Abstract
Simple Summary
Childhood glioblastoma is an aggressive brain tumor in children that has a very poor prognosis. Standard therapy includes surgery, irradiation and chemotherapy with temozolomide. So far, there is no effective drug treatment for pediatric glioblastoma patients. This systematic review aims to outline currently available data on novel pharmacological treatment options. None of the included phase II studies showed any benefit regarding overall survival or a prolongation of stable disease. New genomic technologies discovered the biologic heterogeneity of these tumors, demanding more individualized immunotherapeutic and targeted approaches. Autoimmune modulated therapies and further targeting of tumor-specific receptors provide promising preclinical results. Clinical trials aligned to the tumor characteristics are needed to establish effective new therapeutic approaches.
Abstract
Background: Pediatric glioblastoma (GBM) is an aggressive central nervous system tumor in children that has dismal prognosis. Standard of care is surgery with subsequent irradiation and temozolomide. We aimed to outline currently available data on novel pharmacological treatments for pediatric GBM. Methods: We conducted a systematic literature search in PubMed and Embase, including reports published in English from 2010 to 2021. We included randomized trials, cohort studies and case series. Phase I trials were not analyzed. We followed PRISMA guidelines, assessed the quality of the eligible reports using the Newcastle-Ottawa scale (NOS) and the RoB-2 tool and registered the protocol on PROSPERO. Results: We included 6 out of 1122 screened reports. All six selected reports were prospective, multicenter phase II trials (five single-arm and one randomized controlled trial). None of the investigated novel treatment modalities showed any benefit regarding overall or progression free survival. Conclusions: To date, the role of pharmacological approaches regarding pediatric GBM remains unclear, since no novel treatment approach could provide a significant impact on overall or progression free survival. Further research should aim to combine different treatment strategies in large international multicenter trials with central comprehensive diagnostics regarding subgrouping. These novel treatment approaches should include targeted and immunotherapeutic treatments, potentially leading to a more successful outcome.
Keywords: pediatric glioblastoma, novel therapeutics, treatment resistance, targeted therapy
1. Introduction
Central nervous system (CNS) tumors are the most common form of solid tumors in children and account for the majority of cancer mortality in this age group [1]. Pediatric CNS tumors represent a heterogenous group with different histology, molecular varieties and biological behavior.
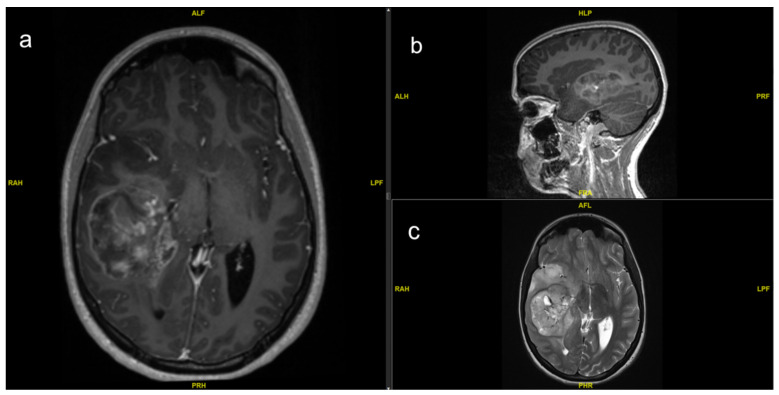
Pediatric glioblastoma (GBM) is most often localized supratentorial, whereas cerebral hemispheres account for approximately half of the cases [2]. Tumor location in the infratentorial region is associated with poor survival [3]. As an important first diagnostic tool, contrast enhanced magnetic resonance imaging (MRI) exhibits either rim or heterogeneous enhancement (Figure 1). Tumors with rim enhancement were shown to have better prognosis [4]. T1-native and contrast enhanced MRI provides information of a necrotic center of the tumor mass and areas with disrupted blood–brain barrier (BBB). T2-weight imaging provides information about the disease progress to peripheral structures due to higher water content in this tissue [5]. Diffusion-weight imaging (DWI) helps to distinguish between cerebral abscess and tumor-suspect lesions [6]. Further options to better classify the lesion, especially in deep located tumors that are difficult to biopsy, are magnetic resonance spectroscopy (MRS) since brain tumors demonstrate a reduced N-acetylaspartate (NAA) and creatine level and increased choline levels [7].
Figure 1.
Preoperative MRI of a 14-year-old girl with right temporal GBM with heterogeneous enhancement in (a) axial and (b) sagittal T1-weight with contrast agent. The tumor mass causes a midline shift and shows central enhancement. (c) Axial T2-sequence with hyperintense signal of infiltrating tumor mass peripheral to enhancing center in the mesial and temporopolar structures.
GBM is classified by the WHO as grade IV glioma, amplifying the aggressiveness and resistance to currently available treatment options [8]. Pediatric GBM accounts for 2.9% of all histologies amongst pediatric CNS tumors in the USA and is most prevalent in children from 10–14 years of age [1].
The poor prognosis of pediatric GBM is reflected by a median survival of only 13–43 months after diagnosis [9,10,11]. The five-year overall survival (OS) for children and adolescents diagnosed with GBM is <20% [12]. Different molecular, and hence outcome behaviors, can be observed in infant and congenital GBM. The definition of infant GBM typically refers to children younger than three to five years of age [13], whereas congenital GBM is defined as presence at birth and represents the most seldom type [14]. Congenital GBM shows the worst prognosis limited to approximately two months due to higher tendency of intracranial hemorrhage [15], while infant GBM tends to differ in clinical outcome from pediatric patients even with incomplete resection with improved five-year OS of 66% [16,17]. However, early relapse in infant GBM is frequent, reflected by a five-year event free survival (EFS) of less than 30% [16,18].
The standard of care for pediatric GBM older than three years of age is gross total resection (GTR) with subsequent irradiation, typically with 50–60 Gy, and temozolomide (TMZ), currently offering the best OS [11,19,20]. At times GTR is not feasible, subtotal resection (STR) is offered, since it was shown to improve OS and progression free survival (PFS) as well [21,22]. TMZ, an inherent part of GBM treatment in adults in combination with irradiation [23], showed lower toxicity than other drug regimens with comparable effectiveness and was therefore added to the standard treatment [24]; although improved outcome could not be shown with the addition of TMZ [25,26]. TMZ is an alkylating agent, which works most effectively in patients with methylated MGMT promoter. However, it was shown that pediatric GBM tumor cells display MGMT promoter methylation significantly less often [26,27] and, therefore, showed a less effective response to TMZ as expected [25,26]. Children younger than three years of age receive surgery as standard treatment and chemotherapy if feasible [28]. Irradiation is not recommended due to severe neurocognitive sequelae and is often not mandatory initially, due to a better response to chemotherapy than older children [16]. Most patients still require irradiation in the relapse situation and long-term sequelae are severe, as these children are often still very young [16].
Pediatric GBM have a high molecular heterogeneity compared to adult GBM but also within its group. Six distinct epigenetic and biological subgroups of pediatric and adult GBM have been defined through DNA methylation studies [29,30]. These six methylation clusters include K27, G34, IDH, receptor tyrosine kinase (RTK) I and II and mesenchymal cluster. They show a distinct age distribution. The K27 (Lys27Met) cluster appeared predominantly in the pediatric population, while the G34 (Gly34Arg) cluster was more frequently detected in adolescents [1]. The most common somatic alterations in infant high-grade glioma (HGG) involve neurotrophin receptor tyrosine kinase 1/2/3 (NTRK1/2/3) genes and have been described in 40% of non-brainstem HGG in infants, including infant GBM [2]. The other clusters were less specific for the pediatric age group [1] (Table 1). Hence, different possible targets have been the focus of research for new therapeutic approaches during the past decade. The K27 and G34 clusters are H3-mutant gliomas. H3K27M induces histone modifications that can be targeted directly by histone deacetylase (HDAC) or demethylation inhibitors [31,32,33]. H3G34R/V gliomas result in an upregulation of MYCN. PDGFRA mutations were identified as major drivers in H3G34R/V mutant gliomas, leading to a further therapeutic target [34]. Synergy was demonstrated for the PDGFRA antagonist dasatinib in combination with the mTOR inhibitor everolimus in pediatric patients [35]. H3- and isocitrate dehydrogenase (IDH) wildtype gliomas show distinct molecular subtypes with mutations such as BRAFV600E or RTK fusions (such as NTRK fusions). BRAF- and mitogen-activated protein kinase kinase (MEK) inhibitors showed promising results in pediatric LGG [36,37,38] while efficacy in HGG is less well understood. Tropomyosin receptor kinase (TRK) fusion-positive gliomas can be targeted with larotrectinib, a highly selective small-molecule inhibitor of TRK fusion positive gliomas [39]. A further subgroup is EGFR amplificated glioma, where newer EGFR inhibitors showed improved BBB penetration with better clinical efficacy in salvage therapy [40,41]. Further novel approaches to pediatric GBM are immunotherapeutic strategies, such as cancer vaccines, monoclonal antibodies, immune checkpoint inhibitors and chimeric antigen receptor (CAR)-T cells.
Table 1.
Subdivision of pediatric GBM into three distinct molecular subgroups.
| Category | Mutation/Cytogenetics | Age Distribution | Tumor Location | Prognosis |
|---|---|---|---|---|
| H3-Mutant |
H3K27M H3G34R/V |
younger children [42] adolescents and young adults [42,43] |
almost exclusively in midline structures (=DMG) [42] cerebral hemispheres [29] |
near 100% mortality [44] better OS than H3K27 mutations [44] |
| IDH-Mutant | IDH 1/2 mutation | older children/young adults [42,43] | cerebral hemispheres, frontal or temporal lobe [29] | better OS than H3-mutants [43] |
| IDH/H3-Wildtype |
BRAFV600E, NF1 mutations, RTK fusions amplifications of EGFR, CDK6, MYCN PDGFRA and MET amplifications |
infants/children/adolescents rare in children and adolescents [29] children and adolescents, rather rare [45] |
supratentorial, commonly hemispheric [29,44] occur throughout the brain [44] hemispheric and midline [42] |
increased survival [42,45] worst OS of WT group [42] poor OS [42,45] |
H3 = histone 3, H3K27M = lysine-to-methionin mutation at position 27 in histone 3.1, 3.2 or 3.3; DMG = diffuse midline glioma, OS = overall survival, H3G34R/V = glycine-to-valin or arginine at position 34 in histone 3.3, IDH = isocitrate dehydrogenase, NF1 = neurofibromatosis type 1, RTK = receptor tyrosine kinase, EGFR = epidermal growth factor receptor, CDK6 = cyclin dependent kinase 6, MYCN = proto-oncogene, WT = wildtype, PDGFRA = platelet derived growth factor receptor alpha.
The aim of this systematic review is to outline currently available data on novel pharmacological treatment options for pediatric GBM.
2. Materials and Methods
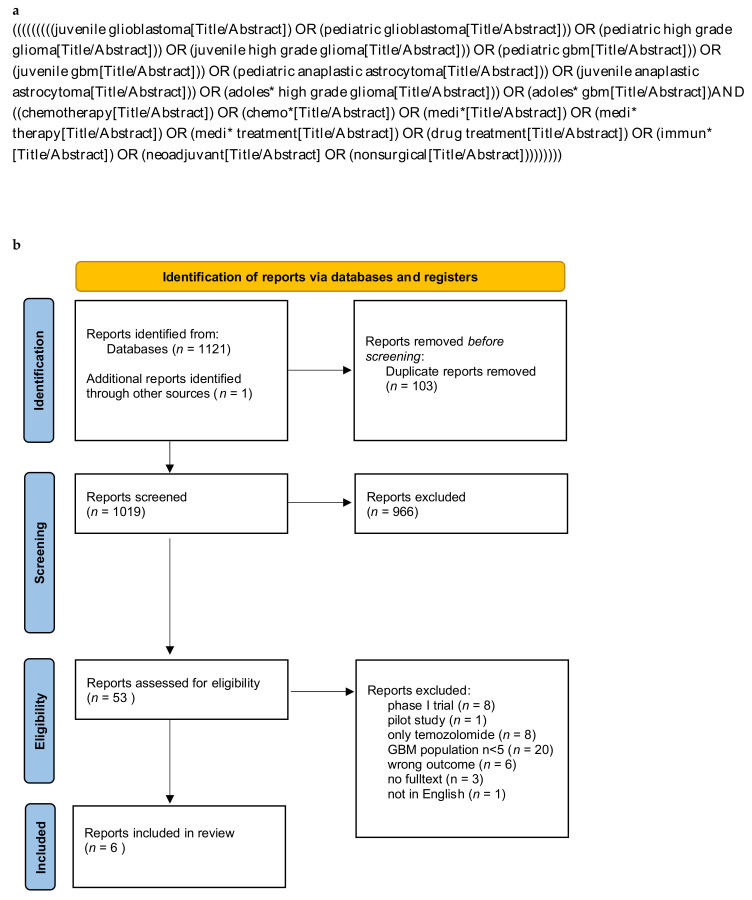
For this systematic review, we searched PubMed and Embase databases and included reports published in English between January 2010 until December 2021. We defined 2010 as the start date of the assessed reports, since while screening the years before, a substantial lack of new treatment modalities next to TMZ was seen, which was implemented as standard treatment for adult GBM in 2005. Our utilized search string included the search items “glioblastoma” and “pediatric” and “drug treatment” and “chemotherapy” (Figure 2a).
Figure 2.
(a) Systematic search string used for Pubmed database. Adjustments of format have been made according to Embase guidelines. (b) PRISMA flow diagram (2020) for systematic reviews.
We included randomized trials, retrospective and prospective cohort studies, and case series including more than five pediatric patients with the diagnosis of GBM. Pediatric age was defined between 0 and 18 years of age. Technical reports, case reports, comments, editorial letters, poster abstracts and reviews were excluded from this report. Phase I trials focusing on pharmacokinetics and estimation of dose tolerance and limitation were excluded, as our intention was to focus on available data on effective treatment options for clinical use in pediatric GBM. Reports including only standard treatment with TMZ, and reports describing HGG without a differentiation into subgroups or without specific baseline characteristics regarding GBM were excluded as well.
After removal of duplicates, which was conducted with the help of the web-based software Rayyan [46], the results were screened by title by two authors, independently (J.W. and N.A.F.). Further, the abstracts were assessed followed by a full text evaluation of the remaining reports. In case of disagreement concerning the in- or exclusion of a report, the senior authors (J.S. and K.S.) made the final decision. One further report was included by screening the references of the other reports. We defined relevant parameters of the studies in reference to PICO standards (Table S1). We extracted the following information from eligible reports: study details (author, year of publication, design and statistics); study population (number of participants, recruitment interval, median age); treatment characteristics (disease status, intervention); outcome measures (EFS, OS, PFS, toxicity and toleration). The primary outcome measure was overall outcome with EFS, PFS or OS. Secondary outcome measures were toxicity and toleration.
The included reports were classified in “newly diagnosed” and “recurrent or refractory” GBM. “Recurrent” is defined by GBM relapse after first- or second-line treatment with initial objective response, whereas “refractory” is defined by progressive GBM failing to respond to treatment. PFS is defined by the length of time during and after treatment without radiological or clinical progression. EFS is defined as the interval between treatment and documentation of clinical or radiological disease progression, complications from disease or treatment, secondary malignancy or death of any cause.
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Files S1 and S2) and is registered in PROSPERO (Prospero-ID: CRD4202232200) (Figure 2b). Quality assessment of the studies was carried out by two reviewers (J.W. and N.A.F.) independently using the Newcastle-Ottawa scale (NOS) [47]. The assessment for the included randomized controlled trial (RCT) [48] was carried out using the RoB-2 tool [49].
3. Results
The systematic search of the databases yielded 1121 reports, while one report was added through screening of references. After removal of duplicates, 1019 report titles were screened and 53 reports assessed for abstract screening. Of these, 23 reports underwent full text evaluation whereof six reports were included in the qualitative analysis [48,50,51,52,53,54] (Figure 2b; Table 2). Four of the included reports investigated novel pharmacologic treatment strategies in therapy refractory or recurrent GBM patients [50,51,52,54], and two in newly diagnosed GBM patients [48,53]. All six selected reports were prospective, multicenter phase Ⅱ trials, five of them single-arm trials [50,51,52,53,54] and one of them a RCT [48]. In total, we included 137 pediatric patients receiving pharmacological treatment for pediatric GBM.
Table 2.
Results of analyzed studies with objectifiable baseline characteristics.
| Publication | Study Type | Recruitment Interval | pGBM Cohort (n) | Age at Study Entry ° (Years) (Median/Range) |
Disease Status | Intervention | Primary Outcome GBM Cohort (EFS; PFS; OS) | Secondary Outcome (Toxicity; Toleration of Treatment) |
Therapeutic Effect | Quality Assessment NOS/RoB-2 (points) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gururangan et al., 2010 [51] |
prospective phase-Ⅱ cohort trial | 10/2006–09/2008 | 8 | 15.7 (5.6–20.1) |
r/r | BEV plus irinotecan | 2/8 SD at >12 weeks, of these 2 patients, median PFS: 8.3 months no sustained OR | 20% toxicity with interruption of treatment | no efficacy in recurrent pGBM | 5 (fair) |
| MacDonald et al., 2013 [50] |
prospective phase-Ⅱ cohort trial | 06/2008–12/2010 | 18 | 14.2 (1.1–20.3) |
r/r | cilengitide | 1/18 SD at 19 months | low toxicity rate, well tolerated | no efficacy in recurrent pGBM | 5 (fair) |
| Robinson et al., 2014 [52] |
multicenter, prospective phase-Ⅱ cohort trial | 01/2005–03/2009 | 9 | 10 (0.6–21) |
r/r | metronomic oral celecoxib, thalidomide, fenofibrate, low dose CPM and etoposide | 1/9 SD at 27 weeks | low toxicity rate, well tolerated | no efficacy in recurrent pGBM | 5 (fair) |
| Wetmore et al., 2016 [54] |
multicenter, prospec-tive phase-Ⅱ cohort trial | 01/2012–06/2013 | 7 | 14.5 (4.7–19.9) |
r/r | sunitinib | Response rate (=CR or PR for at least 8 weeks): 0% | low toxicity rate, well tolerated | closing at interim analysis due to lack of efficiacy | 6 (good) |
| Grill et al., HERBY trial 2018 [48] |
randomized controlled trial | 10/2011–02/2015 | 84 | 11 (3–17) |
newly diagnosed | BEV | HR: 1.37 (95% CI 0.83 to 2.27) for RT plus TMZ compared to RT + TMZ + BEV | no safety concerns; more AEs in BEV-cohort | No measurable effect for unmethylated pGBM | some concerns (RoB-2) |
| Meng-Fen Su et al., 2020 [53] |
multicenter, prospective phase-Ⅱ cohort trial | 09/2009–08/2015 | 11 | 7.9 (3.2–19.9) |
newly diagnosed | VPA and radiation followed by VPA and BEV | median EFS: 10.5 months median OS: 14.9 months |
2 treatment interruptions after addition of BEV; RT and VPA with good tolerance | no improvement of OS | 5 (fair) |
° of the whole HGG cohort; pGBM = pediatric glioblastoma, NOS = Newcastle-Ottawa scale, RoB2 = Risk of Bias 2 tool, r/r = relapsed/refractory disease, PFS = progression free survival, EFS = event free survival, OS = overall survival, OR = objective response, CR = complete remission, PR = partial remission, HR = hazard ratio, CI = confidence interval, RT = radiotherapy, TMZ = temozolomide, BEV = bevacizumab, AEs = adverse events, VPA = valproic acid, CPM = cyclophosphamide.
The investigated medications of the six included prospective studies were bevacizumab (BEV) as monotherapy and in combination with irinotecan [48,51], valproic acid (VPA) [53], cilengitide [50] and an oral combination therapy of thalidomide, celecoxib, fenofibrate, low dose etoposide and cyclophosphamide [52] and sunitinib [54].
The qualitative assessment for the five cohort studies showed a mean Newcastle-Ottawa scale (NOS) rating of 5 ± 0.4 (Table 2). The included RCT [48] was assessed as high quality with some concerns using the RoB-2 tool.
3.1. Newly Diagnosed Pediatric Glioblastoma
Grill et al. concluded a phase II, open-label, randomized, international comparator trial (HERBY trial) with the intervention of the addition of BEV to irradiation and TMZ in pediatric patients with newly diagnosed HGG [48]. Eighty-four (69%) of 121 included HGG patients were diagnosed with a pediatric GBM. EFS for the whole cohort in the BEV plus RT + TMZ was 11.8 months (95% CI 7.8–12.7 months) compared to 8.2 months in the cohort without BEV, showing no benefit for BEV in combination with irradiation and TMZ for GBM [48]. No detailed outcome information for the GBM cohort was made. MGMT methylation status was balanced between the intervention groups [48].
Meng-Fen Su et al. conducted a multi-institutional, single-arm phase Ⅱ clinical trial of irradiation and VPA, followed by maintenance with VPA and BEV in children with newly diagnosed HGG [53]. Out of 38 HGG patients enrolled for the study, 11 (28%) GBM patients were assessed showing a median EFS of 10.5 months and a median OS of 14.9 months. The estimated one-year EFS for all the HGG patients was 24%.
In conclusion, the addition of VPA and BEV to irradiation could not show a significant benefit for newly diagnosed pediatric GBM.
3.2. Recurrent or Refractory Pediatric Glioblastoma
In a phase Ⅱ study, Gururangan, et al. investigated the efficacy of the combination of BEV and irinotecan in children with recurrent HGG [51]. They evaluated 31 patients of which 8 patients (26%) were histologically diagnosed with a pediatric GBM. The primary objective of the study was to determine the objective response (complete response plus partial response) to BEV and irinotecan in recurrent pediatric HGG. Of the whole study cohort, no sustained objective responses were observed. Eight of these 31 patients (25%) showed a sustained stable disease at >12 weeks, while 23 (74%) patients had progressive disease. The group of the sustained stable disease patients included two GBM patients. These two patients showed a median PFS of 8.3 months with a median PFS of the whole glioma-cohort of 4.2 months.
In a phase Ⅱ study, MacDonald, et al. investigated the efficacy of cilengitide in the treatment of recurrent or refractory pediatric HGG [50]. Thirty patients were enrolled. Twenty-four patients were accessible for primary outcome analysis (six excluded due to severe progression prior to first MRI), of which 18 (75%) were diagnosed as GBM. The primary objective was to determine the objective response rate to cilengitide, defined as successful by complete (CR) or partial response (PR) or PFS for at least 12 weeks. Only one patient (4%) of the evaluable 24 patients showed a response with stable disease at 280 days. This response was in a young GBM patient < 3 years of age. For the remaining 23 patients, median time to progression was 28 days. Mortality occurred in 21 patients (87%) with a median time to death of 172 days [50].
Robinson et al. investigated in an open-label, single-arm, multi-institutional phase Ⅱ study the efficacy of an antiangiogenic, metronomic oral drug regimen with thalidomide, celecoxib, fenofibrate and low dose etoposide and cyclophosphamide in recurrent or refractory pediatric cancer [52]. They enrolled 101 pediatric cancer patients, of which 97 commenced treatments. All types of pediatric cancers were included; therefore, patients were categorized in seven strata. GBM patients were included in the HGG strata. Of these, nine were diagnosed with a pediatric GBM, with eight primary GBM and one secondary GBM.
The primary endpoint of the trial was the assessment of the effect of the five-drug regimen given over a period of 27 weeks. Secondary endpoints included OS and PFS. Of the 21 subjects in the HGG strata, 13 patients (62%) showed progressive disease, 7 (33%) stable disease and 1 patient (4%) showed partial remission. Eight out of eight (100%) of primary GBM patients were progressive, one patient showed a stable disease. This patient with a stable disease was diagnosed with a secondary GBM with a prior history of medulloblastoma.
In a multicenter phase Ⅱ trial, Wetmore, et al. evaluated the effect of sunitinib in the treatment of recurrent or refractory pediatric HGG and ependymoma [54]. Thirty patients were enrolled. Seventeen of them were diagnosed with a HGG, while seven of these were diagnosed with GBM. The primary objective of this study was to estimate overall response rate (ORR), defined as complete or partial response for at least eight weeks. The study had to be closed at the time of interim analysis due to missing sustained objective response. The observed response rate in the HGG cohort was 0% (95% Blyth-Still-Casella CI, 0−19.8%). Of the whole cohort of HGG, the median time to progression was 72 days (95% CI 33–84) [54].
In summary, no treatment modality could demonstrate a positive effect on OS or PFS in recurrent or progressive and pretreated pediatric GBM.
4. Discussion
Based on our systematic review of the literature, novel pharmacological treatment options for pediatric GBM showed no benefit in PFS or OS in the setting of newly diagnosed pediatric GBM as well as in recurrent or refractory GBM. Overall, there are only a few phase Ⅱ trials investigating novel drug treatments for pediatric HGG patients with a very limited number of pediatric GBM patients and short follow-up intervals, mainly due to the aggressive course of the disease.
4.1. Newly Diagnosed Pediatric Glioblastoma
We detected two reports investigating BEV and VPA in newly diagnosed pediatric GBM [48,53]. Both of them did not include infant GBM, as they both used concurrent irradiation. The lack of improvement of the outcome with BEV in children is not consistent with data from adult trials showing prolonged PFS [55]. BEV is approved in several countries worldwide for the treatment of relapsed GBM in adults [56]. This finding supports the emerging knowledge of molecular understanding of two different entities of pediatric and adult GBM tumor biologies and makes a translation of efficient treatment strategies from adult patients to pediatric patients impossible [57,58]. An important observation is the pattern of tumor recurrence after BEV treatment. A higher number of patients treated with the addition of BEV also showed, besides local recurrence, distant progression patterns [48,53,59]. The addition of the histone deacetylases (HDAC) inhibitor VPA and BEV to irradiation [53] could not improve the outcome either, although VPA showed a radio sensitizing effect in HGG patients [60,61] and promising results of partial response in a previous phase Ⅰ study in children [32]. The median EFS and OS of Su, et al. is most likely somewhat overestimated, as 36% of the GBM cohort were diagnosed with a mismatch repair deficiency (MMRD) syndrome, clearly exceeding the median EFS with the longest median EFS of 28.5 months [53]. One patient with a Lynch syndrome even showed a sustained complete remission, suggesting further treatment strategies with a HDAC or an alternative angiogenesis inhibitor should be investigated for this subgroup of pediatric GBM patients. MMRD GBM shows a differing genetic profile characterized by a high mutational burden, compared to conventional GBM, which results in different behavior regarding treatment [62]. Immune checkpoint inhibition is a further approach for this small subgroup showing promising results [62].
4.2. Recurrent or Refractory Pediatric Glioblastoma
For the relapsed or refractory setting of pediatric GBM, four reports were identified. The combination treatment of BEV and irinotecan did not show any sustained objective response [51]. These results are in contrast to the observed efficacy of the combination of BEV and irinotecan in adults, similar to the differences already mentioned with the use of single treatment with BEV [63,64,65]. The causes of treatment failure are multifactorial. Possible contributors could be alternative angiogenic pathways and resistance mechanisms maintaining tumor growth [66]. As a single agent, cilengitide showed no efficacy in pediatric GBM patients [50], in contrast to the previous phase I trial [67]. Cilengitide is an alpha(v) integrin antagonist demonstrated to block angiogenesis and showed a tumor regression of GBM cells in vivo [68,69,70]. The efficacy of cilengitide was shown in adult trials [71]. The results of adult trials with a combination of cilengitide, TMZ and irradiation showed significantly better results, especially in patients with methylated MGMT promotor status [71,72]. A synergistic effect of cilengitide and irradiation was also shown [73]. The very limited effect in pediatric patients might be due to the fact that pediatric GBM tumor cells display MGMT promotor methylation significantly less often [26,27]. Another treatment strategy are metronomic low dose treatment schedules of antiangiogenic and cytotoxic agents by suppressing endothelial cell proliferation and affecting the tumor microenvironment by rebuilding an anticancer immune response [74,75,76,77]. The primary endpoint is rather non-progression of disease than an objective tumor reduction and therefore presents another conception of therapy to treat cancer as a chronic disease with maintenance of quality of life [78]. However, the oral combination therapy of thalidomide, celecoxib, low dose etoposide/cyclophosphamide and fenofibrate, a PPAR-alpha agonist, showed an unfavorable response rate [52]. The only GBM patient with stable disease was diagnosed with a secondary GBM and can, therefore, biologically not be compared to primary GBM. The orally bioavailable sunitinib, a tyrosine kinase inhibitor (TKI) showed neither an objective response in pediatric patients [54] nor in adult recurrent GBM [79]. Sunitinib is an inhibitor of PDGFRα-β, vascular endothelial growth factor receptor (VEGFR1-2), fetal liver tyrosine kinase receptor 3 (FLT3) and stem cell factor receptor (KIT) [80]. Several tyrosine kinases, such as PDGFR, KIT and VEGFR are found to be activated in about 30% of pediatric HGG patients [81,82].
There are further reports about novel treatment strategies with even more limited patient numbers, which, therefore, did not qualify for this systematic review. Nimotuzumab, a monoclonal antibody, showed a favorable toxicity profile, even in prolonged use in pediatric HGG [83]. However, looking at the pediatric GBM subgroup of this study, prolonged survival time exceeding 40 months was not apparent, suggesting no benefit in survival time. The checkpoint inhibitor Nivolumab [84] predictably showed that the median survival for PD-L1 positive pediatric HGG patients was significantly higher than in PD-L1 negative patients. The response was, however, only transient and partial [84]. The lack of a significant effect of checkpoint inhibitors in pediatric GBM is also correlated to a known low mutational burden in pediatric GBM cells [85]. This leads to the assumption that a combination therapy with other immunomodulatory approaches, such as a combination of chimeric antigen receptor CAR-T cells and cancer vaccines could possibly better attack these therapeutically challenging tumors.
A patient group not included in the above-mentioned treatment options, besides the metronomic approach, are congenital and infant GBM patients. This subgroup of pediatric patients shows a different tumor biology and disease course with an extremely rapid growth and a highly vulnerable angiogenesis often leading to early and often fatal hemorrhage [15,16]. On the other hand, some cases show noticeable prolonged survival exceeding 24 months, treated by surgery and dose adjusted chemotherapy [28,86]. The vulnerable developing brain of infants under the age of three years leads to the fact that radiotherapy should be avoided, due to serious sequelae, such as developmental delay, endocrine dysfunction and secondary neoplasms of the CNS [15,16]. As the most common somatic alterations in infant HGG involve NTRK genes [17], larotrectinib, as a selective TRK inhibitor, represents a possible targeted therapy option here.
4.3. Novel Therapies within the Scope of Phase Ⅰ Trials and Future Perspectives
Further immunotherapeutic approaches under investigation include therapeutic vaccination, a treatment strategy to redirect T-cells against tumor antigens (Table 3). One category of a tumor vaccine is oncolytic viruses, such as herpes simplex virus (HSV) [87]. A phase I immunovirotherapy trial of oncolytic HSV-1 G207 with stereotactic placement of intratumoral catheters in pediatric HGG led to an increased number of tumor-infiltrating lymphocytes by bypassing the BBB without toxic effect [88]. Further oncolytic viruses under investigation are the parvovirus H-1 [89] and cytomegalovirus (CMV) [90]. After the detection of CMV in the majority of adult GBM cells [91,92], CMV antigens were also detected in approximately 66.7% of pediatric GBM samples [90]. CMV showed to enhance telomerase activity and angiogenesis in adult GBM, making it a possible target for immune-based therapy [93]. As autologous tumor lysate-pulsed dendritic cell vaccinations for pediatric patients with newly diagnosed or recurrent HGG showed feasibility and potential clinical benefit [94], these findings were combined leading to an ongoing trial (NCT03615404) investigating the feasibility of CMV RNA pulsed dendritic cells in children and adults. To our knowledge, to date, no results based on phase Ⅱ trials of these technologies in pediatric GBM patients exist. Another immunotherapeutic approach is CAR-T cells. A current phase I clinical trial is evaluating anti-IL13aR2 CAR-T cells in children 12 years and older with recurrent or relapsed gliomas (NCT0220836).
Table 3.
Currently recruiting trials and published phase I studies on new pharmacological treatment approaches.
| Publication | Study Type | Conditions | Intervention | Outcome | Phase Ⅱ Proposed |
|---|---|---|---|---|---|
| Becher et al., 2017 [96] | multicenter | pediatric solid tumors | perifosine, temsirolimus | well tolerated, no objective response | NA |
| Kieran et al., 2019 [95] | multicenter | BRAF V600E mutation positive pediatric tumors | dabrafenib | well tolerated | yes |
| Friedman et al., 2021 [88] | multicenter | pediatric HGG | HSV-1 G207 | well tolerated, objective change in tumor metabolism | yes |
| McCrea et al., 2021 [97] | multicenter | HGG and DIPG | intraarterial BEV and cetuximab with BBB disruption | well tolerated, little objective effect | yes |
| NCT04295759 | multicenter | pediatric HGG | INCB7839 | recruiting | NA |
| NCT04732065 | multicenter | pediatric brain tumors | ONC206 | recruiting | NA |
| NCT04655404 | multicenter | pediatric HGG | larotrectinib | recruiting | NA |
| NCT03615404 | single center | pediatric brain tumors | CMV-DC with GM-CSF | completed, publication pending | NA |
| NCT02208362 | single center | pediatric and adult glioma | IL13Ralpha2-CAR-T cells | active, not recruiting | NA |
HGG = high grade glioma; DIPG = diffuse intrinsic pontine glioma; BRAF V600E = v-Raf murine sarcoma viral oncogene homolog B1 V600E; BEV = bevacizumab; BBB = blood–brain barrier; INCB7839 = aderbasib; CMV-DC = cytomegalyvirus infected dentritic cells; GM-CSF = granulocyte macrophage colony stimulating factor.
High-throughput genomic technologies discovered the biologic heterogeneity of several pediatric brain tumors [29,80]. This demands more individualized, targeted approaches [80]. Larotrectinib, a highly selective small-molecule inhibitor of tropomyosin receptor kinase (TRK) showed rapid and durable responses with a high tumor control rate and good tolerability in TRK fusion-positive primary central nervous system tumors in adult and pediatric patients [39]. In the pediatric cohort, the 24-week disease control rate was 69% (95% CI, 39−91%) for HGG. A pilot study investigating larotrectinib in newly diagnosed pediatric HGG with NTRK fusion is still recruiting, first results are expected in 2025 (NCT04655404). Kieran, et al. showed in a phase I trial the feasibility of targeting BRAF V600E mutated pediatric solid tumors, amongst others in pediatric GBM patients, with oral dabrafenib [95]. The effect of perifosine and temsirolimus in recurrent pediatric solid tumors by inhibition of the AKT and mTOR pathways axis, proved the feasibility and tolerable toxicity of the agent combination. However, in contrast to preclinical data, no objective response was observed [96]. Further phase I trials investigating the effect of INCB7839, an inhibitor of the ADAM 10 and 17 proteases (a disintegrin and metalloprotease) (NCT04295759) and ONC206, a selective dopamine-2 antagonist (NCT04732065) are ongoing. Evolving strategies to overcome the BBB, such as intraarterial delivery of BVZ and cetuximab are ongoing as well, showing good tolerance while Phase Ⅱ trials are needed to objectify the outcome [97].
Despite conducting a systematic review, several limitations are present in this study. First, we only searched two databases (Pubmed and Embase), while conference abstracts, review protocols, unpublished data and clinical evidence that was not indexed in bibliographic databases were excluded from this study. We further only searched for English literature, which carries a risk of omitting important data published elsewhere. Second, the included trials all comprised this highly heterogenous group of HGG consisting of anaplastic astrocytomas (WHO grade Ⅲ), diffuse midline gliomas and GBM (WHO grade IV). This pooling of patient cohorts is mainly explained through very limited patient numbers with limited observation time due to the aggressive biology of these tumors. However, within the review, we extrapolated the data for pediatric GBM patients wherever possible. Third, the primary outcome for the specific patient group of pediatric GBM varies in the included studies and is at times difficult to compare. Fourth, even within the GBM cohort the clinical and biological parameters, such as the extent of resection, the location of the tumor, previous treatment strategies and the molecular subgroups are highly heterogenous and at times data concerning these variables are not reported. Fifth, the patient cohorts within the studies are rather small, therefore conclusions need to be interpreted with caution. Finally, patients were recruited at different states of the disease and treatment, since the inclusion criteria within the studies were heterogenous, hampering the objective comparison of PFS or OS.
5. Conclusions
The role of pharmacological approaches for the treatment of pediatric GBM remains unsatisfying, since no novel approach led to improvement of PFS or OS. Novel treatment approaches, such as immunotherapeutic approaches and pharmacologically susceptible tumor-specific targets based on molecular biology, present promising preclinical results and should be the focus of future studies. Due to the limited number of patients, the molecular heterogeneity and aggressiveness of the disease, often leading to early death, multicenter, international trials are of paramount importance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14112814/s1, Table S1: Addressing predefined parameters according to PICO for evidence-based medicine; File S1: Prisma 2020 for Abstracts Checklist [98]; File S2: Prisma 2020 Checklist [98].
Author Contributions
Conceptualization, K.S., J.S., N.A.F. and J.W.; methodology, K.S., J.S., N.A.F. and J.W.; writing—original draft preparation, J.W. and N.A.F.; writing—review and editing, J.S. and K.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Protected research time of J.W. was funded by a research grant of Kantonsspital Aarau, grant number 1410.000.113, Switzerland.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ostrom Q.T., Patil N., Cioffi G., Waite K., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology. 2020;22:iv1–iv96. doi: 10.1093/neuonc/noaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fangusaro J. Pediatric High-Grade Gliomas and Diffuse Intrinsic Pontine Gliomas. J. Child Neurol. 2009;24:1409–1417. doi: 10.1177/0883073809338960. [DOI] [PubMed] [Google Scholar]
- 3.Da W., Xueli C., Xiao P., Yian X. Prognostic factors and survival prediction of pediatric glioblastomas: A population-based study. Turk. Neurosurg. 2020;31:873–879. doi: 10.5137/1019-5149.JTN.31915-20.2. [DOI] [PubMed] [Google Scholar]
- 4.Jiao Y., Wang M., Liu X., Wang J., Wang Z., Luo W., Yu Y., Sun H. Clinical Features and Prognostic Factors of Pediatric Glioblastoma: Report of 38 Cases. World Neurosurg. 2021;153:e105–e111. doi: 10.1016/j.wneu.2021.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Rees J., Smirniotopoulos J.G., Jones R.V., Wong K. Glioblastoma multiforme: Radiologic-pathologic correlation. RadioGraphics. 1996;16:1413–1438. doi: 10.1148/radiographics.16.6.8946545. [DOI] [PubMed] [Google Scholar]
- 6.Toh C., Wei K.-C., Chang C.-N., Hsu P.-W., Wong H.-F., Ng S.-H., Castillo M., Lin C.-P. Differentiation of Pyogenic Brain Abscesses from Necrotic Glioblastomas with Use of Susceptibility-Weighted Imaging. Am. J. Neuroradiol. 2012;33:1534–1538. doi: 10.3174/ajnr.A2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gharzeddine K., Hatzoglou V., Holodny A.I., Young R.J. MR Perfusion and MR Spectroscopy of Brain Neoplasms. Radiol. Clin. N. Am. 2019;57:1177–1188. doi: 10.1016/j.rcl.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Louis D.N., Perry A., Reifenberger G., Von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 9.Das K.K., Mehrotra A., Nair A.P., Kumar S., Srivastava A.K., Sahu R.N., Kumar R. Pediatric glioblastoma: Clinico-radiological profile and factors affecting the outcome. Child′s Nerv. Syst. 2012;28:2055–2062. doi: 10.1007/s00381-012-1890-x. [DOI] [PubMed] [Google Scholar]
- 10.Perkins S.M., Rubin J.B., Leonard J.R., Smyth M.D., El Naqa I., Michalski J.M., Simpson J.R., Limbrick D.L., Park T.S., Mansur D.B. Glioblastoma in Children: A Single-Institution Experience. Int. J. Radiat. Oncol. 2011;80:1117–1121. doi: 10.1016/j.ijrobp.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Nikitovic M., Stanic D., Pekmezovic T., Gazibara M.S., Bokun J., Paripovic L., Grujicic D., Saric M., Miskovic I. Pediatric glioblastoma: A single institution experience. Child′s Nerv. Syst. 2015;32:97–103. doi: 10.1007/s00381-015-2945-6. [DOI] [PubMed] [Google Scholar]
- 12.Miller K.D., Ostrom Q.T., Kruchko C., Patil N., Tihan T., Cioffi G., Fuchs H.E., Waite K.A., Jemal A., Siegel R.L., et al. Brain and other central nervous system tumor statistics, 2021. CA A Cancer J. Clin. 2021;71:381–406. doi: 10.3322/caac.21693. [DOI] [PubMed] [Google Scholar]
- 13.Duffner P.K., Horowitz M.E., Krischer J.P., Burger P.C., Cohen M.E., Sanford R.A., Friedman H.S., Kun L.E. The treatment of malignant brain tumors in infants and very young children: An update of the Pediatric Oncology Group experience. Neuro-Oncology. 1999;1:152–161. doi: 10.1093/neuonc/1.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafay-Cousin L., Strother D. Current Treatment Approaches for Infants with Malignant Central Nervous System Tumors. Oncologist. 2009;14:433–444. doi: 10.1634/theoncologist.2008-0193. [DOI] [PubMed] [Google Scholar]
- 15.Ceglie G., Vinci M., Carai A., Rossi S., Colafati G.S., Cacchione A., Tornesello A., Miele E., Locatelli F., Mastronuzzi A. Infantile/Congenital High-Grade Gliomas: Molecular Features and Therapeutic Perspectives. Diagnostics. 2020;10:648. doi: 10.3390/diagnostics10090648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders R.P., Kocak M., Burger P.C., Merchant T.E., Gajjar A., Broniscer A. High-grade astrocytoma in very young children. Pediatr. Blood Cancer. 2006;49:888–893. doi: 10.1002/pbc.21272. [DOI] [PubMed] [Google Scholar]
- 17.Clarke M., Mackay A., Ismer B., Pickles J.C., Tatevossian R.G., Newman S., Bale T.A., Stoler I., Izquierdo E., Temelso S., et al. Infant High-Grade Gliomas Comprise Multiple Subgroups Characterized by Novel Targetable Gene Fusions and Favorable Outcomes. Cancer Discov. 2020;10:942–963. doi: 10.1158/2159-8290.CD-19-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dufour C., Grill J., Lellouch-Tubiana A., Puget S., Chastagner P., Frappaz D., Doz F., Pichon F., Plantaz D., Gentet J., et al. High-grade glioma in children under 5 years of age: A chemotherapy only approach with the BBSFOP protocol. Eur. J. Cancer. 2006;42:2939–2945. doi: 10.1016/j.ejca.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Finlay J.L., Boyett J.M., Yates A.J., Wisoff J., Milstein J.M., Geyer J.R., Bertolone S.J., McGuire P., Cherlow J.M., Tefft M. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Childrens Cancer Group. J. Clin. Oncol. 1995;13:112–123. doi: 10.1200/JCO.1995.13.1.112. [DOI] [PubMed] [Google Scholar]
- 20.Wolff J.E., Driever P.H., Erdlenbruch B., Kortmann R.D., Rutkowski S., Pietsch T., Parker C., Metz M.W., Gnekow A., Kramm C.M. Intensive chemotherapy improves survival in pediatric high-grade glioma after gross total resection: Results of the HIT-GBM-C protocol. Cancer. 2010;116:705–712. doi: 10.1002/cncr.24730. [DOI] [PubMed] [Google Scholar]
- 21.Vanan M.I., Eisenstat D.D. Management of high-grade gliomas in the pediatric patient: Past, present, and future. Neuro-Oncol. Pr. 2014;1:145–157. doi: 10.1093/nop/npu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramm C.M., Wagner S., van Gool S., Schmid H., Sträter R., Gnekow A., Rutkowski S., Wolff J.E.A. Improved Sur-vival after Gross Total Resection of Malignant Gliomas in Pediatric Patients from the HIT-GBM Studies. Anticancer. Res. 2006;26:3773–3779. [PubMed] [Google Scholar]
- 23.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 24.Seidel C., Von Bueren A.O., Bojko S., Hoffmann M., Pietsch T., Gielen G.H., Warmuth-Metz M., Bison B., Kortmann R., Kramm C.M. Concurrent radiotherapy with temozolomide vs. concurrent radiotherapy with a cisplatinum-based polychemotherapy regimen. Strahlenther. und Onkol. 2017;194:215–224. doi: 10.1007/s00066-017-1218-6. [DOI] [PubMed] [Google Scholar]
- 25.Lashford L.S., Thiesse P., Jouvet A., Jaspan T., Couanet D., Griffiths P.D., Doz F., Ironside J., Robson K., Hobson R., et al. Temozolomide in Malignant Gliomas of Childhood: A United Kingdom Children’s Cancer Study Group and French Society for Pediatric Oncology Intergroup Study. J. Clin. Oncol. 2002;20:4684–4691. doi: 10.1200/JCO.2002.08.141. [DOI] [PubMed] [Google Scholar]
- 26.Cohen K.J., Pollack I.F., Zhou T., Buxton A., Holmes E.J., Burger P.C., Brat D.J., Rosenblum M.K., Hamilton R.L., Lavey R.S., et al. Temozolomide in the treatment of high-grade gliomas in children: A report from the Children′s Oncology Group. Neuro-Oncology. 2011;13:317–323. doi: 10.1093/neuonc/noq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buttarelli F.R., Massimino M., Antonelli M., Lauriola L., Nozza P., Donofrio V., Arcella A., Oliva M.A., Di Rocco C., Giangaspero F. Evaluation status and prognostic significance of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in pediatric high grade gliomas. Child’s Nerv. Syst. 2010;26:1051–1056. doi: 10.1007/s00381-010-1191-1. [DOI] [PubMed] [Google Scholar]
- 28.Lu V.M., O’Connor K.P., Himes B.T., Brown D.A., Nesvick C.L., Siada R.G., Niazi T.N., Schwartz J., Daniels D.J. Effect of surgery and chemotherapy on long-term survival in infants with congenital glioblastoma: An integrated survival analysis. J. Neurosurg. Pediatr. 2020;26:563–571. doi: 10.3171/2020.5.PEDS20226. [DOI] [PubMed] [Google Scholar]
- 29.Sturm D., Witt H., Hovestadt V., Khuong-Quang D.-A., Jones D.T.W., Konermann C., Pfaff E., Tönjes M., Sill M., Bender S., et al. Hotspot Mutations in H3F3A and IDH1 Define Distinct Epigenetic and Biological Subgroups of Glioblastoma. Cancer Cell. 2012;22:425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 30.Raabe E.H., Eberhart C.G. Methylome Alterations “Mark” New Therapeutic Opportunities in Glioblastoma. Cancer Cell. 2012;22:417–418. doi: 10.1016/j.ccr.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hummel T.R., Wagner L., Ahern C., Fouladi M., Reid J.M., McGovern R.M., Ames M.M., Gilbertson R.J., Horton T., Ingle A.M., et al. A pediatric phase 1 trial of vorinostat and temozolomide in relapsed or refractory primary brain or spinal cord tumors: A children′s oncology group phase 1 consortium study. Pediatr. Blood Cancer. 2013;60:1452–1457. doi: 10.1002/pbc.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su J.M., Li X.-N., Thompson P., Ou C.-N., Ingle A.M., Russell H., Lau C.C., Adamson P.C., Blaney S.M. Phase 1 Study of Valproic Acid in Pediatric Patients with Refractory Solid or CNS Tumors: A Children′s Oncology Group Report. Clin. Cancer Res. 2010;17:589–597. doi: 10.1158/1078-0432.CCR-10-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashizume R., Andor N., Ihara Y., Lerner R., Gan H., Chen X., Fang D., Huang X., Tom M.W., Ngo V., et al. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat. Med. 2014;20:1394–1396. doi: 10.1038/nm.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C.C., Deshmukh S., Jessa S., Hadjadj D., Lisi V., Andrade A.F., Faury D., Jawhar W., Dali R., Suzuki H., et al. Histone H3.3G34-Mutant Interneuron Progenitors Co-opt PDGFRA for Gliomagenesis. Cell. 2020;183:1617–1633.e22. doi: 10.1016/j.cell.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miklja Z., Yadav V.N., Cartaxo R.T., Siada R., Thomas C.C., Cummings J.R., Mullan B., Stallard S., Paul A., Bruzek A.K., et al. Everolimus improves the efficacy of dasatinib in PDGFRα-driven glioma. J. Clin. Investig. 2020;130:5313–5325. doi: 10.1172/JCI133310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondyli M., Larouche V., Saint-Martin C., Ellezam B., Pouliot L., Sinnett D., Legault G., Crevier L., Weil A., Farmer J.-P., et al. Trametinib for progressive pediatric low-grade gliomas. J. Neuro-Oncol. 2018;140:435–444. doi: 10.1007/s11060-018-2971-9. [DOI] [PubMed] [Google Scholar]
- 37.Kaley T., Touat M., Subbiah V., Hollebecque A., Rodon J., Lockhart A.C., Keedy V., Bielle F., Hofheinz R.-D., Joly F., et al. BRAF Inhibition in BRAFV600-Mutant Gliomas: Results From the VE-BASKET Study. J. Clin. Oncol. 2018;36:3477–3484. doi: 10.1200/JCO.2018.78.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drobysheva A., Klesse L.J., Bowers D., Rajaram V., Rakheja D., Timmons C.F., Wang J., Koral K., Gargan L., Ramos E., et al. Targeted MAPK Pathway Inhibitors in Patients With Disseminated Pilocytic Astrocytomas. J. Natl. Compr. Cancer Netw. 2017;15:978–982. doi: 10.6004/jnccn.2017.0139. [DOI] [PubMed] [Google Scholar]
- 39.Doz F., van Tilburg C.M., Geoerger B., Højgaard M., Øra I., Boni V., Capra M., Chisholm J., Chung H.C., DuBois S.G., et al. Efficacy and safety of larotrectinib in TRK fusion-positive primary central nervous system tumors. Neuro-Oncology. 2021 doi: 10.1093/neuonc/noab196.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makhlin I., Salinas R.D., Zhang D., Jacob F., Ming G.-L., Song H., Saxena D., Dorsey J.F., Nasrallah M.P., Morrissette J.J., et al. Clinical activity of the EGFR tyrosine kinase inhibitor osimertinib in EGFR-mutant glioblastoma. CNS Oncol. 2019;8:CNS43. doi: 10.2217/cns-2019-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X., Chen X., Shi L., Shan Q., Cao Q., Yue C., Li H., Li S., Wang J., Gao S., et al. The third-generation EGFR inhibitor AZD9291 overcomes primary resistance by continuously blocking ERK signaling in glioblastoma. J. Exp. Clin. Cancer Res. 2019;38:1–14. doi: 10.1186/s13046-019-1235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackay A., Burford A., Carvalho D., Izquierdo E., Fazal-Salom J., Taylor K.R., Bjerke L., Clarke M., Vinci M., Nandhabalan M., et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell. 2017;32:520–537. doi: 10.1016/j.ccell.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korshunov A., Ryzhova M., Hovestadt V., Bender S., Sturm D., Capper D., Meyer J., Schrimpf D., Kool M., Northcott P.A., et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015;129:669–678. doi: 10.1007/s00401-015-1405-4. [DOI] [PubMed] [Google Scholar]
- 44.Chatwin H.V., Cruz J.C., Green A.L. Pediatric high-grade glioma: Moving toward subtype-specific multimodal therapy. FEBS J. 2021;288:6127–6141. doi: 10.1111/febs.15739. [DOI] [PubMed] [Google Scholar]
- 45.Korshunov A., Schrimpf D., Ryzhova M., Sturm D., Chavez L., Hovestadt V., Sharma T., Habel A., Burford A., Jones C., et al. H3-/IDH-wild type pediatric glioblastoma is comprised of molecularly and prognostically distinct subtypes with associated oncogenic drivers. Acta Neuropathol. 2017;134:507–516. doi: 10.1007/s00401-017-1710-1. [DOI] [PubMed] [Google Scholar]
- 46.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newcastle—Ottawa Quality Assessment Scale Case Control Studies. [(accessed on 11 April 2022)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK47306/bin/appendixes.app3fm4.pdf.
- 48.Grill J., Massimino M., Bouffet E., Azizi A., McCowage G., Cañete A., Saran F., Le Deley M.-C., Varlet P., Morgan P., et al. Phase II, Open-Label, Randomized, Multicenter Trial (HERBY) of Bevacizumab in Pediatric Patients With Newly Diagnosed High-Grade Glioma. J. Clin. Oncol. 2018;36:951–958. doi: 10.1200/JCO.2017.76.0611. [DOI] [PubMed] [Google Scholar]
- 49.Risk of Bias 2 (RoB 2) Tool|Cochrane Methods. [(accessed on 11 April 2022)]. Available online: https://methods.cochrane.org/risk-bias-2.
- 50.Macdonald T.J., Vezina G., Stewart C.F., Turner D., Pierson C.R., Chen L., Pollack I.F., Gajjar A., Kieran M.W. Phase II study of cilengitide in the treatment of refractory or relapsed high-grade gliomas in children: A report from the Children′s Oncology Group. Neuro-Oncology. 2013;15:1438–1444. doi: 10.1093/neuonc/not058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gururangan S., Chi S.N., Poussaint T.Y., Onar-Thomas A., Gilbertson R.J., Vajapeyam S., Friedman H.S., Packer R.J., Rood B.N., Boyett J.M., et al. Lack of Efficacy of Bevacizumab Plus Irinotecan in Children With Recurrent Malignant Glioma and Diffuse Brainstem Glioma: A Pediatric Brain Tumor Consortium Study. J. Clin. Oncol. 2010;28:3069–3075. doi: 10.1200/JCO.2009.26.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robison N.J., Campigotto F., Chi S.N., Manley P.E., Turner C.D., Zimmerman M.A., Chordas C.A., Werger A.M., Allen J., Goldman S., et al. A phase II trial of a multi-agent oral antiangiogenic (metronomic) regimen in children with recurrent or progressive cancer. Pediatr. Blood Cancer. 2013;61:636–642. doi: 10.1002/pbc.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su J.M., Murray J.C., McNall-Knapp R.Y., Bowers D.C., Shah S., Adesina A.M., Paulino A.C., Jo E., Mo Q., Baxter P.A., et al. A phase 2 study of valproic acid and radiation, followed by maintenance valproic acid and bevacizumab in children with newly diagnosed diffuse intrinsic pontine glioma or high-grade glioma. Pediatr. Blood Cancer. 2020;67:e28283. doi: 10.1002/pbc.28283. [DOI] [PubMed] [Google Scholar]
- 54.Wetmore C., Daryani V.M., Billups C.A., Boyett J.M., Leary S., Tanos R., Goldsmith K.C., Stewart C.F., Blaney S.M., Gajjar A. Phase II evaluation of sunitinib in the treatment of recurrent or refractory high-grade glioma or ependymoma in children: A children′s Oncology Group Study ACNS1021. Cancer Med. 2016;5:1416–1424. doi: 10.1002/cam4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilbert M.R., Dignam J.J., Armstrong T.S., Wefel J.S., Blumenthal D.T., Vogelbaum M.A., Colman H., Chakravarti A., Pugh S., Won M., et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen M.H., Shen Y.L., Keegan P., Pazdur R. FDA Drug Approval Summary: Bevacizumab (Avastin®) as Treatment of Recurrent Glioblastoma Multiforme. Oncology. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 57.Diaz A.K., Baker S.J. The Genetic Signatures of Pediatric High-Grade Glioma: No Longer a One-Act Play. Semin. Radiat. Oncol. 2014;24:240–247. doi: 10.1016/j.semradonc.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones C., Karajannis M.A., Jones D.T.W., Kieran M.W., Monje M., Baker S.J., Becher O.J., Cho Y.-J., Gupta N., Hawkins C., et al. Pediatric high-grade glioma: Biologically and clinically in need of new thinking. Neuro-Oncology. 2016;19:153–161. doi: 10.1093/neuonc/now101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salloum R., DeWire M., Lane A., Goldman S., Hummel T., Chow L., Miles L., Sutton M., Stevenson C., Fouladi M., et al. Patterns of progression in pediatric patients with high-grade glioma or diffuse intrinsic pontine glioma treated with Bevacizumab-based therapy at diagnosis. J. Neuro-Oncol. 2014;121:591–598. doi: 10.1007/s11060-014-1671-3. [DOI] [PubMed] [Google Scholar]
- 60.Camphausen K., Cerna D., Scott T., Sproull M., Burgan W.E., Cerra M.A., Fine H., Tofilon P.J. Enhancement ofin vitro andin vivo tumor cell radiosensitivity by valproic acid. Int. J. Cancer. 2004;114:380–386. doi: 10.1002/ijc.20774. [DOI] [PubMed] [Google Scholar]
- 61.Karagiannis T.C., Kn H., El-Osta A. The Epigenetic Modifier, Valproic Acid, Enhances Radiation Sensitivity. Epigenetics. 2006;1:131–137. doi: 10.4161/epi.1.3.2896. [DOI] [PubMed] [Google Scholar]
- 62.Bouffet E., Larouche V., Campbell B.B., Merico D., De Borja R., Aronson M., Durno C., Krueger J., Cabric V., Ramaswamy V., et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J. Clin. Oncol. 2016;34:2206–2211. doi: 10.1200/JCO.2016.66.6552. [DOI] [PubMed] [Google Scholar]
- 63.Friedman H.S., Prados M.D., Wen P.Y., Mikkelsen T., Schiff D., Abrey L.E., Yung W.A., Paleologos N., Nicholas M.K., Jensen R., et al. Bevacizumab Alone and in Combination With Irinotecan in Recurrent Glioblastoma. J. Clin. Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 64.Kreisl T.N., Kim L., Moore K., Duic P., Royce C., Stroud I., Garren N., Mackey M., Butman J., Camphausen K., et al. Phase II Trial of Single-Agent Bevacizumab Followed by Bevacizumab Plus Irinotecan at Tumor Progression in Recurrent Glioblastoma. J. Clin. Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vredenburgh J.J., Desjardins A., Herndon J.E., Dowell J.M., Reardon D.A., Quinn J.A., Rich J.N., Sathornsumetee S., Gururangan S., Wagner M., et al. Phase II Trial of Bevacizumab and Irinotecan in Recurrent Malignant Glioma. Clin. Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 66.Ellis L.M., Hicklin D.J. Pathways Mediating Resistance to Vascular Endothelial Growth Factor–Targeted Therapy: Fig. 1. Clin. Cancer Res. 2008;14:6371–6375. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 67.MacDonald T.J., Stewart C.F., Kocak M., Goldman S., Ellenbogen R.G., Phillips P., Lafond D., Poussaint T.Y., Kieran M.W., Boyett J.M., et al. Phase I Clinical Trial of Cilengitide in Children With Refractory Brain Tumors: Pediatric Brain Tumor Consortium Study PBTC-012. J. Clin. Oncol. 2008;26:919–924. doi: 10.1200/JCO.2007.14.1812. [DOI] [PubMed] [Google Scholar]
- 68.Yamada S., Bu X.-Y., Khankaldyyan V., Gonzales-Gomez I., McComb J.G., Laug W.E. Effect of the angiogenesis inhibitor cilengitide (emd 121974) on glioblastoma growth in nude mice. Neurosurgery. 2006;59:1304–1312. doi: 10.1227/01.NEU.0000245622.70344.BE. [DOI] [PubMed] [Google Scholar]
- 69.MacDonald T.J., Taga T., Shimada H., Tabrizi P., Zlokovic B.V., Cheresh D.A., Laug W.E. Preferential Susceptibility of Brain Tumors to the Antiangiogenic Effects of an αv Integrin Antagonist. Neurosurgery. 2001;48:151–157. doi: 10.1097/00006123-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 70.Tabatabai G., Tonn J.-C., Stupp R., Weller M. The Role of Integrins in Glioma Biology and Anti-Glioma Therapies. Curr. Pharm. Des. 2011;17:2402–2410. doi: 10.2174/138161211797249189. [DOI] [PubMed] [Google Scholar]
- 71.Stupp R., Hegi M., Gorlia T., Erridge S.C., Perry J., Hong Y.-K., Aldape K.D., Lhermitte B., Pietsch T., Grujicic D., et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–1108. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 72.Stupp R., Hegi M.E., Neyns B., Goldbrunner R., Schlegel U., Clement P.M., Grabenbauer G.G., Ochsenbein A.F., Simon M., Dietrich P.-Y., et al. Phase I/IIa Study of Cilengitide and Temozolomide With Concomitant Radiotherapy Followed by Cilengitide and Temozolomide Maintenance Therapy in Patients With Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2010;28:2712–2718. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 73.Mikkelsen T., Brodie C., Finniss S., Berens M.E., Rennert J.L., Nelson K., Lemke N., Brown S.L., Hahn D., Neuteboom B., et al. Radiation sensitization of glioblastoma by cilengitide has unanticipated schedule-dependency. Int. J. Cancer. 2008;124:2719–2727. doi: 10.1002/ijc.24240. [DOI] [PubMed] [Google Scholar]
- 74.Panigrahy D., Kaipainen A., Butterfield C.E., Chaponis D.M., Laforme A.M., Folkman J., Kieran M.W. Inhibition of tumor angiogenesis by oral etoposide. Exp. Ther. Med. 2010;1:739–746. doi: 10.3892/etm.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klement G., Baruchel S., Rak J., Man S., Clark K., Hicklin D.J., Bohlen P., Kerbel R.S. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J. Clin. Investig. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Antiangiogenic Scheduling of Chemotherapy Improves Efficacy against Experimental Drug-Resistant Cancer—PubMed. [(accessed on 3 April 2022)]; Available online: https://pubmed.ncbi.nlm.nih.gov/10766175/ [PubMed]
- 77.Pasquier E., Kavallaris M., André N. Metronomic chemotherapy: New rationale for new directions. Nat. Rev. Clin. Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 78.Bahl A., Bakhshi S. Metronomic Chemotherapy in Progressive Pediatric Malignancies: Old Drugs in New Package. Indian J. Pediatr. 2012;79:1617–1622. doi: 10.1007/s12098-012-0759-z. [DOI] [PubMed] [Google Scholar]
- 79.Grisanti S., Ferrari V.D., Buglione M., Agazzi G.M., Liserre R., Poliani L., Buttolo L., Gipponi S., Pedersini R., Consoli F., et al. Second line treatment of recurrent glioblastoma with sunitinib: Results of a phase II study and systematic review of literature. J. Neurosurg. Sci. 2019;63:458–467. doi: 10.23736/S0390-5616.16.03874-1. [DOI] [PubMed] [Google Scholar]
- 80.Westerdijk K., Desar I.M., Steeghs N., van der Graaf W.T., van Erp N.P., Dutch Pharmacology and Oncology Group (DPOG) Imatinib, sunitinib and pazopanib: From flat-fixed dosing towards a pharmacokinetically guided personalized dose. Br. J. Clin. Pharmacol. 2020;86:258–273. doi: 10.1111/bcp.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paugh B.S., Qu C., Jones C., Liu Z., Adamowicz-Brice M., Zhang J., Bax D.A., Coyle B., Barrow J., Hargrave D., et al. Integrated Molecular Genetic Profiling of Pediatric High-Grade Gliomas Reveals Key Differences With the Adult Disease. J. Clin. Oncol. 2010;28:3061–3068. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gajjar A., Pfister S., Taylor M., Gilbertson R.J. Molecular Insights into Pediatric Brain Tumors Have the Potential to Transform Therapy. Clin. Cancer Res. 2014;20:5630–5640. doi: 10.1158/1078-0432.CCR-14-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cabanas R., Saurez G., Rios M., Alert J., Reyes A., Valdes J., Gonzalez M.C., Pedrayes J.L., Avila M., Herrera R., et al. Treatment of children with high grade glioma with nimotuzumab. mAbs. 2013;5:202–207. doi: 10.4161/mabs.22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gorsi H.S., Malicki D.M., Barsan V., Tumblin M., Yeh-Nayre L., Milburn M., Elster J.D., Crawford J.R. Nivolumab in the Treatment of Recurrent or Refractory Pediatric Brain Tumors: A Single Institutional Experience. J. Pediatr. Hematol. 2019;41:e235–e241. doi: 10.1097/MPH.0000000000001339. [DOI] [PubMed] [Google Scholar]
- 85.Johnson A., Severson E., Gay L., Vergilio J.-A., Elvin J., Suh J., Daniel S., Covert M., Frampton G.M., Hsu S., et al. Comprehensive Genomic Profiling of 282 Pediatric Low- and High-Grade Gliomas Reveals Genomic Drivers, Tumor Mutational Burden, and Hypermutation Signatures. Oncologist. 2017;22:1478–1490. doi: 10.1634/theoncologist.2017-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Junior A.G., Abreu N.M.P., Leal M.V.B., de Aquino H.L.A., Rodrigues J.P.C., Malveira C.B., Silva Y.P., Coimbra P.P.A. A Case Report of a Rare Pediatric Brain Tumor: Congenital Glioblastoma. Cureus. 2022;14:3229. doi: 10.7759/cureus.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Glod J., Rahme G., Kaur H., Raabe E.H., Hwang E.I., Israel M.A. Pediatric Brain Tumors: Current Knowledge and Therapeutic Opportunities. J. Pediatr. Hematol. 2016;38:249–260. doi: 10.1097/MPH.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Friedman G.K., Johnston J.M., Bag A.K., Bernstock J.D., Li R., Aban I., Kachurak K., Nan L., Kang K.-D., Totsch S., et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021;384:1613–1622. doi: 10.1056/NEJMoa2024947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Josupeit R., Bender S., Kern S., Leuchs B., Hielscher T., Herold-Mende C., Schlehofer J.R., Dinsart C., Witt O., Rommelaere J., et al. Pediatric and Adult High-Grade Glioma Stem Cell Culture Models Are Permissive to Lytic Infection with Parvovirus H-1. Viruses. 2016;8:138. doi: 10.3390/v8050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wakefield A., Pignata A., Ghazi A., Ashoori A., Hegde M., Landi D., Gray T., Scheurer M.E., Chintagumpala M., Adesina A., et al. Is CMV a target in pediatric glioblastoma? Expression of CMV proteins, pp65 and IE1-72 and CMV nucleic acids in a cohort of pediatric glioblastoma patients. J. Neuro-Oncol. 2015;125:307–315. doi: 10.1007/s11060-015-1905-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lucas K.G., Bao L., Bruggeman R., Dunham K., Specht C. The detection of CMV pp65 and IE1 in glioblastoma multiforme. J. Neuro-Oncol. 2010;103:231–238. doi: 10.1007/s11060-010-0383-6. [DOI] [PubMed] [Google Scholar]
- 92.Ghazi A., Ashoori A., Hanley P.J., Brawley V.S., Shaffer D.R., Kew Y., Powell S.Z., Grossman R., Grada Z., Scheurer M.E., et al. Generation of Polyclonal CMV-specific T Cells for the Adoptive Immunotherapy of Glioblastoma. J. Immunother. 2012;35:159–168. doi: 10.1097/CJI.0b013e318247642f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rahbar A., Orrego A., Peredo I., Dzabic M., Wolmer-Solberg N., Strååt K., Stragliotto G., Söderberg-Nauclér C. Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. J. Clin. Virol. 2013;57:36–42. doi: 10.1016/j.jcv.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 94.Lasky J.L., Panosyan E.H., Plant A., Davidson T., Yong W.H., Prins R.M., Liau L.M., Moore T.B. Autologous tumor lysate-pulsed dendritic cell immunotherapy for pediatric patients with newly diagnosed or recurrent high-grade gliomas. Anticancer Res. 2013;33:2047–2056. [PMC free article] [PubMed] [Google Scholar]
- 95.Kieran M.W., Geoerger B., Dunkel I.J., Broniscer A., Hargrave D.R., Hingorani P., Aerts I., Bertozzi A.-I., Cohen K.J., Hummel T.R., et al. A Phase I and Pharmacokinetic Study of Oral Dabrafenib in Children and Adolescent Patients with Recurrent or Refractory BRAF V600 Mutation–Positive Solid Tumors. Clin. Cancer Res. 2019;25:7294–7302. doi: 10.1158/1078-0432.CCR-17-3572. [DOI] [PubMed] [Google Scholar]
- 96.Becher O.J., Gilheeney S.W., Khakoo Y., Lyden D.C., Haque S., De Braganca K.C., Kolesar J.M., Huse J.T., Modak S., Wexler L.H., et al. A phase I study of perifosine with temsirolimus for recurrent pediatric solid tumors. Pediatr. Blood Cancer. 2017;64:e26409. doi: 10.1002/pbc.26409. [DOI] [PubMed] [Google Scholar]
- 97.McCrea H.J., Ivanidze J., O’Connor A., Hersh E.H., Boockvar J.A., Gobin Y.P., Knopman J., Greenfield J.P. Intraarterial delivery of bevacizumab and cetuximab utilizing blood-brain barrier disruption in children with high-grade glioma and diffuse intrinsic pontine glioma: Results of a phase I trial. J. Neurosurg. Pediatr. 2021;28:371–379. doi: 10.3171/2021.3.PEDS20738. [DOI] [PubMed] [Google Scholar]
- 98.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.