Abstract
The production of microcystins (MC) from Microcystis aeruginosa UTEX 2388 was investigated in a P-limited continuous culture. MC (MC-LR, MC-RR, and MC-YR) from lyophilized M. aeruginosa were extracted with 5% acetic acid, purified by a Sep-Pak C18 cartridge, and then analyzed by high-performance liquid chromatography with a UV detector and Nucleosil C18 reverse-phase column. The specific growth rate (μ) of M. aeruginosa was within the range of 0.1 to 0.8/day and was a function of the cellular P content under a P limitation. The N/P atomic ratio of steady-state cells in a P-limited medium varied from 24 to 15 with an increasing μ. The MC-LR and MC-RR contents on a dry weight basis were highest at μ of 0.1/day at 339 and 774 μg g−1, respectively, while MC-YR was not detected. The MC content of M. aeruginosa was higher at a lower μ, whereas the MC-producing rate was linearly proportional to μ. The C fixation rate at an ambient irradiance (160 microeinsteins m−2 s−1) increased with μ. The ratios of the MC-producing rate to the C fixation rate were higher at a lower μ. Accordingly, the growth of M. aeruginosa was reduced under a P limitation due to a low C fixation rate, whereas the MC content was higher. Consequently, increases in the MC content per dry weight along with the production of the more toxic form, MC-LR, were observed under more P-limited conditions.
The bloom of cyanobacterium Microcystis aeruginosa is a ubiquitous phenomenon in eutrophic lakes and reservoirs in many countries of the world. Many strains of Microcystis are known to produce cyanobacterial hepatotoxins called microcystins. The toxin, a soluble peptide, is lethal to many kinds of aquatic organisms and damages zooplankton, fish (14), and the liver of higher animals (2, 22).
Many studies on the effects of environmental factors on microcystin production by cyanobacteria have been conducted with Microcystis (7, 13, 19, 20, 22–24), Anabaena (15, 16), Oscillatoria (17), and Synechocystis (11) species. The toxin of M. aeruginosa is at a maximum at light intensities between 40 and 50 microeinsteins m−2 s−1 (19, 23). The microcystin contents of Anabaena and M. aeruginosa are highest at 25°C (16) and between 20 and 24°C (21), respectively. Lower and higher temperatures decrease their amounts. In contrast, the effects of N and P on the toxin production by cyanobacteria are highly variable (13). Batch-cultured M. aeruginosa decrease in toxicity when N and inorganic C are removed from the medium (2). The concentration of microcystin in Anabaena increases with P (16). Toxin production by Oscillatoria agardhii depends on a low-level concentration of P (0.1 to 0.4 mg of P per liter), and higher concentrations have no additional effect (17). It was recently reported that the net microcystin production rate decreases as the specific cell division rate decreases in N-limited M. aeruginosa cultures (13). However, information about microcystin production related to the nutrient status in cells or in ambient circumstances is still insufficient.
In this study, M. aeruginosa was cultured in a chemostat to produce a culture in a steady state and clarify the toxin production relative to the growth of M. aeruginosa and its nutrient status (with a specific focus on P limitation), both of which seem to be important in eutrophic water.
MATERIALS AND METHODS
M. aeruginosa UTEX 2388, obtained from the University of Texas at Austin, was investigated in a P-limited chemostat (working volume, 800 ml) at dilution rates ranging from 0.1 to 0.8/day at 28°C by using a medium modified from Smith and Wiedeman (18). The composition of the medium was 2,000 μM KNO3, 6 μM Na2HPO4, 300 μM MgSO4 · 7H2O, 100 μM CaCl2 · 2H2O, 5,000 μM HEPES, 170 μM EDTA, 470 μM KOH (85%), 185 μM H3BO3, 100 μM Na2SiO3 · 9H2O, 17.9 μM FeSO4 · 7H2O, and 1 ml of microelements composed of 8.82 g of ZnSO4 · 7H2O, 1.44 g of MnCl2 · 4H2O, 0.17 g of MoO3, 1.57 g of CuSO4 · 5H2O, and 0.4 g of CoCl2 · 6H2O in 1,000 ml of acidified water which included 1 ml of H2SO4 (98%) per 1,000 ml of distilled water. The modification included replacing the Tris buffer with HEPES and adjusting the pH to 8.0. The P concentration was reduced to 6 μM. Illumination was continuous, provided by cool white fluorescence lamps at an average light intensity of 160 microeinsteins m−2 s−1 inside the culture vessel. When the culture reached a steady state, aliquots were used to measure the photosynthesis, cellular components, and microcystins. A 500-ml portion of the culture was centrifuged (Sorvall RC5C) for 10 min at 15,000 × g. The supernatant was used for the analysis of any residual P. At all steady states, no residual P was detectable. Cell pellets were then washed by centrifugation and stored at −65°C for further analysis.
The photosynthesis was measured by using the 14C technique at an ambient light intensity of 160 microeinsteins m−2 s−1. The radioactivity in the cells was measured by a liquid scintillation counter (Beckman LS6000). Chlorophyll a was extracted by using a chloroform-methanol mixture (2:1, vol/vol) and measured with a fluorometer (Turner 450) (25). The cell protein was determined by using bovine serum albumin as a standard (8). The cellular C was determined by using a total organic carbon analyzer (Shimadzu 5000A). The total N and P were determined after persulfate oxidation to nitrate (3) and orthophosphate (9), respectively. The nitrate was determined with a Szechrome NB reagent (26), and the orthophosphate was determined by the phosphomolybdate method (10).
The purification and analysis of the microcystins were carried out by the method developed by Harada et al. (6). First, the lyophilized cells were extracted three times with 50 ml of 5% (vol/vol) acetic acid for 30 min. The extract was centrifuged at 9,300 × g, and the supernatant was then applied to a C18 cartridge (Sep-Pak; Waters Association), eluted with methanol, and finally evaporated. The solution was analyzed on a high-performance liquid chromatograph (Shimadzu CLASS-LC10) fitted with a Nucleosil 5C18 column (Phase Separations) with a methanol–0.05 M phosphate buffer (pH 3.0) (57:43).
RESULTS
P-limited growth.
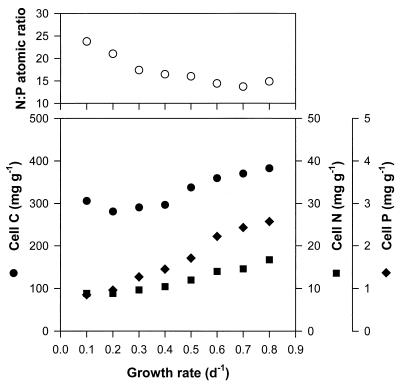
The changes in the cellular C, N, and P contents and the N/P atomic ratio of M. aeruginosa are shown in Fig. 1. On the basis of dry weight, cellular C, N, and P increased with a specific growth rate (μ); in particular, P increased about 3.0-fold with μ from 0.85 mg g−1 at μ of 0.1/day to 2.57 mg g−1 at μ of 0.8/day. The N/P atomic ratio of the steady-state cells was highest at μ of 0.1/day at 24 and gradually decreased with μ until 15 at μ of 0.8/day.
FIG. 1.
N/P atomic ratio and cellular carbon, nitrogen, and phosphorus contents of M. aeruginosa at each μ in a P-limited chemostat.
Microcystin production.
The cell density and microcystin content of the steady-state cells of M. aeruginosa are shown in Table 1. The protein content slightly increased with μ and was highly correlated with μ (Y = 38.0X + 134.0; r2 = 0.746). However, microcystins MC-LR and -RR were at a maximum at μ of 0.1/day at 339 μg g−1 and 774 μg g−1, respectively, and then gradually decreased with μ, whereas no MC-YR was detected.
TABLE 1.
Cell density and microcystin content of M. aeruginosa grown in P-limited Smith and Wiedeman mediuma
| μ/day | Dry wt (mg/liter) | Protein content (mg/g [dry wt]) | MC-LR
|
MC-RR
|
||
|---|---|---|---|---|---|---|
| (μg/g [dry wt]) | (mg/g of protein) | (μg/g [dry wt]) | (mg/g of protein) | |||
| 0.1 | 192 ± 4b | 137 ± 8 | 339.2 ± 4.4 | 2.47 ± 0.03 | 773.5 ± 4.4 | 5.63 ± 0.03 |
| 0.2 | 163 ± 8 | 141 ± 4 | 297.7 ± 6.7 | 2.11 ± 0.05 | 666.5 ± 9.8 | 4.72 ± 0.07 |
| 0.3 | 79 ± 2 | 145 ± 5 | 181.1 ± 9.5 | 1.25 ± 0.07 | 522.9 ± 7.6 | 3.61 ± 0.05 |
| 0.4 | 62 ± 2 | 144 ± 2 | 174.1 ± 2.9 | 1.21 ± 0.02 | 495.4 ± 3.9 | 3.45 ± 0.03 |
| 0.5 | 48 ± 9 | 160 ± 8 | 153.2 ± 6.3 | 0.96 ± 0.04 | 478.9 ± 9.2 | 2.99 ± 0.06 |
| 0.6 | 20 ± 7 | 159 ± 4 | 113.5 ± 3.7 | 0.71 ± 0.02 | 481.3 ± 4.7 | 3.02 ± 0.03 |
| 0.7 | 14 ± 2 | 167 ± 4 | 104.2 ± 3.6 | 0.62 ± 0.02 | 463.6 ± 7.5 | 2.78 ± 0.04 |
| 0.8 | 11 ± 1 | 156 ± 5 | 87.9 ± 5.2 | 0.56 ± 0.03 | 467.0 ± 8.1 | 3.00 ± 0.05 |
MC-YR was not detected.
Each value in the table (except for those in the dry wt column) represents the mean of three measurements ± standard deviation.
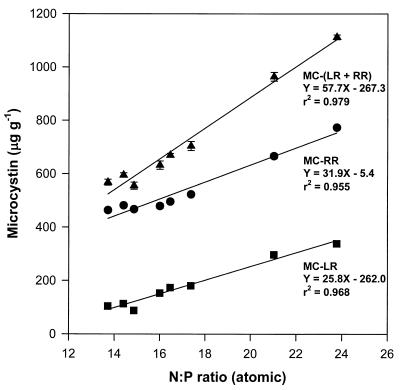
Figure 2 shows the relationship between the N/P atomic ratios and the microcystin contents. The cellular microcystin contents were highly correlated with the cellular N/P atomic ratios as follows: MC-LR (Y = 25.8X − 262.0; r2 = 0.968), MC-RR (Y = 31.9X − 5.4; r2 = 0.955), and MC-LR plus MC-RR (Y = 57.7X − 267.3; r2 = 0.979).
FIG. 2.
Relationship between the N/P atomic ratios and the microcystin [MC-LR, MC-RR, and MC-(LR + RR)] contents of M. aeruginosa. The error bars indicate standard deviation (n = 3).
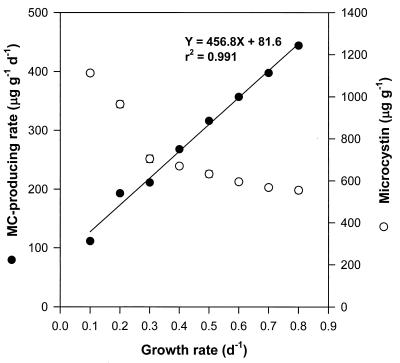
The microcystin-producing rate, derived from multiplying the microcystin content by μ, increased linearly with μ (Y = 456.8X + 81.6; r2 = 0.991), while the total microcystin content per unit of dry weight decreased with μ (Fig. 3). The maximum microcystin-producing rate was about 444 μg g−1 day−1 at μ of 0.8/day. The microcystin-producing rate of the cells increased about 4.0-fold when μ increased from 0.1 to 0.8/day, while the microcystin content decreased to 555 μg g−1 with μ, as a half of 1,118 μg g−1 at μ of 0.1/day. That is, the microcystin contents of the cells at a higher μ were low, while the microcystin-producing rate at a higher μ was higher due to the higher μ.
FIG. 3.
Microcystin-producing rate and microcystin content of M. aeruginosa at each μ in a P-limited chemostat. The error bars indicate standard deviation (n = 3).
Photosynthetic activity.
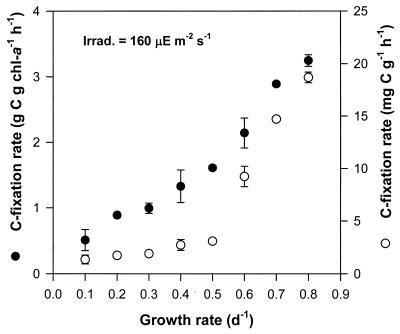
The C fixation rates of the steady-state cells of M. aeruginosa were investigated at an ambient light intensity of 160 microeinsteins m−2 s−1 (Fig. 4). The C fixation rates normalized into a chlorophyll a content increased linearly with μ. On a dry weight basis, the pattern of the C fixation rate versus μ was somewhat different from that on a chlorophyll a basis. The C fixation rates normalized into a dry weight increased with μ in two different patterns: (i) slight increase under μ of <0.5/day and (ii) dramatic increase under μ of >0.6/day.
FIG. 4.
C fixation rate on the basis of chlorophyll a (chl-a) and dry weight of M. aeruginosa at each μ in a P-limited chemostat. The ambient light intensity was 160 microeinsteins m−2 s−1. The error bars indicate standard deviation (n = 3).
Relationship between photosynthesis and microcystin production.
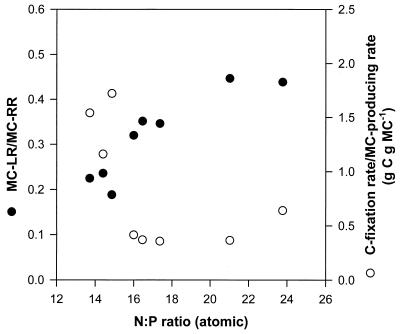
The ratios of MC-LR to MC-RR and the C fixation rate to the microcystin-producing rate are plotted as a function of the N/P atomic ratio of the steady-state cells of M. aeruginosa in Fig. 5. When the N/P atomic ratio was high (severe P-limited condition), the MC-LR content was still lower than the MC-RR content (Table 1; Fig. 2), yet the ratio of MC-LR to MC-RR increased. That is, the degree of the P limitation affected both the microcystin content of M. aeruginosa and the type of microcystin produced.
FIG. 5.
Relationship between the N/P atomic ratios and the ratio of MC-LR to MC-RR or the C fixation rate to the microcystin-producing rate of M. aeruginosa.
The microcystin-producing rates, on the basis of dry weight, increased dramatically with lower C fixation rates (2 to 4 mg of C g−1 h−1); however, with higher C fixation rates (4 to 19 mg of C g−1 h−1) the microcystin-producing rates were much reduced (Fig. 3 and 4). Consequently, there was a tendency of an increase in the ratio of the microcystin-producing rate to the C fixation rate in P-limited conditions (Fig. 5).
DISCUSSION
The cellular C, N, and P constituents of M. aeruginosa were changed as a function of μ in a P-limited chemostat. In particular, the cellular P concentration decreased dramatically with an increasing N/P atomic ratio, which meant further P limitation. As a result, the N/P atomic ratios of M. aeruginosa were within the range of 24 to 15, depending on μ from 0.1 to 0.8/day. In a previous study, Oh and Rhee (12) reported that the optimum N/P atomic ratio of M. aeruginosa was 11, which was determined as the ratio of minimum cell quotas of N to P.
The microcystin content of the steady-state cells of M. aeruginosa decreased with μ, while the protein content slightly increased. That is, the production of microcystin may be uncoupled from general protein synthesis, as previously reported by Utkilen and Gjølme (19).
There have been several reports on the effect of P on microcystin production by cyanobacteria (17, 20, 23). Watanabe and Oishi (23) stated that P-deficient cells have a slightly lower toxicity, yet no difference can be seen between cells grown in 1/10 and 1/20 P conditions. Utkilen and Gjølme (20) reported that phosphate-limited conditions have no effect on the toxin production of M. aeruginosa, whereas iron-limited conditions do. Recently, Sivonen (17) reported that the toxin production of O. agardhii depends on a low-level concentration of P (0.1 to 0.4 mg of P per liter) and higher concentrations have no additional effect. Accordingly, this indicates the possibility of an increase of toxicity in P-limited conditions. In the present experiment, the degree of the P limitation of the cells was directly analyzed, and the microcystin content of the cells at μ of 0.1/day increased to 1,113 μg g−1, double that of 555 μg g−1 at μ of 0.8/day. In other words, more microcystins per unit of dry weight were produced in more P-limited conditions. From the above-mentioned results, it would seem that the controversies may arise from the following. (i) Complete cellular P limitation may not be induced by a supplied P concentration in a batch culture. (ii) There may be a threshold concentration of P which has an effect on the toxin production of cyanobacteria. (iii) There may be many differences among cyanobacterial species and strains.
There is a linear relationship between the microcystin-producing rate and μ (Y = 456.8X + 81.6; r2 = 0.991). According to Watanabe et al. (22), the production rate of MC-RR in Microcystis viridis was 175 μg g−1 day−1 and that of MC-YR in M. aeruginosa M228-12 was 1,130 μg g−1 day−1. In the present experiment, the microcystin-producing rate of M. aeruginosa was highest at 444 μg g−1 day−1 at μ of 0.8/day. Therefore, it would seem that the microcystin-producing rate in a water system is determined by μ of M. aeruginosa that in turn is determined by the concentration of P in the water body.
At an ambient light intensity, there was a biphasic relationship between the C fixation rates based on their dry weights and growth rates. C fixed by photosynthesis is used not only for reproduction but also for cellular maintenance, and some is even excreted (4). The storage and utilization of fixed C may also vary depending on the degree of the P limitation. Therefore, it is not surprising that there was no straightforward relationship between growth and photosynthesis.
The ratio of MC-LR to MC-RR increased under severe P-limited conditions. Unfortunately, the toxicity of MC-LR is more than four times stronger than that of MC-RR judging from the data of the 50% lethal dose with mice (5, 7). According to Kotak et al. (7), MC-LR is one of the most potent microcystins, having a 50% lethal dose of 50 μg kg−1 by intraperitoneal injection in mice. The ratios of the microcystin-producing rate to the C fixation rate were higher at a lower μ. Thus, the growth of M. aeruginosa was reduced under P limitation due to a low C fixation rate, whereas the microcystin content was higher. These results strongly suggest that P is an important factor in the control of both the production of microcystin and the type of microcystin produced. In particular, in the P-limited cells of M. aeruginosa, there were increasing tendencies for both the microcystin content and the more toxic MC-LR, compared to MC-RR. However, in the culture of M. aeruginosa, the microcystin-producing rate was determined by the concentration of P in the water body. Accordingly, these facts should be considered when managing and restoring the water quality in eutrophic lakes where microcystin-producing cyanobacterial bloom occurs frequently.
More P in the culture medium stimulates the growth and microcystin production of M. aeruginosa (Fig. 3). This finding can be extrapolated to M. aeruginosa growing under bloom conditions. That is, the reduction of P in eutrophic waters may lower the growth and microcystin-producing rate of M. aeruginosa, resulting in the reduction of the toxicity. Recently, a similar strategy was reported for Anabaena spp. (16).
The role of toxins in cyanobacteria is still not completely understood (16). It has been suggested that microcystins act as protective compounds against grazing by zooplankton (1). Therefore, it is postulated that, in the ecology of toxic cyanobacteria, M. aeruginosa under P-limited conditions has a more protective function by increasing toxicity against grazing by zooplankton. To confirm the above-mentioned opinion, further experiments are needed along with more information on the relationship between the microcystin content and type of M. aeruginosa and grazing by zooplankton.
REFERENCES
- 1.Carmichael W W. Cyanobacteria secondary metabolites—the cyanotoxins. J Appl Bacteriol. 1992;72:445–459. doi: 10.1111/j.1365-2672.1992.tb01858.x. [DOI] [PubMed] [Google Scholar]
- 2.Codd G A, Poon G K. Cyanobacterial toxins. In: Rogers L J, Gallon J R, editors. Biochemistry of the algae and cyanobacteria. Oxford, England: Clarendon Press; 1988. pp. 283–296. [Google Scholar]
- 3.D'Elia C F, Steudler P A, Corwin N. Determination of total nitrogen in aqueous samples using persulfate digestion. Limnol Oceanogr. 1977;22:760–764. [Google Scholar]
- 4.Fogg G E. The ecological significance of extracellular products of phytoplankton photosynthesis. Bot Mar. 1983;26:3–14. [Google Scholar]
- 5.Harada K-I. Chemistry and detection of microcystins. In: Watanabe M F, Harada K-I, Carmichael W W, Fujiki H, editors. Toxic microcystis. New York, N.Y: CRC Press; 1996. pp. 103–148. [Google Scholar]
- 6.Harada K-I, Matsuura K, Suzuki M, Oka H, Watanabe M F, Oishi S, Dahlem A M, Beasley V R, Carmichael W W. Analysis and purification of toxic peptides from cyanobacteria by reversed-phase high-performance liquid chromatography. J Chromatogr. 1988;448:275–283. doi: 10.1016/s0021-9673(01)84589-1. [DOI] [PubMed] [Google Scholar]
- 7.Kotak B G, Lam A K-Y, Prepas E E, Kenefick S L, Hrudey S E. Variability of the hepatotoxin microcystin-LR in hypereutrophic drinking water lakes. J Phycol. 1995;31:248–263. [Google Scholar]
- 8.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 9.Menzel D W, Corwin N. The measurement of total phosphorus in seawater based on the liberation of organically bound fractions by persulfate oxidation. Limnol Oceanogr. 1965;10:280–282. [Google Scholar]
- 10.Murphy J, Riley J P. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36. [Google Scholar]
- 11.Nascimento S M, Azevedo S M F O. Changes in cellular components in a cyanobacterium (Synechocystis aquatilis f. salina) subjected to different N/P ratios—an ecological study. Environ Toxicol. 1999;14:37–44. [Google Scholar]
- 12.Oh H-M, Rhee G-Y. A comparative study of microalgae isolated from flooded rice paddies: light-limited growth, C fixation, growth efficiency and relative N and P requirement. J Appl Phycol. 1991;3:211–220. [Google Scholar]
- 13.Orr P T, Jones G J. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol Oceanogr. 1998;43:1604–1614. [Google Scholar]
- 14.Penaloza R, Rojas M, Vila I, Zambrano F. Toxicity of a soluble peptide from Microcystis sp. to zooplankton and fish. Freshw Biol. 1990;24:233–240. [Google Scholar]
- 15.Rapala J, Sivonen K. Assessment of environmental conditions that favor hepatotoxic and neurotoxic Anabaena spp. strains cultured under light limitation at different temperatures. Microb Ecol. 1998;36:181–192. doi: 10.1007/s002489900105. [DOI] [PubMed] [Google Scholar]
- 16.Rapala J, Sivonen K, Lyra C, Niemelä S I. Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl Environ Microbiol. 1997;63:2206–2212. doi: 10.1128/aem.63.6.2206-2212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivonen K. Effects of light, temperature, nitrate, orthophosphate, and bacteria on growth of and hepatotoxin production by Oscillatoria agardhii strains. Appl Environ Microbiol. 1990;56:2658–2666. doi: 10.1128/aem.56.9.2658-2666.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith R L, Wiedeman V E. A new alkaline growth medium for algae. Can J Bot. 1964;42:1582–1586. [Google Scholar]
- 19.Utkilen H, Gjølme N. Toxin production by Microcystis aeruginosa as a function of light in continuous cultures and its ecological significance. Appl Environ Microbiol. 1992;58:1321–1325. doi: 10.1128/aem.58.4.1321-1325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Utkilen H, Gjølme N. Iron-stimulated toxin production in Microcystis aeruginosa. Appl Environ Microbiol. 1995;61:797–800. doi: 10.1128/aem.61.2.797-800.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van der Westhuizen A J, Eloff J N. Effect of temperature and light on the toxicity and growth of the blue-green alga Microcystis aeruginosa (UV-006) Planta. 1985;163:55–59. doi: 10.1007/BF00395897. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe M F, Harada K-I, Matsuura K, Watanabe M, Suzuki M. Heptapeptide toxin production during the batch culture of two Microcystis species (Cyanobacteria) J Appl Phycol. 1989;1:161–165. [Google Scholar]
- 23.Watanabe M F, Oishi S. Effects of environmental factors on toxicity of a cyanobacterium (Microcystis aeruginosa) under culture conditions. Appl Environ Microbiol. 1985;49:1342–1344. doi: 10.1128/aem.49.5.1342-1344.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wicks R J, Thiel P G. Environmental factors affecting the production of peptide toxins in floating scums of the cyanobacterium Microcystis aeruginosa in a hypertrophic African reservoir. Environ Sci Technol. 1990;24:1413–1418. [Google Scholar]
- 25.Wood L W. Chloroform-methanol extraction of chlorophyll a. Can J Fish Aquat Sci. 1985;42:38–43. [Google Scholar]
- 26.Wynne D, Rhee G-Y. Effects of light intensity and quality on the relative N and P requirement (the optimum N:P ratio) of marine planktonic algae. J Plankton Res. 1986;8:91–103. [Google Scholar]