Abstract
Folate (vitamin B9) and its biologically active derivatives are well-known antioxidant molecules protecting cells from oxidative degradation. The presence of high glucose, often found in diabetic patients, causes oxidative stress resulting in cellular stress and inflammatory injury. Cells in organs such as the lung are highly prone to inflammation, and various protective mechanisms exist to prevent the progressive disorders arising from inflammation. In the present study, the synthetic form of folate, i.e. folic acid, and active forms of folate, i.e. 5-methyltetrahydrofolate and 10-formyltetrahydrofolate, were evaluated for their antioxidant and antiinflammatory potential against high glucose (50 mM)–mediated oxidative stress and inflammation in BEAS-2B cells, an immortalised bronchial epithelial cell line. High glucose treatment showed a 67% reduction in the viability of BEAS-2B cells, which was restored to the viability levels seen in control cultures by the addition of active folate derivatives to the culture media. The DCFH-DA fluorometric assay was performed for oxidative stress detection. The high glucose–treated cells showed a significantly higher fluorescence intensity (1.81- and 3.8-fold for microplate assay and microscopic observation, respectively), which was normalised to control levels on supplementation with active folate derivatives. The proinflammatory NF-κB p50 protein expression in the active folate derivative–supplemented high glucose–treated cells was significantly lower compared to the folic acid treatment. In support of these findings, in silico microarray GENVESTIGATOR database analysis showed that in bronchiolar small airway epithelial cells exposed to inflammatory condition, folate utilization pathway genes are largely downregulated. However, the folate-binding protein gene, which encodes to the folate receptor 1 (FOLR1), is significantly upregulated, suggesting a high demand for folate by these cells in inflammatory situations. Supplementation of the active folate derivatives 5-methyltetrahydrofolate and 10-formyltetrahydrofolate resulted in significantly higher protection over the folic acid from high glucose–induced oxidative stress and inflammation. Therefore, the biologically active folate derivatives could be a suitable alternative over the folic acid for alleviating inflammatory injury-causing oxidative stress.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11626-022-00691-w.
Keywords: Antioxidant, Diabetes, Folate receptor, Lung, Inflammation
Introduction
Folate (vitamin B9) is a well-known antioxidant molecule where the biologically active derivatives of this vitamin, the tetrahydrofolate (THF) forms, have significantly higher antioxidant activity which is comparable to those of other antioxidant molecules such as ascorbic acid (vitamin C) (Gliszczynska-swiglo 2007). This vitamin plays an inevitable role in one-carbon metabolism, nucleic acid synthesis, and methylation of DNA, apart from acting as co-factor and co-substrate of many reactions (Stanger 2002). Occurrence of neural tube defects (NTD), anaemia, and numerous other adverse health conditions such as cardiovascular disease and cancer have been linked to folate deficiency (Chanarin et al. 1958; Medical Research Council [MRC] Vitamin Study Group 1991; Lee et al. 2011). In nature, the folates exist as polyglutamate forms, and the major forms of food folates are identified as 5-methyltetrahydrofolate (5-MTHF; 5-CH3-H4folate), 5-formyltetrahydrofolate, and 10-formylfolate (Vahteristo et al. 1997; Pfeiffer et al. 2015). In humans, it is mainly seen as 5-MTHF (~ 80% in serum), 5,10-methylene-THF, and 10-formyltetrahydrofolate (10-FTHF; 10-HCO-H4folate) (Stanger 2002). Membrane-bound forms of high-affinity folate-binding proteins mediate the transport of folate compounds across plasma membranes in humans (Henderson 1990). As the cellular folate in the epithelial cell is highly prone to degradation than in the bloodstream, the folate receptor is highly expressed in epithelial cells to meet the increased demand for folate (Weitman et al. 1992). The bronchial epithelial cells are the frontline of cells in the lung that are actively involved in preventing the entrance of foreign molecules and pathogens into the lung (Nicod 1999). Hence, these cells are constantly subjected to inflammatory stress arising from reactive oxygen species (ROS) production that is managed by the supply of antioxidant molecules such as folates. Also, folate helps in the cell division required for replenishing the lung epithelium as the wear and tear of the lung epithelial cells are very high compared to those of the cells of other organs in the body (Hamilton et al. 2001).
ROS are involved in a wide spectrum of diseases, including chronic inflammation. High glucose is one of the major inflammatory agents shown to aggravate the inflammatory condition by causing progressive apoptosis of the tissues such as lung epithelial cells and related disorders (Jansen et al. 2013; Rogliani et al. 2018). The adverse effects of ROS make the person further susceptible to bacterial and viral infections arising through the lung epithelium, including COVID-19, and increase the morbidity risk (Zhu et al. 2020). Folate is often found helpful in preventing and ameliorating the pathogenesis associated with lung disorders (Stidley et al. 2010). A study done amongst smokers with inflammation revealed that those who had maintained a better nutritional uptake and adequate serum folate levels had a better lung function (Leng et al. 2017). Also, short-term supplementation of folic acid in healthy overweight subjects has revealed the potential role of folate as a therapeutic agent for inflammatory ailments (Solini et al. 2006).
Folic acid, the synthetic form of folate, itself is inactive and is converted to its reduced active derivatives in vivo on consumption via a series of enzymatic reactions (Bailey and Ayling 2009). However, the efficiency of enzymatic conversion of folic acid to its active forms in humans is very low (Bailey and Ayling 2009). Methyl THFs are found to be a suitable alternative for folic acid as these are the active form. 5-MTHF and vitamin B12 supplementation increased the bioavailability of active form of folate and reduced free radical cell injury in cystic fibrosis–affected children (Scambi et al. 2009). Likewise, a high ratio of serum 5-MTHF level is positively associated with lung function (Han et al. 2020).
Although the antioxidant and antiinflammatory properties of folate and its biologically active derivatives are known, no information is available on the protective effect of folate supplementation in reducing the proinflammatory oxidative stress due to high glucose in lung epithelial cells. The present study evaluated the protective effect of the active folate derivatives over folic acid in high glucose–induced oxidative stress and inflammation using the human bronchial epithelial BEAS-2B cell line. The two most common natural folates, 5-MTHF and 10-FTHF, were used as the biologically active folate derivatives. The high glucose–treated cells, supplemented with active folate derivatives and folic acid, were investigated and compared for the cell viability, intracellular ROS generation, and NF-κB p50 protein expression. In silico analysis of gene expression levels of the folate-related genes was included in this study to understand the folate demand in inflammatory conditions.
Materials and methods
Chemicals and reagents
Folic acid and 5-MTHF disodium salt were purchased from Sigma (Sigma–Aldrich, Mumbai, India). 10-FTHF was from Schircks Laboratories (Jona, Switzerland). Dulbecco’s modified Eagle medium (DMEM), minimum essential medium Eagle (MEM), phosphate-buffered saline (PBS), penicillin–streptomycin solution, foetal bovine serum (FBS), trypsin–EDTA solution, bovine serum albumin (BSA), and d-glucose were obtained from HiMedia (Mumbai, India). Dichlorofluorescein diacetate (DCFH-DA) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were procured from Sigma (Sigma–Aldrich, India). Antibodies were procured from Cloud-Clone (Cloud-Clone Corporation, Houston, TX).
Cell culture and treatment
The BEAS-2B (ATCC CRL-9609) cell line, derived from human bronchial epithelial cells, was purchased from American Type Culture Collection (ATCC) (date obtained: 07 May 2019; ATCC, Manassas, VA; invoice no. 1920063, Mercury Instruments and Chemicals, Mumbai, India). The data of the authentication performed by the ATCC for the cell line is available in the ATCC STR (short tandem repeats) database. Furthermore, re-authentication (STR analysis) of the cell line was performed by the National Centre for Cell Science (NCCS), Pune, India, on 6 January 2022 (STR profile report, Supplementary Data, Fig. 1). The stocks of the BEAS-2B cells were cryopreserved from the initial passage received and were verified for the absence of mycoplasma contamination using DAPI staining and fluorescent microscopy (Jung et al. 2003). All experiments were performed with mycoplasma-free cells from the original stocks for a maximum of 15 passages. The BEAS-2B cells were maintained in DMEM containing 10% FBS, penicillin 100 IU/mL, and streptomycin 100 µg/mL in a humidified atmosphere containing 5% CO2 in air at 37 °C (Park et al. 2008). The culture medium was changed every 48 h, and the cells were subcultured every week at a 1:20 split ratio by treatment with the trypsin–EDTA solution (do Carmo et al. 2020). Cells were plated for 24 h before different treatments at the indicated concentrations for the various assays. Co-treatments of BEAS-2B cells with high glucose and folates were performed in serum-free media (MEM containing 5 mM glucose). Untreated BEAS-2B cells served as control, and the cells treated with 50 mM d-glucose for 24 h were considered the hyperglycaemic group (Zhang et al. 2019). Working solutions of folic acid and biologically active folate derivatives were made freshly by dissolving each folate form in 0.1 M potassium phosphate (pH 8–8.5) buffer (Osseyi et al. 2001). The folate content in the DMEM medium (0.004 g/L) was considered the base level of folate concentration. The twofold and threefold higher concentrations of folic acid than the base level, i.e. 0.008 and 0.012 g/L, were chosen for the folate supplementation studies. The effective folate concentration was used for further studies with the active folate derivatives.
Cell viability test
Cell viability was measured by the MTT assay according to Park et al. (2008). Cells were seeded on 96-well microtiter plates with 2 × 104 cells in 100 µL media per well. After 24 h of stabilisation, cells were exposed to 50 mM glucose co-treated with folic acid (0.008 and 0.012 g/L) for 24 h. At the end of the exposure, 40 µL of MTT solution (2 mg/mL) was added, and the cells were incubated for 4 h at 37 °C. Media were discarded and the purple formazan crystals formed were dissolved with 200 µL of dimethyl sulfoxide (DMSO), and the absorbance was read at 540 nm using the Epoch microplate spectrophotometer system (BioTek, Winooski, VT). The absorbance is directly proportional to the number of viable cells. The viability of the treated group was expressed as a percentage of the non-treated control group, which was assumed to be 100%. Cell viability assay for the active folate derivatives, i.e. 5-MTHF and 10-FTHF (0.012 g/L), was also carried out in the same manner to ascertain the protective effect of the biologically active forms of folate.
Determination of folate antioxidant activity
The high glucose–induced ROS generation was assessed using the intracellular oxidation of DCFH-DA, a fluorogenic dye (John and Arockiasamy 2021). BEAS-2B cells (1.67 × 105 cells) were plated onto glass coverslips (24 × 24 mm) and incubated overnight. Cells grown to the confluence were co-treated with 50 mM glucose and two different concentrations of folic acid (0.008 g/L and 0.012 g/L) for 24 h, washed with PBS, and then incubated with 20 µM DCFH-DA for 30 min at 37 °C. At the end of DCFH-DA incubation, cells were washed with PBS and lysed with 1 mL of NaOH (1 M) solution, and the aliquots were transferred to a black well plate. Then, the fluorescence of dichlorofluorescein (DCF), the oxidised product of DCFH-DA, was measured using a multimode plate reader (Infinite-M200 Pro, Tecan, Mannedorf, Switzerland) with excitation and emission wavelengths of 485 nm and 530 nm, respectively. Fluorescence intensity correlates to ROS generation. Fluorescence generation was also visualised directly after DCFH-DA incubation using a fluorescence microscope (Olympus CKX53, Tokyo, Japan) with excitation and emission wavelengths of 485 nm and 530 nm, respectively, and an exposure time of 1/100 to 1/130 s, and quantified using ImageJ software (NIH ImageJ: National Institute of Health, Bethesda, MD, version 1.22). The fluorescence intensity was represented as a percentage and was considered as 100% for control. Experiments were repeated with 0.012 g/L active folate derivatives (5-MTHF and 10-FTHF).
Western blot analysis
The BEAS-2B cells were pre-incubated for 24 h for stabilisation. The cells were then treated with glucose (50 mM) in the presence of folic acid and active derivatives of folate (0.012 g/L) for 24 h. After incubation, the cells were collected and washed twice with cold PBS. Protein samples were prepared by cell lysis using RIPA buffer containing protease inhibitor cocktail (1:100 dilution; Sigma–Aldrich, India). The cell lysates were centrifuged at 10,000 rpm (C-30 Plus, REMI, Mumbai, India) and 4 °C for 10 min, and the supernatants were collected and immediately used for analysis. Protein concentration in cell lysate was measured by the Bradford method (Bradford 1976). Aliquots of the lysates (25–30 μg of protein) were separated on 10% SDS–polyacrylamide gels and transferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, CA) with glycine transfer buffer (192 mM glycine, 25 mM Tris–HCl (pH 8.8), and 20% methanol (v/v)). After blocking non-specific sites with 5% BSA in Tris-buffered saline 0.1% polysorbate 20 (TBST), the membrane was incubated overnight at 4 °C with the NF-κB p50 (NF-κB1) primary antibody. Each membrane was further incubated for 1 h with a secondary horseradish peroxidase–linked guinea pig anti-rabbit antibody. Immunoreactive proteins were detected using an enhanced chemiluminescence detection kit (ECL; Bio-Rad, Hercules, CA). Densitometric analysis was carried out to calculate the protein band intensity using Image Lab software (ImageLab, version 4.1, Bio-Rad), and the results were normalised to control antibody, glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
In silico gene expression analysis
Important genes in the folate metabolism (DHFR (dihydrofolate reductase), TYMS (thymidylate synthase), MTHFR (methylenetetrahydrofolate reductase), MTR (5-methyltetrahydrofolate-homocysteine methyltransferase)) as well as the folate uptake–associated gene (FOLR1), in the lung epithelial cell, were analysed for their change in expression under the inflammation-inducing condition. Screening of the targeted gene expression profile was done with the help of GENEVESTIGATOR, the online expression database for the meta-analysis of transcriptomes (Hruz et al. 2008). Briefly, under the human gene panel tool, the perturbation option was chosen for the respective gene selection. The study number HS-00565(2) (smoking study 31/smoking study 30) was selected as the source study for in silico analysis. The selected study in the Genevestigator tool compares the gene expression of tracheal epithelial cells of control group (tracheal epithelial cells from healthy smokers) to gene expression of bronchiolar small airway epithelial cells of an experimental group (bronchiolar small airway epithelial cells from healthy smokers) and displays the RNA expression values which were used for the present study.
Statistical analysis
All data are presented as mean ± SD and are the results of three independent experiments. The significance of the values was analysed by one-way ANOVA followed by Tukey’s test using Prism 5 software. p < 0.05 was considered to be statistically significant.
Results
Effect of folates on cell viability
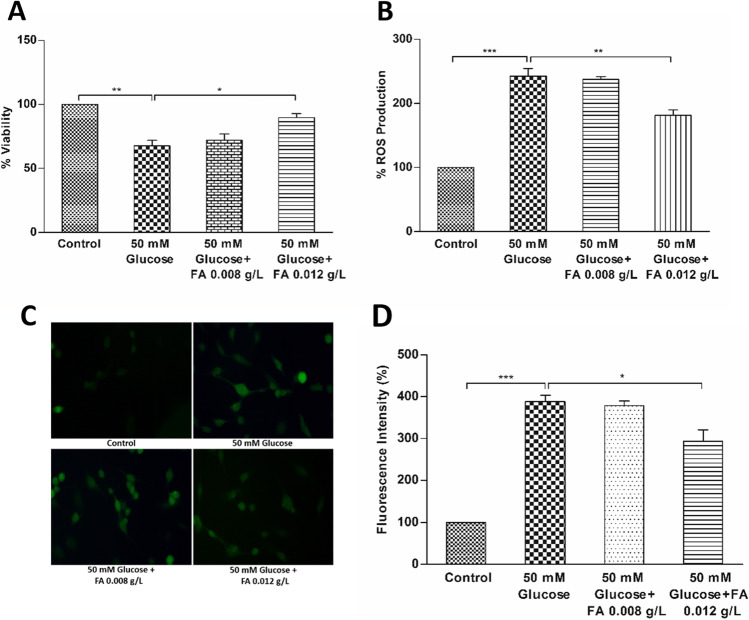
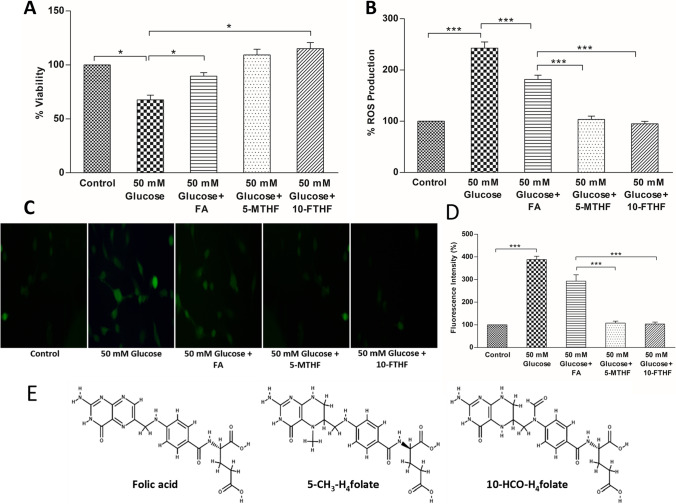
The cell viability data is presented in Fig. 1A and Fig. 2A. The viability of 50 mM glucose–treated BEAS-2B cells significantly decreased to 67% of control. Supplementation of high glucose medium with folic acid at 0.008 g/L and 0.012 g/L restored the cell viability to 72% and 89% respectively of control, suggesting that folic acid offered dose-dependent protection to BEAS-2B cells against high glucose–induced cytotoxicity (Fig. 1A). The viability of cells further improved and restored to the viability levels seen in control cultures on the addition of active folate derivatives (5-MTHF and 10-FTHF at 0.012 g/L) to the culture media (Fig. 2A). Therefore, the result suggests that the supplementation of biologically active folate derivatives offered a better protective effect against the cytotoxicity over folic acid.
Figure 1.
Cell viability and ROS production in high glucose (50 mM)–treated and folic acid (FA) (0.008 and 0.012 g/L)–supplemented BEAS-2B cells. (A) Percentage cell viability of high glucose–treated and folic acid–supplemented cells. (B) Percentage ROS production in high glucose–treated and folic acid–supplemented cells. (C) Microscopic images showing fluorescence intensity of high glucose–treated and folic acid–supplemented cells using DCFH-DA staining. (D) Quantification of ROS production in terms of fluorescence intensity of microscopic images by ImageJ software. The results are presented as mean ± SD (n =3), and the significance of the values was analysed by one-way ANOVA followed by Tukey’s test (p < 0.05). *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 2.
Cell viability and ROS production in BEAS-2B cells grown in high glucose medium and supplemented with 0.012 g/L of folic acid (FA) or active folate derivatives (5-MTHF and 10-FTHF). (A) Percentage cell viability of high glucose–treated and folic acid and folate derivative–supplemented cells. (B) Percentage ROS production in high glucose–treated and folic acid and folate derivative–supplemented cells. (C) Microscopic images showing fluorescence intensity of high glucose–treated and folic acid and active folate derivative–supplemented cells using DCFH-DA staining. (D) Quantification of ROS production in terms of fluorescence intensity of microscopic images by ImageJ software. (E) Chemical structure of folic acid and its active derivatives 5-MTHF (5-CH3-H4folate) and 10-FTHF (10-HCO-H4folate) (retrieved from PubChem). The results are presented as mean ± SD (n =3), and the significance of the values was analysed by one-way ANOVA followed by Tukey’s test (p < 0.05). *p < 0.05, **p < 0.01, ***p < 0.001 (Note: The data for 0.012 g/L FA– supplemented high glucose–treated BEAS-2B cells is
taken from Fig. 1).
Suppression of total ROS generation
The BEAS-2B cells exposed to 50 mM glucose exhibited a 2.4-fold higher fluorescence intensity of oxidised DCF when compared with the control (Fig. 1B), indicating that the treatment with high glucose augmented the ROS generation in the cells. The supplementation of high glucose–treated cells with 0.008 g/L folic acid did not show any significant suppression of ROS generation, and the fluorescence intensity remained similar to high glucose–treated cells. Supplementation of 0.012 g/L folic acid showed a slight suppression (~ 25%) in the fluorescence intensity in high glucose–treated cells, which continued to remain 1.81-fold higher than the control cells (Fig. 1B). On the other hand, the fluorescence intensity of the high glucose–treated cells supplemented with active folate derivatives (5-MTHF and 10-FTHF) at 0.012 g/L was similar to control, suggesting that the folate derivatives were able to prevent the induction of ROS production (Fig. 2B). Observations obtained from the microscopic fluorescence corroborated the results obtained with the fluorescent microplate reader method of DCFH-DA assay (Fig. 1C; Fig. 2C). The high glucose–treated cells showed a 3.8-fold higher fluorescence intensity compared to control. As observed with the microplate reader assay, the fluorescence intensity of the 0.012 g/L folic acid–supplemented high glucose–treated cells showed only a slight suppression of about 24%, which remained 2.9-fold higher than control (Fig. 1D). The fluorescence intensity of the high glucose–treated cells supplemented with 0.012 g/L of both the active folate derivatives was similar to control (Fig. 2D). The results showed that while the folic acid offered little protection against oxidative stress, the active derivatives of folate were highly efficient in ROS quenching and prevented ROS generation in high glucose–treated BEAS-2B cells.
NF-κB protein expression analysis
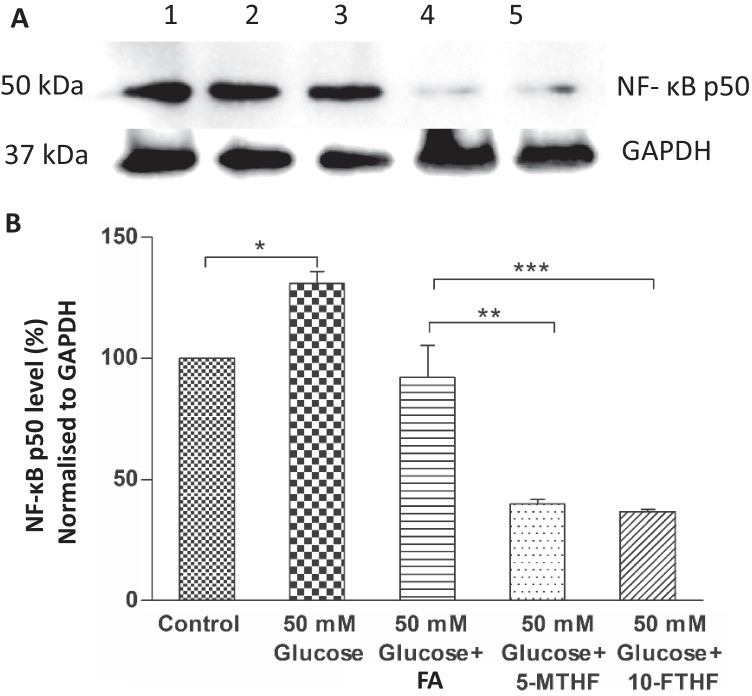
The present study evaluated the effect of folates on the expression of NF-κB P50 protein in high glucose–treated BEAS-2B cells (Fig. 3A). The densitometric analysis of protein bands is presented in Fig. 3B. The NF-κB P50 protein expression was about 31% higher in high glucose–treated cells compared to control. The expression of NF-κB P50 protein in 0.012 g/L folic acid–supplemented high glucose–treated cells was comparable to control. However, the expression of the NF-κB P50 protein in high glucose-treated cells supplemented with 0.012 g/L of 5-MTHF and 10-FTHF was significantly lower (60% and 44% respectively) than the control.
Figure 3.
Inhibitory effect of folates (folic acid (FA), 5-MTHF, and 10-FTHF; 0.012 g/L) supplementation on NF-κB p50 protein expression in high glucose (50 mM)–treated BEAS-2B cells.(A) Western blot analysis to determine NF-κB p50 expression after 24-h treatment; 1. Control, 2. 50 mM Glucose, 3. 50 mM Glucose+FA, 4. 50 mM Glucose+5-MTHF, 5. 50 mM Glucose+10-FTHF. (B) Quantification of NF-κB p50 protein bands normalised to GAPDH using densitometric analysis by Image Lab software. The results are presented as mean ± SD (n =3) and the significance of the values was analysed by one-way ANOVA followed by Tukey’s test (p < 0.05). *p < 0.05, **p < 0.01, ***p < 0.001.
In silico microarray data of folate-related gene expression
Microarray data available on the database Genevestigator was analysed for the expression of folate pathway-related genes under the inflammatory condition in lung epithelial cells. The comparison of the expression of important genes of tracheal epithelial cells with bronchiolar small airway epithelial cells in inflammatory conditions using the Genevestigator study, HS-00565 (2) (smoking study 31/smoking study 30), revealed a six-fold higher expression of FOLR1, an important folate uptake associated gene, in bronchiolar small airway epithelial cells. Except for the MTR (methionine biosynthesis) gene that showed slight upregulation, the other important genes in the folate utilisation pathway, i.e. TYMS (the methyltransferase that contributes to the de novo mitochondrial thymidylate biosynthesis pathway) and MTHFR (catalyses the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a co-substrate for homocysteine remethylation to methionine), were found to be significantly downregulated in bronchiolar small epithelial cells (Fig. 4). Microarray analysis using the Genevestigator tool also showed the maximum expression of the folate transporter gene in the lungs (Supplementary Data, Fig. 2).
Figure 4.
In silico analysis of relative expression of folate pathway–related important genes under the inflammatory condition in bronchiolar small airway epithelial cells compared to tracheal epithelial cells (Genevestigator study number: HS-00565(2)). FOLR1, folate receptor alpha; DHFR, dihydrofolate reductase; TYMS, thymidylate synthase; MTHFR, methylenetetrahydrofolate reductase; MTR, 5-methyltetrahydrofolate-homocysteine methyltransferase. (A) Expression profile presented as heat map generated by Genevestigator. (B) Relative gene expression expressed as fold change. All data were normalised in the Genevestigator platform and the significance of the values is represented as *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
The high glucose level, often associated with diabetic conditions, leads to oxidative stress and is identified as one of the major inflammation-causing agents in the manifestation of diabetic complications (Giugliano et al. 1996). The high glucose treatment has been shown to promote ROS generation and cell apoptosis (Zhang et al. 2019; Wu et al. 2020). In the present study, performed with bronchial epithelial cells, high glucose has been shown to induce oxidative stress as indicated by the decrease in cell viability, and increased fluorescence intensity in DCFH-DA-treated cells. A similar increase in fluorescence intensity due to ROS production has been reported in BEAS-2B cells treated with cerium oxide nanoparticles (Park et al. 2008). The present study has shown that the supplementation of folates has a beneficial effect on reducing oxidative stress and inflammation, which is in accordance with the study by Feng et al. (2011) on evaluating the inhibitory effect of folic acid on lipopolysaccharide-induced inflammation in RAW 264.7 cells. The present study also suggests that biologically active folate derivatives, 5-MTHF and 10-FTHF, provide better protection over folic acid. The higher viability compared to control observed in the cells treated with active folate derivatives (Fig. 2A) could be due to fluctuations between experiments (± SD). However, the observation could also be attributed to the significant protection imparted by the active folate derivatives from ROS, as the antioxidant activity of active folate derivatives is shown to be comparable to that of ascorbic acid (vitamin C) and vitamin E (Gliszczynska-swiglo, 2007). The results are supported by the earlier observation (Gliszczynska-swiglo 2007) on evaluating the antioxidant activity of folate, where the Trolox equivalent antioxidant capacity (TEAC) assessment listed the much-reduced activity of folic acid (0.06 ± 0.01) when compared to the biologically active folate 5-MTHF (0.77 ± 0.04). Folates (pteroylmonoglutamate, PteGlu) are made up of a pterin moiety that is linked to p-aminobenzoic acid coupled to the glutamate moiety (Gliszczynska-swiglo 2007). In folic acid, the pterin moiety is fully oxidised and exists as a fully double-bonded conjugated system, thereby making folic acid electron-deficient, limiting its free radical scavenging activity (Fig. 2E). When the pterin ring is in its reduced form, as seen in the tetrahydrofolates, optimal antioxidant activity is achieved, and it is presumed that an electron-donating effect of the 5-amino groups is a major factor in the antioxidant activity of tetrahydrofolates (Rezk et al. 2003).
Though acute inflammation is beneficial to the host, chronic inflammation may predispose the host to various chronic illnesses. Inflammatory cells produce soluble mediators that activate signal transduction cascades as well as induce changes in transcription factors, such as nuclear factor ΚB (NF-κB), to coordinate immediate cellular stress responses (Reuter et al. 2010; Xu et al. 2021). Hence, the increased NF-κB p50 protein expression in the present study signifies a plausible cause of inflammation by high glucose treatment. A similar increase in NF-κB p50 gene expression was observed by Araújo et al. (2019) in rats with periodontal disease, which was downregulated after treatment with gliclazide, an anti-diabetic medication. The reduced expression of NF-κB p50 protein in high glucose–treated folate-supplemented BEAS-2B cells in the present study indicates the antioxidant potential of folates that can, in turn, turn off cellular stress responses. The canonical and most common functional form of the NF-κB family is the p50-p65 dimer that drives the expression of proinflammatory genes. It has been shown that by targeting NF-κB p50 protein, one can attenuate inflammation through suppression of NF-κB activation (Xia et al. 2004). The present study suggests that a very low expression of NF-κB p50 protein in folate derivative–supplemented cells can potentially inhibit NF-κB activation by interfering with p50-p65 dimerisation, thus suppressing inflammatory gene expression. As the active folate derivatives showed a significantly reduced NF-κB p50 protein expression compared to folic acid, the results suggest that the active folate derivatives could be an effective alternative to folic acid as therapeutic agents for inflammatory conditions.
As folates are prone to oxidative degradation, the stability of the reduced folates decreases with the increase in reactive oxygen species (ROS) in the cells, which necessitates an increased demand for the biologically active form of folate. In silico analysis showed a significant upregulation (sixfold) of folate uptake gene (FOLR1) in bronchiolar small airway epithelial cells in inflammatory condition, indicating a higher demand for folate by these cells when exposed to inflammatory condition. Increased expression of FOLR1 by the bronchiolar small airway epithelial cells that are subjected to oxidative stress or inflammation might be for the uptake of available extracellular folate to alleviate the oxidative stress–induced damage. This high uptake of folate by the cells towards cytoprotection would leave a very scarce supply of available folate for folate utilisation pathways, making these pathway genes to get downregulated. This suggests that very high folate levels are required to alleviate the oxidative stress–induced clinical manifestations. To alleviate such oxidative stress seen in heart diseases and stroke etc., higher doses, up to 5 mg/d of folic acid, have been supplemented, which is fivefold higher than the upper tolerable limit (Bailey and Ayling 2009). As the conversion of folic acid to tetrahydrofolate form by the enzyme DHFR in humans is about 2–3% in comparison to that in rat liver at physiological pH, the high doses of folic acid administered for therapeutic purposes will considerably escalate exposure to circulating unmetabolised folic acid (Bailey and Ayling 2009). At amounts above 200 µg/d, folic acid itself is not fully converted to its active derivatives and appears to be in the unmetabolised form in plasma (Kelly et al. 1997). The results of the present study conform to the earlier studies, which have shown that 5-MTHF is more effective than folic acid (Lamers et al. 2006). As the 5-MTHF has a safe profile for a higher dosage, its use in food ingredients as well as for therapeutic purposes in place of folic acid is being supported (Niederberger et al. 2019).
Dietary intake of antioxidants is a plausible and effective way to augment and fortify endogenous defence systems since many antioxidants act as free radical scavengers and immunomodulators, resulting in cytoprotection. Being an essential vitamin, humans cannot synthesise folate de novo and they depend on plants and bacteria (Basset et al. 2002). Green leafy vegetables, legumes, mushrooms, and the liver are good sources of folates (Strain et al. 2017). The results from the present study suggest that the folate derivatives, i.e. 5-MTHF and 10-FTHF, demonstrate a promising capability to reduce hyperglycaemia-induced oxidative stress and inflammatory response of NF-kB expression in bronchial epithelial BEAS-2B cells under high glucose culture.
Conclusions
In the present study, the folate derivatives 5-MTHF and 10-FTHF were more effective than the folic acid in protecting high glucose–treated BEAS-2B cells. In high glucose–treated cells, supplementation of active folate derivatives restored the cell viability to control level. The fluorescence intensity of the high glucose–treated cells supplemented with both the active folate derivatives was similar to control in DCFH-DA-treated cells. The in silico analysis suggested a higher expression of FOLR1 in bronchiolar small airway epithelial cells under inflammatory conditions, indicating a higher demand for folates by these cells. The active folate derivatives provided better protection against inflammation as the expression of NF-κB p50 in the active folate derivative–supplemented high glucose–treated BEAS-2B cells was lower than in the folic acid–supplemented cells. The study, therefore, suggests that biologically active derivatives of folates could be a potential alternative to folic acid for effective antioxidant and antiinflammatory applications.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
AP acknowledges the CSIR, Govt. of India, for the award of Junior Research Fellowship. The authors thank Director, CSIR-CFTRI, for constant encouragement. The authors thank NCCS, Pune, for their analytical support in cell line authentication. The authors thank Ms. Arya Devi and Ms. Anagha N. for their valuable help and inputs in cell culture–related experiments and Dr. V. Baskaran for his valuable suggestions as a doctoral advisory committee member. The authors are grateful to Dr. Jayadeep A. for the critical evaluation of the manuscript.
Author contribution
Ajana Pathikkal: investigation, formal analysis, and writing—original draft. Bijesh Puthusseri: formal analysis, writing—original draft. Peethambaran Divya: investigation and formal analysis. Sudha Rudrappa: writing—review and editing. Vikas Singh Chauhan: conceptualisation, supervision, formal analysis, writing—review, and editing.
Declarations
Conflict of interest
The authors declare no competing interests.
Contributor Information
Ajana Pathikkal, Email: ajanadihara@gmail.com.
Bijesh Puthusseri, Email: bputhusseri@cftri.res.in.
Peethambaran Divya, Email: divyaptn@gmail.com.
Sudha Rudrappa, Email: sudharudrappa10@gmail.com.
Vikas Singh Chauhan, Email: vikas@cftri.res.in.
References
- Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci. 2009;106(36):15424–15429. doi: 10.1073/pnas.0902072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset G, Quinlivan EP, Ziemak MJ, Diaz de la Garza R, Fischer M, Schiffmann S, Bacher A, Gregory JF, Hanson AD. Folate synthesis in plants: the first step of the pterin branch is mediated by a unique bimodular GTP cyclohydrolase I. Proc Natl Acad Sci. 2002;99(19):12489–12494. doi: 10.1073/pnas.192278499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chanarin I, Mollin DL, Anderson BB. Folic acid deficiency and the megaloblastic anaemias. Proc Roy Soc Med. 1958;51:757–763. doi: 10.1177/003591575805100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo AAd, Morais HBd, Medeiros CACXd, Brito GAdC, Guedes PMM, Hiyari S, Pirih FQ, Araújo RFd Jr (2019) Gliclazide reduced oxidative stress, inflammation, and bone loss in an experimental periodontal disease model. J Appl Oral Sci 27. 10.1590/1678-7757-2018-0211 [DOI] [PMC free article] [PubMed]
- do Carmo MCL, Martins IM, Magalhães AER, Júnior MRM, Macedo JA. Passion fruit (Passiflora edulis) leaf aqueous extract ameliorates intestinal epithelial barrier dysfunction and reverts inflammatory parameters in Caco-2 cells monolayer. Food Res Int. 2020;133:109162. doi: 10.1016/j.foodres.2020.109162. [DOI] [PubMed] [Google Scholar]
- Feng D, Zhou Y, Xia M, Ma J. Folic acid inhibits lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages by suppressing MAPKs and NF-κB activation. Inflamm Res. 2011;60(9):817–822. doi: 10.1007/s00011-011-0337-2. [DOI] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19(3):257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- Gliszczynska-swiglo A. Folates as antioxidants. Food Chemistry. 2007;101(4):1480–1483. doi: 10.1016/j.foodchem.2006.04.022. [DOI] [Google Scholar]
- MRC Vitamin Study Research Group Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. The Lancet. 1991;338(8760):131–137. doi: 10.1016/0140-6736(91)90133-A. [DOI] [PubMed] [Google Scholar]
- Hamilton LM, Davies DE, Wilson SJ, Kimber I, Dearman RJ, Holgate ST (2001) The bronchial epithelium in asthma–much more than a passive barrier. Monaldi Arch Chest Dis=Archivio Monaldi per Le Malattie Del Torace 56(1):48–54 [PubMed]
- Han Y-Y, Forno E, Rosser F, Celedón JC. Serum folate metabolites, asthma, and lung function in a nationwide US study. J Allergy Clin Immunol. 2020;146(1):220–222.e8. doi: 10.1016/j.jaci.2020.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GB. Folate-binding proteins. Ann Rev Nutr. 1990;10(1):319–335. doi: 10.1146/annurev.nu.10.070190.001535. [DOI] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes [Resource Review]. Adv Bioinformatics; Hindawi. 10.1155/2008/420747 [DOI] [PMC free article] [PubMed]
- Jansen F, Yang X, Franklin BS, Hoelscher M, Schmitz T, Bedorf J, Nickenig G, Werner N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc Res. 2013;98(1):94–106. doi: 10.1093/cvr/cvt013. [DOI] [PubMed] [Google Scholar]
- John CM, Arockiasamy S. 3, 5-Dimethoxy-4-benzoic acid (syringic acid) a natural phenolic acid reduces reactive oxygen species in differentiated 3T3-L1 adipocytes. In Vitro Cell Dev Biol Anim. 2021;57(4):386–394. doi: 10.1007/s11626-021-00549-7. [DOI] [PubMed] [Google Scholar]
- Jung H, Wang SY, Ding WH et al (2003) Detection and treatment of mycoplasma contamination in cultured cells. Chang Gung Med J 26:250–258 [PubMed]
- Kelly P, McPartlin J, Goggins M, Weir DG, Scott JM. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr. 1997;65(6):1790–1795. doi: 10.1093/ajcn/65.6.1790. [DOI] [PubMed] [Google Scholar]
- Lamers Y, Prinz-Langenohl R, Brämswig S, Pietrzik K. Red blood cell folate concentrations increase more after supplementation with [6 S]-5-methyltetrahydrofolate than with folic acid in women of childbearing age. Am J Clin Nutr. 2006;84(1):156–161. doi: 10.1093/ajcn/84.1.156. [DOI] [PubMed] [Google Scholar]
- Lee JE, Willett WC, Fuchs CS, Smith-Warner SA, Wu K, Ma J, Giovannucci E. Folate intake and risk of colorectal cancer and adenoma: modification by time. Am J Clin Nutr. 2011;93(4):817–825. doi: 10.3945/ajcn.110.007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng S, Picchi MA, Tesfaigzi Y, Wu G, Gauderman WJ, Xu F, ... Belinsky SA (2017) Dietary nutrients associated with preservation of lung function in Hispanic and non-Hispanic white smokers from New Mexico. Int J Chron Obstruct Pulmon Dis 12:3171 [DOI] [PMC free article] [PubMed]
- Nicod L (1999) Pulmonary defence mechanisms. Respiration 66(1):2–11 [DOI] [PubMed]
- Niederberger KE, Dahms I, Broschard TH, Boehni R, Moser R. Safety evaluation of calcium L-methylfolate. Toxicol Rep. 2019;6:1018–1030. doi: 10.1016/j.toxrep.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osseyi ES, Wehling RL, Albrecht JA. HPLC determination of stability and distribution of added folic acid and some endogenous folates during breadmaking. Cereal Chem J. 2001;78(4):375–378. doi: 10.1094/CCHEM.2001.78.4.375. [DOI] [Google Scholar]
- Park E-J, Choi J, Park Y-K, Park K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology. 2008;245(1–2):90–100. doi: 10.1016/j.tox.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CM, Sternberg MR, Fazili Z, Yetley EA, Lacher DA, Bailey RL, Johnson CL. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J Nutr. 2015;145(3):520–531. doi: 10.3945/jn.114.201210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezk BM, Haenen GRMM, van der Vijgh WJF, Bast A. Tetrahydrofolate and 5-methyltetrahydrofolate are folates with high antioxidant activity. Identification of the antioxidant pharmacophore. FEBS Lett. 2003;555(3):601–605. doi: 10.1016/S0014-5793(03)01358-9. [DOI] [PubMed] [Google Scholar]
- Rogliani P, Ora J, Di Daniele N, Lauro D. Pleiotropic effects of hypoglycemic agents: implications in asthma and COPD. Curr Opin Pharmacol. 2018;40:34–38. doi: 10.1016/j.coph.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Scambi C, De Franceschi L, Guarini P, Poli F, Siciliano A, Pattini P, Biondani A, La Verde V, Bortolami O, Turrini F, Carta F, D’Orazio C, Assael BM, Faccini G, Bambara LM. Preliminary evidence for cell membrane amelioration in children with cystic fibrosis by 5-MTHF and vitamin B12 supplementation: a single arm trial. PLoS ONE. 2009;4(3):e4782. doi: 10.1371/journal.pone.0004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solini A, Santini E, Ferrannini E. Effect of short-term folic acid supplementation on insulin sensitivity and inflammatory markers in overweight subjects. Int J Obes. 2006;30(8):1197–1202. doi: 10.1038/sj.ijo.0803265. [DOI] [PubMed] [Google Scholar]
- Stanger O. Physiology of folic acid in health and disease. Curr Drug Metab. 2002;3(2):211–223. doi: 10.2174/1389200024605163. [DOI] [PubMed] [Google Scholar]
- Stidley CA, Picchi MA, Leng S, Willink R, Crowell RE, Flores KG, Kang H, Byers T, Gilliland FD, Belinsky SA. Multivitamins, folate, and green vegetables protect against gene promoter methylation in the aerodigestive tract of smokers. Can Res. 2010;70(2):568–574. doi: 10.1158/0008-5472.CAN-09-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain JJ, Hughes C, Pentieva K, Ward M, Hoey L, McNulty H (2017) The B-Vitamins. In H. K. Biesalski, A. Drewnowski, J. T. Dwyer, J. J. Strain, P. Weber, & M. Eggersdorfer (Eds.), Sustainable Nutrition in a Changing World (pp. 185–203)
- Vahteristo L, Lehikoinen K, Ollilainen V, Varo P. Application of an HPLC assay for the determination of folate derivatives in some vegetables, fruits and berries consumed in Finland. Food Chemistry. 1997;59(4):589–597. doi: 10.1016/S0308-8146(96)00318-4. [DOI] [Google Scholar]
- Weitman SD, Weinberg AG, Coney LR, Zurawski VR, Jennings DS, Kamen BA. Cellular localization of the folate receptor: potential role in drug toxicity and folate homeostasis. Can Res. 1992;52(23):6708–6711. [PubMed] [Google Scholar]
- Wu F, Wang F, Yang Q, Zhang Y, Cai K, Liu L, Li S, Zheng Y, Zhang J, Gui Y. Upregulation of miRNA-23a-3p rescues high glucose-induced cell apoptosis and proliferation inhibition in cardiomyocytes. In Vitro Cell Dev Biol Anim. 2020;56(10):866–877. doi: 10.1007/s11626-020-00518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y-F, Ye B-Q, Li Y-D, Wang J-G, He X-J, Lin X, Yao X, Ma D, Slungaard A, Hebbel RP. Andrographolide attenuates inflammation by inhibition of NF-κB activation through covalent modification of reduced cysteine 62 of p50. J Immunol. 2004;173(6):4207–4217. doi: 10.4049/jimmunol.173.6.4207. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhang T, Hu X, Xie Y, Wu R, Lian S, Wang J. Exosomal miR-193b-5p as a regulator of LPS-induced inflammation in dairy cow mammary epithelial cells. In Vitro Cell Dev Biol Anim. 2021;57(7):695–703. doi: 10.1007/s11626-021-00596-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xi X, Mei Y, Zhao X, Zhou L, Ma M, Liu S, Zha X, Yang Y. High-glucose induces retinal pigment epithelium mitochondrial pathways of apoptosis and inhibits mitophagy by regulating ROS/PINK1/Parkin signal pathway. Biomed Pharmacother. 2019;111:1315–1325. doi: 10.1016/j.biopha.2019.01.034. [DOI] [PubMed] [Google Scholar]
- Zhu L, She Z-G, Cheng X, Qin J-J, Zhang X-J, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen M-M, … Li H (2020) Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 31(6):1068–1077.e3. 10.1016/j.cmet.2020.04.021 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.