Abstract
Background
The extent to which very young children contribute to the transmission of SARS-CoV-2 is unclear. We aimed to estimate the seroprevalence of antibodies against SARS-CoV-2 in daycare centres that remained open for key workers' children during a nationwide lockdown in France.
Methods
Children and staff who attended one of 22 daycare centres during a nationwide lockdown in France (between March 15 and May 9, 2020) were included in this cross-sectional, multicentre, seroprevalence study. Hospital staff not occupationally exposed to patients with COVID-19, or to children, were enrolled in a comparator group. The primary outcome was SARS-CoV-2 seroprevalence in children, daycare centre staff, and the comparator group. The presence of antibodies against SARS-CoV-2 in capillary whole blood was measured with a rapid chromatographic immunoassay. We computed raw prevalence as the percentage of individuals with a positive IgG or IgM test, and used Bayesian smoothing to account for imperfect sensitivity and specificity of the assay. This study is registered with ClinicalTrials.gov, NCT04413968.
Findings
Between June 4 and July 3, 2020, we enrolled 327 children (mean age 1·9 [SD 0·9] years; range 5 months to 4·4 years), 197 daycare centre staff (mean age 40 [12] years), and 164 adults in the comparator group (42 [12] years). Positive serological tests were observed for 14 children (raw seroprevalence 4·3%; 95% CI 2·6–7·1) and 14 daycare centre staff (7·7%; 4·2–11·6). After accounting for imperfect sensitivity and specificity of the assay, we estimated that 3·7% (95% credible interval [95% CrI] 1·3–6·8) of the children and 6·8% (3·2–11·5) of daycare centre staff had SARS-CoV-2 infection. The comparator group fared similarly to the daycare centre staff; nine participants had a positive serological test (raw seroprevalence 5·5%; 95% CI 2·9–10·1), leading to a seroprevalence of 5·0% (95% CrI 1·6–9·8) after accounting for assay characteristics. An exploratory analysis suggested that seropositive children were more likely than seronegative children to have been exposed to an adult household member with laboratory-confirmed COVID-19 (six [43%] of 14 vs 19 [6%] of 307; relative risk 7·1 [95% CI 2·2–22·4]).
Interpretation
According to serological test results, the proportion of young children in our sample with SARS-CoV-2 infection was low. Intrafamily transmission seemed more plausible than transmission within daycare centres. Further epidemiological studies are needed to confirm this exploratory hypothesis.
Funding
Assistance Publique—Hôpitaux de Paris; Mairie de Paris, Conseil Départemental de Seine Saint Denis.
Translations
For the French translation of the abstract see Supplementary Materials section.
Introduction
Although SARS-CoV-2 infects people of all ages, the available data published so far show that children (ie, individuals aged ≤17 years) account for only 1–8% of laboratory-confirmed cases of COVID-191, 2, 3, 4, 5 and for 2–4% of patients admitted to hospital.5, 6 Furthermore, children rarely require hospital admission,4, 7 admission to an intensive care unit, oxygen therapy, or ventilation.8, 9
The burden of infection among children is therefore difficult to assess if testing is focused on symptomatic patients or those admitted to hospital. Population screening studies that estimate seroprevalence (indicating previous infection) are therefore useful in this context. To the best of our knowledge, few studies have estimated SARS-CoV-2 seroprevalence in children, and only four included a subgroup of preschool children (aged 0–3 years).10, 11, 12, 13 None of the published studies has focused on seroprevalence in daycare centres.
The extent to which children (symptomatic or not) contribute to the transmission of SARS-CoV-2 remains to be determined. At the beginning of the COVID-19 pandemic, children were considered to be potential vectors of transmission because they are known to contribute strongly to the spread of respiratory diseases such as seasonal influenza.14, 15 This premise led to the adoption of preventive measures (including school closures) in many countries. The results of several epidemiological studies suggested that children were not the primary drivers of COVID-19 in their community;16, 17, 18 they were rarely the index case in their households;19 they are less likely than adults to have SARS-CoV-2 infection, with an odds ratio of 0·56 for being an infected contact (compared with adults) in a meta-analysis;20 and the rates of SARS-CoV-2 transmission are low in schools and child-care settings.21 However, the meta-analysis also indicated that the population-level evidence showing that children and adolescents have a lesser role than adults in the transmission of SARS-CoV-2 is still weak.20
Research in context.
Evidence before this study
We searched PubMed and the preprint server medRxiv on Dec 7, 2020, using the terms [“COVID-19” or “SARS-CoV-2”], [“child*” or “pediatric*”], and [“seroprevalence” or “sero-prevalence” or (“prevalence” and “antibodies”) or “seroepidemiology”] for population-screening studies describing the seroprevalence of SARS-CoV-2 infection that included children. We applied no language limitations. References cited by systematic reviews were also checked. After an assessment of the abstracts, 28 relevant publications on population-based studies were identified. Four of the studies had estimated seroprevalence in children aged 5 years or younger. In a national population-based study of 35 883 households in Spain (April, 2020), seroprevalence according to a point-of-care test was 1·1% (95% CI 0·3–3·5) in infants younger than 1 year of age, and 2·1% (1·3–3·4) in children aged 1–4 years. Three other studies found the estimated seroprevalence in children younger than 4 or 5 years of age to be 1·6% (95% CI 0·5–3·0) in Brazil (May, 2020), 0% in the USA (May, 2020), and 20% (13–29) in Iran (April, 2020). None of these studies focused on daycare centres. However, the safety of reopening daycare centres and schools, and the role of young children in the spread of COVID-19, is subject to debate. We completed our search on Dec 7, 2020, by using the terms [“COVID-19” or “SARS-CoV-2”] and [“daycare” or “nursery”] to identify publications describing SARS-CoV-2 infection in daycare centres. We identified two publications. The first was a SARS-CoV-2 carriage study done in Belgium. The researchers did not find any SARS-CoV-2-positive samples between Feb 29 and March 18, 2020. The second was a description of the characteristics of a cluster associated with a single nursery in Poland. The overall PCR positivity rate of the cluster was 27%.
Added value of this study
Our cross-sectional, multicentre, seroprevalence study was done at the end of the first wave of the COVID-19 pandemic in France, in June, 2020, 4–8 weeks after the end of the first lockdown. To the best of our knowledge, this was the first multicentre seroprevalence study to focus on preschool-age children in daycare centres. Estimating seroprevalence in very young children and staff attending daycare centres that remained open during a nationwide lockdown in France might help to understand the extent to which very young children contribute to the spread of SARS-CoV-2. Based on serological results, we found that the proportion of children with SARS-CoV-2 seropositivity was low. In an exploratory analysis, the seroprevalence among daycare centre staff did not differ from that observed in a comparator group of adult hospital workers not exposed to children. The main factor associated with SARS-CoV-2 seropositivity in children was contact with an adult household member with laboratory-confirmed COVID-19.
Implications of all the available evidence
On the basis of these findings, there is no evidence for daycare centres being major foci of viral contagion. Further sero-epidemiological studies are needed to determine the incidence or prevalence, or both, of SARS-CoV-2 infection among children, and to assess the role that children might have in SARS-CoV-2 transmission.
In France, a nationwide lockdown was enforced from March 17 to May 11, 2020, to reduce the burden of COVID-19 on the health-care system. Most daycare centres and all schools were closed during this period. The small number of daycare centres that remained open were for children whose parents had to work during the COVID-19 crisis (ie, health-care professionals and other essential workers). Special precautions were adopted in these daycare centres: use of face masks by the staff; smaller, defined groups of children and staff; systematic measurement of body temperatures; exclusion of children who became feverish or ill; and reinforced hygiene and distancing measures.
We aimed to estimate the seroprevalence of SARS-CoV-2 antibodies in children and staff in the daycare centres that remained open during the lockdown in the Paris region (the most affected region in France) and in two other French cities with a lower incidence (Rouen in Normandy and Annecy in the Alps).
Methods
Study design and participants
We did a cross-sectional, multicentre, seroprevalence study in 22 daycare centres located in the Paris region (n=20) and in the French cities of Annecy (n=1) and Rouen (n=1) in June 2020, 4–8 weeks after the end of France's first nationwide lockdown. The daycare centres were operated by a local authority or a public-sector hospital. Children attending one of the 22 daycare centres during all or part of the nationwide lockdown (from March 15 to May 9, 2020) were eligible for inclusion, as were the staff who worked in these daycare centres during the same period. In each centre, we invited all staff and all parents of enrolled children to participate in the study. Recruitment was stopped after the planned number of participants had been included.
The comparator group (for the daycare centre staff) comprised hospital staff who kept working during the lockdown, were not occupationally exposed to infants, and were not directly exposed to patients with COVID-19. To this end, we recruited a sample of laboratory and administrative staff from six hospitals in the Paris region (n=150), Rouen (n=6), and Annecy (n=8).
The study protocol was approved by an independent ethics committee (CPP IDF III, Paris, France; reference: 2020-AO1540–39). Daycare workers, adults in the comparator group, and children's parents were given information about the study's goals and procedures, and provided their written consent to participate in the study.
This study is registered with ClinicalTrials.gov, NCT04413968.
Outcomes
The primary outcome was SARS-CoV-2 seroprevalence in the three study groups. Secondary outcomes were the proportions of children with a positive nasopharyngeal swab or stool swab in a SARS-CoV-2 RT-PCR test. Exploratory outcomes were factors associated with SARS-CoV-2 infection in the children or the adults (sex, age, medical history, history of symptoms and RT-PCR testing during the lockdown, composition of the family, contact with confirmed or suspected COVID-19 cases, number of days of attendance or work, occupations of children's parents, occupation of adult participants' partners, and the serological status of parents).
Procedures
Eligible participants were enrolled between June 4 and July 3, 2020. An electronic case report form was completed on the day of inclusion. Data on sociodemographic variables, occupation, any personal history of infection, contact with confirmed or suspected COVID-19 cases, clinical symptoms and signs, and previous nasopharyngeal-swab RT-PCR results (if available) were recorded. Data on the general characteristics of the daycare centres were also recorded (geographical location, number of children attending during the lockdown, number of staff, and the number of laboratory-confirmed cases among the children and staff).
Paediatricians collected capillary whole blood specimens (fingersticks) from all participants (ie, children and adults) for testing with a rapid chromatographic immunoassay (Biosynex COVID-19 Ag BSS; Biosynex, Illkirch-Graffenstaden, France) that qualitatively detects IgG and IgM antibodies against SARS-CoV-2. The test had been approved by the French national health authorities. According to the manufacturer's specifications, the sensitivity of the diagnostic test is 91·8% (95% CI 83·8–96·6) and its specificity is 99·2% (97·7–99·8). Tests were considered to be valid only if the control line was present. Positive and negative serologies were defined respectively as the presence and absence of IgM or IgG, or both. The result was given to the parents as soon as it was available.
For detection of SARS-CoV-2 via RT-PCR, a nasopharyngeal swab and a stool swab were obtained from those children whose parents gave their consent for testing. Nasopharyngeal samples were collected with small swabs suitable for children and stored in transport buffer for molecular testing. Stool samples were collected with a swab directly from the children's nappies or using an anal swab and were then conserved in transport buffer. Samples were stored at 4°C if testing was scheduled in the following 1–3 days or at −80°C if testing was scheduled at a later date. The SARS-CoV-2 RT-PCR test was done either locally, with various techniques validated by the French national health authorities and applied on a routine basis for hospital samples (n=248), or as part of a centralised procedure in a hospital laboratory (Avicenne Hospital, Bobigny, France [n=221]; with the Abbott RealTime SARS-CoV-2 assay on a m2000 device [Abbott, Rungis, France]), according to the manufacturer's instructions. In line with international guidelines, all techniques used in the present study detected at least two specific targets for SARS-CoV-2 strains and featured an internal quality control for the extraction and amplification steps. If an invalid result was obtained, stool samples were diluted five-fold (to remove potential polymerase inhibitors) and retested.
For the children's parents and the adult participants' partners, we defined three risk classes for occupational exposure to SARS-CoV-2: health-care professionals working in dedicated COVID-19 units, health-care professionals not working in dedicated COVID-19 units, and those in other occupations.
All parents who worked in a hospital underwent serological testing (an ELISA based on detection of IgG or total Ig antibodies against SARS-CoV-2) in May and June, 2020, as part of a local campaign. The results were collected retrospectively in August, 2020.
Statistical analysis
According to modelling studies, the estimated cumulative incidence of SARS-CoV-2 infection in the general population in the Paris region at the time of the present study was 10%.22 We calculated that the inclusion of 150 participants would enable detection of an increase in seroprevalence (relative to the general population) of 75% (ie, a seroprevalence of 17·5%) and inclusion of 320 participants would enable detection of a 50% increase in seroprevalence (ie, a seroprevalence of 15%), with a power of 80%. For feasibility reasons, we decided to include 320 children, 150 daycare centre staff, and 150 adult comparators. Although the sample size was not computed for this reason, we calculated that a sample size of 150 adults would enable detection of a 100% increase in seroprevalence for daycare centre staff (relative to adult comparators), with a power of 70%.
The raw seroprevalence rate was computed as the percentage of tested participants with IgG or IgM antibodies against SARS-CoV-2. We used Bayesian smoothing to account for imperfect sensitivity and specificity.23
In an exploratory analysis, we used χ2 test, Fisher's exact test, or Wilcoxon's test to compare participants' characteristics as a function of their serological status. We compared seronegative children to seropositive children as a function of the parents' serological status. The relative risk (RR) of a positive serology in children was computed according to whether or not they had been in contact with seropositive adults. We used logistic regression to compute the odds ratios (ORs) of occupational status (daycare workers relative to other occupations) after adjustment for age, sex, and contact with a known COVID-19 case. The threshold for statistical significance was set to p values less than 0·05. All tests were two-sided. Statistical analyses were done with R software, version 4.0.
Role of the funding source
The funding bodies were not involved in study design, data collection, data analysis, or data interpretation, in the writing of the report, or in the decision to submit the manuscript for publication.
Results
22 daycare centres participated in the study: 12 (56%) were operated by a public-sector hospital and ten (44%) were operated by a local council. During the lockdown, the number of children attending each centre per day ranged from eight to 56 (median 24 [IQR 23–28]).
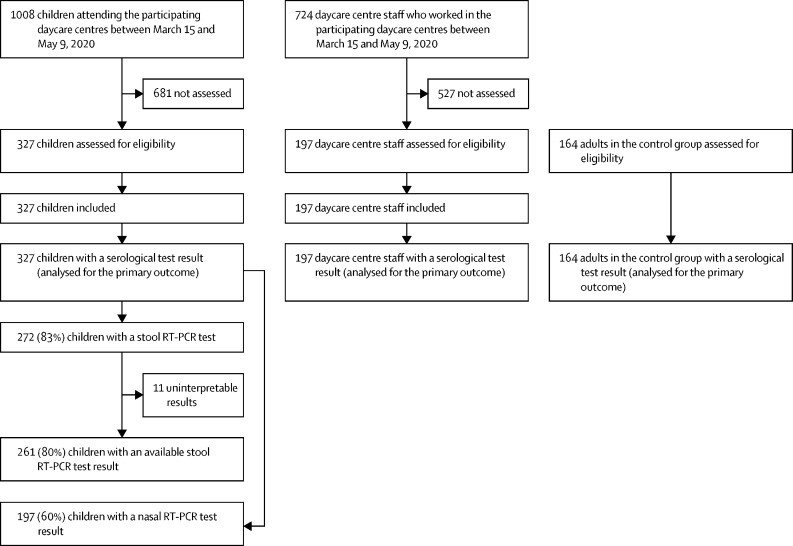
1008 children attending the 22 daycare centres between March 15 and May 9, 2020, were eligible for inclusion in this study; in accordance with the sample size requirements, we included the first 327 (32%) children whose parents consented to participate in the study (figure ). The mean age of the children was 1·9 (SD 0·9) years (range 5 months to 4·4 years), and 167 (51%) of the 327 children were male.
Figure.
Study flowchart
The 197 daycare centre staff members (195 [99%] women) came from 19 daycare centres. The mean age was 40 (SD 12) years.
14 of 327 children and 14 of 197 daycare centre staff were seropositive, resulting in estimated raw seroprevalence rates of 4·3% (95% CI 2·6–7·1) among children and 7·7% (4·2–11·6) among staff (table 1 ). After adjustment for imperfect test sensitivity and specificity, seroprevalence rates were 3·7% (95% credible interval [95% CrI] 1·3–6·8) among children and 6·8% (3·2–11·5) among daycare centre staff. None of the 197 nasal swabs and none of the 261 stool swabs from the children was positive for SARS-CoV-2 in an RT-PCR test.
Table 1.
Estimated prevalence of SARS-CoV-2 infection in the study groups
| Children attending daycare centres (n=327) | Daycare centre staff (n=197) | Comparator group (n=164) | |
|---|---|---|---|
| Serological assay positive for Ig antibodies | 14 (4·3%) | 14 (7·1%) | 9 (5·5%) |
| Serological assay positive for IgG antibodies | 13 (3·9%) | 14 (7·1%) | 8 (4·9%) |
| Serological assay positive for IgM antibodies | 2 (0·6%) | 5 (2·5%) | 4 (2·4%) |
| Raw seroprevalence (95% CI) | 4·3% (2·6–7·1) | 7·7% (4·2–11·6) | 5·5% (2·9–10·1) |
| Corrected seroprevalence (95% CrI)* | 3·7% (1·3–6·8) | 6·8% (3·2–11·5) | 5·0% (1·6–9·8) |
Data are n (%), unless otherwise indicated. 95% CrI=95% credible interval.
Assuming a sensitivity of 92% and a specificity of 99%.
On inclusion, 243 (77%) of 314 children with data on the presence or absence of symptoms were asymptomatic (appendix 2). Children's characteristics by serological status are summarised in table 2 . Contact with a confirmed adult household case of COVID-19 during the lockdown was more frequent in seropositive children than in seronegative children (RR 7·1 [95% CI 2·2–22·4]). There were no other large differences between seropositive and seronegative groups. Importantly, the presence of clinical signs during lockdown and exclusion from the daycare centre were not associated with seropositivity. The 14 seropositive children came from 13 different daycare centres. In the centre with two seropositive cases, the children were attending different sections. Six (43%) of the 14 seropositive children were asymptomatic during the lockdown, and eight (57%) had minor or mild signs of infection (fever, rhinitis, cough, or abdominal signs, or a combination of the above). Serological test results for at least one parent were available for 170 children (table 2), and 28 (17%) of these children had at least one seropositive parent. Of the eight children with a positive serology, symptoms during the lockdown, and seropositive parents, five presented symptoms 4–10 days after their parents did.
Table 2.
Characteristics of the screened children, by serological status
| Seronegative children (n=313) | Seropositive children (n=14) | Relative risk or mean difference (95% CI) | ||
|---|---|---|---|---|
| Sex | ||||
| Female | 155/313 (49%) | 5/14 (36%) | .. | |
| Male | 158/313 (51%) | 9/14 (64%) | 1·3 (0·4 to 3·9) | |
| Age, years | 1·9 (0·9) | 1·7 (0·8) | −0·2 (−0·6 to 0·2) | |
| History of recurrent bronchiolitis or wheezing | 86/312 (28%) | 3/14 (21%) | 0·8 (0·2 to 2·9) | |
| Gestational age at birth, weeks | 39 (1·7) | 39 (1·9) | 0 (−1 to 1) | |
| Birthweight, Z score | −0·05 (1·04) | −0·07 (0·91) | 0 (−0·5 to 0·5) | |
| Ability to walk | 221/311 (73%) | 10 (71%) | 1·0 (0·3 to 3·2) | |
| Attendance at another type of care facility | 61/312 (20%) | 2/14 (14%) | 0·7 (0·2 to 3·4) | |
| Number of adults at home | 2·0 (0·6) | 2·1 (0·6) | 0·1 (−0·2 to 0·4) | |
| Number of children at home, including the study participant | 1·8 (0·8) | 1·5 (0·7) | −0·3 (−0·7 to 0·1) | |
| History of fever (body temperature >38°C) during lockdown* | 70/292 (24%) | 5/14 (36%) | 1·5 (0·5 to 4·5) | |
| History of respiratory signs (dyspnoea, cough, rhinitis, otitis, or conjunctivitis) during lockdown* | 96/291 (33%) | 6/12 (50%) | 1·4 (0·5 to 4·1) | |
| History of abdominal signs (diarrhoea, vomiting, or abdominal pain) during lockdown* | 57/300 (19%) | 2/13 (15%) | 0·8 (0·2 to 3·6) | |
| Exclusion from the daycare centre due to clinical signs during lockdown | 49/311 (16%) | 2 (14%) | 0·9 (0·2 to 4·2) | |
| RT-PCR testing of a nasal swab during lockdown* | 15/313 (5%) | 1 (7%) | 1·5 (0·2 to 12·2) | |
| Positive RT-PCR test during lockdown* | 0 | 0 | .. | |
| Contact with a confirmed case of COVID-19 during lockdown* | 38/307 (12%) | 6/14 (43%) | 3·5 (1·2 to 10·7) | |
| At least one child from the daycare centre | 7/307 (2%) | 1/14 (7%) | 3·2 (0·4 to 27·9) | |
| At least one daycare centre staff member | 7/307 (2%) | 1/14 (7%) | 3·2 (0·4 to 27·9) | |
| At least one other child living with the child | 1/307 (<1%) | 0 | 0 | |
| At least one adult living with the child | 19/307 (6%) | 6/14 (43%) | 7·1 (2·2 to 22·4) | |
| Mean number of days of attendance at a daycare centre during lockdown* | 20 (12) | 19 (12) | −1 (−7 to 5) | |
| Attendance at a daycare centre operated by a hospital† | 250/313 (80%) | 9/14 (64%) | 0·8 (0·3 to 2·5) | |
| Mean number of children attending the centre per day | 29 (11) | 25 (12) | −4 (−10·4 to 2·4) | |
| Parents' occupation | ||||
| At least one health-care worker in a dedicated COVID-19 unit | 94/313 (30%) | 3/14 (21%) | 0·7 (0·2 to 2·6) | |
| At least one health-care worker in a non-COVID-19 unit | 167/313 (53%) | 8/14 (58%) | 1·1 (0·4 to 3·2) | |
| Other occupations | 52/313 (17%) | 3/14 (21%) | 1·3 (0·3 to 4·8) | |
| Serological testing of parents | ||||
| At least one seropositive parent‡ (n=170) | 22/149 (14%) | 6/11 (55%) | 6·1 (1·9 to 19·1) | |
| Serological testing of daycare centre staff§ | ||||
| At least one seropositive staff member (n=312) | 133/299 (44%) | 8/13 (62%) | 1·9 (0·7 to 5·8) | |
Data are n/N (%), or mean (SD), unless otherwise indicated. Missing data for seronegative children: gestational age at birth (n=6), birthweight Z score (n=7), and number of days of daycare centre attendance (n=4).
Between March 15 and May 9, 2020.
Daycare centres not operated by a hospital were operated by a local authority.
SARS-CoV-2 serological status retrospectively determined in an ELISA (based on detection of IgG or total immunoglobulin).
Biosynex COVID-19 BSS test (Biosynex, Illkirch-Graffenstaden, France).
Seropositive children were more likely than seronegative children to have at least one seropositive parent (six [55%] of 11 vs 22 [14%] of 149; RR 6·1 [95% CI 1·9– 19·1]). Children attending a daycare centre with at least one seropositive staff member had a slightly increased risk of being seropositive (RR 1·9 [95% CI 0·7–5·8]; table 2).
On inclusion, none of the 185 daycare centre staff with data on the presence or absence of symptoms was feverish, and 40 (22%) of 185 reported symptoms (appendix 2). There was no increase in the risk of contracting COVID-19 following exposure to a child with laboratory-confirmed COVID-19, but the relative risk following contact with an adult COVID-19 case in the household was large (RR 13·1 [95% CI 0·8–221·1]; table 3 ). The 14 seropositive daycare centre staff came from eight different daycare centres. There were two centres with three seropositive staff members.
Table 3.
Comparison of daycare centre staff and the comparator group
|
Daycare centre staff |
Comparator group |
||||||
|---|---|---|---|---|---|---|---|
| Seronegative individuals (n=183) | Seropositive individuals (n=14) | Relative risk or mean difference (95% CI) | Seronegative individuals (n=155) | Seropositive individuals (n=9) | Relative risk or mean difference (95% CI) | ||
| Sex | |||||||
| Female | 181/183 (99%) | 14/14 (100%) | .. | 121/155 (78%) | 6/9 (67%) | .. | |
| Male | 2/183 (1%) | 0 | 0 | 34/155 (22%) | 3/9 (33%) | 1·5 (0·4 to 6·4) | |
| Age, years | 41 (12) | 41 (15) | 0·0 (−8·0 to 8·0) | 43 (12) | 44 (10) | 1·0 (−5·8 to 7·8) | |
| Number of adults at home, including the study participant | 2·4 (1·2) | 2·0 (0·9) | −0·4 (−0·9 to 0·1) | 2·2 (1·0) | 2·0 (1·0) | −0·2 (−0·9 to 0·5) | |
| Number of children at home | 0·9 (1·1) | 0·8 (1·1) | −0·1 (−0·7 to 0·5) | 1·0 (1·2) | 1·4 (1·1) | 0·4 (−0·3 to 1·1) | |
| History of fever (body temperature >38°C) during lockdown* | 18/180 (10%) | 4/14 (29%) | 2·9 (0·8 to 10·2) | 7/153 (5%) | 1/9 (11%) | 2·5 (0·3 to 22·5) | |
| History of respiratory signs (dyspnoea, cough, rhinitis, otitis, or conjunctivitis) during lockdown* | 61/178 (34%) | 5/13 (39%) | 1·1 (0·3 to 3·3) | 32/144 (22%) | 4/8 (50%) | 2·2 (0·5 to 8·5) | |
| History of abdominal signs (diarrhoea, vomiting, or abdominal pain) during lockdown* | 26/177 (15%) | 5/13 (39%) | 2·5 (0·8 to 8·1) | 19/151 (13%) | 3/9 (33%) | 2·7 (0·6 to 11·8) | |
| History of other signs | 106/183 (58%) | 8/14 (57%) | 1·0 (0·3 to 3·0) | 66/155 (43%) | 8/9 (89%) | 2·1 (0·3 to 17·1) | |
| Loss of appetite | 12/183 (7%) | 5/14 (36%) | 5·4 (1·6 to 18·8) | 8/155 (5%) | 2/9 (22%) | 4·3 (0·8 to 24·2) | |
| Skin signs | 14/183 (8%) | 2/14 (14%) | 1·9 (0·4 to 9·2) | 5/155 (3%) | 0 | 0 | |
| Headache | 91/182 (50%) | 8/14 (57%) | 1·1 (0·4 to 3·4) | 45/155 (29%) | 5/9 (56%) | 1·9 (0·5 to 7·5) | |
| Asthenia | 47/182 (26%) | 7/14 (50%) | 1·9 (0·6 to 5·8) | 39/155 (25%) | 6/9 (67%) | 2·6 (0·6 to 11·1) | |
| Myalgia | 15/183 (8%) | 5/14 (36%) | 4·4 (1·3 to 14·7) | 16/155 (10%) | 4/8 (50%) | 4·3 (1·0 to 17·7) | |
| Anosmia | 2/183 (2%) | 4/14 (29%) | 13·1 (2·8 to 60·1) | 4/155 (3%) | 5/9 (56%) | 21·5 (4·1 to 111·8) | |
| Ageusia | 6/183 (3%) | 4/14 (29%) | 8·7 (2·1 to 35·9) | 3/155 (2%) | 6/9 (67%) | 34·4 (5·7 to 207·6) | |
| Chest pain | 7/183 (4%) | 2/14 (14%) | 3·7 (0·7 to 20·0) | 8/155 (5%) | 3/9 (33%) | 6·5 (1·4 to 30·7) | |
| Joint pain | 16/183 (9%) | 5/14 (36%) | 4·1 (1·2 to 13·7) | 12/155 (8%) | 3/9 (33%) | 4·3 (1·0 to 19·4) | |
| RT-PCR testing of a nasal swab during lockdown* | 36/183 (20%) | 3/14 (21%) | 1·1 (0·3 to 4·1) | 33/155 (21%) | 3/9 (33%) | 1·6 (0·4 to 6·6) | |
| Positive RT-PCR test during lockdown* | 2/36 (6%) | 1/3 (33%) | 5·5 (0·5 to 60·5) | 1/33 (3%) | 3/3 (100%) | 32·0 (1 to 945) | |
| Contact with a confirmed case of COVID-19 during lockdown* | 67/183 (37%) | 7/14 (50%) | 1·4 (0·5 to 4·1) | 60/155 (39%) | 6 (67%) | 1·7 (0·4 to 7·1) | |
| At least one child at work | 10/183 (5%) | 0 | 0 | NA | NA | 0 | |
| At least one adult at work | 41/183 (22%) | 1/14 (7%) | 0·3 (0·0 to 2·5) | 51/155 (33%) | 3/9 (33%) | 1 (0·2 to 4·2) | |
| At least one child living with the adult | 0 | 0 | .. | 2/155 (1%) | 1/9 (11%) | 8·6 (0·7 to 105·3) | |
| At least one other adult living with the adult | 1/183 (1%) | 1/14 (7%) | 13·1 (0·8 to 221·1) | 2/155 (1%) | 2/9 (22%) | 17·2 (2·1 to 140·8) | |
| Partner's occupation | |||||||
| Health-care worker in a dedicated COVID-19 unit | 0 | 0 | NA | 1/109 (1%) | 0 | 0 | |
| Health-care worker in a non-COVID-19 unit | 3/122 (3%) | 0 | 0 | 10/109 (9%) | 0 | 0 | |
| Other occupation | 119/122 (97%) | 8/8 (100%) | 1·1 (0·4 to 2·8) | 98/109 (90%) | 7/7 (100%) | 1·1 (0·1 to 20·8) | |
Data are n/N (%), or mean (SD), unless otherwise indicated. Missing data for seronegative individuals (daycare centre staff): number of adults at home (n=2) and number of children at home (n=2). NA=not applicable.
Between March 15 and May 9, 2020.
There were 164 participants (127 [77%] women and 37 [23%] men) in the comparator group, with a mean age of 43 (SD 12) years. Nine participants were seropositive, resulting in a raw seroprevalence of 5·5% (95% CI 2·9–10·1; table 1). On inclusion, one of the 160 participants with data on the presence or absence of symptoms was feverish, and 20 (12%) reported acute symptoms (appendix 2). Seropositive adults in the comparator group were more likely than seronegative adults to have been exposed to another adult living in the household with confirmed COVID-19 (RR 17·2 [95% CI 2·1–140·8]; table 3).
The corrected seroprevalence rate among daycare centre staff was similar to that in the comparator group (6·8% [95% CrI 3·2–11·5] vs 5·0% [1·6–9·8]). After adjustment for age, sex, and contact with a known COVID-19 case, the odds of a positive serological status for occupational status (ie, being a daycare worker) were 1·5 (95% CI 0·6–3·9).
Discussion
Our results highlight the low SARS-CoV-2 seroprevalence rate among a group of young children attending daycare centres during a nationwide lockdown in France. The seroprevalence rate among children was lower than that reported by various investigators for the general population in the same period in the Paris region (10% [95% CI 9–11] in a multicohort study,24 9% [7–11] in a report by the Direction de la Recherche, des Etudes, de l'Evaluation et des Statistiques [DREES],25 and 7% [5–9] in a nationwide serological surveillance study26), while the seroprevalence rate among the adult participants in our study was similar to that in the general population. This finding is in line with previous studies in which the risk of SARS-CoV-2 infection was lower among children than among adults.20, 27 No seroprevalence estimates have been reported for infants (0–3 years of age) in the Paris region. However, the national seroprevalence rate in France among children younger than 9 years (1·6%) was approximately half that seen in adults (3·3%),26 which is in line with our results. Moreover, the seroprevalence rate among daycare centre staff did not differ from that observed in a group of hospital staff who did not have occupational contact with children or COVID-19-positive patients.
The group of children in our study was considered to be at high risk of contracting COVID-19 from household members (primarily their parents) because of their parents' occupations (health-care workers or other essential workers potentially exposed to SARS-CoV-2). Grouping these children together in a daycare centre during the COVID-19 pandemic was necessary but raised fears of accentuated transmission. Although the virus circulated actively during the lockdown, contact with other children and adults was limited to household members, and strict sanitary measures were introduced and enforced in the daycare centres. A protocol was set up for hosting key workers' children in daycare centres. The children were hosted in small, unchanging groups of six to eight infants per room; the same children were looked after by the same daycare worker all week long. Daycare centre staff had to disinfect indoor surfaces, wear a mask all day long, and comply with social distancing measures, particularly during the lunch break. Parents were instructed on how to screen their children for symptoms that would have prohibited access to the daycare centre and parents were themselves not allowed to enter the daycare centre. Children were excluded from the daycare centre if they were symptomatic. Compliance with these guidelines was not easy but our results suggest that the measures were effective in this particular population. Our results also suggest that exposure to children who had SARS-CoV-2 infection did not result in an increased risk of infection among daycare centre staff, compared with occupationally unexposed adults. Most of the adults were asymptomatic or had minor or mild symptoms during the lockdown. An exploratory analysis comparing seronegative and seropositive adults suggested that the seropositive adults had mostly contracted SARS-CoV-2 infection from another household member.
We did not find any evidence of SARS-CoV-2 transmission within daycare centres. By combining PCR testing with serological testing, we were able to evaluate not only SARS-CoV-2 infection at the time of inclusion in the study but also previous exposure to SARS-CoV-2. None of the children who attended a daycare centre for all or part of the lockdown period tested positive for SARS-CoV-2 RNA; hence, none of the children enrolled at these daycare centres had prolonged or asymptomatic carriage. This finding was in line with the low frequency of symptoms at inclusion (ie, on the day when the sample was collected). SARS-CoV-2 RNA was not detected in any of the stool samples or anal swabs, even though it has been suggested that the virus persists for longer in stools than in the nasopharynx.28, 29
Based on the parents' reports, 43% (six of 14) of seropositive children were asymptomatic, and the remaining eight had only minor or mild symptoms. In exploratory analyses, the presence of symptoms (of any type and at any time) did not seem to be associated with children's serological status, and the main factor associated with SARS-CoV-2 seropositivity in a child was contact with adult household members with laboratory-confirmed COVID-19. Contact with siblings or staff in the daycare centre with confirmed COVID-19 (ie, child-to-child or staff-to-child contact) was not associated with SARS-CoV-2 seropositivity in a child. SARS-CoV-2 seropositivity also seems to be associated with contact with a suspected but non-confirmed case of COVID-19 in an adult household member (results not shown), and the presence of at least one seropositive parent. The association appeared to be independent of the parents' level of occupational exposure to SARS-CoV-2. Moreover, we found that the 14 seropositive children were broadly distributed across 13 different centres and that seropositivity among the children was not associated with the duration of exposure (ie, the number of days attending the daycare centre) or the seropositivity of the daycare centre staff. All of these exploratory analyses constitute additional arguments for intrafamilial transmission rather than transmission at the daycare centre. Our results are in line with a previous report of very few cases of secondary SARS-CoV-2 transmission in a primary school setting in France.17 The available data indicate that children mostly contract COVID-19 at home or through contact with other family members.7, 8, 16, 27, 30 However, these data must be interpreted with caution, since the studies were done during different time periods in countries where schools were closed and strict physical distancing was implemented. Our results suggest that even if young children attend a daycare centre, they are more likely to contract COVID-19 at home, from a household member, than at the daycare centre.
Our study had several limitations. First, our method for selecting participants might have biased our results. If confirmed cases had declined to participate, our study would have underestimated the seroprevalence rate. Conversely, if the participating children or adults had more contact with confirmed or suspected cases or were more frequently suspected of having COVID-19, our study would have overestimated the seroprevalence rate. We did not document the characteristics of non-participating children and staff, or the reasons why some parents did not wish their child to participate in the study (eg, fear of an invasive procedure), even though the daycare centre staff were particularly aware of the value of this type of research during and after the COVID-19 pandemic.
Second, we chose to use a rapid fingertip serological test for its ease of performance and rapid response. This approach helped to ensure a relatively high participation rate among the children. However, the rapid test is less sensitive and less specific than a laboratory test. Hence, we adjusted the seroprevalence rate for imperfect sensitivity and specificity. This correction yielded COVID-19 seroprevalence estimates in adults that were slightly lower than those recorded in the general population in the Paris area.24, 25, 26 Lastly, screening children for an ongoing SARS-CoV-2 infection was difficult because 40% of parents did not consent to the collection of a nasopharyngeal swab. A validated, non-invasive, rapid diagnostic test would be particularly useful in this regard.
The study population had a number of particular features. The participating children were primarily at increased risk of intrafamily transmission due to their parents' occupational exposure to SARS-CoV-2. Furthermore, the child to staff ratio was lower during the lockdown than it would be under normal circumstances, so that rigorous sanitary measures could be implemented for the staff, parents, and children. Unfortunately, we did not measure the levels of compliance with these procedures. Consequently, our results cannot be directly extrapolated to other populations and other time periods.
To investigate occupational contacts with children as a source of COVID-19 exposure among daycare centre staff, we tried to account for exposure in places other than the household (eg, travelling to work and the overall hospital environment) by selecting comparators who had similar but distinct occupational backgrounds. Therefore, our comparator group comprised hospital staff with a similar age range to daycare centre staff, who continued working in the hospital during the nationwide lockdown, and who were not in direct contact with patients who had COVID-19. We determined that laboratory technicians and administrative staff fulfilled these requirements. We found similar seroprevalence estimates in daycare centre staff and the comparator group, and there was no evidence of a strong association between SARS-CoV-2 seropositivity and occupational contact with infants. This finding is in line with a previous study done during April and May, 2020, in the USA, showing that daycare workers whose centres remained open did not have a greater risk of contracting COVID-19 than daycare workers whose centres closed.31
The present results indicate that young children are not super-spreaders of SARS-CoV-2 and that daycare centres are not major foci of viral contagion. Intrafamily transmission was more plausible than transmission within daycare centres. Our exploratory comparison of seropositive and seronegative children suggested that clinical signs are not good decision criteria for PCR testing and that the main criterion should be a suspected or laboratory-confirmed COVID-19 case in an adult household member. However, further epidemiological studies are needed to confirm this hypothesis. The detection of a PCR-positive or seropositive child in a daycare centre does not mean that all the children should be tested. Contact tracing and screening tests must start with parents, other adult household members, and staff at the daycare centre. Further sero-epidemiological studies are needed to determine the extent of SARS-CoV-2 infection among children and to define the role of children in viral transmission.
Data sharing
We are prepared to share the study data (in strict compliance with the French legislation on personnel data) upon request to Coralie Bloch-Queyrat (coralie.bloch-queyrat@aphp.fr).
Acknowledgments
Acknowledgments
The study was funded by the Fondation de l'Assistance Publique - Hôpitaux de Paris pour la recherche (Paris, France), by the Paris City Council (Mairie de Paris, Paris, France) and by the Seine Saint Denis county (Conseil Départemental de Seine Saint Denis, Bobigny, France). We thank Lina Innes Skandri, Mohammed Rahaoui, and the Departement de Recherche Clinique (Hôpital Avicenne). We thank Etienne Carbonnelle and Corinne Levy for helpful discussions. We also thank all the children, daycare centre staff, and parents who participated in the study.
Contributors
EL conceived the concept of this study, designed the study, acquired the data, interpreted the results, and drafted the manuscript. LDP conceived and designed the study, interpreted the results and drafted the manuscript. MC, ML, RB, CG, AN, M-AD, DP, EW, EH, CJ, VG, J-RZ and SB acquired the data and critically reviewed and made substantial contributions to the manuscript. RC conceived the study and critically reviewed and made substantial contributions to the manuscript. P-YB designed the study, did the analyses, interpreted the results, and critically reviewed and made substantial contributions to the manuscript. CB-Q designed the study, acquired the data, interpreted the results and critically reviewed and made substantial contributions to the manuscript. CA designed the study, acquired the data, did the analyses, interpreted the results, and drafted and revised the manuscript. All authors have seen, commented on, and approved the final manuscript. The views expressed are those of the authors and not necessarily those of the funding bodies. EL, CA, P-YB, and CB-Q had full access to all of the data in the study, and verified the data. All authors had final responsibility for the decision to submit for publication.
COVIDOCRECHE collaborators
Lorelei Charbonnier, Anaïs Chosidow, Véronique Hentgen, Oscar Lezcano, Nathalie Mestre, Gaelle Pinto Cardoso, Roselyne Masson, Bahia Rabehi, Anne-Sophie Romain, François Vie le Sage, and Xavier Vuillaume.
Declaration of interests
M-AD reports grants and personal fees from GSK, MSD, and Pfizer; and personal fees from Sanofi, outside the submitted work. CJ reports grants from AP-HP, during the conduct of the study; and personal fees from Novalac, Nestle, and Adare, outside the submitted work. RC reports grants and personal fees from GSK, MSD, and Pfizer, outside the submitted work. J-RZ reports grants, personal fees, and non-financial support from MSD, personal fees and non-financial support from Pfizer, and personal fees from Correvio, outside the submitted work. All other authors declare no competing interests.
Contributor Information
COVIDOCRECHE collaborators:
Lorelei Charbonnier, Anais Chosidow, Véronique Hentgen, Oscar Lescano, Nathalie Mestre, Gaelle Pinto Cardoso, Roselyne Masson, Bahia Rabehi, Anne-Sophie Romain, François Vié le Sage, and Xavier Vuillaume
Supplementary Materials
References
- 1.CDC COVID-19 Response Team Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–446. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control . European Centre for Disease Prevention and Control; Stockholm: 2020. COVID-19 in children and the role of school settings in transmission—first update. Dec 23, 2020. [Google Scholar]
- 5.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parri N, Lenge M, Buonsenso D. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med. 2020;383:187–190. doi: 10.1056/NEJMc2007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029–1046. doi: 10.1007/s00431-020-03684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallal PC, Hartwig FP, Horta BL, et al. SARS-CoV-2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. Lancet Glob Health. 2020;8:e1390–e1398. doi: 10.1016/S2214-109X(20)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutton M, Cieslak P, Linder M. Notes from the field: seroprevalence estimates of SARS-CoV-2 infection in convenience sample—Oregon, May 11–June 15, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1100–1101. doi: 10.15585/mmwr.mm6932a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shakiba M, Nazari SSH, Mehrabian F, Rezvani SM, Ghasempour Z, Heidarzadeh A. Seroprevalence of COVID-19 virus infection in Guilan province, Iran. medRxiv. 2020 doi: 10.1101/2020.04.26.20079244. published online May 1. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayasundara K, Soobiah C, Thommes E, Tricco AC, Chit A. Natural attack rate of influenza in unvaccinated children and adults: a meta-regression analysis. BMC Infect Dis. 2014;14:670. doi: 10.1186/s12879-014-0670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson C, Vynnycky E, Hawker J, Olowokure B, Mangtani P. School closures and influenza: systematic review of epidemiological studies. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee B, Raszka WV., Jr COVID-19 transmission and children: the child is not to blame. Pediatrics. 2020;146 doi: 10.1542/peds.2020-004879. [DOI] [PubMed] [Google Scholar]
- 17.Fontanet A, Grant R, Tondeur L, et al. SARS-CoV-2 infection in primary schools in northern France: a retrospective cohort study in an area of high transmission. medRxiv. 2020 doi: 10.1101/2020.06.25.20140178. published online June 29. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danis K, Epaulard O, Bénet T, et al. Cluster of coronavirus disease 2019 (COVID-19) in the French Alps, February 2020. Clin Infect Dis. 2020;71:825–832. doi: 10.1093/cid/ciaa424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Bloxham CJ, Hulme KD, et al. Children are unlikely to have been the primary source of household SARS-CoV-2 infections. medRxiv. 2020 doi: 10.1101/2020.03.26.20044826. published online March 30. (preprint). [DOI] [Google Scholar]
- 20.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.4573. published online Sept 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macartney K, Quinn HE, Pillsbury AJ, et al. Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health. 2020;4:807–816. doi: 10.1016/S2352-4642(20)30251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salje H, Tran Kiem C, Lefrancq N, et al. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369:208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diggle PJ. Estimating prevalence using an imperfect test. Epidemiol Res Int. 2011;2011 [Google Scholar]
- 24.Carrat F, de Lamballerie X, Rahib D, et al. Seroprevalence of SARS-CoV-2 among adults in three regions of France following the lockdown and associated risk factors: a multicohort study. medRxiv. 2020 doi: 10.1101/2020.09.16.20195693. published online Sept 18. (preprint). [DOI] [Google Scholar]
- 25.Warszawski J, Bajos N, Meyer L, et al. En mai 2020, 4,5 % de la population en France métropolitaine a développé des anticorps contre le SARS-CoV-2. Premiers résultats de l'enquête nationale EpiCov. Direction de la Recherche, des Etudes, de l'Evaluation et des Statistiques. October, 2020. https://drees.solidarites-sante.gouv.fr/IMG/pdf/er1167.pdf
- 26.Vu SL, Jones G, Anna F, et al. Prevalence of SARS-CoV-2 antibodies in France: results from nationwide serological surveillance. medRxiv. 2020 doi: 10.1101/2020.10.20.20213116. published online Oct 21. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somekh E, Gleyzer A, Heller E, et al. The role of children in the dynamics of intra family coronavirus 2019 spread in densely populated area. Pediatr Infect Dis J. 2020;39:e202–e204. doi: 10.1097/INF.0000000000002783. [DOI] [PubMed] [Google Scholar]
- 28.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Q, Xing Y, Shi L, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020;146 doi: 10.1542/peds.2020-0961. [DOI] [PubMed] [Google Scholar]
- 31.Gilliam WS, Malik AA, Shafiq M, et al. COVID-19 transmission in US child care programs. Pediatrics. 2021;140 doi: 10.1542/peds.2020-031971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We are prepared to share the study data (in strict compliance with the French legislation on personnel data) upon request to Coralie Bloch-Queyrat (coralie.bloch-queyrat@aphp.fr).