Abstract
The authors aimed to explore the association between visit‐to‐visit blood pressure variability (BPV) in pregnant women and adverse neonatal outcomes. The study included 52 891 pregnant women. BPV was calculated as standard deviation (SD) and coefficient of variation (CV) of systolic blood pressure (SBP) or diastolic blood pressure (DBP). All participants were divided into four groups by the quartiles of BPV. When comparing the highest quartiles to the lowest quartiles of DBP SD in all participants, the fully adjusted ORs were 1.19 (95% CI 1.11–1.27, p for trend < .001) for fetal distress, 1.32 (95% CI 1.14–1.54, p for trend < .001) for small for gestational age, 1.32 (95% CI 1.06–1.63, p for trend = .003) for 1‐min Apgar score ≤ 7. When comparing the highest quartiles to the lowest quartiles of DBP CV, ORs were 1.22 (95% CI 1.14–1.30, p for trend < .001) for fetal distress, 1.38 (95% CI 1.17–1.61, p for trend < .001) for small for gestational age, 1.43 (95% CI 1.14–1.79, p for trend < .001) for 1‐min Apgar score ≤ 7. ORs for preterm birth and 5‐min Apgar score ≤ 7 were not statistically significant. However, in participants with gestational hypertension or preeclampsia, ORs for preterm birth were 2.80 (95% CI 1.99–3.94, p for trend < .001) in DBP SD and 3.25 (95% CI 2.24–4.72, p for trend < .001) in DBP CV when extreme quartiles were compared. In conclusion, higher visit‐to‐visit BPV was associated with adverse neonatal outcomes.
Keywords: blood pressure variability, fetal distress, neonatal outcomes, preterm birth, small for gestational age
1. INTRODUCTION
Adverse birth outcomes such as fetal distress, small for gestational age, and preterm birth are related to perinatal morbidity and mortality. 1 , 2 , 3 Fetal distress indicates inadequate fetal oxygen supply and it may cause brain damage and even fetal death without immediate intervention. 4 Babies with small for gestational age are reported to have a higher risk of cardiovascular disease in later life. 5 Preterm birth incurs a substantial global burden and leads to various complications such as respiratory distress syndrome, necrotizing enterocolitis, and sepsis. 3 Therefore, it is significant to understand the risk factors linked to these outcomes. Maternal blood pressure has been reported to be a risk factor for adverse birth outcomes. 6 , 7 However, usual blood pressure still has some shortcomings and blood pressure variability (BPV) should not be ignored as another prognostic factor. 8 BPV refers to the fluctuation of blood pressure and it is generally classified as short‐term BPV and long‐term BPV. Short‐term BPV is usually defined as BPV within 24 h obtained by ambulatory blood pressure monitoring while long‐term BPV is defined as visit‐to‐visit BPV. 9 Increasing evidence suggests that BPV is a risk factor for cardiovascular events in the nonpregnant population. 10 , 11 , 12 , 13 Visit‐to‐visit BPV might also have prognostic value in predicting neonatal outcomes but the relationship is still obscure. Previous studies indicated that higher BPV may be associated with pregnancy complications such as gestational hypertension and preeclampsia. 14 , 15 Another study demonstrated that visit‐to‐visit BPV was associated with a higher risk of small for gestational age. 16 However, few studies focused on the association between visit‐to‐visit BPV and fetal outcomes. Considering the above threat and the prognostic value of BPV, our study aims to explore the relationship between gestational visit‐to‐visit BPV and adverse neonatal outcomes.
2. METHOD
2.1. Study design and population
This is a retrospective cohort study. Pregnant women who had regular visits at the International Peace Maternity and Child Health Hospital were recruited. Based on the official records, 56 970 pregnant women gave birth between January 2017 and September 2020. Participants were considered ineligible if they met the following exclusion criteria: (1) stillbirth; (2) multiple pregnancy; (3) with any malignant tumor or history of malignancy; (4) pregestational diabetes mellitus; (5) placental previa; (6) proteinuria before 20 weeks of gestation or renal disease; (6) chronic hypertension before 20 weeks of gestation; (7) participants who had less than six blood pressure measurements. The flow chart of the including and excluding process is shown in Figure 1. Finally, 52 891 participants were included in the analysis.
FIGURE 1.
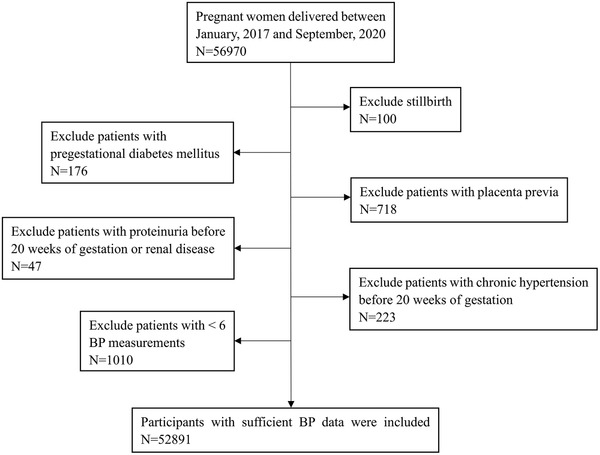
Flow chart of including and excluding
Our study was conducted following the tenets of the Helsinki Declaration and was approved by the ethics committee of the International Peace Maternity and Child Health Hospital. All participants were given written informed consent at the recruitment stage of the study.
2.2. Data collection and description
Data were collected using a standard electronic form. The following elements were extracted from the electronic medical records: prenatal visit‐to‐visit systolic blood pressure (SBP) and diastolic blood pressure (DBP), age, prepregnancy body mass index, past obstetric history, gestational age at delivery, discharge diagnosis, mode of delivery, birth weight, and Apgar scores at 1 and 5 min. Apgar scores were assessed by pediatricians and maternal diseases were diagnosed by senior obstetricians. The Apgar score comprises five elements including activity, pulse, grimace, appearance, and respiration, with a score of 0, 1, or 2 for each element. Apgar score of 8–10 points is considered normal for newborns. Apgar score of 0–3 and 4–7 points indicates severe asphyxia and mild asphyxia, respectively. Therefore, Apgar score ≤7 was regarded as a neonatal outcome in our study. Small for gestational age was defined as birth weight less than the 10th percentile adjusted for gestational age according to local standard. 2 Preterm birth was defined as delivery before 37 gestational weeks. Preeclampsia was diagnosed as hypertension (SBP ≥140 mmHg and/or DBP ≥90 mmHg) after 20 weeks of gestation with proteinuria or hypertension without proteinuria but with the new onset of any of the systemic features including thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, and cerebral or visual symptoms in previously normotensive women. 17 Gestational hypertension was diagnosed as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg without proteinuria or the aforementioned systemic features after 20 weeks of gestation. 17 Gestational diabetes mellitus was diagnosed when any of the following criteria were met based on a 75 g oral glucose tolerance test which is generally performed at around 24–28 weeks of gestation in women without overt diabetes: (1) fasting plasma glucose ≥5.1 mmol/L; (2) at 1 h ≥ 10.0 mmol/L; (3) at 2 h ≥ 8.5 mmol/L. 18 Premature rupture of membranes was defined as spontaneous rupture of membranes before the onset of labor. Maternal anemia was defined as hemoglobin at delivery < 110 g/L. Body mass index was calculated as weight in kilograms divided by the square of height in meters.
2.3. Blood pressure variability
Blood pressure was routinely measured in the sitting position after a 5‐min rest using a calibrated electronic sphygmomanometer following a standardized protocol by trained nurses. Visit‐to‐visit BPV was calculated using two metrics in this study: standard deviation (SD) and coefficient of variation (CV) of SBP and DBP at each antenatal visit. SD and CV were calculated using the following formulas:
| (1) |
| (2) |
The parameter n is the number of blood pressure measurements of each participant taken from antenatal clinic visits. equals mean blood pressure.
2.4. Statistical analysis
Data are expressed as mean ± SD for continuous variables and number (%) for categorical variables. The characteristics of normotensive participants and those with gestational hypertension/preeclampsia were compared using the t‐test for continuous variables and the chi‐square test for categorical variables. All participants were divided into four groups by the quartiles of BPV. The incidence rates of fetal distress, small for gestational age and preterm birth were compared using the chi‐square test in four groups, respectively. Multivariable logistic regression models were used to assess the risk of adverse neonatal outcomes including fetal distress, small for gestational age, preterm birth, and Apgar score ≤7 with BPV as the exposure variable, considering the lowest quartile of BPV as reference. A test for trend was conducted with the use of quartiles of BPV variable as a continuous variable by assigning the median values of the quartiles to the variable. The authors analyzed the independent effects of visit‐to‐visit BPV with several models of adjustment. Model 1 was unadjusted. Model 2 was adjusted for body mass index, age, gravidity, parity, cesarean, in vitro fertilization‐embryo transfer. Model 3 was adjusted for the covariates in Model 2 and hypertensive disorders (normotension, gestational hypertension/preeclampsia), gestational diabetes mellitus, premature rupture of membranes, cephalic presentation, scarred uterus, anemia, mean SBP, and mean DBP. Furthermore, we repeated similar logistic regression analysis in normotensive participants and participants with gestational hypertension/preeclampsia, respectively. Each outcome variable was expressed with an odds ratio (OR) and 95% confidence interval (95% CI). Statistical significance was defined as p < .05 for two‐tailed analysis. Analyses were performed using Statistical Analysis System (SAS) software (version 9.4, SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Baseline characteristics of the included participants
A total of 52 891 participants were eligible for analysis. Table 1 shows the baseline characteristics of the included participants. Blood pressure measurement times were 11.51 on average. In our study, 49 583 participants were normotensive, 1957 patients were diagnosed with gestational hypertension and 1351 were preeclampsia. Gestational weeks at birth varied from 26 to 42, with an average of 39. We observed 10 445 (19.75%) infants with fetal distress, 1422 (2.69%) with small for gestational age, 2402 (4.54%) with preterm birth, 685 (1.30%) with 1‐min Apgar score ≤7, and 67 (.13%) with 5‐min Apgar score ≤ 7. A total of 23 295 (44.04%) participants gave birth by cesarean. Figure 2 shows the gestational SBP and DBP changes in normotensive participants and participants with gestational hypertension/preeclampsia.
TABLE 1.
Baseline characteristics of the included participants
| Total | Normotension | GH/PE | ||
|---|---|---|---|---|
| Variable | (N = 52 891) | (N = 49 583) | (N = 3308) | p |
| Fetal distress, n (%) | 10 445 (19.75) | 9726 (19.62) | 719 (21.74) | .003 |
| Small for gestational age, n (%) | 1422 (2.69) | 1205 (2.43) | 217 (6.56) | <.001 |
| 1‐min Apgar score ≤ 7 | 685 (1.30) | 600 (1.21) | 85 (2.57) | <.001 |
| 5‐min Apgar score ≤ 7 | 67 (.13) | 59 (.12) | 8 (.24) | .054 |
| Preterm birth, n (%) | 2402 (4.54) | 2079 (4.19) | 323 (9.76) | <.001 |
| Placental abruption, n (%) | 167 (.32) | 139 (.28) | 28 (.85) | <.001 |
| Cesarean, n (%) | 23 295 (44.04) | 21 250 (42.86) | 2045 (61.82) | <.001 |
| Gestational diabetes mellitus, n (%) | 7906 (14.95) | 7151 (14.42) | 755 (22.82) | <.001 |
| Scarred uterus, n (%) | 7694 (14.55) | 7301 (14.72) | 393 (11.88) | <.001 |
| Cephalic presentation, n (%) | 50 449 (95.38) | 47 302 (95.40) | 3147 (95.13) | .479 |
| Premature rupture of membranes, n (%) | 12 291 (23.24) | 11 787 (23.77) | 504 (15.24) | <.001 |
| In vitro fertilization‐embryo transfer, n (%) | 3622 (6.85) | 3215 (6.48) | 407 (12.30) | <.001 |
| Anemia, n (%) | 8405 (15.89) | 7929 (15.99) | 476 (14.39) | .015 |
| Age (years) | 31.27 ± 4.00 | 31.23 ± 3.97 | 31.89 ± 4.37 | <.001 |
| Body mass index (kg/m2) | 21.22 ± 2.67 | 21.11 ± 2.55 | 22.97 ± 3.57 | <.001 |
| Gravidity | 1.87 ± 1.10 | 1.87 ± 1.10 | 1.83 ± 1.14 | .038 |
| Parity | 1.32 ± .49 | 1.32 ± .49 | 1.23 ± .44 | <.001 |
| Gestational age (week) | 39.09 ± 1.28 | 39.12 ± 1.25 | 38.58 ± 1.67 | <.001 |
| 1‐min Apgar score | 9.89 ±.54 | 9.90 ± .53 | 9.81 ± .74 | <.001 |
| 5‐min Apgar score | 9.97 ± .25 | 9.96 ± .24 | 9.94 ± .31 | <.001 |
| Birth weight (g) | 3331.72 ± 427.90 | 3340.27 ± 416.97 | 3203.48 ± 551.43 | <.001 |
| Blood pressure measurement times | 11.51 ± 2.14 | 11.52 ± 2.12 | 11.42 ± 2.33 | .009 |
| SBP (mmHg) | 112.01 ± .90 | 111.15 ± 8.24 | 124.96 ± 8.33 | <.001 |
| DBP (mmHg) | 69.92 ± 7.03 | 69.24 ± 6.50 | 80.14 ± 6.88 | <.001 |
| SBP SD (mmHg) | 7.88 ± 2.69 | 7.80 ± 2.65 | 9.12 ± 3.02 | <.001 |
| SBP CV (%) | 7.06 ± 2.43 | 7.04 ± 2.43 | 7.33 ± 2.47 | <.001 |
| DBP SD (mmHg) | 6.30 ± 2.47 | 6.25 ± 2.45 | 7.13 ± 2.58 | <.001 |
| DBP CV (%) | 9.10 ± 3.66 | 9.11 ± 3.67 | 9.02 ± 3.54 | .194 |
Note: Data are presented as number (%) or mean ± SD.
Abbreviations: CV, coefficient of variation; DBP, diastolic blood pressure; GH, gestational hypertension; PE, preeclampsia; SBP, systolic blood pressure; SD, standard deviation.
FIGURE 2.
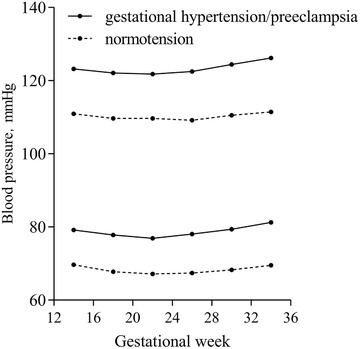
Gestational systolic and diastolic blood pressure changes in normotensive participants and participants with gestational hypertension/preeclampsia
3.2. Visit‐to‐visit BPV and pregnant outcomes
Median value of SBP SD, SBP CV, DBP SD, and DBP CV were 7.46 mmHg, 6.68%, 5.88 mmHg and 8.45%, respectively. Table 2 shows the incidence rates of adverse neonatal outcomes in the four groups compared by the chi‐square test. The incidence rates of fetal distress and small for gestational age were higher in groups with higher DBP SD or DBP CV and the difference reached statistical significance. The results of multivariable logistic regression analysis assessing the relationship between BPV and adverse birth outcomes are presented on Table 3. In all participants, higher BPV was associated with a higher risk of fetal distress and small for gestational age. When comparing the highest quartiles to the lowest quartiles of DBP SD, the fully adjusted ORs were 1.19 (95% CI 1.11–1.27, p for trend < .001) for fetal distress, 1.32 (95% CI 1.14–1.54, p for trend < .001) for small for gestational age, 1.32 (95% CI 1.06–1.63, p for trend = .003) for 1‐min Apgar score ≤ 7. When comparing the highest quartiles to the lowest quartiles of DBP CV, the fully adjusted ORs were 1.22 (95% CI 1.14–1.30, p for trend < .001) for fetal distress, 1.38 (95% CI 1.17–1.61, p for trend < .001) for small for gestational age, 1.43 (95% CI 1.14–1.79, p for trend < .001) for 1‐min Apgar score ≤ 7. ORs for preterm birth and 5‐min Apgar score ≤ 7 were not statistically significant.
TABLE 2.
Comparation of the incidence rates of fetal distress, small for gestational age and preterm birth
| Variables | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p |
|---|---|---|---|---|---|
| SBP SD [mmHg, median (range)] | 5.21 (<6.06) | 6.76 (6.60–7.46) | 8.22 (7.46–9.18) | 10.68 (≥9.18) | |
| N = 13 226 | N = 13 218 | N = 13 225 | N = 13 222 | ||
| Fetal distress, n (%) | 2500 (18.90) | 2579 (19.51) | 2666 (20.16) | 2700 (20.42) | .009 |
| Small for gestational age, n (%) | 283 (2.14) | 371 (2.81) | 327 (2.47) | 441 (3.34) | <.001 |
| Preterm birth, n (%) | 642 (4.85) | 531 (4.02) | 550 (4.16) | 679 (5.14) | <.001 |
| SBP CV [%, median (range)] | 4.66 (<5.41) | 6.05 (5.41–6.68) | 7.38 (6.68–8.24) | 9.58 (≥8.24) | |
| N = 13 222 | N = 13 224 | N = 13 222 | N = 13 223 | ||
| Fetal distress, n (%) | 2524 (19.09) | 2595 (19.62) | 2684 (20.30) | 2642 (19.98) | .081 |
| Small for gestational age, n (%) | 301 (2.28) | 343 (2.59) | 377 (2.85) | 401 (3.03) | .001 |
| Preterm birth, n (%) | 669 (5.06) | 533 (4.03) | 572 (4.33) | 628 (4.75) | <.001 |
| DBP SD [mmHg, median (range)] | 4.40 (<4.72) | 5.30 (4.72–5.88) | 6.54 (5.88–7.37) | 8.64 (≥7.37) | |
| N = 13 227 | N = 13 213 | N = 13 230 | N = 13 221 | ||
| Fetal distress, n (%) | 2330 (17.62) | 2561 (19.38) | 2741 (20.72) | 2813 (21.28) | <.001 |
| Small for gestational age, n (%) | 304 (2.30) | 317 (2.40) | 353 (2.67) | 448 (3.39) | <.001 |
| Preterm birth, n (%) | 659 (4.98) | 537 (4.06) | 542 (4.10) | 664 (5.02) | <.001 |
| DBP CV [%,median(range)] | 5.69 (<6.71) | 7.57 (6.71–8.45) | 9.45 (8.45–10.73) | 12.75 (≥10.73) | |
| N = 13 223 | N = 13 223 | N = 13 223 | N = 13 222 | ||
| Fetal distress, n (%) | 2438 (18.44) | 2525 (19.10) | 2654 (20.07) | 2828 (21.39) | <.001 |
| Small for gestational age, n (%) | 317 (2.40) | 335 (2.53) | 361 (2.73) | 409 (3.09) | .003 |
| Preterm birth, n (%) | 676 (5.11) | 549 (4.15) | 585 (4.42) | 592 (4.48) | .002 |
Abbreviations: CV, coefficient of variation; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
TABLE 3.
Multiple logistic regression models of visit‐to‐visit blood pressure variability with adverse neonatal outcomes
| Outcomes | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p for trend | |
|---|---|---|---|---|---|---|
| Fetal distress | ||||||
| SBP SD | Model 1 | Reference | 1.04 (.98–1.11) | 1.08 (1.02–1.15) | 1.10 (1.04–1.17) | .001 |
| Model 2 | Reference | 1.00 (.94–1.07) | 1.03 (.97‐1.10) | 1.03 (.97‐1.09) | .298 | |
| Model 3 | Reference | 1.00 (.94–1.07) | 1.04 (.97‐1.11) | 1.05 (.98‐1.12) | .115 | |
| SBP CV | Model 1 | Reference | 1.04 (.97–1.10) | 1.08 (1.02‐1.15) | 1.06 (1.00‐1.13) | .049 |
| Model 2 | Reference | 1.03 (.96–1.09) | 1.06 (.99‐1.13) | 1.05 (.98‐1.11) | .141 | |
| Model 3 | Reference | 1.02 (.95–1.08) | 1.05 (.98‐1.12) | 1.05 (.98‐1.12) | .104 | |
| DBP SD | Model 1 | Reference | 1.12 (1.06–1.20) | 1.22 (1.15–1.30) | 1.26 (1.19–1.34) | <.001 |
| Model 2 | Reference | 1.09 (1.02–1.16) | 1.15 (1.08–1.23) | 1.15 (1.08–1.23) | <.001 | |
| Model 3 | Reference | 1.09 (1.02–1.16) | 1.17 (1.10–1.25) | 1.19 (1.11–1.27) | <.001 | |
| DBP CV | Model 1 | Reference | 1.04 (.98–1.11) | 1.11 (1.05–1.18) | 1.20 (1.13‐1.28) | <.001 |
| Model 2 | Reference | 1.04 (.98–1.11) | 1.10 (1.03–1.17) | 1.16 (1.09–1.23) | <.001 | |
| Model 3 | Reference | 1.05 (.98–1.12) | 1.13 (1.05–1.20) | 1.22 (1.14–1.30) | <.001 | |
| Small for gestational age | ||||||
| SBP SD | Model 1 | Reference | 1.32 (1.13–1.54) | 1.16 (.99–1.36) | 1.58 (1.36–1.84) | <.001 |
| Model 2 | Reference | 1.29 (1.10–1.50) | 1.11 (.95–1.31) | 1.49 (1.28–1.73) | <.001 | |
| Model 3 | Reference | 1.25 (1.07–1.46) | 1.07 (.91–1.25) | 1.35 (1.16–1.58) | .001 | |
| SBP CV | Model 1 | Reference | 1.14 (.98–1.34) | 1.26 (1.08–1.47) | 1.34 (1.15–1.56) | <.001 |
| Model 2 | Reference | 1.11 (.95–1.29) | 1.17 (1.01–1.37) | 1.22 (1.05–1.43) | .009 | |
| Model 3 | Reference | 1.12 (.96–1.31) | 1.20 (1.02–1.40) | 1.26 (1.08–1.47) | .004 | |
| DBP SD | Model 1 | Reference | 1.05 (.89–1.23) | 1.17 (1.00–1.36) | 1.49 (1.29–1.73) | <.001 |
| Model 2 | Reference | 1.01 (.86–1.19) | 1.11 (.95–1.30) | 1.40 (1.21–1.63) | <.001 | |
| Model 3 | Reference | 1.00 (.85–1.18) | 1.09 (.93–1.28) | 1.32 (1.14–1.54) | <.001 | |
| DBP CV | Model 1 | Reference | 1.06 (.91–1.24) | 1.14 (.98–1.33) | 1.30 (1.12–1.51) | <.001 |
| Model 2 | Reference | 1.02 (.87–1.19) | 1.07 (.92–1.25) | 1.19 (1.02–1.38) | .014 | |
| Model 3 | Reference | 1.09 (.93–1.27) | 1.19 (1.01–1.39) | 1.38 (1.17–1.61) | <.001 | |
| Preterm birth | ||||||
| SBP SD | Model 1 | Reference | .82 (.73–.92) | .85 (.76–.96) | 1.06 (.95–1.19) | .079 |
| Model 2 | Reference | .82 (.73–.93) | .86 (.77–.97) | 1.08 (.96–1.20) | .045 | |
| Model 3 | Reference | .80 (.71–.90) | .82 (.73–.92) | .96 (.86–1.08) | .994 | |
| SBP CV | Model 1 | Reference | .79 (.70–.89) | .85 (.76–.95) | .94 (.84–1.05) | .675 |
| Model 2 | Reference | .80 (.71–.90) | .87 (.77–.97) | .97 (.86–1.08) | .883 | |
| Model 3 | Reference | .80 (.71–.90) | .88 (.78–.99) | .97 (.86–1.09) | .861 | |
| DBP SD | Model 1 | Reference | .81 (.72–.91) | .82 (.73–.92) | 1.01 (.90–1.13) | .418 |
| Model 2 | Reference | .82 (.73–.92) | .83 (.74–.94) | 1.04 (.93–1.16) | .181 | |
| Model 3 | Reference | .80 (.71–.90) | .79 (.70–.89) | .95 (.85–1.07) | .776 | |
| DBP CV | Model 1 | Reference | .80 (.72–.90) | .86 (.77–.96) | .87 (.78–.97) | .085 |
| Model 2 | Reference | .82 (.73–.92) | .89 (.79–.99) | .91 (.81–1.02) | .352 | |
| Model 3 | Reference | .85 (.76–.96) | .91 (.81–1.03) | .95 (.84–1.08) | .801 | |
| 1‐min Apgar score≤7 | ||||||
| SBP SD | Model 1 | Reference | 1.02 (.83–1.27) | .88 (.71–1.10) | 1.13 (.91–1.39) | .357 |
| Model 2 | Reference | 1.02 (.82–1.26) | .88 (.70–1.09) | 1.12 (.91–1.38) | .383 | |
| Model 3 | Reference | 1.00 (.81–1.24) | .86 (.69–1.07) | 1.06 (.85–1.31) | .755 | |
| SBP CV | Model 1 | Reference | .90 (.73–1.11) | .84 (.67–1.04) | .98 (.80–1.21) | .894 |
| Model 2 | Reference | .92 (.74–1.13) | .86 (.69–1.07) | 1.04 (.84–1.28) | .698 | |
| Model 3 | Reference | .91 (.73–1.12) | .84 (.68–1.05) | 1.00 (.81–1.24) | .972 | |
| DBP SD | Model 1 | Reference | .95 (.76–1.19) | 1.10 (.89–1.37) | 1.35 (1.09–1.66) | <.001 |
| Model 2 | Reference | .95 (.76–1.19) | 1.11 (.89–1.38) | 1.35 (1.09–1.66) | .001 | |
| Model 3 | Reference | .96 (.76–1.20) | 1.10 (.88–1.37) | 1.32 (1.06–1.63) | .003 | |
| DBP CV | Model 1 | Reference | .93 (.74–1.16) | 1.04 (.84–1.29) | 1.29 (1.05–1.59) | .004 |
| Model 2 | Reference | .97 (.77–1.21) | 1.09 (.88–1.36) | 1.37 (1.12–1.70) | <.001 | |
| Model 3 | Reference | .99 (.79–1.24) | 1.12 (.90–1.40) | 1.43 (1.14–1.79) | <.001 | |
| 5–min Apgar score≤7 | ||||||
| SBP SD | Model 1 | Reference | .90 (.48–1.70) | .70 (.35–1.39) | .75 (.38–1.47) | .335 |
| Model 2 | Reference | .89 (.47–1.69) | .69 (.35–1.36) | .73 (.37–1.43) | .297 | |
| Model 3 | Reference | .88 (.46–1.66) | .66 (.33–1.32) | .66 (.33–1.31) | .186 | |
| SBP CV | Model 1 | Reference | .76 (.40–1.46) | .76 (.40–1.46) | .67 (.34–1.31) | .264 |
| Model 2 | Reference | .75 (.39–1.44) | .74 (.38–1.41) | .64 (.32–1.26) | .212 | |
| Model 3 | Reference | .73 (.38–1.40) | .69 (.36–1.34) | .57 (.28–1.14) | .120 | |
| DBP SD | Model 1 | Reference | .50 (.23–1.07) | .90 (.48–1.70) | .95 (.51–1.78) | .721 |
| Model 2 | Reference | .49 (.23–1.06) | .88 (.46–1.66) | .91 (.48–1.71) | .821 | |
| Model 3 | Reference | .49 (.23–1.05) | .85 (.45–1.62) | .84 (.44–1.62) | .987 | |
| DBP CV | Model 1 | Reference | .71 (.34–1.48) | 1.30 (.69–2.44) | .94 (.48–1.86) | .796 |
| Model 2 | Reference | .69 (.33–1.45) | 1.25 (.66–2.36) | .89 (.45–1.77) | .929 | |
| Model 3 | Reference | .67 (.32–1.41) | 1.18 (.61–2.26) | .80 (.39–1.66) | .826 | |
Note: Model 1 was unadjusted. Model 2 was adjusted for body mass index, age, gravidity, parity, cesarean, in vitro fertilization‐embryo transfer. Model 3 was adjusted for covariates in Model 2 and hypertensive disorders (normotension, gestational hypertension/preeclampsia), gestational diabetes mellitus, premature rupture of membranes, cephalic presentation, scarred uterus, anemia, mean SBP and mean DBP.
Abbreviations: CV, coefficient of variation; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
In order to further explore the specific relationship, we classified the whole participants into two subgroups: normotensive participants and patients with gestational hypertension/preeclampsia. Table 4 shows the association between visit‐to‐visit diastolic BPV and adverse neonatal outcomes in different subgroups by multivariable logistic regression analysis with the fully adjusted model. The association between elevated visit‐to‐visit diastolic BPV and small for gestational age and 1‐min Apgar score ≤ 7 in the two subgroups was consistent with that in all participants, respectively. BPV was significantly associated with fetal distress in normotensive participants but the relationship disappeared in patients with gestational hypertension/preeclampsia. In participants with gestational hypertension/preeclampsia, ORs for preterm birth were 2.80 (95% CI 1.99–3.94, p for trend < .001) in DBP SD and 3.25 (95% CI 2.24–4.72, p for trend < .001) in DBP CV when extreme quartiles were compared.
TABLE 4.
Association of visit‐to‐visit blood pressure variability with adverse neonatal outcomes in normotensive participants and patients with gestational hypertension/preeclampsia
| Variables | Quartile | Median (range) | Fetal distress | SGA | Preterm birth | 1‐min Apgar score≤7 |
|---|---|---|---|---|---|---|
| In normotensive participants | ||||||
| DBP SD (mmHg) | Q1 | 4.02 (<4.69) | Reference | Reference | Reference | Reference |
| Q2 | 5.26 (4.69–5.83) | 1.08 (1.01–1.15) | 1.00 (.84–1.18) | .79 (.70–.90) | .90 (.71–1.15) | |
| Q3 | 6.48 (5.83–7.29) | 1.16 (1.09–1.24) | 1.06 (.90–1.25) | .79 (.70–.89) | 1.07 (.85–1.35) | |
| Q4 | 8.53 (≥7.29) | 1.21 (1.13–1.29) | 1.25 (1.06–1.47) | .81 (.71–.92) | 1.24 (.99–1.56) | |
| p for trend | <.001 | .003 | .003 | .019 | ||
| DBP CV (%) | Q1 | 5.70 (< 6.71) | Reference | Reference | Reference | Reference |
| Q2 | 7.58 (6.71–8.45) | 1.06 (.99–1.14) | 1.05 (.89–1.24) | .83 (.73–.94) | .95 (.75–1.21) | |
| Q3 | 9.45 (8.45–10.72) | 1.15 (1.07–1.23) | 1.10 (.93–1.30) | .84 (.74–.95) | 1.06 (.84–1.35) | |
| Q4 | 12.74 (≥10.72) | 1.24 (1.16–1.33) | 1.25 (1.05–1.48) | .79 (.69–.90) | 1.33 (1.05–1.69) | |
| p for trend | <.001 | .008 | .002 | .006 | ||
| In patients with gestational hypertension/preeclampsia | ||||||
| DBP SD (mmHg) | Q1 | 4.46 (< 5.34) | Reference | Reference | Reference | Reference |
| Q2 | 6.02 (5.34–6.77) | .80 (.63–1.01) | 1.09 (.70–1.71) | .97 (.66–1.43) | .95 (.47–1.92) | |
| Q3 | 7.53 (6.77–8.51) | .91 (.72–1.16) | 1.45 (.94–2.23) | 1.28 (.88–1.86) | 1.49 (.77–2.88) | |
| Q4 | 9.96 (≥8.51) | .87 (.68–1.11) | 2.05 (1.35–3.09) | 2.80 (1.99–3.94) | 2.13 (1.14–4.00) | |
| P for trend | .512 | <.001 | <.001 | .005 | ||
| DBP CV (%) | Q1 | 5.39 (< 6.52) | Reference | Reference | Reference | Reference |
| Q2 | 7.47 (6.52–8.52) | .80 (.63–1.02) | 1.49 (.94–2.34) | 1.24 (.85–1.83) | 1.08 (.53–2.17) | |
| Q3 | 9.57 (8.52–10.91) | .79 (.62–1.02) | 2.04 (1.31–3.17) | 1.81 (1.25–2.62) | 1.79 (.93–3.44) | |
| Q4 | 12.92 (≥10.91) | .90 (.69–1.17) | 2.31 (1.46–3.66) | 3.25 (2.24–4.72) | 2.15 (1.09–4.26) | |
| P for trend | .582 | <.001 | <.001 | .011 | ||
Note: Adjusted for body mass index, age, gravidity, parity, cesarean, in vitro fertilization‐embryo transfer, gestational diabetes mellitus, premature rupture of membranes, cephalic presentation, scarred uterus, anemia, mean SBP and mean DBP.
Abbreviations: CV, coefficient of variation; DBP, diastolic blood pressure; Q, quartile; SBP, systolic blood pressure; SD, standard deviation; SGA: small for gestational age.
4. DISCUSSION
In this retrospective cohort study, we found that a higher risk of fetal distress and small for gestational age was associated with the increment of BPV, and the significance did not vanish after adjusting for various confounding factors including mean blood pressure. These data add to the evidence that visit‐to‐visit BPV is a risk factor for adverse neonatal outcomes in pregnant women.
Rothwell and colleagues showed that visit‐to‐visit systolic BPV was a predictive risk factor for stroke independent of mean SBP. 10 Higher visit‐to‐visit systolic BPV was reported to be associated with all‐cause mortality in the general population 11 and related to cardiovascular events in patients with hypertension. 12 A cohort study with 3 285 684 veterans indicated that higher systolic BPV was associated with all‐cause mortality, coronary heart disease, stroke, and end‐stage renal disease. 19 Previous studies generally focused on the impact of BPV in the nonpregnant population. There are only a few reports based on pregnant women with detailed analysis of BPV. Kim and colleagues reported that pregnant women who developed hypertension had higher visit‐to‐visit BPV than the normotensive ones, and participants with higher BPV tended to have a higher risk of pregnancy complications. 15 Our results are generally consistent with the previous studies. Nevertheless, no study by far has explored the relationship between visit‐to‐visit BPV and fetal distress, preterm birth and Apgar score. To the best of our knowledge, this is the first study to demonstrate such a relationship. The Apgar score system has been widely used for the quick assessment of the physical condition and the need for resuscitation of newborns. 20 However, it is warned inappropriate to use the Apgar score alone to diagnose neonatal asphyxia or predict neurologic outcome. 21 Apgar score may also be influenced by a few factors such as preterm birth and gestational age. Despite its limitations, a cohort study with more than one million neonates showed that low 5‐min Apgar score was strongly associated with the risk of neonatal and infant mortality. 22 In our study, higher diastolic BPV was associated with an increased risk of 1‐min Apgar score ≤ 7 when extreme quartiles were compared. However, no significant association was found between systolic BPV and 1‐min or 5‐min Apgar score ≤ 7, which implies that diastolic BPV has a better prognostic value. Fetal distress is a broad concept and it is hard to give its precise definition. It is used when the fetus becomes hypoxia and it often leads to a higher rate of cesarean or operative vaginal delivery. We found higher BPV associated with increased risk of fetal distress and small for gestational age in the fully adjusted model.
The mechanisms demonstrating the association of BPV with neonatal outcomes have not been fully understood. In general, BPV is considered to be the result of complex interactions between extrinsic environmental and intrinsic cardiovascular regulatory mechanisms such as central sympathetic overactivity, reduced arterial/cardiopulmonary reflex, reduced arterial compliance, impairment of hormonal regulation, improper dosing of antihypertensive treatment, and seasonal change. 23 Increased BPV leads to greater stress on blood vessels, endothelial dysfunction and even organ damage. 19 , 24 , 25 , 26 An animal experiment suggested that increased BPV in combination with hypertension aggravates cardiac remodeling and dysfunction in rats via angiotensin II system‐mediated chronic myocardial inflammation. 24 Another animal study in rats demonstrated that BPV has a direct relationship with arterial stiffness and recommended BPV as a parameter for arterial stiffness evaluation. 25 BPV was reported to correlate with markers of endothelial and smooth muscle function, suggesting that BPV may affect vascular health. 27 In a 15‐year retrospective cohort study of 825 hypertensive patients without chronic kidney disease, higher visit‐to‐visit BPV was found to be associated with the slope of estimated glomerular filtration rate. 28 It was also found that higher systolic BPV was related to the loss of residual renal function in peritoneal dialysis patients. 26 Increased BPV may reflect a pathophysiological imbalance of cardiovascular regulation in pregnant women. 29 Placenta is responsible for supplying oxygen to the fetus. Higher BPV may contribute to instability in hemodynamics and placenta dysfunction by subtle damage to the placental vasculature and thus reduce blood flow, oxygen, and nutrients delivery to the fetus, 30 which may increase the risk of fetal distress and small for gestational age.
In the present study, BPV was not a significant risk factor for preterm birth in all participants. Various factors such as polyhydramnios, uterine malformation, and intrauterine infection account for preterm birth. Actually, preterm birth is usually a complex result of multiple factors rather than any one of the overwhelming causes. Hypertensive disorders of pregnancy remain a global public health issue responsible for both maternal and neonatal morbidity and mortality. 31 In the present study, the preterm birth morbidity in normotensive participants and those with gestational hypertension/preeclampsia varied (4.19% vs. 9.76%, p < .001). Furthermore, the status of placental abruption, gestational diabetes mellitus, scarred uterus, premature rupture of membranes, in vitro fertilization‐embryo transfer, age, body mass index, gravidity, and parity distributed differently among normotension group and gestational hypertension/preeclampsia group, with all p values for chi‐square test or t‐test less than .05. We hypothesized that these variables may conceal or interact with the effect of gestational hypertension/preeclampsia. Therefore, subgroup analysis stratified by hypertensive status was conducted. Diastolic BPV was related to the prevalence of small for gestational age in both normotension group and gestational hypertension/preeclampsia group. What should be noted was that the prognostic value of BPV as a risk factor for preterm birth was only observed in gestational hypertension/preeclampsia group but not in normotension group. Mechanisms for the pathogenesis of preeclampsia include endothelial dysfunction, oxidative stress, inflammation impairment, angiogenic imbalance. 32 Preeclampsia also leads to impaired vascular homeostasis. 33 Jieyu and colleagues found that higher visit‐to‐visit BPV was related to increased risk of gestational hypertension and preeclampsia, suggesting that BPV may help identify patients at high risk of gestational hypertension and preeclampsia. 14 Moreover, BPV may reflect the severity of preeclampsia to some extent. Uncontrollable hypertensive disorders of pregnancy especially severe preeclampsia may lead to iatrogenic preterm birth by cesarean section. Therefore, it is reasonable that BPV is associated with preterm birth in patients with gestational hypertension/preeclampsia.
The major strength of this study is the large sample which provided sufficient statistical power. We recruited 52,891 pregnant women from the general population instead of selected participants from clinical trials and thus increased the generalizability of our findings. We also collected detailed information including several covariates, which allowed us to analyze the association between BPV and neonatal outcomes by adjusting several confounding factors. In addition, we separated all the participants into normotensive participants and patients with gestational hypertension/preeclampsia and performed logistic regression analysis in the two subgroups respectively. By selecting patients with gestational hypertension/preeclampsia, we not only chose a population at high risk of neonatal outcomes but also found increased BPV associated with preterm birth.
However, this is a retrospective study and it leads to confounding bias and selection bias, which may distort the effect of visit‐to‐visit BPV on neonatal outcomes. The study did not focus on short‐term BPV and its prognostic value for neonatal outcomes is obscure. Compared with short‐term BPV, visit‐to‐visit BPV might not entirely consist of spontaneous blood pressure variations and it provides limited information on blood pressure profiles. 23 It is unclear whether 24‐h BPV has a better prognostic value for adverse neonatal outcomes than visit‐to‐visit BPV. Yet 24‐h ambulatory blood pressure monitoring is not a routine examination provided in outpatient department and it is costly and inconvenient. Therefore, short‐term BPV is difficult to acquire. In addition, there are no consensual standardized methods or protocols for BPV measurement yet. For example, assessment of visit‐to‐visit BPV such as visit frequency, visit intervals and follow‐up durations varied in different pregnant women and it may lead to measurement bias.
Using BPV along with other prenatal examinations represents an opportunity to improve prognostic assessment tools for neonatal risk. The measurement of visit‐to‐visit BPV is promising to be an important potential target for BP management in pregnant women. A standardized assessment procedure of BPV, such as BPV metrics, visit frequency and visit intervals should be established in future research. Furthermore, it is highly warranted to explore the interventional and therapeutic methods based on the underlying mechanisms of BPV.
5. CONCLUSIONS
The present study showed that higher diastolic BPV, independent of mean blood pressure, is associated with an increased risk of adverse neonatal outcomes including fetal distress, small for gestational age and 1‐min Apgar score ≤ 7 in pregnant women. Our findings suggested that BPV might be a potential marker for the management of blood pressure and prediction of neonatal risks in pregnant women. Further studies are necessary to explore the underlying mechanism of BPV.
CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
AUTHOR CONTRIBUTIONS
Yingjie Gu contributed to statistical analysis and the draft of the manuscript. Haofan Shi contributed significantly to analysis and manuscript preparation. Weijian Zeng and Yulong Zheng helped perform the analysis with constructive discussions. Mengnan Yang and Mengru Sun contributed to data collection. Hong Shi and Wei Gu contributed to the conception of the study and supervised the manuscript.
ACKNOWLEDGMENTS
This study was supported by National Key R&D Program of China (2019YFA0802600) and “Research on Cardiovascular diseases” FRQS – NSFC Collaboration (81861128021). The authors gratefully acknowledge the whole clinical team from the International Peace Maternity and Child Health Hospital for their care of the patients. We would like to express our gratitude to the study participants as well.
Gu Y, Shi H, Zeng W, et al. Association between gestational visit‐to‐visit blood pressure variability and adverse neonatal outcomes. J Clin Hypertens. 2022;24:779–788. 10.1111/jch.14500
Yingjie Gu and Haofan Shi contributed equally to this manuscript.
Contributor Information
Hong Shi, Email: gunxueqiu2011@qq.com.
Wei Gu, Email: krisgu70@163.com.
REFERENCES
- 1. Bullens LM, van Runnard Heimel PJ, van der Hout‐van der Jagt MB, Oei SG. Interventions for intrauterine resuscitation in suspected fetal distress during term labor: a systematic review. Obstet Gynecol Surv. 2015;70(8):524‐539. [DOI] [PubMed] [Google Scholar]
- 2. Katz J, Wu LA, Mullany LC, et al. Prevalence of small‐for‐gestational‐age and its mortality risk varies by choice of birth‐weight‐for‐gestation reference population. PLoS One. 2014;9(3):e92074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raicević S, Cubrilo D, Arsenijević S, et al. Oxidative stress in fetal distress: potential prospects for diagnosis. Oxid Med Cell Longev. 2010;3(3):214‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crispi F, Miranda J, Gratacós E. Long‐term cardiovascular consequences of fetal growth restriction: biology, clinical implications, and opportunities for prevention of adult disease. Am J Obstet Gynecol. 2018;218(2S):S869‐S879. [DOI] [PubMed] [Google Scholar]
- 6. Zhu B, Huang K, Bao W, et al. Dose‐response relationship between maternal blood pressure in pregnancy and risk of adverse birth outcomes: Ma'anshan birth cohort study. Pregnancy Hypertens. 2019;15:16‐22. [DOI] [PubMed] [Google Scholar]
- 7. Rachel B, Steegers EAP, Albert H, Jaddoe VWV. Blood pressure in different gestational trimesters, fetal growth, and the risk of adverse birth outcomes: the generation R study. Am J Epidemiol. 2011;174(7):797. [DOI] [PubMed] [Google Scholar]
- 8. Rothwell PM. Limitations of the usual blood‐pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375(9718):938‐948. [DOI] [PubMed] [Google Scholar]
- 9. Stergiou GS, Parati G, Vlachopoulos C, et al. Methodology and technology for peripheral and central blood pressure and blood pressure variability measurement: current status and future directions ‐ Position statement of the European Society of Hypertension Working Group on blood pressure monitoring and cardiovascular variability. J Hypertens. 2016;34(9):1665‐1677. [DOI] [PubMed] [Google Scholar]
- 10. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895‐905. [DOI] [PubMed] [Google Scholar]
- 11. Paul M, Daichi S, Marcello T, Kristi R, Arnett DK, Suzanne O. The relationship between visit‐to‐visit variability in systolic blood pressure and all‐cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57(2):160. [DOI] [PubMed] [Google Scholar]
- 12. Mehlum MH, Liestøl K, Kjeldsen SE, et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J. 2018;39(24):2243‐2251. [DOI] [PubMed] [Google Scholar]
- 13. Amari Y, Morimoto S, Iida T, et al. Characteristics of visit‐to‐visit blood pressure variability in hemodialysis patients. Hypertens Res. 2019;42(7):1036‐1048. [DOI] [PubMed] [Google Scholar]
- 14. Jieyu L, Yingying C, Tian G, et al. Visit‐to‐visit blood pressure variability is associated with gestational hypertension and pre‐eclampsia. Pregnancy Hypertens. 2019;18:126‐131. [DOI] [PubMed] [Google Scholar]
- 15. Kim S‐A, Lee J‐D, Park JB. Differences in visit‐to‐visit blood pressure variability between normotensive and hypertensive pregnant women. Hypertens Res. 2019;42(1):67‐74. [DOI] [PubMed] [Google Scholar]
- 16. Liu J, Yang L, Teng H, et al. Visit‐to‐visit blood pressure variability and risk of adverse birth outcomes in pregnancies in East China. Hypertens Res. 2021;44(2):239‐249. [DOI] [PubMed] [Google Scholar]
- 17. ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1):1. [DOI] [PubMed] [Google Scholar]
- 18. Association A. Standards of medical care in diabetes – 2016. Diabetes Care. 2016;39:S1‐S106.26696671 [Google Scholar]
- 19. Gosmanova EO, Mikkelsen MK, Molnar MZ, et al. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. 2016;68(13):1375‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li F, Wu T, Lei X, Zhang H, Mao M, Zhang J. The apgar score and infant mortality. PLoS One. 2013;8(7):e69072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watterberg K, Aucott S, Benitz W, et al. The Apgar score. Pediatrics. 2015; 136:819‐822. [DOI] [PubMed] [Google Scholar]
- 22. Iliodromiti S, Mackay DF, Smith GC, Pell JP, Nelson SM. Apgar score and the risk of cause‐specific infant mortality: a population‐based cohort study. Lancet. 2014;384(9956):1749‐1755. [DOI] [PubMed] [Google Scholar]
- 23. Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood‐pressure variability. Nat Rev Cardiol. 2013;10(3):143‐155. [DOI] [PubMed] [Google Scholar]
- 24. Kudo H, Kai H, Kajimoto H, et al. Exaggerated blood pressure variability superimposed on hypertension aggravates cardiac remodeling in rats via angiotensin II system‐mediated chronic inflammation. Hypertension. 2009;54(4):832‐838. [DOI] [PubMed] [Google Scholar]
- 25. Li ZY, Xu TY, Zhang SL, et al. Telemetric ambulatory arterial stiffness index, a predictor of cardio‐cerebro‐vascular mortality, is associated with aortic stiffness‐determining factors. CNS Neurosci Ther. 2013;19(9):667‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jo HA, An JN, Lee JP, Oh KH, Lim CS, Oh YK. Visit‐to‐visit variability in systolic blood pressure is a risk factor for rapid loss of residual renal function in peritoneal dialysis patients. Tohoku J Exp Med. 2015;235(4):295‐304. [DOI] [PubMed] [Google Scholar]
- 27. Diaz KM, Veerabhadrappa P, Kashem MA, et al. Visit‐to‐visit and 24‐h blood pressure variability: association with endothelial and smooth muscle function in African Americans. J Hum Hypertens. 2013;27(11):671‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chia YC, Lim HM, Ching SM. Long‐term visit‐to‐visit blood pressure variability and renal function decline in patients with hypertension over 15 years. J Am Heart Assoc. 2016;5(11):e003825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vermunt JV, Kennedy SH, Garovic VD. Blood pressure variability in pregnancy: an opportunity to develop improved prognostic and risk assessment tools. Curr Hypertens Rep. 2020;22(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shah DA, Khalil RA. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem Pharmacol. 2015;95(4):211‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40(3):213‐220. [DOI] [PubMed] [Google Scholar]
- 32. Costantine MM, Cleary K. Pravastatin for the prevention of preeclampsia in high‐risk pregnant women. Obstet Gynecol. 2013;121(2 Pt 1):349‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]


