Abstract
Azospirillum brasilense Sp7 and its ntrA (rpoN), ntrBC, and ntrC mutants have been evaluated for their capabilities of poly-3-hydroxybutyrate (PHB) accumulation in media with high and low ammonia concentrations. It was observed that the ntrBC and ntrC mutants can produce PHB in both low- and high-C/N-ratio media, while no significant PHB production was observed for the wild type or the ntrA mutant in low-C/N-ratio media. Further investigation by fermentation analysis indicated that the ntrBC and ntrC mutants were able to grow and accumulate PHB simultaneously in the presence of a high concentration of ammonia in the medium, while little PHB was produced in the wild type and ntrA (rpoN) mutant during active growth phase. These results provide the first genetic evidence that the ntrB and ntrC genes are involved in the regulation of PHB synthesis by ammonia in A. brasilense Sp7.
Poly-3-hydroxybutyrate (PHB), a thermoplastic produced by numerous microorganisms as an energy and/or carbon storage material under conditions of nutrient imbalance, has attracted attention for its biodegradability and biocompatibility (2). However, a major limitation in the commercialization of PHB in a wide range of applications is its high production cost (5, 6). Much effort has been devoted to lowering the production cost by developing more efficient fermentation and recovery processes (15); selecting new potential microorganisms, including genetically engineered bacteria (7, 26); metabolic engineering of PHB biosynthetic pathways in higher organisms, such as Saccharomyces cerevisiae (14), insects (33), and plants (34); using alternative cheaper carbon sources (3, 22); and investigating the precise control mechanisms involved in PHB biosynthesis (25, 27).
Intensive studies on the metabolic pathways for PHB biosynthesis and molecular analyses of PHB biosynthesis genes in various bacteria have been conducted in order to understand the mechanisms of PHB biosynthesis and subsequently to construct genetically engineered microorganisms or even plants for more efficient production of PHB. In Ralstonia eutropha (formerly known as Alcaligenes eutrophus), acetyl coenzyme A (acetyl-CoA) is converted to PHB in the following three steps: (i) formation of acetoacetyl-CoA, (ii) stereoselective reduction of acetoacetyl-CoA to d-(−)-3-hydroxybutyryl-CoA, and (iii) ligation of d-(−)-3-hydroxybutyryl to the growing chain of PHB. Since the first phb gene was isolated from Zoogloea ramigera (24), more than 30 different PHB biosynthesis genes have been cloned from various bacteria (15). Some genes involved in the formation of the PHB granule have also been recently characterized (25).
For most PHB-producing bacteria, only little PHB accumulation can be observed during the active growth phase of cells, so a long growth phase is essential for high-density cell cultivation (2). Nutrient limitation is needed for initiation of PHB accumulation, and generally ammonia is considered the critical control factor decoupling the growth of cells and PHB production. However, some bacteria, such as Azotobacter vinelandii strain UWD (obtained by chemical mutagenesis [23]), Alcaligenes latus (8), and Pseudomonas putida KT2442 (9), are able to accumulate large amounts of PHB or polyhydroxyalkanoate (PHA) during exponential growth. The inactivation of inhibition of ammonia of the accumulation of PHB has industrial potential for improvement of process control and productivity (16).
Azospirillum, a genus of free-living nitrogen-fixing bacteria, has been studied intensively in the past decades for its physiological and genetic properties. Some of the species, such as Azospirillum brasilense and Azospirillum lipoferum, are noted for their capabilities of accumulation of intracellular PHB with a relatively high content (up to 88% of the dry biomass) under unbalanced nutrient conditions such as oxygen limitation and a high C/N ratio (12, 28).
In this study, the regulation of PHB production by ammonia was investigated for A. brasilense Sp7 and its ntrA (rpoN), ntrBC, and ntrC mutants. The significant differences in PHB production by the ntrBC and ntrC mutants versus the ntrA (rpoN) mutant and wild type during the exponential growth phase in the presence of a high concentration of ammonia in the medium demonstrate the involvement of the ntrB and ntrC genes in the regulation of PHB biosynthesis by ammonia in A. brasilense Sp7.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. All the strains were routinely grown in MMAB medium (31) at 30°C. Kanamycin (25 μg/ml) was added to the medium when required. Because the ntrB and ntrC genes are organized in one operon, the ntrB mutant, constructed by polar mutation, is an ntrBC mutant (18).
TABLE 1.
A. brasilense strains used in this study
Culture conditions for PHB production.
For test tube cultures of bacteria, 5-ml aliquots of MMAB medium in 20-ml test tubes were each inoculated with 1 loop of bacteria from a fresh plate or 0.15 ml of preculture from another test tube and then incubated at 30°C for 24 h while shaken at 200 rpm. The batch fermentation was performed in a 2-liter O2-stat fermentor as described previously (20). The concentration of dissolved oxygen (DO2) was controlled at a constant level by varying the air flow into the fermentor according to the measured DO2 value so that the air flow rate could be used as an indicator for the oxygen uptake rate (20).
Analytical procedures.
Cell growth was monitored by measuring the optical density at 600 nm with a Perkin-Elmer Lambda 2 UV–visible-spectrum spectrophotometer. Biomass concentration, defined as cell dry weight per milliliter of culture broth, was determined by weighing dry cells with a microbalance (Mettler, Zurich, Switzerland) as described previously (32). l-Malate and ammonia concentrations in the culture broth were determined with test kits from Boehringer Mannheim (Mannheim, Germany). The PHB concentration was determined with a gas chromatograph (HP6890 Plus; Hewlett-Packard, Wilmington, Del.) equipped with an automatic sampler (HP7683; Hewlett-Packard) and a J&W DB-WAX capillary column (0.53 mm by 15 m, 1-μm film thickness) by using benzoic acid as the internal standard (4). Non-PHB biomass was obtained by subtracting the amount of PHB from the biomass, while the PHB content was defined as the ratio of PHB to cell dry weight, expressed as a percentage. All the data in this paper are average values of at least two replicates.
RESULTS
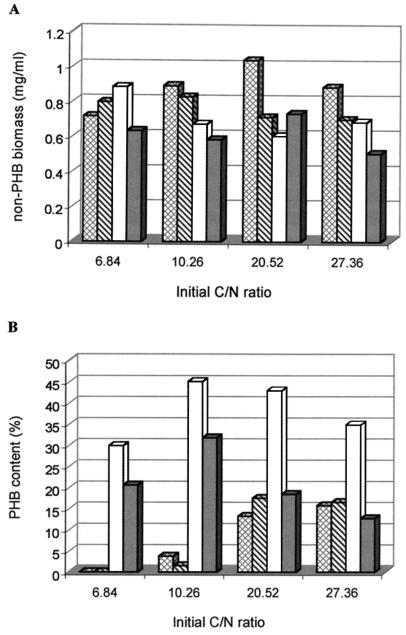
A. brasilense Sp7 and its ntrA (rpoN), ntrBC, and ntrC mutants were grown in MMAB medium with different initial C/N ratios obtained by varying the concentration of malate or NH4Cl. After incubation at 30°C for 24 h, the biomass and PHB concentrations were measured and compared. The results are shown in Fig. 1.
FIG. 1.
Comparison of non-PHB biomass production and PHB content of A. brasilense Sp7 (▩) and its ntrA (▧), ntrBC ( ), and ntrC (■) mutants in MMAB medium with different initial C/N ratios. The concentration of malate was 15 g/liter, while the concentrations of NH4Cl were 3, 2, 1, and 0.75 g/liter, corresponding to the different initial C/N ratios.
PHB production by A. brasilense Sp7 and its ntrA (rpoN) mutant increased with the C/N ratio of the medium, and no PHB could be detected in low-C/N-ratio media because at least half of the initial amount of ammonia was still present in the media when malate was depleted (data not shown). In contrast with the wild type and ntrA (rpoN) mutant, the ntrBC and ntrC mutants were able to synthesize PHB even in low-C/N-ratio media.
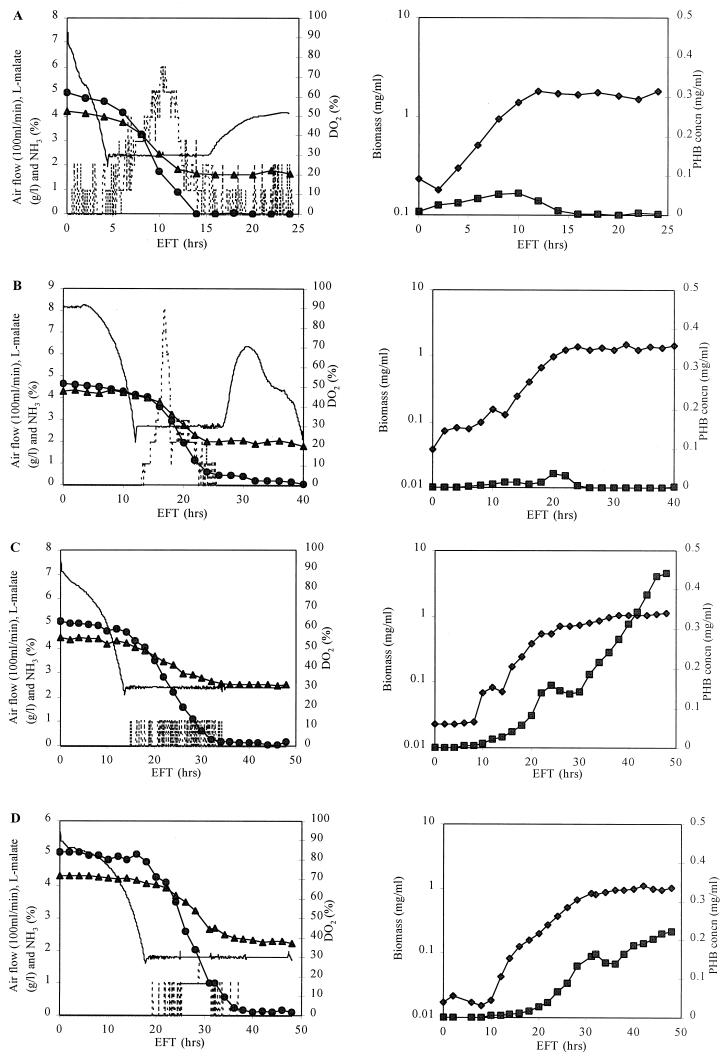
The above results can be interpreted in two ways: (i) PHB biosynthesis coincides with the growth of cells for the ntrBC or ntrC mutant, or (ii) initiation of PHB accumulation occurs at much higher ammonia concentrations in the ntrBC or ntrC mutant. In order to elucidate the involvement of the ntrB and ntrC genes in the regulation of PHB production by ammonia in A. brasilense Sp7, the wild type and mutant strains were grown in a bioreactor, allowing more precise monitoring and control of culture conditions. MMAB medium was supplemented with 10 g of malate and 1.35 g of NH4Cl per liter (initial C/N ratio = 10), and the DO2 concentration was set at 30%, which was reported to be optimal for PHB accumulation in A. brasilense (28). No nitrogen fixation can occur under these culture conditions since the nitrogen fixation process is repressed by the high DO2 concentration and the presence of combined nitrogen. Therefore, the influence of diazotrophic growth can be excluded (9, 10). The results are shown in Fig. 2.
FIG. 2.
Time course of fermentation of A. brasilense Sp7 (A) and its ntrA (rpoN) (B), ntrBC (C), and ntrC (D) mutants. EFT, elapsed fermentation time. Symbols: ——, DO2; –––, air flow; ●, l-malate concentration; ▴, ammonia concentration; ⧫, biomass concentration; ■, PHB concentration.
It can be observed that A. brasilense Sp7 and its ntrA (rpoN) mutant produced only small amounts of PHB in the active growth phase, while no additional PHB accumulated during the stationary phase (Fig. 2A and B). The respiration of cells (indicated by the air flow rate) increased drastically at the end of the exponential growth phase, which is consistent with the results of a previous study (20). Nevertheless, PHB production was not triggered during the stationary phase, since about 20 mM NH4Cl was still present in the medium. For the wild type, the PHB concentration reached its maximum and cell growth entered the stationary phase at 10 h of fermentation even though there was about 4 g of malate per liter left in the medium (Fig. 2A). However, the ntrBC and ntrC mutants not only produced a larger amount of PHB during the growth phase than the wild type but also continued to synthesize PHB in the stationary phase despite a high concentration of ammonia in the medium (Fig. 2C and D). Eventually about 40 and 22% PHB content accumulated in the ntrBC and ntrC mutants, respectively. However, a relatively long lag phase was observed for the ntrBC and ntrC mutants when the DO2 concentration was higher than 30%, and the respiration of the mutants was much lower than that of the wild type, as can be deduced from the air flow. This implies that a high DO2 value might inhibit the growth of the ntrBC and ntrC mutants. The active growth phases are similar for the wild type and the ntrBC and ntrC mutants.
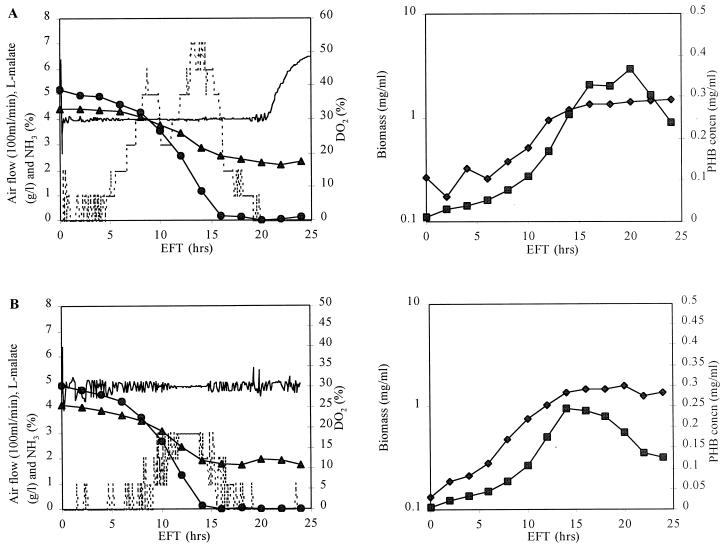
In order to demonstrate and exclude the inhibition influence of a high DO2 concentration on the growth of the ntrBC and ntrC mutants, the DO2 concentration was kept at 30% from the beginning of fermentation by sparging N2 into the fermentor. The results are shown in Fig. 3. It can be observed that the growth properties of the ntrBC and ntrC mutants were similar to that of the wild type, while their PHB production coincided with the active growth. However, the PHB concentration decreased during the stationary phase because the malate was exhausted and PHB was likely used as the alternative carbon source for growth maintenance.
FIG. 3.
Time course of fermentation of the A. brasilense Sp7 ntrBC (A) and ntrC (B) mutants with control of the DO2 concentration from the beginning of fermentation. The symbols and abbreviation are the same as for Fig. 2.
DISCUSSION
The regulatory genes ntrB and ntrC, encoding the two-component sensor-activator regulatory system NtrB-NtrC (13), have been previously characterized in A. brasilense (18). The results of studies on the phenotype of the ntrBC and ntrC mutants indicate that NtrB and NtrC are not strictly required for nitrogen fixation in Azospirillum, although the nitrogenase activity of the ntrC mutant was partially reduced. No significant difference of ammonia uptake rate has been observed for the ntrBC mutant and the wild type of A. brasilense (30). However, NtrC has been shown to be involved in nitrate utilization as a nitrogen source in A. brasilense, and the ntrBC mutant displayed nitrogenase activity which was partially resistant to ammonia inactivation (17). The regulation of the amtB gene, encoding an ammonia transporter, by the Ntr system has been recently demonstrated (30). In P. putida KT2442, which can synthesize PHA during exponential growth when grown on fatty acids, a two-component system homologous to the sensor kinase-response regulator couple LemA-GacA was recently found to be involved in the regulation of PHA synthesis (19). However, no study on the relationship between NtrBC and PHB production has been reported so far.
In this study, some intriguing phenomena from the fermentation data for the ntrBC and ntrC mutants have been observed. Firstly, the respiration of the ntrBC and ntrC mutants diminished greatly compared to that of the wild type, indicating that the ntrBC genes might be involved in the regulation of genes encoding respiratory enzymes. Secondly, the long lag phase of the ntrBC and ntrC mutants (Fig. 2C and D) implies that the ntrBC genes are probably also involved in the regulation of the tolerance of high oxygen concentrations by A. brasilense. Thirdly, the results for PHB production by the ntrBC and ntrC mutants indicate explicitly the involvement of the ntrB and ntrC genes in the regulation of PHB production by ammonia in A. brasilense. The ntrBC and ntrC mutants can produce PHB continuously, whether in the active growth phase or stationary phase or whether or not a high concentration of ammonia is present in the medium. Nevertheless, a transition of PHB production from exponential growth phase to stationary phase can still be observed in the fermentation time courses of the ntrBC and ntrC mutants. Therefore, it can be reasonably concluded that inactivation of the ntrB and ntrC genes not only couples the PHB production and the active growth of cells but also eliminates the inhibition effect of ammonia on PHB biosynthesis by A. brasilense Sp7.
The coupling of PHB production and cell growth has application potential for significant improvement of productivity and facilitation of process control. This study provides evidence of the involvement of the ntrB and ntrC genes in the regulation of PHB production and therefore supports further investigation of this relationship. It will be of interest to identify the target gene(s) of the NtrB-NtrC two-component system.
ACKNOWLEDGMENTS
J.S. is a recipient of a doctoral scholarship from the Research Council, K.U. Leuven. X.P. is a recipient of a scholarship from K.U. Leuven. This study was supported in part by Project OT/95/20 of the K.U. Leuven Research Council.
We thank A. Van Dommelen for her advice, and we also acknowledge C. Elmerich for the gift of the ntrBC and ntrC mutants of A. brasilense Sp7.
REFERENCES
- 1.Albrecht S L, Okon Y. Cultures of Azospirillum. Methods Enzymol. 1980;69:740–749. [Google Scholar]
- 2.Anderson A J, Dawes E A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourque P Y, Pomerleau Y, Groleau D. High-cell-density production of poly-β-hydroxybutyrate (PHB) from methanol by Methylobacterium extorquens: production of high-molecular-mass PHB. Appl Microbiol Biotechnol. 1995;44:367–376. [Google Scholar]
- 4.Braunegg G, Sonnleitner B, Lafferty R M. A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol. 1978;6:29–37. [Google Scholar]
- 5.Byrom D. Polymer synthesis by microorganisms: technology and economics. Trends Biotechnol. 1987;5:246–250. [Google Scholar]
- 6.Choi J, Lee S Y. Process analysis and economic evaluation for poly(3-hydroxybutyrate) production by fermentation. Bioprocess Eng. 1997;17:335–342. [Google Scholar]
- 7.Choi J, Lee S Y, Han K. Cloning of the Alcaligenes latus polyhydroxyalkanoate biosynthesis genes and uses of these genes for enhanced production of poly(3-hydroxybutyrate) in Escherichia coli. Appl Microbiol Biotechnol. 1998;64:4897–4903. doi: 10.1128/aem.64.12.4897-4903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hänggi U J. Pilot scale production of PHB with Alcaligenes latus. In: Dawes E A, editor. Novel biodegradable microbial polymers. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 65–70. [Google Scholar]
- 9.Hartmann A, Burris R H. Regulation of nitrogenase activity by oxygen in Azospirillum brasilense and Azospirillum lipoferum. J Bacteriol. 1987;169:944–948. doi: 10.1128/jb.169.3.944-948.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann A, Fu H, Burris R H. Regulation of nitrogenase activity by ammonium chloride in Azospirillumspp. J Bacteriol. 1986;165:864–870. doi: 10.1128/jb.165.3.864-870.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huisman G W, Wonink E, de Koning G, Preusting H, Witholt B. Synthesis of poly(3-hydroxyalkanoates) by mutant and recombinant Pseudomonasstrains. Appl Microbiol Biotechnol. 1992;38:1–5. [Google Scholar]
- 12.Itzigsohn R, Yarden O, Okon Y. Polyhydroxyalkanoate analysis in Azospirillum brasilense. Can J Microbiol. 1995;41:73–76. [Google Scholar]
- 13.Keener J, Kustu S. Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: role of the conserved amino-terminal domain of NTRC. Proc Natl Acad Sci USA. 1988;85:4976–4980. doi: 10.1073/pnas.85.14.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leaf T A, Peterson M S, Stoup S K, Somers D, Srienc F. Saccharomyces cerevisiaeexpressing bacterial polyhydroxybutyrate synthase produces poly-3-hydroxybutyrate. Microbiology. 1996;142:1169–1180. doi: 10.1099/13500872-142-5-1169. [DOI] [PubMed] [Google Scholar]
- 15.Lee S Y. Bacterial polyhydroxyalkanoates. Biotechnol Bioeng. 1996;49:1–14. doi: 10.1002/(SICI)1097-0290(19960105)49:1<1::AID-BIT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 16.Lee S Y, Chang H N. Production of poly(hydroxyalkanoic acid) Adv Biochem Eng Biotechnol. 1995;52:27–58. doi: 10.1007/BFb0102315. [DOI] [PubMed] [Google Scholar]
- 17.Liang Y Y. Etude du rôle des gènes nifA at ntrBC dans la régulation de l'experssion des gènes nif chez Azospirillum brasilense Sp7. Ph.D. thesis. Paris, France: Institut Pasteur; 1992. [Google Scholar]
- 18.Liang Y Y, Arsène F, Elmerich C. Characterization of the ntrBC genes of Azospirillum brasilenseSp7: their involvement in the regulation of nitrogenase synthesis and activity. Mol Gen Genet. 1993;240:188–196. doi: 10.1007/BF00277056. [DOI] [PubMed] [Google Scholar]
- 19.Madison L L, Huisman G W. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev. 1999;63:21–53. doi: 10.1128/mmbr.63.1.21-53.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchal K, Sun J, Keijers V, Haaker H, Vanderleyden J. A cytochrome cbb3 (cytochrome c) terminal oxidase in Azospirillum brasilenseSp7 supports microaerobic growth. J Bacteriol. 1998;180:5689–5696. doi: 10.1128/jb.180.21.5689-5696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milcamps A, Van Dommelen A, Stigter J, Vanderleyden J, de Bruijn F. The Azospirillum brasilense rpoNgene is involved in nitrogen fixation, nitrate assimilation, ammonium uptake and flagellar biosynthesis. Can J Microbiol. 1996;42:467–478. doi: 10.1139/m96-064. [DOI] [PubMed] [Google Scholar]
- 22.Page W J, Cornish A. Growth of Azotobacter vinelandiiUWD in fish peptone medium and simplified extraction of poly-β-hydroxybutyrate. Appl Environ Microbiol. 1993;59:4236–4244. doi: 10.1128/aem.59.12.4236-4244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page W J, Knosp O. Hyperproduction of poly-β-hydroxybutyrate during exponential growth of Azotobacter vinelandiiUWD. Appl Environ Microbiol. 1989;55:1334–1339. doi: 10.1128/aem.55.6.1334-1339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peoples O P, Masamune S, Walsh C T, Sinskey A J. Biosynthetic thiolase from Zoogloea ramigera. III. Isolation and characterization of the structural gene. J Biol Chem. 1987;262:97–102. [PubMed] [Google Scholar]
- 25.Prieto M A, Bühler B, Jung K, Witholt B, Kessler B. PhaF, a polyhydroxyalkanoate-granule-associated protein of Pseudomonas oleovorans GPo1 involved in the regulatory expression system for phagenes. J Bacteriol. 1999;181:858–868. doi: 10.1128/jb.181.3.858-868.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renner G, Haage G, Braunegg G. Production of short-side-chain polyhydroxyalkanoates by various bacteria from the rRNA superfamily III. Appl Microbiol Biotechnol. 1996;46:268–272. [Google Scholar]
- 27.Slater S, Houmiel K L, Tran M, Mitsky T A, Taylor N B, Padgette S R, Gruys K J. Multiple β-ketothiolases mediate poly(β-hydroxyalkanoate) copolymer synthesis in Ralstonia eutropha. J Bacteriol. 1998;180:1979–1987. doi: 10.1128/jb.180.8.1979-1987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tal S, Okon Y. Production of the reserve material poly-β-hydroxybutyrate and its function in Azospirillum brasilenseCd. Can J Microbiol. 1985;31:608–613. [Google Scholar]
- 29.Tarrand J J, Krieg N R, Dobereiner J. A taxonomic study of the Spirillum lipoferum group, with the description of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilensesp. nov. Can J Microbiol. 1978;24:967–980. doi: 10.1139/m78-160. [DOI] [PubMed] [Google Scholar]
- 30.Van Dommelen A, Keijers V, Vanderleyden J, de Zamaroczy M. (Methyl)ammonium transport in the nitrogen-fixing bacterium Azospirillum brasilense. J Bacteriol. 1998;180:2652–2659. doi: 10.1128/jb.180.10.2652-2659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanstockem M, Michiels K, Vanderleyden J, Van Gool A P. Transposon mutagenesis of Azospirillum brasilense and Azospirillum lipoferum: physical analysis of Tn5 and Tn5-Mob insertion mutants. Appl Environ Microbiol. 1987;53:410–415. doi: 10.1128/aem.53.2.410-415.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang F, Lee S Y. Production of poly(3-hydroxybutyrate) by fed-batch culture of filamentation-suppressed recombinant Escherichia coli. Appl Environ Microbiol. 1997;63:4765–4769. doi: 10.1128/aem.63.12.4765-4769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams M D, Rahn J A, Sherman D H. Production of a polyhydroxyalkanoate biopolymer in insect cells with a modified eukaryotic fatty acid synthase. Appl Environ Microbiol. 1996;62:2540–2546. doi: 10.1128/aem.62.7.2540-2546.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams S F, Peoples O P. Biodegradable plastics from plants. Chemtech. 1996;26:38–44. [Google Scholar]