Abstract
Hypertension is the leading cause of overall mortality in low‐ and middle‐income countries. In Brazil, there is paucity of data on the determinants of incident hypertension and related risk factors. We aimed to determine the incidence of hypertension in a sample from the Brazilian population and investigate possible relationships with body adiposity indexes. We assessed risk factors associated with cardiovascular disease, including adiposity body indexes and biochemical analysis, in a sample from the Baependi Heart Study before and after a 10‐year follow‐up. Hypertension was defined by the presence of systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or the use of antihypertensive drugs. From an initial sample of 1693 participants, 498 (56% women; mean age 38 ± 13 years) were eligible to be included. The overall hypertension incidence was 24.3% (22.3% in men and 25.6% in women). Persons who developed hypertension had higher prevalence of obesity, higher levels for blood pressure, higher frequency of dyslipidemia, and higher body adiposity indexes at baseline. The best prediction model for incident hypertension includes age, sex, HDL‐c, SBP, and Body Mass Index (BMI) [AUC = 0.823, OR = 1.58 (95% CI 1.23‐2.04)]. BMI was superior in its predictive capacity when compared to Body Adiposity Index (BAI), Body Roundness Index (BRI), and Visceral Adiposity Index (VAI). Incident hypertension in a sample from the Brazilian population was 24.3% after 10‐year follow‐up and BMI, albeit the simpler index to be calculated, is the best anthropometric index to predict incident hypertension.
Keywords: blood pressure, body mass index, epidemiology, follow‐up studies, humans, hypertension, logistic models, risk factors
1. INTRODUCTION
Hypertension is a known risk factor for overall mortality worldwide and the leading cause in low‐ and middle‐income countries, such as Brazil. 1 The prevalence of hypertension in the adult Brazilian population was previously estimated to be around 30%. 2 , 3
The American Heart Association has long suggested that hypertension should be high in the priority list of modifiable risk factors for cardiovascular mortality. 4 In this scenario, epidemiological studies investigating the factors associated with the development of cardiovascular diseases, and hypertension, have aimed at identifying risk factors that could predict, and eventually prevent, the development of hypertension. 5 , 6 , 7 , 8 , 9 , 10 , 11 Among the studies, the Framingham Study identified that hypercholesterolemia, glucose intolerance, 5 age, Body Mass Index (BMI), cigarette consumption, family history, and physical inactivity 6 as risk factors associated to the development of arterial hypertension. 7
Body adiposity indexes other than BMI, such as Body Adiposity Index (BAI), Body Roundness Index (BRI), Visceral Adiposity Index (VAI), Waist Circumference (WC), Waist‐to‐Height Ratio (WtHR), Waist‐to‐Hip Ratio (WHR), have been related to increase of blood pressure and prevalence of hypertension. 12 , 13 , 14 The rise in blood pressure associated with increased body adiposity indexes is thought to be related to the accumulation of visceral adipose tissue and adipokine levels, that cause release of inflammatory cytokines, increased oxidative stress, sympathetic dysregulation, and metabolic disorders. 8 , 9 , 15 , 16
Our group previously demonstrated, in a cross‐sectional study in a sample of Brazilian population, that the indicators of adiposity WC, BMI, BAI, and VAI were higher in hypertensive when compared to nonhypertensive individuals; and BMI, along with WC in men, were strongly associated to hypertension. 17 However, few longitudinal assessments of chronic diseases are available in Brazil and there is paucity of data from long‐term longitudinal studies to estimate incident hypertension and its associated risk factors. Furthermore, Brazil has specific characteristics, such as being a highly admixed population, raising the possibility that determinants of hypertension incidence might be different. Therefore, we aimed to determine the incidence of hypertension in a Brazilian sample after a 10‐year follow‐up and investigate factors able to predict the development of the condition focusing on body adiposity indexes.
2. METHODS
2.1. Study design and participants
The Baependi Heart Study is a longitudinal study designed to investigate factors associated with the development of cardiovascular disease in the Brazilian population. It has been taking place in Baependi (752 km2, 19 148 habitants), a small town in the southeast of Brazil, Minas Gerais State, since 2005. The population studied was composed of both genders and the ages ranging from 18 to 102 years.
The baseline enrollment occurred between 2005 and 2006 (1712 participants) 18 and the 10‐year follow‐up visit was conducted between 2015 and 2016 (3423 participants). The eligibility criteria to define participants were previously described. 18 At baseline, participants who had the diagnosis of hypertension were excluded from the current analysis. Participants who were lost to follow‐up were excluded as well.
The study protocol was approved by the ethics committee of the Hospital das Clínicas (SDC: 3485/10/074), University of São Paulo, Brazil, and each person provided informed written consent before participation.
2.2. Clinical and laboratorial characteristics
2.2.1. Questionnaire
A questionnaire was administered to each participant to obtain information related to demographic characteristics, medical history, and environmental exposures. Current smoking status was defined whether smoking had occurred during the last 6 months.
2.2.2. Blood pressure measurement
Blood pressure was measured using a standard digital sphygmomanometer (OMRON,Brazil) on the left arm, after 5 minutes of rest, in the sitting position. Systolic and diastolic blood pressures were calculated from three readings (mean value of all measurements), with a minimal interval of 3 minutes.
2.2.3. Biochemical analysis
Fasting blood glucose (FBG), total cholesterol, triglycerides (TG), lipoprotein fractions as high‐density lipoprotein (HDL‐c), and low‐density lipoprotein (LDL‐c) were assayed by standard techniques applied to 12‐hour fasting blood samples in both cycles. The Friedewald formula was used to estimate LDL‐c.
2.2.4. Anthropometric parameters
Anthropometric parameters were measured according to a standard protocol. 18 Weight was measured on a calibrated digital scale (Filizola), with a maximum load of 180 kg and accuracy of 100 g. Height was measured using a wall mounted stadiometer (Sanny), to the nearest 0.5 cm. WC was measured, in centimeters, at the mean point between the lowest rib margin and the iliac crest with the person standing and at the maximum point of normal expiration. We calculated BMI as body weight (kg) divided by height squared (m2).
Increased WC was defined as ≥ 88 cm for women and ≥ 102 cm for men based on cutoff points advocated by Lean and associates, 1995 19 ]. The calculation of the BAI was based on hip circumference and height (BAI = [hip circumference (cm)/ (height (m) 1.5] – 18) 20 ]. VAI was calculated using the variables WC, BMI, TG and HDL‐c according to the literature 21 assuming VAI = 1 in healthy nonobese persons with normal adipose distribution and normal TG and HDL‐c levels. BRI were calculated using BRI = 364,2 – 365,5 [1 ‐ π –2 WC 2 (m) * Height –2 (m)]1/2. 22 , 23 Based on TG and FBG, the TyG index was calculated using TyG = Ln [(TG (mg/dL) * glucose (mg/dL)/2]. 24 , 25
2.2.5. Disease's diagnosis
Hypertension was defined if the mean systolic blood pressure (SBP) ≥ 140 mmHg and/or means diastolic blood pressure (DBP) ≥ 90 mmHg 26 and/or antihypertensive drug use. Type 2 Diabetes mellitus (T2DM) was diagnosed by the presence of fasting glucose ≥ 126 mg/dL or antidiabetic drug use. Overweight diagnosis was defined when BMI ≥ 25 kg/m2 and < 30 kg/m2, and obesity when BMI ≥ 30 kg/m2.
2.3. Statistics analysis
In both cycles, we accessed clinical characteristics using descriptive statistics. Categorical variables were expressed as percentages and continuous variables were expressed as mean ± SD. The normality of all data was tested with the Kolmogorov–Smirnov test, and characteristics of participants with hypertension and normotensive were compared using t‐test (continuous variables) or chi‐squared test (categorical variables). We used a mixed model logistic regression framework to study predictors of hypertension. Incident hypertension after 10 years was used as the outcome, different variables of adiposity and obesity, plus age and sex were used as exposures. Since we have individuals that shared the same family environment, we added family assignments as a random factor in our model. To evaluate the performance models, Receiver Operational Characteristics (ROC) curves were derived and the area under the curve (AUC) was used to measure the discriminatory power for hypertension. To understand the best cluster of covariates that could explain the incidence of hypertension in 10‐year follow‐up, we explored some cardiometabolic traits based on the literature review. Considering some combinations, the best models found included the following covariates: Age, Sex, and SBP (model 1) and Age, Sex, SBP, and HDL‐c (model 2). Statistical analysis was performed using R software version 3.4.2, with a significance level set at 5%.
3. RESULTS
For this study, 1693 participants recruited at baseline were followed and reassessed 10 years later, and 498 participants (mean age 38 ± 13 years) were eligible to be included in the study (Figure 1). In this sample, the incidence of arterial hypertension was 24.3% (22.3% in men and 25.6% in women) after a 10‐year follow‐up period.
FIGURE 1.
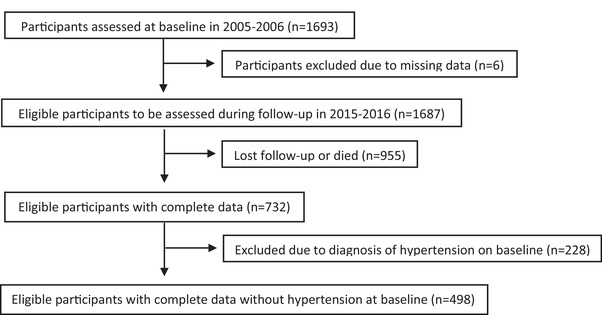
The flow chart of sample selection from the Baependi Heart Study for assessment of incident hypertension
When comparing baseline data from individuals who remained normotensive and individuals that developed hypertension (Table 1), there was significantly higher prevalence of obesity, increased waist circumference, and dyslipidemia in the latter group (p < .05). Individuals who developed hypertension also had higher mean values for systolic and diastolic pressure, triglycerides, and TyG at baseline. Anthropometric indexes of adiposity were also significantly higher (p < .05) at baseline in those that developed hypertension (Table 1). Both groups presented higher means for SBP, DBP, anthropometric indexes of adiposity, in addition to total cholesterol and LDL‐c at follow‐up compared to intragroup baseline values. Additionally, all variables that were different between groups at baseline, except WHR, were also different between groups at follow‐up (Table 1).
TABLE 1.
General characteristics of persons in the general sample at baseline and 10‐year follow‐up according to hypertension development in the Baependi Heart Study
| Baseline | 10‐year follow‐up | |||
|---|---|---|---|---|
| Normotensive (no. = 377) | Developed Hypertension (no. = 121) | Normotensive (no. = 377) | Developed Hypertension (no. = 121) | |
| No. (%) | 75.7 | 24.3 | 75.7 | 24.3 |
| Males (%) | 40.6 | 36.4 | 40.6 | 36.4 |
| Obesity (%) | 4.6 | 15.7 # | 12.0 | 38.0 # ‡ |
| Increased WC (%) | 15.6 | 30.8 # | 31.3 ‡ | 57.9 # ‡ |
| Dyslipidemia treatment (%) | 0.8 | 6.6 # | 8.0 ‡ | 24.6 # ‡ |
| Diabetes treatment (%) | 0.3 | 1.7 | 1.9 | 8.3 # |
| Current smoker (%) | 13.8 | 15.7 | 12.2 | 8.3 |
| Age (years) | 36 ± 13 | 45 ± 14 # | 48 ± 12 ‡ | 55 ± 13 # ‡ |
| SBP (mmHg) | 114.7 ± 10.8 | 124.5 ± 8.3 # | 114.6 ± 11.8 ‡ | 129.7 ± 17.4 # ‡ |
| DBP (mmHg) | 72.7 ± 7.9 | 79.1 ± 6.2 # | 68.8 ± 7.9 ‡ | 76.5 ± 11.3 # ‡ |
| Fasting glucose (mg/dL) | 87.5 ± 19.6 | 91.4 ± 19.4 | 87.9 ± 19.4 | 92.5 ± 18.1 # |
| BMI (kg/m2) | 22.7 ± 3.7 | 25.7 ± 4.4 # | 25.1 ± 4.2 ‡ | 29.0 ± 5.5 # ‡ |
| WC (cm) | 82.6 ± 9.8 | 88.9 ± 11.4 # | 89.2 ± 10.0 ‡ | 98.0 ± 12.7 # ‡ |
| WHR | 0.87 ± 0.08 | 0.89 ± 0.07 # | 1.14 ± 4.33 | 0.95 ± 0.09 ‡ |
| WtHR | 0.50 ± 0.06 | 0.54 ± 0.07 # | 0.54 ± 0.07 ‡ | 0.60 ± 0.08 # ‡ |
| VAI | 3.64 ± 2.32 | 4.42 ± 2.23 # | 5.97 ± 4.63 ‡ | 7.63 ± 4.69 # ‡ |
| BAI | 26.75 ± 5.49 | 29.86 ± 5.98 # | 28.63 ± 6.6 ‡ | 32.12 ± 5.7 # ‡ |
| BRI | 3.40 ± 1.21 | 4.34 ± 1.56 # | 4.27 ± 1.40 ‡ | 5.65 ± 1.78 # ‡ |
| TyG | 4.55 ± 0.26 | 4.65 ± 0.25 # | 4.68 ± 0.27 ‡ | 4.79 ± 0.25 # ‡ |
| Total cholesterol (mg/dL) | 175.7 ± 44.5 | 177.5 ± 41.2 | 202.2 ± 42.3 ‡ | 196.8 ± 37.2 ‡ |
| Triglycerides (mg/dL) | 117.5 ± 60.9 | 135.7 ± 74.37 # | 153.2 ± 83.7 ‡ | 176.56 ± 86.9 # ‡ |
| LDL‐c (mg/dL) | 95.1 ± 41.3 | 97.4 ± 39.13 | 122.8 ± 36.0 ‡ | 118.7 ± 29.1 ‡ |
| HDL‐c (mg/dL) | 57.0 ± 15.4 | 53.5 ± 14.6 # | 47.8 ± 12.0 ‡ | 42.8 ± 11.2 # ‡ |
Continuous data are expressed as mean ± standard deviation. Categorical data are expressed as percentage.
Abbreviations: BMI, body mass index; WC, waist circumference; WHR, waist‐to‐hip ratio; WtHR, waist‐to‐height ratio; VAI, visceral adiposity index; BAI, body adiposity index; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL‐c, low density lipoprotein; HDL‐c, high‐density lipoprotein; BRI, Body roundness index.
Represents p < .05 comparing normotensive vs patients who developed hypertension at the same time‐point (ie, baseline or follow‐up).
Represents p < .05 for intragroup differences comparing follow‐up to baseline values.
First, we used a mixed effects logistic regression model in the overall sample where incident hypertension was the response variable and, sex, age, obesity, and adiposity indexes were covariables resulting in higher AUC for BMI (AUC = 0.752) compared to BAI (AUC = 0.730), WC, and VAI (both with AUC = 0.721). Later, we explored other exploratory covariates in the logistic regression models. The best‐fit model included age, sex, SBP (at baseline), HDL‐c, and BMI (AUC = 0.823), where BMI resulted in an effect size of OR = 1.58 [95% CI 1.23‐2.04]. Further, we explored whether other indexes of body adiposity and obesity could yield better predictive power than BMI (Table 2), however all tested indexes were inferior. The second and third indexes closer to BMI in predictive value were BAI (AUC = 0.818) and BRI (AUC = 0.816).
TABLE 2.
Odds ratio in the binary logistic regression (dependent variable is hypertension)
| Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|
| (Age, Sex, and SBP) | (Age, Sex, SBP, and HDL‐c) | ||||||
| Covariates | OR (95% CI) | AUC | p‐value | OR (95% CI) | AUC | p‐value | |
| Predictors | BMI | 1.57 (1.23‐2.02) | 0.819 | .00035 | 1.58 (1.23‐2.04) | 0.823 | .00039 |
| BAI | 1.40 (1.03‐1.90) | 0.811 | .03342 | 1.41 (1.03‐1.92) | 0.818 | .03341 | |
| BRI | 1.39 (1.08‐1.79) | 0.810 | .01045 | 1.40 (1.08‐1.81) | 0.816 | .01068 | |
| WtHR | 1.39 (1.07‐1.81) | 0.801 | .01380 | 1.39 (1.07‐1.82) | 0.815 | .01487 | |
| WC | 1.03 (1.00‐1.05) | 0.807 | .02644 | 1.03 (1.00‐1.05) | 0.813 | .036968 | |
Abbreviations: OR, Odds Ratio; CI, Confidence Interval; AUC, Area Under Curve; SBP, systolic blood pressure; HDL‐c, high‐density lipoprotein; BMI, body mass index; BAI, body adiposity index; BRI, Body roundness index; WtHR, waist‐to‐height ratio; WC, waist circumference.
4. DISCUSSION
This study is the first performed with a 10‐year follow‐up to determine incident hypertension in Brazil. In our sample, of mostly middle‐aged adults, the incidence of hypertension is approximately 24% in 10 years. We also observed that the best prediction model for incident hypertension includes age, sex, HDL‐c, SBP, and BMI and that BMI remains superior in its predictive ability than other obesity and adiposity indexes.
Hypertension prevalence in the Brazilian population has been previously assessed in several studies, however data are based on population‐based surveys and sometimes representative of cities or states. 2 The estimated prevalence of hypertension from pooled data from different cities in Brazil, in the 2000s, corresponded to 28.7%. 2 The most recent nationwide study (n = 60.202) using self‐reported data estimated the prevalence of hypertension to be of 21.4% above 18 years old. The prevalence increases with age, reaching 55% above 75 years. 3 , 27 Observational studies showed high incidence of hypertension over periods of 5–10‐year follow‐up and one of the reasons for that could be the age increase. 5 Indeed, some studies have estimated the long‐term cumulative incidence of developing hypertension to be unexpectedly high. In the Framingham Heart Study, approximately 90% of adults free of hypertension at age 55 or 65 years developed hypertension during their lifetimes. 5 In this sample of a Brazilian population, incident hypertension was 24.3%, after a 10‐year follow‐up, similar to the study performed at Tujia, a city from China, where in a 9‐year follow‐up, the incidence was 23.4%, 28 and to another study with a sample of North Americans—the Dallas Heart Study—where 25% developed incident hypertension after a follow‐up of 7 years. 29
As expected, in our study, the participants that developed hypertension showed at baseline increased values for adiposity and obesity indexes, as well as for the means of systolic and diastolic blood pressure, TG, and TyG. This has also been reported in other populations such as studies in Thailand 30 and China, 31 , 32 and in some locations in Brazil. 33 , 34 Excessive adiposity, especially abdominal, was previously associated with incident hypertension 12 , 13 , 29 collaborating the present findings. It has been reported that excessive abdominal adiposity and obesity leads to hormonal and inflammatory changes that could lead to alterations in cardiovascular system function and control, contributing to the development of hypertension. 9 , 35 , 36 Therefore, excessive adiposity, especially abdominal, and triglycerides levels are important aspects to consider when planning primary and secondary prevention of hypertension in the Brazilian population. In fact, those are concerns for hypertension prevention once data from the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil) have demonstrated a prevalence of 63.1% excess weight, 61.5% high cholesterol, and 20.3% impaired glucose tolerance. 11 Furthermore, according to the Brazilian Health Ministry, obesity incidence had an increase of 72% in a 13‐year period accounting from 2006 to 2019. 37
Surprisingly, neither diabetes nor glycemia were important cofactors in predicting incident hypertension in our analysis. Those differences may be related to the criteria for diagnosis (adoption of glucose tolerance test, for example), age range of the population, and the design of our study that was not intended to analyze the prevalence of diabetes. Nevertheless, it points out a particular characteristic of the population sample that deserves further investigation.
Recent systematic reviews investigating the association of hypertension and VAI, 14 BRI and ABSI 13 BAI, 38 or other adiposity indexes (BMI, WC, WHR, WHtR). 12 In a systematic review with meta‐analysis, Calderón–García and associates showed that BRI is a good predictor of hypertension for men and women from different populations. 13 Adiposity and anthropometric indexes, in general, are good predictors for hypertension. 12 , 13 , 14 , 38 Deng and associates showed in a systematic review with 38 studies that BMI, WHR, WHtR, and WC, all correlate with incident hypertension, and BMI had the highest prediction ability in adjusted models for the Chinese population assessed in the PURE study. Furthermore, the BMI presented the highest adjusted AUC compared to the other indexes for the overall population, as we also found in our study, although, in the study of Lee and associates (2018) the WHtR presented the highest Odds Ratio. 12 Moliner–Urdiales and associates (2014) have also demonstrated the capability of BMI, BAI, and WHR to predict incident HA (average follow‐up of 9.1 years), with a high Hazard Ratio presented for those with BMI ≥ 30 kg/m2 (2.53 for men, 3.62 for women). 38 In our study, BMI was also the variable with the highest capacity to predict incident hypertension than other indexes that aim to discriminate central adiposity (such as WC, WHR). This may be due to populational characteristics of the study sample that present a more uniform increase in weight (probably with associated increase in percent fat mass) rather than a more centrally distributed adiposity.
Worldwide overweight and obesity are considered a pandemic. 9 In the last years, the elevated BMI have been associated with increased prevalence of hypertension and other cardiovascular diseases as shown by our group and by others. 17 , 28 , 29 , 39 , 40 Hypertension seems to be the most common obesity‐related health problem and visceral obesity seems to be the major associated factor. 12 , 13 , 14 , 15 , 29 Shihab and associates estimate the association of weight and weight change from young adulthood into middle age and through late life (median follow‐up of 46 years) with risk of developing hypertension. 41 Overweight (BMI 25 to < 30) increased risk for incident hypertension (hazard ratio:1.49, 95% CI 1.15‐1.95) and the effect was even larger in obese (BMI ≥ 30) patients (hazard ratio = 2.79, 95% CI 1.30‐6.00) in a model adjusted for time‐dependent characteristics of the population (number of cigarettes smoked, cups of coffee taken, alcohol intake, and physical activity, in addition to parental premature hypertension). Furthermore, the rate of change in BMI increased the risk of incident hypertension in a dose‐response fashion. 41 Lee and associates also demonstrated in a sample from middle‐aged Korean population (aged 40‐69 years) a causal relationship between BMI and hypertension (OR = 1.19, 95% CI: 1.17‐1.21, adjusted for age, sex, geographical area of the population, education, smoking, and alcohol consumption). 42 Recently, Xu and associates (2020) assessing a middle‐aged Chinese sample followed‐up for a median of 12.5 years, demonstrated that, compared with maintaining BMI in the normal range, when BMI changed from normal to overweight there was an increase in the risk for incident hypertension, both for long‐ (HR:1.507, 95% CI: 1.286‐1.767) and short‐term BMI changes (HR: 1.197, 95% CI: 1.019‐1.405). The opposite was true for patients that turned from overweight to normal BMI. Albeit baseline adiposity indexes are highly useful to predict incident hypertension in 10 years, BMI remained the best predictors in our models when compared to other tested proxies. 43
The strength of our study is that it is the first to follow a Brazilian population for 10 years to determine incident hypertension and thoroughly assess the risk factors with direct objective measurements. Nevertheless, there are some limitations such as a population that is restricted to one area of the country, predominantly rural, making it difficult to extrapolate data for the entire nation. Additionally, even though blood pressure measurements were taken in triplicate, there is a chance of “white‐coat hypertension” influencing the data. Despite the baseline sample being about 1712 individuals, in 10‐year follow‐up, the number of participants was reduced to 29% of the initial selection. Additionally, in the baseline, we excluded individuals with blood pressure equal or higher 140/90 mmHg or under the use of medication for hypertension. Then, we need to highlight that these finds can be affected by the reduced sample size. Future studies may test the predictive value of other measurements of body adiposity that may surpass BMI, such as body fat percentage determined by a gold standard method such as dual‐energy X‐ray absorptiometry (DEXA). Nonetheless, although this may be ideal in the research settings, it may not be as easily as BMI to be applicable in clinical assessments.
In summary, our study demonstrated a 24.3% 10‐year incident rate for hypertension in a sample from the Brazilian population. We also show baseline adiposity indexes are highly useful to predict who will develop hypertension in 10 years, and that BMI, albeit the simpler index to be calculated, is the best anthropometric index to predict incident hypertension.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGEMENTS
The authors are grateful to the participants and staff for data collection in the Baependi Heart Study. The current research was supported by grants from São Paulo Research Foundation (FAPESP). This work was supported in part by grant NIH/NHLBI (R01HL141881). ROSA FF received a master's scholarship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES ‐ Coordination for the Improvement of Higher Education Personnel).
Maciel de Oliveira C, França da Rosa F, de Oliveira Alvim R, et al. Body mass index is superior to other body adiposity indexes in predicting incident hypertension in a highly admixed sample after 10‐year follow‐up: The Baependi Heart Study. J Clin Hypertens. 2022;24:731–737. 10.1111/jch.14480
Camila Maciel de Oliveira and Francielle França da Rosa contributed equally to this work.
REFERENCES
- 1. GBD 2017 Risk Factor Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Stu. Lancet (London, England) 2018;392:1923‐1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Picon RV, Fuchs FD, Moreira LB, Riegel G, Fuchs SC. Trends in prevalence of hypertension in Brazil: a systematic review with meta‐analysis. PLoS One. 2012;7:e48255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrade, de Araujo SS, Stopa SR, Brito AS, et al. Prevalência de hipertensão arterial autorreferida na população brasileira: análise da Pesquisa Nacional de Saúde, 2013. Epidemiol Serv Saúde. 2015;24:297‐304. [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Pr. J Am Coll Cardiol. 2018;71:e127‐e248. [DOI] [PubMed] [Google Scholar]
- 5. Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle‐aged women and men: the Framingham Heart Study. JAMA. 2002;287:1003‐1010. [DOI] [PubMed] [Google Scholar]
- 6. Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non‐hypertensive participants in the Framingham Heart Study: a cohort study. Lancet (London, England). 2001;358:1682‐1686. [DOI] [PubMed] [Google Scholar]
- 7. Fuchs FD. Comparação entre medicamentos para tratamento inicial da hipertensão arterial sistêmica. OPAS/OMS ‐ Represent Bras. 2016;1:1‐10. [Google Scholar]
- 8. Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity‐induced hypertension. Hypertens Res. 2010;33:386‐393. [DOI] [PubMed] [Google Scholar]
- 9. Shariq OA, McKenzie TJ. Obesity‐related hypertension: a review of pathophysiology, management, and the role of metabolic surgery. Gland Surg. 2020;9:80‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson PWF, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867‐1872. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt MI, Duncan BB, Mill JG, et al. Cohort profile: longitudinal Study of Adult Health (ELSA‐Brasil). Int J Epidemiol. 2015;44:68‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng G, Yin L, Liu W, et al. Associations of anthropometric adiposity indexes with hypertension risk: a systematic review and meta‐analysis including PURE‐China. Medicine. 2018;97:e13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calderón‐García JF, Roncero‐Martín R, Rico‐Martín S, et al. Effectiveness of Body Roundness Index (BRI) and a Body Shape Index (ABSI) in predicting hypertension: a systematic review and meta‐analysis of observational studies. Int J Environ Res Public Health. 2021;18:11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leite NN, Cota BC, Gotine AREM, Rocha DMUP, Pereira PF, Hermsdorff HHM. Visceral adiposity index is positively associated with blood pressure: a systematic review. Obes Res Clin Pract. 2021;15:546‐556. Elsevier. [DOI] [PubMed] [Google Scholar]
- 15. Saliba LJ, Maffett S. Hypertensive heart disease and obesity: a review. Heart Fail Clin. 2019;15(4):509‐517. [DOI] [PubMed] [Google Scholar]
- 16. Lambert EA, Esler MD, Schlaich MP, Dixon J, Eikelis N, Lambert GW. Obesity‐associated organ damage and sympathetic nervous activity. Hypertension. 2019;73(6):1150‐1159. [DOI] [PubMed] [Google Scholar]
- 17. de Oliveira CM, Ulbrich AZ, Neves FS, et al. Association between anthropometric indicators of adiposity and hypertensionin a Brazilian population: baependi Heart Study. PLoS ONE. 2017;12(10):e0185225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Oliveira CM, Pereira AC, de Andrade M, Soler JM, Krieger JE. Heritability of cardiovascular risk factors in a Brazilian population: baependi Heart Study. BMC Med Genet. 2008;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergman RN, Stefanovski D, Buchanan TA, et al. A better index of body adiposity. Obesity (Silver Spring). 2011;19:1083‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amato MC, Giordano C, Galia M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7:e39504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomas DM, Bredlau C, Bosy‐Westphal A, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity (Silver Spring). 2013;21:2264‐2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride‐glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim Care Diabetes. 2020;14:161‐167. [DOI] [PubMed] [Google Scholar]
- 25. Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey. PLoS One. 2019;14:e0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barroso WKS, Rodrigues CIS, Bartolotto LA, et al. Diretrizes brasileiras de hipertensão arterial‐2020. Arquiv Brasil Cardiol. 2020;116:0‐0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brasil S de V em S. Pesquisa Nacional de Saúde ‐ 2013 ‐ Módulo de Doenças Crônicas. Hipertensão; 2017. http://tabnet.datasus.gov.br/cgi/deftohtm.exe?pns/pnsqa.def. Accessed 8 December 2020. [Google Scholar]
- 28. Liu X, Liu C, Schenck H, Yi X, Wang H, Shi X. The risk factors of 9‐year follow‐up on hypertension in middle‐aged people in tujia‐nationality settlement of china. J Hum Hypertens. 2017;31:838‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chandra A, Neeland IJ, Berry JD, et al. The relationship of body mass and fat distribution with incident hypertension: observations from the dallas heart study. J Am Coll Cardiol. 2014;64:997‐1002. [DOI] [PubMed] [Google Scholar]
- 30. Nguyen Ngoc H, Kriengsinyos W, Rojroongwasinkul N, Aekplakorn W. Association of adiposity indices with hypertension in middle‐aged and elderly thai population: national health examination survey 2009 (NHES‐IV). J Cardiovasc Dev Dis. 2019;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang H, Chen Y, Sun G, Jia P, Qian H, Sun Y. Validity of cardiometabolic index, lipid accumulation product, and body adiposity index in predicting the risk of hypertension in chinese population. Postgrad Med. 2018;130:325‐333. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Z, Shi D, Zhang Q, Wang S, Liu K, Meng Q, et al. Visceral adiposity index (VAI), a powerful predictor of incident hypertension in prehypertensives. Intern Emerg Med. 2018;13:509‐516. [DOI] [PubMed] [Google Scholar]
- 33. Dutra MT, Reis DBV, Martins KG, Gadelha AB. Comparative evaluation of adiposity indices as predictors of hypertension among Brazilian adults. Int J Hypertens. 2018;2018:8396570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gus M, Fuchs SC, Moreira LB, et al. Association between different measurements of obesity and the incidence of hypertension. Am J Hypertens. 2004;17:50‐53. [DOI] [PubMed] [Google Scholar]
- 35. Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res. 2017;122:1‐7. [DOI] [PubMed] [Google Scholar]
- 36. Després J‐P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881‐887. [DOI] [PubMed] [Google Scholar]
- 37. Ministério da Saúde . Diabetes, hipertensão e obesidade avançam entre os brasileiros ‐ Notícia ‐ UNA‐SUS. Ministério da Saúde; 2020. https://www.unasus.gov.br/noticia/diabetes‐hipertensao‐e‐obesidade‐avancam‐entre‐os‐brasileiros [Google Scholar]
- 38. Moliner‐Urdiales D, Artero EG, Sui X, España‐Romero V, Lee DC, Blair SN. Body adiposity index and incident hypertension: the Aerobics Center Longitudinal Study. Nutr Metab Cardiovasc Dis. 2014;24:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sheu WH‐H, Chuang S‐Y, Lee W‐J, Tsai S‐T, Chou P, Chen C‐H. Predictors of incident diabetes, metabolic syndrome in middle‐aged adults: a 10‐year follow‐up study from Kinmen, Taiwan. Diabetes Res Clin Pract. 2006;74:162‐168. [DOI] [PubMed] [Google Scholar]
- 40. Crump C, Sundquist J, Winkleby MA, Sundquist K. Interactive effects of physical fitness and body mass index on the risk of hypertension. JAMA Intern Med. 2016;176:210‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shihab HM, Meoni LA, Chu AY, et al. Body mass index and risk of incident hypertension over the life course: the Johns Hopkins Precursors Study. Circulation. 2012;126(25):2983‐2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee MR, Lim YH, Hong YC. Causal association of body mass index with hypertension using a Mendelian randomization design. Medicine (Baltimore). 2018;97(30):e11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu J, Guo R, Xie Y, et al. Effects of long‐ and short‐term body mass index changes on incident hypertension are different. Nutrition. 2020;74:110755. [DOI] [PubMed] [Google Scholar]


