Abstract
Oxygen activation during oxidation of the lignin-derived hydroquinones 2-methoxy-1,4-benzohydroquinone (MBQH2) and 2,6-dimethoxy-1,4-benzohydroquinone (DBQH2) by laccase from Pleurotus eryngii was examined. Laccase oxidized DBQH2 more efficiently than it oxidized MBQH2; both the affinity and maximal velocity of oxidation were higher for DBQH2 than for MBQH2. Autoxidation of the semiquinones produced by laccase led to the activation of oxygen, producing superoxide anion radicals (Q·− + O2 ↔ Q + O2·−). As this reaction is reversible, its existence was first noted in studies of the effect of systems consuming and producing O2·− on quinone formation rates. Then, the production of H2O2 in laccase reactions, as a consequence of O2·− dismutation, confirmed that semiquinones autoxidized. The highest H2O2 levels were obtained with DBQH2, indicating that DBQ·− autoxidized to a greater extent than did MBQ·−. Besides undergoing autoxidation, semiquinones were found to be transformed into quinones via dismutation and laccase oxidation. Two ways of favoring semiquinone autoxidation over dismutation and laccase oxidation were increasing the rate of O2·− consumption with superoxide dismutase (SOD) and recycling of quinones with diaphorase (a reductase catalyzing the divalent reduction of quinones). These two strategies made the laccase reaction conditions more natural, since O2·−, besides undergoing dismutation, reacts with Mn2+, Fe3+, and aromatic radicals. In addition, quinones are continuously reduced by the mycelium of white-rot fungi. The presence of SOD in laccase reactions increased the extent of autoxidation of 100 μM concentrations of MBQ·− and DBQ·− from 4.5 to 30.6% and from 19.6 to 40.0%, respectively. With diaphorase, the extent of MBQ·− autoxidation rose to 13.8% and that of DBQ·− increased to 39.9%.
The production of extracellular laccase is a common feature of white-rot basidiomycetes (35, 47). These fungi are the only organisms with a demonstrated capacity to both depolymerize and mineralize lignin by an oxidative and nonspecific mechanism (30). Besides laccase, the ligninolytic system of these fungi includes several peroxidases (9, 16, 36, 48), known as lignin peroxidase and manganese peroxidase (MnP), and oxidases that produce the hydrogen peroxide (H2O2) needed for peroxidase activities (18, 29). Another enzyme produced by these fungi, which functions in the degradation of not only lignin but also cellulose, is cellobiose dehydrogenase (11). Laccase catalyzes the one-electron oxidation of a wide range of phenolic compounds and aromatic amines (47). For many years, the participation of laccase in lignin degradation was thought to be limited to the oxidation of phenolic lignin units, which comprise only 10 to 20% of the polymer. However, during the present decade, it has been demonstrated that laccase can also oxidize the nonphenolic lignin units in the presence of certain compounds, known as mediators, that include artificial substrates (5, 8) and fungal metabolites (10). Besides their role in extending the kind and number of lignin units that can be oxidized by the action of laccase, natural mediators are important because ligninolytic enzymes have to act indirectly during the early phases of plant cell wall degradation due to size exclusion limitations (13, 14). Other small molecular agents participating in lignin degradation and produced directly or indirectly by ligninolytic enzymes include manganic ion (Mn3+) (24, 27, 50), the cationic radical of the fungal metabolite veratryl (3,4-dimethoxybenzyl) alcohol (26), and activated oxygen species such as the hydroxyl radical (HO·) and superoxide anion radical (O2·−) (2, 15, 27). Except for O2·−, all of these compounds are able to oxidize lignin units. However, the O2·− produced by white-rot fungi (12) can participate in the production of H2O2 via both dismutation (2 O2·− + 2H+ → H2O2 + O2) and Mn2+ oxidation with concomitant production of Mn3+ (O2·− + Mn2+ + 2H+ → H2O2 + Mn3+) (1). It can also be involved in HO· production through the iron-catalyzed Haber-Weiss reaction (O2·− + H2O2 → HO· + HO− + O2) (4). Furthermore, by reacting with phenoxyl radicals produced from lignin model compounds, it can result in oxidative degradation being favored over coupling reactions (15).
Most enzymatic reactions demonstrating O2·− generation have been carried out with enzymes other than laccase (25, 31, 32, 37, 41). During a comparative study of substrate specificity of the two laccase isoenzymes produced by Pleurotus eryngii, we detected, for the first time, O2·− production in reactions involving benzohydroquinones (38). Although laccase catalyzes the four-electron reduction of O2 to H2O, the semiquinones produced in the one-electron oxidation of hydroquinones are able to autoxidize to a certain extent, reducing O2 to O2·−. For this O2 activation mechanism to be effective, it is essential that hydroquinones are available during lignin degradation and that semiquinones are converted into quinones mainly via autoxidation. As previously rationalized by Schoemaker et al. (44, 45), white-rot fungi can convert all aromatic rings in the lignin polymer to either ring-opened products or quinones-hydroquinones by a combination of oxidative reactions, involving ligninolytic enzymes and active oxygen species, and reductive reactions, carried out by cell-bound systems. This way, lignin mineralization can be accomplished by as-yet-uncharacterized intracellular processes. Another source of quinones is the large amount of white-rot fungi-produced methoxylated and hydroxylated aromatic metabolites (22, 46), which are substrates of the ligninolytic enzymes (23, 34). Quinones are usually reduced to hydroquinones when they are in contact with white-rot fungi mycelium (7, 45). Although it has been postulated that this reaction could lead to the rapid intracellular degradation of quinones (33, 44), a redox cycling process, involving the oxidation of hydroquinones by laccase, was established during the incubation of P. eryngii with several quinones (21). Therefore, it is quite likely that hydroquinones will be present and available for oxidation by laccase and ligninolytic peroxidases under natural conditions of lignin degradation by white-rot fungi. On the other hand, there are many factors controlling the extent of semiquinone autoxidation, including the reduction potential of quinones, which is affected by the nature, number, and position of the substituents (6). Thus, during the oxidation of the hydroquinones produced by P. eryngii from 1,4-benzoquinone, 2-methyl-1,4-benzoquinone, and duroquinone (2,3,5,6-tetramethyl-1,4-benzoquinone) by laccase, the level of O2 activation increased as the number of methyl substituents increased (21). Among these quinones, only 1,4-benzoquinone is a breakdown product of lignin (it is derived from p-hydroxyphenyl lignin units and p-coumarate residues, which are specially abundant in grasses). However, less than 1% of the 1,4-benzosemiquinone produced by P. eryngii laccase isoenzyme I autoxidized (38). For this reason, we planned a new study with hydroquinones derived from guaiacyl and syringyl units of lignin (2-methoxy- and 2,6-dimethoxydroquinone, respectively). The study focused on the extracellular portion of the quinone redox cycling process (activation of oxygen during methoxyhydroquinone oxidation by laccase). Special attention was paid to the possibility of other reactions (besides autoxidation) in which semiquinones are consumed and to factors affecting the extent of the autoxidation reaction.
MATERIALS AND METHODS
Chemicals.
H2O2 (Perhydrol; 30%) was obtained from Merck. Xanthine (Xn), NAD(P)H, and the chelating resin Chelex 100 were purchased from Sigma. 1,4-Benzohydroquinone (BQH2), 2,6-dimethoxy-1,4-benzoquinone (DBQ), and 2-methoxy-1,4-benzohydroquinone (MBQH2) were from Aldrich. 2,6-Dimethoxy-1,4-benzohydroquinone (DBQH2) was prepared from DBQ by reduction with sodium borohydride (3), and 2-methoxy-1,4-benzoquinone (MBQ) was produced from MBQH2 by oxidation with silver oxide (23). All other chemicals used were of analytical grade.
In an attempt to reduce the amounts of trace transition metals present in most laboratory chemicals and reagents, the phosphate buffer and the solutions of the compounds used as substrates in enzymatic reactions were treated with the chelating ion-exchange resin Chelex 100, in accordance with the manufacturer's instructions.
Enzymes.
Laccase (EC 1.10.3.2) from P. eryngii (isoenzyme I) was produced in glucose-ammonium medium and purified by using Sephadex G100 and Mono Q columns as previously described (38). Bovine liver superoxide dismutase (SOD; EC 1.15.1.1) and catalase (EC 1.11.1.6), buttermilk xanthine oxidase (XnO; EC 1.1.3.22), porcine heart diaphorase (EC 1.8.1.4), and rabbit liver cytochrome P450 reductase (EC 1.6.2.4) were obtained from Sigma.
Enzymatic assays.
Laccase activity was determined by spectrophotometrically monitoring product formation, using as substrates 500 μM MBQH2 (ɛ360 = 1,252 M−1 cm−1) and DBQH2 (ɛ397 = 562 M−1 cm−1). Diaphorase and cytochrome P450 reductase were assayed by measuring the oxidation of 200 μM NADH and NADPH, respectively, in the presence of 500 μM MBQ. The extinction coefficient for nucleotides at 340 nm was corrected from 6,220 to 7,120 M−1 cm−1 because MBQ absorbs light of the same wavelength (ɛ340 = 900 M−1 cm−1). All of the above-described reactions were done in 20 mM phosphate buffer, pH 5.0, at room temperature. International units (micromoles per minute) of enzymatic activity were used.
Analytical techniques.
H2O2 levels were estimated by measuring the production of O2 with a Clark-type electrode after addition of 100 U of catalase/ml (heat-denatured catalase was used in blanks). The amount of H2O2 was calculated taking into consideration the stoichiometry of the catalase reaction (2 H2O2:1 O2). The oxygen electrode was calibrated by the same procedure with known amounts of H2O2 from the commercial solution, which were estimated spectrophotometrically (ɛ230 = 81 M−1 cm−1).
Quantitative determinations of MBQH2 and DBQH2 were performed by high-performance liquid chromatography (HPLC), using standard calibration curves for each compound. To stabilize the concentration of hydroquinones until HPLC analysis, the pH was lowered to 2 at the end of the reactions. Samples (20 μl) were injected into a Pharmacia HPLC system equipped with a Spherisorb S50DS2 column (Hichrom). The analyses were done at 30°C at a flow rate of 1 ml min−1 with methanol–10 mM phosphoric acid (20:80) as the eluent. The UV detector operated at 280 nm.
RESULTS
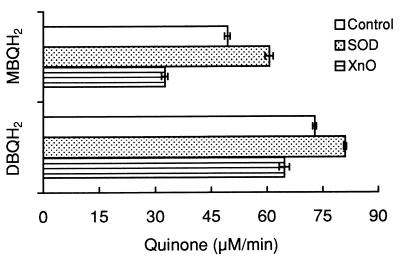
In our previous study of P. eryngii laccase isoenzymes (38), Km and Vmax values of laccase I for BQH2 were found to be 4,600 μM and 21.2 U/mg, respectively. In the present study, for reactions carried out in 20 mM phosphate buffer (pH 5.0), the introduction of methoxyl groups to BQH2 increased not only the Vmax (to 200.0 U/mg [for MBQH2] and 667.5 [for DBQH2]), as expected since methoxyl is a benzene ring-activating group that lowers the reduction potential of hydroquinones, as well as the affinity of the enzyme for its substrate (Km = 190.0 μM [for MBQH2] and 9.9 μM [for DBQH2]). To determine whether the semiquinones produced by laccase were converted into quinones via autoxidation, which is a reversible reaction (Q·− + O2 ↔ Q + O2·−), the effect of systems consuming and producing O2·− (SOD and XnO-Xn, respectively) on quinone production rates was studied. SOD is a useful tool for studying the involvement of O2·− in autoxidation reactions (39, 40). The presence of SOD (which would shift the semiquinone autoxidation equilibrium to the right) during the oxidation of 500 μM concentrations of MBQH2 and DBQH2 by laccase increased the initial rates of production of MBQ and DBQ by 23 and 11%, respectively (Fig. 1). Increasing the O2·− content of the reaction mixture by adding XnO and Xn (thereby shifting the semiquinone autoxidation equilibrium to the left) resulted in 34 and 11% decreases in these rates, respectively. In addition to showing the existence of semiquinone autoxidation reactions, these results indicated that DBQ·− autoxidized to a greater extent than did MBQ·− (the effects of the SOD and XnO-Xn systems on the semiquinone autoxidation reaction were lessened when the equilibrium was shifted more to the right). Autoxidation of semiquinones was confirmed by estimating the production of H2O2 derived from O2·−. The levels of H2O2 (means ± 95% confidence limits) found after oxidation of 500 μM concentrations of MBQH2 and DBQH2 were 4.7 ± 0.2 and 23.9 ± 0.4 μM, respectively. These levels were used to quantify the extent of semiquinone autoxidation, taking into account the stoichiometry of O2·− dismutation (2 O2·−:1 H2O2) and the amount of semiquinones produced by laccase during the entire reaction (500 μM). First, we demonstrated that the O2·− produced during semiquinone autoxidation was not reduced to H2O2 by the hydroquinones used in this study (O2·− + QH2 → H2O2 + Q·−); if it had been, a stoichiometry different from that of O2·− dismutation would have resulted. Since no quinones were observed after incubating 500 μM concentrations MBQH2 and DBQH2 with the XnO-Xn O2·−-generating system, it was assumed that all H2O2 detected after oxidation of methoxyhydroquinones by laccase was produced via O2·− dismutation. Then, it was estimated that only 2 and 10% of the semiquinones produced by laccase from 500 μM concentrations of MBQH2 and DBQH2, respectively, were autoxidized.
FIG. 1.
Effect of SOD and the XnO-Xn system on quinone production rates during oxidation of MBQH2 and DBQH2 by laccase. The reactions were carried out in 20 mM phosphate buffer, pH 5, containing 500 μM hydroquinones (control), 100 U of SOD/ml, 100 mU of XnO/ml, and 250 μM Xn. Heat-denatured enzymes were used in blanks. Means and 95% confidence limits of five replicates are shown.
Based on this limited extent of semiquinone autoxidation, it was evident that semiquinones were transformed into quinones by other mechanisms. The likelihood of semiquinone oxidation by laccase and semiquinone dismutation being these mechanisms was investigated. To test the ability of laccase to oxidize methoxysemiquinones, MBQ·− and DBQ·− were produced from their corresponding quinones with cytochrome P450 reductase (this enzyme catalyzes the monovalent reduction of quinones, using NADPH as an electron donor). Then, the level of H2O2 derived from semiquinone autoxidation was estimated and the effect of laccase on H2O2 levels was evaluated (if laccase were able to oxidize the semiquinones, its presence should decrease H2O2 levels by competing with the autoxidation reaction). The level of H2O2 was estimated once oxidation of 50 μM NADPH was completed, a process monitored spectrophotometrically at 340 nm. Preliminary experiments revealed that the initial concentration of quinones was a crucial factor for semiquinone autoxidation to proceed, probably due to the reversible nature of this reaction (e.g., no H2O2 was generated when 50 μM NADPH and a 400 μM concentration of quinones were used, although NADPH was completely oxidized). The results presented in Table 1 were those obtained with a 20 μM concentration of quinones. The presence of laccase caused 84.4 and 22.7% decreases in the amount of H2O2 produced from MBQ and DBQ, respectively, revealing the ability of laccase to oxidize semiquinones. On the other hand, semiquinone dismutation was found to occur in samples lacking laccase in the above-described cytochrome P450 experiment (Table 1). The existence of this reaction was inferred from the presence of MBQH2 and DBQH2 at the end of reactions involving their corresponding quinones. No hydroquinones were found when laccase was present in the reaction mixture.
TABLE 1.
Production of H2O2 and hydroquinones during reduction of MBQ and DBQ by cytochrome P450 reductase in the absence and presence of laccasea
| Quinone | Mean concn ± 95% confidence limit (μM)
|
|||
|---|---|---|---|---|
| H2O2
|
Hydroquinone
|
|||
| −Laccase | +Laccase | −Laccase | +Laccase | |
| MBQ | 4.1 ± 0.2 | 0.6 ± 0.0 | 13.2 ± 0.9 | 0.0 |
| DBQ | 20.9 ± 1.0 | 16.2 ± 0.7 | 8.5 ± 0.7 | 0.0 |
H2O2 levels were estimated after NADPH was fully oxidized. Reaction mixtures contained 20 mM phosphate buffer (pH 5), 5 mU of cytochrome P450 reductase/ml, 50 μM NADPH, and 20 μM quinones. Laccase experiments (+) were done with 50 mU of enzyme/ml (estimated with 500 μM MBQH2). Samples containing 100 U of catalase/ml were used as blanks.
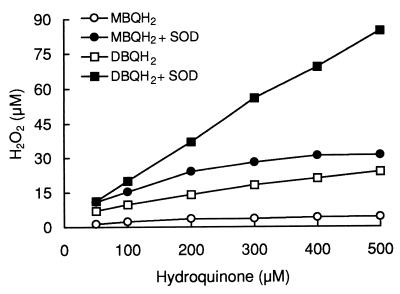
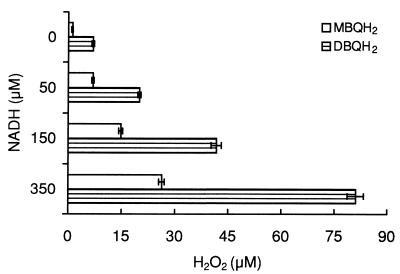
To favor the conversion of semiquinone to quinones via autoxidation over dismutation and laccase oxidation, two experiments resembling more-natural conditions and involving the removal of semiquinone autoxidation products were carried out. First, SOD was added for a faster consumption of O2·−. Using various concentrations of hydroquinones (50 to 500 μM), it was found that the presence of SOD in the laccase reaction mixture increased H2O2 production 6.7- to 8.4-fold (depending on the initial concentration of hydroquinones) in the case of MBQH2 and 1.6- to 3.5-fold in that of DBQH2 (Fig. 2). These results are shown in Table 2 in terms of the extent of semiquinone autoxidation. A negative correlation was found between the initial concentration of hydroquinones, which corresponded with the amount of semiquinones produced by laccase during the entire reaction, and the extent of semiquinone autoxidation. Second, removal of quinones during laccase reactions was achieved by adding diaphorase, a reductase catalyzing the divalent reduction of quinones from NADH oxidation. The final H2O2 levels in 1-ml reaction volumes containing, in addition to laccase and diaphorase, 50 nmol of hydroquinones and various amounts of NADH are shown in Fig. 3 (reaction completion was tested by monitoring NADH oxidation at 340 nm). For the correct interpretation of these results, it should be noted that the amount of hydroquinone oxidized (semiquinone produced) by laccase during the reaction was the quantity present at the beginning of the experiment (50 nmol) plus the amount recycled from the NADH oxidation. Therefore, taking into account the stoichiometry of the diaphorase reaction (1 NADH:1 QH2), it was assumed that 100, 200, and 400 nmol of hydroquinones were oxidized by laccase in samples containing 50, 150, and 350 nmol of NADH, respectively. The complete oxidation of NADH in samples containing an amount larger than that required to reduce the quinone produced by laccase from 50 nmol of hydroquinone demonstrated redox cycling and supported the above assumption. The results shown in Fig. 3 show that H2O2 levels were proportional to the amount of MBQH2 and DBQH2 oxidized by laccase. To evaluate the effect of quinone removal on semiquinone autoxidation, these results were compared with those of the control experiment in Fig. 2, in which quinones accumulated in the reaction mixture (Table 2). Quinone recycling by diaphorase increased MBQ·− and DBQ·− autoxidation 3.0- to 6.5-fold and 2.0- to 3.9-fold, respectively. In addition, quinone recycling kept the extent of semiquinone autoxidation constant (around 14 and 41% in all samples for MBQ·− and DBQ·−, respectively). From these results, it was inferred that quinone accumulation was the factor causing decreased extents of semiquinone autoxidation as the initial concentration of hydroquinones increased in control and SOD experiments. This effect was better shown with MQH·−, whose autoxidation reaction equilibrium was less shifted to the right.
FIG. 2.
Effect of SOD on H2O2 production during oxidation of MBQH2 and DBQH2 by laccase. H2O2 levels were measured after complete oxidation of hydroquinones, which was monitored spectrophotometrically. The compositions of the reaction mixture were as follows: 20 mM phosphate buffer (pH 5), 50 to 500 μM concentrations of hydroquinones, 100 mU of laccase/ml (estimated with 500 μM MBQH2), and 100 U of SOD/ml. Samples containing 100 U of catalase/ml were used as blanks. Means of five replicates are shown (95% confidence limits were less than 5% of the mean).
TABLE 2.
Effect of SOD and diaphorase on extent of semiquinone autoxidationa
| Amt of semiquinone produced (nmol) | % Autoxidation of:
|
|||||
|---|---|---|---|---|---|---|
| MBQ·−
|
DBQ·−
|
|||||
| Control | With SOD | With diaphorase | Control | With SOD | With diaphorase | |
| 50 | 5.2 | 43.6 | 28.0 | 45.1 | ||
| 100 | 4.5 | 30.6 | 13.8 | 19.6 | 40.0 | 39.9 |
| 200 | 3.5 | 24.0 | 14.7 | 14.0 | 37.0 | 41.7 |
| 300 | 2.3 | 18.6 | 12.1 | 37.2 | ||
| 400 | 2.0 | 15.4 | 13.1 | 10.5 | 34.6 | 40.5 |
| 500 | 1.7 | 12.4 | 9.6 | 33.9 | ||
FIG. 3.
Production of H2O2 during oxidation of MBQH2 and DBQH2 by laccase in the presence of diaphorase and various amounts of NADH. H2O2 levels were measured after the oxidation of NADH. The reactions mixtures contained 20 mM phosphate buffer (pH 5), 50 mU of laccase/ml (estimated with 500 μM MBQH2), 50 μM hydroquinones, 150 mU of diaphorase/ml, and 0 to 350 μM NADH. Samples containing 100 U of catalase/ml were used as blanks. Means and 95% confidence limits of five replicates are shown.
DISCUSSION
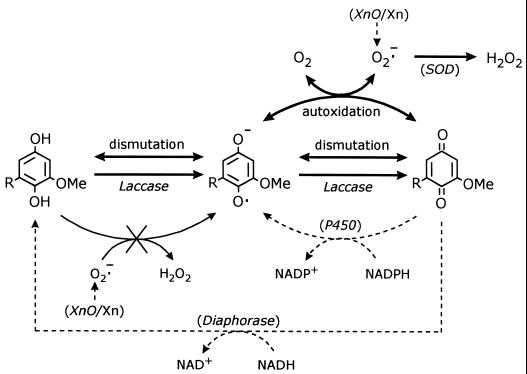
A diagram of the reactions involved in the conversion of the semiquinones produced by laccase into quinones, including the strategies used to demonstrate their existence, is shown in Fig. 4. This conversion can be carried out by three mechanisms: autoxidation, laccase oxidation, and dismutation. Obviously, the contribution of laccase-mediated hydroquinone oxidation to the production of partially reduced oxygen species will depend on the extent of semiquinone autoxidation. This extent was estimated from H2O2 production during laccase reactions after it was demonstrated that other H2O2-producing reactions, such as O2·− reduction by methoxyhydroquinones, did not take place (Fig. 4). The latter reaction, described for certain naphthohydroquinones, leads to a chain reaction in which the O2·− produced during the autoxidation of semiquinones acts as the propagating species (39). This reaction was evidenced with the hydroquinones produced from 2-methyl-1,4-naphthoquinone (menadione) and duroquinone by P. eryngii (21). The buffer and substrate solutions used in the reactions in the present study were treated with the chelating resin Chelex 100 to reduce the levels of trace metal ions, which by reacting with H2O2 or O2·− could lead to H2O2 underestimations. As mentioned above, during the incubation of laccase with 100 μM BQH2, less than 1% of the semiquinone autoxidized (38). The extent of methoxysemiquinone autoxidation was expected to be higher, since electron transfer to O2 is favored by electron-donating substituents, such as methoxyl groups, which decrease the reduction potential of the semiquinone-quinone couple (6). Besides, DBQ·− autoxidation had been described previously in studies concerning the use of DBQ as an anticancer agent (42). As shown in Table 2, the extent of autoxidation of 100 μM concentrations of MBQ·− and DBQ·− rose to 4.5 and 19.6%, respectively. Despite the considerable increase observed, these results revealed that most of the semiquinone produced by laccase during the oxidation of any hydroquinone derived from lignin units was transformed into quinone through dismutation and laccase oxidation. However, in an in vitro laccase reaction, which is needed for demonstration of oxygen activation, the conditions are far from natural. We have focused our attention on the concentrations of semiquinone autoxidation reaction products because they probably entail the main difference in the extent of the reaction under in vitro and in vivo conditions. On the one hand, the O2·− produced in vitro disappeared by spontaneous dismutation. Under more-natural conditions, a faster O2·− consumption is expected because, in addition to undergoing dismutation, it can react with Mn2+, Fe3+ (see above), and radicals produced by ligninolytic enzymes (23). Such a faster consumption of O2·− was simulated by adding SOD to laccase reactions (Fig. 4), and the effects on H2O2 levels and the extent of semiquinone autoxidation are quite well illustrated by the results shown in Fig. 2 and Table 2, respectively. In the case of BQH2, H2O2 levels increased from 0.3 μM to 12 and 34 μM in the presence of SOD and Mn2+, respectively (38). On the other hand, whereas quinones accumulate in in vitro reactions, they are redox cycled in the presence of P. eryngii mycelium (21). Quinone redox cycling has been simulated in the present study by adding diaphorase to laccase reactions (Fig. 4), and the observed increase in the extent of semiquinone autoxidation (Table 2) show quite well the negative effect of quinone accumulation.
FIG. 4.
Scheme of the reactions involved in the conversion of semiquinones into quinones during oxidation of MBQH2 (R=H) and DBQH2 (R=OMe [where Me is a methyl group]) by laccase (bold arrows). The strategies used to demonstrate these reactions are included (dashed arrows and enzymes in brackets).
Besides semiquinone autoxidation, other mechanisms by which white-rot fungi could produce O2·− have been reported. First, in reactions involving lignin peroxidase, O2·− resulted both from the decomposition of a peroxy radical produced during oxidation of a lignin model dimer (25) and from autoxidation of formate radicals (CO2·−) produced during the oxidation of oxalate in the presence of veratryl alcohol (41). Second, MnP has also been linked to the production of O2·−, based on the ability of Mn3+ to produce CO2·− from not only oxalate but also glyoxylate (31, 32). Third, cellobiose dehydrogenase has been described to produce O2·− both directly, via monovalent reduction of oxygen (37), and indirectly, via Fe3+ reduction followed by Fe2+ autoxidation (51). The interest of researchers in studying the origin of O2·− is mainly related to its possible participation in Mn2+ oxidation (38, 41) and the generation of both H2O2 (31, 49) and HO· (41, 51). Since not all white-rot fungi produce the same lignin degradation enzymes, these mechanisms producing O2·− may have special importance in those fungi lacking MnP activities or extracellular oxidases. As mentioned above, laccase is the most widely distributed ligninolytic enzyme among white-rot fungi, and hydroquinones are intermediates in lignin degradation. These facts, together with the results presented in the present paper, establish the production of O2·− from semiquinone autoxidation as a firm alternative to any of the above-described mechanisms.
The results shown here also extend our previous findings on oxygen activation by P. eryngii through quinone redox cycling (21). This process, which mainly occurs as the monovalent reduction of a quinone to a semiquinone by NAD(P)H-dependent reductases followed by the oxidation of the semiquinone by O2, has been studied mostly in mammalian systems due to the human-cytotoxic effects of quinones (28). In P. eryngii, the process is quite peculiar due to the secretion of quinone reduction products, the participation of ligninolytic enzymes, and the extracellular production of reduced oxygen species. Based on the wide substrate specificity of the reductive and oxidative enzymes involved in the redox cycling of quinones, it is quite likely that the reduction of methoxyquinones by P. eryngii, followed by the oxidation of methoxyhydroquinones by laccase, leads to the production of extracellular O2·− on a constant basis. In addition to quinones acting as carriers of electrons from intracellular NAD(P)H to extracellular O2, aromatic aldehydes have being described to play the same role in P. eryngii. After being reduced to alcohols by intracellular reductases, they participate in the divalent reduction of O2 in reactions catalyzed by aryl-alcohol oxidase (17, 19). The simultaneous production of O2·− and H2O2 via the redox cycling of the pertinent fungal metabolites or lignin-derived products probably leads to HO· production via the Haber-Weiss reaction, as has already demonstrated during the redox cycling of menadione by P. eryngii (20). The latter has been recently used to study the effect of extracellular HO· production on the expression of genes encoding ligninolytic peroxidases (43). Besides being useful tools for fundamental studies, these mechanisms of O2 activation could be exploited in some biotechnological applications, such as biopulping, and in edible-mushroom cultivation techniques. An enhanced production of reduced O2 species promoted by redox cycling compounds would favor lignin degradation and competition for the substrate with other microorganisms which are not able to grow under oxidative-stress conditions.
ACKNOWLEDGMENTS
We thank P. Ander (University of Agricultural Science, Uppsala, Sweden) for samples of methoxyhydroquinones.
This research was funded by the project “Evaluation of Enzymatic and Radical-Mediated Mechanisms in Lignin Degradation by Fungi from the Genera Pleurotus and Phanerochaete” (Bio96-0393) of the Spanish Biotechnology Program. The stay of V. Gómez-Toribio at the Centro de Investigaciones Biológicas was supported by a fellowship from the Comunidad Autónoma de Madrid.
REFERENCES
- 1.Archibald F S, Fridovich I. The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys. 1982;214:452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- 2.Backa S, Gierer J, Reitberger T, Nilsson T. Hydroxyl radical activity associated with the growth of white-rot fungi. Holzforschung. 1993;47:181–187. [Google Scholar]
- 3.Baker W. Derivatives of pentahydroxybenzene, and a synthesis of pedicellin. J Chem Soc. 1941;1941:662–670. [Google Scholar]
- 4.Barr D P, Shah M M, Grover T A, Aust S D. Production of hydroxyl radical by lignin peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys. 1992;298:480–485. doi: 10.1016/0003-9861(92)90438-3. [DOI] [PubMed] [Google Scholar]
- 5.Bourbonnais R, Paice M G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990;267:99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- 6.Brunmark A, Cadenas E. Redox and addition chemistry of quinoid compounds and its biological implications. Free Radic Biol Med. 1989;7:435–477. doi: 10.1016/0891-5849(89)90126-3. [DOI] [PubMed] [Google Scholar]
- 7.Buswell J A, Hamp S G, Eriksson K-E. Intracellular quinone reduction in Sporotrichum pulverulentum by a NAD(P)H:quinone oxidoreductase. FEBS Lett. 1979;108:229–232. doi: 10.1016/0014-5793(79)81216-8. [DOI] [PubMed] [Google Scholar]
- 8.Call H P, Mücke I. History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems (Lignozym®- process) J Biotechnol. 1997;53:163–202. [Google Scholar]
- 9.de Jong E, Field J A, Bont J A M. Evidence for a new extracellular peroxidase: manganese inhibited peroxidase from the white-rot fungus Bjerkandera sp. Bos 55. FEBS Lett. 1992;299:107–110. doi: 10.1016/0014-5793(92)80111-s. [DOI] [PubMed] [Google Scholar]
- 10.Eggert C, Temp U, Dean J F D, Eriksson K-E L. A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett. 1996;391:144–148. doi: 10.1016/0014-5793(96)00719-3. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson K-E L, Habu N, Samejima M. Recent advances in fungal cellobiose oxidoreductases. Enzyme Microb Technol. 1993;15:1002–1008. [Google Scholar]
- 12.Faison B D, Kirk T K. Relationship between lignin degradation and production of reduced oxygen species by Phanerochaete chrysosporium. Appl Environ Microbiol. 1983;46:1140–1145. doi: 10.1128/aem.46.5.1140-1145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flournoy D S, Kirk T K, Highley T L. Wood decay by brown-rot fungi: changes in pore structure and cell wall volume. Holzforschung. 1991;45:383–388. [Google Scholar]
- 14.Flournoy D S, Paul J A, Kirk T K, Highley T L. Changes in the size and volume of pores in sweetgum wood during simultaneous rot by Phanerochaete chrysosporium Burds. Holzforschung. 1993;47:297–301. [Google Scholar]
- 15.Gierer J, Yang E Q, Reitberger T. On the significance of the superoxide radical (O2·−/HO2·) in oxidative delignification, studied with 4-t-butylsyringol and 4-t-butylguaiacol. Part I. The mechanism of aromatic ring opening. Holzforschung. 1994;48:405–414. [Google Scholar]
- 16.Glenn J K, Morgan M A, Mayfield M B, Kuwahara M, Gold M H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983;114:1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- 17.Guillén F, Evans C S. Anisaldehyde and veratraldehyde acting as redox cycling agents for H2O2 production by Pleurotus eryngii. Appl Environ Microbiol. 1994;60:2811–2817. doi: 10.1128/aem.60.8.2811-2817.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillén F, Martínez A T, Martínez M J. Substrate specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Eur J Biochem. 1992;209:603–611. doi: 10.1111/j.1432-1033.1992.tb17326.x. [DOI] [PubMed] [Google Scholar]
- 19.Guillén F, Martínez A T, Martínez M J, Evans C S. Hydrogen peroxide-producing system of Pleurotus eryngii involving the extracellular enzyme aryl-alcohol oxidase. Appl Microbiol Biotechnol. 1994;41:465–470. [Google Scholar]
- 20.Guillén F, Martínez M J, Martínez A T. Hydroxyl radical production by Pleurotus eryngii via quinone redox-cycling. In: Messner K, Srebotnik E, editors. Biotechnology in the pulp and paper industry: recent advances in applied and fundamental research. Vienna, Austria: Facultas-Universitätsverlag; 1996. pp. 389–392. [Google Scholar]
- 21.Guillén F, Martínez M J, Muñoz C, Martínez A T. Quinone redox cycling in the ligninolytic fungus Pleurotus eryngii leading to extracellular production of superoxide anion radical. Arch Biochem Biophys. 1997;339:190–199. doi: 10.1006/abbi.1996.9834. [DOI] [PubMed] [Google Scholar]
- 22.Gutiérrez A, Caramelo L, Prieto A, Martínez M J, Martínez A T. Anisaldehyde production and aryl-alcohol oxidase and dehydrogenase activities in ligninolytic fungi from the genus Pleurotus. Appl Environ Microbiol. 1994;60:1783–1788. doi: 10.1128/aem.60.6.1783-1788.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haemmerli S D, Schoemaker H E, Schmidt H W H, Leisola M S A. Oxidation of veratryl alcohol by the lignin peroxidase of Phanerochaete chrysosporium. FEBS Lett. 1987;220:149–154. [Google Scholar]
- 24.Hammel K E, Tardone P J, Moen M A, Price L A. Biomimetic oxidation of nonphenolic lignin models by Mn(III): new observations on the oxidizability of guaiacyl and syringyl substructures. Arch Biochem Biophys. 1989;270:404–409. doi: 10.1016/0003-9861(89)90044-1. [DOI] [PubMed] [Google Scholar]
- 25.Hammel K E, Tien M, Kalyanaraman B, Kirk T K. Mechanism of oxidative Cα-Cβ cleavage of a lignin model dimer by Phanerochaete chrysosporium ligninase. J Biol Chem. 1985;260:8348–8353. [PubMed] [Google Scholar]
- 26.Harvey P J, Schoemaker H E, Palmer J M. Veratryl alcohol as a mediator and the role of radical cations in lignin biodegradation by Phanerochaete chrysosporium. FEBS Lett. 1986;195:242–246. [Google Scholar]
- 27.Joseleau J P, Gharibian S, Comtat J, Lefebvre A, Ruel K. Indirect involvement of ligninolytic enzyme systems in cell wall degradation. FEMS Microbiol Rev. 1994;13:255–264. [Google Scholar]
- 28.Kappus H, Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981;37:1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- 29.Kersten P J, Kirk T K. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J Bacteriol. 1987;169:2195–2201. doi: 10.1128/jb.169.5.2195-2201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirk T K, Farrell R L. Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- 31.Kuan I C, Tien M. Glyoxylate-supported reactions catalyzed by Mn peroxidase of Phanerochaete chrysosporium: activity in the absence of added hydrogen peroxide. Arch Biochem Biophys. 1993;302:447–454. doi: 10.1006/abbi.1993.1238. [DOI] [PubMed] [Google Scholar]
- 32.Kuan I C, Tien M. Stimulation of Mn-peroxidase activity: a possible role for oxalate in lignin biodegradation. Proc Natl Acad Sci USA. 1993;90:1242–1246. doi: 10.1073/pnas.90.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leisola M S A, García S. The mechanism of lignin degradation. In: Coughlan M P, editor. Enzyme systems for lignocellulose degradation. London, United Kingdom: Elsevier Applied Science; 1989. pp. 89–99. [Google Scholar]
- 34.Leonowicz A, Edgehill R U, Bollag J M. The effect of pH on the transformation of syringic and vanillic acids by the laccases of Rhizoctonia practicola and Trametes versicolor. Arch Microbiol. 1984;137:89–96. [Google Scholar]
- 35.Leontievsky A A, Vares T, Lankinen P, Shergill J K, Pozdnyakova N N, Myasoedova N M, Kalkkinen N, Golovleva L A, Cammack R, Thurston C F, Hatakka A. Blue and yellow laccases of ligninolytic fungi. FEMS Microbiol Lett. 1997;156:9–14. doi: 10.1111/j.1574-6968.1997.tb12698.x. [DOI] [PubMed] [Google Scholar]
- 36.Martínez M J, Ruiz-Dueñas F J, Guillén F, Martínez A T. Purification and catalytic properties of two manganese-peroxidase isoenzymes from Pleurotus eryngii. Eur J Biochem. 1996;237:424–432. doi: 10.1111/j.1432-1033.1996.0424k.x. [DOI] [PubMed] [Google Scholar]
- 37.Morpeth F F. Some properties of cellobiose oxidase from the white-rot fungus Sporotrichum pulverulentum. Biochem J. 1985;228:557–564. doi: 10.1042/bj2280557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muñoz C, Guillén F, Martínez A T, Martínez M J. Laccase isoenzymes of Pleurotus eryngii: characterization, catalytic properties, and participation in activation of molecular oxygen and Mn2+ oxidation. Appl Environ Microbiol. 1997;63:2166–2174. doi: 10.1128/aem.63.6.2166-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Öllinger K, Buffinton G D, Ernster L, Cadenas E. Effect of superoxide dismutase on the autoxidation of substituted hydro- and semi-naphthoquinones. Chem-Biol Interact. 1990;73:53–76. doi: 10.1016/0009-2797(90)90108-y. [DOI] [PubMed] [Google Scholar]
- 40.Ordoñez I D, Cadenas E. Thiol oxidation coupled to DT-diaphorase-catalysed reduction of diaziquione. Biochem J. 1992;286:481–490. doi: 10.1042/bj2860481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popp J L, Kalyanaraman B, Kirk T K. Lignin peroxidase oxidation of Mn2+ in the presence of veratryl alcohol, malonic or oxalic acid, and oxygen. Biochemistry. 1990;29:10475–10480. doi: 10.1021/bi00498a008. [DOI] [PubMed] [Google Scholar]
- 42.Roginsky V A, Bruchelt G, Stegmann H B. Fully reversible redox cycling of 2,6-dimethoxy-1,4-benzoquinone induced by ascorbate. Biochemistry (Mosc) 1998;63:200–206. [PubMed] [Google Scholar]
- 43.Ruiz-Dueñas F J, Guillén F, Camarero S, Pérez-Boada M, Martínez M J, Martínez Á. Regulation of peroxidase transcript levels in liquid cultures of the ligninolytic fungus Pleurotus eryngii. Appl Environ Microbiol. 1999;65:4458–4463. doi: 10.1128/aem.65.10.4458-4463.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoemaker H E. On the chemistry of lignin degradation. Rec Trav Chim Pays-Bas Belg. 1990;109:255–272. [Google Scholar]
- 45.Schoemaker H E, Meijer E M, Leisola M S A, Haemmerli S D, Waldner R, Sanglard D, Schmidt H W H. Oxidation and reduction in lignin biodegradation. In: Lewis N G, Paice M G, editors. Plant cell wall polymers. Washington, D. C.: American Chemical Society; 1989. pp. 454–471. [Google Scholar]
- 46.Shimada M, Ohta A, Kurosaka H, Hattori T, Higuchi T, Takahashi M. Roles of secondary metabolism of wood rotting fungi in biodegradation of lignocellulosic materials. In: Lewis N G, Paice M G, editors. Plant cell wall polymers. Washington, D. C.: American Chemical Society; 1989. pp. 412–425. [Google Scholar]
- 47.Thurston C F. The structure and function of fungal laccases. Microbiology (Read) 1994;140:19–26. [Google Scholar]
- 48.Tien M, Kirk T K. Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium Burds. Science. 1983;221:661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- 49.Urzúa U, Kersten P J, Vicuña R. Manganese peroxidase-dependent oxidation of glyoxylic and oxalic acids synthesized by Ceriporiopsis subvermispora produces extracellular hydrogen peroxide. Appl Environ Microbiol. 1998;64:68–73. doi: 10.1128/aem.64.1.68-73.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wariishi H, Valli K, Gold M H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991;176:269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]
- 51.Wood P M. Pathways of production of Fenton's reagent by wood-rotting fungi. FEMS Microbiol Rev. 1994;13:313–320. [Google Scholar]