect (P > 0.001) on biomass formation are shown.
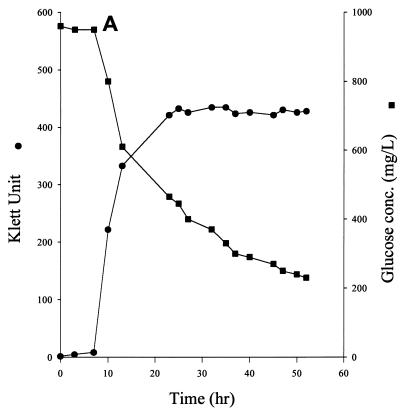
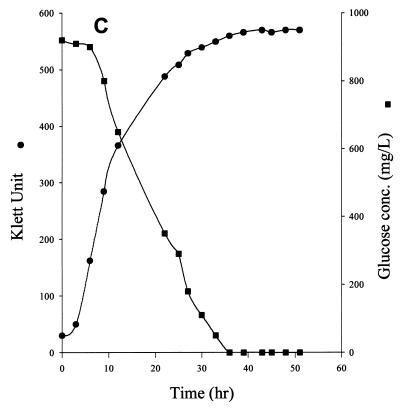
To confirm the glucose component of the above statistical models, we monitored the glucose concentration in the TGY medium. TGY-grown cells consumed glucose steadily. At mid-log phase, the rate of glucose consumption was about 4.9 μmol of glucose/h/108 cells. After the culture reached stationary phase, a significant amount of glucose was still found in the medium (Fig. 3A). Glucose in the medium was gradually depleted about 80 h after stationary phase was reached (data not shown).
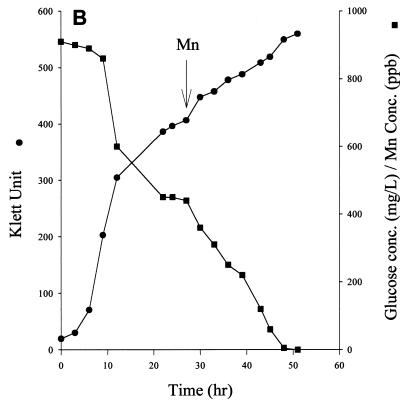
FIG. 3.
Effect of MnCl2 on cell growth and glucose consumption in D. radiodurans. Cultures were grown on TGY medium (A), TGY medium and induced MnCD response after stationary phase was reached (B), and TGY+Mn medium (C).
The addition of Mn to the stationary-phase culture of TGY-grown cells resulted in the rapid disappearance of glucose from the medium. The disappearance of glucose also coincided with MnCD (Fig. 3B). The rate of respiration of the culture during MnCD also increased by 20% (data not shown). Cells grew much faster on TGY+Mn medium than on TGY medium. The mid-log phase culture of TGY+Mn-grown cells consumed glucose rapidly at a rate of about 22 μmol/h/108 cells (Fig. 3C). Glucose was totally depleted when the culture reached stationary phase. The addition of Mn to the stationary-phase culture did not induce further cell growth (data not shown).
The possibility of glucose being transformed into other forms (such as gluconate) or other fermentative products was excluded. Carbohydrate analysis by TLC showed that glucose was the only major carbohydrate found in the medium during the entire growth period (data not shown).
The dual modes of glucose metabolism in D. radiodurans were confirmed by radiorespirometry assay (Fig. 4). Cells growing on TGY medium released CO2 from d-glucose-1-14C glucose slowly (Fig. 4A). About 3 h were needed to detect the peak of 14CO2 released from glucose-1-14C. The peak rate of CO2 production was about 5 μmol/h/108 cells. Only traces of 14CO2 could be detected when d-glucose-3,4-14C was used. These results suggested that TGY-grown cells use PPP to hydrolyze glucose.
FIG. 4.
Radiorespirometry analysis of D. radiodurans growing on TGY medium (A) and TGY+Mn medium (B). Carbon dioxide released after the addition of d-glucose-1-14C or d-glucose-3,4-14C to the culture was trapped by KOH.
TGY+Mn-grown cells (Fig. 4B) released 14CO2 rapidly from d-glucose-3,4-14C. Production of 14CO2 was detected almost immediately after d-glucose-3,4-14C was added to the culture. The optimal rate of CO2 production was about 17.8 μmol/h/108 cells. The TGY+Mn-grown cells also released 14CO2 from d-glucose-1-14C but at a slower rate (about 3 μmol/h/108 cells). The 14CO2 release profiles of MnCD cells were very similar to those of the TGY+Mn-grown cells (data not shown).
The marker enzymes of the PPP (G6PDH) and EMP pathway (ALD) in the cell-free extracts of TGY-, TGY+Mn-, TY-, and TY+Mn-grown cells were analyzed. Results are shown in Table 1. The G6PDH activities in the cell-free extracts of D. radiodurans grown on various media were essentially the same. This enzyme was NADP specific and could not be replaced by NAD. The G6PDH activity in cell-free extracts of D. radiodurans was about fourfold higher than the G6PDH activity found in cell-free extracts of E. coli.
TABLE 1.
Specific activities of G6PDH and fructose 1,6-bisphosphate ALD in cell-free extracts of D. radiodurans grown in TGY, TGY+Mn, TY, and TY+Mn media and E. coli grown on TGY medium
| Cell-free extracts | G6PDH (MU/mg of protein) | ALD (U/mg of protein) |
|---|---|---|
| TGY | 72 ± 10 | 0.039 ± 3 |
| TGY+Mn | 72 ± 9 | 0.084 ± 14 |
| TY | 74 ± 5 | >0.006 |
| TY+Mn | 68 ± 8 | 0.078 ± 11 |
| E. coli | 17 ± 3 | 0.088 ± 5 |
The ALD activity in cell-free extracts of TY-grown D. radiodurans was very low. The ALD activity in the cell-free extracts of TY+Mn- and TGY+Mn-grown D. radiodurans were comparable to the ALD activity in the cell-free extracts of E. coli grown on TGY medium. Interestingly, glucose could also induce the expression of the ALD. The ALD activity of D. radiodurans grown on TGY medium was about 65% that of D. radiodurans grown on TGY+Mn medium.
We used uniformly labeled glucose to analyze the amount of glucose incorporated into the biomass of TGY- and TGY+Mn-grown cells (Table 2). We also compared the effects of other divalent metals on cell growth and glucose incorporation into this organism. At stationary phase, about 12% of the glucose was found in the biomass of TGY-grown cells. This amounts to approximately 4.4% of the glucose incorporated per 108 cells. TGY+Mn-grown cells incorporated slightly less glucose per cell (about 4.1% per 108 cells) than TGY-grown culture. Several metals, including Co, Cu, and Zn, inhibited cell growth and glucose incorporation into the biomass. Interestingly, Ba did not inhibit cell growth but did inhibited glucose incorporation significantly. The inhibition of glucose consumption, but not cell growth, by BaCl2 supported the statistical model (Fig. 1) which predicted that glucose consumption was not essential for biomass formation in this organism. In a parallel study, E. coli grown on TY medium with 5 mM d-glucose-UL-14C incorporated more than 40% of the radioisotope into its biomass.
TABLE 2.
Effect of different divalent metals on the growth and glucose incorporation in D. radioduransa
| Metal (100 μM) | Cell density (108/ml) at stationary phase (%) | % Of glucose incorporated into 108 cells |
|---|---|---|
| Control | 2.70 ± 0.05 (100) | 4.4 |
| Mn | 3.55 ± 0.03 (132)b | 4.1 |
| Zn | 1.48 ± 0.29 (55)c | 2.8d |
| Mg | 2.52 ± 0.30 (93) | 3.9 |
| Ca | 3.11 ± 0.06 (115) | 4.4 |
| Ba | 2.79 ± 0.03 (103) | 2.8d |
| Cu | 1.24 ± 0.03 (46)c | 2.2d |
| Ni | 1.90 ± 0.02 (70) | 3.2 |
| Co | 0.32 ± 0.01 (12)c | 0.9d |
| Fe | 2.48 ± 0.12 (91) | 3.5 |
Cells were grown on 1 ml of half-strength TY medium enriched with 5 mM d-glucose-UL-14C plus the chloride salt of the test metal. Triplicate cultures were grown for 40 h and were washed and courted. Results were analyzed by one-way analysis of variance and were expressed as the means ± standard deviations. The significance level was set at 95%. About 43% of the glucose was incorporated into the biomass of E. coli grown on the control medium.
Mn significantly stimulated the cell growth.
Zn, Cu, and Co inhibited cell growth significantly.
Zn, Ba, Cu, and Co significantly inhibited the incorporation of glucose into the biomass.
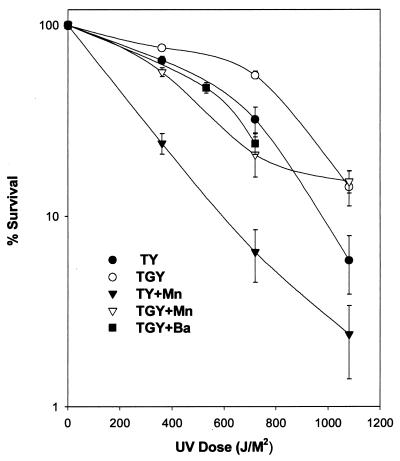
D. radiodurans is known for its resistance to radiation. Chou and Tan (5) showed that MnCD-grown cells were more sensitive to radiation than cells grown without Mn. We examined the UV resistance of D. radiodurans grown on TGY, TGY+Mn, TY, TY+Mn, and TGY+Ba media. Results (Fig. 5) confirmed that cells were more sensitive to UV light when Mn was present in the medium. However, we noticed that cells were more resistant to UV irradiation when glucose was present in the medium. Therefore, TGY-grown cells were the most resistant to UV irradiation, while TY+Mn-grown cells were the most sensitive to UV irradiation. Consistent with the effect of glucose enhancement on radioresistance, cells grown on TGY+Ba medium were also quite sensitive to UV irradiation.
FIG. 5.
Survival rates of D. radiodurans grown on TGY, TY, TGY+Mn, TY+Mn, and TGY+Ba media after UV irradiation.
We examined the amount of 14C from the glucose molecule incorporated into the DNA molecules of TGY-grown and TGY+Mn-grown cells to demonstrate a direct relationship between glucose metabolism and resistance to UV irradiation. TGY-grown D. radiodurans incorporated about 8% of the transported 14C into its DNA. The bacterium incorporated significantly more 14C (18%) into its DNA after the cell was irradiated by UV light. TGY+Mn-grown cells incorporated about 4% of their accumulated 14C into its DNA. The percentage increased to 6% after cells were exposed to UV irradiation (Table 3).
TABLE 3.
Effect of UV irradiation on the incorporation of 14C in D. radioduransa
| Growth medium | UV treatment | Total cpm accumulated per 108 cells | Total cpm found in DNA extracted per 108 cells | % Of 14C incorporated into DNA per cell |
|---|---|---|---|---|
| No | 5,186.5 | 414.3 | 8.0 | |
| TGY | Yes | 2,789.6 | 512.4 | 18.4 |
| No | 8,544.5 | 327.2 | 3.8 | |
| TGY+Mn | Yes | 8,009.1 | 474.6 | 5.9 |
Mid-log-phase cells were transferred to flasks containing 5 ml of fresh TGY medium plus 2 μCi of d-glucose-UL-14C with or without Mn. After 2 h of incubation at 30°C, half of each sample was illuminated with UV light for 5 h. The remaining half of each sample was incubated without UV treatment and was used as the control. Standard procedure was used for DNA extraction. Results were the mean values from triplicate samples (standard derivation was less than 2%).
DISCUSSION
Cell growth is a complex event involving many coordinated metabolic interactions. These interactions are often too complicated to be analyzed simultaneously. A statistical approach, such as the factorial design used in this study, is helpful to determine which few factors, out of many variables, have an important effect on a particular event. We analyzed the various nutrient components of TGY medium with or without Mn for their effects on biomass formation. Our models confirmed that D. radiodurans was a proteolytic organism, but Mn played a major role in biomass formation. Although this statistical model could not provide information on the mechanism of biomass formation, it did suggest that Mn-induced biomass formation was due to its strong interactions with the protein portion in the medium. The model further predicted a marginal participation of glucose in biomass buildup.
Initially, we focused on glucose, because this nutrient was only partially consumed by the cells during normal growth, but was totally depleted after MnCD (Fig. 3B). This observation seems to contradict our model (Fig. 2), which suggested that glucose played a significant, but not major, role in promoting biomass formation in both normal growth and in MnCD. Independent experimental results confirmed that glucose was not needed for MnCD to occur because TY-grown cultures also exhibited MnCD. In addition, the isotope-tracer experiment (Table 2) shows that only a small fraction of glucose was incorporated into the biomass of TGY-grown D. radiodurans. Still less glucose was incorporated into the biomass of TGY+Mn-grown cells. Thus, the induction of glucose consumption by Mn alone could not explain how Mn caused cell division in this organism.
The ability to oxidize but inefficiently incorporate glucose into biomass by D. radiodurans is contradictory to the general idea of glucose metabolism in a living cell. This led us to examine the glucose metabolism of this organism in detail. Raj et al. (25) showed that D. radiodurans has a functional PPP. We confirmed their results and further showed that the enzymatic activity of G6PDH in D. radiodurans was more than fourfold higher than the G6PDH activity in E. coli (Table 1). At mid-log phase, TGY-grown cells consumed glucose at a rate of about 4.9 μmol/h/108 cells (Fig. 3A). The cells also stoichiometrically released CO2 (about 5 μmol/h/108 cells) from the first carbon of the glucose (Fig. 4A) under optimal conditions. The close-to-equal molar ratio of uptake to C1 release suggested that the TGY-grown cells used the PPP exclusively for glucose metabolism.
Addition of Mn to the medium, either before cell inoculation or at stationary phase, resulted in rapid glucose consumption (Fig. 3B and C). These Mn-treated cells released the third and fourth carbons from the glucose molecule preferentially (Fig. 4B). The optimum rate of 14CO2 released from glucose at the third and fourth positions was about 18 μmol/h/108 cells. The Mn-treated cells also released 14CO2 from d-glucose-1-14C simultaneously, but at a slower rate. The optimum rate of 14CO2 production from the first carbon of glucose was about 3 μmol/h/108 cells. These results suggest that Mn-treated cells metabolized glucose via the EMP pathway and the PPP at a 6:1 ratio.
The above in vivo observations were verified by in vitro analysis of the marker enzymes of the EMP pathway (ALD) and PPP (G6PDH). Enzymatic assays (Table 1) showed that Mn alone could fully induce the ALD in D. radiodurans. The Mn-induced ALD activity in the cell-free extracts of D. radiodurans was comparable to the ALD activity found in the cell-free extracts of E. coli. Without Mn and glucose in the medium, D. radiodurans expressed a very low level of ALD activity. Much to our surprise, TGY-grown cells, which did not release CO2 from the third and fourth carbons of glucose in vivo (Fig. 4A), also exhibited the ALD activity. The specific activity of the ALD in the cell-free extracts of TGY-grown cells was about 40% of the ALD activity of the cell-free extracts of TY- or TGY+Mn-grown cells. Addition of Mn to the cell-free extracts of TGY- or TY-grown cells did not stimulate higher ALD activity (data not shown).
D. radiodurans may lack essential metabolic enzymes required to convert carbohydrates to amino acids effectively. Therefore, only a few molecules of glucose were incorporated into the biomass. TLC analysis of the spent media showed that glucose was the only carbohydrate in the medium during the entire growth period. This ruled out the possibility of incomplete oxidation of glucose had occurred. Thus, glucose could only be released mainly as CO2 by enzymes of the tricarboxylic acid cycle, which was fully functional in this organism (25). The potential energy released from glucose oxidation is very large (Gf° = −917.22). D. radiodurans might be able to trap this influx of energy to better utilize the remaining proteins in the medium. This may explain the increased interactions between glucose and other nutrients (Fig. 2B). Disposal of the electrons generated from glucose oxidation by the Mn-treated cells would also create oxidative stress for the cells. This might explain why D. radiodurans exhibited higher SOD and catalase activity after exposure to Mn (5). The inefficient usage of glucose by D. radiodurans might mimic the futile glucose metabolism in Azotobacter vinelandii. The imbalance supply of carbon-nitrogen energy was proposed to be the cause of the low biomass yield of glucose-supported growth of A. vinelandii (15).
Shifting the metabolic mode of glucose utilization would obviously affect the physiological state of the cell. PPP is essential for cell maintenance (2, 4, 10, 24). The products of PPP, ribose-5-P (R5P), glyceraldehyde-3-P (G3P), and NADPH+, are precursors for all deoxyribonucleotide triphosphates. Additionally, NADPH+ may be involved in many biosynthesis and antioxidative reactions. The flux through the PPP and thus the rate of precursors produced is controlled by the reaction rate of G6PDH and is dependent on the availability of its substrate, G6P (22). Therefore, pathways, such as the EMP, that compete for G6P can directly affect the formation of R5P, G3P, and NADPH+. On a nutritional basis, the availability of a suitable nutrient and the ability to secure high concentrations of substrates for the repair enzymes would be of prime importance for successful DNA repair. This is especially true for D. radiodurans, as accelerated DNA degradation appears to be part of the DNA repair process in this organism (29). The high levels of G6PDH in D. radiodurans suggest that the PPP pathway in this organism was very active. One would expect that cells growing on TGY medium would accumulate high levels of R5P, G3P, and NADPH+, which might be used for DNA repair. Experimental data (Table 3) shows that this may be the case. The TGY-grown cells readily incorporated glucose into their DNA when cells were exposed to UV light. The induction of an EMP pathway by Mn might deplete G6P and thus the source of these substrates. This may explain why only 6% of the glucose inside TGY+Mn-grown cells could be incorporated into their DNA after UV illumination (Table 3). This may also explain the increase in UV sensitivities of TGY+Mn-grown cells and those cells grown without glucose (Fig. 5). TGY+Ba-grown cells, which could not use glucose (Table 2), were also more sensitive to UV irradiation (Fig. 5). Figure 5 also reveals that D. radiodurans cultures grown on TGY, TY, TGY+Mn, and TGY+Ba exhibited a shoulder of resistance in which there was little loss of viability to approximately 500 J/M2. However, this typical shoulder of resistance was not detected in cells grown on TY+Mn medium. The reason for this lack of resistance was not clear.
D. radiodurans has been proposed to be used for bioremediation of radiation-contaminated sites. Recently, Lange et al. (13) constructed a recombinant strain of D. radiodurans that can degrade toluene and other organic chemicals. The induction of an active EMP pathway described herein would facilitate biodegradation, as this futile pathway could rapidly digest many carbonaceous chemicals to CO2 without creating significantly greater biomass. Research is in progress to characterize this Mn-induced EMP pathway at the molecular level.
ACKNOWLEDGMENTS
We thank S. T. Tan of the National Tsing Hua University, Taiwan, Republic of China, for the gift of the D. radiodurans IR strain. We are indebted to Professor Larry Steinrauf for reviewing our manuscript.
This work was supported in part by a grant (NSC88-2311-B110-013) from the National Science Council, Republic of China.
REFERENCES
- 1.Anderson A W, Nordan H C, Cain R F, Parrish G, Duggan D. Studies on a radio-resistant Micrococcus. I. Isolation, morphology, cultural characteristics, and resistance to γ-radiation. Food Technol. 1956;10:575–577. [Google Scholar]
- 2.Bannasch P, Klimek F, Mayer D. Early bioenergetic changes in hepatocarcinogenesis: preneoplastic phenotypes mimic responses to insulin and thyroid hormone. J Bioenerg Biomembr. 1997;29:303–313. doi: 10.1023/a:1022438528634. [DOI] [PubMed] [Google Scholar]
- 3.Battista J R. Against all odds: the survival strategies of Deinococcus radiodurans. Annu Rev Microbiol. 1997;51:203–224. doi: 10.1146/annurev.micro.51.1.203. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Yoseph O, Boxer P A, Ross B D. Oxidative stress in the central nervous system: monitoring the metabolic response using the pentose phosphate pathway. Dev Neurosci. 1994;16:328–336. doi: 10.1159/000112127. [DOI] [PubMed] [Google Scholar]
- 5.Chou F I, Tan S T. Manganese (II) induces cell division and increases in superoxide dismutase and catalase activities in an aging deinococcal culture. J Bacteriol. 1990;172:2029–2035. doi: 10.1128/jb.172.4.2029-2035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cody R P, Smith J K. Applied statistics and SAS programming language. 4th ed. Englewood Cliffs, N.J: Prentice-Hall, Inc.; 1997. p. 163. [Google Scholar]
- 7.Daly M J, Minton K W. Interchromosomal recombination in the extremely radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 1995;177:5495–5505. doi: 10.1128/jb.177.19.5495-5505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowdy S, Wearden S. Statistics for research. 2nd ed. New York, N.Y: John Wiley and Sons, Inc.; 1991. p. 407. [Google Scholar]
- 9.Evans D M, Moseley B E B. Identification and initial characterization of a pyrimidine dimer UV endonuclease (UV endonuclease β) from Deinococcus radiodurans, a DNA repair enzyme that requires manganese. Mutat Res. 1985;145:119–128. doi: 10.1016/0167-8817(85)90018-5. [DOI] [PubMed] [Google Scholar]
- 10.Flood M R, Wiebold J L. Glucose metabolism by preimplantation pig embryos. J Reprod Fertil. 1988;84:7–12. doi: 10.1530/jrf.0.0840007. [DOI] [PubMed] [Google Scholar]
- 11.Jagannathan V, Sing L, Damodaran M. Carbohydrate metabolism in citric acid fermentation. IV. Purification and properties of aldolase from Aspergillus niger. Biochem J. 1956;63:94–96. doi: 10.1042/bj0630094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juan J Y, Keeney S N, Gregory E M. Reconstitution of Deinococcus radiodurans aposuperoxide dismutase. Arch Biochem Biophys. 1991;286:257–263. doi: 10.1016/0003-9861(91)90038-k. [DOI] [PubMed] [Google Scholar]
- 13.Lange C C, Wackett L P, Minton K W, Daly M J. Engineering a recombinant Deinococcus radiodurans for organopollutant degradation in radioactive mixed waste environments. Nat Biotechnol. 1998;16:929–933. doi: 10.1038/nbt1098-929. [DOI] [PubMed] [Google Scholar]
- 14.Leibowitz P J, Schwartzberg L S, Bruce A K. The in vivo association of manganese with the chromosome of Micrococcus radiodurans. Photochem Photobiol. 1976;23:45–50. doi: 10.1111/j.1751-1097.1976.tb06769.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu J-K, Lee F-T, Lin C-S, Yao X-Y, Davenport J W, Wong T-Y. Alternative function of the electron transport system in Azotobacter vinelandii: removal of excess reductant by the cytochrome d pathway. Appl Environ Microbiol. 1995;61:3998–4003. doi: 10.1128/aem.61.11.3998-4003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meade H M, Long S R, Ruvkun C B, Brown S E, Ausubel F M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982;149:114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minton K W. DNA repair in the extremely radioresistant bacterium Deinococcus radiodurans. Mol Microbiol. 1994;13:9–15. doi: 10.1111/j.1365-2958.1994.tb00397.x. [DOI] [PubMed] [Google Scholar]
- 18.Minton K W. Repair of ionizing-radiation damage in the radiation resistant bacterium Deinococcus radiodurans. Mutat Res. 1996;363:1–7. doi: 10.1016/0921-8777(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 19.Moseley B E B. Photobiology and radiobiology of Micrococcus (Deinococcus) radiodurans. Photochem Photobiol Rev. 1983;7:223–274. [Google Scholar]
- 20.Murray M G, Thompson W F. Rapid isolation of high-molecular-weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray R G E, Brooks B W. Genus I. Deinococcus. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1035–1043. [Google Scholar]
- 22.Neidhard F C, Ingraham J L, Schaechter M. Physiology of the bacterial cell. Sunderland, Mass: Sinauer Associate Publishers; 1990. [Google Scholar]
- 23.Novitisk J A, Morit R Y. Morphological characterization of small cells resulting from nutrient starvation of a psychrophilic marine vibrio. Appl Environ Microbiol. 1976;32:617–622. doi: 10.1128/aem.32.4.617-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkins S N, Hursting S D, Haines D C, James S J, Miller B J, Phang J M. Chemoprevention of spontaneous tumorigenesis in nullizygous p53-deficient mice by dehydroepiandrosterone and its analog 16-alpha-fluoro-5-androsten-17-one. Carcinogenesis. 1997;18:989–994. doi: 10.1093/carcin/18.5.989. [DOI] [PubMed] [Google Scholar]
- 25.Raj H D, Duryee F L, Deeney A M, Wang C H, Anderson A W, Elliker P R. Utilization of carbohydrates and amino acids by Micrococcus radiodurans. Can J Microbiol. 1960;6:289–298. doi: 10.1139/m60-033. [DOI] [PubMed] [Google Scholar]
- 26.Tan S T, Maxcy R B. Simple method to demonstrate radiation-induced radiation resistance in microbial cells. Appl Environ Microbiol. 1986;51:88–90. doi: 10.1128/aem.51.1.88-90.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan S T, Maxcy R B, Thompson T L. Paper replication method for isolation of radiation-sensitive mutants. Appl Environ Microbiol. 1983;46:233–236. doi: 10.1128/aem.46.1.233-236.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan S T, Maxcy R B. Inactivation and injury of a hemolytic radiation-resistant micrococcus isolated from chicken meat. J Food Sci. 1982;47:1345–1349. [Google Scholar]
- 29.Varghese A F, Day R S. Excision of cytosine-thymine adduct from the DNA of ultraviolet-irradiated Micrococcus radiodurans. Photochem Photobiol. 1970;11:511–517. doi: 10.1111/j.1751-1097.1970.tb06022.x. [DOI] [PubMed] [Google Scholar]
- 30.Wong T Y. Melibiose is hydrolyzed exocellularly by an inducible exo-α-galactosidase in Azotobacter vinelandii. Appl Environ Microbiol. 1990;56:2271–2273. doi: 10.1128/aem.56.7.2271-2273.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]