Abstract
Simple Summary
Up to 30% of breast cancer patients are carriers of pathogenic mutations in breast cancer susceptibility genes. Except for BRCA1/2 genes that account only for 22–30% of hereditary breast cancer, less is known about the remaining genes that are prone to breast cancer. Our aim was to retrospectively evaluate the relationship between the pathogenic mutations (BRCA and non-BRCA), US features, and histopathologic findings of breast cancer patients with and without mutations. We concluded that carrier patients (BRCA, TP53, PALB, CHEK, ATM, RAD) seem to exhibit benign imaging findings on US compared to mutation-negative patients. Furthermore, carrier patients had the majority of tumors with higher histologic grade and a higher proliferation index. BRCA1, TP53, and RAD carriers accounted for up to one third of the ER-negative tumors from the mutation group. Axillary US performed worse in depicting axillary metastatic lymph nodes in carrier patients, compared to negative patients.
Abstract
The purpose of this study is to evaluate the relationship between the pathogenic/likely pathogenic mutations, US features, and histopathologic findings of breast cancer in mutation carriers compared to non-carrier patients. Methods: In this retrospective study, we identified 264 patients with breast cancer and multigene panel testing admitted to our clinic from January 2018 to December 2020. Patient data US findings, US assessment of the axilla, multigene panel tests, histopathology, and immunochemistry reports were reviewed according to the BI-RADS lexicon. Results: The study population was comprised of 40% pathogenic mutation carriers (BRCA1, BRCA2, CHEK2, ATM, PALB, TP 53, NBN, MSH, BRIP 1 genes) and 60% mutation-negative patients. The mean patient age was 43.5 years in the carrier group and 44 years in the negative group. Carrier patients developed breast cancer with benign morphology (acoustic enhancement, soft elastography appearance) compared to non-carriers (p < 0.05). A tendency towards specific US features was observed for each mutation. BRCA1 carriers were associated with BC with microlobulated margins, hyperechoic rim, and soft elastography appearance (p < 0.05). Estrogen receptor (ER)-negative tumors were associated with BRCA1, TP53, and RAD mutations, while BRCA2 and CHEK2 were associated with ER-positive tumors. Conclusions: Patients with pathogenic mutations may exhibit BC with benign US features compared to negative, non-carrier patients. BRCA1, TP53, and RAD carriers account for up to one third of the ER tumors from the carrier group. Axillary US performed worse in depicting involved lymph nodes in carrier patients, compared to negative patients.
Keywords: high-risk population, BRCA, benign appearance breast cancer, CHEK, US
1. Introduction
There are 12 established breast cancer-predisposition genes that are known to have an increased risk of developing cancer [1]. Up to 30% of breast cancer patients are positive for cancer-predisposition genes, evaluated through hereditary multigene testing panels.
Compared to BRCA1/2 mutation carriers, which account for up to 22–30% of the hereditary breast cancer cases, less is known about the other 70% of genetic breast cancer patients [2]. There is an increasing number of studies reporting differences in terms of natural history and treatment response related to each pathogenic mutation. For example, the ATM carrier patients are more prone to develop subcutaneous necrosis and contralateral breast cancer after radiotherapy, which may be a relative contraindication to the standard management [3,4]. These aspects support the idea of different breast cancer sub-types being linked to each mutation and may also be reflected in different breast cancer imaging features. However, except for the BRCA1/2 genes, there are limited studies regarding the other breast-cancer susceptibility genes, on what breast cancer type these patients are prone to, their imaging features, or histopathology characteristics.
Currently, the NCCN recommend mastectomy in breast cancer patients with BRCA mutations and suggest that mastectomy could be offered in high/moderate-risk patients with other pathogenic/likely pathogenic mutations [5]. BRCA1/2, PALB2, TP53, PTEN, CDH1, and STK11 are high penetrant genes, associated with an increased risk of developing breast cancer of >60%, while ATM, CHEK2, and RAD are moderate penetrant genes with a breast cancer risk of 40–60%. Moreover, the American Society of Surgeons recommends genetic testing in all breast cancer patients [6]. Thus, the number of mutation carriers will continue to rise, increasing the need for informed, familiarized breast imagers with their tumor features and possible misdiagnosis characteristics.
Therefore, our study aims to evaluate and compare the relationship between US features and pathologic findings (including aggressiveness markers, molecular type, and axillary lymph node status) of breast cancer that develop in patients who are carriers of pathogenic mutations, versus negative, non-carrier patients.
2. Material and Methods
2.1. Study Population
This retrospective study was approved by the institutional review board of BLINDED* (SR NR 9, from 18 January 2021) and the need for informed consent was waived. Inclusion criteria represented patients with breast cancer, pre-operative breast and axillary US, hereditary multigene testing panels, complete surgery, and pathology reports who presented to our clinic between January 2018 and December 2020.
Exclusion criteria consisted of patients with inadequate or incomplete US images, pathology reports, or genetic tests. Generally, we recommend genetic testing if one of the following criteria are met: breast cancer diagnosed <35 years, bilateral cancer, triple-negative sub-type, one first-degree relative diagnosed with breast cancer <55 years, two second-degree relatives diagnosed with breast cancer <55 years, and if additional melanoma, colon, pancreas, or ovarian cancer is present. Multigene panel testing is a type of genetic testing which analyzes mutations in multiple genes at once. Furthermore, it provides a better understanding of cancer risk compared to single-gene testing.
Up to 48% of the carrier patients and 43% of the mutation-negative patients exhibited symptoms. The remaining presented for breast US by means of an opportunistic screening since there is no organized governmental breast cancer screening in Romania.
A total of 309 patients were identified, and 98 pathogenic mutation carriers and 145 mutation-negative patients were included in the study (Figure 1).
Figure 1.
Study population—inclusion and exclusion criteria.
Patient data including symptoms at the time of diagnosis were also recorded.
2.2. Imaging Technique
Breast US was performed by one radiologist with more than 15 years of experience in breast imaging, using a Hi Vision Ascendus (Hitachi Ltd., Tokyo, Japan) machine with a Wide-Band (6.5–13 MHz) linear probe, and Hologic Supersonic (Aixplorer Mach 30, Aix-en-Provence, France) with a linear probe (5–18 MHz). Greyscale, Color Doppler, and strain elastography (SE) images were retrieved and interpreted by two radiologists with 15 and 4 years of experience and consensus was reached for discordant cases. The two radiologists were blinded to any existing mammography or MRI exams. Greyscale and Color Doppler features were described using the American College of Radiology BI-RADS lexicon (5th edition) [7]. Circumscribed margins, parallel orientation compared to the skin, and posterior enhancement were considered “benign US features”. Microlobulated, spiculated, or indistinct margins, taller than wide orientation, and posterior acoustic shadowing were categorized as “suspicious US features”. The mass homogeneity (homogeneous, heterogeneous) and echogenicity (hypo-, iso-, hyper-echoic, or mixed) were also assessed. The presence of microcalcifications suspected on US was confirmed with mammography for all cases. SE images were classified according to the Ueno–Itoh adaptation of the Tsukuba elasticity score, considering ACR Appropriateness Criteria [8]. All patients with suspect axillary lymph nodes at US (absent fatty hilum, cortical thickness >3 mm, indistinct contour) were considered abnormal on imaging and underwent US-guided core needle biopsy.
2.3. Pathologic and Genetic Data
Pathologic data were reviewed, including the histologic tumor type, size, histologic grade, lymph node status, and immunohistochemistry findings (estrogen and progesterone receptors—ER, PR, HER2 status, ki-67% proliferation index).
Multigene panel testing, including 12 established breast cancer-predisposition genes (BRCA1, BRCA2, TP53, ATM, CHEK2, PALB 2, BARD 1, NBN, MSH, RAD 51C and RAD 51D, BRIP 1), were classified as pathogenic or likely pathogenic according to the ClinVar database and included in the carrier group. Patients positive only for variants of uncertain significance (VUS) were included together with patients that tested negative for all panel genes, in the mutation-negative group.
2.4. Statistical Analysis
Statistical analyses were performed using MedCalc software (version 19.2.6, Ostend, Belgium). To analyze the associations among mutations, clinic-pathologic data, and US findings, the Chi-square or Fisher’s exact test was used. Moreover, the Mann–Whitney U test was used to compare the age, lesion size, and elastography score between patients with and without pathogenic mutations. The agreement between US and surgery in the detection of axillary lymphadenopathy was calculated for each group. p < 0.05 was considered to indicate a statistically significant difference between groups.
3. Results
The carrier group consisted of 98 pathogenic mutation patients divided as follows: 29 BRCA1, 15 BRCA2, and 62 non-BRCA1/2 (15 CHEK2, 15 RAD 51C or D, 7 PALB 2, 6 NBN, 3 TP 53, 3 ATM, 2 BARD 1, 2 MSH 2, 1 BPRIP 1). The mean patient age was 43.5 years (range 30–67 years) in the carrier group and 44 years (range 24–73 years) in the mutation-negative group.
3.1. Associations between Clinico-Pathological Data and Mutation Status
At the time of diagnosis, 47 of 98 (48%) of the carrier group patients and 62 of 145 (43%) mutation-negative patients exhibited symptoms. The remaining presented for breast US by means of an opportunistic screening since there is no organized governmental breast cancer screening in Romania. For both groups, the palpable breast mass was the most common symptom (35/98, 46/145), and only few patients had nipple discharge (10/98, 16/145). In particular, two patients with BRCA1 and CHEK2 mutations presented only with axillary discomfort (2/98, 2%). No significant difference was observed for patient provenience, or symptoms at the time of diagnosis (all p > 0.05).
Invasive ductal carcinoma of no special type (IDC-NST) was the most common type of breast cancer in both groups. The tumor size, lymphadenopathy, lympho-vascular invasion, ER, and HER2 status did not significantly differ (all p > 0.05).
The carrier group had a significantly higher number of unifocal tumors, with higher histologic grade, and higher proliferative index ki-67% (p = 0.03, p < 0.000, and p < 0.001). Additional VUS were found to be associated with 24 out of 98 mutation carrier patients (p < 0.0001) (Table 1).
Table 1.
Clinic-pathological characteristics of the patients.
| Variable | Pathogenic Carrier Group | Mutation-Negative Group | p-Value |
|---|---|---|---|
| Patient age (y), mean (range) | 43.5 (30–67) | 44 (24–73) | 0.644 |
| Patient origin | 0.957 | ||
| Urban | 80 (81.6) | 120 (82.8) | |
| Rural | 18 (18.4) | 25 (17.2) | |
| Symptoms | 0.424 | ||
| Absent | 51 (52) | 83 (57) | |
| Present | 47 (48) | 62 (43) | |
| Breast cancer type | |||
| Invasive ductal carcinoma NST | 88 (89.8) | 120 (82.8) | 0.178 |
| Other * | 10 (10.2) | 25 (17.2) | |
| “In Situ” component | 13 (13.3) | 29 (20) | 0.234 |
| Number of tumors | 0.032 | ||
| Unifocal | 61 (62.2) | 67 (46.2) | |
| Multifocal | 14 (14.3) | 37 (25.5) | |
| Multicentric | 23 (23.5) | 41 (28.3) | |
| Tumor size (mm), mean (range) | 0.884 | ||
| <2 cm | 24 (24.5) | 29 (20) | |
| >2 cm | 74 (75.5) | 116 (80) | |
| Lymph node status | 0.329 | ||
| Negative | 45 (46.9) | 78 (54.2) | |
| Positive | 51 (53.1) | 66 (45.8) | |
| Histologic grade | 0.000 | ||
| Low | 6 (6.1) | 33 (22.8) | |
| Intermediate | 49 (50) | 80 (55.2) | |
| High | 43 (43.9) | 32 (22.1) | |
| Lympho-vascular invasion | 0.927 | ||
| Absent | 72 (73.5) | 105 (72.4) | |
| Present | 26 (26.5) | 40 (27.6) | |
| Immunohistochemistry | |||
| ER+ | 68 (69.4) | 110 (75.8) | 0.3 |
| ER− | 30 (30.6) | 35 (24.1) | |
| HER2+ | 21 (21.4) | 23 (15.8) | |
| HER2− | 77 (78.5) | 122 (84.1) | 0.3 |
| Ki-67% status | 0.001 | ||
| >20% | 77 (78.5) | 84 (60) | |
| <20% | 21 (21.4) | 61 (42) | |
| TNM Stage | 0.002 | ||
| 0 | 4 (4.1) | 3 (2.1) | |
| I | 10 (10.2) | 21 (14.5) | |
| IIA | 38 (38.7) | 59 (40.7) | |
| IIB | 22 (22.4) | 30 (20.7) | |
| IIIA | 15 (15.3) | 27 (18.6) | |
| IIIC | 8 (8.2) | 3 (2.1) | |
| IV | 1 (1) | 2 (1.4) | |
| Variants of uncertain significance (VUS) | 0.000 | ||
| Yes | 24 a (24.4) | 4 (2.7) | |
| No | 74 (75.5) | 141 (97.2) |
* Includes mucinous, metaplastic, papillary carcinoma, and adenoid cystic. a Except for one BRCA1 patient who had concomitant pathogenic CHEK2 and PALB2 mutations and CDH1 VUS, the rest of the patients had two concomitant genetic changes.
3.2. Associations between US Features and Mutation Status
Upon US, the dominant finding was breast mass in both carrier (93%) and mutation-negative (97%) groups, and only few patients exhibited a non-mass appearance (7% and 3%, respectively). No significant difference was observed for shape or orientation (all p > 0.05), with irregular and non-parallel masses as the most frequent finding in both groups (Figure 2).
Figure 2.
60-year-old BRCA2 mutation carrier patient with left breast cancer. There is an irregular, slightly hypoechoic mass with indistinct margins and non-parallel orientation compared to the skin (a), with a hard elastography appearance (TSUKUBA score 5, b). Pathology: IDC-NST, ER/PR-positive, HER2-negative, grade 2, ki67 = 25%.
Margins, echo patterns, and posterior features were found to be statistically different between carrier and mutation-negative groups (p = 0.047, p < 0.0001, and p < 0.0001). Microlobulated margins were found in 10% of the carrier patients and only in 5% of the mutation-negative patients (p = 0.23) (Figure 3).
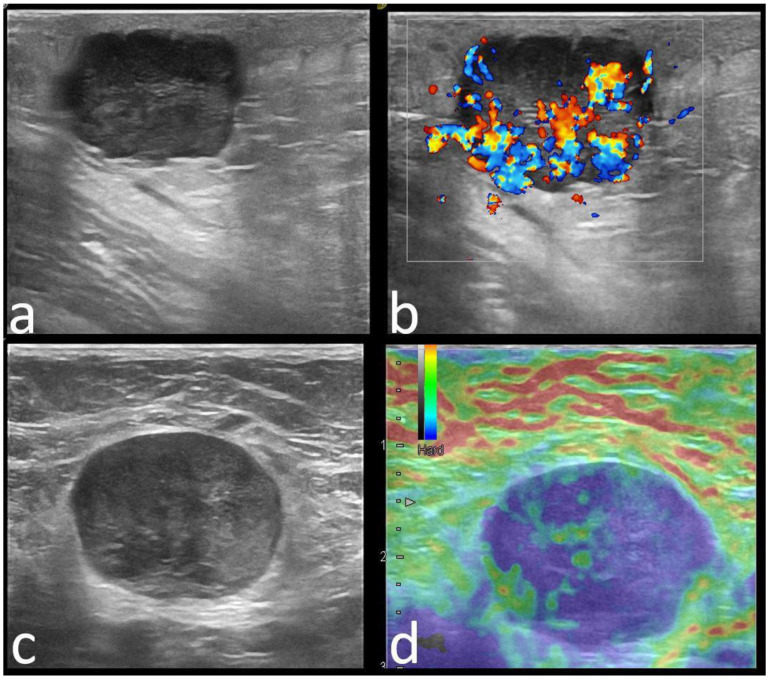
Figure 3.
Upper images: 29-year-old TP53 mutation carrier patient with right breast cancer. There is an oval, hypoechoic mass with microlobulated margins, parallel orientation compared to skin, with acoustic enhancement (a) and internal vascularity (b). Skin invasion was suspected and confirmed later by pathology. Pathology: IDC-NST, ER/PR/HER2-negative, ki67 = 90%. Lower images: 62-year-old PALB2 mutation carrier patient with right breast cancer. There is an oval, hypoechoic mass with circumscribed margins, parallel orientation compared to skin (c), mild acoustic enhancement, and soft elastography appearance (TSUKUBA score 3, d). Pathology: IDC-NST, ER/PR-negative, HER2-positive, grade 2, Ki67 = 80%.
The heterogeneous echo pattern and acoustic enhancement were associated with pathogenic mutation carriers (p < 0.0001, <0.0001), while spiculated margins, hypoechoic pattern, and posterior acoustic shadowing were associated with mutation-negative patients (p = 0.047, <0.0001, and <0.0001) (Figure 4).
Figure 4.
42-year-old RAD51C mutation carrier patient with right breast cancer. There is an oval, hypoechoic mass with indistinct margins (arrows), parallel orientation compared to skin, with internal vascularity and soft elastography appearance (TSUKUBA score 2). Pathology: IDC-NST, ER/PR/HER2-negative, grade 3, Ki67 = 80%.
The presence of calcifications was associated with the carrier group (p = 0.001) and there was a significant difference between the two groups with regard to the calcification type (Figure 5). Consequently, calcifications associated with a mass (30/35) and calcifications alone (3/35) were associated with carriers, while calcifications within ducts (6/23) were associated with mutation-negative patients (p = 0.001).
Figure 5.
Upper images: 66-year-old ATM mutation carrier patient with right breast cancer. There is an irregular, isoechoic mass with indistinct margins, non-parallel orientation compared to skin (a), and soft elastography appearance (TSUKUBA score 3, b). Two punctate microcalcifications are seen within the mass (arrows), confirmed on mammography (not shown). Pathology: IDC-NST, ER/PR-positive, HER2-negative, Ki67 = 14%. Lower images: 43-year-old CHEK2 mutation carrier patient with right breast cancer. There is an irregular, heterogeneous mass with indistinct margins, parallel orientation compared to chest wall, with mild posterior acoustic shadowing, internal vascularity (c), and soft elastography appearance (d, TSUKUBA score 2). Pathology: IDC-NST, ER/PR-positive, HER2-negative, Ki67 = 13%.
Hyperechoic rim was found to be associated with the carrier group (p = 0.001), while the mutation-negative group had no associated features in the majority of cases.
No statistical difference was found regarding the Color Doppler signal and the BI-RADS category between the two groups (all p > 0.05).
SE was statistically different between groups and showed a soft appearance with lower scores (2, 3, or blue-green-red appearance) in patients with mutations and a hard appearance with higher scores (4, 5) in mutation-negative patients (p = 0.029) (Figure 6).
Figure 6.
53-year-old BRCA1 mutation carrier patient with left breast cancer. There is a round, circumscribed, hypoechoic mass, with periphery vessels (a) and a blue-green-red elastography appearance (b). Pathology: IDC-NST, ER/PR/HER2-negative, grade 3, ki67 = 90%.
Axillary US was positive in 37 out of 98 pathogenic mutation carriers and 77 out of 145 negative patients. For the carrier group, the positive axillary US cases corresponding to positive axillary surgery reached a moderate agreement (kappa = 0.48, p = 0.000). For the mutation-negative group, US corresponding to surgery reached a substantial agreement (kappa = 0.656, p < 0.0001) in depicting axillary lymph node involvement (Table 2).
Table 2.
Breast cancer US features in carrier and non-carrier patients.
| US Feature | Pathogenic Carrier Group | Negative, Non-Carrier Group | p-Value |
|---|---|---|---|
| Lesion type | 0.107 | ||
| Mass | 91 (93) | 141 (97) | |
| Non-mass | 7 (7) | 4 (3) | |
| Shape | 0.391 | ||
| Round | 6 (6.1) | 14 (9.7) | |
| Oval | 27 (27.6) | 31 (21.4) | |
| Irregular | 65 (66.3) | 100 (69) | |
| Orientation | 0.861 | ||
| Parallel | 44 (44.9) | 68 (46.9) | |
| Non-parallel | 54 (55.1) | 77 (53.1) | |
| Margins | |||
| Circumscribed | 22 (22.4) | 44 (30.3) | 0.226 |
| Non-circumscribed | |||
| Spiculated | 19 (19.4) | 46 (31.7) | 0.047 |
| Indistinct | |||
| Angular | 21 (21.4) | 19 (13.1) | 0.263 |
| Microlobulated | 10 (10.2) | 8 (5.5) | |
| Echo pattern | 0.000 | ||
| Hypoechoic | 57 (58.2) | 100 (70) | |
| Heterogeneous | 36 (36.7) | 19 (13) | |
| Isoechoic | 5 (5.1) | 26 (17) | |
| Posterior features | 0.000 | ||
| None | 39 (39.8) | 71 (49) | |
| Enhancement | 27 (27.6) | 12 (8.3) | |
| Shadowing | 15 (15.3) | 54 (37.2) | |
| Combined | 17 (17.3) | 8 (5.5) | |
| Calcifications | 0.001 | ||
| Absent | 63 (64.3) | 122 (84.1) | |
| Present * | 35 (35.7) | 23 (15.9) | |
| A | 30 (30.6) | 15 (10.3) | |
| B | 3 (3.1) | 2 (2.1) | |
| D | 2 (2) | 5 (3.4) | |
| Associated features | 0.001 | ||
| None | 59 (60.2) | 115 (79.3) | |
| Hyperechoic Rim | 11 (11.2) | 8 (5.5) | |
| Duct ectasia | 10 (10.2) | 16 (11) | |
| Distortion | 6 (4.1) | 18 (18.4) | |
| Color Doppler signal | 0.696 | ||
| Absent | 9 (9.3) | 17 (11.7) | |
| Present | 88 (90.7) | 128 (88.3) | |
| Strain Elastography | 0.029 | ||
| Soft | 40 (40), 9 BGR | 30 (20.6), 1 BGR | |
| Hard | 58 (60) | 115 (79.3) | |
| BI-RADS | 0.799 | ||
| 4 | 12 (12.2) | 15 (10) | |
| 5 | 86 (87) | 130 (90) | |
| Axillary US | 0.026 | ||
| Negative | 61 (62.2) | 68 (46.9) | |
| Positive | 37 (37.8) | 77 (53.1) |
* A = Calcifications within mass, B = calcifications without mass, D = intraductal calcifications. Soft elastography score = 1, 2, 3 or blue-green-red appearance. Hard elastography appearance = 4 or 5 score. BGR = blue-green-red strain elastography appearance.
3.3. Associations between US Features and Specific Pathogenic Mutations
ER-negative tumors were associated with BRCA1 patients (p < 0.0001) and TP53 patients (p = 0.002) and were also found in the RAD mutations group (33% of tumors, p = 0.36). ER-positive tumors were associated with BRCA2 (p = 0.038) and CHEK2 (p = 0.038) carriers and were also found in PALB2 (85% of tumors, p = 0.15), NBN (100% of tumors, p = 0.18), and ATM (100% of tumors, p = 0.3) patients.
Breast masses with circumscribed margins, hyperechoic rim, and soft elastography appearance were associated with BRCA1 mutations (p < 0.0001, 0.000, and 0.05, respectively) (Figure 6). No statistical difference was noted between patients with other pathogenic mutations, compared to the negative group (all p > 0.05). The pathologic characteristics and US features seen in more than half of each genetic mutation sub-group are summarized in Table 3.
Table 3.
Pathologic characteristics and US features of breast cancer patients associated with pathogenic mutations.
| Variable | BRCA1 | BRCA2 | CHEK2 | RAD Group * | PALB | NBN | TP 53 | ATM |
|---|---|---|---|---|---|---|---|---|
| No. of patients (%) | 29 (29.5) |
15 (15.3) |
15 (15.3) |
15 (15.3) |
7 (7.1) |
6 (6.1) |
3 (3) |
3 (3) |
| Breast cancer type | IDC-NST ** | IDC-NST | IDC-NST | IDC-NST | IDC-NST | IDC-NST | IDC-NST | IDC-NST |
| Molecular sub-type (%) |
ER− (69) |
ER+ (100) |
ER+ (100) |
ER+ (66) ER− (33) |
ER+ (85) |
ER+ (100) |
ER− (100) |
ER+ (100) |
| US features (+/No. of patients) Orientation |
NP (17/29) | NP (9/15) | NP (10/15) | P (7/15) | P (4/7) | P (4/6) | P (2/3) | P (2/3) |
| Margins | C (20/29) | NC (11/15) | NC (11/15) | NC (11/15) | C (5/7) | NC (5/6) | C (3/3) | NC (3/3) |
| Echo pattern | Hypoechoic (19/29) | Hypoechoic (9/15) | Heterogeneous (5/15) | Hypoechoic (11/15) | Hypoechoic (4/7) |
Heterogenous (3/3) |
- | Hypoechoic (2/3) |
| Posterior features | Enhancement (16/29) | Absent (9/15) |
Shadowing (6/15) |
Enhancement (6/15)/Combined pattern (5/15) | No posterior (4/7) |
- | Enhancement (2/3) | - |
| Associated features | Hyperechoic rim (6/29) Soft elastography (11/29) |
Calcifications (7/15) Hard elastography (12/15) |
Calcifications (7/15) | - | - | Calcifications (3/6) Hyperechoic rim (3/6) |
- | Calcifications (2/3) Architectural distortion (2/3) |
* RAD51C, RAD51D; ** IDC-NST = invasive ductal carcinoma no special type; ER+ = estrogen receptor-positive; ER− = estrogen receptor-negative; P = parallel, NP = non-parallel; C = circumscribed margins, NC = non-circumscribed margins. “-” = No predominant US feature.
4. Discussion
In the current study, we found that breast cancer’s histology and US features of pathogenic mutation carriers differ from the mutation-negative patients. Mutation carriers (BRCA and non-BRCA) tend to develop breast cancer with benign morphologic features and more aggressive pathologic characteristics. BRCA1, TP53, and RAD pathogenic mutation carriers account for a large percentage (28.5%) of ER-negative tumors.
Up to 30% of the invasive cancers seen in the high-risk population exhibited benign findings, with round- or oval-shaped masses and smooth margins [8,9,10,11]. Kuhl et al. [12] reported that up to 38% of the genetic breast cancer exhibited benign mammography, US, and MRI features with hypo/anechoic masses with circumscribed margins and parallel orientation. However, out of 13 cancers, 7 had benign features and only 5 of them were BRCA-positive. In contrast, a study reported exclusive malignant phenotypes for 20 BRCA-associated breast cancers, in all imaging modalities [13]. In our study, we had 44 BRCA-associated breast cancers and 54 non-BRCA pathogenic mutation-associated cancers, which exhibited benign morphologic features compared to the 145 mutation-negative breast cancer patients. This could lead to misdiagnosis or delayed diagnosis in these particular patients. Thus, heterogeneous echo pattern, acoustic enhancement, and hyperechoic rim were associated with the pathogenic group. Moreover, we found that spiculated margins, hypoechoic pattern, and posterior acoustic shadowing were features associated with mutation-negative patients (all p < 0.05). The morphologic features might be linked to the presence of pathogenic mutations which led to the development of highly aggressive tumors, compared to negative patients. In relation, we found that carrier patients were associated with a higher histologic grade and a higher proliferative index (all p < 0.05) compared to the mutation-negative group.
High-grade cancers exhibit benign features due to their rapid growth, whereas low/intermediate cancers develop a desmoplastic reaction and appear as spiculated masses [14]. Heterogeneous echo pattern and acoustic enhancement might be secondary to the cystic necrotic areas found within rapid growth tumors and may occasionally be misinterpreted as benign lesions. Hyperechoic rim is usually associated with benign pathology, such as pseudo-angiomatous stromal tumors and myoblastic tumors, being caused by inflammatory peritumoral cells [15]. This particular US aspect may contribute and partially correspond to the MRI rim enhancement reported in these high-risk patients [16].
The presence of calcifications with an accompanying mass was associated with the carrier group (p = 0.001) and found in 30.6% of the lesions. Intraductal calcifications were associated with the mutation-negative group (p = 0.001), corresponding to their higher number of DCIS cases. The high incidence of associated calcifications was previously reported for BRCA1 and BRCA2 patients [11,17], and furthermore suggests that mammography remains an important screening and diagnostic imaging modality in all genetic carrier patients.
The high-grade tumors’ appearance on elastography is still a matter of debate. Ye et al. reported significantly softer high-grade tumors compared to low-grade ones (p < 0.001), while Ganau et al. reported no statistical difference in high- versus low-grade tumors [17,18]. We found that soft SE appearance was associated with the carrier group (p = 0.029). Additionally, the BGR appearance that was previously reported in benign, cystic lesions, was noted in few ER-negative tumors (9, 9% in carriers, versus 1, 0.001% in non-carriers). The first explanation may be found in the presence of abundant necrosis, with a predominant cystic component, seen in these highly aggressive tumors. In addition, in our study, 10% of the tumors were of special sub-types, such as mucinous-, medullary-, or adenoid cystic-type tumors, which may have a minor solid component that could further contribute to this appearance [19,20]. Therefore, we suggest that the BGR aspect seen in solid tumors be re-named as “false BGR” appearance.
As regards to axillary lymphadenopathy, we observed a moderate agreement between axillary US and surgery in carrier patients (kappa = 0.48, p = 0.000) and substantial agreement in mutation-negative patients (kappa = 0.656, p = 0.000). The high histologic grade leading to an increased number of lymph node micro-metastases (<2 mm) could explain the discrepancy in agreement. Our findings advocate that genetic carrier patients are more prone to have false-negative axillary US compared to mutation-negative patients. However, recent data suggest different immunohistochemistry factors to be involved in axillary metastasis in carrier and mutation-negative patients [21].
Up to 24.4% of the pathogenic mutation carriers were also positive for VUS genetic changes. A recent population-based study reported up to 19% of VUS among patients and controls [1]. It remains questionable whether additional VUS have an impact on treatment response or patients’ prognosis, having an uncertain role in the breast cancer pathogenesis.
We performed a separate analysis on each pathogenic mutation group of patients and observed a tendency towards specific US features, found in more than half of the tumors from each subgroup. However, except for the BRCA1 group, no statistical difference was noted among patients, mainly because of the low numbers of cancers (all p > 0.05). To the best of our knowledge, no imaging features were previously reported for non-BRCA mutation-associated breast cancers.
Imaging findings of BRCA-associated breast cancer were previously reported and seem to exhibit benign features on MRI and mammography [8,9,10,11]. One particular study showed the US features of BRCA-associated tumors, which were primarily hypoechoic masses with irregular shape, parallel orientation, and non-circumscribed margins [11]. The same authors compared BRCA1 with BRCA2 tumors and reported a tendency towards benign morphology in BRCA1 patients, due to their association with acoustic enhancement. We found additional benign morphologic features, such as circumscribed margins, hyperechoic rim, and soft elastography appearance, that were associated with BRCA1 patients (all p < 0.005) compared to mutation-negative patients.
Additionally, we found that BRCA1, RAD51C, RAD51D, and TP53 carriers were associated with ER-negative tumors, while BRCA2, CHEK, and ATM carriers developed exclusively ER-positive tumors. Our findings are in agreement with one recently published study [1].
This study has some limitations. First, the study was retrospective and included consecutive genetically tested patients; however, radiologists were aware that a tumor was present and that would have had possible implications on the BI-RADS category assessment. Second, we included only US as an imaging modality. We are a tertiary referral center, where the patients present mainly for biopsy and pre-surgical planning. Thus, mammography and MRI are usually available and evaluated for a second opinion. There were multiple difficulties that prevented us from including them in the current study (e.g., different mammography and MRI machines, different MRI protocols, legal-related policies). Third, for some pathogenic mutations, the number of patients was limited, related to their low incidence. Finally, our study was based on a single institution. A larger, population-based study will be needed in the future to validate our findings.
5. Conclusions
Patients with pathogenic mutations in BRCA and non-BRCA genes (such as TP53, PALB, CHEK, ATM, and RAD) seem to exhibit benign imaging findings on US compared to mutation-negative patients. It remains questionable if the aggressiveness markers, such as high histologic grade, high proliferation index, or ER-negative status, rather than the presence of mutation, tend to exhibit benign morphologic features. BRCA1, TP53, and RAD carriers accounted for up to one-third of the ER-negative tumors from the mutation group. Axillary US performed worse in depicting axillary metastatic lymph nodes in these patients, compared to the mutation-negative patients.
Abbreviations
| BC | breast cancer |
| US | ultrasound |
| BI-RADS | breast imaging and reporting data system |
| ER | estrogen receptor |
| “−” | negative |
| “+” | positive |
| PR | progesterone receptor |
| Ki67% | proliferative index |
| SE | strain elastography |
| ACR | American College of Radiology |
| VUS | variants of uncertain significance |
| BGR | blue-green-red elastography appearance |
Author Contributions
Conceptualization, R.M.P., A.C. and S.D.; Data curation, A.C., M.D., D.F., M.S., D.E. and I.G.; Formal analysis, R.M.P., A.C. and S.D.; Investigation, R.M.P., A.C., M.D., D.F., M.S., D.E., I.G. and S.D.; Methodology, R.M.P., A.C., D.F. and S.D.; Project administration, R.M.P., A.C. and S.D.; Resources, R.M.P., A.C., M.S., D.E. and I.G.; Supervision, A.C.; Validation, A.C.; Writing—original draft, R.M.P., A.C. and S.D.; Writing—review and editing, R.M.P., A.C., M.D., D.F., M.S., D.E., I.G. and S.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Medimages Breast Center (SR NR 9, from 18 January 2021).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data presented in this study is available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was granted by project PDI-PFE-CDI 2021, entitled Increasing the Performance of Scientific Research, Supporting Excellence in Medical Research and Innovation, PROGRES, no. 40PFE/30 December 2022.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hu C., Hart S.N., Gnanaolivu R., Huang H., Lee K.Y., Na J., Gao C., Lilyquist J., Yadav S., Boddicker N.J., et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N. Engl. J. Med. 2021;384:440–451. doi: 10.1056/NEJMoa2005936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filippini S.E., Vega A. Breast cancer genes: Beyond BRCA1 and BRCA2. Front. Biosci. 2013;1:1358–1372. doi: 10.2741/4185. [DOI] [PubMed] [Google Scholar]
- 3.Iannuzzi C.M., Atencio D.P., Green S., Stock R.G., Rosenstein B.S. ATM mutations in female breast cancer patients predict for an increase in radiation-induced late effects. Int. J. Radiat. Oncol. 2002;52:606–613. doi: 10.1016/S0360-3016(01)02684-0. [DOI] [PubMed] [Google Scholar]
- 4.Knappskog S., Chrisanthar R., Løkkevik E., Anker G., Østenstad B., Lundgren S., Risberg T., Mjaaland I., Leirvaag B., Miletic H., et al. Low expression levels of ATM may substitute for CHEK2/TP53 mutations predicting resistance towards anthracycline and mitomycin chemotherapy in breast cancer. Breast Cancer Res. 2012;14:R47. doi: 10.1186/bcr3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCCN Guidelines Version 1.2020. Breast Cancer Risk Reduction. [(accessed on 1 August 2021)]. Available online: https://www2.tri-kobe.org/nccn/guideline/breast/english/breast_risk.pdf.
- 6.Manahan E.R., Kuerer H.M., Sebastian M., Hughes K.S., Boughey J.C., Euhus D.M., Boolbol S.K., Taylor W.A. Consensus Guidelines on Genetic’ Testing for Hereditary Breast Cancer from the American Society of Breast Surgeons. Ann. Surg. Oncol. 2019;26:3025–3031. doi: 10.1245/s10434-019-07549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Orsi C.J., Sickles E.A., Mendelson E.B., Morris A., Creech E.W., Butler F.P., Wiegmann P.G., Chatfield B.M., Meyer W.L., Wilcox A.P. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. American College of Radiology; Reston, VA, USA: 2013. [Google Scholar]
- 8.Mainiero M.B., Lourenco A., Mahoney M.C., Newell M.S., Bailey L., Barke L.D., D’Orsi C., Harvey J.A., Hayes M.K., Huynh P.T., et al. ACR Appropriateness Criteria—Breast Cancer Screening. J. Am. Coll. Radiol. 2016;13:R45–R49. doi: 10.1016/j.jacr.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Schrading S., Kuhl C.K. Mammographic, US, and MR imaging phenotypes of familial breast cancer. Radiology. 2008;246:58–70. doi: 10.1148/radiol.2461062173. [DOI] [PubMed] [Google Scholar]
- 10.Veltman J., Mann R., Kok T., Obdeijn I.M., Hoogerbrugge N., Blickman J.G., Boetes C. Breast tumor characteristics of BRCA1 and BRCA2 gene mutation carriers on MRI. Eur. Radiol. 2008;18:931–938. doi: 10.1007/s00330-008-0851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ha S.M., Chae E.Y., Cha J.H., Kim H.H., Shin H.J., Choi W.J. Association of BRCA Mutation Types, Imaging Features, and Pathologic Findings in Patients With Breast Cancer With BRCA1 and BRCA2 Mutations. Am. J. Roentgenol. 2017;209:920–928. doi: 10.2214/AJR.16.16957. [DOI] [PubMed] [Google Scholar]
- 12.Kuhl C.K., Schmutzler R.K., Leutner C.C., Kempe A., Wardelmann E., Hocke A., Maringa M., Pfeifer U., Krebs D., Schild H.H. Breast MR Imaging Screening in 192 Women Proved or Suspected to Be Carriers of a Breast Cancer Susceptibility Gene: Preliminary Results. Radiology. 2000;215:267–279. doi: 10.1148/radiology.215.1.r00ap01267. [DOI] [PubMed] [Google Scholar]
- 13.Marino M.A., Riedl C.C., Bernathova M., Bernhart C., Baltzer P.A., Helbich T.H., Pinker K. Imaging Phenotypes in Women at High Risk for Breast Cancer on Mammography, Ultrasound, and Magnetic Resonance Imaging Using the Fifth Edition of the Breast Imaging Reporting and Data System. Eur. J. Radiol. 2018;106:150–159. doi: 10.1016/j.ejrad.2018.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaichman J., Marcus J.C., Alsaadi T., El-Khoury M., Meterissian S., Mesurolle B. Sonographic Appearance of Invasive Ductal Carcinoma of the Breast According to Histologic Grade. Am. J. Roentgenol. 2012;199:W402–W408. doi: 10.2214/AJR.11.7374. [DOI] [PubMed] [Google Scholar]
- 15.Mercado C.L., Naidrich S.A., Hamele-Bena D., Fineberg S.A., Buchbinder S.S. Pseudoangiomatous Stromal Hyperplasia of the Breast: Sonographic Features with Histopathologic Correlation. Breast J. 2004;10:427–432. doi: 10.1111/j.1075-122X.2004.21373.x. [DOI] [PubMed] [Google Scholar]
- 16.Noh J.M., Han B.-K., Choi D.H., Rhee S.J., Cho E.Y., Huh S.J., Park W., Park H., Nam S.J., Lee J.E., et al. Association betweenBRCAMutation Status, Pathological Findings, and Magnetic Resonance Imaging Features in Patients with Breast Cancer at Risk for the Mutation. J. Breast Cancer. 2013;16:308–314. doi: 10.4048/jbc.2013.16.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye J., Fenghua L., Jing D., Yifen G. Strain elastography features in invasive breast cancer: Relationship between stiffness and pathological factors. Int. J. Clin. Exp. Med. 2017;10:13290–13297. [Google Scholar]
- 18.Ganau S., Andreu F.J., Escribano F., Martín A., Tortajada L., Villajos M., Baré M., Teixidó M., Ribé J., Sentís M. Shear-wave elastography and immunohistochemical profiles in invasive breast cancer: Evaluation of maximum and mean elasticity values. Eur. J. Radiol. 2015;84:617–622. doi: 10.1016/j.ejrad.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Chiorean A., Duma M., Dudea S., Bolboaca S., Dumitriu D., Eniu D., Sfrangeu S. Typical and Unusual Sonoelastographic Patterns of Breast Cystic Lesions: Impact on BI-RADS Classification. Ultraschall Med. Eur. J. Ultrasound. 2010;33:E138–E144. doi: 10.1055/s-0029-1245699. [DOI] [PubMed] [Google Scholar]
- 20.Pintican R., Duma M., Chiorean A., Fetica B., Badan M., Bura V., Szep M., Feier D., Dudea S. Mucinous versus medullary breast carcinoma: Mammography, ultrasound, and MRI findings. Clin. Radiol. 2020;75:483–496. doi: 10.1016/j.crad.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Pintican R., Duma M.M., Szep M., Feier D., Eniu D., Goidescu I., Chiorean A. The Role of US in Depicting Axillary Metastasis in High-Risk Breast Cancer Patients. J. Pers. Med. 2021;11:1379. doi: 10.3390/jpm11121379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study is available on request from the corresponding author.