Abstract
This meta‐analysis was performed to assess the relationship between Lenvatinib use for malignancy and hypertension (HTN). A total of 2483 patients met inclusion criteria. The relative risk (RR) for all‐grade and high‐grade (≧3) HTN were 2.61 (p ≦ .001) and 3.35 (p≦ .001), respectively, for Lenvatinib compared with other multitarget tyrosine kinase inhibitors or placebo. The cumulative incidence of all‐grade and high‐grade HTN was 70% and 34%, respectively. The studies with median treatment duration (TD) longer than 7.4 months demonstrated a higher incidence of high‐grade HTN than studies with shorter TD (34% vs 28%). The incidence of all levels of HTN increased with TD (68% vs 49%). Trials with median progression‐free survival (PFS) longer than nine months had a higher incidence of both all‐grade (37% vs 28%) and high‐grade (71% vs 48%) HTN. Lenvatinib, a drug commonly used in cancer treatment, is a risk factor for the development of HTN. A longer duration of Lenvatinib treatment was associated with higher frequency of HTN. Further investigation for Lenvatinib of the association between the occurrence of HTN and prognosis will be warranted.
Keywords: hypertension, Lenvatinib, meta‐analysis, solid‐tumors
1. INTRODUCTION
In 2020, there were 19.29 million new cancer cases with 9.96 million deaths worldwide. The treatment of malignancy continues to be a challenge. 1 Tumor growth and metastasis heavily depend on various signaling proteins, including vascular endothelial growth factor (VEGF), play a vital role in this process. 2 Antiangiogenic drugs inhibit the VEGF signal pathway, and have played an increasingly important role in the treatment of malignancy.
Lenvatinib is an oral tyrosine kinase inhibitor (TKI) that mainly acts on vascular endothelial growth factor receptor 1–3 (VEGFR 1–3), fibroblast growth factor receptor 1–4 (FGFR 1–4), platelet‐derived growth factor receptor (PDGFR), rearranged during transfection (RET), and c‐kit proto‐oncogene protein (c‐KIT). 3 Both the SELECT and the REFLECT trials demonstrated the efficacy of Lenvatinib in the treatment of solid tumors. 4 Other studies also confirmed the effectiveness of a combination regimen with Lenvatinib 5 , 6 , 7 ; thus, the Food and Drug Administration (FDA) has approved Lenvatinib for treatment for various solid tumors such as renal cell carcinoma, hepatocellular carcinoma, and thyroid cancer. 8
However, while Lenvatinib demonstrated a significant benefit for solid tumor treatment from different clinical trials, it also showed various adverse effects, the most common of which are hypertension (HTN) and hand‐foot skin reactions. 9 , 10 Uncontrolled HTN is a risk factor for the development cardiovascular and cerebrovascular disease (angina pectoris, myocardial infarction, hemorrhagic stroke and ischemic stroke), all of which have high mortality rates. 11 Several meta‐analyses have focused on HTN in cancer patients treated with Lenvatinib. A meta‐analysis conducted in 2016 analyzed the progression‐free survival (PFS) of Lenvatinib and placebo groups; as well as the incidence of various adverse events in the Lenvatinib group. However, only phase I and phase II studies were included, and the study did not further analyze the relative risk of HTN‐related adverse events in the use of lenvatinib. 12 Two following meta‐analyses published in 2021 also analyzed the efficacy and safety of Lenvatinib in the treatment of tumors, and calculated the incidence of various adverse events, 13 , 14 but did not further analyze the risk of Lenvatinib‐related‐HTN or whether treatment duration (TD), PFS, and overall survival (OS) were related to Lenvatinib‐associated HTN. The goal of our study is to comprehensively assess the risk of developing HTN in patients being treated with Lenvatinib, and to analyze the relationship between Lenvatinib‐related HTN and TD, PFS, and OS.
2. METHODS
2.1. Search strategy
On February 17, 2021, we searched PubMed, Web of Science, Cochrane, and Embase. Our search strategy included the following terms “Lenvatinib,” “Lenvima,” ‘E‐7080,’
“E 7080,” “Neoplasms,” “Neoplas*,” “Tumor,” “Cancer,” “Maligan*,” “Carcinoma*,” and, “Malignant Neoplasm*.” We also searched abstracts published at significant conferences by the American Society of Clinical Oncology and the European Society of Medical Oncology. We included the most complete and up‐to‐date trial report. Only clinical trials published in English were eligible for inclusion. The meta‐analysis was registered in PROSPERO (CRD42021244264).
2.2. Study selection
Trials that met the following criteria were selected for analysis: (i) prospective phase II and III trials of patients with solid tumors, (ii)whether previously treated with lenvatinib monotherapy, and (iii) trials from which could extract sufficient data related to HTN. We excluded phase Ⅰ trials because of dose variability. Case reports, reviews, corresponding letters, and editorials were also excluded.
2.3. Data extraction
Data extraction was performed independently by two independent investigators and resolved together in the case of any dispute, or further seeking help from a third investigator. The information we extracted included the first author's name, year of publication, study design, underlying malignancy, treatment arms and control arms, number of patients enrolled, median age, median OS (MOS), median PFS (MPFS), number of patients available for analysis, and the number of events of all‐ and high‐grade HTN.
Clinical endpoints
To investigate the occurrence and risk of HTN‐associated adverse events in patients using Lenvatinib, we set the study endpoint as HTN and used version 3 or 4 of the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute as standard to collect data, 15 version 4 of hypertension as follows: grade1, prehypertension (systolic blood pressure (BP) 120–130 mmHg or diastolic BP 8089 mmHg); grade 2, stage 1 hypertension (systolic BP 140–159 mmHg or diastolic BP 90–99 mmHg, medical intervention indicated, recurrent or persistent (≥ 24 h), symptomatic increase by > 20 mmHg (diastolic) or to >140/90 mmHg if previously within normal limits); grade 3, stage 2 hypertension (systolic BP ≥ 160 mmHg or diastolic BP ≥ 100 mmHg, medical intervention indicated, more than one drug or more intensive therapy than previously used indicated); grade 4, life‐threatening consequences (e.g., malignant hypertension). The CTCAE version 3 only used in one included trial (Table 1), the main difference of this version is that grade 1,asymptomatic, transient (<24 h) increase in BP of 20 mmHg (diastolic) or to > 150/100 mmHg if previously with normal limits, intervention not indicated; grade 2, recurrent or persistent (>24 h) or symptomatic increase by >20 mmHg (diastolic) or to > 150/100 mmHg if previously within normal limits, monotherapy might be indicated.
TABLE 1.
Baseline characteristics of 18 trials included in the meta‐analysis
| Treatment Arm | No. HTN events | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (year) | Study Design | Histology | Treatment Arm | No. patients | Control Arm | No. patients | Median Age | Median OS (month) | Median PFS (month) | All grade (HTN%) | High grade (HTN%) | CTACE | Ref |
| Vergote (2020) | Phase Ⅱ | EC | Len 24 mg QD | 133 | NA | NA | 62 (38‐80) | 10.6 (8.9‐14.9) | 5.4 (3.7‐6.3) | 65 (48.9%) | 41 (30.8) | 4 | 26 |
| Ueno (2020) | Phase Ⅱ | BTC | Len 24 mg QD | 26 | NA | NA | 64 (41‐78) | 7.35 (4.5‐11.27) | 3.19 (2.79‐7.23) | 22 (84.6) | 10 (38.5%) | 4 | 27 |
| Sato (2020) | Phase Ⅱ | Thymic carcinoma | Len 24 mg QD | 42 | NA | NA | 55.5(49‐65) | NA | 9.3 (7.7‐13.9) | 37 (88.1%) | 27 (64.3%) | 4 | 9 |
| Iwasa (2020) | Phase Ⅱ | CRC | Len 24 mg QD | 30 | NA | NA | 61.5 (42‐78) | 7.4 (6.4‐10.8) | 3.6 (2.6‐3.7) | 24 (80%) | 16 (53.3%) | 4 | 28 |
| Brose (2020) | Phase Ⅱ | RR‐DTC | Len 24 mg QD | 75 | NA | NA | NA | NA | NR (22.1‐NR) | NA | 19 (25.3%) | NA | 29 |
| Len 18 mg QD | 77 | NA | NA | NA | NA | 24.4(14.7‐NR) | NA | 15(19.5%) | NA | ||||
| Gao (2020) | Phase Ⅱ | Thymic carcinoma | Len 24 mg QD | 103 | Placebo | 48 | 60 | NA | 23.9 (12.9‐NE) | NA | 64 (62.1%) | NA | 30 |
| Tchekmedyian (2020) | Phase Ⅱ | ACC | Len 24 mg QD | 32 | NA | NA | 57 (38‐73) | NA | 17.5 (7.2‐NR) | NA | 9 (28.1%) | NA | 31 |
| Takahashi (2019) | Phase Ⅱ | TC | Len 24 mg QD | RR‐DTC:25 | NA | NA | 58(21‐74) | 31.8(31.8‐NR) | 25.8(18.4‐NR) | 24(96%) | 15(60%) | 4 | 32 |
| MTC:9 | NA | NA | 58 (41‐79) | 12.1 (3.8‐NR) | 9.2 (1.8‐NR) | 8 (88.9%) | 2 (22.2%) | 4 | |||||
| ATC:17 | NA | NA | 65 (36‐84) | 10.6 (3.8‐19.8) | 7.4 (1.7‐12.9) | 14 (82.4%) | 5 (29.4%) | 4 | |||||
| Hida (2019) | Phase Ⅱ | LAC | Len 24 mg QD | 25 | NA | NA | 63 (34‐78) | NA | 7.3 (3.6‐10.2) | 17 (68%) | 14 (56%) | NA | 33 |
| Kudo (2018) | Phase III | HCC | Len 12/8 mg QD | 478 | Sorafenib | 476 | 63 (22‐88) | 13.6 (12.1‐14.9) | 7.4 (6.9‐8.8) | 201 (42.1%) | 111 (23.2%) | 4 | 4 |
| Locati (2018) | Phase Ⅱ | ACC | Len 24 mg QD | 28 | NA | NA | 55 (22‐73) | 26.1 (11.1‐NR) | 9 (5.5‐14.2) | 21 (75%) | 5 (17.9%) | 4 | 34 |
| Ikeda (2017) | Phase Ⅱ | HCC | Len 12 mg QD | 46 | NA | NA | 66.5 (37‐80) | 18.7 (12.7‐25.1) | NA | 35 (76.1%) | 25 (54.3%) | 3 | 10 |
| Schlumberger (2016) | Phase Ⅱ | TC | Len 24 mg QD | 59 | NA | NA | 51.6 (22‐74) | 16.6 (16.4‐NE) | 9 (7‐NE) | 30 (50.8%) | 4 (0.7%) | NA | 35 |
| Schlumberger (2015) | Phase III | TC | Len 24 mg QD | 261 | Placebo | 131 | 64 | NE (22‐NE) | 18.3 (15.1‐NE) | 177 (67.8%) | 109 (41.8%) | 4 | 4 |
| Cabanillas (2015) | Phase Ⅱ | TC | Len 24 mg QD | 58 | NA | NA | 63 (34‐77) | NA | 12.6 (9.9‐16.1) | 44 (75.9%) | 6 (10.3%) | NA | 36 |
| Motzer (2015) | Phase Ⅱ | RCC | Len 24 mg QD | 52 | Lenvatinib + everolimus | 51 | 64 (41‐79) | 18.4 (13.3‐NE)) | 7.4 (5.6‐10.2) | 25 (48.1%) | 9 (17.3%) | 4 | 37 |
| Everolimus | 50 | 64 (41‐79) | |||||||||||
| O'Day (2013) | Phase Ⅱ | Melanoma | Len 24 mg QD | 93 | NA | NA | 64 | 9.5 (8.3‐12.9) | 3.7 (2.5‐4) | 55 (59.1%) | 32 (34.4%) | NA | 38 |
| Sherman (2011) | Phase Ⅱ | TC | Len 24 mg QD | 58 | NA | NA | 62 | NA | 12.6 (10.4‐14.1) | 37 (63.8%) | NA | NA | 39 |
EC, endometrial cancer; BTC, biliary tract cancer; CRC, colorectal cancer; RR‐DTC, radioiodine‐refractory differentiated thyroid cancer; ACC, adenoid cystic carcinoma; TC, thyroid cancer; LAC, lung adenocarcinoma; HCC, hepatocellular carcinoma; RCC, renal cell carcinoma; NA, not available; NE, not reached; Len, Lenvatinib.
2.4. Statistical analysis
For all selected studies, we calculated the cumulative incidence and 95% confidence interval (CI) of all grades and high grades of HTN, and for studies with a control group, we also calculated the risk ratio (RR) and its 95% CI to assess the strength of association between Lenvatinib and HTN adverse events. Used Stata 16.0 version (Stata Corporation, Colleges Station, TX, USA) for all calculations, and both the fixedeffects model and the random‐effects model were optional. Regarding the heterogeneity test, we chose I2, and if I2≧50%, we chose the random‐effects model and conversely the fixed‐effects model. The p value for significance was set at .05, and all 95% CIs were provided. In addition, we performed a meta‐analysis to explore further the correlation between PFS and OS and HTN caused by treatment with Lenvatinib. We used Egger's test and the funnel plot method to assess the possible publication bias.
3. RESULTS
3.1. Search results
Four thousand eight hundred eighty‐four articles were initially reviewed, of which 18 met inclusion criteria. A total of 1727 patients were treated with Lenvatinib in these 18 studies, and the specific screening process is presented in Figure 1. The baseline characteristics of the studies included in this study are shown in Table 1. The underlying malignant tumors for these trials included endometrial cancer (one study), biliary tract cancer (one study), thymic carcinoma (two studies), colorectal cancer (one study), thyroid cancer (six studies), lung cancer (one study), hepatocellular carcinoma (two studies), adenoid cystic carcinomas (two studies), renal cell carcinoma (one study), and melanoma (one study). The starting dose and schedule of Lenvatinib were based on the US FDA guideline; different doses were used according to different tumors, but three dosing schedules were mainly used: 24 mg QD, 12 mg QD, and 8 mg QD.
FIGURE 1.
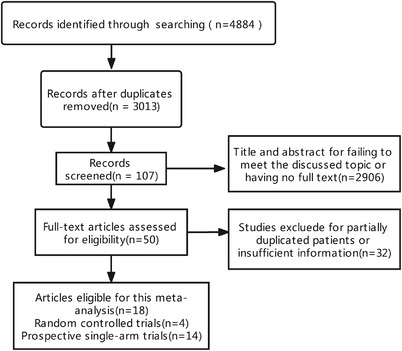
Flow chart of literature search and study selection. A total of 4884 articles were initially retrieved. After carefully reviewed 18 articles reporting the incidence and risk of hypertension with Lenvatinib in treatment of solid tumors
3.2. The overall incidence of HTN
One thousand seven hundred twenty‐seven patients from 18 studies were treated by Lenvatinib monotherapy, and 1440 people were included in the analysis of the incidence of all grades of HTN. A total of 1669 people were included in the analysis of high grades (≧3) of HTN. The reason for the unequal number of patients is that some studies reported the number of patients with all grades of HTN, whereas other studies only reported the number of patients with grade 3 or higher HTN. For the calculation of the cumulative incidence of HTN, we used a random‐effects model, which gave an incidence of all and high grades of HTN of 70% (95%CI: 0.6_0.79, p≦.001) and 34% (95%CI: 0.27_0.42, p≦.001; Figure 2).
FIGURE 2.
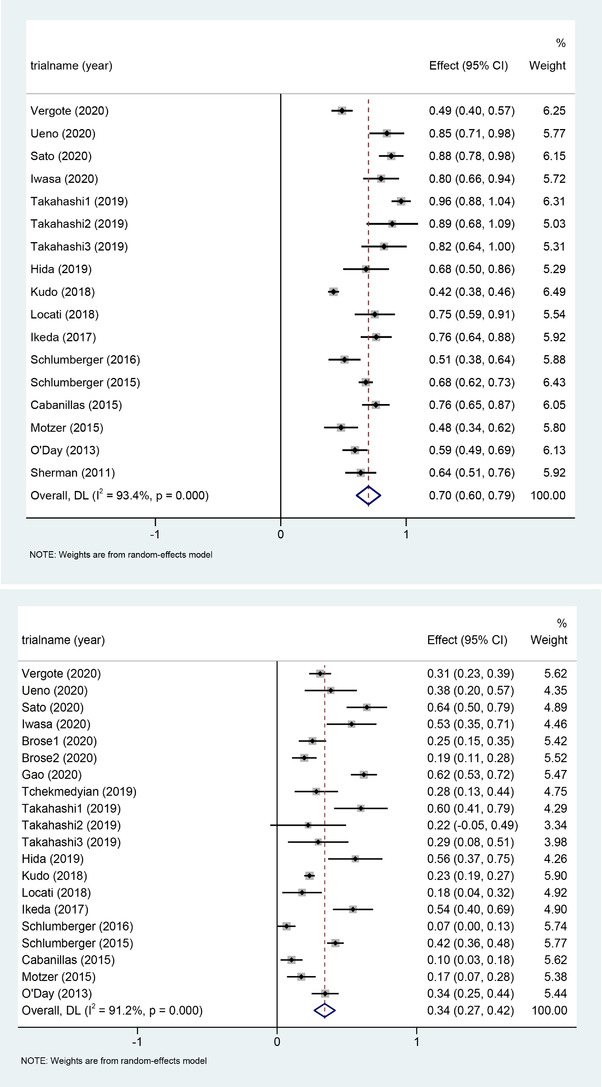
Forest plots of the incidence of all‐grade hypertension (A) and high‐grade hypertension (B) and 95% CI in Lenvatinib‐treated patients
3.3. The risk ratio of hypertension events
Four of the screened studies were controlled studies, and we further analyzed the relative risk of HTN with Lenvatinib. For all grades of HTN, there were 1499 patients from three studies, including four cohorts involved in the analysis, and the resulting RR was 2.61 (95%CI: 1.18_5.78, p = .018; Figure 3). For HTN grade 3 and above, we included 1650 people from four studies, including five subgroups, and the RR obtained was 3.35 (95%CI: 1.52_7.42, p = .003; Figure 3).
FIGURE 3.
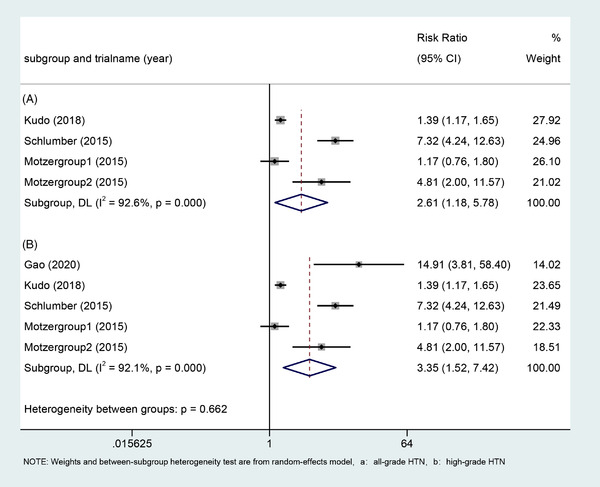
Forest plots of the relative risk of all‐grade hypertension and high‐grade hypertension and 95% CI in Lenvatinib‐treated patients
3.4. Subgroup analysis
To further explore whether the TD, OS, and PFS were related to the risk of Lenvatinib‐related HTN, we conducted a subgroup analysis to calculate the cumulative incidence of HTN according to the median TD (7.4 months), median OS (12.85 months), and median PFS (9 months). The studies with median TD longer than 7.4 months demonstrated a higher incidence of high‐grade HTN compared with studies with shorter TD (34% [95%CI: 0.30_0.38] vs 28% [95%CI: 0.25_0.32], p≦ .001). With the extension of TD, the incidence of all levels of HTN increased. Trials with median PFS longer than nine months also had a higher incidence of both all‐grade and high‐grade HTN (Table 3). However, trials with median OS longer than 12.85 months showed a lower incidence of HTN (Table 3). We also performed a subgroup analysis based on underlying malignancy and found the incidence of Lenvatinib‐related HTN higher in thymic cancer, rectal cancer, and lung adenocarcinoma (Table 2).
TABLE 3.
The relationship between hypertension and prognosis
| All‐grade | High‐grade | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | Study No. | Lenvatnib | No. HTN events | Incidence of HTN (95%CI) | RR of HTN (P) | Study No. | lenvatinib | No. HTN enents | Incidence of HTN (95%CI) | RR of HTN (P) |
| Stratified by treatment duration | ||||||||||
| <7.4 months | 5 | 725 | 354 | 0.49 (0.45,0.52) | 2.25 (0.000) | 5 | 725 | 206 | 0.28 (0.25,0.32) | 1.30 (0.038) |
| ≥7.4 months | 7 | 506 | 345 | 0.68 (0.64,0.72) | 7 | 506 | 172 | 0.34 (0.30,0.38) | ||
| Stratified by OS | ||||||||||
| <12.85 months | 6 | 308 | 188 | 0.61 (0.56,0.66) | 0.61 (0.000) | 6 | 308 | 106 | 0.34 (0.29,0.40) | 0.62 (0.002) |
| ≥12.85 months | 5 | 688 | 336 | 0.49 (0.45,0.53) | 5 | 688 | 169 | 0.25 (0.21,0.28) | ||
| Stratified by PFS | ||||||||||
| <9 months | 6 | 824 | 399 | 0.50 (0.46,0.53) | 2.55 (0.000) | 7 | 858 | 238 | 0.28 (0.25,0.31) | 1.52 (0.000) |
| ≥9 months | 9 | 570 | 402 | 0.70 (0.66,0.74) | 10 | 694 | 256 | 0.37 (0.33,0.40) | ||
TABLE 2.
Incidence of HTN in cancer patients stratified by underlying malignancy
| All‐grade | High‐grade | |||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | Study No. | Lenvatnib | No. HTN events | Incidence of HTN (95%CI) | Study No. | lenvatinib | No. HTN enents | Incidence of HTN (95%CI) |
| Stratified by underlying maglinancy | ||||||||
| EC | 1 | 133 | 65 | 49% (0.40,0.57) | 1 | 133 | 41 | 31% (0.23,0.39) |
| BTC | 1 | 26 | 22 | 85% (0.71,0.98) | 1 | 26 | 10 | 38% (0.20,0.57) |
| Thymic carcinoma | 1 | 42 | 37 | 88% (0.78‐0.98) | 2 | 145 | 91 | 63% (0.55‐0.71) |
| CRC | 1 | 30 | 24 | 80% (0.66,0.94) | 1 | 30 | 16 | 53% (0.35,0.71) |
| TC | 5 | 487 | 334 | 69% (0.64,0.73) | 5 | 581 | 175 | 30% (0.26,0.34) |
| ACC | 1 | 28 | 21 | 75% (0.59,0.91) | 2 | 60 | 14 | 23% (0.13,0.34) |
| HCC | 2 | 522 | 236 | 45% (0.41,0.49) | 2 | 522 | 136 | 26% (0.22,0.30) |
| LAC | 1 | 25 | 17 | 68% (0.50,0.86) | 1 | 25 | 14 | 56% (0.37,0.75) |
| RCC | 1 | 52 | 25 | 48% (0.34,0.62) | 1 | 52 | 9 | 17% (0.07,0.28) |
| Melanoma | 1 | 93 | 55 | 59% (0.49,0.69) | 1 | 93 | 32 | 34% (0.25,0.44) |
3.5. Publication bias and sensitivity analysis
We first used funnel plots to assess the possible publication bias and found that the graph obtained was not completely symmetrical (Figure 4). Therefore, Egger's and Begg's tests were used for quantitative analysis to evaluate the possible publication bias (Figure 5). Both test methods suggested p>.05, so we believe that HTN incidence in this study was not subject to publication bias. With <10 studies reported on RR of HTN, we did not perform an analysis of RR‐related publication bias.
FIGURE 4.
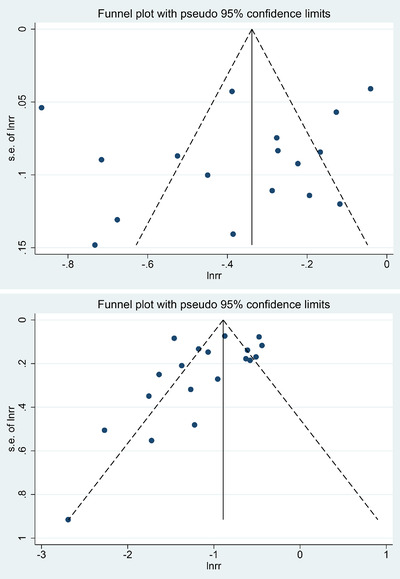
Funnel plots of publication bias of all‐grade hypertension (A) and high‐grade hypertension (B) in Lenvatinib‐treated patients
FIGURE 5.
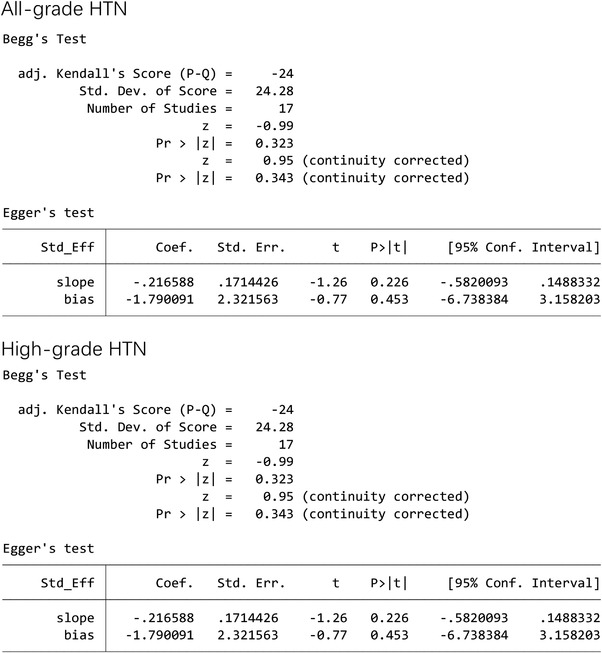
Egger's and Begg's tests for publication bias of all‐grade hypertension and high‐grade hypertension in Lenvatinib‐treated patients
4. DISCUSSION
Our meta‐analysis showed that, RR had a strong correlation with the incidence of both all‐grade (2.61, 95%CI: 1.18_5.78, p = .018 ) and high‐grade HTN (3.35, 95%CI: 1.52_7.42, p = .003) HTN, suggesting that HTN incidence increased during oral administration of Lenvatinib. This is consistent with known literature. 13 Numerous trials involving the treatment of solid tumors with Lenvatinib have reported a higher incidence of Lenvatinib‐associated all‐grade HTN of between 42% and 76%4,. 4 , 10 However, the study by Antonio and associates 3 concluded that Lenvatinib did not statistically differ from sorafenib in the incidence of serious adverse events, including HTN. Further research is required to explore whether the incidence of HTN associated with Lenvatinib is different compared with other TKI drugs.
Lenvatinib is well known for improving the PFS and OS of cancer patients, and the efficacy of Lenvatinib in the treatment of solid tumors has been well‐demonstrated in exciting literature. 16 , 17 , 18 However, these studies did not mention the incidence of Lenvatinib‐related adverse events in relation to TD, PFS, and OS. Therefore, we also performed a subgroup analysis to explore the relationship between duration, PFS, and OS HTN incidence. When median TD was longer than 7.4 months and median PFS was longer than nine months, the incidence of HTN was significantly increased. However, when the median OS was longer than 12.85 months, the incidence of high‐grade HTN decreased (25% [95%CI: 0.21_0.28] vs 34% [95%CI: 0.29_0.40], p≦ .001), and similarly, the incidence of all‐grade HTN also decreased. The reason for this may be that one random controlled trial lacking OS data was excluded from the OS subgroup analysis, which affected the accuracy of the analysis results. For this reason, we further analyzed the research that included both PFS and OS data, and found that the incidence of HTN also decreased with the extension of PFS (all‐grade: 59% [95%CI: 0.53‐0.65] vs 48% [95%CI: 0.45_0.52], p≦.001; high‐grade: 35% [95%CI: 0.30‐0.41] vs 23% [95%CI: 0.19_0.26], p≦.001), we believe this is due to the small sample size included in the study, and RCTs with a relatively large sample size are eliminated. Besides, different tumors have different survival times, and targeted therapy is not the only factor that affects OS, it may lead to differences in data. Our meta‐analysis showed that longer Lenvatinib treatment was associated with higher frequency of HTN, but since the included studies did not measure outcomes in persons with and without Lenvatinib‐induced HTN, it was not possible to conclude whether hypertension was associated with better prognosis. However, it has previously been shown that an increase in early blood pressure is associated with better efficacy and improved prognosis, 19 and the recent SELECT study found that hypertension adverse events occurring with Lenvatinib in the treatment of radioiodinerefractory thyroid cancer (RR‐DTC) were associated with better PFS (18.8 vs 12.9 months; p = .0085) and OS (not‐reached vs 21.7months; p = .0003), suggesting that HTN may be predictive of the efficacy of Lenvatinib in this population. 20 Therefore, based on the above information, we hypothesize that Lenvatinib treatment‐related HTN may be associated with better prognosis in cancer patients, but further large prospective randomized controlled trials are warranted.
Hypertension is a common complication of antiangiogenic agents in the treatment of malignancies, and there have been case reports of acute HTN with the use of TKIs. 21 , 22 However, the specific mechanism by which antiangiogenic drugs such as Lenvatinib cause HTN is not yet understood. Possible mechanisms include multifaceted interactions such as those related to neurostimulators factors, the renin‐angiotensin aldosterone system, the endothelin signaling pathway, and the nitric oxide signaling pathway. 23 , 24 , 25
It should be noted that although the occurrence of HTN may be associated with improved patient prognosis, when HTN occurs, patients should receive antihypertensive treatment as soon as possible to avoid more serious sequela. In addition, for the patients treated with VEGF signaling inhibitors, they should follow recommendations of cardiovascular toxicity management as follows, first, making sure that if they are suffering hypertension prior to treatment, second, measuring blood pressure actively throughout total treatment to assess the risk for potential cardiovascular complications. 23 Timely treatment of HTN can effectively reduce the reduction rate and withdrawal rate of Lenvatinib. So far, there is no internationally recognized recommendation on the choice of drug for Lenvatinib‐related HTN, or how the setting of blood pressure management goals will affect the efficacy of TKIs. AS the literature included in our study did not mention data on further treatment after the occurrence of HTN, analyzing the efficacy of different drugs in the treatment of Lenvatinib‐related HTN was not possible.
Our meta‐analysis has some limitations. The included studies did not describe HTN at baseline, so the incidence of HTN in our articles may be underestimated. We included a large number of phase Ⅱ trials; more reliable randomized controlled trials were lacking, and the small number of included studies precluded analysis according to tumor type and demographic factors, all of which lead to a degree of heterogeneity. Although we further analyzed the relationship between the incidence of HTN with PFS and OS, the presence of various confounders, reduced the validity of the statistics.
In conclusion, despite the above limitations, our meta‐analysis, based on the included studies, indicated a significantly high risk of Lenvatinib‐related HTN. With prolonged TD and PFS, the incidence of both all‐grade and high‐grade HTN is significantly increased. However, whether Lenvatinib treatment‐related hypertension is associated with better prognosis needs to be further demonstrated.
CONFLICTS OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AUTHOR CONTRIBUTIONS
Conception/design: Jinglong Chen and Wei Li. Collection and/or assembly of data: Hongxiao Wu and Yongchao Zhang. Data analysis and interpretation: Hongxiao Wu, Xiaoyan Ding, and Yongchao Zhang. Manuscript writing: Hongxiao Wu. Revision: Jinglong Chen, Wei Li, and Xiaoyan Ding. Final approval of manuscript: Hongxiao Wu, Yongchao Zhang, Xiaoyan Ding, Wei Li, and Jinglong Chen.
ACKNOWLEDGEMENTS
This study is supported by Major Special Projects on AIDS and Viral Hepatitis (grant number 2018ZX10303502‐003).
Wu H, Ding X, Zhang Y, Li W, Chen J. Incidence and risk of hypertension with Lenvatinib in treatment of solid tumors: An updated systematic review and meta‐analysis. J Clin Hypertens. 2022;24:667–676. 10.1111/jch.14463
Contributor Information
Wei Li, Email: weili8989@ccmu.edu.cn.
Jinglong Chen, Email: cjl6412@ccmu.edu.cn.
REFERENCES
- 1. Cancer IAfRo. Global Cancer Observatory. https://gco.iarc.fr/today/data/factsheets/cancers/11‐Liver‐fact‐sheet.pdf 2021, Accessed 2021. [Google Scholar]
- 2. Yang X, Pan X, Cheng X, Kuang Y, Cheng Y. Risk of Hypertension With Sorafenib Use in Patients With Cancer: a Meta‐Analysis From 20,494 Patients. Am J Ther. 2017;24(1). [DOI] [PubMed] [Google Scholar]
- 3. Facciorusso A, Tartaglia N, Villani R, et al. Lenvatinib versus sorafenib as first‐line therapy of advanced hepatocellular carcinoma: a systematic review and meta‐analysis. Am J Transl Res;13(4):2379‐2387. [PMC free article] [PubMed] [Google Scholar]
- 4. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet. 2018;391(10126):1163‐1173. https://10.1016/S0140‐6736(18)30207‐1 [DOI] [PubMed] [Google Scholar]
- 5. Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodinerefractory thyroid cancer. N Engl J Med. 2015;372(7):621‐630. [DOI] [PubMed] [Google Scholar]
- 6. Taylor MH, Lee C‐H, Makker V, et al. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J Clin Oncol. 2020;38(11):1154‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J Clin Oncol. 2020;38(26):2981‐2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38(26):2960‐2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Administration tFaD. U.S. FDA Drugs Database. https://www.drugfuture.com/fda/drugview/206947 2021. Accessed October 3, 2021. [Google Scholar]
- 10. Sato J, Satouchi M, Itoh S, et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol. 2020;21(6):843‐850. [DOI] [PubMed] [Google Scholar]
- 11. Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52(4):512‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aune D, Huang W, Nie J, Wang Y. Hypertension and the Risk of All‐Cause and Cause‐Specific Mortality: an Outcome‐Wide Association Study of 67 Causes of Death in the National Health Interview Survey. Biomed Res Int. 2021;2021:9376134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu C, Ma X, Hu Y, et al. Safety and efficacy profile of lenvatinib in cancer therapy: a systematic review and meta‐analysis. Oncotarget. 2016;7(28):44545‐44557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xie WJ, Zhang S, Su L, Li YH, Zhang Xi, Ran YuGe. The efficacy and safety of lenvatinib in the treatment of solid tumors: an up‐to‐date meta‐analysis. Future Oncol. 2021;17(6):745‐754. [DOI] [PubMed] [Google Scholar]
- 15. Yan Z, Yang M, Lai C‐L. Clinical efficacy of lenvatinib for the treatment of radioiodinerefractory thyroid carcinoma: a systematic review and meta‐analysis of clinical trials. Clin Endocrinol (Oxf). 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Institute NC. the Common Terminology Criteria for Adverse Events, https://ctep.cancer.gov/ 2021. Accessed October 3, 2021.
- 17. Wirth LJ, Brose MS, Sherman EJ, et al. Open‐Label, Single‐Arm, Multicenter, Phase II Trial of Lenvatinib for the Treatment of Patients With Anaplastic Thyroid Cancer. J Clin Oncol. 2021:JCO2003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsuchiya K, Kurosaki M, Sakamoto A, et al. The Real‐World Data in Japanese Patients with Unresectable Hepatocellular Carcinoma Treated with Lenvatinib from a Nationwide Multicenter Study. Cancers (Basel). 2021;13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Capdevila J, Fazio N, Lopez C, et al. Lenvatinib in Patients With Advanced Grade 1/2 Pancreatic and Gastrointestinal Neuroendocrine Tumors: results of the Phase II TALENT Trial (GETNE1509). J Clin Oncol. 2021:JCO2003368. [DOI] [PubMed] [Google Scholar]
- 20. Hamnvik O‐PR, Choueiri TK, Turchin A, et al. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer. 2015;121(2):311‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wirth LJ, Tahara M, Robinson B, et al. Treatment‐emergent hypertension and efficacy in the phase 3 Study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer. 2018;124(11):2365‐2372. [DOI] [PubMed] [Google Scholar]
- 22. Caro J, Morales E, Gutierrez E, Ruilope LM, Praga M. Malignant hypertension in patients treated with vascular endothelial growth factor inhibitors. J Clin Hypertens (Greenwich). 2013;15(3):215‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tlemsani C, Mir O, Boudou‐Rouquette P, et al. Posterior reversible encephalopathy syndrome induced by anti‐VEGF agents. Target Oncol. 2011;6(4):253‐258. [DOI] [PubMed] [Google Scholar]
- 24. Rizzoni D, De Ciuceis C, Porteri E, et al. Use of Antihypertensive Drugs in Neoplastic Patients. High Blood Press Cardiovasc Prev; 2017:127‐132. [DOI] [PubMed] [Google Scholar]
- 25. Bair SM, Choueiri TK, Moslehi J. Cardiovascular complications associated with novel angiogenesis inhibitors: emerging evidence and evolving perspectives. Trends Cardiovasc Med. 2013;23(4):104‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sica DA. Angiogenesis inhibitors and hypertension: an emerging issue. J Clin Oncol. 2006;24(9):1329‐1331. [DOI] [PubMed] [Google Scholar]
- 27. Vergote I, Powell MA, Teneriello MG, et al. Second‐line lenvatinib in patients with recurrent endometrial cancer. Gynecol Oncol. 2020;156(3):575‐582. [DOI] [PubMed] [Google Scholar]
- 28. Ueno M, Ikeda M, Sasaki T, et al. Phase 2 study of lenvatinib monotherapy as second‐line treatment in unresectable biliary tract cancer: primary analysis results. BMC Cancer. 2020;20(1):1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iwasa S, Okita N, Kuchiba A, et al. Phase II study of lenvatinib for metastatic colorectal cancer refractory to standard chemotherapy: the LEMON study (NCCH1503). ESMO Open. 2020;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brose MS, Panaseykin Y, Konda B, et al. A multicenter, randomized, double‐blind, phase II study of lenvatinib (LEN) in patients (pts) with radioiodine‐refractory differentiated thyroid cancer (RR‐DTC) to evaluate the safety and efficacy of a daily oral starting dose of 18 mg vs 24 mg. Ann Oncol. 2020;31. S6. [Google Scholar]
- 31. Gao MG M, Xu Z, Ji Q, et al. A multicenter, randomized, double‐blind, placebo (PBO)‐controlled, phase III trial of lenvatinib (LEN) in patients (pts) with radioiodine‐refractory differentiated thyroid cancer (RR‐DTC) in China. Ann Oncol. 2020;31. S6. [Google Scholar]
- 32. Tchekmedyian V, Sherman EJ, Dunn L, et al. Phase II Study of Lenvatinib in Patients With Progressive, Recurrent or Metastatic Adenoid Cystic Carcinoma. J Clin Oncol. 2019;37(18):1529‐1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takahashi S, Kiyota N, Yamazaki T, et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 2019;15(7):717‐726. [DOI] [PubMed] [Google Scholar]
- 34. Hida T, Velcheti V, Reckamp KL, et al. A phase 2 study of lenvatinib in patients with RET fusion‐positive lung adenocarcinoma. Lung Cancer (Amsterdam, Netherlands). 2019;138:124‐130. [DOI] [PubMed] [Google Scholar]
- 35. DG LL, Calareso G, et al. Phase II study on lenvatinib (LEN) in recurrent and/or metastatic (R/M) adenoid cystic carcinomas (ACC) of the salivary glands (SG) of the upper aereodigestive tract (NCT02860936). J Clin Oncol. 2018. [Google Scholar]
- 36. Schlumberger M, Jarzab B, Cabanillas ME, et al. A Phase II Trial of the Multitargeted Tyrosine Kinase Inhibitor Lenvatinib (E7080) in Advanced Medullary Thyroid Cancer. Clin Cancer Res. 2016;22(1):44‐53. [DOI] [PubMed] [Google Scholar]
- 37. Cabanillas ME, Schlumberger M, Jarzab B, et al. A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine‐refractory, differentiated thyroid cancer: a clinical outcomes and biomarker assessment. Cancer. 2015;121(16):2749‐2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open‐label, multicentre trial. Lancet Oncol. 2015;16(15):1473‐1482. [DOI] [PubMed] [Google Scholar]
- 39. Steven O, Day RG, Kim K, Chmielowski B, et al. A phase II study of the multitargeted kinase inhibitor lenvatinib in patients with advanced BRAF wild‐type melanoma. J Clin Oncol. 2013. [Google Scholar]
- 40. Sherman SI, Jarzab B, Cabanillas ME, et al. A phase II trial of the multitargeted kinase inhibitor E7080 in advanced radioiodine (RAI)‐refractory differentiated thyroid cancer (DTC). J Clin Oncol. 2011;29. 15. Suppl. [Google Scholar]


