Abstract
Many people living with rare disease (RD) report a difficult diagnostic process from the symptom onset until they obtain the definitive diagnosis. The aim of this study was thus to ascertain the diagnostic process in RDs, and explore the determinants related with having to wait for more than one year in this process (defined as “diagnostic delay”). We conducted a case–control study, using a purpose-designed form from the Spanish Rare Diseases Patient Registry for data-collection purposes. A descriptive analysis was performed and multivariate backward logistic regression models fitted. Based on data on 1216 patients living with RDs, we identified a series of determinants associated with experiencing diagnostic delay. These included: having to travel to see a specialist other than that usually consulted in the patient’s home province (OR 2.1; 95%CI 1.6–2.9); visiting more than 10 specialists (OR 2.6; 95%CI 1.7–4.0); being diagnosed in a region other than that of the patient’s residence at the date of symptom onset (OR 2.3; 95%CI 1.5–3.6); suffering from a RD of the nervous system (OR 1.4; 95%CI 1.0–1.8). In terms of time taken to see a specialist, waiting more than 6 months to be referred from the first medical visit was the period of time which most contributed to diagnostic delay (PAR 30.2%). In conclusion, this is the first paper to use a collaborative study based on a nationwide registry to address the diagnostic process of patients living with RDs. While the evidence shows that the diagnostic process experienced by these persons is complex, more studies are needed to determine the implications that this has for their lives and those of their families at a social, educational, occupational, psychological, and financial level.
Keywords: rare diseases, diagnostic odyssey, diagnostic delay, time to diagnosis, diagnostic process, Spain, public health
1. Introduction
Rare diseases (RDs) are those whose prevalence is below 5 cases per 10,000 inhabitants in the European Community and Orphanet has described more than 6000. Diagnostic delay in RDs is a problem which has an impact on the lives, not only of the affected persons and their families, but also of society as a whole. From the time of symptom onset until a definitive diagnosis is received, the RD diagnostic process may involve visits to different specialists, numerous tests (sometimes unnecessary), travel to health centres other than the patient’s usual clinic, moving house, inappropriate treatments, hospitalisations, or the performance of surgical interventions, among other problems [1]. Furthermore, this does not lead to a correct diagnosis in every case, with the ensuing risk of potential iatrogenic effects. These determinants form part of a process which gives rise to increased stress, anxiety, and uncertainty about the patient’s future—something experienced by the whole family—in what has become known as “a diagnostic odyssey” [2,3]. Moreover, there is a lack of sufficiently detailed information about this diagnostic process and its determinants for RDs as a whole, which would allow for the necessary quantification and ensuing implementation of preventive action targeted at resolving the problem, partly or totally. While there are studies, essentially survey-based, which are not supported by related clinical data that would make it possible to validate the data collected, others focus on specific RDs and make no attempt to perform an in-depth analysis of the complete diagnostic process [4,5,6,7,8,9].
Based on the Aarhus Statement, which lays down the key time points and periods of the diagnostic process, the following three time periods can be distinguished [10]: (i) the period running from symptom onset until first medical visit, generally in a primary health care setting; (ii) the period that elapses between first medical visit and referral to specialised care; (iii) the period covering the time from referral to a specialist until reaching a definitive diagnosis, which could be further subdivided into, firstly, the time from referral until being seen by the specialist, and secondly, the time from that first specialist appointment until obtaining the diagnosis.
Among its goals for 2027, the International Rare Diseases Research Consortium (IRDiRC) requires that all known RDs be diagnosed within a maximum of one year from the date on which medical advice on the symptoms is first sought [11]. As there is no standardised definition of diagnostic delay, this Consortium’s guideline can be adopted as a criterion, according to which a wait of more than one year between these two key time points could be regarded as a delay in diagnosis. Similarly, although there is no clear list of causes that contribute to such a diagnostic delay, the most cited of these include: (i) the lack of scientific knowledge surrounding RDs as a whole, which can be frustrating; (ii) the presence of widely varying non-specific symptoms that overlap between different clinical entities; (iii) the time spent in attending the different medical appointments, undergoing tests and obtaining results; (iv) the unavailability of ad hoc diagnostic tests [12]. For their part, health systems add intrinsic difficulties arising from the way they are organised and operate, and their procedures for authorising the transfer of patients between centres, all of which have an impact on this diagnostic delay. Further influential factors are a lack of sufficient staff, difficulties in finding specialised centres, and distances between them, due to them being generally situated in other regions [13].
Accordingly, the aim of this study was to ascertain the diagnostic process to which people living with RDs in Spain are subjected, and to explore the determinants linked to the delay in obtaining a diagnosis of an RD.
2. Materials and Methods
2.1. Study Design
We designed a case–control study using the following operational case definition: any patient whose diagnosis took more than one year from the date of his/her first medical visit, due to symptoms attributable to his/her RD [11]. Controls were defined as patients who managed to obtain a diagnosis of their disease within one year from their first medical visit.
As our sole data-source and inclusion criterion, we used patients who were entered on the Spanish Rare Diseases Patient Registry at the Carlos III Health Institute (Instituto de Salud Carlos III), as of 1 January 2022, and who voluntarily agreed to participate in the study, provided in every case that they were resident in Spain [14]. Study participation was not limited by age, sex, or type of RD (Table S1). In those cases where patients’ age or capacity rendered it impossible for them to answer, a guardian, relative, or caregiver was allowed to answer on their behalf. We excluded patients whose diagnostic process had taken place in another country.
2.2. Form Data Collection
The study data were sourced from a Spanish Rare Diseases Patient Registry form which was purpose-designed for this project and specifically geared to describing the diagnostic process [15]. The following participating institutions actively helped to promote patient registration at the Registry and disseminate the study: the Spanish Rare Disease Federation (Federación Española de Enfermedades Raras/FEDER); the State Reference Centre for the Care of People with Rare Diseases and their families (Centro de Referencia Estatal de Enfermedades Raras/CREER), which comes under the IMSERSO; the Institute of Rare Disease Research.
Computer-assisted web interviewing methodology [16] was completed with questionnaires adapted for visually handicapped persons. To prevent participation biases due to the digital divide and the COVID-19 pandemic, telephone surveys were administered to whoever requested them, as well as face-to-face surveys.
2.3. Measures
For analysis purposes the following variables were included, relating to: (i) the patient: sex, place of residence, type of RD (classified by organ or main system affected as per ICD10 criterion), and the dates (month/year) of symptom onset, first medical visit, and obtaining the definitive diagnosis. The last two dates were used to calculate the variable ‘diagnostic delay’, but where these dates could not be obtained, the qualitative question referring to this same time period was used instead; (ii) symptom onset of patient’s disease: age, decade, type of first medical visit, time elapsed between symptom onset and first medical visit, time elapsed between the latter and referral to the specialist, and time elapsed between referral and attending the first appointment; (iii) process until diagnosis: travel (including destination and number of journeys), changes of address, diagnosis in the same place as symptom onset or first hospital attended, specialists visited and tests performed (including frequency and number of different specialists/tests), hospitalisations and surgical interventions. Data were collected for the period before and immediately after diagnosis, up until one year after diagnosis had been obtained; (iv) diagnosis: time to diagnosis from date of first medical visit, age, place, and definitive or confirmatory test.
2.4. Statistical Analysis
We performed a descriptive analysis including the frequencies of presentation of the variables involved, comparing cases and controls. Univariate logistic regression and a multivariate model were used to calculate the risks of each variable. The dependent variable of the main model was obtaining diagnosis in under (=0) or over a year (=1), and taking under (=0) or over 6 months (=1) from symptom onset to the first medical visit was likewise modelled. The categorical variables were pre-tested as dummy variables. The multivariate model was adjusted by means of a backward process, with the criterion for the elimination of variables being the fact that the 95% confidence intervals (95%CI) of the odds ratios (ORs) of each variable did not include unity and that the difference between the likelihood ratios of the models was greater or not greater than −2logLR. The dummy variables were included in the initial multivariate model if some of their strata in the univariate model showed ORs with 95%CI not containing unity. In both cases, the resulting variables are shown in the final model, with a footnote indicating the variables for which they were adjusted. Two of the authors separately replicated the analyses using the SPSS v27 and Stata 14 software programmes, in order to confirm that the results matched. Lastly, we calculated the Population Attributable Risk (PAR) of taking more than 6 months in each of the 3 time periods, i.e., from symptom onset to first medical visit, from first medical visit to referral to a specialist, and from referral to having the appointment, comparing cases and controls and type of RD [17].
3. Results
Data were obtained on a total of 1232 persons who participated in the Spanish Rare Diseases Patient Registry’s study on diagnostic delay. Application of the exclusion criterion and data-screening yielded complete and consistent information on 1216 people living with an RD (699 cases and 517 controls; 58.8% women).
Table 1 shows the distribution of the variables between cases and controls. The first medical visit due to symptom onset and information about the disease took place in a primary health care setting (either family doctor or paediatrician): 58.2% of cases vs. 41.0% of controls. During the search for diagnosis, 67.7% of cases and 43.5% of controls had to travel to a hospital or specialist other than those in their place of residence. Persons with a diagnostic delay had to travel to a greater extent and more often, whether within their home province, to another province in their home Autonomous Region (AR), to another AR, or to another country. Indeed, twice as many patients with a diagnostic delay were forced to travel to another AR (26.6 vs. 13.2% of controls), mainly to Madrid and Catalonia. Furthermore, RD-related hospitalisations and surgical interventions were also more frequent among persons who experienced a delay in diagnosis (34.8% were hospitalised due to their RD vs. 25.1% of controls). The frequency of visits to specialists and tests performed before obtaining diagnosis was likewise higher among those who experienced diagnostic delay. With regard to the confirmatory test, 38.6% of cases and 21.8% of controls were diagnosed through genetic testing. Lastly, once diagnosis had been obtained, most of the patients with diagnostic delay who were being treated underwent a change in treatment due to the indication furnished by the diagnosis made (59.3%), with this figure being lower among controls (38.0%).
Table 1.
Diagnostic process of people living with an RD in Spain.
| Overall %(n) n = 1216 |
Cases %(n) n = 699 |
Controls %(n) n = 517 |
p Value | ||
|---|---|---|---|---|---|
| Sex | Men | 41.2 (501) | 38.9 (272) | 44.3 (229) | 0.059 |
| Women | 58.8 (715) | 61.1 (427) | 55.7 (288) | ||
| Type of RD | Musculoskeletal system and connective tissue | 9.3 (113) | 8.7 (61) | 10.1 (52) | <0.001 |
| Blood and blood-forming organs and certain disorders involving the immune mechanism | 5.1 (62) | 4.1 (29) | 6.4 (33) | ||
| Endocrine, nutritional and metabolic diseases | 8.1 (99) | 8.9 (62) | 7.2 (37) | ||
| Mental and behavioural disorders | 3.5 (43) | 5 (35) | 1.5 (8) | ||
| Diseases of the nervous system | 25.2 (307) | 28.5 (199) | 20.9 (108) | ||
| Diseases of the eye and adnexa | 16.5 (201) | 12.7 (89) | 21.7 (112) | ||
| Diseases of the circulatory system | 2.5 (31) | 2.1 (15) | 3.1 (16) | ||
| Congenital malformations, deformations and chromosomal abnormalities | 22.4 (272) | 24.3 (170) | 19.7 (102) | ||
| Others | 7.2 (88) | 5.6 (39) | 9.5 (49) | ||
| Symptom onset and medical visits | |||||
| <15 | 44.1 (535) | 47.4 (331) | 39.5 (204) | ||
| Age of symptom onset (years) | 15–29 | 18.8 (228) | 17.6 (123) | 20.3 (105) | 0.027 |
| 30–44 | 21.7 (263) | 21.3 (149) | 22.1 (114) | ||
| >45 | 15.5 (188) | 13.6 (95) | 18 (93) | ||
| Decade of symptom onset | 2010–2021 | 39.9 (484) | 32.9 (229) | 49.4 (255) | <0.001 |
| 2000–09 | 26.1 (316) | 30.3 (211) | 20.3 (105) | ||
| 1990–99 | 12.4 (150) | 12.3 (86) | 12.4 (64) | ||
| 1980–89 | 11.1 (135) | 11.5 (80) | 10.7 (55) | ||
| Until 1979 | 10.6 (128) | 13.1 (91) | 7.2 (37) | ||
| First medical visit due to symptoms | Neonates | 4 (49) | 3.4 (24) | 4.8 (25) | <0.001 |
| Primary care/paediatrics | 50.9 (618) | 58.2 (406) | 41 (212) | ||
| Specialist | 27.8 (338) | 22.5 (157) | 35 (181) | ||
| Emergencies/hospital | 15.6 (189) | 13.9 (97) | 17.8 (92) | ||
| Other | 1.7 (21) | 2 (14) | 1.4 (7) | ||
| Time from symptom onset to first medical visit | <15 days | 40.3 (486) | 38.9 (269) | 42.2 (217) | <0.001 |
| 15 days–1 month | 15.3 (185) | 13.4 (93) | 17.9 (92) | ||
| 1–6 months | 20.8 (251) | 19.8 (137) | 22.2 (114) | ||
| 6–12 months | 7.6 (92) | 8.5 (59) | 6.4 (33) | ||
| >1 year | 15.9 (192) | 19.4 (134) | 11.3 (58) | ||
| Time from first medical visit to referral to specialist | <15 days | 30.1 (304) | 21.9 (123) | 40.3 (181) | <0.001 |
| 15 days–1 month | 16.5 (167) | 11.4 (64) | 22.9 (103) | ||
| 1–6 months | 27.7 (280) | 27.9 (157) | 27.4 (123) | ||
| 6–12 months | 10.9 (110) | 14.6 (82) | 6.2 (28) | ||
| >1 year | 14.8 (150) | 24.2 (136) | 3.1 (14) | ||
| Referral time to specialist until appointment | <15 days | 29.7 (282) | 20.4 (108) | 41.5 (174) | <0.001 |
| 15 days–1 month | 18.9 (179) | 18.5 (98) | 19.3 (81) | ||
| 1–6 months | 34.5 (327) | 35.5 (188) | 33.2 (139) | ||
| 6–12 months | 10.2 (97) | 14.9 (79) | 4.3 (18) | ||
| >1 year | 6.7 (64) | 10.8 (57) | 1.7 (7) | ||
| Travel (searching for diagnosis) | |||||
| Usual hospital as first hospital for provision of healthcare | Yes | 75.1 (881) | 74.9 (511) | 75.4 (370) | 0.867 |
| No | 24.9 (292) | 25.1 (171) | 24.6 (121) | ||
| Province of first hospital and province of diagnosis | Coincide | 78.7 (918) | 72.5 (492) | 87.3 (426) | <0.001 |
| Different | 21.3 (249) | 27.5 (187) | 12.7 (62) | ||
| Travel to a different hospital or specialist | No | 42.6 (518) | 32.3 (226) | 56.5 (292) | <0.001 |
| Yes | 57.4 (698) | 67.7 (473) | 43.5 (225) | ||
| Travel within the same province | No | 62.6 (761) | 54.9 (384) | 72.9 (377) | <0.001 |
| Yes | 37.4 (455) | 45.1 (315) | 27.1 (140) | ||
| No. of journeys to same province | 1 | 17.9 (59) | 13.4 (31) | 28.3 (28) | <0.001 |
| 2–3 | 23.9 (79) | 23.4 (54) | 25.3 (25) | ||
| 4–10 | 35.5 (117) | 33.8 (78) | 39.4 (39) | ||
| >10 | 22.7 (75) | 29.4 (68) | 7.1 (7) | ||
| Travel to a different province, same AR | No | 86.7 (1054) | 84 (586) | 90.5 (468) | <0.001 |
| Yes | 13.3 (161) | 16 (112) | 9.5 (49) | ||
| No. of journeys to another province | 1 | 25.9 (28) | 16 (12) | 48.5 (16) | 0.004 |
| 2–3 | 31.5 (34) | 34.7 (26) | 24.2 (8) | ||
| 4–10 | 32.4 (35) | 36 (27) | 24.2 (8) | ||
| >10 | 10.2 (11) | 13.3 (10) | 3 (1) | ||
| Travel to another AR | No | 79.1 (961) | 73.4 (512) | 86.8 (449) | <0.001 |
| Yes | 20.9 (254) | 26.6 (186) | 13.2 (68) | ||
| No. of journeys to another AR | 1 | 34.6 (56) | 28.3 (34) | 52.4 (22) | 0.007 |
| 2–3 | 34.6 (56) | 34.2 (41) | 35.7 (15) | ||
| 4–10 | 21 (34) | 25 (30) | 9.5 (4) | ||
| >10 | 9.9 (16) | 12.5 (15) | 2.4 (1) | ||
| Travel to another country | No | 98.4 (1195) | 97.9 (683) | 99 (512) | 0.109 |
| Yes | 1.6 (20) | 2.1 (15) | 1 (5) | ||
| No. of journeys to another country | 1 | 54.5 (6) | 50 (4) | 66.7 (2) | 0.78 |
| 2–3 | 36.4 (4) | 37.5 (3) | 33.3 (1) | ||
| 4–10 | 9.1 (1) | 12.5 (1) | 0 (0) | ||
| Change of address related with search for diagnosis | Yes | 3.5 (43) | 3.1 (22) | 4.1 (21) | 0.393 |
| No | 96.5 (1173) | 96.9 (677) | 95.9 (496) | ||
| Province of symptom onset and diagnosis | Same | 76.5 (927) | 70.4 (489) | 84.9 (438) | <0.001 |
| Different | 23.5 (284) | 29.6 (206) | 15.1 (78) | ||
| AR of symptom onset and AR of diagnosis | Same | 83.8 (1015) | 78.3 (544) | 91.3 (471) | <0.001 |
| Different | 16.2 (196) | 21.7 (151) | 8.7 (45) | ||
| Specialists and tests (before diagnosis) | |||||
| No. of visits to specialists | 0–1 (Q1) | 27.3 (320) | 22.1 (151) | 34.4 (169) | <0.001 |
| 2–4 (Q2) | 22.3 (262) | 14.8 (101) | 32.8 (161) | ||
| 5–10 (Q3) | 26.2 (307) | 29.9 (204) | 21 (103) | ||
| >10 (Q4) | 24.2 (284) | 33.1 (226) | 11.8 (58) | ||
| No. of tests performed | 0–2 (Q1) | 25.4 (298) | 21 (143) | 31.6 (155) | <0.001 |
| 3–7 (Q2) | 25.6 (300) | 19.8 (135) | 33.6 (165) | ||
| 8–19 (Q3) | 24.4 (286) | 26.4 (180) | 21.6 (106) | ||
| >19 (Q4) | 24.6 (289) | 32.8 (224) | 13.2 (65) | ||
| No. of different specialists | 0 (Q1) | 18.3 (215) | 16.1 (110) | 21.4 (105) | <0.001 |
| 1–2 (Q2) | 33.5 (393) | 26.2 (179) | 43.6 (214) | ||
| 3–5 (Q3) | 23.1 (271) | 23.9 (163) | 22 (108) | ||
| >5 (Q4) | 25.1 (294) | 33.7 (230) | 13 (64) | ||
| No. of different tests performed | 0–1 (Q1) | 26.3 (308) | 22.3 (152) | 31.8 (156) | <0.001 |
| 2–3 (Q2) | 20.8 (244) | 17.9 (122) | 24.8 (122) | ||
| 4–8 (Q3) | 28.7 (337) | 28.6 (195) | 28.9 (142) | ||
| >8 (Q4) | 24.2 (284) | 31.2 (213) | 14.5 (71) | ||
| RD-related hospitalisations and surgical interventions (before diagnosis) | |||||
| Hospitalisations | No | 62.7 (735) | 56.8 (387) | 70.9 (348) | <0.001 |
| Yes | 30.7 (360) | 34.8 (237) | 25.1 (123) | ||
| Not known if RD-related | 6.6 (77) | 8.4 (57) | 4.1 (20) | ||
| Surgical interventions | No | 77.6 (909) | 72.2 (492) | 84.9 (417) | <0.001 |
| Yes | 17.9 (210) | 21.9 (149) | 12.4 (61) | ||
| Not known if RD-related | 4.5 (53) | 5.9 (40) | 2.6 (13) | ||
| Diagnosis | |||||
| Time to diagnosis from first medical visit (years) | <1 | 42.7 (735) | 100 (507) | <0.001 | |
| 1–3 | 21.7 (257) | 37.8 (258) | |||
| 4–9 | 17.2 (204) | 30 (204) | |||
| >10 | 18.4 (219) | 32.2 (219) | |||
| Age of diagnosis (years) | <5 | 32.9 (400) | 30 (210) | 36.8 (190) | 0.027 |
| 15–29 | 17.8 (216) | 17 (119) | 18.8 (97) | ||
| 30–44 | 25.9 (315) | 27.3 (191) | 24 (124) | ||
| >45 | 23.4 (285) | 25.6 (179) | 20.5 (106) | ||
| Definitive or confirmatory test of diagnosis | Analytical tests | 6.8 (83) | 6.9 (48) | 6.8 (35) | <0.001 |
| Biopsy | 11.8 (143) | 11 (77) | 12.9 (66) | ||
| Medical criterion | 10.2 (124) | 10.4 (73) | 9.9 (51) | ||
| Genetic testing | 31.5 (382) | 38.6 (270) | 21.8 (112) | ||
| Ophthalmological tests | 8.4 (102) | 5.9 (41) | 11.9 (61) | ||
| Neurology tests | 8.2 (99) | 7.9 (55) | 8.6 (44) | ||
| Radiology tests | 19.8 (240) | 16.3 (114) | 24.6 (126) | ||
| More than one test | 1.5 (18) | 1.6 (11) | 1.4 (7) | ||
| DK/NO | 1.8 (21) | 1.4 (10) | 2.1 (11) | ||
| Travel after diagnosis | |||||
| To a different hospital or specialist | Yes | 53.1 (558) | 54.7 (331) | 50.9 (227) | 0.221 |
| No | 46.9 (493) | 45.3 (274) | 49.1 (219) | ||
| Travel within the same province | Yes | 53.2 (306) | 54.5 (186) | 51.3 (120) | 0.441 |
| No | 46.8 (269) | 45.5 (155) | 48.7 (114) | ||
| No. of journeys to same province | 1 | 7.4 (20) | 7 (12) | 8 (8) | 0.971 |
| 2–3 | 23.5 (64) | 23.3 (40) | 24 (24) | ||
| 4–10 | 35.7 (97) | 36.6 (63) | 34 (34) | ||
| >10 | 33.5 (91) | 33.1 (57) | 34 (34) | ||
| Travel to different province in same AR | Yes | 21.7 (125) | 22.3 (76) | 20.9 (49) | 0.700 |
| No | 78.3 (450) | 77.7 (265) | 79.1 (185) | ||
| No. of journeys to another province | 1 | 22.1 (21) | 19.3 (11) | 26.3 (10) | 0.068 |
| 2–3 | 30.5 (29) | 35.1 (20) | 23.7 (9) | ||
| 4–10 | 33.7 (32) | 38.6 (22) | 26.3 (10) | ||
| >10 | 13.7 (13) | 7 (4) | 23.7 (9) | ||
| Travel to another AR | Yes | 43.1 (248) | 42.5 (145) | 44 (103) | 0.722 |
| No | 56.9 (327) | 57.5 (196) | 56 (131) | ||
| No. of journeys to another AR | 1 | 17.9 (32) | 16.7 (18) | 19.7 (14) | 0.577 |
| 2–3 | 36.3 (65) | 33.3 (36) | 40.8 (29) | ||
| 4–10 | 30.2 (54) | 33.3 (36) | 25.4 (18) | ||
| >10 | 15.6 (28) | 16.7 (18) | 14.1 (10) | ||
| Travel to another country | Yes | 4.5 (26) | 4.7 (16) | 4.3 (10) | 0.812 |
| No | 95.5 (549) | 95.3 (325) | 95.7 (224) | ||
| No. of journeys to another country | 1 | 82.4 (14) | 70 (7) | 100 (7) | 0.279 |
| 2–3 | 11.8 (2) | 20 (2) | 0 (0) | ||
| 4–10 | 5.9 (1) | 10 (1) | 0 (0) | ||
| Change of address as a consequence of diagnosis | Yes | 6.6 (72) | 5.8 (36) | 7.7 (36) | 0.206 |
| No | 93.4 (1015) | 94.2 (585) | 92.3 (430) | ||
| RD-related hospitalisations and operations after diagnosis | |||||
| Hospitalisations | Yes | 25.4 (267) | 23.3 (141) | 28.3 (126) | 0.149 |
| No | 72.6 (763) | 74.4 (450) | 70.2 (313) | ||
| Not known if RD-related | 2 (21) | 2.3 (14) | 1.6 (7) | ||
| Surgical interventions | Yes | 20.2 (212) | 19.3 (117) | 21.3 (95) | 0.392 |
| No | 77.6 (816) | 78 (472) | 77.1 (344) | ||
| Not known if RD-related | 2.2 (23) | 2.6 (16) | 1.6 (7) | ||
| Specialists, tests, province of follow-up and treatment after diagnosis | |||||
| No. of specialists before vs. after diagnosis | Similar | 45.1 (493) | 44.4 (276) | 46.1 (217) | 0.049 |
| More | 44.9 (490) | 43.6 (271) | 46.5 (219) | ||
| Fewer | 10 (109) | 11.9 (74) | 7.4 (35) | ||
| No. of tests before vs. after diagnosis | Similar | 36 (393) | 35.6 (221) | 36.4 (172) | 0.210 |
| More | 36.9 (403) | 35.3 (219) | 39 (184) | ||
| Fewer | 27.2 (297) | 29.1 (181) | 24.6 (116) | ||
| Change of treatment after diagnosis | Yes | 35.6 (389) | 38 (236) | 32.4 (153) | 0.095 |
| No | 28.8 (315) | 28.8 (179) | 28.8 (136) | ||
| No previous treatment | 35.6 (389) | 33.2 (206) | 38.8 (183) | ||
| Reason for change of treatment | Having a diagnosis | 50.9 (194) | 59.3 (137) | 38 (57) | <0.001 |
| Knowing the disease course of the RD | 27.3 (104) | 20.3 (47) | 38 (57) | ||
| More complete medical/social report | 9.2 (35) | 7.8 (18) | 11.3 (17) | ||
| Other | 12.6 (48) | 12.6 (29) | 12.7 (19) | ||
Q: quartile; RD: rare disease; AR: autonomous region; DK/NO: don’t know/no opinion.
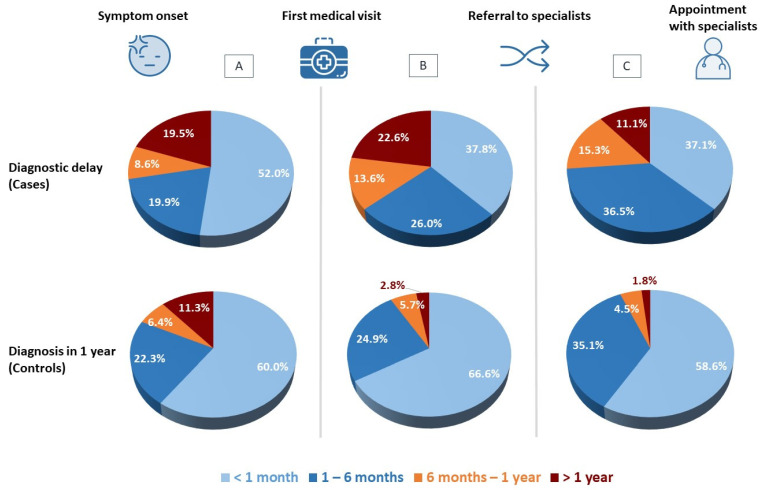
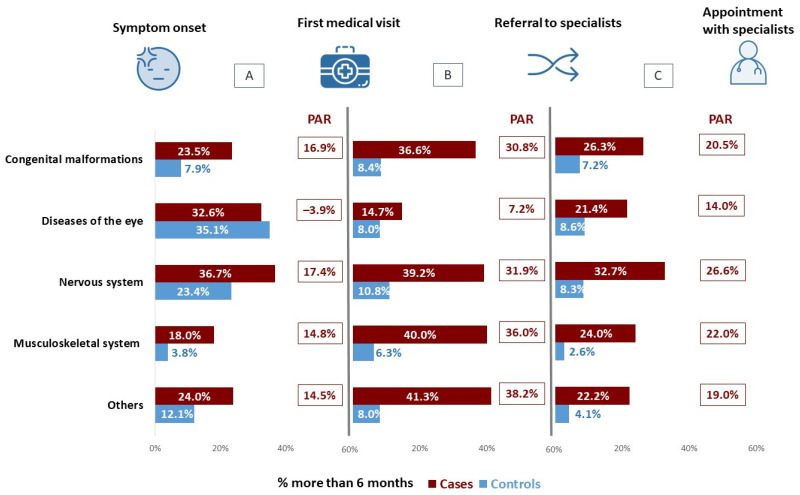
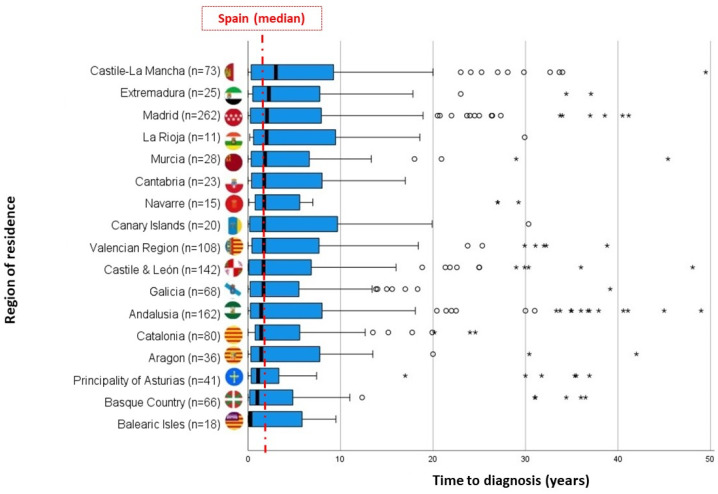
On comparing the times elapsed between RD symptom onset, attending a first medical visit, being referred to the specialist, and having the first appointment with that specialist, persons who experienced diagnostic delay were observed to take longer in every case (Figure 1). This pattern was maintained on analysing by type of RD, especially among persons affected by diseases of the nervous system (Figure 2). For RDs as a whole, the length of time between first medical visit and referral to a specialist is the period that displays the highest attributable risk (PAR 30.2%), followed by the time between referral and appointment with the specialist (PAR 21.4%) and the time taken by patients to first seek medical advice about their symptoms (PAR 12.6%). With regard to the time taken from first medical visit to diagnosis, the great dispersion of data meant that it was impossible to observe differences by AR of residence (p = 0.745; Figure 3).
Figure 1.
Time periods elapsed: (A) from symptom onset to first medical visit; (B) from first medical visit to referral to specialist; (C) from referral to appointment with specialist.
Figure 2.
Percentage of RD-affected persons who took more than 6 months: (A) from symptom onset to first medical visit; (B) from first medical visit to referral to specialist; (C) from referral to appointment with specialist. PAR: Population Attributable Risk.
Figure 3.
Time to diagnosis (years) by AR of RD symptom onset. Box-plot. The whisker plot at left shows (Xmin, Q1). The first part of the box (Q1, Q2). The second part of the box (Q2, Q3). The line inside the box represents the median of each AR, and the vertical red line, the median for Spain. The whisker plot at right is given by (Q3, Xmax). Circles: outliers that exceed the length of the IQR box by 1.5 units. Asterisks: outliers that exceed the length of the IQR box by 3 units.
According to the multivariate model shown in Table 2, the risk of taking more than 6 months to seek medical advice about symptoms was 8 times higher among persons who had initial manifestations of their RD before 1980 (OR 8.2; 95%CI 4.9–13.7), and more than twice as high among those with symptom onset in the 3 decades from 1980 to 2009. In terms of age of symptom onset, adults over the age of 15 years had a higher risk (OR 2.3; 95%CI 1.6–3.3). When analysed by type of RD, a higher risk of taking more than 6 months to seek medical advice about symptoms was observed among persons affected by diseases of the nervous system (OR 1.8; 95%CI 1.3–2.4), followed by diseases of the eye and adnexa (OR 1.7, 95%CI 1.2–2.5). Conversely, shorter times were recorded for patients affected by diseases of the musculoskeletal system and connective tissue (OR 0.5, 95%CI 0.3–0.8).
Table 2.
Determinants associated with taking more than 6 months to attend the first medical visit from time of RD symptom onset.
| Variable | Category | OR | 95%CI |
|---|---|---|---|
| Age at symptom onset | Adult (>15 years) | 2.3 | 1.6–3.3 |
| Decade of symptom onset | 2010–2021 * | ||
| 2000–09 | 2.2 | 1.5–3.2 | |
| 1990–99 | 2.6 | 1.7–4.1 | |
| 1980–89 | 2.4 | 1.4–4.0 | |
| Until 1979 | 8.2 | 4.9–13.7 | |
| Type of RD | Diseases of the musculoskeletal system and connective tissue | 0.5 | 0.3–0.8 |
| Diseases of the nervous system | 1.8 | 1.3–2.4 | |
| Diseases of the eye and adnexa | 1.7 | 1.2–2.5 |
* Reference group; Variables are shown whose 95%CI do not contain the value 1. Model adjusted for age of symptom onset, decade of symptom onset, and type of RD; OR: Odds ratio; RD: rare disease; n: 1182 (2.6% missing values). Hosmer-Lemeshow test p = 0.292.
In the case of risk of diagnostic delay, the multivariate model (Table 3) showed this to be higher among persons who first sought medical advice from their primary health care provider (OR 2.5; 95%CI 1.9–3.3). When the diagnostic process began, a higher risk of experiencing a delay in diagnosis was observed for persons who had to travel to hospitals or specialists other than those usually consulted in their home province (OR 2.1; 95%CI 1.6–2.9), and for those who had to travel to a different AR (OR 1.7; 95%CI 1.1–2.5). Furthermore, persons who were diagnosed in any AR other than that in which symptom onset occurred, likewise registered a higher risk of experiencing diagnostic delay (OR 2.3; 95%CI 1.5–3.6). As was to be expected, the higher the frequency of visits to specialists, the longer the diagnostic delay, especially in cases where patients consulted specialists more than 10 times (OR 2.6; 95%CI 1.7–4.0). Similarly, time to diagnosis increased where patients underwent tests of different types (OR 1.3; 95%CI 1.2–1.5) and/or some RD-related surgical intervention, before obtaining the definitive diagnosis (OR 1.8; 95%CI 1.3–2.5). There was evidence to show that having been diagnosed through genetic testing, albeit being positive in terms of obtaining the diagnosis, increased time to diagnosis (OR 2.1; 95%CI 1.5–2.8). When it came to the type of RD, persons affected by diseases of the nervous system had a higher risk (OR 1.4; 95%CI 1.0–1.8) and those affected by diseases of the eye and adnexa had a lower risk of diagnostic delay (OR 0.7; 95%CI 0.5–0.9).
Table 3.
Determinants associated with diagnostic delay for RDs in Spain.
| Variable | Category | OR | 95%CI |
|---|---|---|---|
| Type of RD | Diseases of the nervous system | 1.4 | 1.0–1.8 |
| Diseases of the eye and adnexa | 0.7 | 0.5–0.9 | |
| First medical visit by symptom | Primary care | 2.5 | 1.9–3.3 |
| Travel within the same province | Yes | 2.1 | 1.6–2.9 |
| Travel to another AR | Yes | 1.7 | 1.1–2.5 |
| AR of symptom onset and AR of diagnosis | Different | 2.3 | 1.5–3.6 |
| Surgical interventions | Yes | 1.8 | 1.3–2.5 |
| No. of visits to specialists | 0–1 (Q1) * | ||
| 2–5 (Q2) | 0.8 | 0.6–1.1 | |
| 6–10 (Q3) | 1.6 | 1.1–2.5 | |
| +10 (Q4) | 2.6 | 1.7–4.0 | |
| Ratio tests performed | Ratio | 1.3 | 1.2–1.5 |
| Definitive or confirmatory test of diagnosis | Genetic testing | 2.1 | 1.5–2.8 |
* Reference group; Variables are shown whose 95%CI do not contain the value 1. Model adjusted for sex, type of RD, age of symptom onset, first medical visit due to symptoms, travel within same province, travel to another AR, number of journeys within same province, province of symptom onset, and province of diagnosis, AR of first hospital and AR of diagnosis, number of visits to specialists, number of different specialists, ratio of tests performed (frequency/different), hospitalisations, surgical interventions, definitive or confirmatory test of diagnosis; OR: Odds ratio; Q: quartile; RD: rare disease; AR: autonomous region; n: 1150 (5.4% missing values). Hosmer-Lemeshow test p = 0.702.
4. Discussion
Based on Spanish Rare Diseases Patient Registry data, this study is the first to give a detailed account of the diagnostic process experienced by patients living with RDs, and to estimate the PAR of the time that elapses between symptom onset, making a first medical visit, being referred to a specialist, and finally being attended to by the specialist.
This study has highlighted some of the determinants of diagnostic delay, such as the type of RD, where the natural history of the individual diseases which make up each group influences the average obtained, though the low number of cases of each of these diseases would not allow for a robust and stable estimate to be obtained for any given RD. Persons affected by diseases of the nervous system display a higher risk of experiencing delays, due to the fact that many of these diseases are of great diagnostic complexity and that many of their clinical manifestations overlap, requiring more specific tests to arrive at a disease-specific diagnosis. Another determinant is making the first medical visit to primary health care to seek advice about symptoms. Furthermore, persons who experience diagnostic delay have a higher likelihood of being diagnosed in a region other than that in which they reside, and having to travel to other hospitals or specialists both within and outside their home AR. It is also more likely that they will have to visit specialists more often, that they will have to undergo more medical or even surgical tests, and that the definitive test of their diagnosis will be genetic. Our study thus corroborates the fact that persons who experience diagnostic delay undergo a more complex diagnostic process than those who are diagnosed within less than a year. A further finding is that the more recent the date of symptom onset is with respect to the date of study, the shorter the diagnostic delay, as other studies also report [5,18,19,20]. This may be related to the progressive improvement in knowledge and means, including genetic testing, for reaching a diagnosis, though recall bias may also have an influence (better recall of recent than of past events).
The period of time that elapses between symptom onset and first medical visit may vary depending on: the severity of the symptoms, the capacity of the RD-affected person or caregiver to recognise these, and, especially, the type of RD [21,22]. Other factors highlighted as having an influence are the decade and age of symptom onset, with the time between symptom onset and first medical visit being longer in the case of diseases of appearance at adult age. When symptoms begin, most persons visit their primary health care provider, a standard step in the Spanish public health system, since specialist care is provided on referral from this first healthcare level. Nevertheless, a higher attributable risk of diagnostic delay is shown, not by time periods that depend on the patient, but rather by those that depend on the health care system. Specifically, taking more than 6 months between first medical visit and referral to a specialist may be affected by high patient-to-physician ratios or the short amount of time allowed for consultation. In addition to this, there is the elapse of more than 6 months between the order of referral to the specialist and actually attending the appointment, which is linked to the way the Spanish public health system operates, since shorter waits are reported in other countries [21]. A number of studies stress the fact that taking less time to seek medical advice is associated with a shorter time to diagnosis [21,23], and that patients who are initially referred to specialists are diagnosed more quickly [6].
According to our study, the fact of residing in a given AR in a decentralised health system exerts an influence via the variable which reflects the need to travel to other regions to obtain a diagnosis. During the diagnostic process, more than half of all persons had to travel to hospitals or specialists other than those usually consulted: 20.9% to another AR, and 42.6% more than four times. This finding is similar to that of the ENSERio study, which indicated that 24.6% travelled to another AR, and 41.2% had to do so more than five times [5]. In the European context, according to EurordisCare2, 25% had to travel to another region, 2% had to travel to another country, and 10% of people living with an RD were finally forced to move house after obtaining the diagnosis [4]. Other studies highlight the fact that such journeys are associated with a longer time to diagnosis [6,7,8,9], though it is true to say that this association is not always found [21,24,25]. Studies on specific RDs, such as Poland Syndrome, indicate that risk of diagnostic delay increases 3.5-fold in cases where diagnosis is received in a region other than the home region [26]. These journeys, which are associated with a longer diagnostic delay, arise particularly when the RD is complex and requires specialised referral centres, which are usually concentrated in certain ARs, such as Madrid and Catalonia, among others [27]. Travel or even changes of address involve an important investment of time and money, and also have effects of an emotional nature.
As was to be expected, persons who experienced diagnostic delay visited more physicians, with 24.2% visiting as many as 10 or more during their search for diagnosis. Other studies also quantify this for RDs as a whole, with an average of 7.3 medical visits and 14 tests before obtaining diagnosis [28]. Eurordis indicates that 24% of families in Spain visited more than five physicians, and 10% visited more than ten, though it notes differences between countries: the Polish and Danish patients surveyed needed more visits than did their French, Swiss and Dutch counterparts [29]. In the UK, around 7 out of 10 patients made more than three medical visits, and 22% made six or more such visits [30,31]. At paediatric ages, 38.0% consulted six or more different physicians, and 11.1% consulted ten or more [32]. In general, persons with more complex, acute, and numerous symptoms visit more specialists, something that tends to be associated with the great number of medical visits and taking longer to obtain the diagnosis. Although the performance of certain diagnostic tests shortens this time [6,21], in general the number of tests performed is significantly higher among persons who experience diagnostic delay. In terms of the consequences, a greater number of medical visits and tests performed entail a higher financial cost for the person affected by RD, his/her family, and the National Health System. Other possible consequences are hospitalisations and surgical interventions that are experienced by patients before being diagnosed, which prove to be more frequent among persons with diagnostic delay. The natural history of the disease means that certain actions or surgical interventions geared to correcting a specific problem or improving the patient’s quality of life may sometimes become a priority, thereby possibly making the diagnostic process even longer. Furthermore, almost one-third of persons were diagnosed through genetic testing; although this is a useful tool for obtaining diagnoses, such tests are extremely time-consuming because not everyone has already described mutations, making it advisable to shorten the time taken to obtain results.
It is well known that healthcare systems are not always prepared to solve complex cases’ diagnosis problems as the ones some RD present. In order to face this, some solutions have been proposed: (i) using panels of genes with known effects that allow for testing if a patient has one of the known variants and can be diagnosed with a simple analysis; (ii) creating multidisciplinary units focus on RD; (iii) establishing networks of reference centres in which experts in different RD areas or groups work together to accelerate the diagnosis.
When it comes to comparing ours with other studies which analyse diagnostic delay by reference to symptom onset [33,34], it should be borne in mind that our study follows the IRDiRC indications, by establishing the date of first medical visit in order to ascertain the delay [11]. Similarly, it should be stressed that this study also furnished data on time elapsed from symptom onset.
With respect to limitations, it is possible that the lack of representation of some RDs may affect the results shown, since RDs are addressed as a whole. Furthermore, the heterogeneity of the natural history of the RDs included might also exert an influence. Another limitation of the study is the change in case definition, clinical practice and genetic diagnosis of RD during the study period. However, this limitation is a constant within the framework of a group of diseases that are characterized by high heterogeneity even when they are not entirely new and unknown. Insofar as form is concerned, participation was affected by the restrictive measures implemented during the COVID-19 pandemic, so that the digital divide was partially reduced. Lastly, though the response rate was in line with what is to be expected for these types of studies, the fact that persons with diagnostic delay might have a greater interest in participating means that there may be a greater number of cases than controls.
Lastly, this study’s main strength lies in its description of the diagnostic process and its determinants in Spain, being the first of its kind to use a nationwide registry open to any RD, with confirmed diagnoses in clinical reports, as its data source. Likewise, it enjoyed wide-ranging participation by RD-affected persons and their families, thanks to their being highly motivated to contribute to research. In addition, the participation of older persons and those who were visually handicapped was facilitated by using specially adapted forms along with telephone and/or in-person surveys, with the aim of mitigating possible selection biases.
5. Conclusions
This study is the first to address the diagnostic process of people living with RDs in Spain, based on data sourced from a national patient registry, in collaboration with FEDER and CREER. It shows that the diagnostic process undergone by people living with RDs is complex, particularly among those who experience diagnostic delay. In addition, it identified some of the determinants associated with diagnostic delay, such as making the first medical visit to the general practitioner, having to travel within the same province or to another AR, visiting more than 10 specialists, or being diagnosed in a region other than that of residence. This study also highlights the fact that the period of time posing the highest attributable risk of diagnostic delay is that of referral to the specialist, something that depends on the health system. Future in-depth studies are needed to continue the work of determining these factors in different types of RDs, along with the implications which this diagnostic process has for the lives of persons affected by RDs and their families, at a social, educational, occupational, psychological, and financial level.
Acknowledgments
We should like to thank the following: all RD-affected patients and their families who participated in this study; FEDER, CREER, the technical staff of the Carlos III Health Institute and medical societies that endorsed, promoted and supported the registration of new patients on the Spanish Patient Rare Diseases Registry. We are grateful to Beatriz Arconada and Begoña Ruiz for their valuable contributions that made this investigation possible. Also to the Undiagnosed Diseases Network International (UDNI). Finally to Michael Benedict for language support.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19116456/s1, Table S1: Table showing the complete list of included RD in the study.
Author Contributions
Conceptualization, V.A.-F., A.A.-D., A.A.-G., G.A.-M. and M.P.d.l.P.; methodology, V.A.-F., G.A.-M., J.B.-L. and M.P.d.l.P.; software, G.A.-M., M.G.-M. and J.B.-L.; validation, J.B.-L., G.A.-M., M.G.-M., A.A.-D., A.A.-G., V.A.-F. and M.P.d.l.P.; formal analysis, J.B.-L., G.A.-M., M.G.-M. and M.P.d.l.P.; investigation, J.B.-L., G.A.-M., M.G.-M., A.A.-D., A.A.-G., M.P.d.l.P. and V.A.-F.; resources, V.A.-F. and M.P.d.l.P.; data curation, M.G.-M. and J.B.-L.; writing—original draft preparation, J.B.-L. and V.A.-F.; writing—review and editing, V.A.-F., J.B.-L., G.A.-M., M.G.-M., A.A.-D., A.A.-G. and M.P.d.l.P.; visualization, J.B.-L., V.A.-F., M.G.-M., G.A.-M. and M.P.d.l.P.; supervision, V.A.-F. and M.P.d.l.P.; project administration, V.A.-F.; funding acquisition, V.A.-F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Spanish Rare Diseases Patient Registry and the present study were approved by the Committee for Ethical Research of the Institute of Health Carlos III (CEI PI 74_2016 and CEI PI 58_2021-v2).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study through the Spanish Rare Diseases Patient Registry.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the Spanish State Research Agency, State R&D Program Oriented to the Challenges of the Society, project no. RTI2018-094035-A-I00. J.B-L enjoys a Grant PRE2019-091508 funded by MCIN/AEI/10.13039/501100011033 by “ESF Investing in your future”.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kole A., Faurisson F. Rare Diseases Social Epidemiology: Analysis of Inequalities. In: Posada de la Paz M., Groft S., editors. Rare Diseases Epidemiology. Advances in Experimental Medicine and Biology. Volume 686. Springer; Dordrecht, The Netherlands: 2010. [DOI] [PubMed] [Google Scholar]
- 2.Black N., Martineau F., Manacorda T. Diagnostic Odyssey for Rare Diseases: Exploration of Potential Indicators. Policy Innovation Research Unit. 2015. [(accessed on 30 March 2022)]. Available online: https://piru.ac.uk/assets/files/Rare%20diseases%20Final%20report.pdf.
- 3.Carmichael N., Tsipis J., Windmueller G., Mandel L., Estrella E. “Is it going to hurt?”: The impact of the diagnostic odyssey on children and their families. J. Genet. Counsel. 2015;24:325–335. doi: 10.1007/s10897-014-9773-9. [DOI] [PubMed] [Google Scholar]
- 4.Eurordis Survey of the Delay in Diagnosis for 8 Rare Diseases in Europe (‘EurordisCare2′) 2007. [(accessed on 30 March 2022)]. Available online: https://www.eurordis.org/sites/default/files/publications/Fact_Sheet_Eurordiscare2.pdf.
- 5.FEDER (Federación Española de Enfermedades Raras) CREER (Centro de Referencia Estatal de Atención a Personas con Enfermedades Raras y sus Familias) Estudio Sobre Situación de Necesidades Sociosanitarias de las Personas con Enfermedades Raras en España: Estudio ENSERio: Datos 2016–2017. 1st ed. FEDER; Madrid, Spain: 2018. [Google Scholar]
- 6.Sreih A.G., Cronin K., Shaw D.G., Young K., Burroughs C., Kullman J., Machireddy K., McAlear C.A., Merkel P.A. Diagnostic delays in vasculitis and factors associated with time to diagnosis. Orphanet J. Rare Dis. 2021;16:184. doi: 10.1186/s13023-021-01794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palese F., Sartori A., Logroscino G., Pisa F.E. Predictors of diagnostic delay in amyotrophic lateral sclerosis: A cohort study based on administrative and electronic medical records data. Amyotroph. Lateral Scler. Front. Degener. 2019;20:176–185. doi: 10.1080/21678421.2018.1550517. [DOI] [PubMed] [Google Scholar]
- 8.Walter A.L., Baty F., Rassouli F., Bilz S., Brutsche M.H. Diagnostic precision and identification of rare diseases is dependent on distance of residence relative to tertiary medical facilities. Orphanet J. Rare Dis. 2021;16:131. doi: 10.1186/s13023-021-01769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jog M., Chouinard S., Hobson D., Grimes D., Chen R., Bhogal M., Simonyi S. Causes for treatment delays in dystonia and hemifacial spasm: A Canadian survey. Can. J. Neurol. Sci. 2011;38:704–711. doi: 10.1017/S0317167100012270. [DOI] [PubMed] [Google Scholar]
- 10.Weller D., Vedsted P., Rubin G., Walter F.M., Emery J., Scott S., Campbell C., Andersen R.S., Hamilton W., Olesen F., et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer. 2012;106:1262–1267. doi: 10.1038/bjc.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin C.P., Cutillo C.M., Lau L.P.L., Jonker A.H., Rath A., Julkowska D., Thomson D., Terry S.F., de Montleau B., Ardigò D., et al. Future of Rare Diseases Research 2017–2027: An IRDiRC Perspective. Clin. Transl. Sci. 2018;11:21–27. doi: 10.1111/cts.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chazal P.E., Chalandon A.S., Aymé S., Deleuze C. Diagnostic Delay in Rare Diseases: A Documented List of (296) Rare Diseases for Which Delayed Diagnosis Would Be Especially Detrimental, Based on the French Situation (PREPRINT). Research Square. 2020. [(accessed on 30 March 2022)]. Available online: https://www.researchsquare.com/article/rs-32308/v1.
- 13.Faviez C., Chen X., Garcelon N., Neuraz A., Knebelmann B., Salomon R., Lyonnet S., Saunier S., Burgun A. Diagnosis support systems for rare diseases: A scoping review. Orphanet J. Rare Dis. 2020;15:94. doi: 10.1186/s13023-020-01374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spanish Ministry of Health and Consumer Affairs (Ministerio de Sanidad y Consumo) Orden SCO/1730/2005, de 31 de Mayo, por la que se Crean y Suprimen Ficheros de Datos de Carácter Personal Gestionados por el Departamento. [(accessed on 30 March 2022)]. Boletín Oficial del Estado, 138, sec. III, de 10 de Junio de 2005, 19987 a 19989. Available online: https://www.boe.es/eli/es/o/2005/05/31/sco1730.
- 15.Spanish Rare Diseases Patient Registry. [(accessed on 30 March 2022)]. Available online: https://registroraras.isciii.es/Comun/Inicio.aspx.
- 16.Sowa P., Pędziński B., Krzyżak M., Maślach D., Wójcik S., Szpak A. The Computer-Assisted Web Interview Method as Used in the National Study of ICT Use in Primary Healthcare in Poland—Reflections on a Case Study. Stud. Log. Gramm. Rhetor. 2015;43:137–146. doi: 10.1515/slgr-2015-0046. [DOI] [Google Scholar]
- 17.Merletti F.C., Solkolne C.K., Vineis P. Epidemiología y Estadística. In: Stellman J.M., McCann M., Warshaw L., Brabant C., Finklea J., Messite J., Coppée G.H., Sauter S.L., Hunt V.R., Spiegel J., editors. Enciclopedia de Salud y Seguridad en el Trabajo. Volume 1. Ministerio de Trabajo y Asuntos Sociales, International Labour Organization; Madrid, Spain: 1998. [Google Scholar]
- 18.Radin M., Foddai S.G., Barinotti A., Cecchi I., Rubini E., Sciascia S., Roccatello D. Reducing the diagnostic delay in Antiphospholipid Syndrome over time: A real world observation. Orphanet J. Rare Dis. 2021;16:280. doi: 10.1186/s13023-021-01906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bygum A. Hereditary angio-oedema in Denmark: A nationwide survey. Br. J. Dermatol. 2009;161:1153–1158. doi: 10.1111/j.1365-2133.2009.09366.x. [DOI] [PubMed] [Google Scholar]
- 20.Takala J.H., Kautiainen H., Malmberg H., Leirisalo-Repo M. Wegener’s granulomatosis in Finland in 1981–2000: Clinical presentation and diagnostic delay. Scand. J. Rheumatol. 2008;37:435–438. doi: 10.1080/03009740802238366. [DOI] [PubMed] [Google Scholar]
- 21.Berges A.J., Lina I.A., Chen L., Ospino R., Davis R., Hillel A.T. Delayed Diagnosis of Idiopathic Subglottic Stenosis. Laryngoscope. 2022;132:413–418. doi: 10.1002/lary.29783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kachko L., Efrat R., Ben Ami S., Mukamel M., Katz J. Complex regional pain syndromes in children and adolescents. Pediatr. Int. 2008;50:523–527. doi: 10.1111/j.1442-200X.2008.02625.x. [DOI] [PubMed] [Google Scholar]
- 23.Hoyer N., Prior T.S., Bendstrup E., Wilcke T., Shaker S.B. Risk factors for diagnostic delay in idiopathic pulmonary fibrosis. Respir. Res. 2019;20:103. doi: 10.1186/s12931-019-1076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blankart C.R. Does healthcare infrastructure have an impact on delay in diagnosis and survival? Health Policy. 2012;105:128–137. doi: 10.1016/j.healthpol.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Roll K. The influence of regional health care structures on delay in diagnosis of rare diseases: The case of Marfan Syndrome. Health Policy. 2012;105:119–127. doi: 10.1016/j.healthpol.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Baldelli I., Gallo F., Crimi M., Fregatti P., Mellini L., Santi P., Ciliberti R. Experiences of patients with Poland syndrome of diagnosis and care in Italy: A pilot survey. Orphanet J. Rare Dis. 2019;14:269. doi: 10.1186/s13023-019-1253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministry of Health, Consumer Affairs and Social Welfare Statistical Site of the National Health System (Ministerio de Sanidad, Consumo y Bienestar Social. Portal Estadístico del Sistema Nacional de Salud-SNS) [(accessed on 30 March 2022)]. Available online: https://www.sanidad.gob.es/estadEstudios/sanidadDatos/tablas/tabla22.htm.
- 28.Shen F., Liu H. Incorporating Knowledge-Driven Insights into a Collaborative Filtering Model to Facilitate the Differential Diagnosis of Rare Diseases. AMIA Annu. Symp. Proc. 2018;2018:1505–1514. [PMC free article] [PubMed] [Google Scholar]
- 29.Kole A., Faurisson F. The voice of 12000 patients. Experiences and Expectations of Rare Disease Patients on Diagnosis and Care in Europe. Eurordis. 2009. [(accessed on 30 March 2022)]. Available online: https://www.eurordis.org/es/publication/voice-12000-patients.
- 30.Limb L., Nutt S., Sen A. Experiences of Rare Diseases: An Insight from Patients and Families. 2010. [(accessed on 30 March 2022)]. Rare Diseases UK. Available online: https://www.raredisease.org.uk/media/1594/rduk-family-report.pdf.
- 31.Muir E. The Rare Reality—An Insight into the Patient and Family Experience of Rare Disease. 2016. [(accessed on 30 March 2022)]. Rare Disease UK. Available online: https://www.raredisease.org.uk/media/1588/the-rare-reality-an-insight-into-the-patient-and-family-experience-of-rare-disease.pdf.
- 32.Zurynski Y., Deverell M., Dalkeith T., Johnson S., Christodoulou J., Leonard H., Ellliot E.J. Australian children living with rare diseases: Experiences of diagnosis and perceived consequences of diagnostic delays. Orphanet J. Rare Dis. 2017;12:68. doi: 10.1186/s13023-017-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanherpe P., Fieuws S., D’Hont A., Bleyenheuft C., Demaerel P., De Bleecker J., Van den Bergh P., Baets J., Remiche G., Verhoeven K., et al. Late- onset Pompe disease (LOPD) in Belgium: Clinical characteristics and outcome measures. Orphanet J. Rare Dis. 2020;15:83. doi: 10.1186/s13023-020-01353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balashova M.S., Tuluzanovskaya I.G., Glotov O.S., Glotov A.S., Barbitoff Y.A., Fedyakov M.A., Alaverdian D.A., Ivashchenko T.E., Romanova O.V., Sarana A.M., et al. The spectrum of pathogenic variant of the ATP7B gene in Wilson disease in the Russian Federation. J. Trace Elem. Med. Biol. 2020;59:126420. doi: 10.1016/j.jtemb.2019.126420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.