Abstract
The feasibility of biologically removing nitrate from groundwater was tested by using cyanobacterial cultures in batch mode under laboratory conditions. Results demonstrated that nitrate-contaminated groundwater, when supplemented with phosphate and some trace elements, can be used as growth medium supporting vigorous growth of several strains of cyanobacteria. As cyanobacteria grew, nitrate was removed from the water. Of three species tested, Synechococcus sp. strain PCC 7942 displayed the highest nitrate uptake rate, but all species showed rapid removal of nitrate from groundwater. The nitrate uptake rate increased proportionally with increasing light intensity up to 100 μmol of photons m−2 s−1, which parallels photosynthetic activity. The nitrate uptake rate was affected by inoculum size (i.e., cell density), fixed-nitrogen level in the cells in the inoculum, and aeration rate, with vigorously aerated, nitrate-sufficient cells in mid-logarithmic phase having the highest long-term nitrate uptake rate. Average nitrate uptake rates up to 0.05 mM NO3− h−1 could be achieved at a culture optical density at 730 nm of 0.5 to 1.0 over a 2-day culture period. This result compares favorably with those reported for nitrate removal by other cyanobacteria and algae, and therefore effective nitrate removal from groundwater using this organism could be anticipated on large-scale operations.
Nitrate (NO3−) concentrations in groundwater have increased globally (26). Wastewater, fertilizers, and livestock farming are major sources of nitrate in groundwater supplies (18). Groundwater in many locations is used as a supply for drinking water, and high nitrate concentrations present a potential risk to public health, particular to infants (13). In the United States, the Environmental Protection Agency has set a maximum contaminant level (MCL) for nitrate in drinking water of 0.71 mM (10 mg of NO3−-N liter−1) (35). Similar MCLs for nitrate have been established in Canada and Europe. As a result, many public and private groundwater supply wells have been shut off as drinking water sources because their nitrate levels exceed the MCL (26). As water demands in many urban and agricultural areas increase, technology for treating nitrate-contaminated groundwater is becoming increasingly urgent.
Nitrate removal from groundwater may be accomplished by bacterially mediated denitrification, or chemically and physically based technologies (26). These treatment processes, however, require input of external energy sources (e.g., electricity or organic carbon) and/or chemical additives and generate concentrated waste streams that must be disposed. Therefore, they are often problematic and expensive (8, 20). Since nitrate may be taken up effectively by photosynthetic microorganisms, such as cyanobacteria, which require mostly fixed nitrogen, inorganic carbon, and light for growth, the use of photosynthetic organisms would minimize the need of chemicals and fossil fuels for nitrate removal, thus leading to an efficient resource recovery and recycling. However, limited information on engineering photosynthetic system for treating nitrate-contaminated drinking water supplies is available.
With cyanobacteria, nitrate is taken up by a common high-affinity transport system involving the NrtABCD permease (an ABC-type transporter) and to a lesser extent enters the cells by diffusion (11, 32, 33). Once inside the cell, nitrate is reduced to nitrite by nitrate reductase, and nitrite is further reduced to ammonium by nitrite reductase (19). Ammonium is then incorporated into carbon skeletons mainly through the operation of the glutamine synthetase-glutamate synthase cycle (11). Fixed nitrogen storage components, such as C-phycocyanin (5) and/or cyanophycin (31), may be formed and accumulated in the cells under certain physiological conditions. Regulation of nitrate assimilation has been found to be controlled by several regulatory products that affect expression of the nrtABCD, narB (encoding nitrate reductase), and nirA (encoding nitrite reductase) genes that together form the nirA-nrtABCD-narB operon in several cyanobacteria (19, 41). Nitrate uptake can be influenced by availability of phosphate ion (PO43−), which has an important role in cellular energetics as part of ATP (adenosine triphosphate) and which influences the activity of many enzymes required for cell metabolism, including the nitrate reduction process (2, 19). Also, nitrate uptake can be affected by spectral quality and light intensity (3, 38), temperature (32, 42), and cell density and nitrate level (28, 30), as well as physiological acclimation of the culture (30).
Microalgal treatment of wastewater has been investigated for over 4 decades as an environmentally sound alternative to remove nutrients and heavy metals from wastewater sources. However, very few investigations have considered applying this technology to the treatment of drinking water (8, 20, 42). The chemical composition (e.g., the pH, dissolved inorganic carbon, nutrient levels, and metals) of groundwater differs from that of wastewater, and the feasibility of growing microalgae (a term used for cyanobacteria and unicellular algae) in groundwater has yet to be evaluated. The objectives of the present study were, first, to determine whether groundwater can sustain cyanobacterial development and, second, to determine the rate of nitrate removal from the water. Third, the study was designed to determine how nitrate uptake and cyanobacterial growth could be affected by environmental and biotic factors. Several cyanobacterial species were employed as a working model for the study because of their rapid nitrate uptake and high growth rate (19) and their ability to be genetically manipulated (44), which may lead to performance improvements of cyanobacteria for drinking water remediation.
MATERIALS AND METHODS
Organisms and culture conditions.
Three strains of cyanobacteria were tested in this study: Synechocystis sp. strain PCC 6803, Synechocystis minima CCAP 1480/4, and Synechococcus sp. strain PCC 7942. Axenic stock cultures were maintained in BG-11 growth medium (45). Unless stated otherwise, all cultures were maintained at 32°C and 160 μmol of photons m−2 s−1.
Experimental system.
The groundwater used in this study was obtained from wells in the Phoenix, Ariz., metropolitan area. Typical chemical composition of groundwater was as follows: 1.5 to 2.3 mM NO3−, a negligible amount of dissolved phosphorus, 1,500 mg of total dissolved solids liter−1, 2.5 to 3.3 mM bicarbonate, and a pH of 7.4 ± 0.3. Groundwater was passed through a microfilter (0.45-μm-pore-size nylon membrane; Cole-Parmer, Vernon Hills, Ill.) and stored in a Nalgene container at 4°C until it was used as a growth medium.
Growth experiments were carried out in glass columns (2.6-cm inner diameter, 45-cm length, 320-ml culture capacity) inserted in a 32°C water bath. Mixing of cultures was provided by bubbling compressed air through a capillary (inner diameter, 1 mm) that ended near the bottom of the column (22). Unless stated otherwise, the aeration rate was 0.6 volumes of air per volume of culture suspension per minute (vvm). Light was provided by a panel of fluorescent lamps on one side of the water bath. Light intensities ranging from 6 to 180 μmol of photons m−2 s−1 were adjusted by covering the surface of the glass columns by shade nets. The illumination intensity was measured with a light meter (LiCor model LI-189).
To initiate nutrient deprivation, a continuous culture of Synechococcus sp. strain PCC 7942 was propagated in a flat-plate bioreactor similar to that described by Hu et al. (21) at a constant dilution rate of 0.18 day−1. Before batch culture experiments were started, cells were harvested from this continuous-culture system by centrifugation and washed once with distilled water. All experiments were run at least in triplicate. Samples were collected three to four times a day, and cell density and nutrient concentrations were monitored accordingly.
Growth analysis.
Cell density, monitored as the optical density at 730 nm (OD730) of cyanobacterial suspensions, was measured with a spectrophotometer (UV-160; Shimadzu, Kyoto, Japan). Increments in optical density during certain time intervals were used to calculate the specific growth rate (μ) by the equation μ = (ln X2 − ln X1)/(t2 − t1), where X1 and X2 are the optical densities at times t1 and t2, respectively.
Nutrient analysis.
Cyanobacterial suspensions were passed through a filter (0.45-μm-pore-size nylon membrane; Cole-Parmer) to remove cells and some other organic particles, and the filtrates were stored at −20°C until elemental analysis was performed. Analyses were done with a TRAACS 800 continuous-flow analyzer, by using industrial method no. 824-87T for NO3− and method no. J-004-88C for PO43− determinations (BRAN+LUEBBE, Chicago, Ill.).
Nutrient and irradiance dependence of growth.
The specific growth rate as a function of nutrient availability was fitted to the kinetic growth model of Dugdale (10) by using a rectangular hyperbola function described by the equation μ = μmax S/(KS + S), where μmax is the maximum specific growth rate, S is the nutrient concentration (e.g., PO43− or NO3−), and Ks is the nutrient concentration at which half-maximal growth occurs. The specific growth rate as a function of irradiance was also fitted to a hyperbolic curve described by the equation μ = μmax I(Ik + I), where Ik and I are the half saturation and incident irradiance, respectively (16).
RESULTS
Cyanobacterial growth in groundwater.
The viability of cyanobacteria in groundwater was determined. Three fairly well characterized species of cyanobacteria that do not fix nitrogen (Synechocystis sp. strain PCC 6803, Synechocystis minima CCAP 1480/4, and Synechococcus sp. strain PCC 7942) were precultured in BG-11 growth medium. Cells were harvested in the exponential growth phase and inoculated into glass columns containing groundwater. All three strains were found to grow quite well under these conditions, with doubling times during exponential growth between 10 and 20 h. During the time course of cultivation of Synechococcus sp. strain PCC 7942, the nitrate concentration in the groundwater decreased with a half-life of about 12 h. About 30 h after the start of the experiment, nitrate was virtually undetectable in the medium, indicative of an efficient nitrate uptake by the cells. Synechococcus sp. strain PCC 7942 was among the fastest growers in this experiment, and therefore it was used in all subsequent experiments reported in this paper.
Light intensity dependence of growth and nitrate removal.
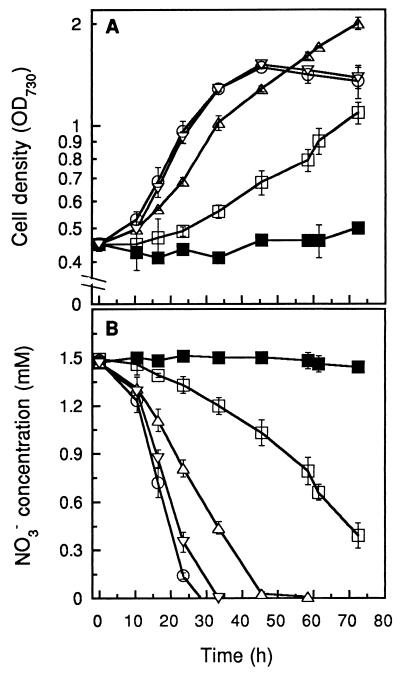
Cell growth and nitrate uptake rates as a function of light intensity were investigated for Synechococcus sp. strain PCC 7942. Maximal photoautotrophic growth rates of this strain increased with light intensity, until reaching a saturation level (Fig. 1A). The light intensity (Ik) at which the specific growth rate of the cells is half the maximal value was estimated to be 50 μmol of photons m−2 s−1. Prolonged exposure of the culture with relatively low cell density (OD730, <0.1) to 180 μmol of photons m−2 s−1 was found to cause photobleaching of the cells (data not shown). Nitrate uptake for the same experiment is presented in Fig. 1B. The rate of nitrate uptake closely paralleled the growth rate, with conditions of most vigorous growth coinciding with conditions for the highest nitrate uptake rate. On the basis of these results, an irradiance of 160 μmol of photons m−2 s−1, causing nearly maximal growth and nitrate uptake rates, was chosen for subsequent experiments.
FIG. 1.
Influence of light intensity on growth of Synechococcus sp. strain PCC 7942 cultures (A) and nitrate removal (B). Light intensities tested were 6 (■), 12 (□), 50 (▵), 100 (○), and 180 (▿) μmol of photons m−2 s−1. Cultures were inoculated with cells precultured in BG-11 medium which were harvested during the exponential growth phase and washed twice with distilled water. Groundwater contained 1.5 mM nitrate, the aeration rate was ca. 0.7 vvm, and the culture temperature was 32°C (error bars indicate standard deviations; n = 4).
Long-term survival of cyanobacteria in groundwater.
Growth of Synechococcus sp. strain PCC 7942 eventually ceased after being maintained in continuous culture in groundwater for about 7 days. To identify the limiting nutrient, 0.32 mM sulfur (S) and magnesium (Mg), 100 μM phosphate (P), and 1.0 ml of BG-11 trace element mix liter−1 or, as a control, BG-11 growth medium were added to the Synechococcus sp. strain PCC 7942 culture, and growth was monitored. Phosphate was the limiting nutrient: whereas addition of 0.32 mM MgSO4 or 1.0 ml of trace element mix liter−1 (both concentrations are equal to those present in BG-11 growth medium) did not improve growth, supplementation with 150 μM PO43− alone caused the culture to resume growth, albeit at a significantly lower specific growth rate than was observed before nutrient limitation (data not shown). A similar growth rate was also found in the culture that was transferred to BG-11 growth medium, indicating that many cells lost much of their viability during the period they were maintained in groundwater.
After addition of 150 μM PO43− to groundwater, Synechococcus sp. strain PCC 7942 cells were able to grow and take up both nitrate and phosphate, and the culture could go through at least four additional divisions in the continuous-culture mode. Thereafter, growth as well as phosphate and nitrate uptake ceased, although ca. 80 μM PO43− and 1.2 mM NO3− remained in the medium (data not shown). At this time, addition of 0.3 ml of a trace element mix liter−1 was sufficient to recover cell growth. However, recovery did not occur upon addition of any of the macronutrients (Mg, S, N, or P) alone. It was clear that, after nitrate and phosphate, one or more trace elements became limiting for long-term growth of Synechococcus sp. strain PCC 7942 in groundwater.
Phosphate requirement for cell growth and nitrate uptake.
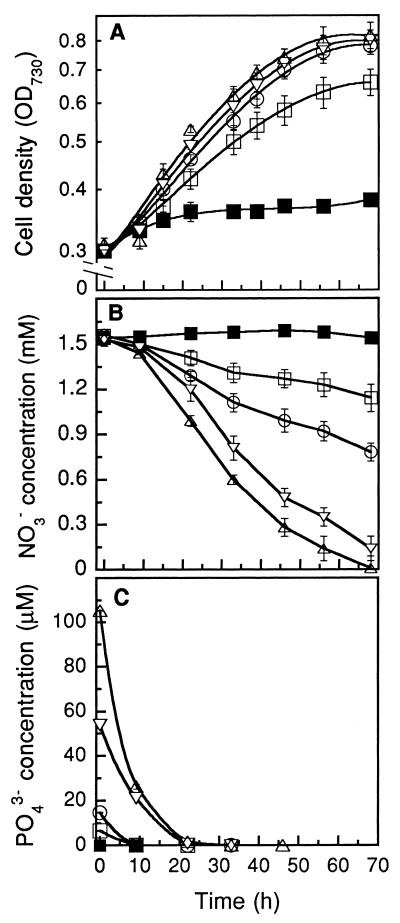
To determine how much phosphate is minimally needed for continued growth at satisfactory rates, phosphate-deprived Synechococcus sp. strain PCC 7942 cells were transferred to groundwater with ambient nitrate (1.53 mM NO3−) and to which 0.3 ml of BG-11 trace element mix liter−1 and various phosphate concentrations (0, 6.5, 14.6, 53.6, or 105.2 μM PO43−) had been added. Figure 2 shows the growth of the culture as well as nitrate and phosphate levels remaining in the medium as a function of the added PO43− concentration. While little growth was observed without external PO43− addition, addition of even modest amounts of phosphate (6.5 to 14.6 μM PO43−) resulted in resumption of growth at a significant rate. Little growth enhancement was observed upon a further increase of the phosphate concentration above 53.6 μM (Fig. 2A). The phosphate concentration (Ks) leading to half the maximal specific growth rate was 9 μM.
FIG. 2.
Changes in cell density (A) and external NO3− (B) and external PO43− (C) concentrations in the medium during the course of cultivation of Synechococcus sp. strain PCC 7942 in groundwater in the presence of various concentrations of phosphate. Cells had been previously deprived of PO43− by propagating the cells in groundwater without addition of phosphate for a week. The initial PO43− concentrations were 0.16 μM (■) (the phosphate concentration in the groundwater used), 6.6 μM (□), 14.7 μM (○), 53.7 μM (▿), and 105.3 μM (▵). The light intensity was 160 μmol m−2 s−1, the culture temperature was 32°C, and the aeration rate was ca. 0.7 vvm (error bars indicate standard deviations; n = 4).
As could be expected from a culture with little growth, no nitrate removal from the medium was detected in a phosphate-deprived culture (Fig. 2B). Nitrate uptake was restored quickly after phosphate addition. However, even though the growth rate was close to maximal after addition of low-phosphate concentrations (≥15 μM), cellular nitrate removal rates from the medium continued to increase significantly with increasing phosphate concentration, reaching a maximum at the highest phosphate concentration assayed in this experiment (105.2 μM) (Fig. 2B). Added phosphate was simultaneously taken up rapidly by the cells (Fig. 2C). Even 105.2 μM PO43−, the highest level of phosphate tested, was completely removed from the medium within the first 20 h of cultivation.
Not only was addition of phosphate required for uptake of nitrate and simultaneous growth, but it also modified the pigment composition of cells. A relatively high level of phycobilisome in the cells was observed upon phosphate deprivation (no growth) and decreased upon addition of low levels of phosphate. The dependency of dissolved phosphate concentrations on phycobilisome levels reflects that these pigment-protein complexes break down to facilitate some cell growth under nutrient-limiting conditions and then increase significantly with addition of higher phosphate levels. The amount of chlorophyll a also declined upon addition of low phosphate concentrations and then increased again upon addition of more phosphate, but this change was somewhat smaller than that in the level of phycobilisomes.
Growth and nitrate uptake as a function of nitrate availability.
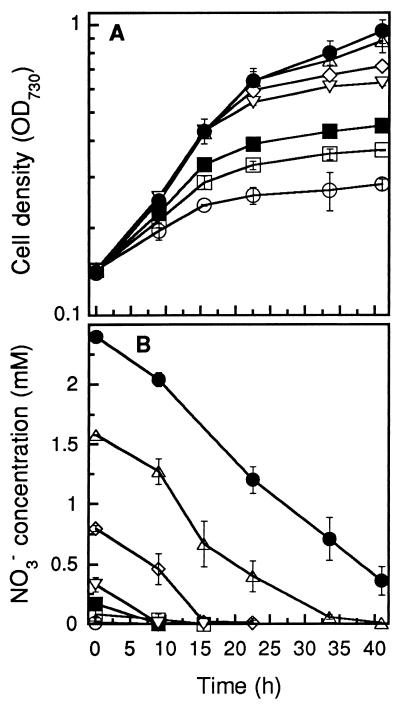
To determine nitrate uptake and cell growth of Synechococcus sp. strain PCC 7942 at various initial nitrate concentrations, tap water, which had an ionic composition similar to that of groundwater except for a much lower NO3− level, was used as growth medium after supplementation with 100 μM PO43− and various amounts of NaNO3. By using nitrate-deprived Synechococcus sp. strain PCC 7942 cells as an inoculum, growth was determined for different initial NO3− concentrations. The simulated groundwater (tap water plus NO3−) sustained growth rates of cyanobacteria identical to those in groundwater. The growth rate clearly depended on the nitrate concentration in the medium (Fig. 3A). The maximal specific growth rate as a function of nitrate concentration showed typical saturation kinetics (10), and the initial nitrate concentration (Ks) at which the growth rate was half maximal was 0.2 mM. Nitrate removal from the medium (taken up by the cells) occurred at a significant rate upon addition of nitrate (Fig. 3B) and then was almost independent of the initial nitrate concentration when it was above 0.32 mM. However, higher maximal cell densities were observed in cultures with higher initial NO3− concentrations. As shown in Fig. 1, that cells continued growing even after nitrate had been deprived from the medium, indicating a rapid accumulation of internal fixed-nitrogen reserves.
FIG. 3.
Effect of initial NO3− concentrations in tap water on growth of (A) and NO3− removal (B) by Synechococcus sp. strain PCC 7942. Nitrate-deprived cells to be used for inoculation were prepared by growing Synechococcus sp. strain PCC 7942 in modified BG-11 medium without fixed nitrogen for 40 h. The initial NO3− concentrations were 0.007 mM (○), 0.09 mM (□), 0.17 mM (■), 0.32 mM (▿), 0.82 mM (◊), 1.61 mM (▵), and 2.36 mM (●). The light intensity was 160 μmol m−2 s−1, the culture temperature was 32°C, and the aeration rate was ca. 0.7 vvm (error bars indicate standard deviations; n = 3).
Effect of nitrate level of cells in inoculum.
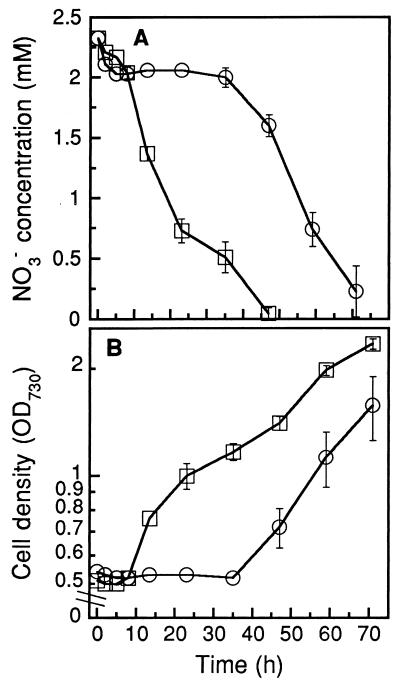
Rapid nitrate uptake has often been observed in nitrate-deprived cells over a time scale of minutes (19, 30). To determine whether nitrate-deprived or nitrate-sufficient cells were more appropriate for rapid, consistent, and complete removal of nitrate from groundwater, nitrate uptake and cell growth were monitored in parallel in both types of cells (Fig. 4). Nitrate-deprived cells were generated by culturing in nitrate-free BG-11 medium for 48 h, whereas nitrate-sufficient cells had been grown for 24 h in normal BG-11 medium. Before inoculation, both types of cells were harvested and washed twice with deionized water.
FIG. 4.
Effect of the fixed-nitrogen level of cells in an inoculum on nitrate uptake (A) and growth (B) of Synechococcus sp. strain PCC 7942. Nitrate-sufficient cells (□) were obtained from an inoculum that had been propagated in BG-11 medium for 24 h. Nitrate-depleted cells (○) originated from a culture that had been maintained in nitrate-free BG-11 growth medium for 48 h. The groundwater used for this experiment contained 2.3 mM NO3−, and PO43− had been added to a final concentration of 100 μM. The light intensity was 160 μmol m−2 s−1, the culture temperature was 32°C, and the aeration rate was ca. 0.7 vvm (error bars indicate standard deviations; n = 3).
In the nitrate-deprived culture, a rapid nitrate uptake (0.08 mM h−1) was observed during the first 2 h of incubation (Fig. 4A). However, after this short period of nitrate uptake, no decline in nitrate concentration was detected in the medium for the next 30 h, corresponding to a lag phase in cell growth of the same length (Fig. 4B). At about 35 h after inoculation, rapid uptake of nitrate and cell growth resumed. In contrast, the nitrogen-sufficient culture exhibited a lower initial nitrate uptake rate (0.05 mM h−1). However, the latter showed a much shorter lag phase in both nitrate uptake and growth than the former. After the lag phase had been overcome, the rate of nitrate uptake by cells was independent of whether cells were nitrate deprived or nitrate sufficient.
Nitrate uptake optimization.
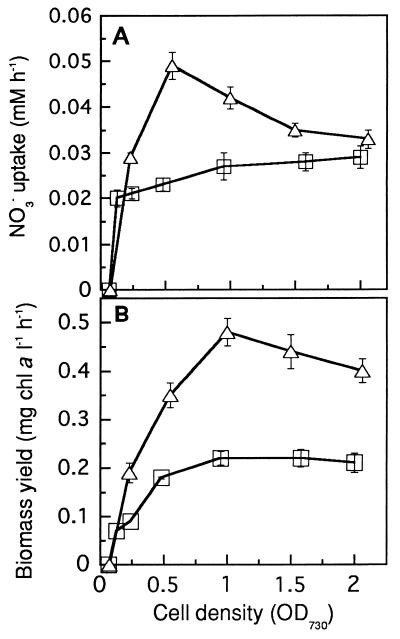
Nitrate uptake and reduction require energy and reducing power; therefore, aeration of the culture, which provides CO2 and improves light availability of individual cells for photosynthesis, was likely to be important for most efficient nitrate uptake. Figure 5A shows the average nitrate uptake during a cultivation period of 45 h as a function of aeration rate and initial cell density. Indeed, a higher aeration rate yielded higher nitrate uptakes. The maximum rate of nitrate uptake was close to 0.05 mM h−1 in the culture with an initial cell density of 0.5 to 1.0 OD730, and declined when more concentrated cultures that were closer to stationary phase were used. Similarly, the total amount of biomass produced increased with aeration rate and with inoculum size, up to a maximal level (Fig. 5B).
FIG. 5.
NO3− uptake and biomass yield as affected by culture aeration and initial cell density. NO3− uptake rates (A) and biomass yields (B) were the average values calculated from a cultivation period of 45 h. To prepare inocula with various initial cell concentrations, cells were precultured in BG-11 medium for 24 h, concentrated by centrifugation, and washed twice with deionized water. The x axis indicates the cell density right after inoculation. The aeration rate was set up at two levels, 0.3 (□) and 0.8 (▵) vvm. The light intensity was 160 μmol m−2 s−1, and the culture temperature was 32°C (error bars indicate standard deviations; n = 4).
DISCUSSION
Nitrate removal by cyanobacteria.
Our results demonstrate the potential of cyanobacteria to efficiently remove and utilize nitrate from groundwater. The rate with which this uptake by cyanobacterial cultures proceeds is such that within one or several days the nitrate level in groundwater can be reduced from severalfold above MCL to levels that are acceptable for drinking water standards. Moreover, no nitrite or ammonia was detected in the cultures (data not shown), indicating that the levels of these harmful intermediates are less than 0.1 mg liter−1. The cyanobacterial strains tested in this study are rather comparable in their ability to grow in groundwater and in their nitrate uptake efficiency. However, we have observed that cyanobacteria that can fix nitrogen from the air (for example, Nostoc and Anabaena species) were very inefficient in uptake of nitrate when nitrate was present in the medium at relatively low concentrations. Moreover, the tested nitrogen-fixing cyanobacteria excreted ammonium into the medium (data not shown), which is known to be common for many nitrogen-fixing cyanobacteria (4).
The obvious advantages of using non-nitrogen-fixing cyanobacteria to efficiently remove nitrate from groundwater include its minimal requirement for chemical additions and the supply of solar energy rather than of fossil fuel energy (physical-chemical nitrate removal) or organic carbon (bacterial nitrate reduction). Moreover, potentially valuable biomass is produced as a by-product. However, before practical applications can be considered, several issues need to be further investigated. These include (i) optimization of nitrate uptake under outdoor conditions, (ii) possible toxicity of cyanobacterial products that may be excreted, and (iii) scale-up potential for continuous-flow reactor systems.
Nitrate removal from groundwater versus wastewater.
Nitrate-contaminated groundwater differs from most wastewater in terms of the levels of other macronutrients, including phosphate. Whereas groundwater usually has little phosphate, wastewater generally contains a significant amount of this compound (9, 43). Indeed, the phosphate level apparently limits cyanobacterial growth in groundwater, influencing not only the overall growth rate but also the final cell density that the cyanobacterial culture reaches. The first few cell divisions after transfer to groundwater probably depend mostly on cell-internal phosphate reserves that have been carried over from the artificial growth medium (such as BG-11). Long-term growth and survival of the cells, however, require addition of phosphate.
The role of phosphate and trace elements.
Synechococcus sp. strain PCC 7942 requires addition of about 9 μM phosphate to grow at half-maximal rate. This appears to be higher by an order of magnitude than the phosphate concentration required for some other species (12, 36). Factors that may contribute to this difference include the species used and the mode in which the cultures are grown (batch versus continuous culture) as well as the cellular phosphorus level at which the assay is conducted. The amount of nitrate versus phosphate that is required to restore a half-maximal growth rate differs in this strain by a factor of 22 [(Ks = 200 μM NO3−)/(Ks = 9 μM PO43−)], and this ratio is within the range common for many algae and cyanobacteria, at which one nutrient limitation changes over to the other (17, 38). Interestingly, the phosphate concentration needed for half-maximal rate of nitrate uptake was higher than the concentration sufficient for maximal growth rates (15 and 9 μM, respectively). This may reflect the requirement for cell vigor and health for optimal nitrate uptake. Indeed, as shown in Fig. 4, under fully phosphate-sufficient conditions, the amount of pigments was larger than at lower phosphate levels. This difference presumably may lead to a difference in the photosynthetic activity. Since nitrate uptake and assimilation require ATP and reducing equivalents, photosynthetic activity is likely to be a prerequisite for efficient nitrate uptake and reduction. It is important to note that the Synechococcus sp. strain PCC 7942 culture was able to take up all phosphate from the medium within 20 h, even at the highest phosphate concentration available (105 μM). This time frame is similar to that needed for uptake of nitrate, and the addition of small amounts of phosphate to the culture will not lead to secondary contamination of treated water by residual phosphate.
Besides phosphate, our results indicate that a deficiency in trace elements can also affect the long-term viability of cyanobacteria in groundwater. However, it is not known which specific trace element(s) is in short supply in our study. Obviously, the requirement for trace elements will depend much on the specific composition of the groundwater supply. Supplementation of 0.3 ml of the BG-11 trace elements liter−1 every few days was sufficient for long-term maintenance of cyanobacterial cultures in groundwater, and therefore trace elements are not a major limiting factor that would impact larger-scale applications of cyanobacterial cultures.
Nitrate-starved cells versus nitrate-sufficient cells.
It has been known that nitrogen-deprived cells generally show higher inorganic nitrogen uptake rates than nitrogen-sufficient cells in the short term (14, 30). However, after an initial nitrate uptake in nitrogen-deprived cells, these cells entered a lag phase in which no nitrate uptake occurred. Furthermore, the length of the lag phase increased with the time that cells were deprived of nitrate. In contrast, nitrogen-sufficient cells continued to take up nitrate. Therefore, results generated from short-term nitrate uptake experiments cannot be reliably used to determine conditions for optimal, sustained nitrate uptake rates. For long-term studies, the overall uptake rates of nitrate and perhaps also of other forms of fixed nitrogen are closely related to the growth rate of the cells, and a fixed nitrogen-sufficient inoculum, rather than nitrogen-deprived one, is preferred because of the lengthy lag phase observed after nitrogen deprivation. In addition, nitrogen deprivation may cause cell autolysis (7) and excretion of secondary metabolites (4, 6).
Light requirement.
The nitrate assimilation process is ultimately driven by light energy, which provides, through photosynthesis, ATP and reducing equivalents for nitrate uptake and reduction. On the other hand, too much light can also be a source of photooxidative damage. At low irradiance (approximately 6 μmol of photons m−2 s−1), photosynthesis and respiration occur at roughly the same rate (light compensation point), and a lack of significant nitrate uptake is not unexpected. At about 50 μmol of photons m−2 s−1, the growth rate is half maximal. The nitrate uptake rate as a function of light intensity follows a similar pattern as the growth curve, consistent with the requirement of photosynthesis for efficient nitrate uptake (27). The light requirement for Synechococcus sp. strain PCC 7942 growth appeared to be quantitatively similar to what has been reported for many algal and cyanobacterial species (15).
At a high light intensity (250 μmol of photons m−2 s−1 and above, depending on the density of the culture), photooxidative stress is introduced in cyanobacteria due to absorption of excess light than cannot be used productively for photosynthesis. Upon exposure to 180 μmol of photons m−2 s−1, the Synechococcus sp. strain PCC 7942 culture was bleached and killed within 48 h if the initial cell OD730 were below 0.1. This is consistent with earlier data that attributed the light-induced demise of a low-density culture mainly to photooxidative damage (1, 23). Therefore, from a practical standpoint, a scaled-up bioreactor powered by solar energy will need to have a relatively long light path so that cells are generally shielded by each other in order to prevent photo-bleaching. In this context, aeration can mainly affect the light regime to which individual cells in the culture is exposed (39), thereby ensuring the maximal photosynthetic activity while at the same time diminishing the inhibitory effect associated with high light intensities (24).
Application potential.
Synechococcus sp. strain PCC 7942 and other non-nitrogen-fixing cyanobacteria appear to be very suitable to remove nitrate from groundwater or other similar types of water sources. When maintained at an optimal cell density (OD730, 0.5∼1.0) and at an incident light intensity of 180 μmol of photons m−2 s−1 at 32°C, the average nitrate uptake rate was 0.05 mM NO3− h−1 (Fig. 5A). For an initial nitrogen concentration of 1.5 mM NO3−, this uptake rate would reduce nitrate levels below the MCL limit (0.71 mM NO3−) within 14 h and allow complete removal of this nutrient within 30 h. These values compare favorably with those reported in the literature (Table 1).
TABLE 1.
Comparison of nitrate uptake rates in various species of algae and cyanobacteria and in different culture systems
| Species | Free-living or immobilized | Continuous or batch mode | Continuous light or light-dark | NO3− uptake rate (μM h−1) | Reference |
|---|---|---|---|---|---|
| Phormidium laminosum | Free-living | Batch | Light-dark | 0.25∼0.5a | 14 |
| Chlorella vulgaris | Immobilized | Batch | Continuous | 7.4 | 25 |
| Phormidium laminosum | Immobilized | Continuous | Continuous | 14.0 | 42 |
| Synechococcus sp. strain PCC 7942 | Free-living | Batch | Continuous | 51.8 | Present study |
This is an estimated figure based on two assumptions: (i) chlorophyll a constitutes 1.5% of the dry weight of cells and (ii) the cell density for the maximal nitrate uptake rate is 500 to 1,000 mg liter−1 at a light intensity of 100 μmol of photons m−2 s−1. The actual nitrate uptake rate reported by Garbisu et al. (14) was 33.4 nmol (μg of chlorophyll)−1 h−1.
Efficient, long-term nitrate uptake may be readily achieved through manipulation of several factors, such as cell density (i.e., inoculum size in case of batch culture and steady-state cell density in case of continuous culture mode). Since the nitrate uptake rates are a function of both the specific growth rate and cell density, a high nitrate uptake rate would not be achieved with either a too low or a too high cell density culture. This is obviously different from the phenomenon observed on a short time scale (tens of minutes), when a higher rate of nitrate uptake on a volumetric basis is associated with a higher initial cell density (28). The conditions for the most efficient long-term nitrate uptake clearly include a rapidly growing, healthy, phosphate-sufficient cyanobacterial culture.
In summary, several species of cyanobacteria exhibit rapid uptake of nitrate and growth in groundwater that is supplemented with phosphate and trace elements. Based on these results, long-term steady-state removal of nitrate from groundwater by growing cyanobacteria in a continuous-flow photobioreactor system is feasible and attractive.
ACKNOWLEDGMENTS
This work was funded by an Arizona State University Project Ingenhousz grant for collaborative research.
We are grateful to Tom Colella for his kind assistance in determining nitrate and phosphate concentrations.
REFERENCES
- 1.Abeliovich A, Shilo M. Photooxidative death in blue-green algae. J Bacteriol. 1972;111:682–689. doi: 10.1128/jb.111.3.682-689.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahlgren G. Phosphorus as growth-regulating factor relative to other environmental factors in cultured algae. Hydrobiologia. 1988;170:191–210. [Google Scholar]
- 3.Azuara M P, Aparicio P J. In vivo blue-light activation of Chlamydomonas reinhardtii nitrate reductase. Plant Physiol. 1983;71:286–290. doi: 10.1104/pp.71.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boussiba S. Ammonia assimilation and its biotechnological aspects in cyanobacteria. In: Rai A K, editor. Cyanobacterial nitrogen metabolism and environmental biotechnology. New Delhi, India: Narosa Publishing House; 1997. pp. 35–72. [Google Scholar]
- 5.Boussiba S, Richmond A. C-phycocyanin as a storage in the blue-green alga Spirulina platensis. Arch Microbiol. 1980;125:143–147. [Google Scholar]
- 6.Brussaard C P D, Noordeloos A A M, Riegman R. Autolysis kinetics of the marine diatom Ditylum brightwellii (Bacillariophyceae) under nitrogen and phosphorus limitation and starvation. J Phycol. 1997;33:980–987. [Google Scholar]
- 7.Daley R J, Brown S R. Chlorophyll, nitrogen, and photosynthetic patterns during growth and senescence of two blue-green algae. J Phycol. 1973;9:395–401. [Google Scholar]
- 8.de la Noüe J, Laliberté G, Proulx D. Algae and waste water. J Appl Phycol. 1992;4:247–254. [Google Scholar]
- 9.Droste R L. Water components and quality standards. In: Droste R L, editor. Theory and practice of water and wastewater treatment. New York, N.Y: John Wiley & Sons, Inc.; 1997. pp. 181–216. [Google Scholar]
- 10.Dugdale R C. Nutrient limitation in the sea: dynamics, identification and significance. Limnol Oceanogr. 1967;12:685–695. [Google Scholar]
- 11.Flores E, Herrero A. Assimilatory nitrogen metabolism and its regulation. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer; 1994. pp. 487–517. [Google Scholar]
- 12.Fuhs G W. Phosphorus content and rate of growth in the diatom Cyclotella nana and Thalassiosira fluviatiilis. J Phycol. 1969;5:312–321. doi: 10.1111/j.1529-8817.1969.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 13.Gangolli S D, van den Brandt P A, Feron V J, Janzowsky C, Koeman J H, Speijers G J A, Spiegelhalder B, Walker R, Wishnok J S. Nitrate, nitrite, and N-nitroso compounds. Eur J Pharmacol Environ Toxicol Pharmacol Sect. 1994;292:1–38. doi: 10.1016/0926-6917(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 14.Garbisu C, Hall D O, Serra J. Nitrate and nitrite uptake by free-living and immobilized N-starved cells of Phormidium laminosum. J Appl Phycol. 1992;4:139–148. [Google Scholar]
- 15.Geider R J, Osborne B A, Raven J A. Light dependence of growth and photosynthesis in Phaeodactylum tricornutum (Bacillariophyceae) J Phycol. 1985;21:609–619. [Google Scholar]
- 16.Goldman J C. Outdoor algal mass cultures. II. Photosynthetic yield limitations. Water Res. 1979;13:119–136. [Google Scholar]
- 17.Goldman J C, McCarthy J J, Peavey D G. Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature. 1979;279:210–215. [Google Scholar]
- 18.Hem J D. Study and interpretation of the chemical characteristics of nature water. USGS water supply paper 2254. 3rd ed. Washington, D.C.: U.S. Government Printing Office; 1992. [Google Scholar]
- 19.Herrero A, Flores E. Nitrate metabolism. In: Rai A K, editor. Cyanobacterial nitrogen metabolism and environmental biotechnology. New Delhi, India: Narosa Publishing House; 1997. pp. 1–33. [Google Scholar]
- 20.Hoffmann J P. Wastewater treatment with suspended and nonsuspended algae. J Phycol. 1998;34:757–763. [Google Scholar]
- 21.Hu Q, Guterman H, Richmond A. A flat inclined modular photobioreactor (FIMP) for outdoor mass cultivation of photoautotrophs. Biotechnol Bioeng. 1996;51:51–60. doi: 10.1002/(SICI)1097-0290(19960705)51:1<51::AID-BIT6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Hu Q, Guterman H, Richmond A. Physiological characteristics of Spirulina platensis cultured at ultrahigh cell densities. J Phycol. 1996;32:1066–1073. [Google Scholar]
- 23.Hu Q, Richmond A. Optimizing the population density of Isochrysis galbana grown outdoors in a glass column photobioreactor. J Appl Phycol. 1994;6:391–396. [Google Scholar]
- 24.Hu Q, Richmond A. Productivity and photosynthetic efficiency of Spirulina platensis affected by light intensity, cell density and rate of mixing in a flat plate photobioreactor. J Appl Phycol. 1996;8:139–145. [Google Scholar]
- 25.Jeanfils J, Canisius M F, Burlion N. Effect of nitrate concentrations on growth and nitrate uptake by free-living and immobilized Chlorella vulgaris cells. J Appl Phycol. 1993;5:369–374. [Google Scholar]
- 26.Kapoor A, Viraraghavan T. Nitrate removal from drinking water—review. J Environ Eng. 1997;123:371–380. [Google Scholar]
- 27.Lara C, Romero J M. Distinctive light and CO2 fixation requirements of nitrate and ammonium utilization by the cyanobacterium Anacystis nidulans. Plant Physiol. 1986;81:686–688. doi: 10.1104/pp.81.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau P S, Tam N F Y, Wong Y S. Effect of algal density on nutrient removal from primary settled wastewater. Environ Pollut. 1995;8:59–66. [Google Scholar]
- 29.Lau P S, Tam N F Y, Wong Y S. Wastewater nutrients removal by Chlorella vulgaris: optimization through acclimation. Environ Technol. 1996;17:183–189. [Google Scholar]
- 30.Lavoie A, de la Noüe J. Hyperconcentrated cultures of Scenedesmus obliquus: a new approach for wastewater biological tertiary treatment? Water Res. 1985;19:1437–1442. [Google Scholar]
- 31.Lawry N H, Simon R D. The normal and induced occurrence of cyanophycin inclusion-bodies in several blue-green algae. J Phycol. 1982;18:391–399. [Google Scholar]
- 32.Lomas M, Glibert P M, Berg G M. Characterization of nitrogen uptake by natural population of Aureococcus anophagefferens (Chrysophyceae) as a function of incubation duration, substrate concentration, light and temperature. J Phycol. 1996;32:907–916. [Google Scholar]
- 33.Luque I, Flores E, Herrero A. Nitrate and nitrite transport in the cyanobacterium Synechococcus sp. PCC 7942 are mediated by the same permease. Biochim Biophys Acta. 1994;1184:296–298. [Google Scholar]
- 34.Omata T, Andriesse X, Hirano A. Identification and characterization of a gene cluster involved in nitrate transport in the cyanobacterium Synechococcus sp. PCC 7942. Mol Gen Genet. 1993;236:193–202. doi: 10.1007/BF00277112. [DOI] [PubMed] [Google Scholar]
- 35.Pontius F W. Nitrate and cancer: is there a link. J Am Water Works Assoc. 1993;85:12–14. [Google Scholar]
- 36.Rhee G Y. Phosphate uptake under nitrate limitation by Scenedesmus sp. and its ecological implications. J Phycol. 1974;10:170–175. [Google Scholar]
- 37.Rhee G Y, Gotham I J. Optimal N:P ratios and coexistence of planktonic algae. J Phycol. 1980;16:486–489. [Google Scholar]
- 38.Rhee G Y, Gotham I J. The effect of environmental factors on phytoplankton growth: light and the interactions of light with nitrate limitation. Limnol Oceanogr. 1981;26:649–659. [Google Scholar]
- 39.Richmond A. Outdoor mass culture of microalgae. In: Richmond A, editor. Handbook of microalgal mass culture. Boca Raton, Fla: CRC Press; 1986. pp. 285–330. [Google Scholar]
- 40.Sawayama S, Rao K K, Hall D O. Nitrate and phosphate ion removal from water by Phormidium laminosum immobilized on hollow fibers in a photobioreactor. Appl Microbiol Biotechnol. 1998;49:463–468. [Google Scholar]
- 41.Suzuki I, Sugiyama T, Omata T. Primary structure and transcriptional regulation of the gene for nitrite reductase from the cyanobacterium Synechococcus sp. PCC 7942. Plant Cell Physiol. 1993;34:1311–1320. [Google Scholar]
- 42.Talbot P, de la Noüe J. Tertiary treatment of wastewater with Phormidium bohneri (Schmidle) under various light and temperature conditions. Water Res. 1993;27:153–159. [Google Scholar]
- 43.Tam N F Y, Lau P S, Wong Y S. Wastewater inorganic N and P removal by immobilized Chlorella vulgaris. Water Sci Technol. 1994;30:369–374. [Google Scholar]
- 44.Vermaas W. Molecular genetics of the cyanobacterium Synechocystis sp. PCC 6803: principles and possible biotechnology applications. J Appl Phycol. 1996;8:263–273. [Google Scholar]
- 45.Vonshak A. Laboratory techniques for the cultivation of microalgae. In: Richmond A, editor. Handbook of microalgal mass culture. Boca Raton, Fla: CRC Press; 1986. pp. 117–146. [Google Scholar]