Abstract
We aimed to review the data available to evaluate the long-term consequences of coronavirus disease 2019 (COVID-19) at 6 months and above. We searched relevant observational cohort studies up to 9 February 2022 in Pubmed, Embase, and Web of Science. Random-effects inverse-variance models were used to evaluate the Pooled Prevalence (PP) and its 95% confidence interval (CI) of long-term consequences. The Newcastle–Ottawa quality assessment scale was used to assess the quality of the included cohort studies. A total of 40 studies involving 10,945 cases of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection were included. Of the patients, 63.87% had at least one consequence at the 6 month follow-up, which decreased to 58.89% at 12 months. The most common symptoms were fatigue or muscle weakness (PP 6–12 m = 54.21%, PP ≥ 12 m = 34.22%) and mild dyspnea (Modified Medical Research Council Dyspnea Scale, mMRC = 0, PP 6–12 m = 74.60%, PP ≥ 12 m = 80.64%). Abnormal computerized tomography (CT; PP 6–12 m = 55.68%, PP ≥ 12 m = 43.76%) and lung diffuse function impairment, i.e., a carbon monoxide diffusing capacity (DLCO) of < 80% were common (PP 6–12 m = 49.10%, PP ≥ 12 m = 31.80%). Anxiety and depression (PP 6–12 m = 33.49%, PP ≥ 12 m = 35.40%) and pain or discomfort (PP 6–12 m = 33.26%, PP ≥ 12 m = 35.31%) were the most common problems that affected patients’ quality of life. Our findings suggest a significant long-term impact on health and quality of life due to COVID-19, and as waves of ASRS-CoV-2 infections emerge, the long-term effects of COVID-19 will not only increase the difficulty of care for COVID-19 survivors and the setting of public health policy but also might lead to another public health crisis following the current pandemic, which would also increase the global long-term burden of disease.
Keywords: COVID-19, long-term consequence, systematic review, meta-analysis
1. Introduction
Caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the pandemic of coronavirus disease 2019 (COVID-19) is currently still the greatest global public health challenge. Reported to the World Health Organization (WHO), globally, as of 5 April 2022, there have been more than 490 million confirmed cases of COVID-19, including more than 6 million deaths [1]. However, the natural history, clinical course, and long-term effects are still not fully understood [2]. While the majority of patients recover from COVID-19, for a significant number of people, the virus poses a range of serious long-term effects or complications, regardless if they are men or women, hospitalized or not, young or old, or even children [2,3]. On 6 October 2021, the WHO developed a clinical case definition of the post-COVID-19 condition by Delphi consensus: the post-COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis [4,5].
The post-COVID-19 condition, also known as long COVID-19, has become an important target for research and clinical practice. However, the prevalence of long COVID-19 is not the same in current studies due to the different research methods, but it seems to be significant. Statistics from Altea (a network for sharing evidence-based information on the long-term effects of COVID-19) showed that around a quarter of people who have had COVID-19 continue to experience symptoms for at least a month, and one in 10 are still unwell after 12 weeks [6,7]. Luiza’s systematic review showed that the frequency of long-term COVID-19 in the acute phase or 3–24 weeks after discharge ranged from 4.7% to 80% [8]. A meta-analysis of more than 50 long-term effects of COVID-19 showed that 80% of SARS-CoV-2 infected patients developed at least one long-term symptom, but the study defined long COVID-19 as only 14–110 days after infection with the virus, which was a little short and might overestimate the prevalence of longer-term symptoms [9].
According to the WHO, common symptoms of long COVID-19 include, but are not limited to, fatigue, shortness of breath, and cognitive impairment, and generally have an impact on daily functioning [3,4,10]. These symptoms might be new after recovery from an acute COVID-19 episode, or persistent from the initial infection [3,4,10]. Previous studies have shown that the health effects of long COVID-19 may be multi-system, including not only non-specific general symptoms but also respiratory, cardiovascular, blood, kidney, gastrointestinal, neurological, and metabolic system effects, and even thrombosis, retinal abnormalities, male erectile dysfunction, and other complications [8,11,12]. In addition, COVID-19 might be related to long-term decreased quality of life and mental health issues [13,14,15,16], a meta-analysis suggested that post-acute COVID-19 syndrome was associated with poor quality of life and persistent symptoms, including fatigue, dyspnea, anosmia, sleep disturbances, and worse mental health [15]. A recent study based on the United Kingdom Biobank (aged 51–81) reported that SARS-CoV-2 was associated with structural changes in the brain, such as changes in the frontal cortex and parahippocampal gyrus, tissue damage in areas linked to primary olfactory cortex function, and a reduction in global brain size [17]. However, it still has not been determined whether these changes were related to any long-term functional effects or whether they will return to baseline over time [18].
With the re-emergence of new waves of SARS-CoV-2 infection, long COVID-19 is expected to produce another public health crisis on the heels of the current pandemic [11]. Therefore, it is imperative to emphasize this situation and increase the awareness of medical professionals, patients, the public, and policymakers [11]. Although there have been more and more studies on the long-term effects of COVID-19, they have mostly been limited to specific systems and the conclusions were distinguished. Previous reviews have been limited to three or six months or less after the onset of acute COVID-19 and limited to specific systems, making it difficult to fully assess the longer-term effects of COVID-19. Thus, we aimed to assess the long-term effects of COVID-19 at 6 months and above to provide a more comprehensive and scientific basis for the care and rehabilitation of COVID-19 survivors, the surveillance of these patients, and setting public health policy for healthcare facilities.
2. Methods
2.1. Search Strategy and Selection Criteria
We searched studies without language restrictions in the PubMed, Embase, and Web of Science databases up to 9 February 2022 with the following search terms: (COVID-19 OR SARS-CoV-2 OR coronavirus OR long COVID-19 OR post COVID-19) AND (long-term effect OR sequelae OR consequences) AND (cohort OR follow-up OR retrospective OR prospective). We used EndNoteX8.2 (Thomson Research Soft, Stanford, CA, USA) to manage records, screen, and exclude duplicates. This study was strictly performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). This study was registered on PROSPERO (CRD42022309720).
We included observational cohort studies that examined the long-term consequences of COVID-19 at 6 months and above. The following studies were excluded: (1) irrelevant to the subject of the meta-analysis, such as studies that did not use SARS-CoV-2 infection as the exposure; (2) insufficient data to calculate the prevalence of long-term COVID-19 consequences; (3) duplicate studies or overlapping participants; (4) reviews, editorials, conference papers, case series/reports, secondary analysis or animal experiments; (5) qualitative designs; and (6) studies that did not clarify the identification of COVID-19. For example, the confirmed diagnosis of COVID-19 via a reverse-transcription polymerase chain reaction (rt-PCR) test, serologic test, or other means was not mentioned in the text.
Studies were identified by two investigators (MYR and DJ) independently following the criteria above, while discrepancies were solved by consensus or with a third investigator (LQ).
2.2. Data Extraction
The following data were extracted from the selected studies: (1) basic information of the studies, including the first author, publication time, and country where the study was conducted; (2) characteristics of the study population, including the sample size, median age, gender, follow-up period, smoking status, severity of COVID-19, underlying diseases, admission to hospital or intensive care unit (ICU), and length of stay (LOS); (3) clinical features of COVID-19, including the number of cases with general COVID-19-related symptoms, respiratory symptoms, cardiovascular symptoms, gastrointestinal symptoms, and neurological symptoms, as well as the results of a pulmonary functional test (PFT) and chest computerized tomography (CT); (4) the number of cases with psychiatric problems; and (5) the number of cases with problems in 5 dimensions of the European Quality of Life Five-Dimension Five-Level Scale (EQ-5D-5L), which is an instrument developed for describing and valuing health-related quality of life by the EuroQol Group in 1987. A template was used for the primary data extraction, as shown in Supplementary Table S1.
The data extraction and determination of information eligibility were conducted by two investigators (MYR and DJ) independently following the criteria above, while discrepancies were solved by consensus or with a third investigator (LQ).
2.3. Quality Assessment and Risk of Bias
We used the Newcastle–Ottawa quality assessment scale to evaluate the risk of bias in the included cohort studies. Cohort studies were classified as having a low (≥7 stars), moderate (5–6 stars), or high risk of bias (≤4 stars), with an overall quality score of 9 stars. We used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to evaluate the evidence quality of the long-term consequences of COVID-19.
Quality assessment was conducted by two investigators (MYR and DJ) independently, while discrepancies were solved by consensus or with a third investigator (LQ).
2.4. Data Synthesis and Statistical Analysis
We performed a meta-analysis to estimate the Pooled Prevalence (PP) and its 95% confidence interval (CI) of the long-term consequences of COVID-19 at 6 months and above. We performed subgroup analyses by the follow-up period (6–12 months and ≥12 months), severity of COVID-19 (non-severe and severe; the non-severe group included mild and moderate COVID-19, and the severe group included severe and critical COVID-19), whether patients were hospitalized (inpatients and outpatients), and gender. Random-effects or fixed-effects models were used to pool the rates and adjusted estimates across studies separately, based on the heterogeneity among estimates (I²). Fixed-effects models were used if I² ≤ 50%, which represents low to moderate heterogeneity, and random-effects models were used if I² ≥ 50%, representing substantial heterogeneity. The D-L method was used to estimate the tau square in the case of random-effects models. Publication bias was assessed by Harbord’s modified test. All analyses were performed using Stata version 16.0 (Stata Corp, College Station, TX, USA).
3. Results
3.1. Basic Characteristics
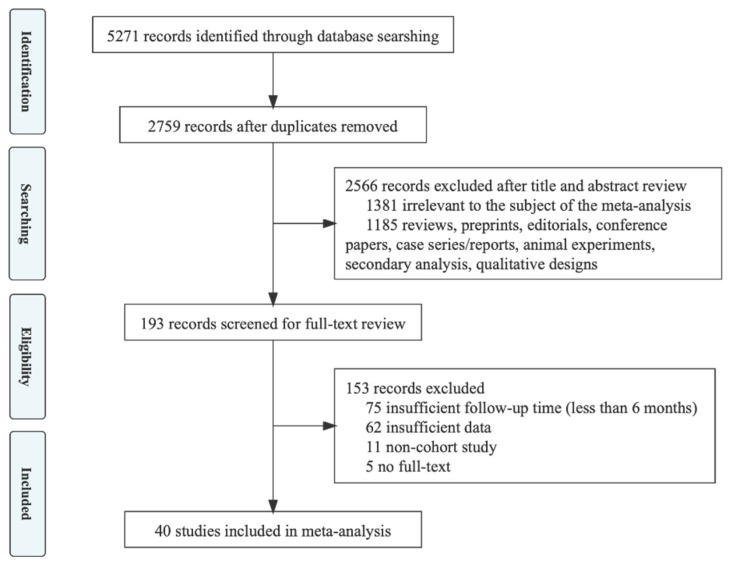
In the initial literature search, 5271 potential articles were identified up to 9 February 2022 (1459 in PubMed, 1894 in Embase, 1918 in Web of Science. A total of 2512 duplicates were excluded. After reading the titles and abstracts, 2566 articles were excluded based on the inclusion and exclusion criteria. Among the 193 studies under full-text review, 153 studies were excluded. Eventually, 40 studies were included in this meta-analysis based on the inclusion criteria [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. The literature retrieval flow chart is shown in Figure 1.
Figure 1.
Flowchart of the study selection.
The included studies were observational cohort studies describing the long-term consequences of COVID-19 at follow-up 6 months and above, which involved 10,945 cases of SARS-CoV-2 infection. A total of 26 studies described COVID-19 consequences at 6–12 months’ follow-up and 19 studies described COVID-19 consequences at 12 months and above. The majority of the included studies were of great methodological rigor (i.e., 7–9 stars on the Newcastle–Ottawa Scale); only 2 included studies had 6 stars, mainly due to the insufficient comparability between the exposed cohort and unexposed cohort. The characteristics of the included studies are shown in Supplementary Table S2.
3.2. Pooled Prevalence of COVID-19 Symptoms at 6 Months and Above
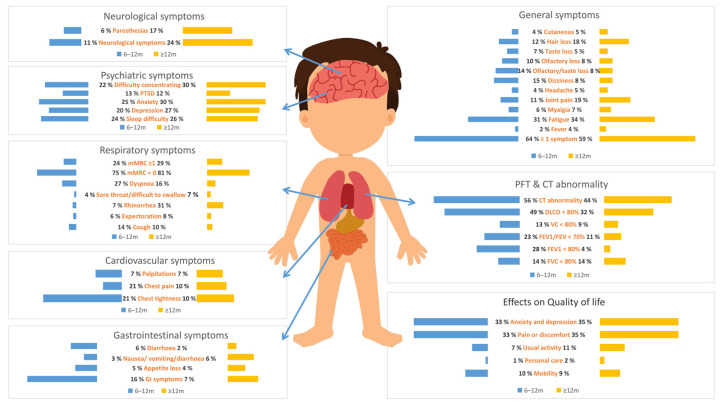
A total of 63.87% (95% CI, 53.64–74.09%) of COVID-19 patients reported at least one symptom at 6 to 12 months, which dropped to 58.89% (95% CI, 45.87–71.91%) at 12 months and above. COVID-19 patients are at risk for long-term symptoms from multiple systems, as shown in Table 1 and Figure 2.
Table 1.
Pooled prevalence of COVID-19 consequences at follow-up 6 months and above.
| Consequences | 6–12 Months | 12 Months and Above | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Studies | Patients n/N |
PP (%) |
95% CI (%) |
p-Value | I2 | Number of Studies | Patients n/N |
PP (%) |
95% CI (%) |
p-Value | I2 | |
| General symptoms | ||||||||||||
| ≥1 Symptom | 13 | 4051/6477 | 63.87 | 53.64–74.09 | <0.05 | 98.70% | 8 | 1230/2290 | 58.89 | 45.87–71.91 | <0.05 | 97.20% |
| Fever | 7 | 64/3403 | 2.07 | 0.32–3.82 | <0.05 | 93.50% | 7 | 12/778 | 3.53 | −0.45–7.50 | >0.05 | 70.40% |
| Chill | 2 | 129/815 | 13.0 3 | −0.33–26.39 | >0.05 | 97.20% | ||||||
| Fatigue | 10 | 793/3000 | 30.94 | 20.21.41.66 | <0.05 | 98.20% | 14 | 822/3248 | 34.22 | 23.75–44.70 | <0.05 | 98.00% |
| Muscle weakness | 2 | 34/847 | 4.2 | 1.68–6.72 | <0.05 | 15.40% | - | - | - | - | - | - |
| Myalgia or joint pain | - | - | - | - | - | - | 2 | 187/503 | 34.52 | 9.01–60.02 | <0.05 | 97.50% |
| Fatigue or muscle weakness | 3 | 1949/3459 | 54.21 | 45.16–63.27 | <0.05 | 96.40% | - | - | - | - | - | - |
| Limited mobility | 3 | 83/943 | 21.81 | −4.17–47.78 | <0.05 | 97.90% | - | - | - | - | - | - |
| Myalgia | 9 | 271/4988 | 6.34 | 3.89–8.79 | <0.05 | 93.90% | 9 | 128/2368 | 6.59 | 4.05–9.13 | <0.05 | 79.80% |
| Joint pain | 6 | 396/3900 | 11.25 | 7.53–14.98 | <0.05 | 92.60% | 8 | 320/2058 | 18.73 | 12.24 –25.22 | <0.05 | 91.60% |
| Headache | 8 | 174/5134 | 3.68 | 2.20–5.15 | <0.05 | 89.70% | 5 | 93/1787 | 5.24 | 3.47–7.01 | <0.05 | 37.00% |
| Dizziness | 5 | 263/3289 | 14.96 | 9.72 –20.19 | <0.05 | 95.40% | 3 | 100/1607 | 8.14 | 3.82–12.46 | <0.05 | 77.70% |
| Olfactory or taste loss | 4 | 161/1556 | 14.38 | 8.40–20.36 | <0.05 | 90.20% | 2 | 21/259 | 8.21 | 2.84–13.58 | <0.05 | 54.60% |
| Olfactory loss | 8 | 491/4507 | 10.07 | 5.47–14.68 | <0.05 | 97.20% | 8 | 127/2004 | 8.22 | 5.21–11.23 | <0.05 | 70.00% |
| Taste loss | 8 | 338/4507 | 7.48 | 4.46–10.50 | <0.05 | 94.70% | 7 | 88/2308 | 4.55 | 2.45–6.65 | <0.05 | 78.00% |
| Hair loss | 7 | 712–4485 | 11.58 | 4.08–19.08 | <0.05 | 98.90% | 4 | 255–1807 | 18.42 | 9.21–27.63 | <0.05 | 94.30% |
| Cutaneous | 7 | 150/4200 | 3.87 | 2.32–5.43 | <0.05 | 84.50% | 5 | 99/2121 | 4.5 | 3.42–5.58 | <0.05 | 16.40% |
| Respiratory symptoms | ||||||||||||
| Respiratory symptoms | - | - | - | - | - | - | 2 | 79/241 | 32.7 | 3.97–61.43 | <0.05 | 96.20% |
| Cough | 12 | 381/3241 | 13.85 | 9.00–18.70 | <0.05 | 96.40% | 9 | 81/973 | 9.54 | 5.26–13.81 | <0.05 | 83.10% |
| Expectoration | 3 | 36/554 | 6.45 | 1.01–11.90 | <0.05 | 86.60% | 4 | 30/488 | 7.97 | 1.23–14.71 | <0.05 | 88.50% |
| Rhinorrhea | 2 | 13/267 | 7.44 | –5.43–20.30 | >0.05 | 89.00% | 2 | 68/210 | 30.93 | 11.60–50.26 | <0.05 | 90.10% |
| Sore throat or difficulty swallowing | 7 | 233/4885 | 4.43 | 2.49–6.37 | <0.05 | 92.60% | 6 | 78/1870 | 7.33 | 3.19–11.48 | <0.05 | 78.20% |
| Dyspnea | 12 | 717/3173 | 27.06 | 18.67–35.44 | <0.05 | 97.60% | 8 | 127/1129 | 16.43 | 9.66–23.20 | <0.05 | 91.90% |
| mMRC = 0 | 5 | 3491/3673 | 74.5 | 66.94–82.06 | <0.05 | 91.50% | 3 | 1042/1448 | 80.64 | 62.87–98.42 | <0.05 | 97.70% |
| mMRC ≥ 1 | 5 | 3491/3673 | 24.49 | 21.17–27.81 | <0.05 | 76.30% | 4 | 510/1622 | 29.1 | 10.64–47.56 | <0.05 | 98.10% |
| Cardiovascular symptoms | ||||||||||||
| Chest tightness | 2 | 200/815 | 21.18 | 4.94–37.43 | <0.05 | 97.00% | 3 | 24/278 | 10.24 | 0.77–19.71 | <0.05 | 62.30% |
| Chest pain | 9 | 265/5572 | 4.78 | 2.88–6.68 | <0.05 | 92.20% | 5 | 117–2009 | 7.76 | 2.60–12.91 | <0.05 | 93.50% |
| Back pain | 2 | 20/478 | 7.19 | −3.04–17.42 | >0.05 | 84.30% | - | - | - | - | - | - |
| Palpitations | 5 | 303/3604 | 7.19 | 3.68–10.71 | <0.05 | 93.40% | 7 | 173/2299 | 6.79 | 3.81–9.78 | < 0.05 | 86.00% |
| Gastrointestinal symptoms | ||||||||||||
| GI symptoms | 4 | 87/1049 | 15.62 | 4.91–26.34 | <0.05 | 96.80% | 5 | 71/1178 | 6.57 | 2.48–10.65 | <0.05 | 90.60% |
| Loss of appetite | 7 | 310/5106 | 4.65 | 1.98–7.32 | <0.05 | 96.70% | 5 | 58/1666 | 3.87 | 1.86–5.88 | <0.05 | 47.10% |
| Nausea, vomiting or diarrhea | 4 | 126/3699 | 3.47 | 1.41–5.52 | <0.05 | 91.80% | 4 | 34/1550 | 5.86 | 0.73–11.00 | <0.05 | 84.80% |
| Nausea | - | - | - | - | - | - | 2 | 8/63 | 10.55 | 0.75–20.35 | <0.05 | 41.70% |
| Vomiting | 2 | 16/996 | 2.01 | 1.03–2.98 | <0.05 | 0 | - | - | - | - | - | - |
| Diarrhea | 8 | 146/2272 | 6 | 2.86–9.15 | <0.05 | 94.10% | 4 | 8/397 | 2.18 | –0.56–4.91 | >0.05 | 45.90% |
| Stomachache | 2 | 50/865 | 6.52 | 2.48–10.57 | <0.05 | 40.70% | - | - | - | - | - | - |
| Constipation | 2 | 22/865 | 6.01 | −3.69–15.71 | >0.05 | 84.50% | - | - | - | - | - | - |
| Altered bowel habits | - | - | - | - | - | - | 2 | 27/165 | 16.17 | 10.56–21.78 | <0.05 | 0.00% |
| Neurological symptoms | ||||||||||||
| Neurological symptoms | 3 | 232/1803 | 10.81 | 0.40–21.21 | <0.05 | 98.60% | 4 | 167/634 | 23.85 | 11.42–36.29 | <0.05 | 92.70% |
| Polyneuropathy | 2 | 32/847 | 7.48 | −2.95–17.91 | >0.05 | 79.30% | - | - | - | - | - | - |
| Paresthesias | 4 | 68/1305 | 6.24 | 2.24–10.24 | <0.05 | 93.60% | 4 | 127/679 | 17.42 | 6.90–27.95 | <0.05 | 92.70% |
| Disorientation or confusion | 3 | 37/1237 | 2.7 | 0.31–5.09 | <0.05 | 88.10% | - | - | - | - | - | - |
| Forgetfulness | 2 | 131/815 | 18.65 | 5.23–32.08 | <0.05 | 94.80% | - | - | - | - | - | - |
| Memory loss | 3 | 89/850 | 10.65 | 1.86–19.43 | <0.05 | 96.50% | - | - | - | - | - | - |
| Visual impairment | 3 | 30/760 | 8.11 | −0.22–16.45 | >0.05 | 89.40% | - | - | - | - | - | - |
| Hearing impairment | 2 | 15/815 | 1.76 | 0.86–2.67 | <0.05 | 0.00% | - | - | - | - | - | - |
| Psychiatric symptoms | ||||||||||||
| Sleep difficulty | 9 | 1146/5121 | 24.11 | 14.67–33.56 | <0.05 | 98.90% | 5 | 476/2120 | 26.31 | 15.73–36.89 | <0.05 | 96.20% |
| GAD-7 score ≥ 10 | 2 | 62/639 | 10.8 | 8.26–13.34 | <0.05 | - | - | - | - | - | - | - |
| Depression | 6 | 301/1968 | 20.16 | 10.36–29.97 | <0.05 | 97.30% | 5 | 196/737 | 27.26 | 16.23–38.30 | <0.05 | 92.30% |
| Anxiety | 6 | 374/1970 | 25.19 | 13.88–36.49 | <0.05 | 97.60% | 5 | 213/737 | 29.78 | 16.29–43.27 | <0.05 | 94.70% |
| PTSD | 3 | 73/522 | 13.41 | 4.30–22.51 | <0.05 | 88.70% | 3 | 68/523 | 11.57 | 0.50–22.64 | <0.05 | 95.70% |
| Difficulty concentrating | 3 | 111/719 | 22.47 | 4.49–40.44 | <0.05 | 96.90% | 3 | 100/ 376 | 29.47 | 19.80–39.14 | <0.05 | 69.50% |
| PFT | ||||||||||||
| FVC < 80% | 4 | 45/441 | 13.66 | 5.23–22.09 | <0.05 | 64.90% | 5 | 43/ 374 | 12.78 | 3.99–21.56 | <0.05 | 87.10% |
| FEV1 < 80% | 2 | 11/46 | 28.03 | 1.04–55.03 | <0.05 | 66.80% | 3 | 28/216 | 13.81 | 5.57–22.05 | <0.05 | 59.50% |
| FEV1/FEV < 70% | 2 | 8/46 | 22.86 | 8.95–36.77 | <0.05 | - | 3 | 9/ 223 | 4.01 | −1.37–9.39 | >0.05 | 67.10% |
| VC < 80% | 2 | 43/323 | 13.27 | 9.57–16.97 | <0.05 | 0.00% | 2 | 22/199 | 11.05 | 6.70–15.41 | <0.05 | 0.00% |
| TLC < 80% | - | - | - | - | - | - | 4 | 28/285 | 9.28 | 4.28–14.27 | <0.05 | 49.40% |
| DLCO < 80% | 4 | 223/510 | 49.1 | 33.27–64.92 | <0.05 | 90.60% | 6 | 115/371 | 31.8 | 18.65–44.95 | <0.05 | 88.10% |
| CT results | ||||||||||||
| CT abnormality | 4 | 291/627 | 55.68 | 26.75–84.62 | <0.05 | 98.40% | 4 | 139/330 | 43.76 | 7.78–79.74 | <0.05 | 98.30% |
| GGO | 5 | 102/408 | 21.25 | 9.79–32.71 | <0.05 | 89.10% | 4 | 74/292 | 21.35 | 8.30–34.39 | <0.05 | 87.80% |
| Consolidation | 4 | 11/325 | 2.56 | 0.78–4.35 | <0.05 | 5.50% | 2 | 2/112 | 2.13 | –0.79–5.04 | >0.05 | - |
| Reticular pattern | 4 | 36/290 | 11.3 | 3.29–19.30 | <0.05 | 80.30% | 3 | 7/195 | 3.93 | 1.07–6.79 | <0.05 | 0.00% |
| Fibrosis | 3 | 108/272 | 66.28 | 52.35–80.21 | <0.05 | 63.00% | 3 | 29/209 | 13.88 | 6.04–21.72 | <0.05 | 56.40% |
| Crazy paving pattern | 3 | 0/211 | - | - | - | - | - | - | - | - | - | - |
| Air bronchogram | 3 | 0/211 | - | - | - | - | - | - | - | - | - | - |
| Bronchiectasis | 3 | 57/315 | 16.19 | −1.82–34.21 | >0.05 | 96.50% | 2 | 15/180 | 7.39 | −5.54–20.33 | >0.05 | 91.90% |
| Traction bronchiectasis | 2 | 16/93 | 17.63 | −3.45–38.70 | >0.05 | 86.20% | - | - | - | - | - | - |
| Nodules | 2 | 20/166 | 9 | −5.43–23.44 | >0.05 | 92.70% | 3 | 87/209 | 38.56 | 18.95–58.17 | <0.05 | 87.20% |
| Irregular interface | 2 | 12/93 | 12.88 | 6.07–19.68 | <0.05 | 0.00% | - | - | - | - | - | - |
| Parenchymal band | 2 | 30/93 | 32.53 | 16.45–48.60 | <0.05 | 64.90% | - | - | - | - | - | - |
| Pleural effusion | 3 | 2/284 | 1.69 | −0.63–4.02 | >0.05 | - | - | - | - | - | - | - |
| Pericardial effusion | 2 | 16/170 | 13.56 | 7.38–19.74 | <0.05 | - | - | - | - | - | - | - |
| Lymphadenopathy | 2 | 5/170 | 4.24 | 0.60–7.87 | <0.05 | - | - | - | - | - | - | - |
| Interlobular septal thickening | 4 | 63/294 | 21.75 | 6.66–36.84 | <0.05 | 91.60% | 3 | 14/195 | 7.33 | 1.68–12.98 | <0.05 | 53.80% |
| Lines and bands | - | - | - | - | - | - | 2 | 60/191 | 30.97 | −0.91–62.85 | >0.05 | 96.30% |
| Quality of life evaluation (EQ-5D-5L) | ||||||||||||
| Mobility | 4 | 278/3421 | 10.36 | 6.88–13.83 | <0.05 | 90.90% | 2 | 132/1436 | 9.18 | 7.68–10.67 | <0.05 | 0.00% |
| Personal care | 4 | 40/3422 | 0.94 | 0.11–1.77 | <0.05 | 83.20% | 2 | 23/1436 | 1.6 | 0.95–2.25 | <0.05 | 0.00% |
| Usual activity | 4 | 129/3413 | 6.68 | 3.61–9.76 | <0.05 | 96.30% | 2 | 52/1436 | 10.76 | −8.04–29.55 | >0.05 | 97.30% |
| Pain or discomfort | 4 | 989/3415 | 33.26 | 27.01–39.51 | <0.05 | 92.60% | 2 | 441/1436 | 35.31 | 22.38–48.24 | <0.05 | 90.60% |
| Anxiety and depression | 4 | 882/3418 | 33.49 | 23.87–43.12 | <0.05 | 97.20% | 2 | 406/1436 | 35.4 | 16.39–54.41 | <0.05 | 95.60% |
| 6MWT (distance lower than expected %) | 3 | 1086/3258 | 17.33 | 10.64–24.02 | <0.05 | 95.60% | - | - | - | - | - | - |
Abbreviations: PP, pooled prevalence; CI, confidence interval; mMRC, Modified Medical Research Council Dyspnea Scale; GI, gastrointestinal; GAD-7, generalized anxiety disorder-7; PTSD, post-traumatic stress disorder; PFT, pulmonary functional test; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; FEV1/FEV, forced expiratory volume in one second/forced expiratory volume; VC, vital capacity; TLC, total lung capacity; DLCO, carbon monoxide diffusing capacity; CT, computerized tomography; GGO, ground-glass opacity; EQ-5D-5L, European Quality of Life Five-Dimension Five-Level Scale; 6 MWT, 6-min walk test.
Figure 2.
Pooled prevalence of COVID-19 consequences at 6 months and above.
At 6 to 12 months, there were 9 symptoms with a PP of more than 20%, including mMRC = 0 (PP = 74.5%, 95% CI, 66.94–82.06%), fatigue or muscle weakness (PP = 54.21%, 95% CI, 45.16–63.27%), fatigue (PP = 30.94%, 95% CI, 20.21.41.66%), dyspnea (PP = 27.06%, 95% CI, 18.67–35.44%), anxiety (PP = 25.19%, 95% CI, 13.88–36.49%), mMRC ≥ 1 (PP = 24.49%, 95% CI, 21.17–27.81%), sleep difficulty (PP = 24.11%, 95% CI, 14.67–33.56%), difficulty concentrating (PP = 22.47%, 95% CI, 4.49–40.44%), limited mobility (PP = 21.81%, 95% CI, −4.17–47.78%), chest tightness (PP = 21.18%, 95% CI, 4.94–37.43%), and depression (PP = 20.16%, 95% CI, 10.36–29.97%). Of these nine symptoms, most were respiratory and psychiatric consequences.
At more than 12 months’ follow-up, there were nine symptoms with a PP of more than 20%, including mMRC = 0 (PP = 80.64%, 95% CI, 62.87–98.42%), myalgia or joint pain (PP = 34.52%, 95% CI, 9.01–60.02%), fatigue (PP = 34.22%, 95% CI, 23.75–44.70%), respiratory symptoms (PP = 32.7%, 95% CI, 3.97–61.43%), rhinorrhea (PP = 30.93%, 95% CI, 11.60–50.26%), anxiety (PP = 29.78%, 95% CI, 16.29–43.27%), difficulty concentrating (PP = 29.47%, 95% CI, 19.80–39.14%), sleep difficulty (PP = 26.31%, 95% CI, 15.73–36.89%), and neurological symptoms (PP = 23.83%, 95% CI, 11.42–36.29%). Three out of the nine symptoms above were psychiatric consequences.
3.3. Pooled Prevalence of Pulmonary Functional Test Results after COVID-19 at 6 Months and Above
Lung function tests showed that some participants had varying degrees of reduction in lung function after COVID-19 at 6 months and above. For example, during the follow-up at 6 to 12 months, FEV1/FEV < 70% occurred in 22.86% of participants (95% CI, 8.95–36.77%). Abnormal pulmonary diffuse function (DLCO < 80%) was noteworthy, which occurred in 49.1% of the participants (95% CI, 33.27–64.9%) at 6–12 months’ follow-up and above and 31.8% (95% CI, 18.65–44.95%) at 12 months’ follow-up and above. In addition, some of this reduction seemed to taper off over time between two follow-ups; the PP of FEV1 < 80% reduced from 28.03% (95% CI, 1.04–55.03%) to 13.81% (95% CI, 5.57–22.05%), and the PP of FVC < 80% reduced from 13.66% (95% CI, 5.23–22.09%) to 12.78% (95% CI, 3.99–21.56%). The analysis results are shown in Table 1 and Figure 2.
3.4. Pooled Prevalence of CT Results after COVID-19 at 6 Months and Above
CT results were abnormal in 55.68% of participants at 6–12 months’ follow-up (95% CI, 26.75–84.62%), reduced to 43.76% at 12 months’ follow-up and above (95% CI, 7.78–79.74%). Fibrosis was most common at 6–12 months’ follow-up (PP = 66.28%, 95% CI, 52.35–80.21%), followed by parenchymal band (PP = 32.53%, 95% CI, 16.45–48.60%), interlobular septal thickening (PP = 21.75%, 95% CI, 6.66–36.84%), and GGO (PP = 21.25%, 95% CI, 9.79–32.71%). At 12 months’ follow-up and above, nodules were most common (PP = 38.56%, 95% CI, 18.95%–58.17%), followed by GGO (PP = 21.35%, 95% CI, 8.30–34.39%). In addition, we observed some reduction in the PP of abnormal CT results over time, such as fibrosis, which decreased significantly from 66.28% to 13.88% (95% CI, 6.04–21.72%), as well as reticular pattern and interlobular septal thickening between two follow-ups. The PP of other abnormal CT results was less than 20%. More analysis results are shown in Table 1 and Figure 2.
3.5. The Impact of COVID-19 on Quality of Life
Assessed by the EQ-5D-5L test, the quality of life of people with COVID-19 was affected in the long term, as shown in Table 2. Pain or discomfort and anxiety and depression were the most common, and personal care problems were the least common. At 6 to 12 months’ follow-up, 33.26% (95% CI, 27.01–39.51%) of patients had pain or discomfort problems, 33.49% (95% CI, 23.87–43.12%) had anxiety or depression problems, and only 0.94% (95% CI, 0.11–1.77%) were affected in personal care. At more than 12 months’ follow-up, 35.31% (95% CI, 22.38–48.24%) of patients had pain or discomfort problems, 35.4% (95% CI, 16.39–54.41%) had anxiety or depression problems, and 1.6% (95% CI, 0.95–2.25%) were affected in personal care.
Table 2.
Gender differences in consequences of long-term COVID-19.
| Consequences | Study Number | Male n/N |
Female n/N |
OR | 95% CI | p-Value | I2 |
|---|---|---|---|---|---|---|---|
| ≥1 symptom | 5 | 977/1790 | 1113/1749 | 0.64 | 0.55–0.75 | <0.05 | 0.0% |
| General symptoms | |||||||
| Fever | 3 | 28/1435 | 32/1370 | 0.79 | 0.46–1.33 | >0.05 | 0.0% |
| Fatigue | 7 | 851/1971 | 942/1850 | 0.69 | 0.60–0.79 | <0.05 | 0.0% |
| Muscle weakness | 2 | 529/1284 | 639/1168 | 0.80 | 0.19–3.42 | >0.05 | 92.5% |
| Limited mobility | 3 | 61/619 | 71/577 | 0.76 | 0.51–1.15 | >0.05 | 0.0 |
| Myalgia | 3 | 79/1435 | 93/1370 | 0.79 | 0.49–1.27 | >0.05 | 36.9% |
| Headache | 3 | 26/1435 | 62/1370 | 0.40 | 0.25–0.65 | <0.05 | 0.0% |
| Dizziness | 2 | 70/907 | 77/843 | 0.79 | 0.55–1.14 | >0.05 | 0.0% |
| Olfactory or taste loss | 4 | 223/1487 | 251/1413 | 0.85 | 0.69–1.04 | >0.05 | 0.0% |
| Olfactory loss | 2 | 109/1007 | 119/1001 | 0.92 | 0.70–1.21 | >0.05 | 0.0% |
| Taste loss | 2 | 69/1007 | 90/1001 | 0.71 | 0.42–1.21 | >0.05 | 46.1% |
| Hair loss | 2 | 176/1007 | 192/1001 | 0.36 | 0.03–3.93 | >0.05 | 67.5% |
| Respiratory symptoms | |||||||
| Cough | 3 | 108/629 | 112/614 | 0.79 | 0.58–1.09 | >0.05 | 0.0% |
| Sore throat or difficulty swallowing | 3 | 68/1435 | 78/1370 | 0.79 | 0.57–1.11 | >0.05 | 0.0% |
| Dyspnea | 2 | 161/481 | 128/411 | 0.82 | 0.31–2.16 | >0.05 | 75.6% |
| mMRC = 0 | 2 | 786/1023 | 709/987 | 1.34 | 1.01–1.77 | <0.05 | 30.6% |
| mMRC ≥ 1 | 2 | 237/1023 | 278/955 | 0.64 | 0.36–1.11 | >0.05 | 78.6% |
| Cardiovascular symptoms | |||||||
| Chest pain | 3 | 66/1284 | 62/1168 | 0.96 | 0.67–1.37 | >0.05 | 0.0% |
| Gastrointestinal symptoms | |||||||
| GI symptoms | 3 | 101/618 | 105/576 | 0.83 | 0.61–1.13 | >0.05 | 0.0% |
| Loss of appetite | 2 | 73/1284 | 73/1168 | 0.92 | 0.66–1.30 | >0.05 | 0.0% |
| Nausea, vomiting or diarrhea | 3 | 78/1435 | 104/1370 | 0.65 | 0.41–1.03 | >0.05 | 38.7% |
| Diarrhea | 2 | 36/579 | 50/571 | 0.60 | 0.38–0.95 | <0.05 | 0.0% |
| Neurological symptoms | |||||||
| Paresthesias | 2 | 49/566 | 62/534 | 0.99 | 0.35–2.76 | >0.05 | 78.3% |
| Psychiatric symptoms | |||||||
| Sleep difficulty | 4 | 325/1474 | 365/1377 | 0.75 | 0.52–1.07 | >0.05 | 52.6% |
| Depression | 3 | 93/776 | 112/641 | 0.54 | 0.37–0.79 | <0.05 | 21.3% |
| Anxiety | 3 | 104/775 | 152/640 | 0.41 | 0.31–0.56 | <0.05 | 0.0% |
| Quality of life evaluation (EQ-5D-5L) | |||||||
| Mobility | 2 | 69/1046 | 92/1005 | 0.70 | 0.51–0.91 | <0.05 | 0.0% |
| Personal care | 2 | 6/1047 | 7/1005 | 0.82 | 0.27–2.45 | >0.05 | 0.0% |
| Usual activity | 2 | 25/1041 | 45/1000 | 0.52 | 0.31–0.85 | <0.05 | 0.0% |
| Pain or discomfort | 2 | 266/1044 | 316/1000 | 0.74 | 0.61–0.90 | <0.05 | 0.0% |
| Anxiety and depression | 2 | 200/1046 | 300/1001 | 0.55 | 0.45–0.68 | <0.05 | 0.0% |
Abbreviations: CI, Confidence interval; OR, odds ratio; mMRC, Modified Medical Research Council Dyspnea Scale; GI, gastrointestinal; EQ-5D-5L, European Quality of Life Five-Dimension Five-Level Scale.
3.6. Gender Differences in Consequences of Long-Term COVID
Compared to females, males with COVID-19 were less likely to develop long-term COVID-19 symptoms (OR = 0.64, 95% CI, 0.55–0.75), but only a fraction of our analysis results were statistically significant (p < 0.05), including fatigue (OR = 0.69, 95% CI, 0.60–0.79), headache (OR = 0.40, 95% CI, 0.25–0.65) and diarrhea (OR = 0.60, 95% CI, 0.38–0.95). Compared to females, males with COVID–19 were more likely to experience dyspnea symptoms (mMRC = 0, OR = 1.34, 95% CI, 1.01–1.77).
In addition, female COVID-19 survivors appeared to be more likely than males to have psychological symptoms and quality of life issues. For example, there was a lower risk of anxiety (OR = 0.41, 95% CI, 0.31–0.56) and depression (OR = 0.54, 95% CI, 0.37–0.79) in male than in female COVID-19 patients. Assessed by the EQ-5D-5L, compared to female COVID-19 patients, there was a lower risk for males to experience quality of life problems, such as mobility (OR = 0.70, 95% CI, 0.51–0.91), usual activity (OR = 0.52, 95% CI, 0.31–0.85), pain or discomfort (OR = 0.74, 95% CI, 0.61–0.90), and anxiety and depression (OR = 0.55, 95% CI, 0.45–0.68). The analysis results are shown in Table 2.
3.7. Quality Evaluation, Risk of Bias, and Publication Bias
We evaluated the quality of all 40 included studies according to the Newcastle–Ottawa quality assessment scale, 38 of them were of good quality and had a low risk of bias (≥7 stars), and 2 were of moderate quality and moderate risk of bias (6 stars), as shown in Supplementary Table S3. We evaluated the publication bias of the included studies based on Harbord’s modified test, and the p values of Harbord’s modified test for all the meta-analyses were higher than 0.1, indicating that there was no publication bias.
3.8. GRADE Evidence Evaluation
We evaluated the evidence quality of all long-term health consequences of COVID-19 using the GRADE approach. The 40 included studies were all observational studies. After a detailed evaluation of 75 long-term COVID-19 consequences at 6–12 months’ follow-up, a total of 3 outcomes were identified as high-quality evidence, 19 outcomes were identified as moderate-quality evidence, and the remaining 53 outcomes were identified as low-quality evidence. After the assessment of 57 long-term COVID-19 consequences at 12 months’ follow-up and above, a total of 4 outcomes were identified as high-quality evidence, 17 outcomes were identified as moderate-quality evidence, and the remaining 36 outcomes were identified as low-quality evidence. The detailed results are shown in Supplementary Tables S4 and S5.
4. Discussion
Nowadays, COVID-19 continues to ravage the world, and although the infection of the pandemic Omicron variant may be mild [29,59], that does not mean we should relax our guard. Currently, the data on the effects of COVID-19 are growing rapidly. These data suggested that even if COVID-19 patients fully recover, they may face the risk of a variety of mid- and long-term effects [60]. Our systematic review and meta-analysis of 40 cohort studies involving 10,945 cases of SARS-CoV-2 infection provide the pooled prevalence (PP) of long-term consequences of COVID-19 at 6 months and above, and we compared subgroups stratified by follow-up period, severity of COVID-19, and gender. Understanding the long-term sequelae of COVID-19 is key to early intervention, treatment, and vaccination deployment. Previous studies have looked at the COVID -19 consequences at three months or longer [61]. Our study included a longer follow-up period of 6 months or more and a more comprehensive scope, including general, cardiovascular, respiratory, gastrointestinal, and psychiatric system symptoms, as well as the evaluation of medical imaging, lung function, and quality of life.
Consistent with previous studies, the proportion of patients with at least one symptom was as high as 60% at 6 months’ follow-up, and showed a decreasing trend over time [59]. However, it should not be ignored that the proportion of patients with at least one symptom was still more than 50% when followed up at 12 months or more. In Lombardo’s study, the proportion was higher, at more than 80%, but other studies have reported a lower proportion (about 40%) [29,59]. This suggests that COVID-19 may lead to sustained effects on organs, and the inconsistent results of 12-month follow-up studies suggest that more original studies on the long-term sequelae of COVID-19 are needed.
Available data analyses have shown that respiratory symptoms were common in long COVID-19, and a high PP of persistent dyspnea is of concern. A French study found that hyperventilation syndrome was common in COVID-19 patients (34%) [60], which may be related to the occurrence of persistent dyspnea. People with COVID-19 could suffer from varying degrees of respiratory damage. The available data showed that mild dyspnea was one of the most common symptoms in long-term COVID, and the proportions of CT abnormity and abnormal pulmonary diffuse function were reduced over time, which indicates that lung damage could be improved. In addition, we should also consider the impact of underlying respiratory conditions. In one meta-analysis, COPD patients with COVID-19 had a greater risk of severe disease than the non-COPD group (calculated Risk Ratio, RR = 1.88, 95% CI, 1.4–2.4) [61]. Another study found that COPD was associated with persistent symptoms at 12 months and above (OR = 10.74, p < 0.05) [59]. For people with such underlying diseases, COVID-19 sequelae may increase their burden.
The PPs of diffuse lung function impairment (DLCO < 80%) and pulmonary fibrosis were higher at long-term follow-up, but it is encouraging that this lung damage caused by COVID-19 did not appear to develop over time. In this study, diffuse lung function impairment decreased from 50% at 6–12 months to 30% at 12 months at least, and pulmonary fibrosis decreased from 66% to 14%. A study of COVID-19 patients discharged for 12 months showed no further development of pulmonary fibrosis and progressive pulmonary interstitial changes during long-term follow-up [42]. However, it should be cautioned that the repair of pulmonary fibrosis injury may bring a great burden to patients [62].
Health-related quality of life (HRQoL) is an important indicator to evaluate the impact of diseases on patients’ physical, psychological, and social fields [63], and the EQ-5D-5L questionnaire is one of the most commonly used tools [64]. Our results suggest that COVID-19 patients may have long-term problems with quality of life and mental well-being, and that women are more likely to be affected than men. This could be because women, more than men, tend to take care of the family and the housework, and the job and income loss have caused women to face an economic crisis at the same time, as well as facing a larger burden of unpaid care [65]. In addition, women’s exposure to domestic violence has increased because of social restrictions and isolation [66].
Since the long-term effects of COVID-19 are still unclear, the best way to reduce the consequences is to avoid infection, for which vaccination is important. In addition, improving COVID-19 screening and diagnosis capabilities can help the detection and treatment as early as possible. We should pay more attention to women’s mental health and give them more psychological support, even interventions when necessary, since they are more likely to have psychological problems compared to men. In addition to the original research on the long-term effects of COVID-19, articles on the effects of vaccines on the consequences of COVID-19 are also needed.
There is currently a lack of RCTs to evaluate interventions for the long-term impact of COVID-19. This study focused on the meta-analysis of the clinical features of the long-term impacts of COVID-19. Research studies on intervention for long-term effects of COVID-19 are recommended in the future to provide evidence-based medical evidence of high GRADE quality for the development of clinical guidelines.
Our study has some limitations. First, due to limited data, some COVID-19 consequences could only analyze the PP at either 6–12 months’ follow-up or at 12 months and more, not both. In addition, the heterogeneity of the PP for long-term COVID-19 effects was high, which may be related to age and gender differences.
5. Conclusions
Our results show that 63.87% of COVID-19 patients had at least one type of COVID-19 consequences at 6–12 months’ follow-up after recovery or discharge, and 58.89% of patients continued to suffer at 12 months’ follow-up and above. The most common symptoms were fatigue or muscle weakness (6–12 m: PP = 54.21%, ≥ 12 m: PP = 34.22%) and mild dyspnea (mMRC = 0) (6–12 m: PP = 74.60%, ≥12 m: PP = 80.64%). Anxiety and depression (6–12 m: PP = 33.49%, ≥12 m: PP = 35.40%) and pain or discomfort (6–12 m: PP = 33.26%, ≥12 m: PP = 35.31%) became the two most common problems affecting patients’ quality of life. Our findings suggest significant long-term impacts of COVID-19 on health and quality of life, and as waves of ASRS-CoV-2 infections emerge, the long-term effects of COVID-19 will not only increase the difficulty of the care for COVID-19 survivors and setting public health policy but also might lead to another public health crisis following the current pandemic, which would also increase the global long-term burden of disease. Therefore, the long-term effects of COVID-19 should not be ignored, and it is crucial to provide a more comprehensive and scientific basis for COVID-19 survivors to guide long-term care, rehabilitation, surveillance, and prevention measures, and to set public health policy for healthcare facilities.
Abbreviations
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | Coronavirus disease 2019 |
| PP | Pooled prevalence |
| CI | Confidence interval |
| OR | Odds ratio |
| ICU | Intensive care unit |
| LOS | Length of stay |
| COPD | Chronic obstructive pulmonary diseases |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PFT | Pulmonary functional test |
| WHO | World Health Organization |
| UK | United Kingdom |
| CT | Computerized tomography |
| DLCO | Carbon monoxide diffusing capacity |
| mMRC | Modified Medical Research Council Dyspnea Scale |
| GI | Gastrointestinal |
| GAD-7 | Generalized anxiety disorder-7 |
| PTSD | Post-traumatic stress disorder |
| 6 MWT | 6-min walk test |
| EQ-5D-5L | European Quality of Life Five-Dimension Five-Level Scale |
| GGO | Ground-glass opacity |
| FVC | Forced vital capacity |
| FEV1 | Forced expiratory volume in one second |
| VC | Vital capacity |
| FEV1/FEV | Forced expiratory volume in one second/forced expiratory volume |
| TLC | Total lung capacity |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluation |
| RR | Risk Ratio |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19116865/s1, Table S1: Template of primary data extraction; Table S2: Characteristic of the included studies; Table S3: Risk of bias and quality of included studies assessed by Newcastle-Ottawa quality assessment Scale (NOS); Table S4: GRADE evidence evaluation results of COVID-19 consequences in 6–12 months follow-up; Table S5: GRADE evidence evaluation results of COVID-19 consequences in 12 months and above follow-up.
Author Contributions
Y.M. and J.D. contributed equally as first authors. J.L. and M.L. contributed equally as correspondence authors. J.L. and M.L. conceived and designed the study. Y.M., J.D. and Q.L. carried out the literature searches, extracted the data, and assessed the study quality. J.D. and Y.M. performed the statistical analysis and wrote the manuscript. J.L., M.L., Q.L., M.D., Y.M. and J.D. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author by request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was funded by the National Natural Science Foundation of China (72122001; 71934002) and the National R&D Key project (2021ZD0114101, 2021ZD0114104, 2021ZD0114105). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the paper. No payment was received by any of the co-authors for the preparation of this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization . WHO Coronavirus (COVID-19) Dashboard. World Health Organization; Geneva, Switzerland: 2022. [Google Scholar]
- 2.World Health Organization . WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—30 October 2020. World Health Organization; Geneva, Switzerland: 2020. [(accessed on 6 April 2022)]. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---30-october-2020. [Google Scholar]
- 3.World Health Organization . A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. World Health Organization; Geneva, Switzerland: 2021. [(accessed on 6 April 2022)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1. [Google Scholar]
- 4.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Royal Society Long COVID: What Is It, and What Is Needed? 2020. [(accessed on 6 April 2022)]. Available online: https://royalsociety.org/-/media/policy/projects/set-c/set-c-long-covid.pdf.
- 6.World Health Organization . Altea: A Network for Sharing Evidence-Based Information on the Long-Term Effects of COVID-19. World Health Organization; Geneva, Switzerland: 2022. [(accessed on 6 April 2022)]. Available online: https://cdn.who.int/media/docs/default-source/science-translation/case-studies-1/cs2_altea.pdf?sfvrsn=fded8c90_4. [Google Scholar]
- 7.Altea Network Symptoms Overview. 2022. [(accessed on 6 April 2022)]. Available online: https://www.altea-network.com/en/long-covid/symptoms-overview/
- 8.Cabrera Martimbianco A.L., Pacheco R.L., Bagattini Â.M., Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: A systematic review. Int. J. Clin. Pract. 2021;75:e14357. doi: 10.1111/ijcp.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P.A., Cuapio A., Villapol S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—7 October 2021. World Health Organization; Geneva, Switzerland: 2021. [(accessed on 6 April 2022)]. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---7-october-2021. [Google Scholar]
- 11.Garg M., Maralakunte M., Garg S., Dhooria S., Sehgal I., Bhalla A.S., Vijayvergiya R., Grover S., Bhatia V., Jagia P., et al. The Conundrum of ‘Long-COVID-19’: A Narrative Review. Int. J. Gen. Med. 2021;14:2491–2506. doi: 10.2147/IJGM.S316708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Z., Yang M., Lai C.L. Long COVID-19 Syndrome: A Comprehensive Review of Its Effect on Various Organ Systems and Recommendation on Rehabilitation Plans. Biomedicines. 2021;9:966. doi: 10.3390/biomedicines9080966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnúsdóttir I., Lovik A., Unnarsdóttir A., McCartney D., Ask H., Kõiv K., Christoffersen L., Johnson S., Hauksdóttir A., Fawns-Ritchie C., et al. Acute COVID-19 severity and mental health morbidity trajectories in patient populations of six nations: An observational study. Lancet Public Health. 2022;7:E406–E416. doi: 10.1016/S2468-2667(22)00042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Y., Xu E., Al-Aly Z. Risks of mental health outcomes in people with COVID-19: Cohort study. BMJ. 2022;376:e068993. doi: 10.1136/bmj-2021-068993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik P., Patel K., Pinto C., Jaiswal R., Tirupathi R., Pillai S., Patel U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J. Med. Virol. 2022;94:253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walia N., Walia N., Lat J.O., Tariq R., Tyagi S., Qazi A.M., Salari S.W., Jafar A., Kousar T., Bieniek S. Post-acute sequelae of COVID-19 and the mental health implications. Discoveries. 2021;9:e140. doi: 10.15190/d.2021.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L.R., Griffanti L., Duff E., et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Leary K. Brain Pathology of COVID-19. 2022. [(accessed on 6 April 2022)]. Available online: https://www.nature.com/articles/d41591-022-00043-x>.
- 19.Du Y.Y., Zhao W., Zhou X.L., Zeng M., Yang D.H., Xie X.Z., Huang S.H., Jiang Y.J., Yang W.H., Guo H., et al. Survivors of COVID-19 exhibit altered amplitudes of low frequency fluctuation in the brain: A resting-state functional magnetic resonance imaging study at 1-year follow-up. Neural Regen. Res. 2022;17:1576–1581. doi: 10.4103/1673-5374.327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L., Xu X., Zhang L., Zheng D., Liu Y., Feng B., Hu J., Lin Q., Xi X., Wang Q., et al. Post-traumatic Stress Disorder Symptoms and Quality of Life of COVID-19 Survivors at 6-Month Follow-Up: A Cross-Sectional Observational Study. Front. Psychiatry. 2021;12:782478. doi: 10.3389/fpsyt.2021.782478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindahl A., Aro M., Reijula J., Mäkelä M.J., Ollgren J., Puolanne M., Järvinen A., Vasankari T. Women report more symptoms and impaired quality of life: A survey of Finnish COVID-19 survivors. Infect. Dis. 2022;54:53–62. doi: 10.1080/23744235.2021.1965210. [DOI] [PubMed] [Google Scholar]
- 22.Mazza M.G., Palladini M., De Lorenzo R., Bravi B., Poletti S., Furlan R., Ciceri F., Vai B., Bollettini I., Melloni E.M.T., et al. One-year mental health outcomes in a cohort of COVID-19 survivors. J. Psychiatr. Res. 2022;145:118–124. doi: 10.1016/j.jpsychires.2021.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tessitore E., Handgraaf S., Poncet A., Achard M., Höfer S., Carballo S., Marti C., Follonier C., Girardin F., Mach F., et al. Symptoms and quality of life at 1-year follow up of patients discharged after an acute COVID-19 episode. Swiss Med. Wkly. 2021;151:w30093. doi: 10.4414/smw.2021.w30093. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Duarte Á., Rivera-Izquierdo M., Guerrero-Fernández de Alba I., Pérez-Contreras M., Fernández-Martínez N.F., Ruiz-Montero R., Serrano-Ortiz Á., González-Serna R.O., Salcedo-Leal I., Jiménez-Mejías E., et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: The ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19:129. doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong L., Li Q., Cao X., Xiong H., Huang M., Yang F., Liu Q., Meng D., Zhou M., Wang G., et al. Dynamic changes of functional fitness, antibodies to SARS-CoV-2 and immunological indicators within 1 year after discharge in Chinese health care workers with severe COVID-19: A cohort study. BMC Med. 2021;19:163. doi: 10.1186/s12916-021-02042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan Y., Zhu Y., Wang S., Jia S., Gao Y., Lu Y., Zhou C., Liang R., Sun D., Wang X., et al. SARS-CoV-2 immunity and functional recovery of COVID-19 patients 1-year after infection. Signal Transduct. Target. Ther. 2021;6:368. doi: 10.1038/s41392-021-00777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mainous A.G., Rooks B.J., Wu V., Orlando F.A. COVID-19 Post-acute Sequelae Among Adults: 12 Month Mortality Risk. Front. Med. 2021;8:2351. doi: 10.3389/fmed.2021.778434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F., Tao M., Shang L., Liu Y., Pan G., Jin Y., Wang L., Hu S., Li J., Zhang M., et al. Assessment of Sequelae of COVID-19 Nearly 1 Year After Diagnosis. Front. Med. 2021;8:717194. doi: 10.3389/fmed.2021.717194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellan M., Baricich A., Patrucco F., Zeppegno P., Gramaglia C., Balbo P.E., Carriero A., Amico C.S., Avanzi G.C., Barini M., et al. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci. Rep. 2021;11:22666. doi: 10.1038/s41598-021-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faverio P., Luppi F., Rebora P., Busnelli S., Stainer A., Catalano M., Parachini L., Monzani A., Galimberti S., Bini F., et al. Six-Month Pulmonary Impairment after Severe COVID-19: A Prospective, Multicentre Follow-Up Study. Respiration. 2021;100:1078–1087. doi: 10.1159/000518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y., Yang C., An X., Xiong Y., Shang Y., He J., Qiu Y., Zhang N., Huang L., Jia J., et al. Follow-up study on COVID-19 survivors one year after discharge from hospital. Int. J. Infect. Dis. 2021;112:173–182. doi: 10.1016/j.ijid.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eloy P., Tardivon C., Martin-Blondel G., Isnard M., Turnier P.L., Marechal M.L., CabiÉ A., Launay O., Tattevin P., Senneville E., et al. Severity of self-reported symptoms and psychological burden 6-months after hospital admission for COVID-19: A prospective cohort study. Int. J. Infect. Dis. 2021;112:247–253. doi: 10.1016/j.ijid.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caruso D., Guido G., Zerunian M., Polidori T., Lucertini E., Pucciarelli F., Polici M., Rucci C., Bracci B., Nicolai M., et al. Post-acute sequelae of COVID-19 pneumonia: Six-month chest CT follow-up. Radiology. 2021;301:E36–E405. doi: 10.1148/radiol.2021210834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peghin M., Palese A., Venturini M., De Martino M., Gerussi V., Graziano E., Bontempo G., Marrella F., Tommasini A., Fabris M., et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin. Microbiol. Infect. 2021;27:1507–1513. doi: 10.1016/j.cmi.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maestrini V., Birtolo L.I., Francone M., Galardo G., Galea N., Severino P., Alessandri F., Colaiacomo M.C., Cundari G., Chimenti C., et al. Cardiac involvement in consecutive unselected hospitalized COVID-19 population: In-hospital evaluation and one-year follow-up. Int. J. Cardiol. 2021;339:235–242. doi: 10.1016/j.ijcard.2021.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu M., Lv F., Zheng Y., Xiao K. A prospective cohort study on radiological and physiological outcomes of recovered COVID-19 patients 6 months after discharge. Quant. Imaging Med. Surg. 2021;11:4181–4192. doi: 10.21037/qims-20-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nehme M., Braillard O., Chappuis F., Courvoisier D.S., Guessous I. Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID-19 in an outpatient setting. Ann. Intern. Med. 2021;174:1252–1260. doi: 10.7326/M21-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., Hu P., Guo L., Liu M., Xu J., et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darcis G., Bouquegneau A., Maes N., Thys M., Henket M., Labye F., Rousseau A.F., Canivet P., Desir C., Calmes D., et al. Long-term clinical follow-up of patients suffering from moderate-to-severe COVID-19 infection: A monocentric prospective observational cohort study. Int. J. Infect. Dis. 2021;109:209–216. doi: 10.1016/j.ijid.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lombardo M.D.M., Foppiani A., Peretti G.M., Mangiavini L., Battezzati A., Bertoli S., Martinelli Boneschi F., Zuccotti G.V. Long-Term Coronavirus Disease 2019 Complications in Inpatients and Outpatients: A One-Year Follow-up Cohort Study. Open Forum Infect. Dis. 2021;8:ofab384. doi: 10.1093/ofid/ofab384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao K., Yang H., Liu B., Pang X., Du J., Liu M., Liu Y., Jing X., Chen J., Deng S., et al. Antibodies Can Last for More Than 1 Year After SARS-CoV-2 Infection: A Follow-Up Study From Survivors of COVID-19. Front. Med. 2021;8:967. doi: 10.3389/fmed.2021.684864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu X., Liu X., Zhou Y., Yu H., Li R., Zhan Q., Ni F., Fang S., Lu Y., Ding X., et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menges D., Ballouz T., Anagnostopoulos A., Aschmann H.E., Domenghino A., Fehr J.S., Puhan M.A. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PLoS ONE. 2021;16:e0254523. doi: 10.1371/journal.pone.0254523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fayol A., Livrozet M., Boutouyrie P., Khettab H., Betton M., Tea V., Blanchard A., Bruno R.M., Hulot J.S. Cardiac performance in patients hospitalized with COVID-19: A 6 month follow-up study. ESC Heart Fail. 2021;8:2232–2239. doi: 10.1002/ehf2.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han X., Fan Y., Alwalid O., Li N., Jia X., Yuan M., Li Y., Cao Y., Gu J., Wu H., et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299:E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M., Lv F., Huang Y., Xiao K. Follow-Up Study of the Chest CT Characteristics of COVID-19 Survivors Seven Months After Recovery. Front. Med. 2021;8:212. doi: 10.3389/fmed.2021.636298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bai T., Zhou D., Yushanjiang F., Wang D., Zhang D., Liu X., Song J., Zhang J., Hou X., Ma Y. Alternation of the Autonomvous System Is ssociated with Pulmonary Sequelae in Patients With COVID-19 After Six Months of Discharge. Front. Physiol. 2021;12:80595. doi: 10.3389/fphys.2021.805925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xianyu Y., Wang M., Yue F., Xu X., Yang H., Zhao D., Hu K. One-year follow-up of 18 women who infected COVID-19 while pregnant. J. Med. Virol. 2022;94:2302–2306. doi: 10.1002/jmv.27628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Augustin M., Schommers P., Stecher M., Dewald F., Gieselmann L., Gruell H., Horn C., Vanshylla K., Di Cristanziano V., Osebold L., et al. Post-COVID syndrome in non-hospitalised patient with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health-Eur. 2021;6:100122. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Veenendaal N., Van der Meulen I.C., Onrust M., Paans W., Dieperink W., Van der Voort P.H.J. Six-Month Outcomes in COVID-19 ICU Patients and Their Family Members: A Prospective Cohort Study. Healthcare. 2021;9:865. doi: 10.3390/healthcare9070865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gamberini L., Mazzoli C.A., Prediletto I., Sintonen H., Scaramuzzo G., Allegri D., Colombo D., Tonetti T., Zani G., Capozzi C., et al. Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study. Respir. Med. 2021;189:106665. doi: 10.1016/j.rmed.2021.106665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Becker C., Beck K., Zumbrunn S., Memma V., Herzog N., Bissmann B., Gross S., Loretz N., Mueller J., Amacher S.A., et al. Long COVID 1 year after hospitalisation for COVID-19: A prospective bicentric cohort study. Swiss Med. Wkly. 2021;151:w30091. doi: 10.4414/smw.2021.w30091. [DOI] [PubMed] [Google Scholar]
- 54.Peluso M.J., Kelly J.D., Lu S., Goldberg S.A., Davidson M.C., Mathur S., Durstenfeld M.S., Spinelli M.A., Hoh R., Tai V., et al. Persistence, Magnitude, and Patterns of Postacute Symptoms and Quality of Life Following Onset of SARS-CoV-2 Infection: Cohort Description and Approaches for Measurement. Open Forum Infect. Dis. 2022;9:ofab640. doi: 10.1093/ofid/ofab640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erber J., Wiessner J.R., Zimmermann G.S., Barthel P., Burian E., Lohofer F., Martens E., Mijocevic H., Rasch S., Schmid R.M., et al. Longitudinal Assessment of Health and Quality of Life of COVID-19 Patients Requiring Intensive Care-An Observational Study. J. Clin. Med. 2021;10:5469. doi: 10.3390/jcm10235469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molhave M., Leth S., Gunst J., Jensen-Fangel S., Ostergaard L., Wejse C., Agergaard J. Long-Term Symptoms among Hospitalized COVID-19 Patients 48 Weeks after Discharge-A Prospective Cohort Study. J. Clin. Med. 2021;10:5298. doi: 10.3390/jcm10225298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fortini A., Rosso A., Cecchini P., Torrigiani A., Lo Forte A., Carrai P., Alessi C., Fabbrizzi F., Lovicu E., Sbaragli S., et al. One-year evolution of DLCO changes and respiratory symptoms in patients with post COVID-19 respiratory syndrome. Infection. 2022;50:513–517. doi: 10.1007/s15010-022-01755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim Y., Bitna-Ha, Kim S.W., Chang H.H., Kwon K.T., Bae S., Hwang S. Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea. BMC Infect. Dis. 2022;22:93. doi: 10.1186/s12879-022-07062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fumagalli C., Zocchi C., Tassetti L., Silverii M.V., Amato C., Livi L., Giovannoni L., Verrillo F., Bartoloni A., Marcucci R., et al. Factors associated with persistence of symptoms 1 year after COVID-19: A longitudinal, prospective phone-based interview follow-up cohort study. Eur. J. Intern. Med. 2022;97:36–41. doi: 10.1016/j.ejim.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouteleux B., Henrot P., Ernst R., Grassion L., Raherison-Semjen C., Beaufils F., Zysman M., Delorme M. Respiratory rehabilitation for COVID-19 related persistent dyspnoea: A one-year experience. Respir. Med. 2021;189:106648. doi: 10.1016/j.rmed.2021.106648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almehmadi M., Alqahtani A.S., Quaderi S., Mandal S., Hurst J.R. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS ONE. 2020;15:e0233147. doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Testa M.A., Simonson D.C. Assessment of quality-of-life outcomes. New Engl. J. Med. 1996;334:835–840. doi: 10.1056/NEJM199603283341306. [DOI] [PubMed] [Google Scholar]
- 64.Buchholz I., Janssen M.F., Kohlmann T., Feng Y.S. A Systematic Review of Studies Comparing the Measurement Properties of the Three-Level and Five-Level Versions of the EQ-5D. PharmacoEconomics. 2018;36:645–661. doi: 10.1007/s40273-018-0642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witteveen D., Velthorst E. Economic hardship and mental health complaints during COVID-19. Proc. Natl. Acad. Sci. USA. 2020;117:27277–27284. doi: 10.1073/pnas.2009609117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McLean S.A., McIntosh J.E. The mental and physical health of family mental health practitioners during COVID-19: Relationships with family violence and workplace practices. Aust. J. Psychol. 2021;73:395–404. doi: 10.1080/00049530.2021.1934118. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author by request.