Abstract
Cumulative evidence has pointed out cannabinoid CB2 receptors (CB2r) as a potential therapeutic key target for treating alcohol use disorder (AUD). This review provides the most relevant results obtained from rodent and human studies, including an integrative section focused on the involvement of CB2r in the neurobiology of alcohol addiction. A literature search was conducted using the electronic databases Medline and Scopus for articles. The search strategy was as follows: “Receptor, Cannabinoid, CB2” AND “Alcohol-Related Disorders” AND “human/or patients”; “Receptor, Cannabinoid, CB2” AND “Alcohol” OR “Ethanol” AND “rodents/or mice/or rats”. Pharmacological approaches demonstrated that the activation or blockade of CB2r modulated different alcohol-addictive behaviors. Rodent models of alcoholism revealed significant alterations of CB2r in brain areas of the reward system. In addition, mice lacking CB2r (CB2KO) show increased alcohol consumption, motivation, and relapse alterations. It has been stressed that the potential neurobiological mechanisms underlying their behavioral effects involve critical elements of the alcohol reward system. Interestingly, recent postmortem studies showed CNR2 alterations in brain areas of alcoholic patients. Moreover, although the number of studies is limited, the results revealed an association between some genetic alterations of the CNR2 and an increased risk for developing AUD. This review provides evidence that CB2r may play a role in alcohol addiction. Clinical studies are necessary to figure out whether CB2r ligands may prove useful for the treatment of AUD in humans.
Keywords: cannabinoid CB2 receptors, CB2KO, alcohol, reward system, pharmacological studies
1. Introduction
Alcohol use disorder (AUD) represents the seventh most common leading risk factor for premature death and disability worldwide, accounting for more than 3 million deaths annually (5.3% of all deaths) [1]. The situation worsens in the age range of 15–49 years, being the leading cause of disability. Comorbidity with psychiatric disorders occurs as alcohol dependence progresses, significantly increasing disability, morbidity, and mortality rates associated with AUD [2]. Despite these devastating data, treatment options for treating AUD are scarce and present limited efficacy. Currently, there are only four medications approved with different mechanisms of action: acamprosate, disulfiram, naltrexone, and nalmefene [3,4]. These drugs, especially naltrexone and acamprosate, are effective in certain patients. However, it is disturbing that approximately 70% of patients relapse within the first year of treatment [5,6]. Unfortunately, the FDA and EMA has approved no new drugs for almost the last decade.
The development of new and effective treatment options for AUD encompasses identifying the underlying neurobiological mechanisms that are still pending despite the efforts made. In this respect, a growing and compelling body of evidence shows that cannabinoid receptors (CB1r and CB2r) play a relevant role in AUD. Cumulative pieces of evidence have supported the role of CB1r in the regulation of ethanol intake, the establishment of tolerance/dependence, and the vulnerability to relapse, mainly through animal studies [7,8,9,10,11]. Consequently, pharmacological modulation of CB1r employing CB1r-agonists or -antagonists attracted strong interest due to its therapeutic potential [9,12,13,14,15,16]. However, CB1r agonists have been associated with essential side effects, such as psychotomimetic effects and abuse and dependence problems [17,18]. In addition, increased anxiety and suicidal ideation rates were observed in obese patients treated with the CB1r-antagonist SR141716A, marketed as rimonabant, reasons for which it had to be withdrawn from the market [19,20,21,22]. These facts led to the exploration of additional lines of research, among which CB2r highlights for lacking such undesirable effects and being involved in the addictive properties of alcohol.
CB2r, initially considered the peripheral cannabinoid receptor, has been expressed in the central nervous system, playing a relevant role in modulating several processes [23,24,25,26,27,28,29,30]. This cannabinoid receptor is a G protein-coupled receptor that inhibits adenylyl cyclase’s activity through its Gi/Goα subunits [31,32]. Interestingly, in some cells, such as human leukocytes, CB2r are coupled to Gαs activating, in this case, the adenylyl cyclase and, therefore, increasing cAMP [33,34]. Indeed, CB2r are also known to activate the MAPK-ERK pathway through Gβγ subunits, inducing changes in cell migration [35,36,37].
This cannabinoid receptor CB2r is widely expressed through the brain, from cortical areas to the cerebellum, including limbic regions, such as the hippocampus (HIP) and amygdala (AMY), and regions within the reward system, such as the ventral tegmental area (VTA) and nucleus accumbens (NAcc) [23,25,38,39]. Despite some controversy, the progress in antibodies against CB2r have allowed identifying the expression of this cannabinoid receptor in neurons within the prefrontal cortex, dorsal striatum, NAcc, VTA, HIP, and cerebellum [28,29,38,39,40,41,42,43,44]. In more detail, CB2r is expressed in dopaminergic [38,42,45,46,47,48], glutamatergic [46,49], and GABAergic neurons [50,51]. In addition, CB2r has been identified in astrocytes [38,52,53] and microglia [44,54,55,56], in both basal and activated states, downregulating the gene expression of inflammatory mediators [57,58,59]. The expression of CB2r in brain regions belonging to classical neuronal circuits involved in drug addiction, such as the VTA, NAcc, AMY, and HIP, has promoted further studies to understand its potential role in these pathological conditions.
This review is aimed at providing information from animal studies, including pharmacological and genetic manipulations, and human studies, supporting the role of CB2r in alcohol addiction. Furthermore, we are reviewing the neurobiological mechanisms underlying the modulatory effects of CB2r on alcohol addiction. Finally, we provide concluding remarks highlighting the next steps needed for achieving the goal that compounds acting on CB2r from entering into clinical practice soon.
2. Results
2.1. Evidence from Rodent Studies
2.1.1. Changes of CB2r Induced by Ethanol in Brain Regions of the Reward System
The involvement of CB2r in AUD has been studied in several animal models simulating different stages of alcohol addiction. The reduction in the gene encoding for CB2r (CNR2) gene expression in the ventral midbrain of mice developing increased ethanol preference was the first evidence of functional changes in this receptor. In contrast, no alteration of CNR2 was found in the ventral midbrain of mice with lower preference (Table 1) [60].
Table 1.
CB2r alterations in brain regions of rodent models exposed to different regimens of alcohol addiction.
| Species | Experimental Design | Results | References |
|---|---|---|---|
| Male C57/Bj6 mice | Free access to EtOH for 15 days (2–32% alcohol concentration, p.o.) | ↓ CNR2 in the midbrain of mice with higher preference for alcohol solution at a concentration of 16–32% | [60] |
| Male and female APPswe/PS1dE9 (AZ) and WT C57BL/6J mice | Intermittent alcohol exposure during adolescence: 2.5 g/kg, i.p., for 4 days per week, 4 weeks | ↑ CNR2 and CB2r protein expression in the HIP of WT mice at 6 and 12 months of age ↓ CNR2 in the HIP of AZ mice at 12 months of age, without changes in the protein expression |
[61] |
| Male Swiss mice | Treatment with EtOH: 2 g/kg/d, i.p., for 21 days, and mice classification as EtOH-High or EtOH-Low according to their locomotor activity after the last administration | At the end of the treatment: ↓ CB2r protein expression in the IL and HIP of EtOH-Low mice On the 5th day of withdrawal: ↑ CB2r protein expression in the Acbsh and BLA in both groups. ↑ CB2r protein expression in the CG1 and Acbco in EtOH-High group ↑ CB2r protein expression in the CeA and Dis in the EtOH-High group EtOH challenge (1.4 g/kg) after withdrawal: ↓ CB2r protein expression in the AMY and striatum in both groups |
[62] |
| Male Wistar rats | Intermittent alcohol exposure during adolescence: 3 g/kg, i.p. for 4 days per week, 4 weeks | ↓ CNR2 in the striatum and HIP in adulthood | [63] |
| Male Wistar rats | Exposure to a two-bottle choice paradigm during adolescence: EtOH 10% (v/v) 24 h/4 days for 4 weeks. | ↑ CNR2 in the HIP in adulthood ↓ CNR2 in the AMY in adulthood |
[64] |
| Male Wistar rats | Chronic continuous ethanol diet at 10% (w/v) for 15 days or intermittent ethanol consumption at 10% (w/v), 5 days per week, 3 weeks |
↓ CNR2 in the AMY at 24 h in both groups | [65] |
| Male and female Wistar rats | Exposure to a chronic mild stress animal model, followed by a two-bottle choice paradigm with 20% of ethanol solution | ↓ CB2r protein levels in the hippocampal formation of females No changes in males |
[66] |
| Male Wistar rats | Exposure to an early restraint stress followed by an intermittent alcohol administration of 3 g/kg, 4 days a week for 4 weeks during adolescence. | ↑ CB2r protein expression in the AMY | [67] |
EtOH, ethanol; p.o., oral administration; CNR2, gene encoding for cannabinoid receptor 2; APPswe/PS1dE9, transgenic Alzheimer disease mice; AZ, Alzheimer disease; i.p., intraperitoneal administration; CB2r, cannabinoid receptor 2; HIP, hippocampus; AMY, amygdala; IL, infralimbic cortex; Acbsh, nucleus accumbens shell; BLA, basolateral amygdala; CG1, anterior cingulate cortex; Acbco, nucleus accumbens core; CeA, central nucleus of amygdala; Dis, dorsolateral striatum. ↓: decrease; ↑: increase.
Since then, changes in CB2r have been detected in brain regions of rodents exposed to different patterns of ethanol consumption. Sanchez-Marin et al. [63] identified a significant reduction of CNR2 expression in the striatum and HIP of adult rats exposed to a forced intermittent ethanol intoxication (binge drinking) (3 g/kg; i.p.; 4 consecutive days × 4 weeks) during adolescence. These results highlight the involvement of CB2r in the long-lasting emotional disturbances (increased anxiety-like behaviors and cognitive impairments) induced by ethanol intoxication during adolescence. Later, the same authors identified a significant increase and decrease of CNR2 expression in the HIP and AMY, respectively, of adult rats exposed to an intermittent voluntary ethanol consumption during adolescence (EtOH 10% (v/v); 4 days/24 h/4 w). These alterations were accompanied by an increase of glial fibrillary acidic protein (GFAP) and allograft inflammatory factor 1 (AIF-1) in the medial prefrontal cortex (mPFC) and HIP, respectively, crucial brain areas for the development of alcohol addiction [64].
A recent study developed by Ledesma et al. [61] has focused on analyzing the impact of ethanol binge drinking during adolescence on cognitive disturbances. This study showed that pre-exposure to ethanol (2.5 g/kg, i.p.) during adolescence increased CNR2 and CB2r protein expression in the HIP of wild-type (WT) mice at 6- and 12-months of age. Interestingly, in the transgenic mice model of Alzheimer’s disease, APPswe/PS1dE9 mice, CNR2 expression reduced at 6-months of age while statistical significance was notified at 12-months. The authors concluded that the increased levels of CB2r in WT could be a protective mechanism for ethanol-induced cognitive impairments since WT-EtOH mice at 6-months of age did not display learning impairments. On the contrary, the reduction of CB2r in APPswe/PS1dE9 mice appears to be a negative hallmark for displaying cognitive impairments.
More recently, CB2r receptor availability and its correlation with ethanol-induced locomotor sensitization have been evaluated. For this purpose, mice were treated with ethanol (EtOH, 2 g/kg/d; i.p.) for 21 days and classified into EtOH-high or -low groups, according to their behavioral variability on locomotor activity on the 21st day of acquisition. A significant decrease in CB2r protein expression was detected in the infralimbic cortex and HIP (CA1, CA2, and CA3 fields) of the EtOH-low group. Interestingly, mice were subjected to 5 days of EtOH withdrawal in an additional independent experiment after completion of the acquisition phase. An increase of CB2r protein levels was found in the basolateral nucleus of the amygdala (BLA) and the nucleus accumbens shell (Acbsh) (both groups), in the anterior cingulate cortex (CG1) and nucleus accumbens core (Acbco) (only in EtOH-low), and CeA (central nucleus of amygdala) and dorsolateral striatum (Dis) (only in EtOH-high). The authors concluded that CB2r upregulation might be correlated with withdrawal aspects of EtOH-sensitized mice, pointing out the need for further studies to clarify this aspect. In the third set of experiments, changes in CB2r protein levels were analyzed in mice injected with EtOH (1.4 g/kg) (EtOH challenge) after the withdrawal period. In this case, CB2r were downregulated in the AMY and striatum of both EtOH groups, regardless of motor sensitization. Moreover, in all experimental groups (including controls), CB2r was reduced in the prefrontal cortex and HIP. The results showed that CB2r downregulation appeared to be related to the development of motor sensitization [62].
Additional studies evaluated CNR2 alterations induced by ethanol withdrawal. Serrano et al., [65] studied the effects of single or repeated-ethanol withdrawal periods on CNR2 in the AMY of rats. Briefly, rodents were exposed to a chronic continuous ethanol diet (10% w/v; 15 days) or an intermittent ethanol consumption (10% w/v; 5-days/week × 3 weeks), evaluating changes in CNR2 expression at 6 h and 24 h after last ethanol exposure. No significant alterations were found in either group’s CNR2 expression at 6 h. Surprisingly, a small but non-significant reduction was observed at 24 h in rats exposed to the continuous ethanol diet, and a statistically significant decrease in the ethanol intermittent consumption group. These results revealed that exposure to repeated withdrawal periods induced more broad and robust alterations in CNR2 in the AMY, suggesting its potential implication in negative motivational states and enhanced stress responsivity associated with alcohol dependence and withdrawal.
Epidemiological data and clinical and preclinical studies indicate that exposure to stressful situations increases vulnerability to alcohol consumption. Although several studies have assessed this correlation, only a few have examined the involvement of CB2r. In this respect, Marco et al. [66] identified reduced levels of CB2r protein expression in the hippocampal formation of female Wistar rats with high ethanol consumption and preference after previous exposure to chronic mild stress (CMS) during 6 weeks. No changes were observed in the frontal cortex. Interestingly, male Wistar rats exposed to the same paradigm did not display ethanol preference nor increased ethanol consumption, suggesting significant sex-dependent vulnerability to ethanol after CMS model exposure. Additionally, other studies demonstrated that the combination of restraint stress with intermittent alcohol exposure (3 g/kg ethanol; p.o.; 4 days/week for 4 weeks) during adolescence significantly increases CB2r in the AMY of young adults [67].
In summary, evidence achieved to date showed that ethanol modified the gene and protein expression of CB2r across the brain. Some of these studies obtained opposite changes suggesting that several factors, such as the strain of rodent, the pattern (acute vs. chronic) and route (oral, i.p., etc.) of ethanol administration, the animal model (conditioned place preference (CPP), ethanol-self administration), and the techniques used to measure CNR2 and CB2r protein expression, may influence the alterations observed. Moreover, advanced techniques, such as single-cell gene expression, RNAscope in situ hybridization (ISH), and immunohistochemistry (IHC) would be of great interest to further characterize cell-specific changes of CNR2 and CB2r protein expression induced by ethanol in neurons, microglia, and astroglia.
2.1.2. Genetic Studies in Rodents
Further support of the role of CB2r on the vulnerability to ethanol consumption has been obtained using genetically-modifies mice.
On the one hand, mice lacking CB2r (CB2KO) showed higher sensitivity to withdrawal, as indicated by an increased handling-induced convulsion score after an acute high dose of ethanol (Table 2). Moreover, CB2KO mice presented higher voluntary ethanol intake and preference, ethanol-conditioned place preference, increased motivation to drink, and higher levels of ethanol consumption in the oral ethanol self-administration paradigm. These behavioral alterations were accompanied by significant changes in the gene expression of critical targets underlying alcohol addiction, such as the mu-opioid receptor (Oprm1), reduced in the NAcc of naïve CB2KO mice. Interestingly, acute ethanol administration increased tyrosine hydroxylase (TH) in the VTA at all doses tested in CB2KO mice. In contrast, a significant reduction was observed in WT at 0.5 and 1 g/kg of ethanol doses. Interestingly enough, Oprm1 in the NAcc of CB2KO significantly increased after ethanol administration (1 and 2 g/kg). In contrast, no modification was observed in WT mice. The conclusion that can be drawn from this study is that deletion of CB2r influences ethanol-induced effects in TH and Oprm1 in brain targets of the mesolimbic system that may contribute, at least in part, to the higher sensitivity to ethanol’s rewarding effects observed in CB2KO mice [68].
Table 2.
Summary of the experimental studies evaluating the influence of the genetic manipulation of CB2r in animal models of alcohol addiction.
| Genetic Manipulation | Species | Experimental Design | Results | References |
|---|---|---|---|---|
| CB2KO | Mice | Sensitivity to acute EtOH-induced hypothermia HIC CPP two-bottle choice Oral ethanol self-administration paradigms |
↓ rectal temperature ↑ HIC ↑ conditioned place preference ↑ EtOH consumption and preference in the two-bottle choice paradigm ↑ EtOH consumption and motivation |
[68] |
| CB2KO | Mice | CPP | ↑ conditioned place preference | [69] |
| CB2KO | Mice | Continuous and intermittent forced EtOH drinking under single and group-housing conditions | Continuous forced drinking: Individually housed CB2KO < grouped CB2KO Individually housed WT < grouped WT Intermittent forced drinking: individually housed CB2KO = grouped CB2KO individually housed WT > grouped WT |
[70] |
| DAT-CNR2-/- conditional knockout | Mice | EtOH-induced CPP evaluation after restraint stress. | ↓ conditioned place preference | [71] |
EtOH, ethanol; CNR2, gene encoding for cannabinoid receptor 2; CB2KO, mice lacking the cannabinoid receptor 2; WT: wild-type; DAT, dopaminergic neurons in the ventral tegmental area; CPP, conditioned place preference; HIC, handling-induced convulsions. ↓: decrease; ↑: increase; <: less than; >: more than; =: as well as.
Similarly, in an additional study, the absence of CB2r in mice enhanced ethanol conditioning in the conditioned place preference (CPP) paradigm. However, no changes were observed in the two-bottle choice paradigm between CB2KO and WT mice [69]. These results contrast with those provided by Ortega-Alvaro et al. [68]. Such discrepancies could be related to the genetic background strain of the KO mice used (C57Bl/6J and CD1, respectively). Furthermore, Powers and colleagues carried out the two-bottle choice paradigm 3 weeks after the CPP, that modified CB2r expression in WT due to the repeated ethanol exposure in CPP. These modifications may explain why no differences between the CB2KO and WT mice were observed in the CPP by Powers et al. [69].
Likewise, the involvement of CB2r in modulating the effects of stressful situations on alcohol consumption has been addressed by additional studies. In this respect, Pradier et al. [70] aimed to determine if CB2r has a role in ethanol consumption induced by social isolation. To this aim, ethanol intake was evaluated in CB2KO and WT mice housed individually or in the group. Both strains did not differ in the forced drinking paradigm, i.e., ethanol intake was lower in individually housed mice when compared to grouped mice, regardless of the genotype. However, apparent differences were observed in the intermittent forced drinking model. CB2KO grouped and individually housed mice did not differ in ethanol intake whereas for WT ethanol consumption was higher in individually housed mice when compared to grouped animals. Altogether, these results supported the modulatory function of CB2r in the social environmental-induced alcohol intake.
Moreover, the involvement of CB2r in modulating the impact of acute stress exposure on ethanol consumption has also been addressed using a knockout mouse lacking the CB2r in midbrain dopaminergic neurons (DAT-CNR2 conditional knockout mice). Under basal conditions, alcohol preference was lower in DAT-CNR2-/- mice compared with controls. Interestingly, these differences were more pronounced when mice were exposed to an acute stress protocol. Control mice showed a significant enhanced preference in the CPP paradigm that was not present in DAT-CNR2-/- mice. These results supported the idea that the absence of CB2r in dopaminergic neurons decreases alcohol consumption, even after acute stress, supporting the involvement of CB2r in alcohol dependence [71].
Together, these studies show that CB2r regulates rewarding ethanol effects since full CB2KO mice showed increased ethanol preference, motivation and consumption in different animal models. Moreover, full and conditioned CB2KO mice revealed that CB2r also modulates the impact of stress exposure on ethanol intake.
2.1.3. Pharmacological Studies in Rodents
To date, several pharmacological studies have been carried out in rodents to assess how the activation or blockade of CB2r modulates alcohol consumption, motivation, and relapse (Table 3). The first study revealed that systemic CB2r-agonist JWH015 (20 mg/kg, i.p.) administration induced a marked increase in ethanol intake in mice previously exposed to the CMS model. Interestingly, the CB2r-antagonist AM630 (3 mg/kg, i.p.) induced just the opposite effects, preventing the development of alcohol preference (Table 3) [60].
Table 3.
Summary of the pharmacological studies in rodents studying the role of CB2r in animal models of alcohol addiction.
| Drug | Pattern of Administration | Species | Experimental Design | Results | References |
|---|---|---|---|---|---|
| JWH015 CB2r agonist |
20 mg/kg, i.p. | C57BL/6J mice | CPP under CMS condition | ↑ Voluntary ethanol consumption | [60] |
| β-caryophyllene CB2r agonist | 25, 50 and 100 mg/kg | C57BL/6J mice | CPP VC |
↓ CPP ↓ Voluntary ethanol consumption and preference |
[72] |
| JWH133 CB2r agonist |
10 and 20 mg/kg, i.p. | HS/Ibg mice | CPP VC |
No differences | [69] |
| JWH133 CB2r agonist |
5 mg/kg, i.p. | C57BL/6J mice | CPP | ↓ CPP | [73] |
| JWH133 CB2r agonist |
1 mg/kg, i.p. | C57BL/6J mice | OESA | ↓ Motivation to drink ethanol | [74] |
| JWH133 CB2r agonist |
1 mL per 100 g of body weight, i.p. | C57BL/6J mice | CPP | ↓ Motivation to drink ethanol | [75] |
| JWH133 CB2r agonist |
0.2mg/kg, i.p. | Wistar rats | Free access EtOH 10%, 2 weeks | ↑ number of BrdU+ cells in the subventricular zone of the DG | [76] |
| AM630 CB2r antagonist |
3 mg/kg, i.p. | C57BL/6J mice | CPP under CMS condition | ↓ Motivation to drink ethanol | [60] |
| AM630 CB2r antagonist |
10 and 20 mg/kg, i.p. | HS/Ibg mice C57BL/6J mice |
CPP CV |
No differences | [69] |
| AM630 CB2r antagonist |
1 mg/kg, i.p. | C57BL/6J mice | OESA | ↑ Motivation to drink ethanol | [74] |
CPP: conditioned place preference, VC: ethanol voluntary consumption, OESA: oral ethanol self-administration, CMS: chronic mild stress. ↓: decrease; ↑: increase.
Similarly, Al Mansouri et al. [72] demonstrated that β-caryophyllene, a selective CB2r-agonist, significantly reduced voluntary alcohol consumption and CPP in male C57BL/6J mice. These effects were blocked by the administration of the antagonist AM630, supporting the role of CB2r as the target involved in the effects of β-caryophyllene.
Likewise, Liu et al. [73] showed that JWH133 (5 mg/kg, i.p.) completely blocked alcohol-induced CPP. Similar effects occurred following the administration of the CB2r-agonist, JWH133, which reduced alcohol and food rewarding behaviors in C57BL/6 mice during the acquisition phase [75]. In contrast, Powers et al. [69] failed to observe any effect on alcohol consumption and CPP in HS/Ibg mice treated with CB2r-agonist JWH133 (10 and 20 mg/kg; i.p.) or CB2r-antagonist AM630 (10 and 20 mg/kg; i.p.). Discrepancies regarding the effects of pharmacological regulation of rCB2r on alcohol consumption may be due to various experimental circumstances, such as using different mouse strains (HS/Ibg or C57BL/6J) or the dose, route, or schedule of administration of cannabinoid compounds.
Pharmacological modulation of CB2r by the antagonist AM630 (1 mg/kg, i.p.) significantly increased the motivation to drink and the level of alcohol consumption in the oral operant self-administration paradigm [74]. Furthermore, this study showed how the agonist JWH133 (1 mg/kg, i.p.) produced opposite effects, significantly reducing alcohol self-administration. These changes were accompanied by bidirectional modifications in the gene expression of brain targets related to regulating the reinforcing effects of alcohol, such as TH in the VTA and Oprm1, CNR1 and CNR2 in the NAcc (for details, see below).
In another experimental paradigm in which rats were fed with liquid diets of ethanol (10%) for 2 weeks, treatment with JWH133 specifically counteracted the impairment of adult neural progenitor cell (NPC) proliferation induced by ethanol. In detail, JWH133 increased the number of BrdU+ cells in the subgranular zone of the dentate gyrus, subventricular area of the lateral ventricles, and hypothalamus [76].
Indeed, a recent study revealed that the CB2r-agonist JWH133 (0.2 mg/kg) increased astrocytes and microglia in the HIP of rats exposed to a model of subchronic (2 weeks) ethanol diet (11% v/v) exposure. These results suggested a role of CB2r in astrocytes and microglial recruitment and activation following specific neurotoxic stimuli [77].
Altogether, the majority of the pharmacological studies carried out to date revealed that the agonism of CB2r can reduce ethanol consumption, preference, and motivation to drink in several animal models. Moreover, CB2r activation is proposed to induce a neuroprotective effect against ethanol neurotoxicity, through its modulatory effects on microglia and astroglia. Interestingly, key targets of the opioidergic and dopaminergic systems would play a role in such effects.
2.2. Evidence from Human Studies
The role of CB2r in AUD in humans has not been extensively studied. Onaivi’s group provided the first reference [60]. This study described for the first time an association between the Q63R polymorphism in the CNR2 (glutamine in amino acid position 63 substituted by arginine) and alcoholism in a population of Japanese patients. In short, this study indicated that this single nucleotide polymorphism (SNP) might be a risk factor for alcoholism, at least in Japanese people. More recently, one genome-wide association study (GWAS) found a positive association cluster around the 3′ untranslated region (3′UTR) of the CNR2 and AUD [73].
Interestingly, our group recently carried out a postmortem study analyzing CNR2 in the dorsolateral prefrontal cortex (DLPFC) and NAcc of patients with alcohol dependence (unpublished data), showing an observed reduction in both regions. Conversely, another study found an increase CNR2 in human monocyte-derived dendritic cells from alcohol users [78].
Altogether, these data suggested that CB2r is involved in AUD in humans and emphasized the need for future large-scale studies of alcohol-dependent patients to determine its potential therapeutic role in AUD.
2.3. Involvement of CB2r in the Neurobiology of Alcohol Addiction
2.3.1. CB2r and the Alcohol Brain’s Reward System
Drugs of abuse, such as alcohol, activate the mesolimbic dopaminergic system that mediates reinforcing and motivational actions [79]. Mainly, the rewarding effects of alcohol occurred by the interaction between the opioidergic and dopaminergic mesolimbic systems, involving the increased firing of dopamine neurons in the VTA [74] and a subsequent increase of dopamine release into the NAcc [80]. Furthermore, other systems, such as the cholinergic glutamatergic and GABAergic systems and molecular targets involved in neuroinflammation, have been proposed to display a relevant role in alcohol actions. The following sections describe the available information on the role of CB2r in regulating dopaminergic, opioidergic and cholinergic neurotransmissions in the mesocorticolimbic circuit, neuroinflammatory mediators activated by alcohol consumption and excitatory-inhibitory balance, and, consequently, its involvement in the modulation of alcohol rewarding properties.
2.3.2. CB2r and the Dopaminergic System
A crucial step to deepen the functional role of CB2r in alcohol reward was to improve the understanding of its interaction with the dopaminergic system. Double confocal immunohistochemical analyses revealed that CB2r are expressed in neurons and astrocytes of the NAcc and VTA of CD1 male mice [38,81]. Similarly, Zhang et al. confirmed these findings by in situ hybridization and immunohistochemical assays detecting CNR2 gene expression and CB2r immunostaining in VTA dopaminergic neurons [46]. CB2r was also found in VTA dopaminergic neurons of WT mice, whereas it was absent in conditional knockout mice in which CNR2 was selectively suppressed in these neurons (DAT-Cnr2 cKO mice) [73].
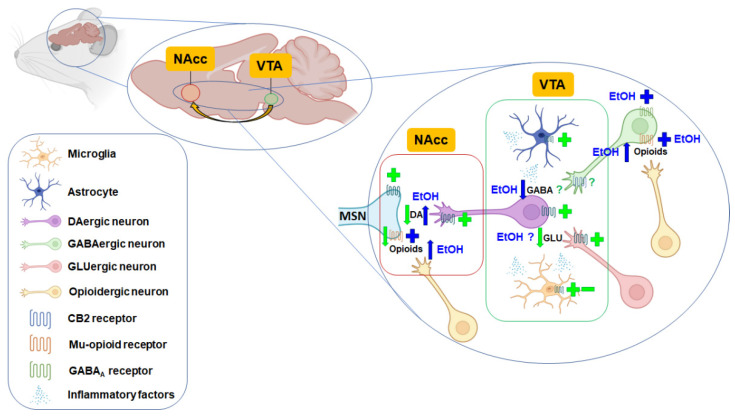
CB2r immunoreactive cells and dopamine D2 receptor (D2Dr) were present in the NAcc and VTA of WT mice [38]. Recently, CB2r was also identified in striatal medium spiny neurons that express dopamine D1 or D2 receptors by RNAscope ISH assay [82]. Interestingly, repeated CB2r activation induced a significant D2Dr upregulation by involving GRK5, β-Arrestin 2, and ERK1/2 protein signaling [83]. Altogether, these results suggest possible functional cooperation between CB2r and dopamine receptors (Figure 1).
Figure 1.
Graphic diagram illustrating the proposed mechanisms involved in ethanol actions (blue) and CB2r-mediated regulation of the mesolimbic DA system (green) according to studies performed in rodents. Ethanol increases the release of dopamine in the nucleus accumbens (NAcc) mainly by increasing the release of endogenous opioids acting on mu-opioid receptors and inhibiting GABAergic neurotransmission. The activation of CB2r induces different actions depending on its location: (1) reduction of dopamine (DA) release from ventral tegmental area (VTA) DAergic neurons projecting to the NAcc; (2) reduction of glutamate (GLU) release from GLUergic neurons projecting to VTA DAergic neurons; (3) modulation of inflammatory factors’ release from microglia or astrocytes, and (4) reduction of mu-opioid receptors density in the NAcc. According to available evidence, the role of CB2r in regulating GABA release from GABAergic terminals projecting to VTA DAergic neurons is not entirely clear. MSN: GABAergic inhibitory medium spiny neuron. Created with Biorender.com. +: activation/agonism; -: inhibition/antagonism; ↓: reduction; ↑: increase.
In vivo microdialysis studies were performed to evaluate if the genetic manipulation of CB2r in mice could modulate extracellular dopamine levels in the NAcc of mice. Xi et al. revealed that the deletion of CB2r (CB2KO mice) was not associated with any change in basal concentrations of extracellular dopamine between WT and CB2KO mice [84]. On the other hand, delta-9-tetrahydrocannabinol (THC) produced a dose-dependent decrease in extracellular dopamine in the NAcc of WT mice. In contrast, the opposite effect was obtained in CB2KO mice, suggesting that activation of the CB2r inhibits DA release in the NAcc [80]. Our group also carried out microdialysis experiments to investigate the effect of CB2r overexpression (CB2xP mice) on NAcc dopamine release. No differences were present between WT and CB2xP mice [38].
Despite CB2r deletion or overexpression not modifying NAcc dopamine levels, systemic administration of the selective CB2r agonist, JWH133 (3, 10, 20 mg/kg, i.p.), significantly and dose-dependently lowered extracellular NAcc dopamine in WT mice, but not in CB2KO mice. In addition, this reduction was blocked by the selective CB2r antagonist AM630 (10 mg/kg, i.p.), suggesting that CB2r is involved in the inhibitory effect of JWH133 on DA release.
Interestingly, to determine if this inhibitory effect was mediated by activation of brain or peripheral CB2r, intranasal or intra-NAcc local administration of JWH133 was assessed. Both routes of administration produced a significant reduction in extracellular dopamine levels suggesting the participation of brain CB2r located in the NAcc [84]. On the other hand, systemic or local administration of a CB2r inverse agonist, Xie2-64, into the NAcc reduced extracellular dopamine levels in a dose-dependent manner in rats [85].
To further explore the CB2r-mediated modulation of the dopaminergic tone in the VTA, the effects of JWH133 administration on neuronal dopamine firing were evaluated by electrophysiological methods. For that purpose, three different approaches were used: (1) perforated patch-clamp recording in single dissociated VTA dopaminergic neurons; (2) cell-attached patch-clamp in slice preparations (ex vivo); and (3) single-unit recording in anesthetized mice (in vivo). Interestingly, under these three experimental conditions, JWH133 significantly reduced the neuronal firing, effects that were blocked by AM630 [39]. A recent study further explored the underlying mechanisms of CB2r-mediated VTA dopaminergic neuronal function regulation. Employing patch-clamp recording in mouse VTA slices and dissociated VTA dopaminergic neurons, Ma and colleagues suggested that CB2r modulates the excitability mainly through an intrinsic mechanism, including reducing intracellular cAMP while enhancing M-type K+ currents. They also pointed out that although JWH133 reduces presynaptic glutamate release probability and results in a decreased glutamatergic synaptic transmission in VTA dopaminergic neurons, this may play a marginal role in the reduction of neuronal firing since pharmacological blockade of synaptic transmission failed to prevent the JWH133-induced inhibitory effect [45].
Another experimental approach to evaluate how CB2r manipulation could modulate dopaminergic transmission is the analysis of TH gene expression changes, the rate-limiting enzyme of dopamine synthesis. CB2xP mice showed increased TH and higher dopamine transporter (DAT) gene expression levels in the VTA [38]. In contrast, CB2KO mice presented a significant decrease in TH gene expression [81]. Similarly, in DAT-CNR2 cKO mice, TH protein levels were modified in midbrain areas [86]. Interestingly, acute ethanol administration downregulated TH gene expression (0.5, 1, and 2 g/kg, p.o.) in the VTA of WT mice but induced a remarkable upregulation in CB2KO mice (1 and 2 g/kg, p.o.) [68]. Furthermore, pharmacological blockade of CB2r with AM630 (1 mg/kg, i.p.) increased TH gene expression in the VTA, whereas the administration of JWH133 (1 mg/kg, i.p.) produced a downregulation [74].
Overall, there is sufficient evidence suggesting that functional regulation of the CB2r can modify dopaminergic activity in the mesolimbic system, partly responsible for modulating alcohol’s reinforcing and motivational effects.
2.3.3. CB2r and the Opioidergic System
Crosstalk between the endocannabinoid and opioidergic system has been widely studied [87,88,89,90]. However, very sparce information is available regarding the specific interaction between opioid receptors and CB2r and its regulation of alcohol reward. In this respect, valuable information through genetic and pharmacological approaches has been provided. A significant decrease in the expression of the gene encoding for mu-opioid receptors (Oprm1) was seen in the NAcc of CB2xP mice [38], while the opposite effect was observed in the NAcc of CB2KO mice [68]. In addition, acute ethanol administration did not change Oprm1 gene expression in the NAcc of WT mice, whereas a very significant increase appeared in CB2KO mice (1 and 2 g/kg, p.o.). Interestingly, the activation of CB2r by JWH133 (1 mg/kg, i.p.) significantly decreased Oprm1 levels while the blockade with AM630 (1 mg/kg, i.p.) induced an upregulation [75]. Thus, it could be argued that a lower CB2r functionality (genetic deletion (CB2−/−) or pharmacological blockade (AM630)) leads to an increased expression of Oprm1. This effect might, at least in part, represent the basis of the higher vulnerability to alcohol reward. Future studies are needed to elucidate how CB2r interacts with the opioidergic system in the mesolimbic circuit and its involvement in regulating the reinforcing effects of alcohol.
2.3.4. CB2r and the Cholinergic System
Alcohol, like nicotine, requires neuronal nicotinic acetylcholine receptors (nAChRs) to display its rewarding effects within the mesolimbic system. Yorgason et al. demonstrated that the release of dopamine in the NAcc by EtOH is mediated by cholinergic interneurons and atypical GABAA receptors [91]. Interestingly, the same group reported that low ethanol concentrations induced positive allosteric effects at nAChRs, which was correlated with an increase in spontaneous DA release [92]. The fact that both drugs share the latter molecular pathway has been proposed to contribute to the increased comorbidity of alcohol and nicotine dependence [93].
A close interaction between CB2r and nAChRs has been identified in different animal models of nicotine exposure and reward. One of the first studies evaluating the role of cannabinoid receptors in nicotine-induced discriminative behavior demonstrated that neither CB1r nor CB2r were involved [94]. Some years later, the actions of cannabinoid receptor activation by WIN55,212-2, a CB1/CB2r agonist, on nicotine self-administration were explored. WIN55,212-2 significantly increased nicotine self-administration and induced dose-dependent reinstatement, mediated by CB1r but not CB2r [95]. Likewise, Gamaleddin et al. concluded that CB2r were not involved in mediating nicotine self-administration or nicotine-induced seeking behavior [96].
However, a subsequent study showed that genetic deletion (CB2KO mice) and pharmacological blockade of CB2r (CB2r antagonism) abolished nicotine self-administration, CPP, and withdrawal. Interestingly, immunohistochemical analyses revealed that CB2r colocalized with nicotinic receptor α3 and α4 subunits in neurons of the NAcc and VTA. Thus, it is tempting to hypothesize that the presence of CB2r is necessary for the mediation of nicotine effects [81]. Accordingly, another study also found that CB2r is essential for nicotine-induced CPP in mice, but not for nicotine withdrawal syndrome [97]. Finally, a recent study suggested that CB2r located in the CA3 region of the HIP interacts with muscarinic acetylcholine receptors, modulating memory consolidation processes, a relevant aspect of drug addiction [98].
Although very little information is available, an interaction between the cholinergic system and CB2r may be occurring in the mesolimbic system, responsible for mediating the rewarding effects of nicotine. Considering that tobacco/cannabis smoking plus alcohol consumption tends to be very common, further studies elucidating the precise role of CB2r in this co-use exposure are necessary.
2.3.5. CB2r and Neuroinflammatory Mediators Activated by Alcohol Consumption
The innate immune system has been associated with areas of the mesolimbic system and different processes involved in addictive behaviors [99,100,101]. During the last years, a growing number of studies have revealed a significant association between systemic and brain inflammation with substance use disorders [102,103], suggesting a critical role in regulating such behaviors.
Although, the functions of the immune system in defensive and repair of damage and exposure to pathogens are well established, its involvement in the regulation of brain functions, such as long-term potentiation (LTP), memory, synaptic plasticity [104,105], and emotional regulation [106], has been elucidated only recently. These physiological functions are closely related to pathways associated with the reward system and alcohol disorders.
One of the leading and most studied receptors involved in the synthesis and release of pro-inflammatory cytokines through the nuclear factor kappa B (NF-κB) pathway is toll-like receptor 4 (TLR4). Lippai et al. showed how mice deficient in this receptor (TLR4-KO) were protected from NF-κB activation induced by chronic alcohol administration [107]. In addition, the protective effect of the opioid antagonist nalmefene on the acquisition of alcohol preference in a stepwise drinking paradigm is not observed in TLR4-KO mice [108]. Interestingly, opioid agonists are postulated to non-stereoselectively activate TLR4 signaling pathways in the absence of LPS-mediated stimulation [109]. These findings highlight the involvement of the innate immune system in the mechanisms underlying the process of the development of alcohol dependence.
Concerning CB2r, Zoppi et al. proved the involvement of the CB2r in a model of stress-induced neuroinflammation. Specifically, an increase in tumor necrosis factor alpha (TNFα) mRNA expression was found in the prefrontal cortex, which decreased in mice treated with JWH-133. Interestingly, the use of genetically modified mice revealed that mice overexpressing CB2r in the central nervous system, CB2xP, presented reduced levels of TNFα gene expression in the PFC but increased levels in CB2KO mice. Similar results were shown regarding the expression of the p65 subunit of the transcription factor NF-κB. The fact that the basal level of this pathway was seen in CB2KO mice suggested that these mice exhibited a basal state of neuroinflammation [110].
On the other hand, Pan et al. observed a shift of microglia towards an anti-inflammatory phenotype in the hippocampus and cortex by administering the agonist JWH-133 in a pneumococcal meningitis paradigm [111]. In animal models of alcoholism, the administration of the CB2r agonist AM1241 promoted liver regeneration in a thioacetamide (TAA)-induced liver injury model by suppressing TLR4/miR-155/NF-κB p65 pathway [112]. Jing et al. showed similar results in a spinal cord ischemia model, where pretreatment with the CB2r agonist JWH-133 blocked the TLR4 MyD88/NF-κB signaling pathway [113]. Moreover, the activation of CB2r located on microglial cells or astrocytes in either the VTA, NAcc, or both, has been proposed to inhibit NAcc DA release indirectly by releasing cytokines and inflammatory factors, thereby inhibiting cocaine self-administration and cocaine-enhanced locomotion (Figure 1).
These studies suggest an important relationship between the CB2r and the processes associated with neuroinflammation in alcohol-related neurobiological and behavioral alterations. Thus, pharmacological modulation of this receptor and its potential therapeutic effects in AUD could be mediated, in part, by the improvement of inflammatory parameters.
2.3.6. CB2r and Excitatory-Inhibitory Balance
Recent studies point out that CB2r are expressed in GABAergic [50,114] and glutamatergic [40,49,115,116,117] neurons. This fact suggests the relevant role that CB2r could play in regulating the balance of excitatory and inhibitory synaptic inputs in distinct brain regions, including those involved in drug reward. Emerging evidence demonstrates that CB2r activation modulates synaptic transmission in the rat medial entorhinal cortex [50], excitatory hippocampal autaptic neurons [118], and hippocampal pyramidal neurons [41]. Indeed, suppression of GABAergic inhibition could be induced by the CB2r agonist JWH133 and reversed by the CB2r antagonist AM630 [50]. Likewise, activation of CB2r inhibited GABA-A receptor-mediated currents [51]. Moreover, chronic treatment with a CB2r agonist increased excitatory transmission in glutamatergic as opposed to GABAergic synapses [41].
The firing of VTA dopaminergic neurons is tightly controlled by excitatory and inhibitory synaptic innervations. Thus, it is tempting to hypothesize that a possible modulation by CB2r could modify the functionality of these inputs, and therefore the reinforcing and motivational effects of alcohol. Interestingly, acute activation of CB2r induced a reduction in glutamatergic synaptic transmission in VTA dopaminergic neurons, including reduced presynaptic glutamate release probability. However, activation of CB2r induced minimal effects on either presynaptic GABA release or postsynaptic GABA-A receptor function in VTA dopaminergic neurons [45]. Similarly, another study demonstrated that VTA GABAergic neurons were insensitive to JWH133 administration [46].
In conclusion, there is limited information on how CB2r-mediated regulation of the excitatory/inhibitory balance may, in turn, modulate the rewarding and non-rewarding effects of alcohol in the brain. Future studies will be necessary to elucidate the underlying mechanisms.
3. Discussion
Despite several reviews exploring the potential role of the endocannabinoid system in AUD, mainly through the pharmacological modulation of the CB1r, few studies have focused on the CB2r. This cannabinoid receptor has emerged as a crucial target in several diseases affecting the central nervous system, including psychiatric and drug-use disorders.
Although CB2r occur at lower levels than CB1r, they can be found in neurons, microglia and astroglia in different brain regions. Interestingly, recent studies identified CB2r in dopaminergic, GABAergic, and glutamatergic neurons. The studies carried out to date demonstrated alterations in CB2r, at gene and protein level, in different brain regions of the reward system and related areas, including the AMY and HIP, by acute and chronic ethanol consumption. Moreover, changes in CB2r have also been found in different animal models of withdrawal and, more importantly, in mouse models evaluating the effects of stress on alcohol consumption. Later on, both pharmacological and genetic studies in rodents further suggested the involvement of CB2r in ethanol consumption and vulnerability since mice lacking the CB2r displayed increased vulnerability for alcohol consumption and motivation to drink, and treatment with CB2r agonists reduced alcohol consumption. Mechanistic studies pointed out the potential interactions between CB2r and dopaminergic, opioidergic, and cholinergic systems. Similarly, further studies are needed to elucidate the crosstalk between CB2r and other brain networks, including GABA and glutamatergic systems, and the involvement of microglia and astroglia in the central effects of alcohol.
4. Material and Methods
A literature search was conducted using the electronic databases Medline and Scopus for articles in English from the database inception to 15th November 2021. The search terms included both keywords and subject headings. The search strategy was as follows: “Receptor, Cannabinoid, CB2” AND “Alcohol-Related Disorders” AND “human/or patients”; “Receptor, Cannabinoid, CB2” AND “Alcohol” OR “ethanol” AND “rodents/or mice/or rats”.
5. Conclusions and Future Perspectives
Altogether, this data shows that CB2r functional manipulation may regulate ethanol motivation and consumption vulnerability. Interestingly, genetic and pharmacological studies revealed interactions between CB2r and the key targets involved in the alcohol reward system. Indeed, CB2r-agonism might become an approach for treating AUD that deserves further exploration. Future studies (e.g., with selective CB2r ligands or CB2r knockout and knockin mice) are necessary to evaluate ethanol rewarding aspects (e.g., conditioned place preference, voluntary consumption, or relapse). Moreover, considering the broad expression of CB2r in neurons, glia, and astroglia, further neurobiological studies, for instance, single-cell and RNAseq, would be interesting to characterize the mechanism of action CB2r-compounds at the single-cell level.
Acknowledgments
We thank all participants in this study. Thanks to Biorender for Figure 1.
Author Contributions
M.S.G.-G. and J.M. conceived the presented idea; M.S.G.-G. took the lead in writing the manuscript; F.N., A.G., D.N., Á.M, and T.F. wrote the manuscript in consultation with M.S.G.-G. and J.M. All authors provided critical feedback and helped shape the literature research, analysis and manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
The preparation of the manuscript was supported by “Red Primaria de adicciones” (RD21/0009/0008), “Ministerio de Sanidad, Delegación del Gobierno para el Plan Nacional Sobre Drogas” (PNSD, 2019I012 to J.M.), and “Ministerio de Ciencia e Innovación programa Ramón y Cajal” (RYC201722666 to T.F).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global Status Report on Alcohol and Health. World Health Organization (WHO); Geneva, Italy: 2018. [Google Scholar]
- 2.Castillo-Carniglia A., Keyes K.M., Hasin D.S., Cerdá M. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatry. 2019;6:1068–1080. doi: 10.1016/S2215-0366(19)30222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams S.H. Medications for treating alcohol dependence. Am. Fam. Physician. 2005;72:1775–1780. [PubMed] [Google Scholar]
- 4.Goh E.T., Morgan M.Y. Review article: Pharmacotherapy for alcohol dependence—The why, the what and the wherefore. Aliment. Pharmacol. Ther. 2017;45:865–882. doi: 10.1111/apt.13965. [DOI] [PubMed] [Google Scholar]
- 5.Finney J.W., Hahn A.C., Moos R.H. The effectiveness of inpatient and outpatient treatment for alcohol abuse: The need to focus on mediators and moderators of setting effects. Addiction. 1996;91:1773–1796; discussion 1803–1820. doi: 10.1111/j.1360-0443.1996.tb03801.x. [DOI] [PubMed] [Google Scholar]
- 6.Swift R.M. Drug Therapy for Alcohol Dependence. N. Engl. J. Med. 1999;340:1482–1490. doi: 10.1056/NEJM199905133401907. [DOI] [PubMed] [Google Scholar]
- 7.Getachew B., Hauser S.R., Dhaher R., Katner S.N., Bell R.L., Oster S.M., McBride W.J., Rodd Z.A. CB1 receptors regulate alcohol-seeking behavior and alcohol self-administration of alcohol-preferring (P) rats. Pharmacol. Biochem. Behav. 2011;97:669–675. doi: 10.1016/j.pbb.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varodayan F.P., Soni N., Bajo M., Luu G., Madamba S.G., Schweitzer P., Parsons L.H., Roberto M. Chronic ethanol exposure decreases CB1receptor function at GABAergic synapses in the rat central amygdala. Addict. Biol. 2016;21:788–801. doi: 10.1111/adb.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hungund B.L., Szakall I., Adam A., Basavarajappa B., Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J. Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 10.Vinod K.Y., Maccioni P., Garcia-Gutierrez M.S., Femenia T., Xie S., Carai M.A.M., Manzanares J., Cooper T.B., Hungund B.L., Colombo G. Innate difference in the endocannabinoid signaling and its modulation by alcohol consumption in alcohol-preferring sP rats. Addict. Biol. 2012;17:62–75. doi: 10.1111/j.1369-1600.2010.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Femenía T., García-Gutiérrez M.S., Manzanares J. CB1 Receptor Blockade Decreases Ethanol Intake and Associated Neurochemical Changes in Fawn-Hooded Rats. Alcohol. Clin. Exp. Res. 2010;34:131–141. doi: 10.1111/j.1530-0277.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- 12.Alen F., Moreno-Sanz G., de Tena A.I., Brooks R.D., López-Jimenez A., Navarro M., López-Moreno J.A. Pharmacological activation of CB1 and D2 receptors in rats: Predominant role of CB1 in the increase of alcohol relapse. Eur. J. Neurosci. 2008;27:3292–3298. doi: 10.1111/j.1460-9568.2008.06302.x. [DOI] [PubMed] [Google Scholar]
- 13.Colombo G., Serra S., Vacca G., Carai M., Gessa G. Endocannabinoid system and alcohol addiction: Pharmacological studies. Pharmacol. Biochem. Behav. 2005;81:369–380. doi: 10.1016/j.pbb.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Hauser S.R., Katner S.N., Waeiss R.A., Truitt W.A., Bell R.L., McBride W.J., Rodd Z.A. Selective breeding for high alcohol preference is associated with increased sensitivity to cannabinoid reward within the nucleus accumbens shell. Pharmacol. Biochem. Behav. 2020;197:173002. doi: 10.1016/j.pbb.2020.173002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubio M., Fernández-Ruiz J., de Miguel R., Maestro B., Walker J.M., Ramos J.A. CB1 receptor blockade reduces the anxiogenic-like response and ameliorates the neurochemical imbalances associated with alcohol withdrawal in rats. Neuropharmacology. 2008;54:976–988. doi: 10.1016/j.neuropharm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Nowak K.L., Vinod K.Y., Hungund B.L. Pharmacological manipulation of cb1 receptor function alters development of tolerance to alcohol. Alcohol. Alcohol. 2005;41:24–32. doi: 10.1093/alcalc/agh217. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y., Hitchcock S.A. Targeting cannabinoid agonists for inflammatory and neuropathic pain. Expert Opin. Investig. Drugs. 2007;16:951–965. doi: 10.1517/13543784.16.7.951. [DOI] [PubMed] [Google Scholar]
- 18.Taylor B.N., Mueller M., Sauls R.S. StatPearls (Internet) StatPearls Publishing; Treasure Island, FL, USA: 2021. Cannaboinoid Antiemetic Therapy. [PubMed] [Google Scholar]
- 19.Christensen R., Kristensen P.K., Bartels E.M., Bliddal H., Astrup A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 20.Moreira F.A., Crippa J.A.S. The psychiatric side-effects of rimonabant. Rev. Bras. Psiquiatr. 2009;31:145–153. doi: 10.1590/S1516-44462009000200012. [DOI] [PubMed] [Google Scholar]
- 21.Le Foll B., Gorelick D.A., Goldberg S.R. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology. 2009;205:171–174. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sam A.H., Salem V., Ghatei M.A. Rimonabant: From RIO to Ban. J. Obes. 2011;2011:432607. doi: 10.1155/2011/432607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Gutierrez M.S., Pérez-Ortiz J.M., Gutiérrez-Adán A., Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB2 receptors. Br. J. Pharmacol. 2010;160:1773–1784. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Gutiérrez M.S., Ortega-Álvaro A., Busquets-García A., Pérez-Ortiz J.M., Caltana L., Ricatti M.J., Brusco A., Maldonado R., Manzanares J. Synaptic plasticity alterations associated with memory impairment induced by deletion of CB2 cannabinoid receptors. Neuropharmacology. 2013;73:388–396. doi: 10.1016/j.neuropharm.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 25.Gong J.-P., Onaivi E.S., Ishiguro H., Liu Q.-R., Tagliaferro P.A., Brusco A., Uhl G.R. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 26.García-Gutiérrez M.S., Manzanares J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J. Psychopharmacol. 2011;25:111–120. doi: 10.1177/0269881110379507. [DOI] [PubMed] [Google Scholar]
- 27.Onaivi E.S. Neuropsychobiological Evidence for the Functional Presence and Expression of Cannabinoid CB2 Receptors in the Brain. Neuropsychobiology. 2006;54:231–246. doi: 10.1159/000100778. [DOI] [PubMed] [Google Scholar]
- 28.Onaivi E.S., Ishiguro H., Gong J.-P., Patel S., Meozzi P.A., Myers L., Perchuk A., Mora Z., Tagliaferro P.A., Gardner E., et al. Brain Neuronal CB2 Cannabinoid Receptors in Drug Abuse and Depression: From Mice to Human Subjects. PLoS ONE. 2008;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onaivi E.S., Ishiguro H., Gong J.-P., Patel S., Meozzi P.A., Myers L., Perchuk A., Mora Z., Tagliaferro P.A., Gardner E., et al. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann. N. Y. Acad. Sci. 2008;1139:434–449. doi: 10.1196/annals.1432.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onaivi E.S., Ishiguro H., Gu S., Liu Q.-R. CNS effects of CB2 cannabinoid receptors: Beyond neuro-immuno-cannabinoid activity. J. Psychopharmacol. 2012;26:92–103. doi: 10.1177/0269881111400652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouaboula M., Poinot-Chazel C., Marchand J., Canat X., Bourrie B., Rinaldi-Carmona M., Calandra B., Le Fur G., Casellas P. Signaling Pathway Associated with Stimulation of CB2 Peripheral Cannabinoid Receptor. Involvement of Both Mitogen-Activated Protein Kinase and Induction of Krox-24 Expression. Eur. J. Biochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- 32.Soethoudt M., Grether U., Fingerle J., Grim T.W., Fezza F., De Petrocellis L., Ullmer C., Rothenhäusler B., Perret C., Van Gils N., et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat. Commun. 2017;8:13958. doi: 10.1038/ncomms13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhee M.-H., Bayewitch M., Avidor-Reiss T., Levy R., Vogel Z. Cannabinoid Receptor Activation Differentially Regulates the Various Adenylyl Cyclase Isozymes. J. Neurochem. 1998;71:1525–1534. doi: 10.1046/j.1471-4159.1998.71041525.x. [DOI] [PubMed] [Google Scholar]
- 34.Börner C., Smida M., Höllt V., Schraven B., Kraus J. Cannabinoid Receptor Type 1- and 2-mediated Increase in Cyclic AMP Inhibits T Cell Receptor-triggered Signaling. J. Biol. Chem. 2009;284:35450–35460. doi: 10.1074/jbc.M109.006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nogueras-Ortiz C., Roman-Vendrell C., Mateo-Semidey G.E., Liao Y.-H., Kendall D.A., Yudowski G.A. Retromer stops beta-arrestin 1–mediated signaling from internalized cannabinoid 2 receptors. Mol. Biol. Cell. 2017;28:3554–3561. doi: 10.1091/mbc.e17-03-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoemaker J.L., Ruckle M.B., Mayeux P.R., Prather P.L. Agonist-Directed Trafficking of Response by Endocannabinoids Acting at CB2 Receptors. J. Pharmacol. Exp. Ther. 2005;315:828–838. doi: 10.1124/jpet.105.089474. [DOI] [PubMed] [Google Scholar]
- 37.Correa F., Mestre L., Docagne F., Guaza C. Activation of cannabinoid CB2 receptor negatively regulates IL-12p40 production in murine macrophages: Role of IL-10 and ERK1/2 kinase signaling. Br. J. Pharmacol. 2005;145:441–448. doi: 10.1038/sj.bjp.0706215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aracil-Fernández A., Trigo J.M., García-Gutiérrez M.S., Álvaro A.O., Ternianov A., Navarro D., Robledo P., Berbel P., Maldonado R., Manzanares J. Decreased Cocaine Motor Sensitization and Self-Administration in Mice Overexpressing Cannabinoid CB2 Receptors. Neuropsychopharmacology. 2012;37:1749–1763. doi: 10.1038/npp.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Sickle M.D., Duncan M., Kingsley P.J., Mouihate A., Urbani P., Mackie K., Stella N., Makriyannis A., Piomelli D., Davison J.S., et al. Identification and Functional Characterization of Brainstem Cannabinoid CB 2 Receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 40.Stempel A.V., Stumpf A., Zhang H.-Y., Özdoğan T., Pannasch U., Theis A.-K., Otte D.-M., Wojtalla A., Rácz I., Ponomarenko A., et al. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron. 2016;90:795–809. doi: 10.1016/j.neuron.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J., Li Y. Chronic activation of CB2 cannabinoid receptors in the hippocampus increases excitatory synaptic transmission. J. Physiol. 2015;593:871–886. doi: 10.1113/jphysiol.2014.286633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan C., Xi Z.-X. Progress in brain cannabinoid CB2 receptor research: From genes to behavior. Neurosci. Biobehav. Rev. 2019;98:208–220. doi: 10.1016/j.neubiorev.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onaivi E.S., Ishiguro H., Gong J.P., Patel S., Perchuk A., Meozzi P.A., Myers L., Mora Z., Tagliaferro P., Gardner E., et al. Discovery of the Presence and Functional Expression of Cannabinoid CB2 Receptors in Brain. Ann. N. Y. Acad. Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- 44.Liu Q.R., Pan C.H., Hishimoto A., Li C.Y., Xi Z.X., Llorente-Berzal A., Viveros M.P., Ishiguro H., Arinami T., Onaivi E.S., et al. Species differences in cannabinoid receptor 2 (CNR2): Identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009;8:519–530. doi: 10.1111/j.1601-183X.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma Z., Gao F., Larsen B., Gao M., Luo Z., Chen D., Ma X., Qiu S., Zhou Y., Xie J., et al. Mechanisms of cannabinoid CB2 receptor-mediated reduction of dopamine neuronal excitability in mouse ventral tegmental area. eBioMedicine. 2019;42:225–237. doi: 10.1016/j.ebiom.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H.Y., Gao M., Liu Q.-R., Bi G.-H., Li X., Yang H.-J., Gardner E.L., Wu J., Xi Z.-X. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc. Natl. Acad. Sci. USA. 2014;111:E5007–E5015. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia M.C., Cinquina V., Palomo-Garo C., Rábano A., Fernández-Ruiz J. Identification of CB2 receptors in human nigral neurons that degenerate in Parkinson’s disease. Neurosci. Lett. 2015;587:1–4. doi: 10.1016/j.neulet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 48.López-Ramírez G., Sánchez-Zavaleta R., Ávalos-Fuentes A., Sierra J.J., Paz-Bermúdez F., Leyva-Gómez G., Vila J.S., Cortés H., Florán B. D2autoreceptor switches CB2receptor effects on [3H]-dopamine release in the striatum. Synapse. 2019;74:e22139. doi: 10.1002/syn.22139. [DOI] [PubMed] [Google Scholar]
- 49.Sánchez-Zavaleta R., Cortés H., Avalos-Fuentes J.A., García U., Vila J.S., Erlij D., Florán B. Presynaptic cannabinoid CB2 receptors modulate [3H]-Glutamate release at subthalamo-nigral terminals of the rat. Synapse. 2018;72:e22061. doi: 10.1002/syn.22061. [DOI] [PubMed] [Google Scholar]
- 50.Morgan N.H., Stanford I.M., Woodhall G.L. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology. 2009;57:356–368. doi: 10.1016/j.neuropharm.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 51.Sadanandan S.M., Kreko-Pierce T., Khatri S.N., Pugh J.R. Cannabinoid type 2 receptors inhibit GABAA receptor-mediated currents in cerebellar Purkinje cells of juvenile mice. PLoS ONE. 2020;15:e0233020. doi: 10.1371/journal.pone.0233020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García-Gutiérrez M.S., Navarrete F., Navarro G., Reyes-Resina I., Franco R., Lanciego J.L., Giner S., Manzanares J. Alterations in Gene and Protein Expression of Cannabinoid CB2 and GPR55 Receptors in the Dorsolateral Prefrontal Cortex of Suicide Victims. Neurotherapeutics. 2018;15:796–806. doi: 10.1007/s13311-018-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franklin A., Stella N. Arachidonylcyclopropylamide increases microglial cell migration through cannabinoid CB2 and abnormal-cannabidiol-sensitive receptors. Eur. J. Pharmacol. 2003;474:195–198. doi: 10.1016/S0014-2999(03)02074-0. [DOI] [PubMed] [Google Scholar]
- 55.López A., Aparicio N., Pazos M.R., Grande M.T., Barreda-Manso M.A., Cuesta I.B., Vázquez C., Amores M., Ruiz-Pérez G., García-García E., et al. Cannabinoid CB2 receptors in the mouse brain: Relevance for Alzheimer’s disease. J. Neuroinflamm. 2018;15:158. doi: 10.1186/s12974-018-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Núñez E., Benito C., Tolón R.M., Hillard C.J., Griffin W.S.T., Romero J. Glial expression of cannabinoid CB2 receptors and fatty acid amide hydrolase are beta amyloid–linked events in Down’s syndrome. Neuroscience. 2008;151:104–110. doi: 10.1016/j.neuroscience.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 57.Komorowska-Müller J.A., Rana T., Olabiyi B.F., Zimmer A., Schmöle A.-C. Cannabinoid Receptor 2 Alters Social Memory and Microglial Activity in an Age-Dependent Manner. Molecules. 2021;26:5984. doi: 10.3390/molecules26195984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tao Y., Jiang B., Feng Z., Yang L., Tang J., Chen Q., Zhang J., Tan Q., Feng H., Chen Z. Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model. Brain Behav. Immun. 2016;58:118–129. doi: 10.1016/j.bbi.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 59.Benito C., Tolón R.M., Pazos M.R., Núñez E., Castillo A.I., Romero J. Cannabinoid CB2 receptors in human brain inflammation. J. Cereb. Blood Flow Metab. 2008;153:277–285. doi: 10.1038/sj.bjp.0707505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishiguro H., Iwasaki S., Teasenfitz L., Higuchi S., Horiuchi Y., Saito T., Arinami T., Onaivi E.S. Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. Pharmacogenomics J. 2007;7:380–385. doi: 10.1038/sj.tpj.6500431. [DOI] [PubMed] [Google Scholar]
- 61.Ledesma J.C., Rodríguez-Arias M., Gavito A.L., Sánchez-Pérez A.M., Viña J., Medina Vera D., Rodríguez de Fonseca F., Miñarro J. Adolescent binge-ethanol accelerates cognitive impairment and beta-amyloid production and dysregulates endocannabinoid signaling in the hippocampus of APP/PSE mice. Addict. Biol. 2021;26:e12883. doi: 10.1111/adb.12883. [DOI] [PubMed] [Google Scholar]
- 62.Coelhoso C.C., Engelke D.S., Filev R., Silveira D.X., Mello L.E., Junior J.G.D.S. Temporal and Behavioral Variability in Cannabinoid Receptor Expression in Outbred Mice Submitted to Ethanol-Induced Locomotor Sensitization Paradigm. Alcohol. Clin. Exp. Res. 2013;37:1516–1526. doi: 10.1111/acer.12130. [DOI] [PubMed] [Google Scholar]
- 63.Sánchez-Marín L., Pavon F.J., Decara J., Suarez J., Gavito A., Castilla-Ortega E., De Fonseca F.R., Serrano A. Effects of Intermittent Alcohol Exposure on Emotion and Cognition: A Potential Role for the Endogenous Cannabinoid System and Neuroinflammation. Front. Behav. Neurosci. 2017;11:15. doi: 10.3389/fnbeh.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sánchez-Marín L., Gavito A., Decara J., Pastor A., Castilla-Ortega E., Suarez J., de la Torre R., Pavon F., de Fonseca F.R., Serrano A. Impact of intermittent voluntary ethanol consumption during adolescence on the expression of endocannabinoid system and neuroinflammatory mediators. Eur. Neuropsychopharmacol. 2020;33:126–138. doi: 10.1016/j.euroneuro.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Serrano A., Rivera P., Pavon F.J., Decara J., Suárez J., de Fonseca F.R., Parsons L.H. Differential Effects of Single Versus Repeated Alcohol Withdrawal on the Expression of Endocannabinoid System-Related Genes in the Rat Amygdala. Alcohol. Clin. Exp. Res. 2012;36:984–994. doi: 10.1111/j.1530-0277.2011.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marco E.M., Ballesta J.A., Irala C., Hernández M.-D., Serrano M.E., Mela V., López-Gallardo M., Viveros M.-P. Sex-dependent influence of chronic mild stress (CMS) on voluntary alcohol consumption; study of neurobiological consequences. Pharmacol. Biochem. Behav. 2017;152:68–80. doi: 10.1016/j.pbb.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Sánchez-Marín L., Flores-López M., Pastor A., Gavito A.L., Suárez J., de la Torre R., Pavón F.J., de Fonseca F.R., Serrano A. Acute stress and alcohol exposure during adolescence result in an anxious phenotype in adulthood: Role of altered glutamate/endocannabinoid transmission mechanisms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;113:110460. doi: 10.1016/j.pnpbp.2021.110460. [DOI] [PubMed] [Google Scholar]
- 68.Ortega-Álvaro A., Ternianov A., Aracil-Fernández A., Navarrete F., García-Gutiérrez M.S., Manzanares J. Role of cannabinoid CB2 receptor in the reinforcing actions of ethanol. Addict. Biol. 2015;20:43–55. doi: 10.1111/adb.12076. [DOI] [PubMed] [Google Scholar]
- 69.Powers M.S., Breit K.R., Chester J.A. Genetic Versus Pharmacological Assessment of the Role of Cannabinoid Type 2 Receptors in Alcohol Reward-Related Behaviors. Alcohol. Clin. Exp. Res. 2015;39:2438–2446. doi: 10.1111/acer.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pradier B., Erxlebe E., Markert A., Rácz I. Interaction of cannabinoid receptor 2 and social environment modulates chronic alcohol consumption. Behav. Brain Res. 2015;287:163–171. doi: 10.1016/j.bbr.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 71.Canseco-Alba A., Schanz N., Ishiguro H., Liu Q.-R., Onaivi E.S. Behavioral Evaluation of Seeking and Preference of Alcohol in Mice Subjected to Stress. BIO-PROTOCOL. 2018;8:e3061. doi: 10.21769/BioProtoc.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al Mansouri S., Ojha S., Al Maamari E., Al Ameri M., Nurulain S.M., Bahi A. The cannabinoid receptor 2 agonist, β-caryophyllene, reduced voluntary alcohol intake and attenuated ethanol-induced place preference and sensitivity in mice. Pharmacol. Biochem. Behav. 2014;124:260–268. doi: 10.1016/j.pbb.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 73.Liu Q.-R., Canseco-Alba A., Zhang H.-Y., Tagliaferro P., Chung M., Dennis E., Sanabria B., Schanz N., Escosteguy-Neto J.C., Ishiguro H., et al. Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci. Rep. 2017;7:17410. doi: 10.1038/s41598-017-17796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Navarrete F., García-Gutiérrez M.S., Manzanares J. Pharmacological regulation of cannabinoid CB2 receptor modulates the reinforcing and motivational actions of ethanol. Biochem. Pharmacol. 2018;157:227–234. doi: 10.1016/j.bcp.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 75.Martín-Sánchez A., Warnault V., Montagud-Romero S., Pastor A., Mondragón N., De La Torre R., Valverde O. Alcohol-induced conditioned place preference is modulated by CB2 cannabinoid receptors and modifies levels of endocannabinoids in the mesocorticolimbic system. Pharmacol. Biochem. Behav. 2019;183:22–31. doi: 10.1016/j.pbb.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 76.Rivera P., Blanco E., Bindila L., Alen F., Vargas A., Rubio L., Pavon F.J., Serrano A., Lutz B., De Fonseca F.R., et al. Pharmacological activation of CB2 receptors counteracts the deleterious effect of ethanol on cell proliferation in the main neurogenic zones of the adult rat brain. Front. Cell Neurosci. 2015;9:379. doi: 10.3389/fncel.2015.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rivera P., Fernández-Arjona M., Silva-Peña D., Blanco E., Vargas A., López-Ávalos M.D., Grondona J.M., Serrano A., Pavón F.J., de Fonseca F.R., et al. Pharmacological blockade of fatty acid amide hydrolase (FAAH) by URB597 improves memory and changes the phenotype of hippocampal microglia despite ethanol exposure. Biochem. Pharmacol. 2018;157:244–257. doi: 10.1016/j.bcp.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 78.Agudelo M., Yndart A., Morrison M., Figueroa G., Muñoz K., Samikkannu T., Nair M.P. Differential expression and functional role of cannabinoid genes in alcohol users. Drug Alcohol Depend. 2013;133:789–793. doi: 10.1016/j.drugalcdep.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koob G., Volkow N. Neurocircuitry of Addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Chiara G., Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Navarrete F., Rodriguez-Arias M., Martin-García E., Navarro D., García-Gutiérrez M.S., Aguilar M.A., Aracil-Fernández A., Berbel P., Miñarro J., Maldonado R., et al. Role of CB2 Cannabinoid Receptors in the Rewarding, Reinforcing, and Physical Effects of Nicotine. Neuropsychopharmacology. 2013;38:2515–2524. doi: 10.1038/npp.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H.-Y., De Biase L., Chandra R., Shen H., Liu Q.-R., Gardner E., Lobo M.K., Xi Z.-X. Repeated cocaine administration upregulates CB2 receptor expression in striatal medium-spiny neurons that express dopamine D1 receptors in mice. Acta Pharmacol. Sin. 2021;43:876–888. doi: 10.1038/s41401-021-00712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Franklin J.M., de Souza R.K.B., Carrasco G.A. Cannabinoid 2 receptors regulate dopamine 2 receptor expression by a beta-arrestin 2 and GRK5-dependent mechanism in neuronal cells. Neurosci. Lett. 2021;753:135883. doi: 10.1016/j.neulet.2021.135883. [DOI] [PubMed] [Google Scholar]
- 84.Xi Z.X., Peng X.Q., Li X., Song R., Zhang H.Y., Liu Q.R., Yang H.J., Bi G.H., Li J., Gardner E.L. Brain cannabinoid CB receptors modulate cocaine’s actions in mice. Nat. Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jordan C.J., Feng Z.-W., Galaj E., Bi G.-H., Xue Y., Liang Y., McGuire T., Xie X.-Q., Xi Z.-X. Xie2-64, a novel CB2 receptor inverse agonist, reduces cocaine abuse-related behaviors in rodents. Neuropharmacology. 2020;176:108241. doi: 10.1016/j.neuropharm.2020.108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Canseco-Alba A., Schanz N., Sanabria B., Zhao J., Lin Z., Liu Q.-R., Onaivi E.S. Behavioral effects of psychostimulants in mutant mice with cell-type specific deletion of CB2 cannabinoid receptors in dopamine neurons. Behav. Brain Res. 2019;360:286–297. doi: 10.1016/j.bbr.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamamoto T., Takada K. Role of Cannabinoid Receptor in the Brain as It Relates to Drug Reward. Jpn. J. Pharmacol. 2000;84:229–236. doi: 10.1254/jjp.84.229. [DOI] [PubMed] [Google Scholar]
- 88.Childers S.R., Fleming L., Konkoy C., Marckel D., Pacheco M., Sexton T., Ward S. Opioid and Cannabinoid Receptor Inhibition of Adenylyl Cyclase in Brain. Ann. N. Y. Acad. Sci. 1992;654:33–51. doi: 10.1111/j.1749-6632.1992.tb25954.x. [DOI] [PubMed] [Google Scholar]
- 89.Manzanares J., Corchero J., Romero J., Fernández-Ruiz J.J., Ramos J.A., Fuentes J.A. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol. Sci. 1999;20:287–294. doi: 10.1016/S0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- 90.Scavone J., Sterling R., Van Bockstaele E. Cannabinoid and opioid interactions: Implications for opiate dependence and withdrawal. Neuroscience. 2013;248:637–654. doi: 10.1016/j.neuroscience.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yorgason J.T., Wadsworth H.A., Anderson E.J., Williams B.M., Brundage J.N., Hedges D.M., Stockard A.L., Jones S.T., Arthur S.B., Hansen D.M., et al. Modulation of dopamine release by ethanol is mediated by atypical GABAA receptors on cholinergic interneurons in the nucleus accumbens. Addict. Biol. 2022;27:e13108. doi: 10.1111/adb.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao F., Chen D., Ma X., Sudweeks S., Yorgason J.T., Gao M., Turner D., Eaton J.B., McIntosh J.M., Lukas R.J., et al. Alpha6-containing nicotinic acetylcholine receptor is a highly sensitive target of alcohol. Neuropharmacology. 2019;149:45–54. doi: 10.1016/j.neuropharm.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vergara V.M., Weiland B.J., Hutchison K.E., Calhoun V.D. The Impact of Combinations of Alcohol, Nicotine, and Cannabis on Dynamic Brain Connectivity. Neuropsychopharmacology. 2018;43:877–890. doi: 10.1038/npp.2017.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zaniewska M., McCreary A.C., Przegaliński E., Filip M. Evaluation of the role of nicotinic acetylcholine receptor subtypes and cannabinoid system in the discriminative stimulus effects of nicotine in rats. Eur. J. Pharmacol. 2006;540:96–106. doi: 10.1016/j.ejphar.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 95.Gamaleddin I., Wertheim C., Zhu A.Z., Coen K.M., Vemuri K., Makryannis A., Goldberg S.R., Le Foll B. Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict. Biol. 2012;17:47–61. doi: 10.1111/j.1369-1600.2011.00314.x. [DOI] [PubMed] [Google Scholar]
- 96.Gamaleddin I., Zvonok A., Makriyannis A., Goldberg S.R., Le Foll B. Effects of a Selective Cannabinoid CB2 Agonist and Antagonist on Intravenous Nicotine Self Administration and Reinstatement of Nicotine Seeking. PLoS ONE. 2012;7:e29900. doi: 10.1371/journal.pone.0029900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ignatowska-Jankowska B.M., Muldoon P.P., Lichtman A.H., Damaj M.I. The cannabinoid CB2 receptor is necessary for nicotine-conditioned place preference, but not other behavioral effects of nicotine in mice. Psychopharmacology. 2013;229:591–601. doi: 10.1007/s00213-013-3117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nasehi M., Forouzanmehr E., Khakpai F., Zarrindast M.-R. Possible interaction between the ventral hippocampal cannabinoid CB2 and muscarinic acetylcholine receptors on the modulation of memory consolidation in mice. NeuroReport. 2020;31:174–183. doi: 10.1097/WNR.0000000000001381. [DOI] [PubMed] [Google Scholar]
- 99.Nennig S., Schank J. The Role of NFkB in Drug Addiction: Beyond Inflammation. Alcohol Alcohol. 2017;52:172–179. doi: 10.1093/alcalc/agw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crews F.T., Walter T.J., Coleman L.G., Vetreno R.P. Toll-like receptor signaling and stages of addiction. Psychopharmacology. 2017;234:1483–1498. doi: 10.1007/s00213-017-4560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Namba M.D., Leyrer-Jackson J.M., Nagy E.K., Olive M.F., Neisewander J.L. Neuroimmune Mechanisms as Novel Treatment Targets for Substance Use Disorders and Associated Comorbidities. Front. Neurosci. 2021;15:650785. doi: 10.3389/fnins.2021.650785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Loftis J.M., Huckans M. Substance use disorders: Psychoneuroimmunological mechanisms and new targets for therapy. Pharmacol. Ther. 2013;139:289–300. doi: 10.1016/j.pharmthera.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martinez P., Lien L., Zemore S., Bramness J.G., Neupane S.P. Circulating cytokine levels are associated with symptoms of depression and anxiety among people with alcohol and drug use disorders. J. Neuroimmunol. 2018;318:80–86. doi: 10.1016/j.jneuroim.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tancredi V., D’Antuono M., Cafè C., Giovedì S., Buè M.C., D’Arcangelo G., Onofri F., Benfenati F. The Inhibitory Effects of Interleukin-6 on Synaptic Plasticity in the Rat Hippocampus Are Associated with an Inhibition of Mitogen-Activated Protein Kinase ERK. J. Neurochem. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- 105.Schneider H., Pitossi F., Balschun D., Wagner A., Del Rey A., Besedovsky H. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc. Natl. Acad. Sci. USA. 1998;95:7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Femenia T., Qian Y., Arentsen T., Forssberg H., Heijtz R.D. Toll-like receptor-4 regulates anxiety-like behavior and DARPP-32 phosphorylation. Brain, Behav. Immun. 2018;69:273–282. doi: 10.1016/j.bbi.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 107.Lippai D., Bala S., Csak T., Kurt-Jones E.A., Szabo G. Chronic Alcohol-Induced microRNA-155 Contributes to Neuroinflammation in a TLR4-Dependent Manner in Mice. PLoS ONE. 2013;8:e70945. doi: 10.1371/journal.pone.0070945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Montesinos J., Gil A., Guerri C. Nalmefene Prevents Alcohol-Induced Neuroinflammation and Alcohol Drinking Preference in Adolescent Female Mice: Role of TLR4. Alcohol. Clin. Exp. Res. 2017;41:1257–1270. doi: 10.1111/acer.13416. [DOI] [PubMed] [Google Scholar]
- 109.Zhang P., Yang M., Chen C., Liu L., Wei X., Zeng S. Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility. Front. Immunol. 2020;11:1455. doi: 10.3389/fimmu.2020.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zoppi S., Madrigal J.L., Caso J.R., García-Gutiérrez M.S., Manzanares J., Leza J.C., García-Bueno B. Regulatory role of the cannabinoid CB2receptor in stress-induced neuroinflammation in mice. Br. J. Pharmacol. 2014;171:2814–2826. doi: 10.1111/bph.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pan S.D., Grandgirard D., Leib S.L. Adjuvant Cannabinoid Receptor Type 2 Agonist Modulates the Polarization of Microglia Towards a Non-Inflammatory Phenotype in Experimental Pneumococcal Meningitis. Front. Cell. Infect. Microbiol. 2020;10:588195. doi: 10.3389/fcimb.2020.588195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ali A.M., El-Tawil O.S., Al-Mokaddem A.K., Abd El-Rahman S.S. Promoted inhibition of TLR4/miR-155/ NFkB p65 signaling by cannabinoid receptor 2 agonist (AM1241), aborts inflammation and progress of hepatic fibrosis induced by thioacetamide. Chem. Biol. Interact. 2021;336:109398. doi: 10.1016/j.cbi.2021.109398. [DOI] [PubMed] [Google Scholar]
- 113.Jing N., Fang B., Li Z. Tian A Exogenous activation of cannabinoid-2 receptor modulates TLR4/MMP9 expression in a spinal cord ischemia reperfusion rat model. J. Neuroinflamm. 2020;17:101. doi: 10.1186/s12974-020-01784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lanciego J.L., Barroso-Chinea P., Rico A.J., Conte-Perales L., Callén L., Roda E., Gómez-Bautista V., López I.P., Lluis C., Labandeira-García J.L., et al. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J. Psychopharmacol. 2010;25:97–104. doi: 10.1177/0269881110367732. [DOI] [PubMed] [Google Scholar]
- 115.Li Y., Kim J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience. 2015;311:253–267. doi: 10.1016/j.neuroscience.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Y., Kim J. Deletion of CB2 cannabinoid receptors reduces synaptic transmission and long-term potentiation in the mouse hippocampus. Hippocampus. 2016;26:275–281. doi: 10.1002/hipo.22558. [DOI] [PubMed] [Google Scholar]
- 117.Den Boon F.S., Chameau P., Houthuijs K., Bolijn S., Mastrangelo N., Kruse C.G., Maccarrone M., Wadman W.J., Werkman T.R. Endocannabinoids produced upon action potential firing evoke a Cl− current via type-2 cannabinoid receptors in the medial prefrontal cortex. Pflugers Arch. 2014;466:2257–2268. doi: 10.1007/s00424-014-1502-6. [DOI] [PubMed] [Google Scholar]
- 118.Atwood B.K., Straiker A., Mackie K. CB2 cannabinoid receptors inhibit synaptic transmission when expressed in cultured autaptic neurons. Neuropharmacology. 2012;63:514–523. doi: 10.1016/j.neuropharm.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]