Abstract
Breast cancer is the most commonly diagnosed cancer among women and it is estimated that about 30% of newly diagnosed cancers in women will be breast cancers. While advancements in treating breast cancer have led to an average 5-year survival rate of 90%, many survivors experience cognitive impairments as a result of chemotherapy treatment. Doxorubicin, cyclophosphamide, and docetaxel (TAC) are commonly administered as breast cancer treatments; however, there are few studies that have tested the cognitive effects of TAC. In the current study, 12-week-old female C57BL/6 mice received 4 weekly intraperitoneal injections of either saline or a combination therapy of doxorubicin and cyclophosphamide followed by 4 weekly docetaxel injections. Four weeks after the last injection, mice were tested for hippocampus-dependent cognitive performance in the Y-maze and the Morris water maze. During Y-maze testing, mice exposed to TAC exhibited impairment. During the water maze assessment, all animals were able to locate the visible and hidden platform locations. However, mice that received the TAC presented with a significant impairment in spatial memory retention on the probe trial days. TAC treatment significantly decreases the dendritic complexity of arborization in the dentate gyrus region of the hippocampus. In addition, comparative proteomic analysis revealed downregulation of proteins within key metabolic and signaling pathways associated with cognitive dysfunction, such as oxidative phosphorylation, ephrin signaling, and calcium signaling.
Keywords: Cyclophosphamide, Doxorubicin, Docetaxel, Cognition
1. Introduction
Breast cancer is one of the most commonly diagnosed cancers and the primary cause of mortality due to cancer in women around the world (Miller et al., 2016a; Akram et al., 2017a). In developed countries, such as the US, early cancer diagnoses are increasing due to scientific advancements that have improved diagnostic methods. Improved treatment methods have also resulted in an increase of 5-year survival rates up to levels as high as 90% (Miller et al., 2016b; Akram et al., 2017b). Despite the advancements in survivorship, there is still much to be understood about the long-term effects that current treatment methods have on the quality of life of survivors. For instance, many breast cancer survivors experience cognitive impairments, such as difficulties in memory, attention, processing speed, and executive function after receiving chemotherapy treatment (Seigers and Fardell, 2011). Due to the untargeted method of administration of chemotherapy patients are at risk for potential side effects in other organ systems unrelated to their cancer, such as the central nervous system. This condition of chemotherapy-induced cognitive impairment, or “chemobrain,” has been of significant interest to researchers, because at present our current understanding of the mechanisms that cause this condition is still limited. In the context of chemobrain one of the most intensively studied regions of the brain, in both animals and humans, is the hippocampus (Inagaki et al. 2007; Dubois et al. 2014; Peukert et al. 2020; Yang and Moon 2015). The hippocampus is significant due to its strong connection to other brain regions (higher cortical brain structures and limbic system) and its role as a large integrating organ, which encodes and consolidates memory information from multiple brain regions (Schultz and Engelhardt 2014). Additionally, the hippocampus is connected to the default mode network and thus plays a critical role in memory retrieval and spatial memory retention (Ranganath and Ritchey 2012; Nadel, Hoscheidt, and Ryan 2013). There have been numerous studies that have investigated the role of drug treatment-induced structural brain changes within the hippocampus and the implications of these changes have on memory and emotional-related processes (Maguire and Mullally 2013; Encinas et al. 2011). Chemotherapy in particular has been associated with reduction in hippocampal volume and neurogenesis in addition to cognitive impairment (Inagaki et al. 2007; Dietrich et al. 2006; Janelsins et al. 2010; Christie et al. 2012; McElroy et al. 2020; Anderson et al. 2020).
The use of chemotherapy to treat breast cancer has a long history dating as far back as the 1940s. Some of the most commonly used chemotherapeutics used to treat breast cancer are doxorubicin, cyclophosphamide, docetaxel, epirubicin, fluorouracil, capecitabine, gemcitabine, paclitaxel, and methotrexate (Lee and Nan, 2012a). Treatment regimens for these chemotherapeutic agents are given both singularly and in combination. The first combination therapy for breast cancer was administered in the 1960s using methotrexate and thioTEPA (Greenspan et al., 1963). Some other common examples of combination therapies are AC (doxorubicin and cyclophosphamide) and CMF (Cyclophosphamide, Methotrexate, and Fluorouracil) (Lee and Nan, 2012b). Today, combination therapy has become increasingly more common due to benefits such as lower dosage requirements, decreased likelihood of tumor resistance, and ability to address different molecular targets in carcinogenesis. Despite these benefits, combination therapy also comes with an increased risk of side effects, such as inflammation, neutropenia, and cognitive impairments.
Currently, one of the most common combination therapies for breast cancer is docetaxel, doxorubicin, and cyclophosphamide (TAC). The TAC regimen was first introduced in the late 1990s and was revolutionary because it was the first combination to demonstrate the efficacy of docetaxel in the adjuvant setting (Martin, 2006). Docetaxel is in a class of compounds called taxanes discovered in the 1950s (Guenard, Gueritte-Voegelein, and Potier, 1993). Its mechanism of action is to disrupt microtubule function (Lee and Nan, 2012c). Docetaxel can be used to treat breast cancer, prostate cancer, head and neck cancer, and non-small cell lung cancer (Petrylak et al., 2004; Tripathy, 2006; Potter, 2007). Doxorubicin is an anthracycline compound that was discovered by isolating the compound from the bacteria Streptomyces (Lee and Nan, 2012d). Its mechanism of action is to inhibit DNA and RNA synthesis by intercalation between base pairs, blocking replication by inhibiting topoisomerase II activity, and creating iron-mediated free oxygen radicals that damage the DNA and cell membranes of cancer cells (Lee and Nan, 2012e). Alone, it can be used to treat certain types of thyroid cancers (Gottlieb and Hill, 1974). Finally, cyclophosphamide is an alkylating agent that was derived from mustard gas and approved for medical use in the 1950s (DeVita and Chu, 2008). Its mechanism of action is to crosslink DNA strands at guanine bases, thus preventing DNA from uncoiling during replication (Emadi, Jones, and Brodsky, 2009). The purpose of this study is to investigate the effects of a clinically relevant regimen of TAC on cognition, dendritic structure, and protein regulation within the hippocampus of female mice.
2. Results
2.1. Food Consumption & Body Weight
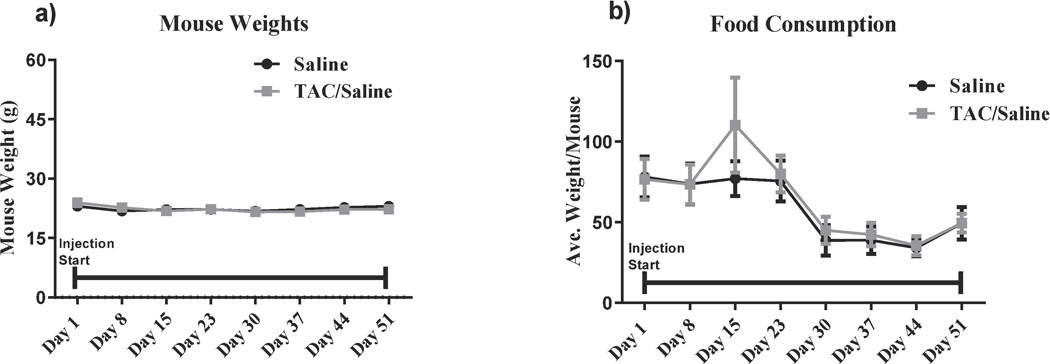
Food consumption and body weights were tracked during injections, no significant changes in food consumption were identified (F (7,42)= 1.16, P = 1.16; Fig. 1a). For body weight, there were a significant differences between treatment-by-week interaction (F (7,42)= 3.82, P <0.001; Fig. 1b) however, Holm- Sidak post-hoc analysis did not reveal any significant changes in body weights.
Figure 1. Mouse Weights and Food Consumption over Chemotherapy Injection Period.
Mouse weight and food consumption did not significantly change between the TAC-treated animals and saline-treated animals during the entire injection period. Indicating that the treatment does not affect the overall health of the mice.
2.2. YMaze
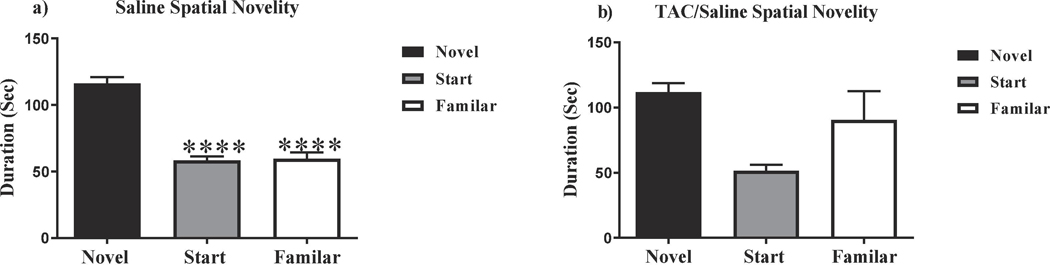
In order to assess potential deficits in short term memory and exploratory activity, all animals were subjected to Y maze testing. The Y maze paradigm takes advantage of the fact that rodents are naturally curious creatures; thus, they will naturally orient themselves towards a novel stimulus. The preference for the novel arm represents normal spatial recognition. Saline-treated animals spent significantly more time in the novel arm when compared to the start and familiar arms, indicating normal spatial recognition (F(2,27) = 61.08, P < .0001; Fig. 2a). TAC-treated animals were able to differentiate between the Start and Familiar arm but were unable to distinguish between the Novel and Familiar arms (F(2,29) = 4.514, P = .0196; Fig. 2b), which indicated that these animals had impaired spatial recognition memory induced by the TAC regimen.
Figure 2: Y maze.
(a) Saline-treated mice spent significantly more time exploring the novel arm during the testing phase of the Y maze, indicating no short-term memory deficits. (b) TAC-treated mice were not able to distinguish between the three Y maze arms, indicating that the TAC regimen induces short-term memory deficits **** P< .0001.
2.3. Morris Water Maze
Spatial learning and memory retention were assessed with the 5-day MWM testing paradigm. First, we assessed the mean velocity to the platform. The repeated-measures ANOVA revealed there was no significant treatment-by-day interactions in the mean velocity (F(4, 72) = 0.77, P = .54). Next, we assessed latency, which is the amount of time it takes the animal to reach the platform. The repeated-measures ANOVA revealed there was no significant treatment-by-day interactions in the mean latency (F(4, 90) = 1.71, P = .16). For distanced moved, analysis revealed there was no significant treatment-by-day interactions in the distance moved (F(4, 72) = 0.54, P = .0.70) during the visible-platform training (days 1 and 2), all experimental groups swam similar distances to the platform. In addition, all groups showed daily improvements in their ability to locate during the hidden-platform training (days 3–5).
Probe trials
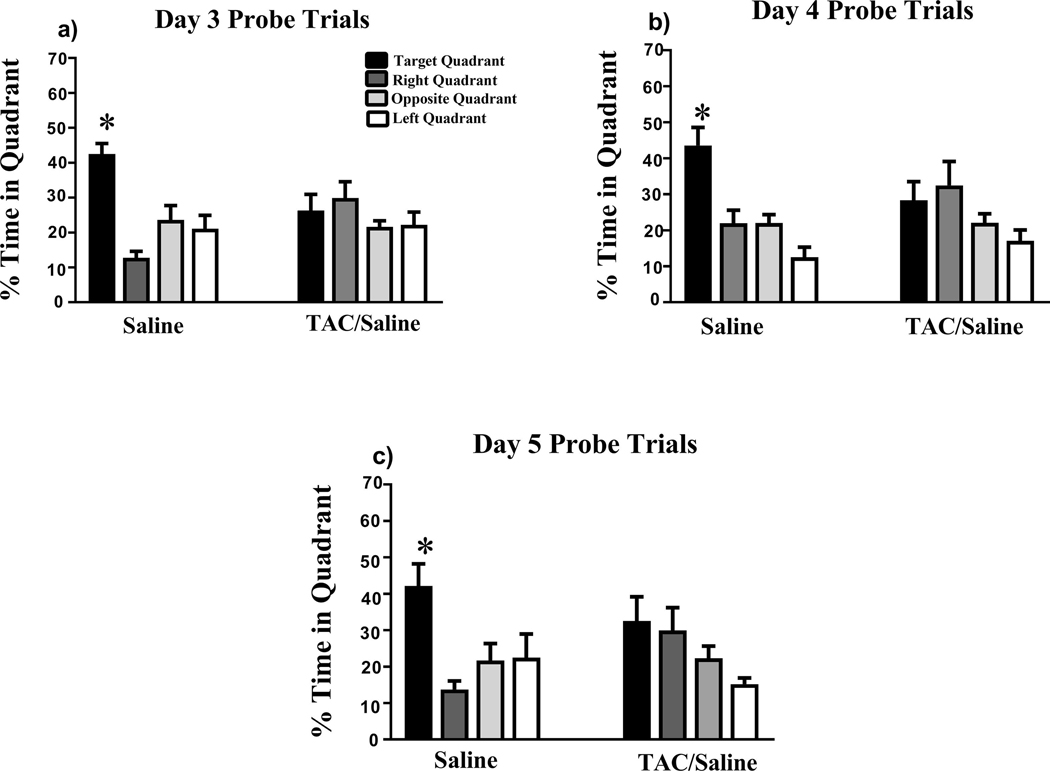
During the probe trial assessment days, the platform was removed after the hidden platform trials in order to assess spatial memory retention. The saline group exhibited significant preference for the target quadrant on days 3 (F(3,36) = 14.15, P < .0001; Fig. 3a ), 4 (F(3,36) = 13.37, P < .0001; Fig. 3b ), and 5 (F(3,36) = 5.459, P < .0001; Fig. 3c). In contrast, the TAC group did not show memory retention on days 3 (F(3,36) = 1.008, P = .4004; Fig. 4a), 4 (F(3,36) = 2.130, P = .1135; Fig. 3b), or 5 (F(3,36)) = 2.548, P = .0711; Fig. 3c).
Figure 3. Spatial memory retention during probe trials on days 3–5 of Morris water-maze testing.
(a-c) During all days of the probe trials, the saline group was able to locate the target quadrant, whereas the TAC group was unable to distinguish the target quadrant on any day of testing. Mean ± SEM; *P < .05.
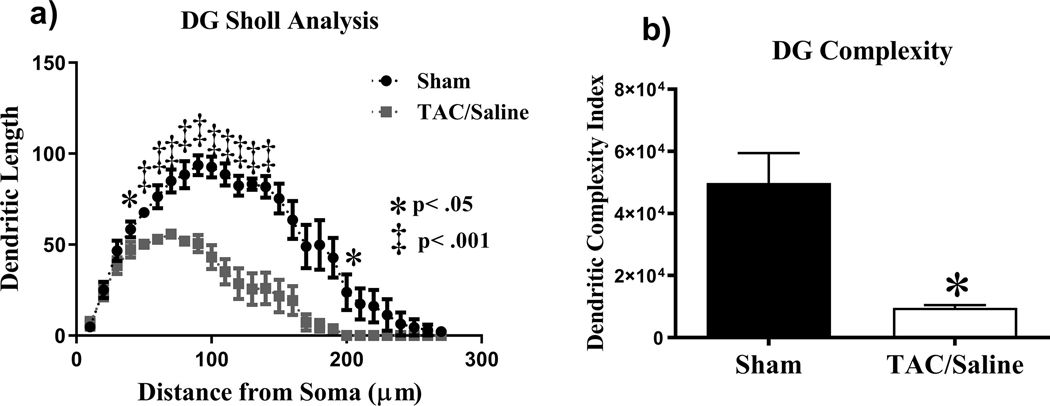
Figure 4. Sholl analysis and dendritic complexity throughout the DG region of the hippocampus.
(a-b) TAC significantly affected dendritic length and complexity. Mean ± SEM *P < .05.
2.4. Changes in dendritic spine density and morphology
Dentate Gyrus
Sholl analysis was performed in order to investigate the effects of the TAC regimen on dendritic length as a function of increasing 10 |im intervals from the soma. In the DG cells, there was a significant interaction between treatment and dendritic Sholl length (F(25,200) = 3.958, P < .0001; Fig. 4a). Holm-Sidak post-hoc analysis revealed that TAC groups decreased dendritic arborization at 40 μm from the soma (P < .05) and 50–140 μm (P < .001) and at 200 μm from the soma (P < .05). TAC treatment was also shown to significantly compromise overall dendritic complexity (t = 4.046, P < .05; Fig. 4b) when number of branch points, arborizations and ends were quantified. A quantitative analysis was also conducted on changes in dendritic spine density of different types of spines. We found that there were no significant changes in thin spines (t = 0.08, P = .93), stubby spines (t = 0.09, P = .92), or mushroom spines (t = 0.83, P = .41) as a consequence of the TAC regimen.
Cornu Ammonis 1 (CA1) pyramidal neurons
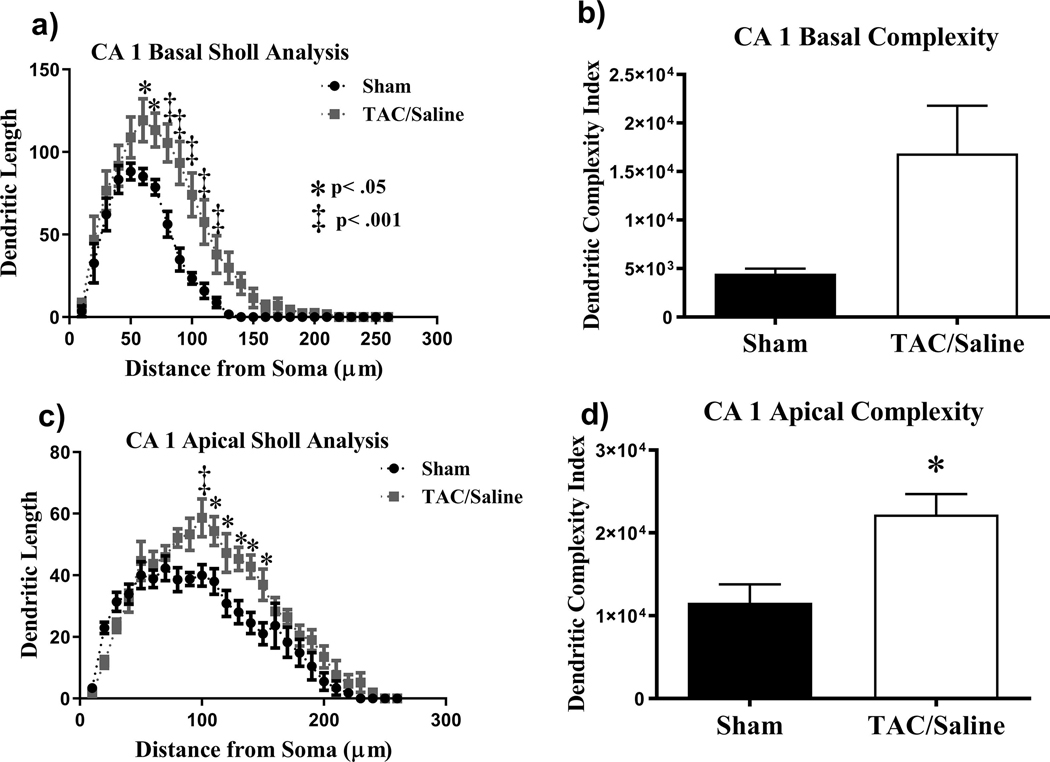
Similar analyses were conducted on the apical and basal regions of CA1 pyramidal neurons. In the basal CA1 neurons there was a significant interaction between treatment and length (F(25,200) = 3.95, P < .0001; Fig. 5a). Holm-Sidak’s revealed that the TAC group increased dendritic arborization at 60–70 μm (P < .05; Fig. 5a) and 80–120 μm (P < .001; Fig. 5a). However, there were no significant changes observed in dendritic complexity (t = 2.475, P = .07; Fig. 5b) when number of branch points, arborizations and ends were quantified. When assessing changes in spine density there were no significant effects of TAC on thin spines (t = 0.52, P = .60), stubby spines (t = 0.28, P = .78), and mushroom spines (t = 0.86, P = .40).
Figure 5. Sholl analysis and dendritic complexity throughout the CA1 pyramidal neurons.
(a) Dendritic length measured by Sholl analysis in CA1 basal revealed an increased in arborization at 60–120 |im from the soma. (b) TAC did not significantly affect complexity. (c) TAC significantly increased dendritic length in the CA1 apical. (d) Complexity was significantly increased in TAC-treated mice. Mean ± SEM (n=10); *P < .05, ** P < .01,
The apical CA1 neurons exhibited similar trends to the basal CA1. There was also a significant interaction (F(25,200) = 3.11, P < .0001; Fig.5c) between Sholl length and treatment (Fig. 5c). Further post-hoc analysis revealed that the TAC group increased dendritic arborization at 100 |im (P < .001; Fig.5c) and 110 – 150 |im (P < .05; Fig.5c). However, there was a significant difference between the saline and TAC groups in dendritic complexity (t = 3.107, P < .05; Fig 5d). Similar trends in spine morphology were observed in the apical CA1 pyramidal neurons as the basal CA1 with TAC having no significant effect on thin spines (t =0.24, P = .81), stubby spines (t = 0.28, P = .77), or mushroom spines (t = 1.12, P = .29).
2.5. Proteomics
Identification of proteins differentially expressed relative to cognitive impairment
In order to identify specific proteins and pathways associated with memory deficits in the TAC group as well as putative candidates that may underlie the behavioral and neuronal deficits observed in “chemo brain,” TAC-treated hippocampal sample cell lysates were analyzed using proteomic analysis relative to the saline group. A set of 195 proteins out of a total of 6,074 identified proteins were differentially expressed between TAC-treated and saline-treated animals with P < 0.05 and a fold change greater than 2 (Tables 1 and 2, Online Resource 1). Of these 195 proteins, 17 were highlighted for their association to cognitive impairment, neuronal function, and a significant log ratio fold change over or equal to +/− 2.
Table 1.
Top down regulated proteins differentially expressed associated with cognitive decline. A comparison of TAC-treated mice versus saline-treated mice identified 192 proteins as meeting statistical significance for differential expression
| Protein | Description | Location | Type | Fold Change (log ratio) |
|---|---|---|---|---|
| D3Z0L4 | MICOS complex subunit | Mitochondrion inner Membrane | Other | −6.79 |
| A2AER7 | Pqbpl protein | Nucleus | Other | −4.49 |
| B1AW58 | Calcium/calmodulin-dependent protein kinase ID | Nucleus | Kinase | −2.64 |
| Q9CWB7 | Glutaredoxin-like protein C5orf63 homolog | Mitochondrion | Other | −2.46 |
| Q9DBM2 | Peroxisomal bifunctional enzyme (PBE) (PBFE) [Includes: Enoyl- CoA hydratase/3,2-trans-enoyl-CoA isomerase (EC 4.2.1.17) (EC 5.3.3.8); 3-hydroxyacyl-CoA dehydrogenase (eC 1.1.1.35)] | Peroxisome | Enzyme | −2.45 |
| Q9JJV5 | Voltage-dependent calcium channel gamma-3 subunit (Neuronal voltage- gated calcium channel gamma-3 subunit) (Transmembrane AMPAR regulatory protein gamma-3) (TARP gamma-3) | Membrane | Ion channel | −2.40 |
| Q8C4C4 | Repulsive guidance molecule A | Brain | Other | −2.28 |
| Q8BKL6 | Gamma-tubulin complex component | Cytoskeleton | Other | −2.20 |
| Q62159 | Rho-related GTP-binding protein RhoC | Plasma Membrane | Other | −2.16 |
| P21952 | POU domain, class 3, transcription factor 1 (Octamer-binding protein 6) (Oct-6) (Octamer-binding transcription factor 6) (OTF-6) (POU domain transcription factor SCIP) | Nucleus | Other | −2.14 |
| Q8VCF0 | Mitochondrial antiviral-signaling protein (MAVS) (CARD adapter inducing interferon beta) (Cardif) (Interferon beta promoter stimulator protein 1) (IPS-1) (Virus-induced- signaling adapter) (VISA) | Mitochondrion and Peroxisome | Other | −2.10 |
Table 2.
Top up regulated proteins differentially expressed associated with cognitive decline. A comparison of TAC-treated mice versus saline-treated mice identified 192 proteins as meeting statistical significance for differential expression.
| Protein | Description | Location | Type | Fold Change (log ratio) |
|---|---|---|---|---|
| Q5JC28 | Epidermal growth factor receptor pathway substrate 15 isoform B (Epidermal growth factor receptor substrate 15) | Other | 2.26 | |
| Q8CBF3 | Ephrin type-B receptor 1 (EC 2.7.10.1) | Plasma membrane, Endosomes, Dendrites | 3.73 | |
| D6RDE8 | Desumoylating isopeptidase 1 | Other | 2.19 | |
| Q7TQF2 | F-box only protein 10 | Other | 2.69 | |
| Q61112 | 45 kDa calcium-binding protein (Cab45) (Stromal cell-derived factor 4) (SDF-4) | Golgi apparatus | 3.71 | |
| A0A140LIJ4 | Dynein, axonemal, heavy chain 14 | Cytoskeleton | 5.00 |
Proteins, pathways, and protein networks affected by TAC treatment
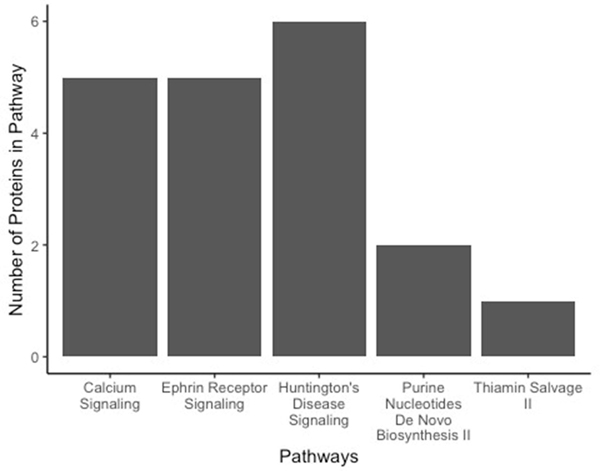
In order to gain insight on the potential pathways and networks involved in mediating these cognitive deficits, IPA analysis was conducted on all 195 proteins that were identified to be significantly differentially expressed between TAC-treated and saline-treated animals. IPA is a web-based software application that was used to extract meaningful biological information from our list of differentially expressed proteins. IPA was able to determine the top 5 canonical pathways affected by TAC treatment (calcium signaling, ephrin receptor signaling, Huntington’s disease signaling, purine nucleotides de novo biosynthesis II, and thiamine salvage II; Table 3). These top 5 pathways include proteins that have been disrupted due to TAC treatment (Figure 6). Additionally, all of the disrupted pathways have associations with neuronal function as well as learning and memory. IPA was also able to predict a nondirectional protein interaction map in the form of networks with associated functions (Table 4). These protein networks provide information on the interaction between proteins with similar functions (e.g., cell-to-cell signaling, cellular movement, and organismal injuries) while also providing indicators of the dysregulation of proteins within the network as a result of TAC treatment (Figure 7). Additional canonical pathways and protein networks that were disrupted due to TAC treatment can be found in supplementary material (Online Resource 2 and 3).
Table 3.
Top five pathways associated with memory deficits in TAC-treated mice. Overlapping proteins that met statistical significance for differential expression in TAC-treated animals (TAC vs. Saline) comparisons were uploaded into Ingenuity Pathway Analysis (IPA) to identify enriched pathways.
| Top Canonical Pathways | P-value |
|---|---|
| Purine Nucleotides De Novo Biosynthesis II | 1.84e-03 |
| Huntington’s Disease Signaling | 2.95e-03 |
| Ephrin Receptor Signaling | 4.37e-03 |
| Thiamin Salvage III | 5.91e-03 |
| Calcium Signaling | 7.65e-03 |
Figure 6.
Number of proteins determined to be dysregulated within each of top 5 pathways associated with memory deficits in TAC-treated mice.
Table 4.
Top five IPA protein networks associated with TAC treatment
| Network 1 | Associated network functions: cell to cell signaling and interaction, cellular movement, organismal injury and abnormalities Number of “focus molecules” contained in the network: 20 IPA p-score: 38* Network proteins: ANGPT1,APOH,ATF2,Akap9,Ap1,C18orf25,CG,Calcineurin protein(s),Creb,ERK1/2,GUCY1A1,HBA1/HBA2,HDL,IL12 (complex),JUND,KLF13,LDL,MAP2K1/2,MYL3, Mek,Mlc,Notch,PDE8B,PDGF BB,PDLIM1,PENK,PTN,Pka catalytic subunit,RHOC,SCGN,SERPINA1,TRPC6,TSC22D 3,TSH,UBQLN4 |
| Network 2 | Associated network functions: : Cellular Assembly and Organization, Cellular Function and Maintenance, Cellular Movement Number of “focus molecules” contained in the network: 20 IPA p-score: 38* Network proteins: 26s Proteasome, APC, Actin, Akt, Alpha catenin,Ap2 alpha,Ck2,DENND1A,DNM1,EPHA7,EPHB1,EPS 15,EXOSC5,FAM110B,GTPase,Hsp70,IFN Beta,Ifn,IgG2a,MAVS,N4BP 1,NUMA1,PHLDB1,RGMA,S100A10,SIPA1L1,SIPA1L3,SYNPO,TJP 2,TXLNA,Tgf beta,Ubiquitin,XPO6,caspase,tubulin |
| Network 3 | Associated network functions: Energy Production, Molecular Transport, Nucleic Acid Metabolism Number of “focus molecules” contained in the network: 16 IPA p-score: 29* Network proteins: APP,ATOX1,BCS1L,C11orf98,CCZ1/CCZ1B,CLOCK,CUL5,DCUN1D3,DNAH 11,DNAH14,DNAL1,DNALI1,ERBB2,ESR2,ETNPPL,FAM160A2,FSIP1,GABRA4,GART,GCC2,GPR3,HNF4A,ITM2C,KIAA1211L,LSM14B,MT-ATP6,NDUFA3,PEAK1,PPAT,PRPF39,RAI2,SOWAHC,TEN1,TSPAN 13,UBE2F |
| Network 4 | Associated network functions: Cancer, Cell Cycle, Protein Synthesis Number of “focus molecules” contained in the network: 15 IPA p-score: 26* Network proteins: AK2,CHCHD3,DIP2B,DNAJC11,EHHADH,EIF6,IPO8,ISLR2,KLHL13,KYAT3,MAGEE2,MRI1,MRPL20,MRPL21,MRPL35,MRPL39,MYDGF,NDUFA10,NTRK1,NUDT3,PANK4,PEAK1,PIGT,RANBP6,RHOT2,SAE1,SRSF11,TRAF6,UBA2,ULK2,UPF2,WBP11,WDR36,ZNF207,ZNF629 |
| Network 5 | Associated network functions: Developmental Disorder, Hereditary Disorder, Metabolic Disease Number of “focus molecules” contained in the network: 15 IPA p-score: 26* Network proteins: ADSSL1,AGAP3,ANKHD1/ANKHD 1 |
Using a proprietary algorithm, IPA generates these networks and adds proteins not contained within the original dataset. Additionally, IPA calculates a p-score, which indicates the probability of finding a given number of focus molecules in a given network selected randomly from IPA’s Global Molecular Network. The p-score (-log10 (p-value)) is calculated by the Fisher’s exact test.
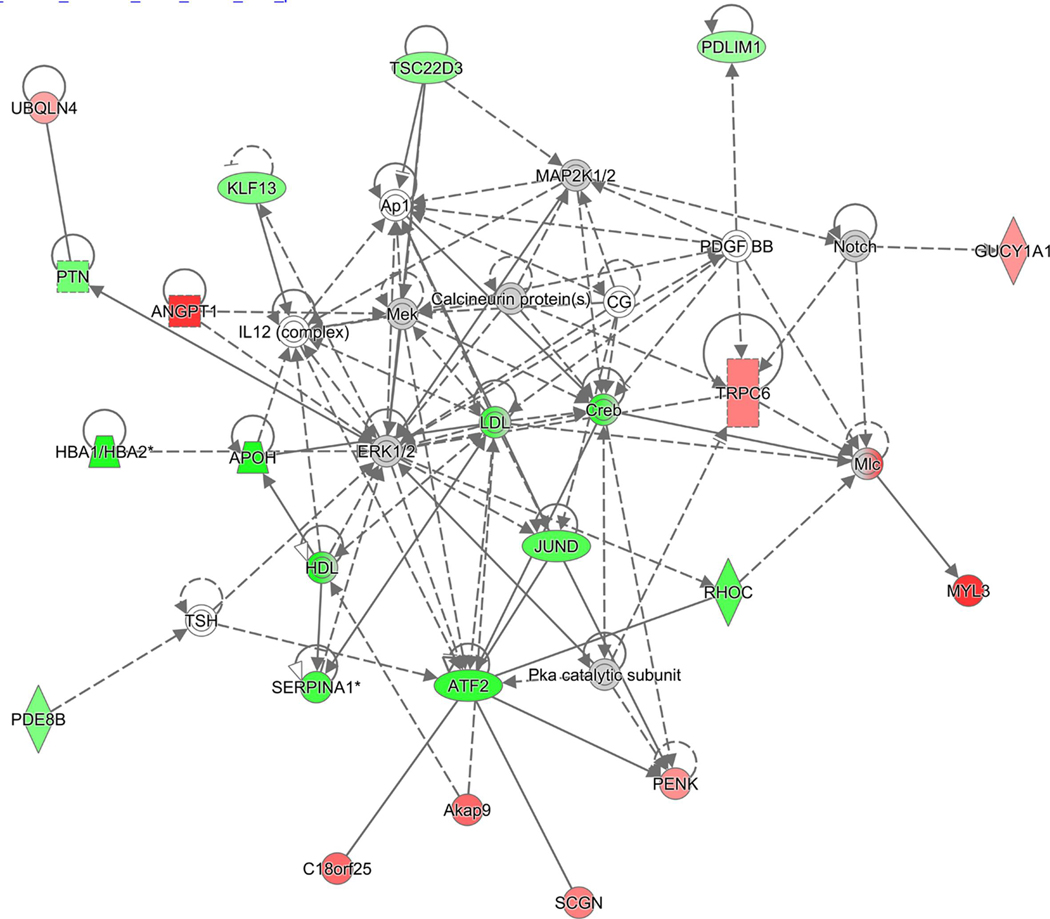
Figure 7. Graphic representation of mouse hippocampus protein network 1, identified by IPA as being affected by TAC.
The color of the node depicts differential expression. Red represents upregulated proteins. Green represents downregulated proteins. The intensity of the color denotes the degree of regulation where brighter colors are more regulated. Gray node color reflects proteins that were found in the data set but were insignificant expression wise. Uncolored nodes were not identified as differentially expressed in our data, but were incorporated into the computational network based on evidence stored in the Ingenuity Knowledge Base. Known direct and indirect interactions between network proteins, as well as the direction of the interaction, are indicated by arrows or blocked lines.
3. Discussion
In this study, we examined the effects of a TAC regimen on cognition, hippocampal dendritic morphology, and dysregulation of protein pathways. During the Y maze behavioral test, we observed that the TAC group was unable to distinguish the novel arm from the start and familiar arms, indicating that these mice experienced deficits in short-term memory retention when compared to the saline group. In the MWM, we observed a significant deficit in spatial memory retention in the TAC group when compared to the saline group. The saline group spent significantly more time in the target quadrant during the probe trials. The TAC group was unable to distinguish the target quadrant from the other quadrants during the probe trials. Finally, we observed decreases in dendritic complexity in the hippocampus of the TAC group compared to the saline group.
Chemotherapy-induced cognitive impairment has also been characterized in cancer-free rodent models. The AC combination has recently become a more relevant topic within research due to its potential effects on cognition. Kitamura et al. (2015) found that when male rats were treated with AC, they experienced impairments in hippocampal-dependent spatial cognition (based on assessments from the light-dark test) and anxiety-like behavior (based on assessments from location recognition test). Salas-Ramirez et al. (2015) observed similar hippocampal- dependent spatial memory deficits in female rodents when treated with AC.
Several rodent studies have found that taxanes (e.g., docetaxel) induce cognitive dysfunction, such as decline in hippocampal-dependent spatial memory and depression-like behaviors (Fardell, Vardy, and Johnston, 2013; Callaghan and O’Mara, 2015). Since the late 1990s, the use of taxanes has become more prevalent in breast cancer treatment. Currently, docetaxel and similar taxanes have become integrated into standard combination therapy regimens for the treatment of breast cancer. As such, understanding the effects that TAC has on cognition is extremely important.
There are a few in vivo studies that have studied the impact of TAC on cognitive deficits. Shi et al. (2019) discovered that TAC-treated mice spent significantly less time in the target quadrant of the MWM, indicating deficits in hippocampal-dependent spatial memory. We observed similar results in this study. Additionally, we used the Y maze paradigm to assess hippocampal-dependent short-term memory and observed a significant decrease in spatial recognition memory in the TAC-treated animals.
Many studies have observed a cognitive decline after chemotherapy; however, the mechanisms behind this phenomenon have remained elusive. Previous studies have found that changes in dendritic complexity impact cognitive function. Increases in complexity have been associated with improved cognitive function whereas decreases have been associated with cognitive declines (Dickstein et al., 2010; Groves et al., 2017). In this study, we measured dendritic morphology by assessing differences in dendritic branch points within the hippocampus after TAC treatment. Developing dendrites form connections with other neurons in a process called “dendritic arborization.” The length and pattern of these determines the amount of synaptic inputs that a dendrite is able to process. In previous studies, we have found that cognitive decline as a result of chemotherapy to be associated with cognitive decline (McElroy et al. 2020; Anderson et al. 2020). Consistent with previous studies, we found that dendrites within the DG of the hippocampus from the TAC group had significantly fewer branch points than that of the saline group. Fewer branch points typically infers decreases in dendritic complexity and, consequently, fewer synaptic connections resulting in cognitive deficits. Reduction in synaptic connections or plasticity within the hippocampus can be morphological indicators of cognitive impairment as such changes can be observed in neurological disorders (Cheung and Ip 2011).
The DG has been implicated as the region of new information input into the hippocampus, thus acting as a preprocessor of information that will be further processed in the circuitry of the hippocampus. The CA1, on the other hand, is where information exits the hippocampus. Interestingly, unlike previous studies we observed that throughout the cornu CA1 of the hippocampus there were significant increases in branch points and dendritic complexity.
A considerable amount of neurogenesis occurs within the DG compared to the CA1 (Gonçalves et al., 2016). Thus, a plausible explanation for this observation is that the DG is more sensitive to TAC than the CA1, or it is evidence of some type of compensatory mechanism within the hippocampus. Previous studies have observed differences in dendritic complexity between subregions of the hippocampus. One such study found that prenatal stress induced decreases in hippocampal dendritic complexity in the CA1 for both male and female rodents. However, in the DG they observed increases in complexity for males and a decrease for females (Bock et al., 2011).
Recently, with the introduction of ‘omics,’ there has been a shift in the approach towards cancer research to use molecular technologies to detect gene-based changes in the structure or function that may be impacting diagnosis, prognosis, and treatment of cancer. Currently, there are a few studies that have used omics technology as a tool to understand how chemotherapy may be inducing negative side effects (e.g., chemo brain at the molecular level). In this study, we used proteomic analysis to investigate which hippocampal regulatory protein processes may be disrupted as a result of TAC. Using the IPA analysis, we were able to identify significantly differentially expressed proteins that may be related to cognitive impairment (Table 1, 2). For example, we found that the MICOS complex subunit was down regulated, which is important for inner mitochondrial membrane structure; and thus, could potentially contribute to cognitive impairment induced by mitochondrial dysfunction (Friedman et al., 2015). We also see epidermal growth factor receptor substrate 15 upregulated, which is associated with postsynaptic neurotransmitter receptor internalization (Lin and Man, 2014).
IPA determined the top 5 dysregulated canonical pathways likely to be responsible for deficits in cognitive function (Table 3, Fig. 7). This included pathways such as purine metabolism and ephrin and calcium signaling, which have all been implicated in having a role in neuropathology (Brini et al., 2014; Ansoleaga et al., 2015; Dines and Lamprecht, 2016). Additionally, the IPA-network functions tool identified the 5 top affected protein networks that will be important for interpreting molecular effects of TAC on the hippocampus (Table 4). For example, the central node protein of network 1 (Fig. 7), cAMP-responsive element binding protein (CREB), has been implicated as a multifaceted regulator of neuronal plasticity and as having a role in both learning and memory (Sakamoto, Karelina, and Obrietan, 2011). CREB has also been found to regulate neuroprotection via the regulation of reactive oxygen species (ROS) detoxification. Another interesting central node protein in network 1, activating transcription factor 2 (ATF2) was downregulated in TAC group in our study and has been implicated in a number of mouse studies to have a role in neurological development, neuronal migration, and apoptotic neuronal cell death (Pearson et al., 2005). Together, this proteomic analysis has revealed that TAC induces dysregulation in a number of protein pathways and networks. These findings suggest that TAC may induce cognitive impairment through a number of compromised biological functions. Some potential limitations of this study are our drug administration method and the use of a rodent model in order to examine cognitive functioning processes. In breast cancer patients, chemotherapeutics are typically administered via intravenous (IV) injections in order to infuse drugs directly into the bloodstream. Administering drugs via IP may result in the drugs being first hepatically metabolized before reaching systemic circulation. Therefore, there is potential for the animals IP treated in this study to have differential responses to the drugs compared to IV. However, we believe IP to be a viable method (also less physically traumatic to the animals) as it has been shown to have therapeutic bioavailability and for the purposes of this study we were primarily concerned with the “proof of concept” on the effect of TAC on cognition than the pharmacokinetics for clinical translation (Al Shoyaib, Archie, and Karamyan 2019). Both humans and rodents differ considerably in their neuroanatomic architecture as humans are a higher order complex organisms comparatively. Even among rodent models there are notable differences between species as well as in between strains (Bohlen et al. 2014; Moy et al. 2008). For instance, with respect to neurogenesis in the hippocampus it has been found that the rate of neurogenesis in adult rats is larger than in mice, new cells matured 2 weeks earlier, twice as likely to escape cell death, and were ten times as likely to be activated during learning than in mice (Snyder et al. 2009). In terms of cognitive assessment rats have been found to be more consistently stable over time than mice, because they are less affected by non-cognitive distractions like stress and thigmotaxis (Ellenbroek and Youn 2016). Our choice of a mice model was done because of the amount of more established transgenic models that would allow for the potential for future mechanic studies in order to study the effects of TAC in different genetic backgrounds, however these differences among animal models are important to note. Additionally, we understand that cognition is a process that is extremely complicated not just limited to hippocampal-dependent function. In future studies, we would like to evaluate other dimensions of cognition by performing behavioral assays that target other cortical areas of the brain, such as the IntelliCage (Kiryk et al. 2020).
4. Conclusion
In conclusion, in adult female mice, the effects of TAC were clearly demonstrated with regard to learning and memory through the use of several behavioral assessments. Additionally, we analyzed changes in dendritic morphology and proteomic analysis of the TAC group. These data indicate that TAC induces cognitive dysfunction. In future studies, we would like to further assess the mechanisms underlying cognitive decline, such as oxidative stress. Understanding the relationship between chemotherapeutic agents and dysregulated molecular processes underlying cognitive decline will provide insight into potential prevention strategies. Because the posttreatment life expectancy of patients with breast cancer is increasing quite dramatically, it is important to consider their quality of life. Therefore, developing strategies to ameliorate chemotherapy-induced cognitive deficits is imperative to ensure the welfare of patients.
5. Methods
5.1. Animals
Thirty-two 12-week-old CB57BL/6J female mice purchased from Jackson labs were used, with 16 mice per group. 12-week old mice were chosen because they are mature adult age comparatively to humans and this avoided potential health complications with aging due to the long treatment period of 8 weeks. The mice were group housed (n=5/cage) under a constant 12- hour light: 12-hour dark cycle in a climate-controlled environment. Food and water were provided ad libitum. Mice housed together were the same treatment group and allowed one week of acclimation prior to injections. This study was approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences.
5.2. Chemotherapeutic Injections
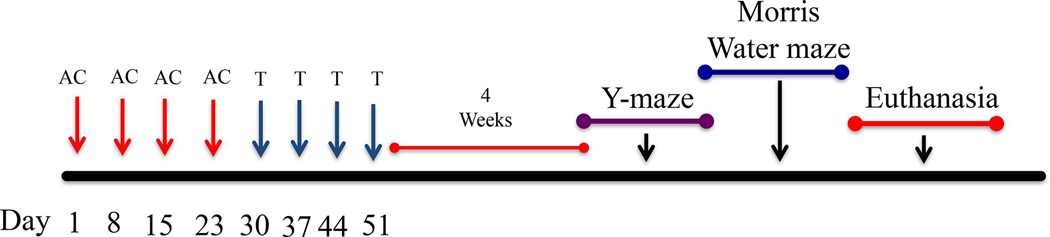
Mice were given an intraperitoneal (IP) injection weekly for 4 weeks of either saline (0.9% sodium chloride) or of a combination of Doxorubicin (2mg/kg) and Cyclophosphamide (50 mg/kg). The AC regimen was given over four weeks totaling four injections (Day 1, 8, 15, and 22) (Fig. 8). The next cycle of chemotherapy included weekly intraperitoneal injections of Docetaxel (8 mg/kg) for 4 weeks. This constituted the TAC regimen (Fig. 8). Weight and food consumption of each animal were recorded throughout the entire injection period (8 weeks) in order to assess if the treatment itself induced health decline in the animals (Fig. 1).
Figure 8. Graphical Representation of Experimental Design.
Behavioral testing was conducted on 12-week-old female CB57BL/6J mice.
Dosages were selected based on standard human chemotherapeutic doses. Patients receive four cycles of AC (doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2) followed by four cycles of docetaxel (25–35 mg/m2). To translate the clinical dose to animals, the body surface area normalization was used. Previous work by Flanigan et al (2018). showed that doxorubicin (2 mg/kg) and cyclophosphamide (50 mg/kg) was sufficient to cause changes in hippocampal morphology without acute toxicity. The dosages of Cyclophosphamide, doxorubicin and docetaxel translate to human equivalent doses that are below clinical dose. Drugs were diluted with sterile saline and stored per the manufacturer’s instructions. Drugs were mixed immediately prior to injections.
5.3. Behavioral Scheme
5.3.1. Y-Maze
In order to assess potential short-term spatial memory deficits induced by the TAC regimen, the Y maze behavioral paradigm was used. The Y maze consists of three arms (each 45 cm x 15 cm x 30 cm), which includes the start arm, the familiar arm, and the novel arm. The test is conducted in two trials, 4 hours apart, so that the mice are allowed to explore the arena. During the training trial, the mice were able to explore the start and familiar arms for 5 mins. During the testing trial the mice were allowed to explore all three of the arms of the arena. Spatial novelty for both treatment groups was determined by measuring the time spent in the novel arm and comparing it to the time spent in the start and familiar arms. The behavioral analysis was performed by recording all sessions using a charged-coupled device video camera positioned above the maze and the EthoVision XT video tracking system and software (version 11; Noldus Information Technology, Wageningen, Netherlands).
5.3.2. Morris Water Maze (MWM)
In order to measure the effects of the TAC regimen on hippocampal-dependent spatial learning and memory, the Morris water maze (MWM) paradigm was used. The maze consists of a circular pool (140 cm in diameter) filled with opaque water (24 °C). Mice were trained to locate a visible platform (luminescence at 200 lux). The behavioral analysis consisted of 3 trials completed over a period of 5 days. The EthoVision XT video tracking system and software (Noldus Information Technology) was used to record the distance moved and the average velocity of the animals in the visible platform, hidden platform, and probe trials.
The first trial, or the visible platform trial, measures the ability of the animal to learn a task. During this trial (days 1 and 2), each treatment group was run through 2 sessions (spaced 2 hours apart) per day for a total of 4 sessions. Each session consisted of 3 trials with the start location and platform location moving to different quadrants with each trial. Each animal was released into a quadrant and trained to locate the platform using visual cues positioned around the pool.
The second trial, or the hidden platform trial, measures the acquisition of spatial learning. This trial was performed days 3–5consistent with the visible platform trial; however, the water level was raised 1.5 cm to submerge the platform. For both the visible and hidden platform trials, the tests were terminated once the mouse found the platform or swam around the maze for 60 seconds. If the mouse was unable to find the platform, the examiner would place their finger in the maze to guide the mouse to the platform. The mouse remained in the maze until it stayed on the platform for 10 seconds.
The last trial, or the probe trial, was conducted one hour after the last run on each day of hidden platform trial (i.e., three separate probe trials days 3–5). The mice were released in the start location (placed in a quadrant opposite of the target quadrant, i.e., platform location) and were allowed to swim around the maze for 60 seconds. The amount of time spent in both of these quadrants was recorded and compared.
5.4. Tissue Preparation
The mice were sacrificed by cervical dislocation followed by decapitation. The brains were removed and dissected. The left hemisphere was dissected down to the olfactory bulb, cerebellum, hippocampus, prefrontal cortex, and cortex in cold phosphate buffered saline (1X PBS). The samples were snap frozen in liquid nitrogen followed by storage at −80°C. The right hemisphere was stored in Golgi solution
5.5. Molecular Assays
5.5.1. Golgi-Cox Staining
In order to quantify changes in dendritic morphology of TAC treated animals, the Golgi-Cox staining method was used and performed with the superGolgi Kit (Bioenno Tech, LLC, Santa Anna, CA). After sacrificing the animals, the brains were harvested. 5 Half brains per treatment group were processed for analysis. First, the samples were impregnated by immersing them in 5 to 10 mL of a mercuric chloride solution at room temperature for 2 weeks in darkness. The samples were then rinsed and immersed in the kit post-impregnation buffer for 2 full days (the buffer was renewed after the first day). The half brains were cut with a microtome into 150 μm sections in 1X phosphate buffered saline (PBS). The sections were placed in a 6-well plate and washed with 1 mL 0.01M PBS buffer (pH 7.4) with 0.3% Triton X-100 (PBS-T) while shaking at room temperature for 30 minutes. PBS-T was removed from the wells and replaced with 1 mL of an ammonium hydroxide solution; the samples were incubated while shaking at room temperature for 18 minutes. The ammonium hydroxide solution was discarded and the samples were immersed in 1 mL of poststaining buffer and incubated while shaking at room temperature for 18 minutes . Next, the poststaining buffer was removed, and the sections were washed 3 times for 10 minutes with PBST. The sections were then placed on 1% gelatin-coated slides and allowed to dry overnight at room temp. The mounted sections were washed in 100% ethanol 3 times while shaking for 5 minutes. Finally, the sections were washed 3 times for 5 minutes with xylene and cover slipped with Permount mounting medium (Thermo Fisher Scientific, Waltham, MA).
5.5.2. Dendritic Spine Density and Morphology
Dendritic spines were analyzed from coded Golgi impregnated brain sections of the dorsal hippocampus. The spines on the dendrites of the DG, CA1, and CA3 were examined. Neurons were chosen for analysis if they exhibited the following criteria: presence of nontruncated dendrites, consistent and dark Golgi staining along the entire extent of the dendrites, and relative isolation from neighboring neurons to avoid interference with analysis (Titus et al., 2007). Five dendritic segments, 20 nm in length, per neuron were analyzed; 5 to 7 neurons per brain were analyzed. Neurons were traced using the Neurolucida software program (MBF Bioscience, Williston, VT) and a 1003 oil immersion objective on a computerized stage.
5.5.3. Dendritic Morphology Quantification
Quantification of dendritic morphology (Sholl analysis, dendritic complexity index [DCI], total dendritic length, and number of branch points) was performed with Neurolucida Explorer (MBF Bioscience), the analytical software companion for Neurolucida. First, in order to characterize the morphological characteristics of the neurons, Sholl analysis was performed. Sholl analysis assesses the amount and distribution of an arbor at increasing distances from the neural soma (Sholl, 1953). For this study, the distance between each radius was set to 20 μm and the length of each dendritic branch within each progressively larger circle was counted from the soma. This was done in order to quantify the amount and distribution of dendritic material.
Following the Sholl analysis, the branch-point analysis was performed. Branch point analysis is a method of quantifying the number of bifurcations of dendrites and the order of points (Sholl, 1953; Pillai et al., 2012). Lower branch-point orders characterize proximal regions of the branch tree, whereas larger orders characterize distal regions. Branch point analysis was also used to assess the complexity of the dendritic tree. The DCI was calculated with the following equation in the DG and the CA1 and CA3: DCI = Σ (branch tip orders + number of branch tips) × (total dendritic length/total number of primary dendrites).
5.6. Behavior and Golgi Statistical Analysis
Data were expressed as Mean (+/−) SEM. All statistical analyses were conducted using GraphPad Prism 6.0 software (La Jolla, CA), and P < .5 was considered significant. For measures of dendritic intersections, mixed factors analysis of variance (ANOVA) tested for the effects of radiation (between the subjects variable) and distance from the cell body (the Sholl radius and the repeated measures variable) followed by Holm-Sidak post hoc tests, when appropriate. A one-way ANOVA followed by Holm-Sidak post hoc test was used to evaluate statistical differences between sham and chemotherapy treated groups in the Y-maze. In the MWM, visible and hidden platform learning curves were analyzed using a 2-way repeated measures ANOVA. The Holm correction was used to control for multiple comparisons. Separate analyses were conducted for the visible and hidden platform learning curves. For analysis of performance in the MWM probe trials, a one-way ANOVA was used along with Holm-Sidak post hoc test, when appropriate. Differences were considered to be statistically significant when P < .05.
5.7. Proteomics
5.7.1. Tissue preparation
The day after the last TAC injection, a small cohort of mice (n = 4) was sacrificed for immediate molecular analysis. The hippocampus was removed and placed in 400 μl of RIPA lysis buffer (10 mM Tris-Cl pH 8.0, 1 mM EDTA, 0.5 M EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl). The tissue was homogenized on ice, incubated for 30 minutes on ice, and then centrifuged at 20,000 × g for 10 minutes at 4 °C. The supernatant was transferred to a new microcentrifuge tube and stored at −80 °C until processing. The Compat- Able Protein Assay Preparation Reagent Kit (Thermo Fisher Scientific) was used to eliminate EGTA as an interfering substance for the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Protein was separated by SDS-PAGE using the 4–15% Criterion TGX Precast Midi Protein Gel, 18-well, 30μL (Bio-Rad, Hercules, CA) run at 120V for 75 minutes. The gel was stained using Coomassie blue staining (Bio-Rad). The samples were processed by the UAMS Proteomics Core for further mass spectrometry analysis.
5.7.2. Liquid chromatography with tandem mass spectrometry analysis
Each SDS-PAGE gel lane was sectioned into 12 segments of equal volume. Each segment was subjected to in-gel trypsin digestion. The gel slices were destained in 50% methanol (Thermo Fisher Scientific) and 100 mM ammonium bicarbonate (Sigma-Aldrich, St. Louis, MO) followed by reduction in 10 mM Tris[2-carboxyethyl] phosphine (Thermo Fisher Scientific) and alkylation in 50 mM iodoacetamide (Sigma-Aldrich). The gel slices were dehydrated in acetonitrile (Thermo Fisher Scientific), followed by the addition of 100 ng porcine sequencing grade modified trypsin (Promega, Madison, WI) in 100 mM ammonium bicarbonate (Sigma- Aldrich) and incubated at 37 °C for 12 to 16 hours. Peptide products were acidified in 0.1% formic acid (Thermo Fisher Scientific). Tryptic peptides were separated by reverse phase chromatography using the XSelect CSH C18 2.5 ^m resin (Waters Corporation, Milford, MA) on an in-line 150 × 0.075 mm column using the nanoAcquity UPLC system (Waters Corporation). Peptides were eluted using a 30-minute gradient from 97:3 to 67:33 buffer A:B ratio (buffer A is 0.1% formic acid, 0.5% acetonitrile; buffer B is 0.1% formic acid, 99.9% acetonitrile). Eluted peptides were ionized by electrospray (2.15 kV) followed by tandem mass spectrometry (MS/MS) analysis using higher-energy collisional dissociation (HCD) on an Orbitrap Fusion Tribrid mass spectrometer (FTMS; Thermo Fisher Scientific) in top-speed data- dependent mode. MS data were acquired using the FTMS analyzer in profile mode at a resolution of 240,000 over a range of 375 to 1500 m/z. Following HCD activation, MS/MS data were acquired using the ion trap analyzer in centroid mode and normal mass range with precursor mass-dependent normalized collision energy between 28.0 and 31.0.
5.7.3. Data analysis
Proteins were identified and quantified by searching the UniprotKB database restricted to Mus musculus using MaxQuant (version 1.6.5.0; Max Planck Institute, Munich, Germany). The database search parameters included the MS1 reporter type, trypsin digestion with up to two missed cleavages, fixed modifications for carbamidomethyl of cysteine, variable modifications for oxidation on methionine and acetyl on N-terminus, the precursor ion tolerance of 5 ppm for the first search and 3 ppm for the main search, and label-free quantitation with iBAQ. Peptide and protein identifications were accepted using the 1.0% false discovery rate (FDR) identification threshold. Protein probabilities were assigned by the Protein Prophet algorithm (Nesvizhskii et al., 2003).
MaxQuant iBAQ intensities for each sample were median normalized so the medians of each sample were equal to the sample with the maximum median. Median normalized iBAQ intensities were imported into Perseus version 1.6.1.3 (Max Planck Institute) to perform log2 transformation and impute the missing values using a normal distribution with a width of 0.3 and a downshift of 2 standard deviations. The linear models for microarray data Bioconductor package was used to calculate differential expression among the experimental conditions using the lmFit and eBayes options (Ritchie et al., 2015). Proteins were considered to be significantly different with a fold change greater than 2 and an FDR adjusted P < 0.05. Differentially expressed proteins were analyzed using Ingenuity Pathway Analysis (IPA; QIAGEN, Germantown, MD) to identify pathways.
Supplementary Material
Funding:
This work was supported by Grant under NIH P20 GM109005 (ARA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors would like to acknowledge the University of Arkansas for Medical Sciences Proteomics Core Facility and the Arkansas Children’s Research Institute Systems Biology Bioinformatics Core supported by National Institute of General Medical Sciences grant P20GM121293.
Footnotes
Conflict of Interest: None of the authors has competing financial interests or other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akram Muhammad, Iqbal Mehwish, Daniyal Muhammad, and Asmat Ullah Khan. 2017a. “Awareness and Current Knowledge of Breast Cancer.” Biological Research 50 (1): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram Muhammad, Iqbal Mehwish, Daniyal Muhammad, and Asmat Ullah Khan. 2017b. “Awareness and Current Knowledge of Breast Cancer.” Biological Research 50 (1): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyaib Al, Abdullah Sabrina Rahman Archie, and Karamyan Vardan T.. 2019. “Intraperitoneal Route of Drug Administration: Should It Be Used in Experimental Animal Studies?” Pharmaceutical Research 37 (1): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson Julie E., Trujillo Madison, Taylor McElroy, Groves Thomas, Alexander Tyler, Kiffer Frederico, and Allen Antiño R.. 2020. “Early Effects of Cyclophosphamide, Methotrexate, and 5-Fluorouracil on Neuronal Morphology and Hippocampal-Dependent Behavior in a Murine Model.” Toxicological Sciences: An Official Journal of the Society of Toxicology 173 (1): 156–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansoleaga Belén, Mariona Jové Agatha Schlüter, Paula Garcia-Esparcia, Jesús Moreno, Pujol Aurora, Pamplona Reinald, Manuel Portero-Otín, and Isidre Ferrer. 2015. “Deregulation of Purine Metabolism in Alzheimer’s Disease.” Neurobiology of Aging 36 (1): 68–80. [DOI] [PubMed] [Google Scholar]
- Bock J, Sriti Murmu M, Biala Y, Weinstock M, and Braun K. 2011. “Prenatal Stress and Neonatal Handling Induce Sex-Specific Changes in Dendritic Complexity and Dendritic Spine Density in Hippocampal Subregions of Prepubertal Rats.” Neuroscience 193 (October): 34–43. [DOI] [PubMed] [Google Scholar]
- Bohlen Martin, Hayes Erika R., Bohlen Benjamin, Bailoo Jeremy D., Crabbe John C., and Wahlsten Douglas. 2014. “Experimenter Effects on Behavioral Test Scores of Eight Inbred Mouse Strains under the Influence of Ethanol.” Behavioural Brain Research 272 (October): 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini Marisa, Cali Tito, Ottolini Denis, and Carafoli Ernesto. 2014. “Neuronal Calcium Signaling: Function and Dysfunction.” Cellular and Molecular Life Sciences. 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan Charlotte K., and O’Mara Shane M.. 2015. “Long-Term Cognitive Dysfunction in the Rat Following Docetaxel Treatment Is Ameliorated by the Phosphodiesterase-4 Inhibitor, Rolipram.” Behavioural Brain Research 290 (September): 84–89. [DOI] [PubMed] [Google Scholar]
- Cheung Zelda H., and Ip Nancy Y.. 2011. “From Understanding Synaptic Plasticity to the Development of Cognitive Enhancers.” The International Journal of Neuropsychopharmacology / Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum 14 (9): 1247–56. [DOI] [PubMed] [Google Scholar]
- Christie Lori-Ann, Acharya Munjal M., Parihar Vipan K., Nguyen Anna, Martirosian Vahan, and Limoli Charles L.. 2012. “Impaired Cognitive Function and Hippocampal Neurogenesis Following Cancer Chemotherapy.” Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 18 (7): 1954–65. [DOI] [PubMed] [Google Scholar]
- DeVita Vincent T., and Chu Edward. 2008. “A History of Cancer Chemotherapy.” Cancer Research. 10.1158/0008-5472.can-07-6611. [DOI] [PubMed] [Google Scholar]
- Dickstein Dara L., Brautigam Hannah, Stockton Steven D., Schmeidler James, and Hof Patrick R.. 2010. “Changes in Dendritic Complexity and Spine Morphology in Transgenic Mice Expressing Human Wild-Type Tau” Brain Structure and Function. 10.1007/s00429-010-0245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich Joerg, Han Ruolan, Yang Yin, Margot Mayer-Proschel, and Mark Noble. 2006. “CNS Progenitor Cells and Oligodendrocytes Are Targets of Chemotherapeutic Agents in Vitro and in Vivo.” Journal of Biology 5 (7): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dines Monica, and Lamprecht Raphael. 2016. “The Role of Ephs and Ephrins in Memory Formation.” The International Journal of Neuropsychopharmacology / Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum 19 (4). 10.1093/ijnp/pyv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Lapinte N, Villier V, Lecointre C, Roy V, Tonon M-C, Gandolfo P, Joly F, Hilber P, and Castel H. 2014. “Chemotherapy-Induced Long-Term Alteration of Executive Functions and Hippocampal Cell Proliferation: Role of Glucose as Adjuvant.” Neuropharmacology 79 (April): 234–48. [DOI] [PubMed] [Google Scholar]
- Ellenbroek Bart, and Youn Jiun. 2016. “Rodent Models in Neuroscience Research: Is It a Rat Race?” Disease Models & Mechanisms 9 (10): 1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadi Ashkan, Jones Richard J., and Brodsky Robert A.. 2009. “Cyclophosphamide and Cancer: Golden Anniversary.” Nature Reviews. Clinical Oncology 6 (11): 638–47. [DOI] [PubMed] [Google Scholar]
- Encinas Juan M., Hamani Clement, Lozano Andres M., and Enikolopov Grigori. 2011. “Neurogenic Hippocampal Targets of Deep Brain Stimulation.” The Journal of Comparative Neurology 519 (1): 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardell Joanna E., Vardy Janette, and Johnston Ian N.. 2013. “The Short and Long Term Effects of Docetaxel Chemotherapy on Rodent Object Recognition and Spatial Reference Memory.” Life Sciences. 10.1016/jjfs.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Flanigan Timothy J., Anderson Julie E., Elayan Ikram, Allen Antiño R., and Ferguson Sherry A.. 2018. “Effects of Cyclophosphamide And/or Doxorubicin in a Murine Model of Postchemotherapy Cognitive Impairment.” Toxicological Sciences: An Official Journal of the Society of Toxicology 162 (2): 462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman Jonathan R., Mourier Arnaud, Yamada Justin, McCaffery J. Michael, and Jodi Nunnari. 2015. “MICOS Coordinates with Respiratory Complexes and Lipids to Establish Mitochondrial Inner Membrane Architecture.” eLife 4 (April). 10.7554/eLife.07739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves J. Tiago J. Tiago Gon9alves, Schafer Simon T., and Gage Fred H.. 2016. “Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior.” Cell. 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Gottlieb JA, and Hill CS Jr. 1974. “Chemotherapy of Thyroid Cancer with Adriamycin. Experience with 30 Patients.” The New England Journal of Medicine 290 (4): 193–97. [DOI] [PubMed] [Google Scholar]
- Greenspan EM, Fieber M, Lesnick G, and Edelman S. 1963. “Response of Advanced Breast Carcinoma to the Combination of the Antimetabolite, Methotrexate, and the Alkylating Agent, Thio-TEPA.” Journal of the Mount Sinai Hospital, New York 30 (May): 246–67. [PubMed] [Google Scholar]
- Groves Thomas R., Farris Ryan, Anderson Julie E., Alexander Tyler C., Kiffer Frederico, Carter Gwendolyn, Wang Jing, Boerma Marjan, and Allen Antiño R.. 2017. “5-Fluorouracil Chemotherapy Upregulates Cytokines and Alters Hippocampal Dendritic Complexity in Aged Mice.” Behavioural Brain Research 316 (January): 215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenard Daniel, Francoise Gueritte-Voegelein, and Pierre Potier. 1993. “Taxol and Taxotere: Discovery, Chemistry, and Structure-Activity Relationships.” Accounts of Chemical Research. 10.1021/ar00028a005. [DOI] [Google Scholar]
- Inagaki Masatoshi, Yoshikawa Eisho, Matsuoka Yutaka, Sugawara Yuriko, Nakano Tomohito, Akechi Tatsuo, Wada Noriaki, Imoto Shigeru, Murakami Koji, and Uchitomi Yosuke. 2007. “Smaller Regional Volumes of Brain Gray and White Matter Demonstrated in Breast Cancer Survivors Exposed to Adjuvant Chemotherapy.” Cancer 109 (1): 146–56. [DOI] [PubMed] [Google Scholar]
- Janelsins Michelle C., Roscoe Joseph A., Berg Michel J., Thompson Bryan D., Gallagher Mark J., Morrow Gary R., Heckler Charles E., Jean-Pierre Pascal, Opanashuk Lisa A., and Gross Robert A.. 2010. “IGF-1 Partially Restores Chemotherapy-Induced Reductions in Neural Cell Proliferation in Adult C57BL/6 Mice.” Cancer Investigation 28 (5): 544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryk Anna, Janusz Artur, Zglinicki Bartosz, Turkes Emir, Knapska Ewelina, Konopka Witold, Lipp Hans-Peter, and Kaczmarek Leszek. 2020. “IntelliCage as a Tool for Measuring Mouse Behavior - 20 Years Perspective.” Behavioural Brain Research 388 (June): 112620. [DOI] [PubMed] [Google Scholar]
- Kitamura Yoshihisa, Hattori Sayo, Yoneda Saori, Watanabe Saori, Kanemoto Erika, Sugimoto Misaki, Kawai Toshiki, et al. 2015. “Doxorubicin and Cyclophosphamide Treatment Produces Anxiety-like Behavior and Spatial Cognition Impairment in Rats: Possible Involvement of Hippocampal Neurogenesis via Brain-Derived Neurotrophic Factor and Cyclin D1 Regulation.” Behavioural Brain Research 292 (October): 184–93. [DOI] [PubMed] [Google Scholar]
- Lee Jun H., and Nan Anjan. 2012a. “Combination Drug Delivery Approaches in Metastatic Breast Cancer.” Journal of Drug Delivery. 10.1155/2012/915375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Jun H., and Nan Anjan. 2012b. “Combination Drug Delivery Approaches in Metastatic Breast Cancer.” Journa of Drug Delivery. 10.1155/2012/915375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Jun H., and Nan Anjan. 2012c. “Combination Drug Delivery Approaches in Metastatic Breast Cancer.” Journal of Drug Delivery. 10.1155/2012/915375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Jun H., and Nan Anjan. 2012d. “Combination Drug Delivery Approaches in Metastatic Breast Cancer.” Journal of Drug Delivery. 10.1155/2012/915375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Jun H., and Nan Anjan. 2012e. “Combination Drug Delivery Approaches in Metastatic Breast Cancer.” Journal of Drug Delivery. 10.1155/2012/915375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Amy, and Man Heng-Ye. 2014. “Endocytic Adaptor Epidermal Growth Factor Receptor Substrate 15 (Eps15) Is Involved in the Trafficking of Ubiquitinated a-Amino-3-Hydroxy-5- Methyl-4-Isoxazolepropionic Acid Receptors.” The Journal of Biological Chemistry 289 (35): 24652–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire Eleanor A., and Mullally Sinéad L.. 2013. “The Hippocampus: A Manifesto for Change.” Journal of Experimental Psychology. General 142 (4): 1180–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin Miguel. 2006. “Docetaxel, Doxorubicin and Cyclophosphamide (the TAC Regimen): An Effective Adjuvant Treatment for Operable Breast Cancer.” Women’s Health. 10.2217/17455057.2A527. [DOI] [PubMed] [Google Scholar]
- McElroy Taylor, Brown Taurean, Kiffer Fred, Wang Jing, Byrum Stephanie D., Oberley-Deegan Rebecca E., and Allen Antiño R.. 2020. “Assessing the Effects of Redox Modifier MnTnBuOE-2-PyP 5+ on Cognition and Hippocampal Physiology Following Doxorubicin, Cyclophosphamide, and Paclitaxel Treatment.” International Journal of Molecular Sciences 21 (5). 10.3390/ijms21051867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Kimberly D., Siegel Rebecca L., Chun Chieh Lin, Angela B. Mariotto, Kramer Joan L., Rowland Julia H., Stein Kevin D., Alteri Rick, and Jemal Ahmedin. 2016a. “Cancer Treatment and Survivorship Statistics, 2016.” CA: A Cancer Journal for Clinicians 66 (4): 271–89. [DOI] [PubMed] [Google Scholar]
- Miller Kimberly D., Siegel Rebecca L., Chun Chieh Lin, Mariotto Angela B., Kramer Joan L., Rowland Julia H., Stein Kevin D., Alteri Rick, and Jemal Ahmedin. 2016b. “Cancer Treatment and Survivorship Statistics, 2016.” CA: A Cancer Journal for Clinicians 66 (4): 271–89. [DOI] [PubMed] [Google Scholar]
- Moy Sheryl S., Nadler Jessica J., Young Nancy B., Nonneman Randal J., Segall Samantha K., Andrade Gabriela M., Crawley Jacqueline N., and Magnuson Terry R.. 2008. “Social Approach and Repetitive Behavior in Eleven Inbred Mouse Strains.” Behavioural Brain Research 191 (1): 118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel Lynn, Hoscheidt Siobhan, and Ryan Lee R.. 2013. “Spatial Cognition and the Hippocampus: The Anterior-Posterior Axis.” Journal of Cognitive Neuroscience 25 (1): 22–28. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii Alexey I., Keller Andrew, Kolker Eugene, and Aebersold Ruedi. 2003. “A Statistical Model for Identifying Proteins by Tandem Mass Spectrometry.” Analytical Chemistry 75 (17): 4646–58. [DOI] [PubMed] [Google Scholar]
- Pearson AG, Curtis MA, Waldvogel HJ, Faull RLM, and Dragunow M. 2005. “Activating Transcription Factor 2 Expression in the Adult Human Brain: Association with Both Neurodegeneration and Neurogenesis.” Neuroscience 133 (2): 437–51. [DOI] [PubMed] [Google Scholar]
- Petrylak Daniel P., Tangen Catherine M., Hussain Maha H. A., Lara Primo N. Jr, Jones Jeffrey A., Taplin Mary Ellen, Burch Patrick A., et al. 2004. “Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer.” The New England Journal of Medicine 351 (15): 1513–20. [DOI] [PubMed] [Google Scholar]
- Peukert Xenia, Steindorf Karen, Schagen Sanne B., Runz Adrian, Meyer Patric, and Zimmer Philipp. 2020. “Hippocampus-Related Cognitive and Affective Impairments in Patients With Breast Cancer-A Systematic Review.” Frontiers in Oncology 10 (February): 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai Anup G., de Jong Danielle, Sofia Kanatsou, Krugers Harm, Knapman Alana, Heinzmann Jan-Michael, Holsboer Florian, Landgraf Rainer, Joels Marian, and Touma Chadi. 2012. “Dendritic Morphology of Hippocampal and Amygdalar Neurons in Adolescent Mice Is Resilient to Genetic Differences in Stress Reactivity.” PloS One 7 (6): e38971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter David. 2007. “Faculty of 1000 Evaluation for Cisplatin and Fluorouracil Alone or with Docetaxel in Head and Neck Cancer.” F1000 - Post-Publication Peer Review of the Biomedical Literature. https://doi.org/10.34107f.1097013.552978 [Google Scholar]
- Ranganath Charan, and Ritchey Maureen. 2012. “Two Cortical Systems for Memory-Guided Behaviour.” Nature Reviews. Neuroscience 13 (10): 713–26. [DOI] [PubMed] [Google Scholar]
- Ritchie Matthew E., Phipson Belinda, Wu Di, Hu Yifang, Law Charity W., Shi Wei, and Smyth Gordon K.. 2015. “Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies.” Nucleic Acids Research. 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Kensuke, Karelina Kate, and Obrietan Karl. 2011. “CREB: A Multifaceted Regulator of Neuronal Plasticity and Protection.” Journal of Neurochemistry 116 (1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Ramirez Kaliris Y., Bagnall Ciara, Frias Leslie, Abdali Syed A., Ahles Tim A., and Hubbard Karen. 2015. “Doxorubicin and Cyclophosphamide Induce Cognitive Dysfunction and Activate the ERK and AKT Signaling Pathways.” Behavioural Brain Research 292 (October): 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz Christian, and Engelhardt Maren. 2014. “Anatomy of the Hippocampal Formation.” Frontiers of Neurology and Neuroscience 34 (April): 6–17. [DOI] [PubMed] [Google Scholar]
- Seigers Riejanne, and Fardell Joanna E.. 2011. “Neurobiological Basis of Chemotherapy- Induced Cognitive Impairment: A Review of Rodent Research.” Neuroscience & Biobehavioral Reviews. 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Shi Dong-Dong, Huang Yu-Hua, Cora Sau Wan Lai, Dong Celia M., Ho Leon C., Wu Ed X., Li Qi, et al. 2019. “Chemotherapy-Induced Cognitive Impairment Is Associated with Cytokine Dysregulation and Disruptions in Neuroplasticity.” Molecular Neurobiology 56 (3): 2234–43. [DOI] [PubMed] [Google Scholar]
- Sholl DA 1953. “Dendritic Organization in the Neurons of the Visual and Motor Cortices of the Cat.” Journal of Anatomy 87 (4): 387–406. [PMC free article] [PubMed] [Google Scholar]
- Snyder Jason S., Choe Jessica S., Clifford Meredith A., Jeurling Sara I., Hurley Patrick, Brown Ashly, Kamhi J. Frances, and Cameron Heather A.. 2009. “Adult-Born Hippocampal Neurons Are More Numerous, Faster Maturing, and More Involved in Behavior in Rats than in Mice.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 29 (46): 14484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus ADJ, Shankaranarayana Rao BS, Harsha HN, Ramkumar K, Srikumar BN, Singh SB, Chattarji S, and Raju TR. 2007. “Hypobaric Hypoxia-Induced Dendritic Atrophy of Hippocampal Neurons Is Associated with Cognitive Impairment in Adult Rats.” Neuroscience 145 (1): 265–78. [DOI] [PubMed] [Google Scholar]
- Tripathy D. 2006. “3–49 Adjuvant Docetaxel or Vinorelbine With or Without Trastuzumab for Breast Cancer.” Breast Diseases: A Year Book Quarterly. 10.1016/s1043-321x(06)80537-0. [DOI] [Google Scholar]
- Yang Miyoung, and Moon Changjong. 2015. “Effects of Cancer Therapy on Hippocampus- Related Function.” Neural Regeneration Research 10 (10): 1572–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.