Abstract
Background:
Ideal cardiovascular health (CVH) is associated with a lower incidence of cardiovascular disease (CVD). Extra-coronary calcification (ECC) - measured at the aortic valve (AVC), mitral annulus (MAC), ascending thoracic aorta (ATAC) and descending thoracic aorta (DTAC) - is an indicator of systemic atherosclerosis. This study examined whether favorable CVH was associated with a lower risk of ECC.
Methods:
We analyzed data from MESA participants aged 45–84 years without CVD at baseline. ECC was measured by non-contrast cardiac CT scan at baseline and after an average of 2.4 years. Prevalent ECC was defined as an Agatston score >0 at the baseline scan. Incident ECC was defined as Agatston score >0 at the follow-up scan among participants with Agatston score = 0 at the baseline scan. Each CVH metric (smoking, physical activity, body mass index, diet, blood pressure, total cholesterol and blood glucose) was scored 0–2 points, with 2 indicating “ideal”, 1 “intermediate” and 0 “poor”. The aggregated CVH score was 0–14 points (0–8: inadequate; 9–10: average; 11–14: optimal). We used Poisson and linear mixed-effect regression models to examine the association between CVH and ECC adjusted for sociodemographic factors.
Results:
Of 6,504 participants, 53% were women with mean age (SD) of 62 (10) years. Optimal and average CVH scores were associated with lower ECC prevalence, incidence and extent. For example, optimal CVH scores were associated with 57%, 56%, 70% and 54% lower risk of incident AVC, MAC, ATAC and DTAC, respectively. In addition, optimal and average CVH scores were associated with lower ECC progression at 2 years, although these associations were only significant for MAC and DTAC.
Conclusions:
In this multi-ethnic cohort, favorable CVH was associated with a lower risk of extra-coronary atherosclerosis. These findings emphasize the importance of primordial prevention as an intervention to reduce the burden of CVD.
Keywords: Atherosclerosis, calcification, cardiovascular disease, ideal cardiovascular health metrics, Life’s Simple 7, risk factors, prevention, valvular heart disease, pathologic calcification
Introduction
The American Heart Association introduced the cardiovascular health (CVH) metrics to monitor and assess ideal CVH defined as the absence of disease and the presence of seven key health factors and behaviors [1–4]. These seven metrics have been utilized globally as surveillance tools to measure CVH along with clinical and subclinical cardiovascular disease (CVD) outcomes in the general population [5, 6]. Several studies have demonstrated an inverse association between favorable CVH and measures of clinical and subclinical CVD [7–21]. For example, a prior study found that adults with moderate and high CVH scores had 43% and 71% lower odds of having prevalent coronary artery calcification (CAC) (Agatston scores greater than zero), compared to adults with low CVH scores [10]. However, only a few studies have examined the relationship between the CVH metrics and extra-coronary calcification (ECC) measured solely by aortic valve calcification. The results of these studies showed that higher CVH scores were associated with a lower prevalence and incidence of aortic valve calcification and aortic stenosis [22, 23].
Therefore, it is unknown how favorable CVH affects calcification at other extra-coronary sites. Although several risk factors that are associated with the development of CAC similarly increase the risk of ECC, these risk factors may not always extend across vascular beds. Given the risk of CVD and mortality are significantly higher with calcification in multiple extra-coronary sites [24, 25], the aim of this study was to analyze data from the Multi-Ethnic Study of Atherosclerosis (MESA) to explore whether favorable CVH is associated with the presence and progression of ECC in the aortic valve (AVC), mitral annulus (MAC), ascending thoracic aorta (ATAC) and descending thoracic aorta (DTAC). Our findings could provide insight on how the CVH metrics could be optimized to reduce the risk of calcification at multiple extra-coronary sites. We hypothesized that favorable CVH will be associated with a lower prevalence and incidence of ECC as well as lower ECC extent and progression, regardless of sex or race/ethnicity.
Methods
Transparency and Openness Promotion
The data, methods and materials used to conduct this study will be made available to other researchers for the purposes of reproducing or expanding on the results upon application to and approval by the MESA publications and presentations committee. Requests for the use of MESA data can also be done through the National Heart, Lung, Blood Institute (NHLBI) Biologic Specimen and Data Repository Coordinating Center (BioLINCC) (https://biolincc.nhlbi.nih.gov/studies/mesa/.)
Study population
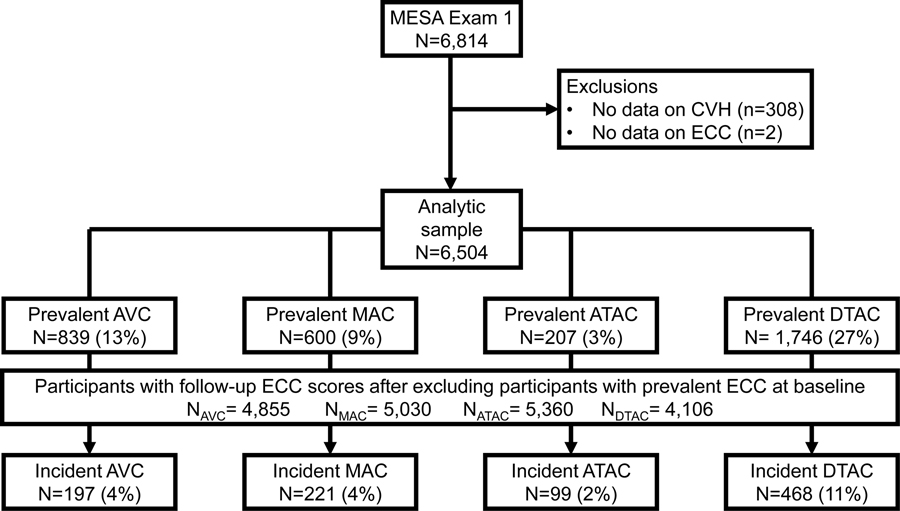
The methodology of the MESA study has been described elsewhere [26]. The study was designed to investigate the characteristics of subclinical CVD and the risk factors that predict progression to clinical CVD. Between July 2000 and August 2002, MESA recruited 6,814 men and women between the ages of 45 and 84 years with no prior history of clinical CVD at the time of enrollment. The 6 recruitment centers in the United States were Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY and St Paul, MN. Study participants were 38% White, 12% Chinese American, 28% Black and 22% Hispanic adults. All participants provided informed consent and the study protocol was approved by the institutional review boards of the recruitment centers. Baseline data were collected using standardized questionnaires, physical examinations, and fasting laboratory blood tests. In this study, we included 6,504 participants from the baseline examination after excluding participants with missing information for the CVH score and ECC (n=310) (Figure 1).
Figure 1.
Legend. Flowchart of study participants.
Abbreviations: ATAC, ascending thoracic aorta calcification; AVC, aortic valve calcification; CVH, cardiovascular health; DTAC, descending thoracic aorta calcification; ECC, extra-coronary calcification; MAC, mitral annular calcification.
Exposure: Cardiovascular health
The American Heart Association defines “ideal CVH” as follows: non-smoking; body mass index (BMI), <25kg/m2; physical activity at goal levels; a healthy diet consistent with current guidelines; total cholesterol <200mg/dL (without lipid-lowering medications); blood pressure <120/<80mmHg (without anti-hypertensive medications); and fasting blood glucose <100mg/dL (without anti-diabetic medications) [1]. Data on smoking status were obtained from self-report questionnaires and defined as non-smokers (participants who reported they had never smoked or quit >12 months), former smokers (participants who quit within the last 12 months) and current smokers. Using the measured weights and heights of participants, BMI was calculated and reported in kg/m2. A self-report survey instrument adapted from the Cross-Cultural Activity Participation Study [27] was used to assess physical activity. The survey contained 28 questions on time and frequency of activities during a week in the past month. The total minutes of moderate and vigorous exercise were estimated in metabolic equivalent of task/minute/week [28].
MESA used a 120-item validated food frequency questionnaire adapted from the Insulin Resistance Atherosclerosis Study instrument [29, 30] to collect data on dietary habits. Based on recommended dietary guidelines, a healthy diet comprised of fruits and vegetables, fish, whole grains, intake of sodium <1500mg/day and sugar-sweetened beverages ≤450 kcal (36 oz.)/week [1]. 12-hour fasting blood samples were collected to measure total cholesterol (mg/dL) and blood glucose (mg/dL) levels. Three blood pressure readings were taken from participants after 5 minutes of rest in a seated position and the average of the last 2 readings was used in the analysis. A CVH score was created from the seven metrics by a scoring system that assigned points to the metrics described in the section for statistical analysis [28, 31].
Outcome: Extra-coronary calcification
The details of the MESA cardiac computed tomography (CT) protocol have been previously described [32]. Between 2000 and 2002, two consecutive baseline non-contrast cardiac CT scans that were ECG-gated to the R-R interval were obtained from each study participant [32]. The Imatron C-150XL electron-beam CT scanner (GE-Imatron, San Francisco, California) was utilized at 3 study sites while the 4 slice-multidetector row CT scanner was utilized at the other 3 sites [32]. High concordance has been reported between both CT scanner types with a kappa statistic of 0.94–0.96 [33, 34]. After an average of 2.4 years, study participants were randomly assigned for follow-up CT scans at either exam 2 (2002–2004) or exam 3 (2004–2005). The Agatston scoring method [35] was used to quantify the presence of calcification at 4 extra-coronary sites from the: 1) aortic valve to just before the aortic root, 2) level of the mitral annulus, 3) ascending thoracic aorta and 4) descending thoracic aorta. Based on prior research [36], prevalent ECC was defined as an Agatston score >0 at the baseline scan and incident ECC was defined as Agatston score >0 at the follow-up scan among participants with Agatston score = 0 at the baseline scan.
Covariates
Baseline sociodemographic factors included as covariates were age, sex, race/ethnicity, education, income, health insurance and MESA field center. Age was assessed as a continuous variable. Sex was categorized as male and female. Participants self-identified as either White, Chinese-American, Black or Hispanic adults. Education and income were dichotomized in the baseline characteristics as ≥bachelor’s degree or <bachelor’s degree and ≥$40,000 or <$40,000, respectively.
Statistical analyses
We performed all analyses using STATA statistical software version 15.0 and considered a p value <0.05 on a 2-tailed test as statistically significant. The baseline characteristics of study participants were grouped by the CVH scores. As previously described in prior research, the total CVH score (0–14 points) was derived from the CVH metrics and each metric was scored 0–2 points, with 2 indicating “ideal”, 1 “intermediate” and 0 “poor” [1, 31]. A score of 0–8 points was inadequate, 9–10 points was average and 11–14 points was optimal, based on the methodology of previous studies [28, 37, 38]. We reported frequencies with percentages for categorical variables and means with standard deviation (SD) for continuous variables. The chi-square and ANOVA tests were used to determine whether statistically significant differences were present between the baseline characteristics of study participants across the categories of the CVH score (inadequate, average, and optimal). We utilized the chi-square test when the baseline variable was categorical and the ANOVA test was employed when the baseline variable was continuous.
We used Poisson regression models with robust variance estimation to examine the associations of the CVH scores with prevalent and incident ECC. For prevalent ECC measured at the baseline scan, we used regression models to examine the risk of having any prevalent ECC at baseline compared to the absence of ECC. Incident ECC was assessed only among participants with zero ECC at the baseline scan and regression models were used to evaluate the risk of having any ECC compared to the remaining participants with zero ECC at the follow-up scan. The prevalence and incidence ratios generated from the Poisson regression models were presented with their respective 95% confidence intervals (CI). The first model was unadjusted while the second model adjusted for age, sex, race/ethnicity, education, income, health insurance and field center. We used linear mixed-effects models with robust variance estimation to examine the associations of the CVH scores with ECC extent at baseline and progression at 2 years. ECC was modeled as a continuous variable and natural log transformed for this analysis (expressed as ln[ECC+1]). The percent difference and percent change generated from the linear mixed-effects models were presented with their respective 95% CI. The 2 models fitted were the same as the models for the Poisson regression. Linear mixed-effects models examine the cross-sectional effects of covariates on ECC in combination with the longitudinal effects while taking into consideration participant-specific random slopes and intercepts.
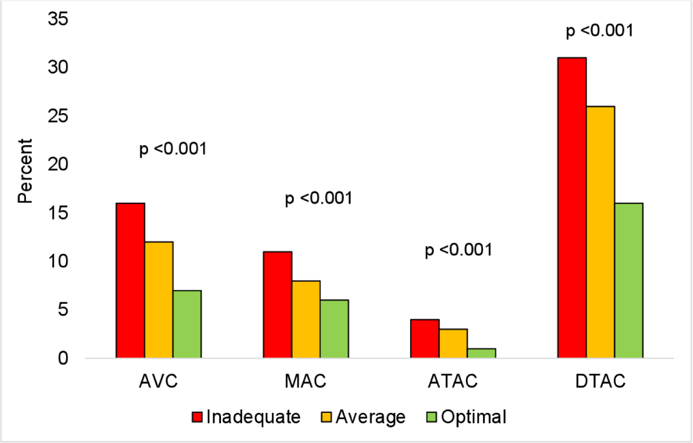
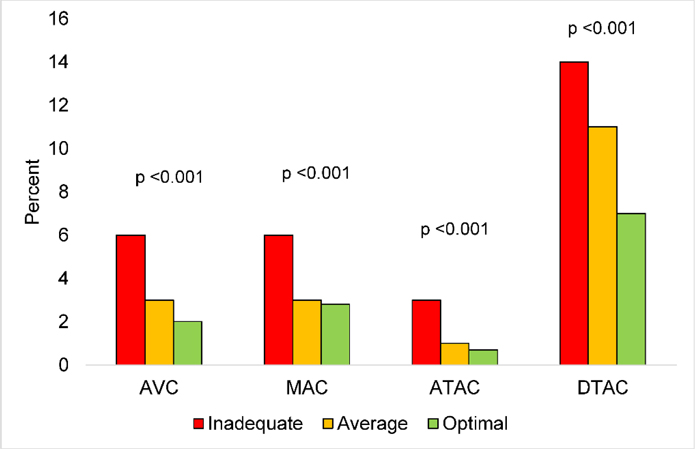
Furthermore, we examined the associations of the individual CVH metrics with prevalent and incident ECC using Poisson regression models and we used linear mixed-effects models to estimate the associations of the individual CVH metrics with ECC extent and progression at 2 years. The model was adjusted for age, sex, race/ethnicity, education, income, health insurance and field center. The distribution of participants with prevalent and incident ECC by CVH scores was presented in Figures 2 & 3. In Figures 4–6, we presented CT images of severe AVC, mild AVC and zero AVC in study participants with inadequate, average and optimal CVH scores, respectively. We tested for the interaction of CVH with sex and race/ethnicity by including cross-product terms in the adjusted models and we performed stratified analysis by subgroups where interaction was statistically significant. To account for multiple testing, a Bonferroni adjusted two-sided p-value <0.002 was considered as statistically significant for the analysis that examined the associations between the CVH metrics and ECC. This adjusted p-value was derived from 0.05 divided by 28 comparisons (7 exposure variables × 4 outcome variables).
Figure 2.
Legend. Distribution of participants with prevalent ECC by CVH scores.
Red, inadequate scores; Orange, average scores; Green, optimal scores.
Abbreviations: ATAC, ascending thoracic aorta calcification; AVC, aortic valve calcification; CVH, cardiovascular health; DTAC, descending thoracic aorta calcification; ECC, extra-coronary calcification; MAC, mitral annular calcification.
Figure 3.
Legend. Distribution of participants with incident ECC by CVH scores.
Red, inadequate scores; Orange, average scores; Green, optimal scores.
Abbreviations: ATAC, ascending thoracic aorta calcification; AVC, aortic valve calcification; CVH, cardiovascular health; DTAC, descending thoracic aorta calcification; ECC, extra-coronary calcification; MAC, mitral annular calcification.
Figure 4.
Legend. Severe AVC in a study participant with inadequate CVH scores.
Abbreviations: AVC, aortic valve calcification; CVH, cardiovascular health.
Figure 6.
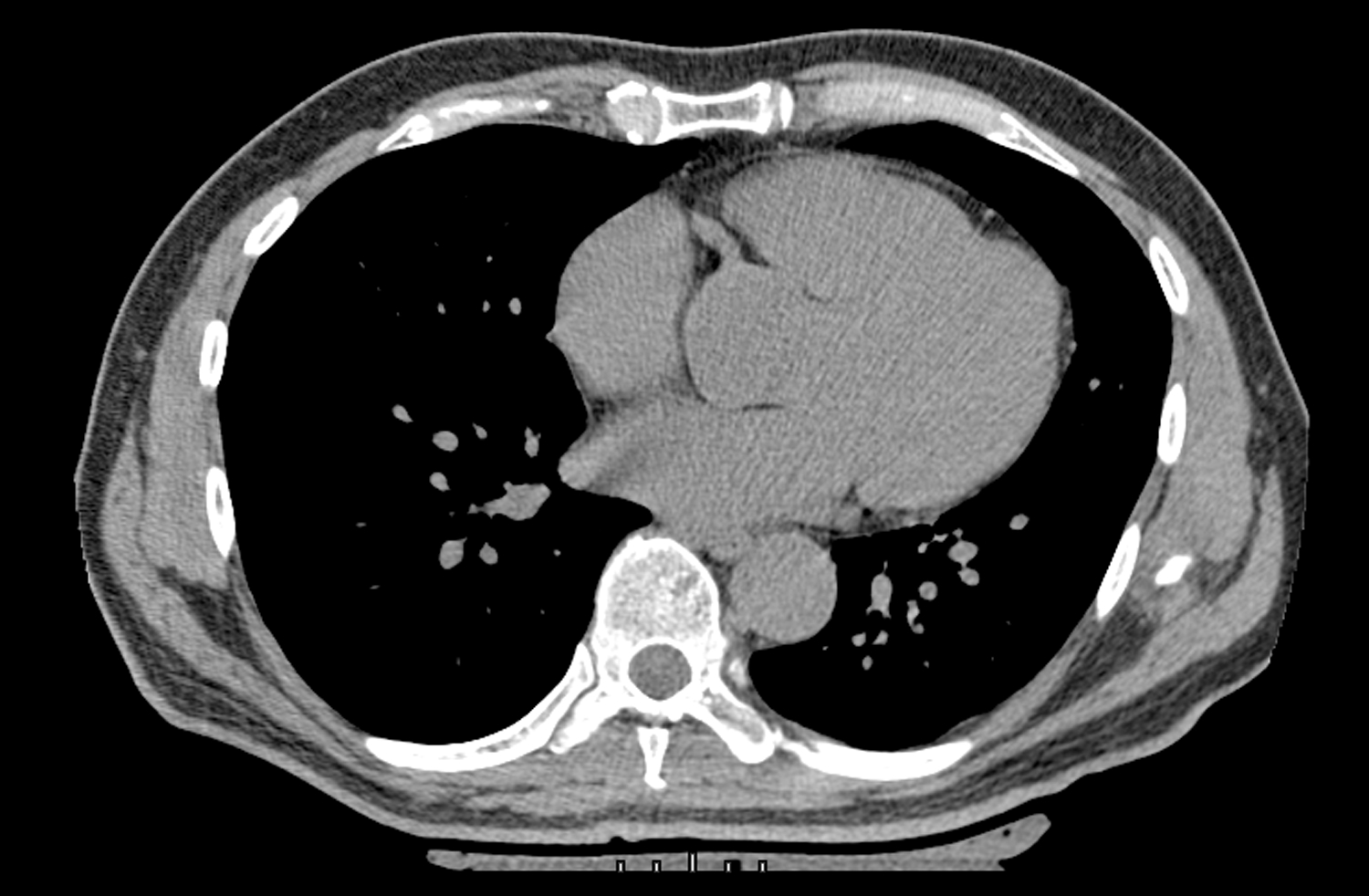
Legend. Zero AVC in a study participant with optimal CVH scores.
Abbreviations: AVC, aortic valve calcification; CVH, cardiovascular health.
Results
The mean age (SD) of study participants at baseline was 62 (10) years and 53% were women. A review of baseline characteristics revealed sociodemographic factors and mean ECC score differed by CVH score except for sex and health insurance status. Mean ECC score decreased across CVH scores from inadequate to optimal scores (Table 1).
Table 1.
Characteristics of study participants in the Multi-Ethnic Study of Atherosclerosis
| Total | Inadequate | Average | Optimal | P value | |
|---|---|---|---|---|---|
| N = 6,504 | n = 3,079 | n = 2,119 | n = 1,306 | ||
|
| |||||
| Age, years | 62 (10) | 63 (10) | 62 (11) | 60 (10) | <0.001 |
| Sex | 0.799 | ||||
| Women | 3,430 (53%) | 1,614 (52%) | 1,117 (53%) | 690 (54%) | |
| Men | 3,074 (47%) | 1,465 (48%) | 1,002 (47%) | 607 (46%) | |
| Race/Ethnicity | <0.001 | ||||
| White | 2,537 (39%) | 979 (32%) | 906 (43%) | 652 (50%) | |
| Chinese American | 796 (12%) | 216 (7%) | 319 (15%) | 261 (20%) | |
| Black | 1,715 (26%) | 1,042 (34%) | 474 (22%) | 199 (15%) | |
| Hispanic | 1,456 (22%) | 842 (27%) | 420 (20%) | 194 (15%) | |
| Education | <0.001 | ||||
| ≥ Bachelor’s degree | 2,331 (36%) | 796 (26%) | 834 (39%) | 701 (54%) | |
| < Bachelor’s degree | 4,173 (64%) | 2,283 (74%) | 1,285 (61%) | 605 (46%) | |
| Income | <0.001 | ||||
| ≥$40,000 | 3,214 (49%) | 1,272 (41%) | 1,125 (53%) | 817 (63%) | |
| <$40,000 | 3,290 (51%) | 1,807 (59%) | 994 (47%) | 489 (37%) | |
| Health insurance | 0.392 | ||||
| Yes | 5,923 (91%) | 2,791 (91%) | 1,944 (92%) | 1,188 (91%) | |
| No | 581 (9%) | 288 (9%) | 175 (8%) | 118 (9%) | |
| AVC, Agatston units | 25 (202) | 34 (249) | 23 (178) | 10 (78) | 0.001 |
| MAC, Agatston units | 48 (430) | 65 (497) | 36 (350) | 27 (370) | 0.008 |
| ATAC, Agatston units | 8 (136) | 13 (189) | 4 (61) | 2 (32) | 0.011 |
| DTAC, Agatston units | 212 (848) | 264 (971) | 206 (837) | 96 (452) | <0.001 |
Abbreviations: ATAC, ascending thoracic aorta calcification; AVC, aortic valve calcification; DTAC, descending thoracic aorta calcification; MAC, mitral annular calcification.
Data were presented as n (%) or mean (SD).
In comparison to inadequate CVH scores, participants with average and optimal scores had a lower prevalence and incidence of ECC (Table 2). For example, in the adjusted model, participants with optimal CVH scores had 50% (60%, 39%), 31% (45%, 13%), 70% (84%, 47%) and 34% (41%, 27%) lower prevalence of AVC, MAC, ATAC and DTAC, respectively. Additionally, participants with optimal CVH scores had 57% (73%, 33%), 56% (71%, 35%), 70% (86%, 38%) and 54% (64%, 41%) lower risk of incident AVC, MAC, ATAC and DTAC, respectively. These associations were slightly attenuated in comparison to the unadjusted models except for incident MAC and DTAC.
Table 2.
Associations of the cardiovascular health score with ECC prevalence and incidence
| Model 1 | Model 2 | Model 1 | Model 2 | |
|---|---|---|---|---|
|
| ||||
| Prevalent ECC | Prevalence ratio (95% CI) | |||
|
| ||||
| AVC | MAC | |||
| Inadequate | Reference | Reference | Reference | Reference |
| Average | 0.75 (0.65, 0.86)* | 0.75 (0.66, 0.86)* | 0.76 (0.64, 0.91) † | 0.77 (0.65, 0.90) † |
| Optimal | 0.44 (0.36, 0.54)* | 0.50 (0.40, 0.61)* | 0.58 (0.46, 0.73)* | 0.69 (0.55, 0.87) † |
| ATAC | DTAC | |||
| Inadequate | Reference | Reference | Reference | Reference |
| Average | 0.65 (0.48, 0.88) † | 0.69 (0.51, 0.95) ‡ | 0.84 (0.77, 0.92)* | 0.80 (0.74, 0.87)* |
| Optimal | 0.23 (0.13, 0.40)* | 0.30 (0.16, 0.53)* | 0.60 (0.53, 0.68)* | 0.66 (0.59, 0.73)* |
|
| ||||
| Incident ECC | Incidence rate ratio (95% CI) | |||
|
| ||||
| AVC | MAC | |||
| Inadequate | Reference | Reference | Reference | Reference |
| Average | 0.53 (0.38, 0.73)* | 0.53 (0.39, 0.73)* | 0.47 (0.34, 0.64)* | 0.45 (0.33, 0.61)* |
| Optimal | 0.41 (0.27, 0.63)* | 0.43 (0.27, 0.67)* | 0.45 (0.30, 0.66)* | 0.44 (0.29, 0.65)* |
| ATAC | DTAC | |||
| Inadequate | Reference | Reference | Reference | Reference |
| Average | 0.51 (0.33, 0.81) † | 0.52 (0.33, 0.82) † | 0.73 (0.60, 0.88) † | 0.68 (0.57, 0.83)* |
| Optimal | 0.26 (0.12, 0.54)* | 0.30 (0.14, 0.62) † | 0.48 (0.37, 0.62)* | 0.46 (0.36, 0.59)* |
Abbreviations: ATAC, ascending thoracic aorta calcification; AVC, aortic valve calcification; CI, confidence interval; DTAC, descending thoracic aorta calcification; ECC, extra-coronary calcification; MAC, mitral annular calcification.
CVH score ranged from 0–14 points: inadequate score, 0–8; average, 9–10; optimal, 11–14.
Prevalence ratios and incidence rate ratios were derived from Poisson regression with robust variance estimation.
Prevalent ECC was defined as Agatston score >0 at baseline. Incident ECC was defined as Agatston score >0 at exam 2/3 among participants with Agatston score =0 at baseline.
Model 1 was unadjusted.
Model 2 was adjusted for age, sex, race/ethnicity, education, income, health insurance and field center.
Statistically significant results are in bold font.
P <0.001
P <0.01
P <0.05.
Sample size for incident ECC = 5,520. Incident rate ratios were adjusted for time between scans.
Average and optimal CVH scores were associated with lower ECC extent at baseline although the associations were attenuated in the adjusted model (Table 3). For example, optimal CVH scores were associated with 24% (30%, 17%), 13% (21%, 6%), 10% (14%, 6%) and 42% (49%, 33%) lower AVC, MAC, ATAC and DTAC extent at baseline, respectively. We also observed that optimal CVH scores were associated with 8% (12%, 4%) and 15% (20%, 10%) lower MAC and DTAC progression at 2 years, respectively.
Table 3.
Associations of the cardiovascular health score with ECC extent and progression
| Model 1 | Model 2 | Model 1 | Model 2 | |
|---|---|---|---|---|
|
| ||||
| Extent at baseline | Percent difference (95 % CI) | |||
|
| ||||
| AVC | MAC | |||
| Inadequate | Reference | Reference | Reference | Reference |
| Average | −17 (−24, −10)* | −14 (−20, −7)* | −15 (−22, −8)* | −13 (−19, −6) † |
| Optimal | −33 (−38, −27)* | −24 (−30, −17)* | −22 (−28, −15)* | −13 (−21, −6) † |
| ATAC | DTAC | |||
| Inadequate | Reference | Reference | Reference | Reference |
| Average | −9 (−13, −5)* | −8 (−12, −3)* | −26 (−36, −24)* | −24 (−33, −14)* |
| Optimal | −14 (−17, −10)* | −10 (−14, −6)* | −53 (−60, −46)* | −42 (−49, −33)* |
|
| ||||
| Progression at 2 years | Percent change (95% CI) | |||
|
| ||||
| AVC | MAC | |||
| Inadequate | Reference | Reference | Reference | Reference |
| Average | −2 (−6, 2) | −2 (−6, 2) | −8 (−11, −4)* | −8 (−11, −4)* |
| Optimal | −4 (−8, 0) | −4 (−8, 0) | −8 (−12, −4)* | −8 (−12, −4)* |
| ATAC | DTAC | |||
| Inadequate | Reference | Reference | Reference | Reference |
| Average | −1 (−5, 2) | −1 (−5, 2) | −7 (−13, −1) ‡ | −7 (−13, −1) ‡ |
| Optimal | −3 (−6, 1) | −3 (−6, 0) | −15 (−20, −10)* | −15 (−20, −10)* |
Abbreviations: ATAC, ascending thoracic aorta calcification; AVC, aortic valve calcification; CI, confidence interval; DTAC, descending thoracic aorta calcification; ECC, extra-coronary calcification; MAC, mitral annular calcification.
CVH score ranged from 0–14 points: inadequate score, 0–8; average, 9–10; optimal, 11–14.
ECC was expressed as natural log transformed (ECC + 1).
Percent difference and percent change were calculated from [Exp (β) −1]*100, derived from linear mixed-effects regression models.
Model 1 was unadjusted.
Model 2 was adjusted for age, sex, race/ethnicity, education, income, health insurance and field center.
Statistically significant results are in bold font.
P <0.001
P <0.01
P <0.05.
Furthermore, ideal levels of the CVH metrics were mostly associated with a lower prevalence of ECC except for ideal levels of physical activity and diet. For example, ideal blood pressure was associated with 36% (46%, 23%) and 39% (45%, 32%) lower prevalence of AVC and DTAC, respectively (Table 4). Ideal levels of the CVH metrics were mostly associated with a lower risk of incident ECC except for ideal diet. For example, ideal levels of the smoking and blood pressure metrics were associated with 38% (52%, 20%) and 56% (66%, 44%) lower risk of incident DTAC, respectively (Table 5).
Table 4.
Multivariable-adjusted associations of CVH metrics with prevalent ECC
| AVC | MAC | ATAC | DTAC | |
|---|---|---|---|---|
|
| ||||
| Prevalence ratios (95% CI) | ||||
|
| ||||
| Smoking | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 1.01 (0.55, 1.87) | 0.49 (0.17, 1.41) | 0.86 (0.28, 2.61) | 0.78 (0.53, 1.14) |
| Ideal | 0.86 (0.70, 1.07) | 0.76 (0.59, 0.98)* | 0.41 (0.29, 0.60)* | 0.67 (0.60, 0.76)* |
|
| ||||
| Physical activity | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0.93 (0.77, 1.13) | 0.95 (0.76, 1.20) | 0.94 (0.64, 1.37) | 1.03 (0.93, 1.15) |
| Ideal | 0.89 (0.77, 1.03) | 0.94 (0.79, 1.12) | 0.69 (0.51, 0.95) ‡ | 0.98 (0.90, 1.07) |
|
| ||||
| Body mass index | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0.86 (0.75, 0.99) ‡ | 0.82 (0.69, 0.97) ‡ | 0.90 (0.65, 1.26) | 0.95 (0.87, 1.03) |
| Ideal | 0.70 (0.59, 0.83)* | 0.68 (0.56, 0.84)* | 1.04 (0.71, 1.54) | 0.97 (0.88, 1.06) |
|
| ||||
| Diet | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 1.03 (0.91, 1.17) | 0.97 (0.83, 1.12) | 0.92 (0.70, 1.22) | 1.07 (1.00, 1.16) |
| Ideal | 0.94 (0.50, 1.77) | 1.22 (0.67, 2.23) | 1.70 (0.65, 4.47) | 0.97 (0.70, 1.31) |
|
| ||||
| Total cholesterol | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0.80 (0.68, 0.95) † | 1.10 (0.89, 1.38) | 0.65 (0.46, 0.93) ‡ | 0.96 (0.87, 1.06) |
| Ideal | 0.60 (0.51, 0.71)* | 0.92 (0.73, 1.15) | 0.48 (0.33, 0.71)* | 0.80 (0.72, 0.88)* |
|
| ||||
| Blood pressure | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0.83 (0.72, 0.96) ‡ | 0.94 (0.79, 1.12) | 0.81 (0.60, 1.11) | 0.83 (0.76, 0.90)* |
| Ideal | 0.64 (0.54, 0.77)* | 0.80 (0.65, 0.98) ‡ | 0.50 (0.32, 0.76) † | 0.61 (0.55, 0.68)* |
|
| ||||
| Blood glucose | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0.87 (0.71, 1.05) | 0.79 (0.62, 1.01) | 1.13 (0.72, 1.78) | 0.95 (0.85, 1.07) |
| Ideal | 0.72 (0.61, 0.85)* | 0.66 (0.53, 0.81)* | 0.93 (0.62, 1.36) | 0.82 (0.74, 0.91)* |
Abbreviations: ATAC, ascending thoracic aorta calcification; AVC, aortic valve calcification; CI, confidence interval; CVH, cardiovascular health; DTAC, descending thoracic aorta calcification; ECC, extra-coronary calcification; MAC, mitral annular calcification.
Prevalence ratios were derived from Poisson regression with robust variance estimation.
Prevalent ECC was defined as Agatston score >0 at baseline.
Model was adjusted for age, sex, race/ethnicity, education, income, health insurance and field center.
Statistically significant results at P <0.002 are in bold font.
Results in italics were statistically significant at:
P <0.01
P <0.05.
Table 5.
Multivariable-adjusted associations of CVH metrics with incident ECC
| AVC | MAC | ATAC | DTAC | |
|---|---|---|---|---|
|
| ||||
| Incidence rate ratios (95% CI) | ||||
|
| ||||
| Smoking | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0.34 (0.05, 2.36) | 0.64 (0.16, 2.63) | 0.00 (0.00, 0.00) | 1.03 (0.54, 1.97) |
| Ideal | 0.65 (0.44, 0.97) ‡ | 0.70 (0.46, 1.04) | 0.66 (0.36, 1.21) | 0.62 (0.48, 0.80)* |
|
| ||||
| Physical activity | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0.58 (0.37, 0.93) ‡ | 0.70 (0.46, 1.07) | 1.48 (0.82, 2.69) | 0.80 (0.62, 1.04) |
| Ideal | 0.70 (0.51, 0.96) ‡ | 0.76 (0.57, 1.03) | 1.03 (0.61, 1.72) | 0.77 (0.64, 0.93) † |
|
| ||||
| Body mass index | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0.85 (0.62, 1.15) | 0.61 (0.46, 0.82) † | 0.65 (0.42, 1.00) ‡ | 0.86 (0.70, 1.05) |
| Ideal | 0.55 (0.37, 0.82) † | 0.43 (0.30, 0.61)* | 0.45 (0.25, 0.81) † | 0.86 (0.69, 1.07) |
|
| ||||
| Diet | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0.99 (0.75, 1.31) | 1.05 (0.80, 1.37) | 1.07 (0.70, 1.64) | 0.99 (0.83, 1.17) |
| Ideal | 0.00 (0.00, 0.00) | 2.03 (0.98, 4.21) | 0.80 (0.11, 5.64) | 0.48 (0.17, 1.33) |
|
| ||||
| Total cholesterol | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0.85 (0.57, 1.26) | 0.71 (0.50, 1.00) ‡ | 0.89 (0.52, 1.54) | 0.86 (0.67, 1.09) |
| Ideal | 0.59 (0.39, 0.89) ‡ | 0.56 (0.39, 0.80) † | 0.53 (0.29, 0.96 ) ‡ | 0.71 (0.56, 0.91) † |
|
| ||||
| Blood pressure | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0.99 (0.73, 1.36) | 0.83 (0.61, 1.14) | 0.59 (0.35, 0.98) ‡ | 0.73 (0.60, 0.88) † |
| Ideal | 0.64 (0.44, 0.94) ‡ | 0.58 (0.40, 0.85) † | 0.35 (0.18, 0.66) † | 0.44 (0.34, 0.56)* |
|
| ||||
| Blood glucose | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0.71 (0.45, 1.12) | 0.75 (0.49, 1.14) | 0.62 (0.32, 1.21) | 0.89 (0.66, 1.20) |
| Ideal | 0.59 (0.40, 0.87) † | 0.46 (0.32, 0.66)* | 0.58 (0.34, 1.00) ‡ | 0.68 (0.52, 0.87) † |
Abbreviations: ATAC, ascending thoracic aorta calcification; AVC, aortic valve calcification; CI, confidence interval; CVH, cardiovascular health; DTAC, descending thoracic aorta calcification; ECC, extra-coronary calcification; MAC, mitral annular calcification.
Incidence rate ratios were derived from Poisson regression with robust variance estimation.
Incident ECC was defined as Agatston score >0 at exam 2/3 among participants with Agatston score =0 at baseline.
Model was adjusted for age, sex, race/ethnicity, education, income, health insurance, field center and time between scans.
Statistically significant results at P <0.002 are in bold font.
Results in italics were statistically significant at:
P <0.01
P <0.05.
Sample size for incident ECC = 5,520.
The trend was similar for ECC extent at baseline and progression at 2 years with some exceptions. Ideal levels of BMI and blood pressure were associated with 17% (24%, 9%) and 17% (23%, 9%) lower AVC extent at baseline, respectively (Table 6). An 8% (12%, 4%), 10% (13%, 6%) and 12% (18%, 6%) lower risk of MAC progression at 2 years were associated with ideal levels of BMI, blood pressure and blood glucose, respectively (Table 7). We observed a graded reduction in the distribution of participants with prevalent and incident ECC across CVH scores (Figures 2 & 3). Participants with inadequate scores had a higher proportion of prevalent and incident ECC in comparison to participants with average and optimal scores.
Table 6.
Multivariable-adjusted associations of CVH metrics with ECC extent
| AVC | MAC | ATAC | DTAC | |
|---|---|---|---|---|
|
| ||||
| Percent difference (95% CI) | ||||
|
| ||||
| Smoking | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | −6 (−28, 23) | −18 (−35, 4) | −5 (−23, 18) | −38 (−61, −1) ‡ |
| Ideal | −3 (−12, 6) | −7 (−15, 2) | −13 (−19, −6)* | −44 (−52, −34)* |
|
| ||||
| Physical activity | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | −2 (−12, 9) | −6 (−16, 4) | −2 (−8, 5) | 3 (−13, 22) |
| Ideal | −7 (−14, 2) | −7 (−15, 1) | −7 (−11, −2) ‡ | −7 (−19, 6) |
|
| ||||
| Body mass index | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | −8 (−16, 0) | −12 (−19, −4) † | −2 (−6, 3) | −3 (−14, 10) |
| Ideal | −17 (−24, −9)* | −18 (−25, −10)* | 2 (−4, 8) | 3 (−11, 20) |
|
| ||||
| Diet | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 1 (−6, 9) | −1 (−8, 6) | −2 (−6, 2) | 7 (−4, 20) |
| Ideal | −13 (−33, 13) | 3 (−30, 48) | 9 (−16, 40) | −17 (−51, 40) |
|
| ||||
| Total cholesterol | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | −8 (−18, 3) | 10 (−1, 23) | −7 (−14, 0) | −1 (−17, 18) |
| Ideal | −16 (−24, −6) † | 6 (−4, 17) | −10 (−16, −4) † | −15 (−28, 0) |
|
| ||||
| Blood pressure | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | −14 (−22, −6) † | −7 (−16, 2) | −4 (−9, 1) | −36 (−44, −26)* |
| Ideal | −17 (−23, −9)* | −10 (−17, −2) ‡ | −7 (−11, −3) † | −46 (−53, −38)* |
|
| ||||
| Blood glucose | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | −8 (−21, 8) | −13 (−26, 2) | 1 (−8, 10) | −7 (−26, 17) |
| Ideal | −15 (−26, −4) ‡ | −21 (−31, −9) † | −1 (−8, 6) | −24 (−38, −8) † |
Abbreviations: ATAC, ascending thoracic aorta calcification; AVC, aortic valve calcification; CI, confidence interval; CVH, cardiovascular health; DTAC, descending thoracic aorta calcification; ECC, extra-coronary calcification; MAC, mitral annular calcification.
ECC was expressed as natural log transformed (ECC + 1).
Percent difference was calculated from [Exp (β) −1]*100, derived from linear mixed-effects regression models.
Model was adjusted for age, sex, race/ethnicity, education, income, health insurance and field center.
Statistically significant results at P <0.002 are in bold font.
Results in italics were statistically significant at:
P <0.01
P <0.05.
Table 7.
Multivariable-adjusted associations of CVH metrics with ECC progression
| AVC | MAC | ATAC | DTAC | |
|---|---|---|---|---|
|
| ||||
| Percent change (95% CI) | ||||
|
| ||||
| Smoking | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | −5 (−11, 2) | 3 (−12, 21) | −6 (−17, 6) | 3 (−24, 38) |
| Ideal | −4 (−8, 2) | 1 (−4, 7) | 2 (−2, 7) | −2 (−10, 6) |
|
| ||||
| Physical activity | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 1 (−4, 7) | −6 (−11, −1) ‡ | 0 (−5, 6) | −8 (−15, 1) |
| Ideal | 0 (−5, 5) | −3 (−8, 2) | 0 (−4, 4) | −5 (−11, 2) |
|
| ||||
| Body mass index | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | −1 (−6, 3) | −4 (−9, 1) | −3 (−6, 1) | −3 (−9, 3) |
| Ideal | −3 (−7, 1) | −8 (−12, −4)* | −2 (−6, 2) | 0 (−7, 7) |
|
| ||||
| Diet | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 2 (−2, 5) | 1 (−2, 5) | 1 (−2, 4) | 3 (−2, 9) |
| Ideal | 0 (−7, 6) | 18 (−4, 46) | −5 (−25, 19) | −9 (−28, 16) |
|
| ||||
| Total cholesterol | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | −1 (−7, 5) | −1 (−8, 5) | 2 (−3, 7) | −3 (−12, 5) |
| Ideal | −1 (−6, 5) | −5 (−11, 1) | 0 (−5, 4) | −9 (−16, −1) ‡ |
|
| ||||
| Blood pressure | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0 (−4, 5) | −6 (−11, −1) ‡ | −4 (−8, 0) ‡ | −16 (−22, −10)* |
| Ideal | −3 (−7, 1) | −10 (−13, −6)* | −4 (−7, −1) ‡ | −26 (−30, −21)* |
|
| ||||
| Blood glucose | ||||
|
| ||||
| Poor | Reference | Reference | Reference | Reference |
| Intermediate | 0 (−8, 9) | −8 (−15, 1) | −5 (−11, 2) | −11 (−21, 1) |
| Ideal | −2 (−9, 5) | −12 (−18, −6)* | −6 (−11, 0) ‡ | −29 (−27, −10)* |
Abbreviations: ATAC, ascending thoracic aorta calcification; AVC, aortic valve calcification; CI, confidence interval; CVH, cardiovascular health; DTAC, descending thoracic aorta calcification; ECC, extra-coronary calcification; MAC, mitral annular calcification.
ECC was expressed as natural log transformed (ECC + 1).
Percent change was calculated from [Exp (β) −1]*100, derived from linear mixed-effects regression models.
Model was adjusted for age, sex, race/ethnicity, education, income, health insurance and field center.
Statistically significant results at P <0.002 are in bold font.
Results in italics were statistically significant at:
P <0.01
P <0.05.
We found significant interaction by race/ethnicity for the association of CVH with incident AVC (p = 0.033), AVC extent/progression (p = 0.044) and DTAC extent/progression (p<0.001). Stratified analysis showed that Hispanic participants with optimal scores had a 79% (95%, 6%) lower risk for incident AVC (Table S1). In addition, White participants with optimal scores had the lowest magnitude of AVC and DTAC extent at baseline. However, the magnitude of AVC and DTAC progression at 2 years was lowest for Hispanic participants with optimal CVH scores (Table S2). We also found significant interaction by sex for the association between CVH and AVC extent/progression (p = 0.013). The stratified analysis showed that men had a lower AVC extent at baseline compared to women (−31% vs. −19%) (Table S3).
Discussion
Summary of results
In this multi-ethnic cohort of adults free of CVD at baseline, favorable CVH as measured by the CVH scores and metrics was associated with lower prevalence and incidence of ECC as well as lower ECC extent and progression at 2 years. In stratified analysis, White participants with optimal CVH scores had the lowest AVC and DTAC extent at baseline followed by Chinese-American participants. In addition, Hispanic participants with optimal CVH scores had significantly lower risk of incident AVC and DTAC progression at 2 years. Although men and women with optimal CVH scores had lower AVC extent at baseline, the magnitude was much lower for men.
Comparison to previous studies
The findings of prior research on the relationship between CVH and ECC are comparable to the findings of our study, although these studies focused on one measure of ECC. The investigators from the Atherosclerosis Risk in Communities Study found that participants who achieved the lowest percentage of the CVH score in mid to late life had a higher prevalence of aortic sclerosis and stenosis in later life after adjusting for age, sex and race [22]. In the EPIC-Norfolk study, participants in the highest quartile of the CVH score had a lower risk and event rate for calcific aortic valve stenosis compared to participants in the lowest quartile after adjusting for age and sex [Relative Risk: 0.45 (0.31–0.65), event rate of 0.8% vs 2.9%] [23]. Traditional CVD risk factors are also linked to greater risk of ECC. Previous studies from MESA found that after adjusting for sociodemographic characteristics and other CVD risk factors, the relative risk of prevalent AVC for participants with elevated blood pressure was between 35% and 44% [39]. Current smokers had 45% greater odds of having prevalent MAC [40] and diabetes was associated with greater TAC progression [41].
Explanation of results
The greater burden of calcification in extra-coronary sites associated with poorer CVH scores may be the result of high levels of inflammatory biomarkers and endothelial dysfunction [22]. Of note, DTAC burden was greatest, a finding that is not peculiar to this study and may be associated with a larger change in pulse pressure and compliance found in DTAC compared to other vascular beds, although the exact mechanism is still under investigation [42].
Our observation that ideal levels of the metrics for smoking, BMI, cholesterol, blood pressure and blood glucose were protective against the development of ECC is not surprising and the mechanisms driving the associations have been well documented in prior studies [43–47]. However, we observed that the protective effect was not consistent across all 4 vascular beds which may suggest that the risk factors for calcification in extra-coronary sites do not always overlap. In addition, the differential magnitude of the extent and rate of progression of vascular calcification may be a contributing factor. In contrast to these findings, we did not have sufficient evidence to support the findings of prior research that showed ideal physical activity is associated with a lower risk of atherosclerosis [48]. Our finding may have been influenced by lack of statistical power as well as a much smaller chance of rejecting the null hypothesis (0.2% vs 5%). Additionally, inaccuracies associated with dietary recall may have contributed to the null results observed for the association between the dietary metric and ECC. The differential associations observed between the CVH metrics and ECC suggest that further research is required to improve our understanding of the effect of risk factors on calcification at extra-coronary sites.
The mechanisms that could explain the racial/ethnic differences in the associations between CVH and ECC are still unknown but ongoing research suggest that social determinants of health may be primary drivers of health disparities across racial and ethnic groups, more so than true genetic differences [49]. Given that men with optimal CVH scores had lower AVC extent at baseline compared to women, the differential impact of sex on the association between favorable CVH and lower ECC burden needs further exploration.
Implications
The burden of ECC is substantial. Depending on the extra-coronary site, method of analysis and age of the population, the prevalence of ECC ranges from 7.5% to 55% for asymptomatic men and women [50] with calcification at multiple extra-coronary sites significantly increasing the risk for CVD and cardiac-related mortality [24, 25]. Given the heavy clinical and economic burden associated with CVD [3], our study finding of an association between favorable CVH and a lower risk of ECC underscores the importance of encouraging the public to improve their CVH by adopting healthier lifestyle to attain favorable CVH. This prevention strategy has the potential to reduce the development of ECC which will ultimately lower the burden of CVD.
Strengths and limitations
This study has strengths. We analyzed data using a diverse population-based cohort with a prospective study design. Additionally, data collection was carried out with highly standardized methodology for many CVD risk factors and measures of ECC. However, we also have some limitations. The use of self-report questionnaires to collect data on the CVH metrics for smoking, physical activity and diet may have led to misclassification from recall bias. A single measurement of CVH at baseline may not be representative of the future CVH of participants. The inclusion of only participants without CVD at enrollment may have weakened the observed associations because participants with higher risk of ECC may have been excluded. For some of the associations examined, the time between baseline and exam 2/3 ECC assessments may not have been adequate to measure ECC incidence and progression. Data were not available for measures of calcification in other vascular beds such as the aortic arch and iliac arteries, so we could not examine the associations with the CVH metrics. Our study findings may not be generalizable to other populations because the selection of participants in MESA was non-randomized. Lastly, we cannot make causal inferences or rule out residual confounding because of the observational study design.
Conclusions
In this multi-ethnic community-based cohort study of adults free of CVD at baseline, favorable CVH was significantly associated with a lower risk of calcification at multiple extra-coronary sites suggesting that optimizing CVH may lower the risk of subclinical disease across various vascular beds. In addition, our findings emphasize the importance of primordial prevention as an intervention strategy to reduce the burden of CVD. Future studies could explore the utility of the CVH score to predict and quantify the risk of subclinical and clinical polyvascular disease.
Supplementary Material
Figure 5.
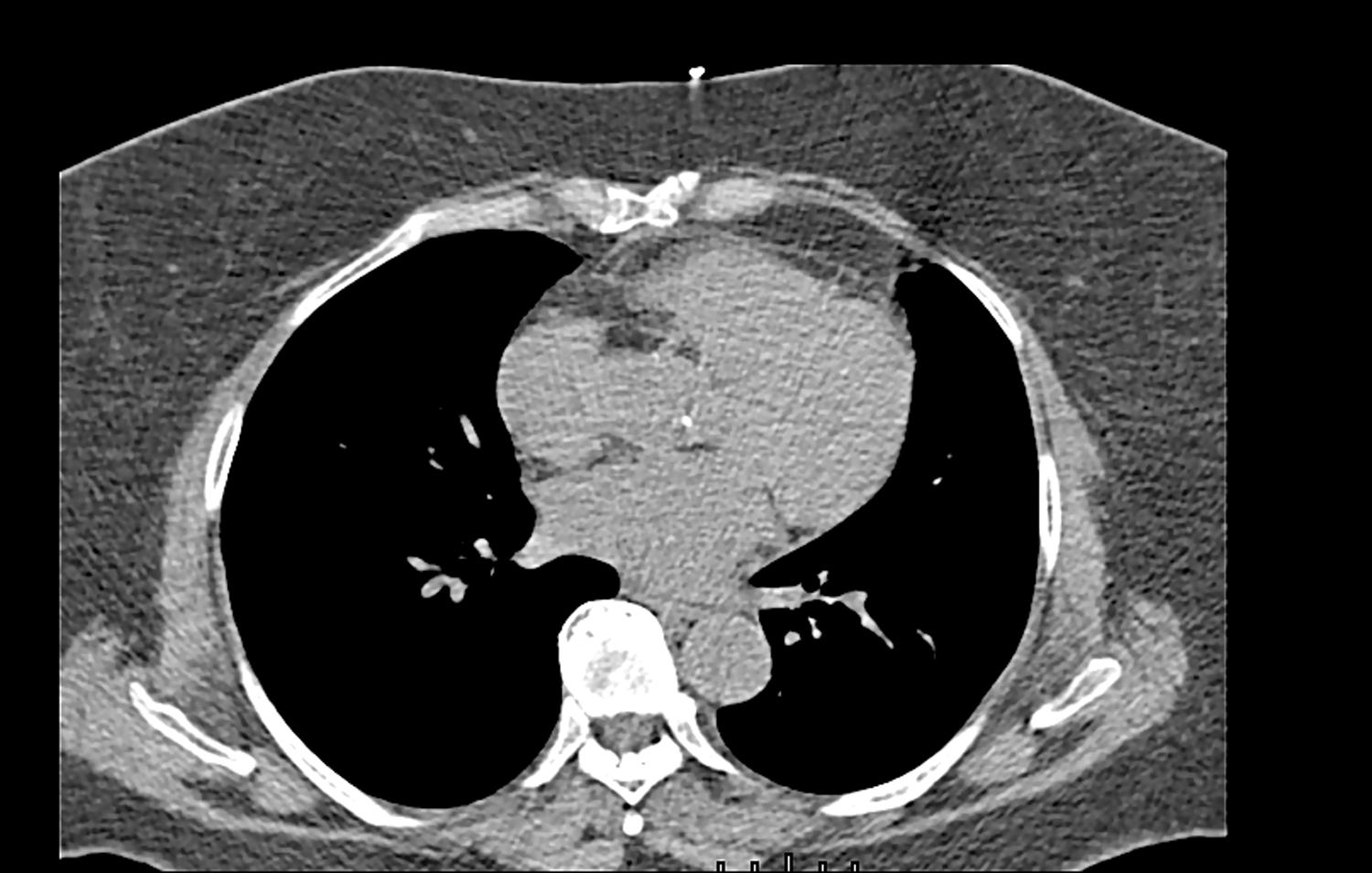
Legend. Mild AVC in a study participant with average CVH scores.
Abbreviations: AVC, aortic valve calcification; CVH, cardiovascular health.
Clinical Perspective.
Extra-coronary calcification is a marker of systemic atherosclerosis. The presence of extra-coronary calcification is associated with a significantly increased risk of clinical cardiovascular disease, particularly with calcification at multiple extra-coronary sites. The heavy socioeconomic burden associated with cardiovascular disease makes prevention a top public health priority. The construct of ideal cardiovascular health was introduced by the American Heart Association and it employs the concept of primordial prevention to reduce the incidence of cardiovascular disease by promoting the attainment of ideal levels of the cardiovascular health metrics. In this study, we found that participants with favorable cardiovascular health had a lower risk of extra-coronary calcification measured at the aortic valve, mitral annulus, ascending thoracic aorta and descending thoracic aorta. In addition, our findings showed that the magnitude of risk differed across the extra-coronary sites. Therefore, future research could evaluate the utility of the cardiovascular health metrics in the creation of a risk score to predict and quantify future clinical cardiovascular disease among patients with extra-coronary calcification. Furthermore, in the management of patients with or without overt cardiovascular disease, continued education should be provided on the importance of attaining and preserving ideal cardiovascular health. This can be achieved by the use of educational tools such as My Life Check®, an interactive website that helps people assess and monitor their cardiovascular health so they understand their risk of cardiovascular disease.
Acknowledgements
The authors thank the other investigators, the staff, and the MESA participants for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding sources
The MESA study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. Dr. Michos is additionally funded by the Amato Fund for Women’s Cardiovascular Health at Johns Hopkins University.
Non-standard Abbreviations and Acronyms
- ATAC
Ascending thoracic aorta calcification
- AVC
Aortic valve calcification
- BMI
Body mass index
- ECC
Extra-coronary calcification
- DTAC
Descending thoracic aorta calcification
- MAC
Mitral annular calcification
- MESA
Multi-Ethnic Study of Atherosclerosis
- CAC
Coronary artery calcification
- CT
Computed tomography
- CVH
Cardiovascular health
- CVD
Cardiovascular disease
Footnotes
References
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010; 121:586–613. [DOI] [PubMed] [Google Scholar]
- 2.Labarthe D, Lloyd-Jones DM. 50×50×50. Circulation. 2018; 138:968–970. [DOI] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021; 143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 4.Michos ED, Khan SS. Further understanding of ideal cardiovascular health score metrics and cardiovascular disease. Expert Rev Cardiovasc Ther. 2021; 19:607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angell SY, McConnell MV, Anderson CAM, Bibbins-Domingo K, Boyle DS, Capewell S, Ezzati M, de Ferranti S, Gaskin DJ, Goetzel RZ, et al. The American Heart Association 2030 Impact Goal: A Presidential Advisory From the American Heart Association. Circulation. 2020; 141:e120–e138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roger VL, Sidney S, Fairchild AL, Howard VJ, Labarthe DR, Shay CM, Tiner AC, Whitsel LP, Rosamond WD, American Heart Association Advocacy Coordinating Committee. Recommendations for Cardiovascular Health and Disease Surveillance for 2030 and Beyond: A Policy Statement From the American Heart Association. Circulation. 2020; 141:e104–e119 [DOI] [PubMed] [Google Scholar]
- 7.Ogunmoroti O, Michos ED, Aronis KN, Salami JA, Blankstein R, Virani SS, Spatz ES, Allen NB, Rana JS, Blumenthal RS, et al. Life’s Simple 7 and the risk of atrial fibrillation: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2018; 275:174–181. [DOI] [PubMed] [Google Scholar]
- 8.Ogunmoroti O, Oni E, Michos ED, Spatz ES, Allen NB, Rana JS, Virani SS, Blankstein R, Aronis KN, Blumenthal RS, et al. Life’s Simple 7 and Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017; 6:e005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osibogun O, Ogunmoroti O, Spatz ES, Fashanu OE, Michos ED. Ideal cardiovascular health and resting heart rate in the Multi-Ethnic Study of Atherosclerosis. Prev Med. 2020; 130:105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polonsky TS, Ning H, Daviglus ML, Liu K, Burke GL, Cushman M, Eng J, Folsom AR, Lutsey PL, Nettleton JA, et al. Association of Cardiovascular Health With Subclinical Disease and Incident Events: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017; 6:e004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benschop L, Schalekamp-Timmermans S, Schelling SJC, Steegers EAP, van Lennep JER et al. Early Pregnancy Cardiovascular Health and Subclinical Atherosclerosis. J Am Heart Assoc. 2019; 8:e011394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Niu JY, Zhao ZY, Li M, Xu M, Lu JL, Wang TG, Chen YH, Wang SY, Meng D, et al. Ideal Cardiovascular Health is Inversely Associated with Subclinical Atherosclerosis: A Prospective Analysis. Biomed Environ Sci. 2019; 32:260–271. [DOI] [PubMed] [Google Scholar]
- 13.Shpilsky D, Bambs C, Kip K, Patel S, Aiyer A, Olafiranye O, Reis SE, Erqou S. Association between ideal cardiovascular health and markers of subclinical cardiovascular disease. Clin Cardiol. 2018; 41:1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enserro DM, Vasan RS, Xanthakis VS. Twenty-Year Trends in the American Heart Association Cardiovascular Health Score and Impact on Subclinical and Clinical Cardiovascular Disease: The Framingham Offspring Study. J Am Heart Assoc. 2018; 7: e008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Alvira JM, Fsuster V, Pocock S, Sanz J, Fernández-Friera L, Laclaustra M, Fernández-Jiménez R, Mendiguren J, Fernández-Ortiz A, Ibáñez B et al. Predicting Subclinical Atherosclerosis in Low-Risk Individuals: Ideal Cardiovascular Health Score and Fuster-BEWAT Score. J Am Coll Cardiol. 2017; 70:2463–2473. [DOI] [PubMed] [Google Scholar]
- 16.Wang YQ, Wang C, Zhu L, Yuan H, Wu L, Chen Z. Ideal cardiovascular health and the subclinical impairments of cardiovascular diseases: a cross-sectional study in central south China. BMC Cardiovasc Disord. 2017; 17:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talegawkar SA, Jin Y, Kandula NR, Kanaya AM. Cardiovascular health metrics among South Asian adults in the United States: Prevalence and associations with subclinical atherosclerosis. Prev Med. 2017; 96:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaye B, Mustafic H, Laurent S, Perier M, Thomas F, Guibout C, Tafflet M, Pannier B, Boutouyrie P, Jouven X et al. Ideal Cardiovascular Health and Subclinical Markers of Carotid Structure and Function: The Paris Prospective Study III. Arterioscler Thromb Vasc Biol. 2016; 36:2115–2124. [DOI] [PubMed] [Google Scholar]
- 19.Laitinen TT, Pahkala K, Magnussen CG, Oikonen M, Viikari JSA, Sabin MA, Daniels SR, Heinonen OJ, Taittonen L, Hartiala O, et al. Lifetime measures of ideal cardiovascular health and their association with subclinical atherosclerosis: The Cardiovascular Risk in Young Finns Study. Int J Cardiol. 2015; 185:186–191. [DOI] [PubMed] [Google Scholar]
- 20.Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, Wang TJ, Tofler G, Vasan RS. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014; 130:1676–1683. [DOI] [PubMed] [Google Scholar]
- 21.Osibogun O, Ogunmoroti O, Ferraro RA, Ndumele CE, Burke GL, Larson NB, Bielinski SJ, Michos ED. Favorable cardiovascular health is associated with lower hepatocyte growth factor levels in the Multi-Ethnic Study of Atherosclerosis. Front Cardiovasc Med. 2022; 8: 760281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sengeløv M, Cheng S, Biering-Sørensen T, Matsushita K, Konety S, Solomon SD, Folsom AR, Shah AM et al. Ideal Cardiovascular Health and the Prevalence and Severity of Aortic Stenosis in Elderly Patients. J Am Heart Assoc. 2018; 7:e007234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrot N, Boekholdt SM, Mathieu P, Wareham NJ, Khaw KT, Arsenault BJ. Life’s simple 7 and calcific aortic valve stenosis incidence in apparently healthy men and women. Int J Cardiol. 2018;269:226–228. doi: 10.1016/j.ijcard.2018.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tison GH, Blaha MJ, Nasir K. Atherosclerosis imaging in multiple vascular beds--enough heterogeneity to improve risk prediction? Atherosclerosis. 2011; 214: 261–263. [DOI] [PubMed] [Google Scholar]
- 25.Tison GH, Guo M, Blaha MJ, McClelland RL, Allison MA, Szklo M, Wong ND, Blumenthal RS, Budoff MJ, Nasir K, et al. Multisite extracoronary calcification indicates increased risk of coronary heart disease and all-cause mortality: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2015; 9: 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002; 156:871–881. [DOI] [PubMed] [Google Scholar]
- 27.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999; 8:805–813. [DOI] [PubMed] [Google Scholar]
- 28.Unger E, Diez-Roux AV, Lloyd-Jones DM, Mujahid MS, Nettleton JA, Bertoni A, Badon S, Ning H, Allen NB. Association of neighborhood characteristics with cardiovascular health in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014; 7:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990; 43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 30.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999; 9:314–324. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd-Jones DM. Improving the cardiovascular health of the US population. JAMA. 2012; 307:1314–1316. [DOI] [PubMed] [Google Scholar]
- 32.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005; 234:35–43. [DOI] [PubMed] [Google Scholar]
- 33.Budoff MJ, Takasu J, Katz R, Mao S, Shavelle DM, O’Brien KD, Blumenthal RS, Carr JJ, Kronmal R. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006; 13:166–172. [DOI] [PubMed] [Google Scholar]
- 34.Budoff MJ, Katz R, Wong ND, Nasir K, Mao SS, Takasu J, Kronmal R, Detrano RC, Shavelle DM, Blumenthal RS, et al. Effect of scanner type on the reproducibility of extracoronary measures of calcification: the multi-ethnic study of atherosclerosis. Acad Radiol. 2007; 14:1043–1049. [DOI] [PubMed] [Google Scholar]
- 35.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15:827–832. [DOI] [PubMed] [Google Scholar]
- 36.Ezeigwe A, Fashanu OE, Zhao D, Budoff MJ, Otvos JD, Thomas IC, Mora S, Tibuakuu M, Michos ED. The novel inflammatory marker GlycA and the prevalence and progression of valvular and thoracic aortic calcification: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2019; 282: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogunmoroti O, Allen NB, Cushman M, Michos ED, Rundek T, Rana JS, Blankstein R, Blumenthal RS, Blaha MJ, Veledar E, et al. Association Between Life’s Simple 7 and Noncardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2016; 5:e003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osibogun O, Ogunmoroti O, Mathews L, Okunrintemi V, Tibuakuu M, Michos ED. Greater Acculturation is Associated With Poorer Cardiovascular Health in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2021;10:e019828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O’Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006; 113:2113–2119. [DOI] [PubMed] [Google Scholar]
- 40.Kanjanauthai S, Nasir K, Katz R, Rivera JJ, Takasu J, Blumenthal RS, Eng J, Budoff MJ. Relationships of mitral annular calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2010; 213: 558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katz R, Budoff MJ, O’Brien KD, Wong ND, Nasir K. The metabolic syndrome and diabetes mellitus as predictors of thoracic aortic calcification as detected by non-contrast computed tomography in the Multi-Ethnic Study of Atherosclerosis. Diabet Med. 2016; 33: 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmad MM, Pir SHA, Muhammad MN, Hussaini S, Kiani IA, Ahmad MN, Razzaque I, Syed MN, Ullah R, Suhail Allaqaband, et al. Influence of Differential Calcification in the Descending Thoracic Aorta on Aortic Pulse Pressure. J Patient Cent Res Rev. 2017; 4:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai J, Jan Y, Yun C, Sung K, Liu C, Kuo J, Hung C, Wu T, Lin J, Hou C et al. Associations of cigarette smoking and burden of thoracic aortic calcification in asymptomatic individuals: A dose-response relationship. PLoS One. 2020; 15:e0227680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schinzari F, Tesauro M, Bertoli A, Valentini A, Veneziani A, Campia U, Cardillo C. Calcification biomarkers and vascular dysfunction in obesity and type 2 diabetes: influence of oral hypoglycemic agents. Am J Physiol Endocrinol Metab. 2019; 317:E658–E666. [DOI] [PubMed] [Google Scholar]
- 45.Kottke BA, Pineda AA, Case MT, Orsuzar AM, Brzys KA. Hypercholesterolemia and atherosclerosis: present and future therapy including LDL-apheresis. J Clin Apher. 1988; 4:35–46. [DOI] [PubMed] [Google Scholar]
- 46.Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995. 25:155–161. [DOI] [PubMed] [Google Scholar]
- 47.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002. 287: 2570–2581. [DOI] [PubMed] [Google Scholar]
- 48.Kamimura D, Cain-Shields LR, Clark D, Oshunbade AA, Ashley KE, Guild CS, Loprinzi PD, Newton R, Blaha MJ, Suzuki T et al. Physical Activity, Inflammation, Coronary Artery Calcification, and Incident Coronary Heart Disease in African Americans: Insights From the Jackson Heart Study. Mayo Clin Proc. 2021; 96:901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nasir K, Katz R, Takasu J, Shavelle DM, Detrano R, Lima JA, Blumenthal RS, O’Brien K, Budoff MJ. Ethnic differences between extra-coronary measures on cardiac computed tomography: multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. 2008; 198:104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shavelle D, Takasu J. Chapter 8: Extra-Coronary Calcium. In: Shibane JS, Budoff MJ, eds. Cardiac CT Imaging: Diagnosis of Cardiovascular Disease. 2nd ed. Springer-Verlag London Limited; 2006:107–122. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.