Abstract
Simple Summary
For 657 cases of segment or less repeat liver resection with results of plasma albumin and bilirubin levels and platelet counts before and 3 months after surgery, the indicators were compared before and after surgery. There were 268 open repeat after open and 224 cases laparoscopic repeat after laparoscopic liver resection. The background factors and liver functional indicators before and after surgery, and the changes were compared between both groups. Plasma levels of albumin (p = 0.006) and total bilirubin (p = 0.01) were decreased, and ALBI score (p = 0.001) indicated worse liver function after surgery. Though laparoscopic group had poorer performance status and liver function, changes of the values and overall survivals were similar between both groups. Plasma levels of albumin and bilirubin and ALBI score could be the liver functional indicators for liver functional deterioration after liver resection. The laparoscopic group with poorer conditions showed a similar deterioration of liver function and overall survival to the open group.
Abstract
Whether albumin and bilirubin levels, platelet counts, ALBI, and ALPlat scores could be useful for the assessment of permanent liver functional deterioration after repeat liver resection was examined, and the deterioration after laparoscopic procedure was evaluated. For 657 patients with liver resection of segment or less in whom results of plasma albumin and bilirubin levels and platelet counts before and 3 months after surgery could be retrieved, liver functional indicators were compared before and after surgery. There were 268 patients who underwent open repeat after previous open liver resection, and 224 patients who underwent laparoscopic repeat after laparoscopic liver resection. The background factors, liver functional indicators before and after surgery and their changes were compared between both groups. Plasma levels of albumin (p = 0.006) and total bilirubin (p = 0.01) were decreased, and ALBI score (p = 0.001) indicated worse liver function after surgery. Laparoscopic group had poorer preoperative performance status and liver function. Changes of liver functional values before and after surgery and overall survivals were similar between laparoscopic and open groups. Plasma levels of albumin and bilirubin and ALBI score could be the indicators for permanent liver functional deterioration after liver resection. Laparoscopic group with poorer conditions showed the similar deterioration of liver function and overall survivals to open group.
Keywords: laparoscopic liver resection, repeat liver resection, liver function, liver functional deterioration, overall survival
1. Introduction
The treatment options for hepatocellular carcinoma (HCC) are liver resection (LR) [1], liver transplantation [2], transarterial chemoembolization, local ablation therapy [3], and currently emerging systemic (immune-) chemotherapy using kinase inhibitors and immune checkpoint inhibitor [4,5]. Although some treatments provide the hope for a cure of the current HCC [3,6,7,8], most patients of HCC with underlying chronic liver disease (CLD) are developing metachronous multicentric HCCs from its preneoplastic background. When considering treatments for the patients, not only the oncological therapeutic effects to the current tumor, but also the post-treatment residual liver function for the future HCC treatments should be taken into account. The strategy of combination therapy during the long treatment history of HCC patients, depending on each patient’s tumor condition and liver function at each time, is needed [9,10]. Although the strategy should be planned with liver functional assessments of the deterioration after treatments, there is currently no good tool for the assessment.
We (ILLS-Tokyo collaborator group) conducted international multi-institutional propensity score-based studies for laparoscopic repeat LR (LRLR) with patients with HCC, comparing to open repeat LR (ORLR) [11,12]. In the study [11], the overall survival curves after LRLR and ORLR were clearly separated with the better tendency in LRLR (not significant with p-value of 0.086), although the disease-free survival curves were identical and overlapped. We speculated that overall survival after LRLR was better since less liver functional damage of LRLR [13] made the repeat treatments more accessible and the number of deceased patients due to liver insufficiency decreased.
Recently, ALBI score [14,15] calculated with plasma albumin and total bilirubin levels and ALPlat score [16] calculated with plasma albumin level and blood platelet counts were proposed as the indicators of liver functional reserve for the preoperative evaluation of LR. In this study, we examined whether plasma albumin level, total bilirubin level, blood platelet counts, ALBI score, and ALPlat score could be useful as liver functional indicators for the assessment of permanently settled liver functional deterioration 3 months after repeat LR (RLR) and, using the indicators, evaluated that the extent of liver functional deterioration after LRLR compared to ORLR.
2. Methods
2.1. Participating Centers and Registered Patients
The present study involved 42 high-volume liver surgery centers around the world that provided data from patients who underwent RLR for HCC between January 2007 and December 2017. Institutional Review Board (IRB) approval was obtained from the coordinating center, with a data transfer agreement and IRB approval having been provided by all centers.
The centers registered 1582 patients, including 934 and 648 treated by ORLR and LRLR. Each case was discussed under a multidisciplinary setting in each center, and each patient provided informed consent for the procedure. The detail of registered patients’ number from each center in original patient group was described in a previous study [10].
This study conformed to the ethical guidelines of Declaration of Helsinki and was retrospective in nature. Approval from the ethics committee of each institution was obtained (HM20-094 for primary investigator’s institution, FHU).
2.2. Selection of Patients and Data Collection
For 1582 registered patients, the results of usual laboratory blood examination were examined. A total of 875 patients, in whom the results of plasma albumin level, total bilirubin level, and blood platelet counts before and 3 months after surgery could be retrieved, were extracted. Background factors of the patients with ORLR or LRLR are described in Table 1. Then, 657 patients, who underwent segment or less resection, were selected for the first study searching indicators for liver functional change 3 months after RLR in order to eliminate the impact of decreased liver volume after LR.
Table 1.
Background factors of all patients (n = 875) with ORLR or LRLR before RLR.
| ORLR, n = 450 | LRLR, n = 425 | p Value | |
|---|---|---|---|
| Age (years old) | 66.07 ± 10.77 | 68.03 ± 10.60 | 0.007 * |
| Sex (male:female) | 355:95 | 322:103 | 0.270 |
| BMI | 22.98 ± 3.43 | 23.98 ± 3.96 | <0.001 * |
| Performance status (0:1:2) | 411:37:1 | 365:55:5 | 0.016 * |
| Size of tumor (mm) | 23.59 ± 17.52 | 20.49 ± 10.74 | 0.002 * |
| Number of tumors (1:2:3:>4) | 315:87:23:25 | 335:70:13:7 | 0.003 * |
| Tumor location (AL:PS) | 159:107 | 145:77 | 0.223 |
|
Extent of resection
(Segment or less: Section: 2 or more sections) |
329:72:49 | 382:33:10 | <0.001 * |
| Albumin (g/dL) | 4.09 ± 0.41 | 4.01 ± 0.48 | 0.006 * |
| Total Bilirubin (mg/dL) | 0.73 ± 0.32 | 0.76 ± 0.35 | 0.095 |
| Platelet (X104/microL) | 14.77 ± 6.11 | 13.93 ± 5.10 | 0.026 * |
| Presence of fibrosis (NL:CH:LF:LC) | 73:56:114:202 # | 49:39:120:21 3 ## | 0.056 |
| Child–Pugh score (5:6:7:>8) | 393:46:9:2 | 322:84:14:5 | <0.001 * |
RLR: repeat liver resection, ORLR: open repeat liver resection, LRLR: laparoscopic repeat liver resection. Data are shown as mean ± SD or number of cases. *: statistically significant. #: There are 5 missing data, ##: There are 4 missing data.
The following data were obtained as background factors: patient characteristics (age, sex, body mass index (BMI), and preoperative performance status (PS)); indicators of preoperative liver function (presence of liver fibrosis, plasma total bilirubin level (mg/dL), plasma albumin level (g/dL), blood platelet count (/microL), Child–Pugh score)); tumor characteristics (number, size (mm), and location (anterolateral or posterosuperior segments)); surgical procedures (ORLR or LRLR) and the previous LR procedure (open or laparoscopic).
In addition, the results 3 months after RLR of plasma albumin level, total bilirubin level, and blood platelet counts were obtained.
2.3. Analysis of the Indicators of Liver Function before and 3 Months after RLR
The results before and 3 months after RLR of plasma albumin level, total bilirubin level, and blood platelet counts were compared in the selected 657 patients. Furthermore, calculated ALBI scores [14,15] and ALPlat scores [16] before and after RLR were compared (Table 2).
Table 2.
Analysis of the indicators of liver function before and 3 months after repeat liver resection.
| Pre-Operative Data | Post-Operative Data | p Value | |
|---|---|---|---|
| Albumin (g/dL) | 4.04 ± 0.45 | 3.97 ± 0.53 | 0.006 * |
| Total Bilirubin (mg/dL) | 0.76 ± 0.33 | 0.81 ± 0.40 | 0.010 * |
| Platelet (×104/microL) | 14.07 ± 5.02 | 14.12 ± 5.20 | 0.862 |
| ALBI score | −2.73 ± 0.40 | −2.65 ± 0.48 | 0.001 * |
| AlPlat score | 504.49 ± 70.46 | 498.24 ± 77.05 | 0.125 |
Data are shown as mean ± SD. *: statistically significant.
2.4. Comparison between the Patients Who Underwent ORLR after Previous Open LR (OO group) and LRLR after Previous Laparoscopic LR (LL Group): Background Factors, Indicators for Liver Function before RLR, Their Changes after RLR, and Overall Survival after RLR
There were 268 patients who underwent ORLR after previous open LR (OO group) and 224 patients who underwent LRLR after previous laparoscopic LR (LL group) among selected 657 patients with segment or less RLR. Selected patients’ numbers for the final analysis, comparing ORLR and LRLR in the present study, from each center are in the description of Table 3
Table 3.
Comparison between OO group and LL group: Background factors, indicators for liver function before RLR, and after RLR.
| Before LR | OO | LL | p Value |
|---|---|---|---|
| Age (years old) | 67.37 ± 10.36 | 68.62 ± 9.96 | 0.176 |
| Sex (male:female) | 214:54 | 167:57 | 0.194 |
| BMI | 22.94 ± 3.44 | 23.96 ± 3.98 | 0.002 * |
| Performance status (1:2:3) | 250:17:1 | 194:29:1 | 0.043 * |
| Number of tumors (1:2:3:>4) | 188:58:14:8 | 176:38:6:4 | 0.209 |
| Size of tumor (mm) | 20.93 ± 15.21 | 19.00 ± 9.52 | 0.089 |
| Tumor location (AL:PS) | 159:107 | 145:77 | 0.223 |
| Albumin (g/dL) | 4.09 ± 0.39 | 3.94 ± 0.49 | <0.001 * |
| Total Bilirubin (mg/dL) | 0.73 ± 0.31 | 0.75 ± 0.35 | 0.698 |
| Platelet (×104/microL) | 14.58 ± 4.89 | 13.57 ± 5.41 | 0.031 * |
| ALBI score | −2.78 ± 0.34 | −2.65 ± 0.46 | <0.001 * |
| AlPlat score | 514.32 ± 61.09 | 490.43 ± 79.95 | <0.001 * |
| Presence of fibrosis (NL:CH:LF:LC) | 48:41:70:106 | 21:23:63:114 | 0.006 * |
| Child-Pugh score (5:6:7:>8) | 239:25:4:0 | 160:53:7:4 | <0.001 * |
| 3 months after LR | |||
| Albumin (g/dL) | 4.03 ± 0.47 | 3.89 ± 0.55 | 0.003 * |
| Total Bilirubin (mg/dL) | 0.77 ± 0.36 | 0.80 ± 0.39 | 0.461 |
| Platelet (X104/microL) | 14.77 ± 5.09 | 13.68 ± 5.56 | 0.025 * |
| ALBI score | −2.71 ± 0.42 | −2.59 ± 0.52 | 0.003 * |
| AlPlat score | 510.10 ± 69.08 | 486.70 ± 83.60 | 0.001 * |
Data are shown as mean ± SD or number of cases. *: statistically significant. OO group: Cases who underwent open repeat liver resection after previous open liver resection. LL group: Cases who underwent laparoscopic repeat liver resection after previous laparoscopic liver resection. RLR: repeat liver resection, LR; liver resection, BMI: body mass index, AL: tumors located anterolateral segments (segments 2–6), PS: tumors located posterosuperior segments (segments1,7,8), NL: normal liver, CH:chronic hepatitis, LF: liver fibrosis, LC: liver cirrhosis.
The factors listed before (background factors, indicators for liver function, ALBI score, and ALPlat score) RLR; plasma albumin level, total bilirubin level, blood platelet counts, ALBI score, and ALPlat score 3 months after RLR were compared between LL and OO groups.
Changes of the values before and after RLR in albumin, bilirubin, platelet, ALBI score, and ALPlat score were compared between LL and OO groups.
Overall survival after RLR was compared between LL and OO groups.
2.5. Statistical Analyses
Data are expressed as mean ± standard deviation or as the number of patients. Between-group differences in categorical variables were analyzed by Pearson’s Chi-squared test or Fisher’s exact test with Yates correction, as appropriate. Between group differences in continuous parametric variables were analyzed by un-paired Student’s t-test or ANOVA, and between-group differences in continuous non-parametric variables were analyzed by Mann–Whitney or Kruskal–Wallis test. Survival was plotted by the Kaplan–Meier method, and between-group differences were analyzed by log-rank test. Statistical analyses were performed with the use of SPSS Statistics 25 (IBM Corp., Armonk, NY, USA) or R 3.3.4 (R Foundation for Statistical Computing, Vienna, Austria). p < 0.05 was considered statistically significant.
3. Results
3.1. Analyses of the Indicators for Liver Function before and 3 Months after RLR
Plasma levels of albumin (4.04 ± 0.45 vs. 3.97 ± 0.53 g/dL, p = 0.006) was significantly decreased and total bilirubin (0.76 ± 0.33 vs. 0.81 ± 0.40 mg/dL, p = 0.01) was significantly increased 3 months after RLR compared to those values before RLR. The difference in blood platelet counts was not significant (14.07 ± 5.02 vs. 14.12 ± 5.20 × 104/microL, p = 0.862). Consequently, ALBI score (−2.73 ± 0.40 vs. −2.65 ± 0.48, p = 0.001) indicated significantly worse liver function 3 months after RLR, but not ALPlat score (504.49 ± 70.46 vs. 498.24 ± 77.05, p = 0.125).
3.2. Comparison between OO Group and LL Group: Background Factors, Indicators for Liver Function before RLR, and Those, Their Changes, and Overall Survivals after RLR
There was significantly higher BMI and poorer PS in the LL group. The LL group had significantly higher incidence of liver fibrosis and Child–Pugh score before RLR, although there were no significant differences between OO and LL groups in tumor-related factors, such as tumor number, size, and location. In addition, there were significant differences before and also after RLR in plasma level of albumin (OO vs. LL before RLR: 4.09 ± 0.39 vs. 3.94 ± 0.49 g/dL, p < 0.001; OO vs. LL after RLR: 4.03 ± 0.47 vs. 3.89 ± 0.55 g/dL, p = 0.003), blood platelet count (14.58 ± 4.89 vs. 13.57 ± 5.41 × 104/microL, p = 0.031; 14.77 ± 5.09 vs. 13.68 ± 5.56 × 104/microL, p = 0.025), ALBI score (−2.78 ± 0.34 vs. −2.65 ± 0.46, p < 0.001; −2.71 ± 0.42 vs. −2.59 ± 0.52, p = 0.003), and ALPlat score (514.32 ± 61.09 vs. 490.43 ± 79.95, p < 0.001; 510.10 ± 69.08 vs. 486.70 ± 83.60, p = 0.001) between LL vs. OO groups. (Table 3)
All the changes of values before and after RLR in albumin, bilirubin, platelet, ALBI score, and ALPlat score were similar without significant differences between LL and OO groups. (Table 4)
Table 4.
Comparison between OO group and LL group: Changes in indicators for liver function before and after RLR.
| OO | LL | p Value | |
|---|---|---|---|
| Change of Alb (g/dL) | 0.068 ± 0.40 | 0.054 ± 0.42 | 0.710 |
| Change of Total Bilirubin (mg/dL) | −0.036 ± 0.34 | −0.049 ± 0.33 | 0.653 |
| Change of Platelet (×104/microL) | −0.19 ± 4.26 | −0.11 ± 3.34 | 0.830 |
| Change of ALBI score | −0.064 ± 0.35 | −0.063 ± 0.38 | 0.969 |
| Change of ALPlat score | 4.23 ± 53.46 | 3.73 ± 53.59 | 0.919 |
Data are shown as mean ± SD. OO group: Cases who underwent open repeat liver resection after previous open liver resection. LL group: Cases who underwent laparoscopic repeat liver resection after previous laparoscopic liver resection.
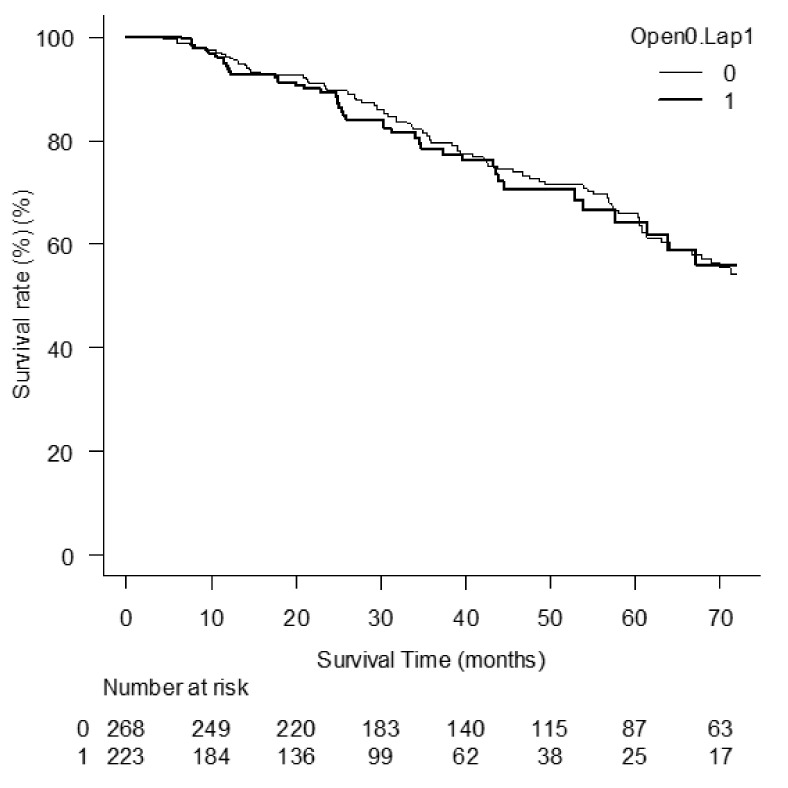
There was no significant difference in overall survival after RLR between LL and OO groups. (Figure 1, p = 0.576).
Figure 1.
Overall survival after RLR between LL and OO groups.
OO patients were registered from Clinica Universitaria de Navarra = 2 (number of patients), Wakayama Medical University Hospital = 13, Osaka City University = 30, Queen Mary Hospital = 34, Shizuoka Cancer Center = 47, University of Pittsburgh = 2, University Hospital Reina Sofia = 1, Kitazato University = 7, Komagome Hospital = 5, Osaka City General Hospital = 5, Kurume University = 18, Kurashiki Central Hospital = 18, National Cancer Center Hospital East = 37, Kansai Rosai Hospital = 4, Tokyo Medical and Dental University = 22, Toho University = 6, Fujita Health University Hospital = 4, Keio University = 3 and LL from Seoul National University Bundang Hospital = 2, Clinica Universitaria de Navarra = 2, Wakayama Medical University Hospital = 4, Osaka City University = 15, Queen Mary Hospital = 5, Fujita Health University Bantane Hospital = 15, Shizuoka Cancer Center = 3, Kitazato University = 1, Komagome Hospital = 4, Koo Foundation Sun Yat-Sen Cancer Center = 2, Far-Eastern Memorial Hospital = 20, Osaka City General Hospital = 32, Kurume University = 1, Kurashiki Central Hospital = 23, National Cancer Center Hospital East = 4, Tulane University = 1, Institute Mutualiste Montsouris = 10, Kansai Rosai Hospital = 33, Tokyo Medical and Dental University = 1, Toho University = 4, Asan Medical Center = 3, Fujita Health University Hospital = 22, and Keio University = 17.
4. Discussion
The present study showed that the plasma level of albumin, that of total bilirubin, and ALBI score indicated significantly worsened liver function 3 months after RLR comparing to the preoperative values. Although ALBI [14,15] and ALPlat [16] scores are advocated for liver functional evaluation before HCC treatments including LR, there are no established assessment indicators for the permanent deterioration of liver function settled stable 3 months after treatments. These factors, plasma level of albumin, that of total bilirubin, and ALBI score, could be the candidate indicators for the assessments of liver functional permanent deterioration after LR. Using these indicators, evaluation for the extent of liver functional deterioration after LRLR compared to ORLR were also performed in present study.
With the original patient group for the present study, we conducted international multi-institutional studies for LRLR to HCC patients, compared to ORLR [11,12]. The studies showed that LRLR is feasible and has short-term advantages of less intraoperative blood loss and less morbidity for selected patients. In the first study [11], the overall survival curves after LRLR and ORLR were clearly separated with the better tendency in LRLR, although the disease-free survival curves were identical. Overall survival of HCC patients with CLD after LR is determined not only by the recurrence of the resected HCC, but also by metachronous multicentric HCCs and liver insufficiency [8,9]. During the long and repeated treatment history of patients with HCC, they should have enough residual liver function after each treatment which makes them possible to undergo repeat combination treatments. We hypothesized that overall survival after LRLR was better since less deterioration of liver function after LRLR [12], in addition to less adhesion, made the repeat treatments more accessible and the number of deceased patients due to liver insufficiency decreased.
The main advantages of LLR for repeat treatments are thought to be less adhesion after LR and less damage to the liver and surrounding structures, such as collateral vessels [17], using the laparoscopic direct approach to the surgical area [18,19,20], sometimes without complete dissection of adhesion. Those could work not only on the technical aspects during LR, but also on the liver function after treatments resulting in less deterioration. Both possible advantages were verified by simple comparison of OO (open repeat LR after open LR) and LL (laparoscopic repeat LR after laparoscopic LR) groups, excluding the patients who underwent both open and laparoscopic procedures, in the present study. Selecting the resections of segmentectomy or less were for minimizing the impact on the deterioration from the decreased functional liver volume. There was no difference in tumor number, size, and location (in anterolateral segments or posterosuperior segments) between OO and LL groups. Thereafter, tumor and surgical factors are similar in both groups compared. On the other hand, the LL group had patients with poorer general (poorer PS and higher BMI) and liver condition (more fibrosis, lower albumin and platelet, worse ALBI/ALPLat/Child–Pugh scores) compared to the OO group. LL group patients with poorer liver and general conditions and similar tumor and surgical factors showed similar deterioration of liver function and resulted in similar overall survival to OO group patients. It could be translated that LL group patients could have gone through repeat LR well, despite the fact that they were allocated to LRLR due to the fear of liver decompensation and morbidity after ORLR. It may show the advantage of LLR, that it could prolong the overall survival of the HCC patients with CLD as a powerful local therapy which can be applied repeatedly with minimal deterioration of liver function.
The deterioration of liver function by each HCC treatment is usually smaller and more difficult to detect than years-long deterioration by CLD, except major hepatectomies which remove a large volume of functional liver. The present study showed that plasma level of albumin, that of total bilirubin, and ALBI score are the possible indicators for the assessment of liver functional permanent deterioration after LR. However, LR should have heaviest damage on liver function among the treatment options and, also, the evaluation of each individual case in different condition should be more difficult. Therefore, further investigations are needed for the assessment of liver functional change after each treatment during repeated treatments for the patients with metachronous multicentric HCCs and CLD.
Author Contributions
Z.M., L.A., G.B. (Giulio Belli), F.R. (Francesca Ratti), T.T.C., C.M.L., S.T., S.K., Y.O. (Yukiyasu Okamura), K.U., K.M., H.S., K.H., K.K., N.G., K.C., A.K., Y.T., Y.O. (Yoshiaki Ohmura), M.U., T.O., K.S.S., Y.K. (Yutaro Kato), A.S., A.B., H.N., M.Y., D.C., N.A.H., A.L., H.K., Y.O. (Yuichiro Otsuka), K.H.K., H.-D.C., C.C.-W.L., Y.O. (Yusuke Ome), Y.S., R.I.T., G.B. (Giammauro Berardi), F.R. (Fernando Rotellar), G.C.W., D.A.G., O.S., T.Y., T.K., Y.K. (Yusuke Kumamoto)., H.-S.H., E.E., I.D., D.F., B.G., J.F.B., R.C., J.B., N.O., J.L., B.E., M.S., Y.A., M.A.H., M.A., M.T. and G.W. collected the data at their various institutions and discussed results. Z.M., L.A. and G.B. (Giammauro Berardi) designed this study, collected the data, and drafted this paper. D.C., G.W. and M.T. co-organized the international study and discussed results during the drafting of this paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study conformed to the ethical guidelines of Declaration of Helsinki and was retrospective in nature. Approval from the ethics committee of each institution was obtained (HM20-094 for primary investigator’s institution, FHU). Informed consent was obtained from all subjects.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
All authors declare no conflict of interest nor sources of funding related to this study.
Funding Statement
This study has no related funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Capussotti L., Ferrero A., Viganò L., Polastri R., Tabone M. Liver resection for HCC with cirrhosis: Surgical perspectives out of EASL/AASLD guidelines. Eur. J. Surg. Oncol. 2009;35:11–15. doi: 10.1016/j.ejso.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Hwang S., Lee S.G., Belghiti J. Liver transplantation for HCC: Its role: Eastern and Western perspectives. J. Hepatobiliary Pancreat. Sci. 2010;17:443–448. doi: 10.1007/s00534-009-0241-0. [DOI] [PubMed] [Google Scholar]
- 3.Lau W.Y., Leung T.W., Yu S.C., Ho S.K. Percutaneous local ablative therapy for hepatocellular carcinoma: A review and look into the future. Ann. Surg. 2003;237:171–179. doi: 10.1097/01.SLA.0000048443.71734.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Cheng A.L., Baron A., Han G., Lopez C., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 5.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Cheng A.L., Kudo M., Merle P., Li D., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferro V., Regalia E., Doci R., Andreola S., Pulvirenti A., Bozzetti F., Gennari L., Montalto F., Ammatuna M., Morabito A. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 7.Ryder S.D. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;52:1–8. doi: 10.1136/gut.52.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahbari N.N., Mehrabi A., Mollberg N.M., Müller S.A., Koch M., Büchler M.W., Weitz J. Hepatocellular carcinoma: Current management and perspectives for the future. Ann. Surg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 9.El-Serag H.B., Marrero J.A., Rudolph L., Reddy K.R. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 10.Llovet J.M., Fuster J., Bruix J. The Barcelona approach: Diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115–S120. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 11.Morise Z., Aldrighetti L., Belli G., Ratti F., Belli A., Cherqui D., Alzoubi M., Lo M., Kubo S., Monden K., et al. ILLS-Tokyo Collaborator group. Laparoscopic repeat liver resection for hepatocellular carcinoma: A multicentre propensity score-based study. Br. J. Surg. 2020;107:889–895. doi: 10.1002/bjs.11436. [DOI] [PubMed] [Google Scholar]
- 12.3Miyama A., Morise Z., Aldrighetti L., Belli G., Ratti F., Cheung T.-T., Lo C.-M., Tanaka S., Kubo S., Okamura Y., et al. Multicenter Propensity Score-Based Study of Laparoscopic Repeat Liver Resection for Hepatocellular Carcinoma: A Subgroup Analysis of Cases with Tumors Far from Major Vessels. Cancers. 2021;13:3187. doi: 10.3390/cancers13133187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morise Z., Ciria R., Cherqui D., Chen K.H., Belli G., Wakabayashi G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J. Hepatobiliary Pancreat. Sci. 2015;22:342–352. doi: 10.1002/jhbp.215. [DOI] [PubMed] [Google Scholar]
- 14.Johnson P.J., Berhane S., Kagebayashi C., Satomura S., Teng M., Reeves H.L., Toyoda H., Fox R., Palmer D., Lai P., et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinato D.J., Sharma R., Allara E., Yen C., Arizumi T., Kubota K., Carr B.I., Kim Y.-W., Kudo M., Guerra V., et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J. Hepatol. 2017;66:338–346. doi: 10.1016/j.jhep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto G., Taura K., Ikai I., Fujikawa T., Nishitai R., Kaihara S., Uemoto S., Okuda Y., Tanabe K., Nishio T., et al. ALPlat criterion for the resection of hepatocellular carcinoma based on a predictive model of posthepatectomy liver failure. Surgery. 2020;167:410–416. doi: 10.1016/j.surg.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Kanazawa A., Tsukamoto T., Shimizu S., Kodai S., Yamamoto S., Yamazoe S., Ohira G., Nakajima T. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J. Hepatobiliary Pancreat. Sci. 2013;20:512–517. doi: 10.1007/s00534-012-0592-9. [DOI] [PubMed] [Google Scholar]
- 18.Tomishige H., Morise Z., Kawabe N., Nagata H., Ohshima H., Kawase J., Arawaka S., Isetani M., Yoshida R. Caudal approach to pure laparoscopic posterior sectionectomy under the laparoscopy-specific view. World J. Gastrointest. Surg. 2013;5:173–177. doi: 10.4240/wjgs.v5.i6.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakabayashi G., Cherqui D., Geller D.A., Han H.S., Kaneko H., Buell J.F. Laparoscopic hepatectomy is theoretically better than open hepatectomy: Preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J. Hepatobiliary Pancreat. Sci. 2014;21:723–731. doi: 10.1002/jhbp.139. [DOI] [PubMed] [Google Scholar]
- 20.Soubrane O., Schwarz L., Cauchy F., Perotto L.O., Brustia R., Bernard D., Scatton O. A conceptual technique for laparoscopic right hepatectomybased on facts and oncologic principles: The caudal approach. Ann. Surg. 2015;261:1226–1231. doi: 10.1097/SLA.0000000000000737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.