Abstract
Purpose of the review:
Human Immunodeficiency Virus (HIV)-associated neurocognitive disorders (HAND) continues to be prevalent in people living with HIV despite antiretroviral therapy. However, understanding disease mechanisms and identifying therapeutic avenues has been challenging. One of the challenges is that HAND is a heterogeneous disease and that patients identified with similar impairments phenotypically may have very different underlying disease processes. As the NeuroAIDS field is re-evaluating the approaches used to identify patients with HIV-associated neurological impairments we propose the subtyping of patients into biotypes based on viral and immune pathogenesis.
Recent findings:
Here we review the evidence supporting subtyping patients with HIV-associated neurological complications into four biotypes: (1) Macrophage-mediated HIV encephalitis, (2) CNS viral escape, (3), T cell-mediated HIV encephalitis, and (4) HIV protein-associated encephalopathy.
Summary:
Subtyping patients into subgroups based on biotypes has emerged as a useful approach for studying heterogeneous diseases. Understanding biotypes of HIV-associated neurocognitive impairments may therefore enable better understanding of disease mechanisms, allow for the development of prognostic and diagnostic markers, and could ultimately guide therapeutic decisions.
Keywords: HIV, HIV-associated neurological disorders, biotypes, NeuroAIDS, encephalitis, immune reconstitution inflammatory syndrome
Introduction
Subtyping patients into groups based on biological disease features, or biotypes, has emerged as a useful approach for studying heterogeneous diseases. Biotypes based on immune specificities have long been established in rheumatic diseases and are reliable predictors of prognosis and treatment response [1, 2]. Biotypes are also being applied to neurologic diseases including cancer related cognitive impairment [3] and autism spectrum disorder, where outcomes are improved when biotype-specific therapies are implemented [4]. Further, biotypes identified by imaging studies in depression were shown to predict response to treatment [5]. Understanding biotypes of neurocognitive impairments may therefore enable better disease trajectory prediction and could ultimately guide therapeutic decisions.
Human immunodeficiency virus (HIV)-associated neurocognitive disorders (HAND), is a heterogeneous disease which occurs in a subset of people living with HIV, even those well controlled on antiretroviral therapy (ART) [6]. Patients with HAND have neurocognitive and motor function deficits which are classified as Asymptomatic Neurocognitive Impairment (ANI), HIV-associated Mild Neurocognitive Disorder (MND), and HIV-Associated Dementia (HAD) [7]. These impairments represent a major quality of life issue for patients, however understanding disease mechanisms and identifying therapeutic avenues has been challenging. One reason for this is that the defined phenotypes (ANI, MND, and HAD) are themselves heterogeneous. The bulk of patients with HAND have ANI may be functionally cognitively normal. The large number of patients categorized as having ANI is likely due to outdated criteria resulting in an over-estimation of the true burden of neurocognitive deficits in people living with HIV [8**, 9*]. Including patients with ANI within the umbrella of HAND may therefore mask important biological findings as these patients may not be impaired. Further, patients classified as having MND or HAD have very different diseases, disease trajectory, and disease manifestations and can be subtyped into profiles based on types of deficits [10-14]. Therefore, defining disease mechanisms within MND or HAD may be obscured by disease heterogeneity. This is further complicated by the fact that not all patients are treated with antiretroviral drugs and when treatment is initiated, it may be at different stages of the illness which would further contribute to the heterogeneity of the disease. The choice of antiretroviral drugs and their variable penetration into the brain can further impact the neuropathogenesis of the infection. As in other diseases, clarity may be found by further sub-setting patients into biotypes.
Biotypes of HIV-Associated Cognitive Impairments
Although multiple approaches to subtyping patients with HAND are possible, including clinical assessment and presence of symptoms [8] or imaging findings [15], in this manuscript we focus on the biotypes of HIV-associated cognitive impairments based on viral and immune pathogenesis. Specifically, we propose four distinct biotypes of HAND that may have some overlap: (1) Macrophage-mediated HIV encephalitis, (2) CNS viral escape, (3) T cell-mediated HIV encephalitis, and (4) HIV protein-associated encephalopathy (Table 1). Subtyping patients into these categories may facilitate a better understanding of disease mechanisms that underlie each phenotype, ultimately leading to interventions that target the virologic or immunologic process driving the neurologic damage and impairments.
Table 1.
Key immune, viral, and pathology features of proposed biotypes of HIV-associated neurologic impairments.
| Biotype | Macrophage- mediated HIV encephalitis |
CNS viral escape | T cell-mediated HIV encephalitis |
HIV protein- associated encephalopathy |
|---|---|---|---|---|
| Clinical features | Subacute subcortical dementia | Headache, tremors, cognitive impairment, confusion, focal neurologic deficits, and seizures | Diverse symptoms. Can include sensory and visual changes, headache, confusion, cognitive impairment, seizures and coma | Slowly progressive cognitive and psychomotor impairments |
| ART status | Untreated. | Treated. Associated with low CNS drug penetration, poor drug compliance, and drug resistant viral mutations | Treated. | Treated. |
| Immune profile | CD4 T cells <200/mm3; elevated markers of macrophage activation in CSF | Asymptomatic: none; secondary and symptomatic: lymphocytic pleocytosis | Low CD4+ T cell nadir (<100 cells/μL) prior to ART, rapid immune restoration after initiation of ART; Lymphocytic pleocytosis(CD4+ or CD8+); microglial activation | Neuroinflammation, lymphocytic infiltration is possible. Microglial and astrocyte activation. |
| Viral profile | Viral load elevated in blood and CSF | Viral load elevated in CSF; ART resistant mutations | Often associated with opportunistic infections; HIV can be present in CSF or brain | Undetectable viral loads, but viral proteins (Tat, Nef, gp120, Vpr, and Gag) detectable in CSF |
| Pathology | Macrophage infiltration; multinucleated giant cells infected with HIV; astrocyte infection, neurodegeneration | Lymphocytic infiltrate into the CNS. | CD4+ or CD8+ immune infiltrate that can be both perivascular and diffuse into the parenchyma | Neuronal loss, Aβ and Tau deposits |
| Neuroimaging | Diffuse periventricular hyperintensities in white matter | White matter hyperintensities with deep brain nuclei involvement and enhancement | diffuse white matter hyperintensities with mild edema | Brain atrophy |
| Treatment | ART with CNS penetration | Changes to ART to enhance CNS penetration or overcome viral mutations. Treatment of secondary infection. | Treatment of opportunistic infection and corticosteroids. | None currently available. |
Aβ - Amyloid beta, ART – Antiretroviral therapy, CSF - Cerebrospinal fluid, CNS – Central nervous system
Macrophage-mediated HIV encephalitis
In individuals who have not been treated with antiretroviral drugs, macrophage infiltration and the presence of multinucleated giant cells is considered the hallmark of HIV infection in the brain [16]. These cells are predominantly in the perivascular region and some but not all are productively infected with HIV. In some individuals, perivascular astrocytes also contain the virus, however these cells have a restricted or latent viral infection [17]. There is also evidence of microglial cell activation, compromise of the blood brain barrier as indicated by leakage of serum proteins, and neuronal injury. Macrophage activation markers such CCL-2, tumor necrosis factor (TNF)-α, and neopterin can be detected in the cerebrospinal fluid, the dynamics of which change over the course of disease [18*]. MRI scans show atrophy of the brain with periventricular diffuse hyperintensities on T2 weighted images or FLAIR sequences (Figure 1 and reviewed in [19, 20]). These individuals often have severe cognitive impairment and bradykinesia or Parkinsonism and present with a subcortical dementia. They are usually severely immunosuppressed (CD4 cell counts <200 cells/mm3) with high viral loads. If they remain untreated, they may die within a few months from the onset of the dementing illness. This syndrome develops in nearly 20-30% of untreated immunosuppressed individuals. It has been termed HIV encephalitis, AIDS dementia complex or HIV-associated dementia (HAD) in the literature [16, 21]. However, we propose that the term, macrophage-mediated viral encephalitis may be more appropriate to distinguish it from other forms of encephalitis seen in individuals treated with antiretroviral drugs.
Figure 1.
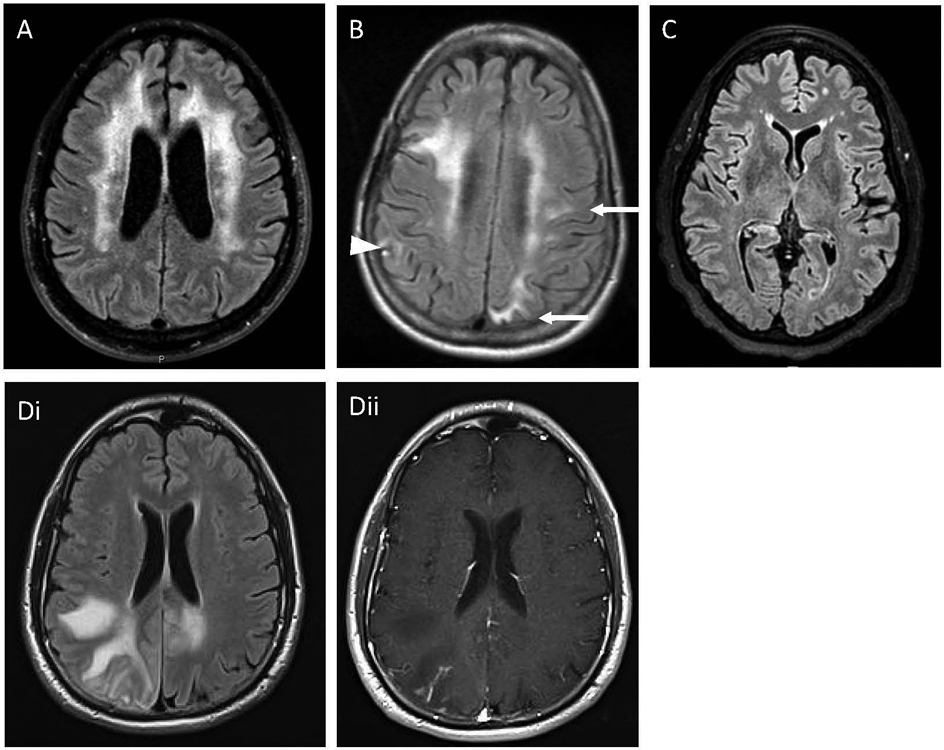
Magnetic resonance imaging of brain showing distinct biotypes of HAND: (A) Periventricular high signal intensities in the white matter that spares the U fibers and the juxta cortical fibers. The ventricles are enlarged. This is representative of a patient with HIV associated dementia which would correspond to macrophage-mediated HIV encephalitis. (B) Periventricular high signal intensities that extend to the juxta cortical fibers (arrows) and the cortex (arrowhead). This represents a patient with HIV associated immune reconstitution inflammatory syndrome which corresponds to a T cell mediated HIV-encephalitis. (C) Diffuse cortical atrophy with preservation of the subcortical structures which was progressive over several years. This represents a patient well controlled on long-term antiretroviral therapy and corresponds to HIV-protein associated encephalopathy. (D) Patient with HIV infection and progressive multifocal leukoencephalopathy. (Di) Focal areas of high signal intensity lesions that extend to the U fibers and juxta cortical regions. (Dii) enhancement with gadolinium in the center of the lesion which corresponds to T cell encephalitis in the setting of an opportunistic infection.
CNS viral escape
CNS viral escape is characterized by high viral load in the CNS despite low serum viral loads and has been defined as asymptomatic, secondary, and symptomatic [22]. Asymptomatic viral escape has no evidence of brain injury or inflammation and may be a transitory finding [22, 23]. Both secondary and symptomatic viral escape phenotypes are associated with CSF pleocytosis [22] although etiology of the inflammation may be driven by different processes. Secondary CSF escape occurs during a CNS co-infection, such as syphilis or herpes viruses which may result in increased trafficking of CD4+ T cells into the CNS, some of which may be latently infected with HIV [22, 24]. The inflammation associated with symptomatic viral escape likely occurs due to the presence of HIV itself [22, 25-27]. CSF viral escape is an important contributor to the development of HIV-associated CD8+ T cell encephalitis, accounting for 68% of patients in a recently examined cohort [28**], suggesting that this process is mediated by immune responses directed towards HIV. Elevated WBC counts in the CSF may even predict viral escape and cognitive decline [22].
HIV viral escape in symptomatic patients can occur due to low penetration of ART into the CNS, poor adherence or compliance to ART, or mutations that confer resistance [22, 25]. Drug resistant mutations may arise more frequently in the CNS as there might be enhanced viral replication in this compartment. Recent studies documented elevated levels of soluble CD30, a marker of ongoing HIV-1 transcriptional activity, in the CSF despite ART [29*]. This contrasts sharply with ART induced decreases in soluble CD30 in the serum, suggesting that there may be ongoing viral replication in the CNS compartment despite ART. Which cells produce the virus detected in the CSF is an area of ongoing investigation. HIV establishes CNS infection early in disease [30] and has been documented primarily in microglia [31, 32], but also in astrocytes [17]. Some emerging evidence suggests that virus detected in the CSF during viral escape contains CD26, a marker expressed on activated T cells, and therefore may be from CD4+ T cells [33*]. However, it is unknown if the T cells are trafficking in from the periphery or are brain-resident. Further, CD26 is expressed in regions of the human CNS with high levels in the meningeal endothelial cells [34] and is present in neurons and activated glia in rodent models [35].
Patients with symptomatic viral escape have a wide spectrum of symptoms including headache, tremors, cognitive impairment, confusion, focal neurologic deficits, and seizures [25, 36, 37]. These symptoms most often occur months to years after being stable on ART [22, 25]. Brain atrophy in viral escape is rare and neuroimaging in patients most commonly demonstrate white matter hyperintensities (Figure 1) with deep brain nuclei involvement and enhancement in some patients [22, 25]. Patients with symptomatic viral escape benefit from changes to ART regimens that provide better CNS penetration or to compensate for viral mutations [22, 25, 38]. Although similar clinical manifestations can occur in patients with secondary viral escape, these patients may benefit from therapies targeting the co-infection or steroids that help to control inflammation [39].
T cell mediated HIV encephalitis
T cell mediated HIV encephalitis is characterized by a CD4+ or CD8+ immune infiltrate that can be perivascular and diffuse into the parenchyma [40, 41]. This biotype encompasses both immune reconstitution inflammatory syndrome (IRIS), which can be driven by CD4+ or CD8+ T cells, and CD8+ T cell encephalitis. Patients with T cell mediated HIV encephalitis present with a wide range of clinical symptoms and signs including headache, confusion, cognitive impairment, and seizures [42]. This clinical syndrome overlaps with symptomatic viral escape described above. Neuroimaging can show diffuse white matter hyperintensities with mild edema (Figure 1 and reviewed in [43]). IRIS specifically occurs after ART initiation (reviewed in [44-46]) whereas HIV associated CD8 encephalitis can occur at any time and has been observed in patients who are well controlled on ART, treatment naïve, or as an IRIS event [28, 47, 48].
Risk factors for this biotype include low CD4+ T cell nadir (<100 cells/μL), the presence of opportunistic infections, and a rapid immune restoration after initiation of ART [45, 46]. The immune pathogenesis of this biotype is underscored by the increased risk of development of IRIS in patients treated with integrase inhibitors, which cause a rapid decline in viral loads [49-51]. This viral load decrease is accompanied by a sustained, hyperactive, and dysregulated immune response most often directed at opportunistic pathogens, residual HIV, or less frequently to self-antigens [52-56]. Metabolic alterations, including increased glycolysis and altered amino acid and lipid metabolism, which are correlated with immune activation, have been noted in patients that develop IRIS prior to the initiation of ART and during the IRIS event [57, 58]. Overactivation of both the innate and adaptive immune responses have been noted with particular activation of monocytes [59] and antigen specific CD4+ T-cells [52, 53, 60, 61] which results in an over production of proinflammatory cytokines and chemokines, further driving inflammation.
In patients with opportunistic infections, antigen specific T cells can infiltrate the CNS [54]. In contrast, little is known about the antigen specificity of the T cells that infiltrate the brain in the absence of an opportunistic infection. In four patients where T cell receptor sequencing from the CNS was performed there was no evidence of a dominant clone [28]. Even in the absence of HIV CSF escape it may be that there is an influx of HIV-specific CD8+ T cells, as well as non-specific T cells, into the CNS as has been reported in acute infections [62] and IRIS [53]. Although not specifically examined in the context of T cell mediated viral encephalitis, brain resident T cells may also influence the disease process. In murine models, brain resident viral specific T cells have been shown to induce reactive gliosis during antigen restimulation [63*] and a recent cohort study of neuropathological findings in patients with HIV-associated CD8 encephalitis revealed that in addition to T-cell infiltration microglial activation is a common feature of this disease [28]. Microglial activation, in turn, can recruit additional T cells into the brain, driving widespread inflammation.
Importantly, patients with this biotype may benefit from immune modulatory therapies. While the immune response is important for containing the HIV or underlying opportunistic infections, the immune response during T cell encephalitis contributes to CNS damage and therefore dampening the response with corticosteroids can significantly reduce the incidence of death [28]. Other immune modulatory therapies, such as the use of maraviroc, a CCR5 inhibitor, have been suggested to have clinical utility. However, in a small study, IRIS associated with PML was not prevented nor was disease course influenced by CCR5 inhibition when compared to patients who received corticosteroids only [64].
HIV protein associated encephalopathy
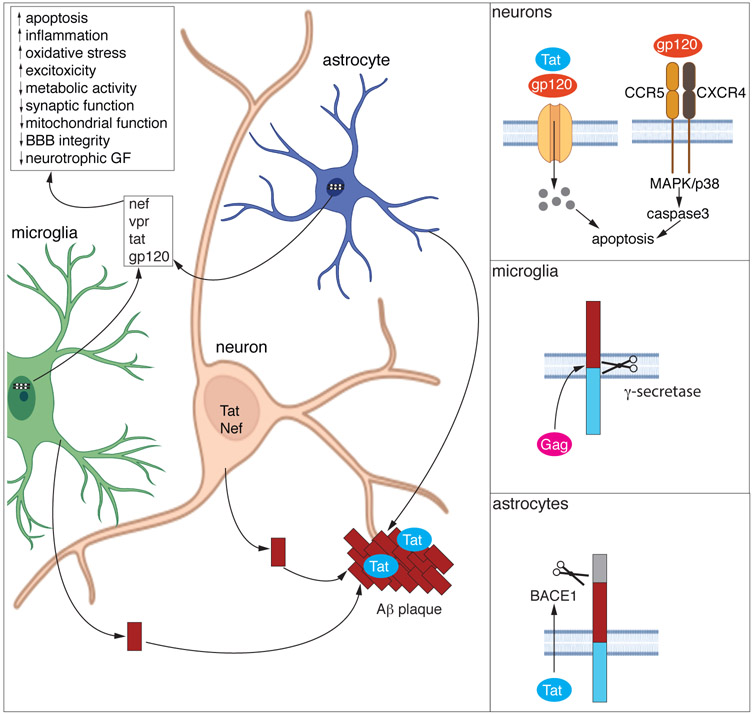
HIV can also induce CNS damage through the production and release of toxic proteins and by driving proteinopathy processes. Once integrated, current ART does not impair translation of viral proteins, therefore proteins such as Tat, Nef, gp120, Vpr, and Gag, which modify CNS cell viability and functioning, are still produced, even from defective proviruses [56, 65, 66*]. Patients with viral protein associated encephalopathy are typically well controlled on ART with no detectable virus in the blood or CSF, but HIV proteins such as Tat can be detected in the CSF and the presence of this protein is associated with cognitive impairments [56, 65]. The production of viral proteins in the brain can directly and indirectly damage the CNS by several mechanisms including inducing apoptosis, causing synaptic loss, impairing metabolic pathways, driving inflammation, and inducing oxidative stress (Figure 2) [56, 67-73]. Further, the production of viral proteins in the periphery can result in endothelial dysfunction which contributes to the development of cardiovascular disease (reviewed in [74]) that can result in ischemic stress in the CNS.
Figure 2. Viral protein mediated neurotoxicity and proteinopathy.
Once integrated, provirus from microglia and astrocytes continue to produce viral proteins, some of which are secreted into the extracellular environment or released in exosomes. These proteins can cause neurotoxicity by direct and indirect mechanisms. Extensive investigations have found numerous mechanisms by which viral proteins indirectly damage the CNS (summarized in the main panel) and broadly include excitotoxicity, metabolic alterations, proinflammatory processes, and blood brain barrier impairments. Direct neuronal toxicity has been demonstrated for both Tat and gp120 (top inset). These proteins can induce neuronal apoptosis by engaging with the NMDA receptor and allowing for calcium flux into the cell leading to apoptosis. Additionally, gp120 can bind to CXCR4 and CCR5 resulting in activation of p38 mitogen-activated protein kinase (MAPK) resulting in apoptosis.
Viral proteins can also drive proteinopathies including amyloid-beta (Aβ) accumulation. Aβ can be produced from microglia, astrocytes, and neurons and viral proteins increase the synthesis and secretion of Aβ from all these cell types. In neurons, both Tat and Nef stimulate the secretion of Aβ (main figure). In microglia, Gag increases APP cleavage by γ-secretase resulting in an increase in secretion of Aβ from microglia (middle inset). Tat drives the expression of APP and beta-secretase 1 (BACE1) in astrocytes which results in increased release of Aβ from these cells (bottom inset). The released Aβ from all these cell types can form protein aggregates that are stabilized in a complex with Tat and exert enhanced neurotoxicity as compared to Aβ alone (main panel).
In addition to inflammation and direct neurotoxicity, viral proteins can also induce proteinopathy. Amyloid beta (Aβ) [75, 76] and Tau [77, 78] deposits are present in the brains from patients with HIV and deposition of Aβ is correlated with HIV disease duration and not age [79], suggesting the virus contributes to the deposition of this protein. Viral proteins induce the production of Aβ and its secretion (Figure 2). Gag drives secretase-dependent cleavage of amyloid precursor protein (APP) which amplifies the production of Aβ in microglia [80] and Tat drives the enhanced expression of β-site cleaving enzyme, APP, and Aβ in astrocytes [81]. Both Tat [82] and Nef [83] stimulate the secretion of Aβ from neurons. Tat also inhibits neprilysin and thus prevents the degradation of Aβ [84, 85]. Tat may also stimulate the phosphorylation of Tau into its pathogenic isoforms [77]. Further, viral proteins can increase the toxicity of protein aggregates. For example, Tat can complex with Aβ forming multifibrillar structures which have increased toxicity as compared to Aβ alone [71]. Understanding the contribution and mechanisms behind proteinopathies driven by viral proteins is critical for therapeutic development. For example, in in vitro models, Aβ deposition and neurotoxicity induced by Gag could be prevented with gamma-secretase inhibitors [80]. Additionally, as the production of viral proteins from integrated virus is not targeted by ART, the development of adjunctive therapies inhibiting the expression or biological function of these proteins is needed.
Conclusion
Despite rigorous efforts and extensive research, little progress has been made in our ability to predict, prevent, or treat HIV-associated neurocognitive impairments except for optimization of ART. One large barrier to these advancements is that despite similar clinical phenotypes, patients with HAND may have highly divergent disease processes. In this review we suggest that classifying patients based on viral and immune pathogenesis may clarify important disease mechanisms and elucidate pathways for therapeutic targeting. It also helps identify some clinical phenotypes that are based on distinct underlying pathophysiological processes. Further deep phenotyping of these biotypes is necessary to understand the underlying mechanisms and clinical manifestations. This could provide clues for the proper diagnosis and treatment of these conditions.
Key points.
HIV-associated neurocognitive disorders is a heterogenous disease
Biotypes of HIV-associated neurocognitive impairments based on pathogenesis may elucidate key disease mechanisms
There are four distinct pathological biotypes driven in part by the choice, timing of initiation, and duration of treatment with antiretroviral drugs
Each of these biotypes have overlapping yet distinct clinical and neuro-radiological features, prognostic features and require unique modes of intervention
Acknowledgements:
The authors would like to acknowledge Dr. Michael C. Haffner for assistance in preparation of Figure 2. Figure 2 was created with BioRender.com.
Financial support and sponsorship:
Supported by intramural funds from NINDS (NS003130).
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
References
- 1.Malmstrom V, Catrina AI, Klareskog L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat Rev Immunol. 2017;17(1):60–75. [DOI] [PubMed] [Google Scholar]
- 2.Leslie D, Lipsky P, Notkins AL. Autoantibodies as predictors of disease. J Clin Invest. 2001;108(10):1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kesler SR, Petersen ML, Rao V, Harrison RA, Palesh O. Functional connectome biotypes of chemotherapy-related cognitive impairment. J Cancer Surviv. 2020;14(4):483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong SJ, Valk SL, Di Martino A, Milham MP, Bernhardt BC. Multidimensional Neuroanatomical Subtyping of Autism Spectrum Disorder. Cereb Cortex. 2018;28(10):3578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wager TD, Woo CW. Imaging biomarkers and biotypes for depression. Nat Med. 2017;23(1):16–7. [DOI] [PubMed] [Google Scholar]
- 6.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(5):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nightingale S, Dreyer AJ, Saylor D, Gisslen M, Winston A, Joska JA. Moving on From HAND: Why We Need New Criteria for Cognitive Impairment in Persons Living With Human Immunodeficiency Virus and a Proposed Way Forward. Clin Infect Dis. 2021;73(6):1113–8. **A critical assessment of the way HAND is diagnosed and the other factors that can contribute to the determination of HAND, and in particular, asymptomatic neurocognitive impairment. The authors propose a more rigorous and meaningful way to classify patients based on clinical history and not solely on cognitive testing that requires symptomatic impairments.
- 9. Ciccarelli N Considerations on nosology for HIV-associated neurocognitive disorders: it is time to update? Infection. 2020;48(1):37–42. * Review the discusses the limitations of the Frascati criteria for diagnosis of HAND and how these limitations may overestimated the true burden of neurocognitive impairments in patients with HIV.
- 10.Rubin LH, Saylor D, Nakigozi G, Nakasujja N, Robertson K, Kisakye A, et al. Heterogeneity in neurocognitive change trajectories among people with HIV starting antiretroviral therapy in Rakai, Uganda. J Neurovirol. 2019;25(6):800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devlin KN, Giovannetti T. Heterogeneity of Neuropsychological Impairment in HIV Infection: Contributions from Mild Cognitive Impairment. Neuropsychol Rev. 2017;27(2):101–23. [DOI] [PubMed] [Google Scholar]
- 12.Brouillette MJ, Yuen T, Fellows LK, Cysique LA, Heaton RK, Mayo NE. Identifying Neurocognitive Decline at 36 Months among HIV-Positive Participants in the CHARTER Cohort Using Group-Based Trajectory Analysis. PLoS One. 2016;11(5):e0155766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez D, Power C, Gill MJ, Koenig N, Vega R, Fujiwara E. Empiric neurocognitive performance profile discovery and interpretation in HIV infection. J Neurovirol. 2019;25(1):72–84. [DOI] [PubMed] [Google Scholar]
- 14.Rubin LH, Maki PM, Springer G, Benning L, Anastos K, Gustafson D, et al. Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology. 2017;89(15):1594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ances BM, Hammoud DA. Neuroimaging of HIV-associated neurocognitive disorders (HAND). Curr Opin HIV AIDS. 2014;9(6):545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19(6):525–35. [DOI] [PubMed] [Google Scholar]
- 17.Li GH, Henderson L, Nath A. Astrocytes as an HIV Reservoir: Mechanism of HIV Infection. Curr HIV Res. 2016;14(5):373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gisslen M, Keating SM, Spudich S, Arechiga V, Stephenson S, Zetterberg H, et al. Compartmentalization of cerebrospinal fluid inflammation across the spectrum of untreated HIV-1 infection, central nervous system injury and viral suppression. PLoS One. 2021;16(5):e0250987. * In this cross-sectional study inflammatory biomarkers were analyzed in CSF and serum from patients with HIV including patients who were untreated with and without HIV-associated dementia. Comparisons of biomarkers were made to patients with HIV on ART, elite controllers, and uninfected controls. Over time markers in the CSF of untreated individuals changed from a lymphocytic profile to one depicting activated macrophage responses and increased CNS injury.
- 19.McArthur JC, Johnson TP. Chronic inflammation mediates brain injury in HIV infection: relevance for cure strategies. Curr Opin Neurol. 2020;33(3):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(4):234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White DA, Taylor MJ, Butters N, Mack C, Salmon DP, Peavy G, et al. Memory for verbal information in individuals with HIV-associated dementia complex. HNRC Group. J Clin Exp Neuropsychol. 1997;19(3):357–66. [DOI] [PubMed] [Google Scholar]
- 22.Ferretti F, Gisslen M, Cinque P, Price RW. Cerebrospinal Fluid HIV Escape from Antiretroviral Therapy. Curr HIV/AIDS Rep. 2015;12(2):280–8. [DOI] [PubMed] [Google Scholar]
- 23.Eden A, Nilsson S, Hagberg L, Fuchs D, Zetterberg H, Svennerholm B, et al. Asymptomatic Cerebrospinal Fluid HIV-1 Viral Blips and Viral Escape During Antiretroviral Therapy: A Longitudinal Study. J Infect Dis. 2016;214(12):1822–5. [DOI] [PubMed] [Google Scholar]
- 24.Hagberg L, Price RW, Zetterberg H, Fuchs D, Gisslen M. Herpes zoster in HIV-1 infection: The role of CSF pleocytosis in secondary CSF escape and discordance. PLoS One. 2020;15(7):e0236162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manesh A, Barnabas R, Mani S, Karthik R, Abraham OC, Chacko G, et al. Symptomatic HIV CNS viral escape among patients on effective cART. Int J Infect Dis. 2019;84:39–43. [DOI] [PubMed] [Google Scholar]
- 26.Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26(14):1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50(5):773–8. [DOI] [PubMed] [Google Scholar]
- 28. Lucas SB, Wong KT, Nightingale S, Miller RF. HIV-Associated CD8 Encephalitis: A UK Case Series and Review of Histopathologically Confirmed Cases. Front Neurol. 2021;12:628296. ** Recent case series, the largest to date, of HIV-associated CD8+ T cell encephalitis confirmed by histopathology (biopsy or post-mortem). This manuscript finds that similar pathology can be driven by HIV in the CNS, CSF viral escape, or IRIS, suggesting that similar phenotypes can be driven by different underlying pathogenic mechanisms.
- 29. Peluso MJ, Thanh C, Prator CA, Hogan LE, Arechiga VM, Stephenson S, et al. Cerebrospinal fluid soluble CD30 elevation despite suppressive antiretroviral therapy in individuals living with HIV-1. J Virus Erad. 2020;6(1):19–26. * This cross-sectional study demonstrated that soluble CD30, a marker of HIV replication, was consistently elevated in the CSF despite ART. This is in contrast to serum soluable CD30 which decreases during ART. These data suggest that persistent CNS HIV-1 infection may occur and that soluable CD30 may be a useful marker of this process.
- 30.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512(7512):74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallet C, De Rovere M, Van Assche J, Daouad F, De Wit S, Gautier V, et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front Cell Infect Microbiol. 2019;9:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong ME, Jaworowski A, Hearps AC. The HIV Reservoir in Monocytes and Macrophages. Front Immunol. 2019;10:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lustig G, Cele S, Karim F, Derache A, Ngoepe A, Khan K, et al. T cell derived HIV-1 is present in the CSF in the face of suppressive antiretroviral therapy. PLoS Pathog. 2021;17(9):e1009871. * This study investigated the cellular origin of HIV detected in the CSF from patients with CSF viral escape. The authors quantified CD26, a marker of T cells, on virions isolated from the CSF. As elevated levels of CD26 were found, it suggests the virus in the CSF during viral escape comes from T cells.
- 34.Raha AA, Chakraborty S, Henderson J, Mukaetova-Ladinska E, Zaman S, Trowsdale J, et al. Investigation of CD26, a potential SARS-CoV-2 receptor, as a biomarker of age and pathology. Biosci Rep. 2020;40(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiraly K, Kozsurek M, Lukacsi E, Barta B, Alpar A, Balazsa T, et al. Glial cell type-specific changes in spinal dipeptidyl peptidase 4 expression and effects of its inhibitors in inflammatory and neuropatic pain. Sci Rep. 2018;8(1):3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan TY, De Zan V, Gregg A, Alagaratnam J, Gerevini S, Antinori A, et al. The symptomatology of cerebrospinal fluid HIV RNA escape: a large case-series. AIDS. 2021;35(14):2341–6. [DOI] [PubMed] [Google Scholar]
- 37.Mastrangelo A, Turrini F, de Zan V, Caccia R, Gerevini S, Cinque P. Symptomatic cerebrospinal fluid escape. AIDS. 2019;33 Suppl 2:S159–S69. [DOI] [PubMed] [Google Scholar]
- 38.Dravid AN, Gawali R, Betha TP, Sharma AK, Medisetty M, Natrajan K, et al. Two treatment strategies for management of Neurosymptomatic cerebrospinal fluid HIV escape in Pune, India. Medicine (Baltimore). 2020;99(24):e20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss JJ, Spudich S, Barakat L. VZV myelitis with secondary HIV CSF escape. BMJ Case Rep. 2021;14(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rushing EJ, Liappis A, Smirniotopoulos JD, Smith AB, Henry JM, Man YG, et al. Immune reconstitution inflammatory syndrome of the brain: case illustrations of a challenging entity. J Neuropathol Exp Neurol. 2008;67(8):819–27. [DOI] [PubMed] [Google Scholar]
- 41.Miller RF, Isaacson PG, Hall-Craggs M, Lucas S, Gray F, Scaravilli F, et al. Cerebral CD8+ lymphocytosis in HIV-1 infected patients with immune restoration induced by HAART. Acta Neuropathol. 2004;108(1):17–23. [DOI] [PubMed] [Google Scholar]
- 42.Post MJ, Thurnher MM, Clifford DB, Nath A, Gonzalez RG, Gupta RK, et al. CNS-immune reconstitution inflammatory syndrome in the setting of HIV infection, part 1: overview and discussion of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome and cryptococcal-immune reconstitution inflammatory syndrome. AJNR Am J Neuroradiol. 2013;34(7):1297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowen L, Nath A, Smith B. CNS immune reconstitution inflammatory syndrome. Handb Clin Neurol. 2018;152:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vinhaes CL, Araujo-Pereira M, Tiburcio R, Cubillos-Angulo JM, Demitto FO, Akrami KM, et al. Systemic Inflammation Associated with Immune Reconstitution Inflammatory Syndrome in Persons Living with HIV. Life (Basel). 2021;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang CC, Sheikh V, Sereti I, French MA. Immune reconstitution disorders in patients with HIV infection: from pathogenesis to prevention and treatment. Curr HIV/AIDS Rep. 2014;11(3):223–32. [DOI] [PubMed] [Google Scholar]
- 46.French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48(1):101–7. [DOI] [PubMed] [Google Scholar]
- 47.Cheema A, Mathias K, Bui C, Dunham SR, Goodman JC, El Sahly HM. CD8 Encephalitis in a Treatment-Naive and a Virologically Suppressed Patient with HIV. Can J Neurol Sci. 2019;46(6):773–5. [DOI] [PubMed] [Google Scholar]
- 48.Morioka H, Yanagisawa N, Sasaki S, Sekiya N, Suganuma A, Imamura A, et al. CD8 Encephalitis Caused by Persistently Detectable Drug-resistant HIV. Intern Med. 2016;55(10):1383–6. [DOI] [PubMed] [Google Scholar]
- 49.Wijting IEA, Wit F, Rokx C, Leyten EMS, Lowe SH, Brinkman K, et al. Immune reconstitution inflammatory syndrome in HIV infected late presenters starting integrase inhibitor containing antiretroviral therapy. EClinicalMedicine. 2019;17:100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Psichogiou M, Basoulis D, Tsikala-Vafea M, Vlachos S, Kapelios CJ, Daikos GL. Integrase Strand Transfer Inhibitors and the Emergence of Immune Reconstitution Inflammatory Syndrome (IRIS). Curr HIV Res. 2017;15(6):405–10. [DOI] [PubMed] [Google Scholar]
- 51.Dutertre M, Cuzin L, Demonchy E, Pugliese P, Joly V, Valantin MA, et al. Initiation of Antiretroviral Therapy Containing Integrase Inhibitors Increases the Risk of IRIS Requiring Hospitalization. J Acquir Immune Defic Syndr. 2017;76(1):e23–e6. [DOI] [PubMed] [Google Scholar]
- 52.Ravimohan S, Tamuhla N, Nfanyana K, Steenhoff AP, Letlhogile R, Frank I, et al. Robust Reconstitution of Tuberculosis-Specific Polyfunctional CD4+ T-Cell Responses and Rising Systemic Interleukin 6 in Paradoxical Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. Clin Infect Dis. 2016;62(6):795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahnke YD, Greenwald JH, DerSimonian R, Roby G, Antonelli LR, Sher A, et al. Selective expansion of polyfunctional pathogen-specific CD4(+) T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood. 2012;119(13):3105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aly L, Yousef S, Schippling S, Jelcic I, Breiden P, Matschke J, et al. Central role of JC virus-specific CD4+ lymphocytes in progressive multi-focal leucoencephalopathy-immune reconstitution inflammatory syndrome. Brain. 2011;134(Pt 9):2687–702. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Zhao N, Yang J, Wen Y. Case Report: Orbital Myositis and Myasthenia Gravis as Symptoms of Immune Reconstitution Inflammatory Syndrome in a Patient With Human Immunodeficiency Virus Infection. Front Immunol. 2020;11:595068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A. 2013;110(33):13588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pei L, Fukutani KF, Tiburcio R, Rupert A, Dahlstrom EW, Galindo F, et al. Plasma Metabolomics Reveals Dysregulated Metabolic Signatures in HIV-Associated Immune Reconstitution Inflammatory Syndrome. Front Immunol. 2021;12:693074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammoud DA, Boulougoura A, Papadakis GZ, Wang J, Dodd LE, Rupert A, et al. Increased Metabolic Activity on 18F-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography in Human Immunodeficiency Virus-Associated Immune Reconstitution Inflammatory Syndrome. Clin Infect Dis. 2019;68(2):229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akilimali NA, Muema DM, Specht C, Chang CC, Moosa MS, Levitz SM, et al. Cryptococcosis-Associated Immune Reconstitution Inflammatory Syndrome Is Associated With Dysregulation of IL-7/IL-7 Receptor Signaling Pathway in T Cells and Monocyte Activation. J Acquir Immune Defic Syndr. 2019;80(5):596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsu DC, Breglio KF, Pei L, Wong CS, Andrade BB, Sheikh V, et al. Emergence of Polyfunctional Cytotoxic CD4+ T Cells in Mycobacterium avium Immune Reconstitution Inflammatory Syndrome in Human Immunodeficiency Virus-Infected Patients. Clin Infect Dis. 2018;67(3):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Antonelli LR, Mahnke Y, Hodge JN, Porter BO, Barber DL, DerSimonian R, et al. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood. 2010;116(19):3818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kessing CF, Spudich S, Valcour V, Cartwright P, Chalermchai T, Fletcher JL, et al. High Number of Activated CD8+ T Cells Targeting HIV Antigens Are Present in Cerebrospinal Fluid in Acute HIV Infection. J Acquir Immune Defic Syndr. 2017;75(1):108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Prasad S, Hu S, Sheng WS, Chauhan P, Lokensgard JR. Recall Responses from Brain-Resident Memory CD8(+) T Cells (bTRM) Induce Reactive Gliosis. iScience. 2019;20:512–26. * This study examined the pathology associated with the restimulation of antigen specific brain-resident memory T cells in a murine model. The authors found that these T cells could activate glia and induce neurotoxicity. This is important as chronic inflammation, including microglial activation, has been linked to the development of HAND.
- 64.Bernard-Valnet R, Moisset X, Maubeuge N, Lefebvre M, Ouallet JC, Roumier M, et al. CCR5 Blockade in Inflammatory PML and PML-IRIS Associated With Chronic Inflammatory Diseases' Treatments. Neurol Neuroimmunol Neuroinflamm. 2022;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henderson LJ, Johnson TP, Smith BR, Reoma LB, Santamaria UA, Bachani M, et al. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS. 2019;33 Suppl 2:S145–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Imamichi H, Smith M, Adelsberger JW, Izumi T, Scrimieri F, Sherman BT, et al. Defective HIV-1 proviruses produce viral proteins. Proc Natl Acad Sci U S A. 2020;117(7):3704–10. * This manuscript demonstrates that HIV proteins can be produced even from defective proviruses in vitro and from patients on ART. This is of particular importance as the bulk of proviruses are defective. These viral proteins can then drive inflammation and in the CNS may contribute directly to neurotoxicity.
- 67.Zhou Y, Liu J, Xiong H. HIV-1 Glycoprotein 120 Enhancement of N-Methyl-D-Aspartate NMDA Receptor-Mediated Excitatory Postsynaptic Currents: Implications for HIV-1-Associated Neural Injury. J Neuroimmune Pharmacol. 2017;12(2):314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li W, Huang Y, Reid R, Steiner J, Malpica-Llanos T, Darden TA, et al. NMDA receptor activation by HIV-Tat protein is clade dependent. J Neurosci. 2008;28(47):12190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alirezaei M, Watry DD, Flynn CF, Kiosses WB, Masliah E, Williams BR, et al. Human immunodeficiency virus-1/surface glycoprotein 120 induces apoptosis through RNA-activated protein kinase signaling in neurons. J Neurosci. 2007;27(41):11047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dickens AM, Yoo SW, Chin AC, Xu J, Johnson TP, Trout AL, et al. Chronic low-level expression of HIV-1 Tat promotes a neurodegenerative phenotype with aging. Sci Rep. 2017;7(1):7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hategan A, Bianchet MA, Steiner J, Karnaukhova E, Masliah E, Fields A, et al. HIV Tat protein and amyloid-beta peptide form multifibrillar structures that cause neurotoxicity. Nat Struct Mol Biol. 2017;24(4):379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee MH, Amin ND, Venkatesan A, Wang T, Tyagi R, Pant HC, et al. Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J Neurovirol. 2013;19(5):418–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hammond JW, Qiu WQ, Marker DF, Chamberlain JM, Greaves-Tunnell W, Bellizzi MJ, et al. HIV Tat causes synapse loss in a mouse model of HIV-associated neurocognitive disorder that is independent of the classical complement cascade component C1q. Glia. 2018;66(12):2563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anand AR, Rachel G, Parthasarathy D. HIV Proteins and Endothelial Dysfunction: Implications in Cardiovascular Disease. Front Cardiovasc Med. 2018;5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Achim CL, Adame A, Dumaop W, Everall IP, Masliah E, Neurobehavioral Research C. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4(2):190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19(4):407–11. [DOI] [PubMed] [Google Scholar]
- 77.Ohene-Nyako M, Nass SR, Hahn YK, Knapp PE, Hauser KF. Morphine and HIV-1 Tat interact to cause region-specific hyperphosphorylation of tau in transgenic mice. Neurosci Lett. 2021;741:135502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111(6):529–38. [DOI] [PubMed] [Google Scholar]
- 79.Morgello S, Cortes EP, Gensler G, Meloni G, Jacobs MM, Murray J, et al. HIV disease duration, but not active brain infection, predicts cortical amyloid beta deposition. AIDS. 2021;35(9):1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chai Q, Jovasevic V, Malikov V, Sabo Y, Morham S, Walsh D, et al. HIV-1 counteracts an innate restriction by amyloid precursor protein resulting in neurodegeneration. Nat Commun. 2017;8(1):1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sil S, Hu G, Liao K, Niu F, Callen S, Periyasamy P, et al. HIV-1 Tat-mediated astrocytic amyloidosis involves the HIF-1alpha/lncRNA BACE1-AS axis. PLoS Biol. 2020;18(5):e3000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. HIV-1 protein-mediated amyloidogenesis in rat hippocampal cell cultures. Neurosci Lett. 2010;475(3):174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khan MB, Lang MJ, Huang MB, Raymond A, Bond VC, Shiramizu B, et al. Nef exosomes isolated from the plasma of individuals with HIV-associated dementia (HAD) can induce Abeta(1-42) secretion in SH-SY5Y neural cells. J Neurovirol. 2016;22(2):179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daily A, Nath A, Hersh LB. Tat peptides inhibit neprilysin. J Neurovirol. 2006;12(3):153–60. [DOI] [PubMed] [Google Scholar]
- 85.Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19(2):127–35. [DOI] [PubMed] [Google Scholar]