Abstract
Pregnant women are at increased risk of adverse outcomes resulting from SARS-CoV-2 infection, including preeclampsia and preterm birth. Pregnancy imprints specific maternal immune responses that can modulate host susceptibility to microbial infection, and therefore recent studies have focused on the humoral response against SARS-CoV-2 in pregnant women. However, the pregnancy-specific cellular immune responses triggered by SARS-CoV-2 infection are poorly understood. Herein, we undertook an extensive in vitro investigation to determine the cellular immune responses to SARS-CoV-2 particles and proteins/peptides in pregnant women. First, we show that SARS-CoV-2 particles do not alter the pregnancy-specific oxidative burst of neutrophils and monocytes. Yet, SARS-CoV-2 particles/proteins shift monocyte activation from the classical to intermediate states in pregnant, but not non-pregnant, women. Furthermore, SARS-CoV-2 proteins, but not particles or peptide pools, mildly enhance T-cell activation during pregnancy. As expected, B-cell phenotypes are heavily modulated by SARS-CoV-2 particles in all women; yet, pregnancy itself further modified such responses in these adaptive immune cells. Lastly, we report that pregnancy itself governs cytokine responses in the maternal circulation, of which IFN-β and IL-8 are diminished upon SARS-CoV-2 challenge. Collectively, these findings highlight the differential in vitro responses to SARS-CoV-2 in pregnant and non-pregnant women, and shed light on the immune mechanisms implicated in COVID-19 during pregnancy.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1) is still an ongoing global catastrophe (2, 3). Nearly 400 million people have been infected and over 5.5 million have died globally (2). Notably, women are more likely to exhibit reduced COVID-19 severity and mortality compared to men (4, 5). However, pregnant women are not included in such a disparity, given their increased risk of adverse outcomes associated with COVID-19 including admission to the intensive care unit, mechanical ventilation, and death (6–8). Furthermore, pregnant women with COVID-19 are more likely to undergo obstetrical complications (9–11) as evidenced by a correlation between the severity of SARS-CoV-2 infection in pregnancy and the risk of preeclampsia (9), preterm birth (9, 10), and stillbirth (11). Yet, SARS-CoV-2 is not the only virus to which pregnant women are more susceptible (7, 12). Increased risk of adverse outcomes in pregnant women has also been reported for other viruses such as influenza, SARS-CoV-1, and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) (13–15). Importantly, neonates born to women exposed to viruses during pregnancy can subsequently develop chronic diseases during adulthood, as evidenced by the increased prevalence of cardiovascular disease in adults born to women affected by the Spanish flu in 1918 (16, 17). Therefore, elucidating the underlying mechanisms leading to such heightened susceptibility during pregnancy is timely.
Pregnancy is a unique immunological state in which the maternal immune system undergoes complex and tightly regulated adaptations to allow the mother to tolerate the semi-allogeneic fetus (18–21) and vice versa (22–24). This is contrary to the historical view that pregnancy was an immunosuppressive state (25–27). Indeed, the innate immune limb of pregnant women is strengthened in its capacity to deal with viral or bacterial infections, showing increased granulocyte number and oxidative burst as well as altered phenotypes in basal and stimulated conditions (28–30). On the other hand, pregnant women display decreased numbers of T and B cells in the peripheral blood (30); yet, they exhibit enhanced immune cellular components of innate and adaptive origin such as homeostatic macrophages (31, 32) and regulatory T cells (33–36). Hence, pregnancy itself imprints specific maternal immune responses (37), which can dictate susceptibility or resistance to microbes. Notably, recent studies have characterized the humoral (i.e., antibody-mediated) response toward SARS-CoV-2 antigens in pregnant and non-pregnant women (38–40). However, the pregnancy-specific cellular immune responses implicated in SARS-CoV-2 infection are poorly understood.
In the current study, we performed an extensive in vitro investigation to evaluate the cellular immune responses to SARS-CoV-2 particles and proteins/peptides in pregnant women and compare such to non-pregnant women. First, we explored whether SARS-CoV-2 particles alter reactive oxygen species (ROS) production by neutrophils and monocytes as a readout of the acute innate host response. Next, monocyte phenotypes and activation status in response to SARS-CoV-2 particles and proteins/peptides were characterized using in vitro assays coupled with flow cytometry. Furthermore, T-cell activation, as evidenced by the expression of surface markers and effector molecules, and B-cell phenotypes were also evaluated in response to SARS-CoV-2 particles and proteins/peptides. Finally, using multiplex immunoassays, cytokine release was determined in peripheral blood mononuclear cells (PBMCs) and in whole blood TruCulture® systems in response to SARS-CoV-2 particles and Spike (S) protein, respectively.
MATERIALS AND METHODS
Human subjects, clinical specimens, and definitions
Peripheral blood samples were collected from healthy non-pregnant and pregnant women. All women were recruited into research protocols of the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), U.S. Department of Health and Human Services, Wayne State University (Detroit, MI, USA), and the Detroit Medical Center (DMC) (Detroit, MI, USA). The collection and use of human materials for research purposes were approved by the Institutional Review Boards of Wayne State University School of Medicine and Detroit Medical Center. All participating women provided written informed consent prior to sample collection. The maternal peripheral blood of pregnant women was collected in the third trimester prior to the administration of any medication. The non-pregnant group included healthy women of reproductive age from the same community as the pregnant patients. Women who self-reported as testing positive for SARS-CoV-2, having COVID-19, or receiving a vaccine were excluded from the study.
Reactive oxygen species (ROS) production by neutrophils and monocytes
Peripheral blood samples were collected from individuals by venipuncture into EDTA-containing tubes. After collection, 50 µL of whole blood was stimulated with 50 µL of ROS assay mix containing a 1:250 dilution of ROS assay stain in ROS assay buffer (ROS assay Kit; Cat# 88–5930, eBioscience, San Diego, CA, USA) together with 1 µL of phorbol myristate acetate (PMA; 3 µg/mL; Cat# P1585–1MG, Millipore Sigma, Burlington, MA, USA) or 1 µL of 1X phosphate buffered saline (PBS) (Cat# 10–010-023, Fisher Scientific, Durham, NC, USA) as an unstimulated control. The cells were incubated at 37°C with 5% CO2 for 60 min. Following incubation, erythrocytes were lysed using Ammonium-Chloride-Potassium (ACK) lysing buffer (Quality Biological Inc., Gaithersburg, MD, USA), and the resulting leukocytes were collected after centrifugation at 300 x g for 5 min. Leukocytes were resuspended in 0.5 mL of 1X PBS and acquired using the BD LSRFortessa flow cytometer (BD Biosciences, San Jose, CA, USA) and FACSDiva 9.0 software (BD Biosciences) to measure ROS production by neutrophils and monocytes. Data analysis and plots were performed using FlowJo software version 10 (FlowJo, Ashland, OR, USA).
Stimulation of PBMCs with heat-inactivated SARS-CoV-2 particles
PBMCs were isolated from blood samples collected in EDTA-containing tubes using the density gradient reagent Lymphoprep (Cat# 07801, Stemcell Technologies Inc., Vancouver, Canada), according to the manufacturer’s instructions. Mononuclear cell suspensions were cultivated in complete RPMI 1640 medium (Cat# 11875–093, Thermo Fisher Scientific, Life Technologies Limited, Paisley, UK) [enriched with 5% human serum (Cat# H3667, Sigma-Aldrich, St Louis, MO, USA) and 1% Penicillin-Streptomycin (Cat# 15140122, Thermo Fisher Scientific)] at 1×106 cells/mL in a six-well culture plate. Heat-inactivated SARS-CoV-2 culture fluid (41) (containing viral particles and hereafter termed “SARS-CoV-2 particles”) (Isolate USA-WA1/2020; Cat# 0810587CFHI; ZeptoMetrix Corporation, Buffalo NY, USA) was added to the PBMC culture at 1×106 50% Tissue Culture Infectious Dose (TCID50)/mL, and culture supernatants from Vero E6 cells alone were added as controls. Cells were incubated at 37°C and 5% CO2 for 48 h with the addition of a protein transport inhibitor cocktail (Cat# 00–4980-93, Thermo Fisher Scientific) for the last 4 h of incubation. Adherent cells were gently lifted from the plate and collected. Cell suspensions were transferred into 5 mL Falcon tubes and centrifuged at 300 x g for 5 min at 4°C. Cell supernatants were harvested and stored at −80°C for cytokine determinations and cell pellets were used for immunophenotyping.
Stimulation of PBMCs with SARS-CoV-2 proteins
PBMCs were isolated as described above and cultivated in complete RPMI medium in a 12-well culture plate at 1×106 cells/mL for monocyte stimulation or in a 24-well culture plate at 2.5×106 cells/mL for T-cell stimulation. SARS-CoV-2 Nucleocapsid Protein (N protein, Cat# 40588-V08B), SARS-CoV-2 Spike Protein [S protein receptor binding domain (RBD), Cat# 40592-V08B], SARS-CoV-2 Spike Protein (S1 Subunit, Cat# 40591-V08B1), SARS-CoV-2 Spike Protein (S1+S2 extracellular domain, Cat# 40589-V08B1), and SARS-CoV-2 (2019-nCoV) Spike S1 (S1 protein mutant D614G, Cat# 40591-V08H3) (all from Sino Biological, Wayne, PA, USA) were added to their respective cell culture well at final concentrations of 20nM. A non-protein control, a positive control for T-cell activation [phytohemagglutnin (PHA; 5 μg/mL; Cat# L8902, Sigma-Aldrich)], and a positive control for monocytes [lipopolysaccharide of Escherichia coli (LPS; 100 ng/mL Cat#tlrl-peklps, Invivogen, San Diego, CA, USA)] were also included. Plates were incubated at 37°C and 5% CO2 for 24 h (for monocytes) or 48 h (for T cells). Protein transport inhibitor cocktail was added to the wells for the last 4 h of incubation. Cells were then collected, transferred into 5 mL Falcon tubes, and centrifuged at 300 x g for 5 min at 4°C. Cell supernatants were stored at −80°C for cytokine determinations and cell pellets were used for immunophenotyping.
Stimulation of PBMCs with SARS-CoV-2 peptides
PBMCs were isolated as described above and cultivated in complete RPMI medium in a 12-well culture plate at 1×106 cells/mL for monocyte stimulation or in a 24-well culture plate at 2.5×106 cells/mL for T-cell stimulation. PepTivator SARS-CoV-2 Prot_S (Cat# 130–126-700), PepTivator SARS-CoV-2 Prot_S1 (RBD) (Cat# 130–127-041), PepTivator SARS-CoV-2 Prot_N (Cat# 130–126-698), PepTivator SARS-CoV-2 Prot_M (Cat# 130–126-702), and PepTivator CEF MHC Class I Plus – premium grade (Cat# 130–098-426; peptide-specific positive control) (all from Miltenyi Biotec, Bergisch Gladbach, Germany) were added to their respective cell culture well at final concentrations of 0.6 nmol/mL. A non-peptide control, a positive control for T-cell activation (PHA; 5 μg/mL), and a positive control for monocytes (LPS; 100 ng/mL) were also included. Plates were incubated at 37°C and 5% CO2 for 24 h for monocytes and 48 h for T cells. Protein transport inhibitor cocktail was added to the cell cultures for the last 4 h of incubation. Cells were then collected, transferred into 5 mL Falcon tubes, and centrifuged at 300 x g for 5 min at 4°C. Cell supernatants were stored at −80°C for cytokine determinations and cell pellets were used for immunophenotyping.
Immunophenotyping of PBMCs after stimulation
After stimulation, PBMCs pellets were washed and resuspended in 1X PBS followed by incubation with Fixable Viability Stain 510 (Cat# 564406, BD Biosciences) for 15 min at room temperature in the dark. Then, the cells were washed and subsequently incubated with specific extracellular fluorochrome-conjugated anti-human mAbs (Table I) for 30 min at 4 °C in the dark. After washing, the cells were fixed and permeabilized with either the BD Cytofix/Cytoperm kit (Cat# 554714, BD Biosciences) or the eBioscience Foxp3/Transcription Factor Staining Buffer Set (Cat# 00–5523-00, Thermo Fisher Scientific, Life Technologies Corporation, Carlsbad, CA, USA) prior to staining with intracellular Abs (Table I). Leukocytes were then washed and resuspended in 0.5 mL of FACS staining buffer (Cat# 554656, BD Biosciences) and acquired using the BD LSRFortessa flow cytometer and FACSDiva 9.0 software. Data analysis and plots were performed using the FlowJo software version 10. Immunophenotyping included the identification of: general leukocyte populations (monocytes, T cells, and B cells), monocyte subsets, T-cell subsets, and B-cell subsets. Specifically, classical monocytes (CD14hiCD16−), intermediate monocytes (CD14hiCD16+ cells), non-classical monocytes (CD14loCD16+), and CD14loCD16− cells were identified as well as CD4+ and CD8+ T cells, CD19+ B cells, and their respective memory or activation markers.
Table I.
List of antibodies used for immunophenotyping.
| Antigen | Fluorochrome | Clone | Company | Isotype |
|---|---|---|---|---|
| CD14 | BUV395 | MφP9 | BD Biosciences Cat#563561 | Mouse BALB/c IgG2b, κ |
| CD16 | APC-H7 | 3G8 | BD Biosciences Cat#560195 | Mouse CDF1 IgG1, κ |
| CCR5 (CD195) | BV711 | 2D7/CCR5 | BD Biosciences Cat#563395 | Mouse C57BL/6 IgG2a, κ |
| CD181 (CXCR1) | PE-Cy5 | 5A12 | BD Biosciences Cat#551081 | Mouse IgG2b, κ |
| CD182 (CXCR2) | APC | 6C6 | BD Biosciences Cat#551127 | Mouse IgG1, λ |
| CD142 (Tissue Factor) | BUV737 | HTF-1 | BD Biosciences Cat#748835 | Mouse IgG1, κ |
| CD162 (PSGL) | BV786 | KPL-1 | BD Biosciences Cat#743483 | Mouse BALB/c IgG1, κ |
| CCR2 | BV650 | LS132.1D9 | BD Biosciences Cat#747849 | Mouse BALB/c IgG2a, κ |
| IL-6 | PE-CF594 | MQ2–13A5 | BD Biosciences Cat#563543 | Rat IgG1 |
| IL-8 | BV421 | G265–8 | BD Biosciences Cat#563310 | Mouse IgG2b |
| TNF | BV605 | MAb11 | Biolegend Cat#502936 | Mouse IgG1, κ |
| CXCL10 | Alexa Fluor 488 | 4NY8UN | Invitrogen Cat#53–9744-42 | Mouse / IgG2b, κ |
| IL-1RA | PE | AS17 | BD Biosciences Cat#340525 | Mouse IgG1 |
| CD3 | PerCP-Cy5.5 | UCHT1 | BD Biosciences Cat#560835 | Mouse BALB/c IgG1, κ |
| CD4 | BUV737 | SK3 | BD Biosciences Cat# 612748 | Mouse BALB/c IgG1, κ |
| CD8 | BUV395 | RPA-T8 | BD Biosciences Cat#563795 | Mouse IgG1, κ |
| CD25 | PE-Cy7 | M-A251 | BD Biosciences Cat#557741 | Mouse BALB/c IgG1, κ |
| CD45RA | Alexa Fluor 700 | HI100 | BD Biosciences Cat#560673 | Mouse IgG2b, κ |
| CCR7 | PE | 3D12 | BD Biosciences Cat#552176 | Rat IgG2a, κ |
| CD154 (CD40L) | FITC | TRAP1 | BD Biosciences Cat#558988 | Mouse BALB/c IgG1, κ |
| CD137 (4–1BB) | BV421 | 4B4–1 | BD Biosciences Cat#564091 | Mouse BALB/c IgG1, κ |
| CD69 | PE-Cy5 | FN50 | BD Biosciences Cat#555532 | Mouse IgG1, κ |
| CD134 (OX40) | APC-Cy7 | Ber-ACT35 | Biolegend Cat#350022 | Mouse IgG1, κ |
| CD95 (FAS) | APC | DX2 | BD Biosciences Cat#558814 | Mouse C3H/Bi IgG1, κ |
| Granzyme B | PE-CF594 | GB11 | BD Biosciences Cat#562462 | Mouse BALB/c IgG1, κ |
| IFN-γ | BV650 | 4S.B3 | BD Biosciences Cat#563416 | Mouse BALB/c IgG1, κ |
| IL-10 | BV786 | JES3–9D7 | BD Biosciences Cat# 564049 | Rat IgG1 |
| Ki67 | BV711 | B56 | BD Biosciences Cat#563755 | Mouse IgG1, κ |
| CD19 | Alexa Fluor 488 | HIB19 | BD Biosciences Cat# 557697 | Mouse IgG1, κ |
| CD20 | PerCP-Cy5.5 | 2H7 | BD Biosciences Cat#560736 | Mouse C57BL/6 IgG2b, κ |
| CD23 | Alexa Fluor 700 | M-L233 | BD Biosciences Cat#563673 | Mouse IgG1, κ |
| CD27 | APC-H7 | M-T271 | BD Biosciences Cat# 560222 | Mouse BALB/c IgG1, κ |
| CD86 | PE-CF594 | 2331(FUN-1) | BD Biosciences Cat#562390 | Mouse BALB/c IgG1, κ |
| CD80 | BV650 | 2D10.4 | BD Biosciences Cat# 751725 | Mouse IgG1, κ |
| CD40 | PE-Cy7 | 5C3 | BD Biosciences Cat# 561215 | Mouse IgG1, κ |
| CD43 | BV605 | 1G10 | BD Biosciences Cat#563378 | Mouse IgG1, κ |
| CD22 | PE-Cy5 | HIB22 | BD Biosciences Cat#555425 | Mouse IgG1, κ |
| CD24 | BUV395 | ML5 | BD Biosciences Cat#563818 | Mouse IgG2a, κ |
| CD38 | BUV737 | HB7 | BD Biosciences Cat#612824 | Mouse IgG1, κ |
| CD138 | BV711 | MI15 | BD Biosciences Cat#563184 | Mouse BALB/c IgG1, κ |
| CD5 | BV421 | UCHT20 | BD Biosciences Cat#562646 | Mouse BALB/c IgG1, κ |
| IgD | PE | IA6–2 | BD Biosciences Cat#555779 | Mouse BALB/c IgG2a, κ |
| IgM | APC | G20–127 | BD Biosciences Cat#551062 | Mouse IgG1, κ |
| IgG | BV786 | G18–145 | BD Biosciences Cat#564230 | Mouse IgG1, κ |
| Isotype | BV711 | X40 | BD Biosciences Cat#563044 | Mouse BALB/c IgG1, κ |
| Isotype | PE-Cy5 | MOPC-21 | BD Biosciences Cat#555750 | Mouse IgG1, κ |
| Isotype | APC | MOPC-21 | BD Biosciences Cat#555751 | Mouse IgG1, κ |
| Isotype | PE-CF594 | R3–34 | BD Biosciences Cat#562309 | Rat IgG1, κ |
| Isotype | BV421 | 27–35 | BD Biosciences Cat#562748 | Mouse C.SW IgG2b, κ |
| Isotype | BV605 | MOPC-21 | Biolegend Cat#400162 | Mouse IgG1, κ |
| Isotype | Alexa Fluor 488 | eBMG2b | Invitrogen Cat#53–4732-80 | Mouse / IgG2b, κ |
| Isotype | PE | MOPC-21 | BD Biosciences Cat#555749 | Mouse IgG1, κ |
| Isotype | PE-CF594 | X40 | BD Biosciences Cat#562292 | Mouse BALB/c IgG1, κ |
| Isotype | BV650 | X40 | BD Biosciences Cat#563231 | Mouse BALB/c IgG1, κ |
| Isotype | BV786 | R3–34 | BD Biosciences Cat#563847 | Rat IgG1, κ |
Whole blood stimulation using TruCulture®
Peripheral blood samples were collected from women by venipuncture into heparin-containing tubes. One mL of whole blood was directly placed into the TruCulture® (Myriad RBM, Inc., Austin, TX, USA) (42) tubes containing SARS-CoV-2 Spike Protein (Part# 782–001411) or null tubes (Part# 782–001086) as a control. The TruCulture® tubes were gently inverted 10 times to mix the whole blood and culture media then incubated for 48 h at 37°C in a heating block. After incubation, cell supernatants were collected separately into cryovials and stored at −80°C.
Determination of cytokine concentrations in culture supernatants
Cell culture supernatants and TruCulture® tubes supernatants were used for the determination of cytokine concentrations. The V-PLEX Proinflammatory Panel 1 (human), and U-PLEX Biomarker Group 1 (human) multiplex immunoassays (Meso Scale Discovery, Rockville, MD, USA) were used to measure the concentrations of IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and TNF (Pro-inflammatory Panel 1) as well as IFN-β, IFN-γ, I-TAC, TRAIL, IL-17E/IL-25, IL-17F, IL-21, IL-22, IL-23, IL-27, IL-29/IFN-λ1, IL-31, and IL-33 (Biomarker Group 1), according to the manufacturer’s instructions. Plates were read using the MESO QuickPlex SQ 120 (Meso Scale Discovery) and analyte concentrations were calculated with the Discovery Workbench 4.0 (Meso Scale Discovery). The sensitivities of the assays were: 0.21–0.62 pg/mL (IFN-γ), 0.01–0.17 pg/mL (IL-1β), 0.01–0.29 pg/mL (IL-2), 0.01–0.03 pg/mL (IL-4), 0.05–0.09 pg/mL (IL-6), 0.03–0.14 pg/mL (IL-8), 0.02–0.08 pg/mL (IL-10), 0.02–0.89 pg/mL (IL-12p70), 0.03–0.73 pg/mL (IL-13), 0.01–0.13 pg/mL (TNF), 3.1 pg/mL (IFN-β), 1.7 pg/mL (IFN-γ), 1.5 pg/mL (I-TAC), 0.66 pg/mL (TRAIL), 0.58 pg/mL (IL-17E/IL-25), 155 pg/mL (IL-17F), 1.2 pg/mL (IL-21), 0.13 pg/mL (IL-22), 1.4 pg/mL (IL-23), 9.6 pg/mL (IL-27), 1.2 pg/mL (IL-29/IFN-λ1), 7.3 pg/mL (IL-31), and 0.59 pg/mL (IL-33).
Tissue Factor concentrations in culture supernatants
Cell culture supernatants and supernatants from TruCulture® tubes were collected and stored as described above. The IMUBIND Tissue Factor ELISA kit (BioMedica Diagnostics Inc., Windsor, NS, Canada) was used to measure the concentrations of Tissue Factor in the supernatants according to the manufacturer’s instructions. Plates were read using the SpectraMax iD5 (Molecular Devices, San Jose, CA, USA) and analyte concentrations were calculated with the SoftMax Pro 7 software (Molecular Devices). The sensitivity of the assay was 22.411 pg/mL.
Statistical analysis
Statistical analyses were performed using the R language and environment. For the comparison of flow cytometry data and cytokine intensities between study groups, linear mixed effects models were fit to account for repeated observations from each patient. For cytokine mean fluorescence intensities, an offset was added to the data to ensure positive values and the data were log10 transformed to improve normality. The flow cytometry data were modeled as proportions. A false discovery rate-adjusted p-value < 0.05 was considered statistically significant. For heatmap representation of immunophenotyping results, flow cytometry data were transformed into Z-scores by subtracting the mean and dividing by the standard deviation.
RESULTS
Study design
This study included peripheral blood samples collected from a largely African-American cohort of women in the third trimester of pregnancy as well as healthy non-pregnant women of reproductive age. We exposed peripheral whole blood or PBMCs to inactivated SARS-CoV-2 particles, proteins, and peptide pools in vitro to thoroughly explore the systemic cellular immune responses triggered by this coronavirus in pregnant and non-pregnant women. Characterization of monocyte, T-cell, and B-cell subsets using flow cytometry revealed that both non-pregnant and pregnant women displayed strong responses to SARS-CoV-2 stimulation as indicated by altered phenotypes (presented in Extended Data). Here, we solely focused on differences in cellular phenotypes between the non-pregnant and pregnant groups (shown as black asterisks throughout the figures) to investigate pregnancy-specific immune responses to in vitro challenge with SARS-CoV-2 particles and proteins/peptides.
Pregnancy-specific production of reactive oxygen species is not modified in response to SARS-CoV-2 particles
Innate immune cells (e.g., neutrophils and monocytes) have been shown to undergo heightened activation during pregnancy (28–30); therefore, it is likely that such immune cells act as a first line of defense against microbial infections during this period. We collected peripheral whole blood from pregnant and non-pregnant women and performed in vitro stimulation with inactivated SARS-CoV-2 particles to evaluate ROS production by neutrophils and monocytes (Fig. 1A). Consistent with prior reports (28, 29), pregnancy itself resulted in increased ROS production by peripheral monocytes and neutrophils (Fig. 1B). However, stimulation with SARS-CoV-2 particles did not modify ROS production by either pregnant or non-pregnant neutrophils and monocytes (Fig. 1B). These data show that the pregnancy-specific production of ROS by neutrophils and monocytes is not modified by SARS-CoV-2 particles, suggesting that such a component of innate immunity may be unresponsive to this coronavirus.
Figure 1. Monocyte and neutrophil responses to SARS-CoV-2 particles in pregnant and non-pregnant women.
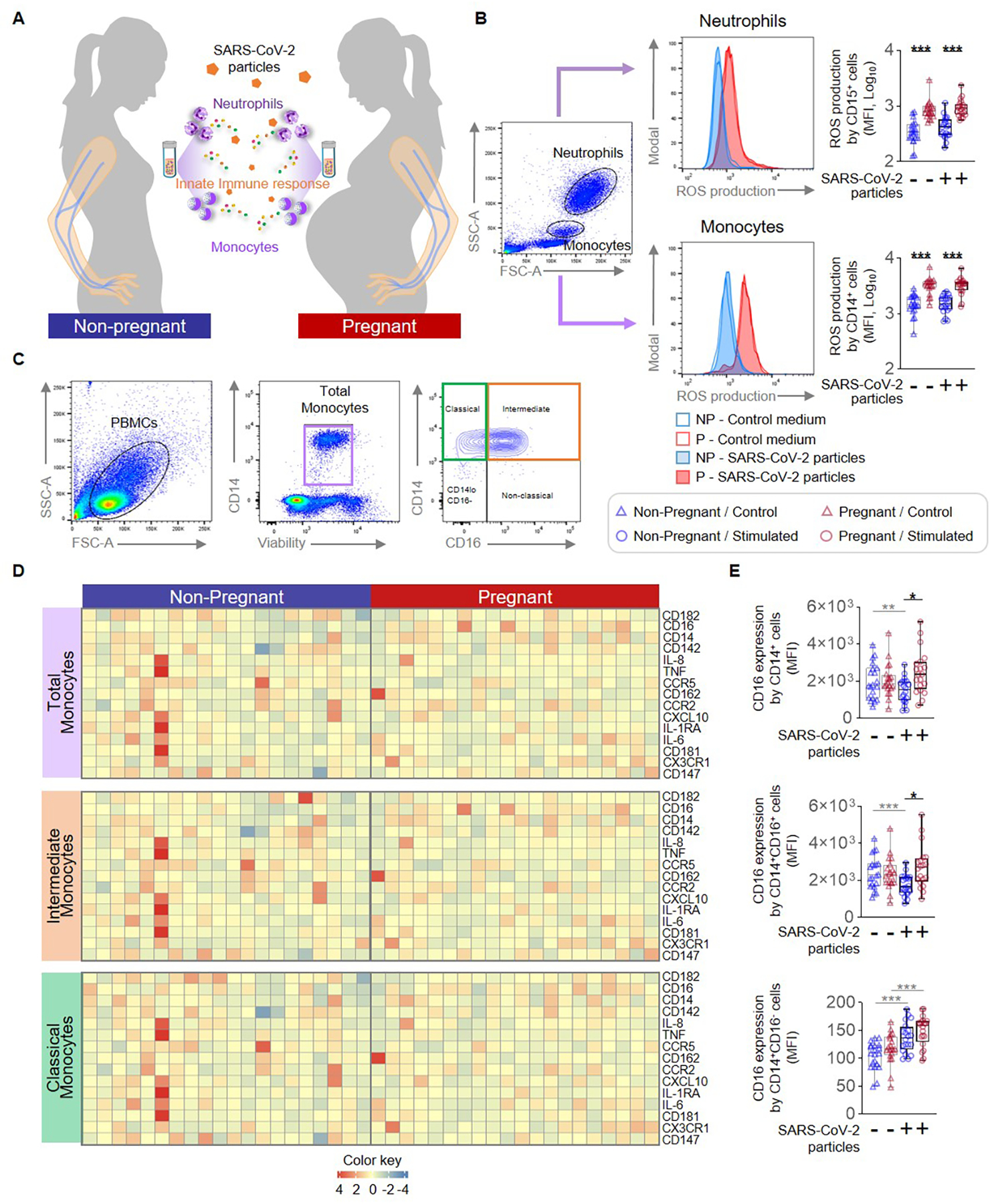
(A) Peripheral blood samples were collected from non-pregnant (n = 20, indicated in blue) and pregnant (n = 20, indicated in red) women to isolate peripheral blood mononuclear cells (PBMCs) for in vitro stimulation with SARS-CoV-2 particles. Flow cytometry was performed to determine the expression of activation markers by monocyte subsets and neutrophils. (B) (Left) Flow cytometry gating strategy to measure the production of reactive oxygen species (ROS) in neutrophils and monocytes stimulated with SARS-CoV-2 particles (filled histograms) or control medium (open histograms). (Right) Quantification of ROS production by neutrophils or monocytes after stimulation with SARS-CoV-2 particles (circles) or control medium (triangles). (C) Flow cytometry gating strategy for immunophenotyping of monocyte subsets. Monocytes were initially gated as viable CD14+ cells, followed by gating for classical (CD14hiCD16−), intermediate (CD14hiCD16+), non-classical (CD14loCD16+), and CD14loCD16− monocytes. (D) Heatmap representations showing the expression of activation markers by total, intermediate, and classical monocytes after stimulation with SARS-CoV-2 particles. (E) Mean fluorescence intensity (MFI) of CD16 expression by total, intermediate, and classical monocytes from pregnant (red symbols) and non-pregnant (blue symbols) women in response to SARS-CoV-2 particles (circles) or control medium (triangles). *p < 0.05; **p < 0.01; ***p < 0.001. (+) Stimulated, (−) Control.
Pregnancy imprints specific monocyte responses to SARS-CoV-2 particles and proteins
Monocytes are conventionally divided into several major subsets: classical (CD14hiCD16−), intermediate (CD14hiCD16+), and non-classical (CD14loCD16+), each of which displays distinct functionality (43). Thus, we next investigated whether SARS-CoV-2 particles differentially affected specific monocyte subsets in pregnant women. Few non-classical monocytes were observed in our study samples, and therefore we focused on the phenotypes of the total, intermediate, and classical monocyte subsets (Fig. 1C). We evaluated the expression of specific surface markers, cytokines/chemokines, and receptors by total, intermediate, and classical monocytes in response to stimulation with SARS-CoV-2 particles (Fig. 1D and Supplemental Fig. 1A). While SARS-CoV-2 particles reduced CD16 expression by total and intermediate monocytes from non-pregnant women, it tended to increase the expression of this marker by pregnancy-derived monocytes (Fig. 1E). CD16 expression in response to SARS-CoV-2 particle stimulation was elevated in total and intermediate monocytes from pregnant women compared to those from non-pregnant women (Fig. 1E). In addition, SARS-CoV-2 particle stimulation increased the typically minimal expression of CD16 by classical monocytes from both non-pregnant and pregnant women, suggesting that this coronavirus promotes a transition from classical to intermediate monocytes (Fig. 1E).
The SARS-CoV-2 virus has four major structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N) (44, 45) (Fig. 2A). The S protein (which includes an S1 and an S2 subunit) is critical for viral entry into host cells, and thus remains an attractive target for anti-viral agents (45). The N protein is highly conserved, produced in abundance during infection, and strongly immunogenic (46). Therefore, we utilized the S and N proteins, the S1 and S2 subunits, and a mutant S1 (S1D614G) with increased infectious capacity (47, 48) to elucidate whether monocytes exhibit distinct responses to different SARS-CoV-2 proteins (Fig. 2A). Heatmaps display the expression of surface markers, cytokines/chemokines, and receptors by stimulated total monocytes (Fig. 2B). Decreased expression of CD181 was observed on N protein-stimulated total monocytes from pregnant women compared to those from non-pregnant controls (Fig. 2C), and an increase in expression of CX3CR1 was observed in response to S protein stimulation (Fig. 2D). Given that intermediate monocytes appeared to be most activated by SARS-CoV-2 stimulation (Fig. 1E), we also determined the specific effects of viral proteins on this monocyte subset as shown in the heatmap representations (Fig. 3A). Consistent with responses observed in total monocytes (Fig. 2B&C), intermediate monocytes displayed decreased expression of CD181 in response to N protein (Fig. 3B) and increased CX3CR1 expression in response to S protein (Fig. 3C) in pregnant compared to non-pregnant women. Similarly, classical monocytes (Fig. 4A) derived from pregnant women also showed reduced expression of CD181 in response to N protein (Fig. 4B) but did not show changes in the expression of CX3CR1 in response to the S protein (Fig. 4C).
Figure 2. Total monocyte response to SARS-CoV-2 proteins in pregnant and non-pregnant women.
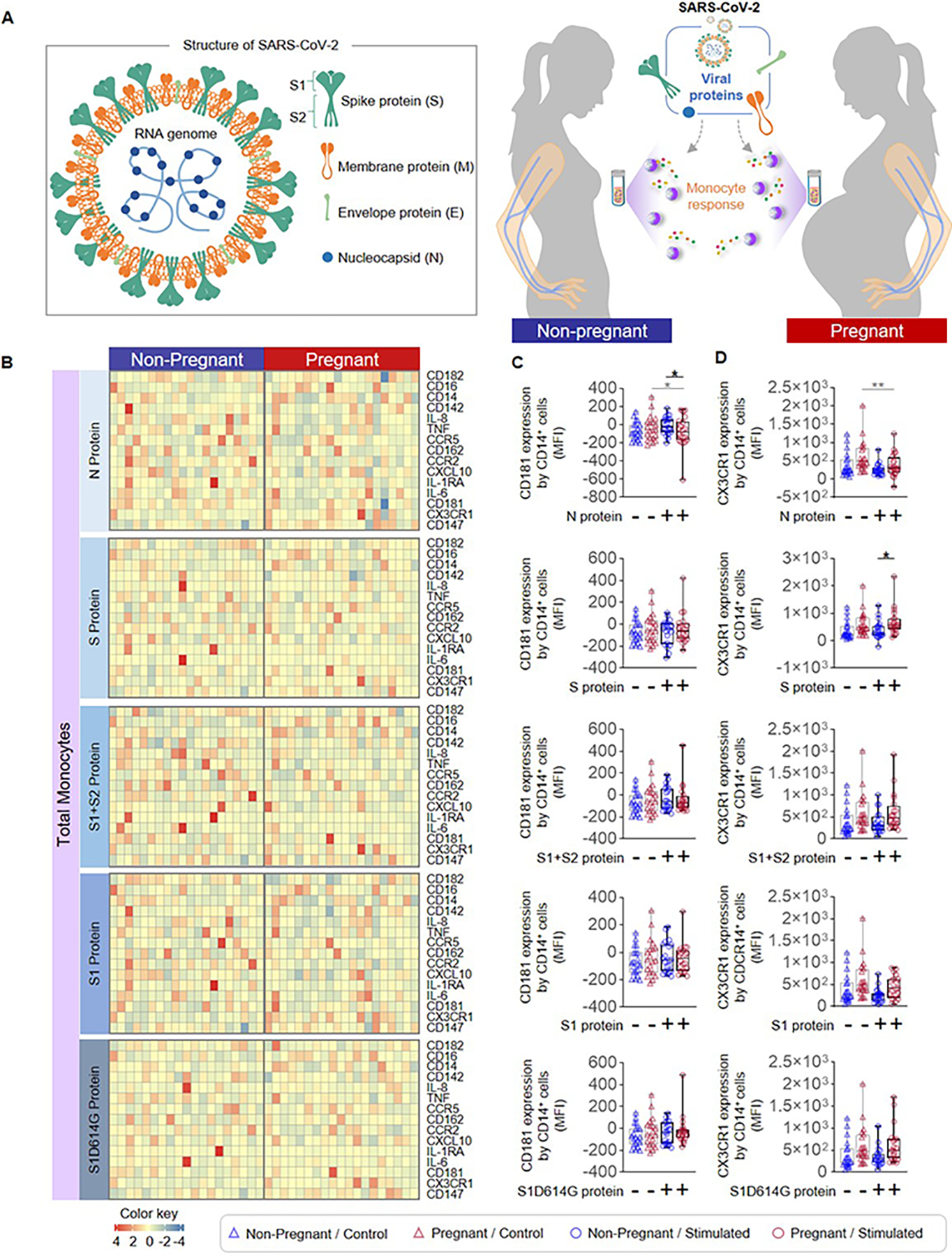
(A) (Left) Schematic representation of SARS-CoV-2 structure showing the spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins as well as the S protein subunits S1 and S2. (Right) Peripheral blood samples were collected from non-pregnant (n = 20, indicated in blue) and pregnant (n = 20, indicated in red) women to isolate peripheral blood mononuclear cells (PBMCs) for in vitro stimulation with SARS-CoV-2 proteins. Flow cytometry was performed to determine the expression of activation markers by total monocytes. (B) Heatmap representations showing expression of activation markers by total monocytes after stimulation with SARS-CoV-2 proteins or the mutant S1 variant S1D614G. Mean fluorescence intensity (MFI) of (C) CD181 expression and (D) CX3CR1 expression by total monocytes from pregnant (red symbols) and non-pregnant (blue symbols) women in response to SARS-CoV-2 proteins (circles) or control (triangles). *p < 0.05; **p < 0.01. (+) Stimulated, (−) Control.
Figure 3. Intermediate monocyte response to SARS-CoV-2 proteins in pregnant and non-pregnant women.
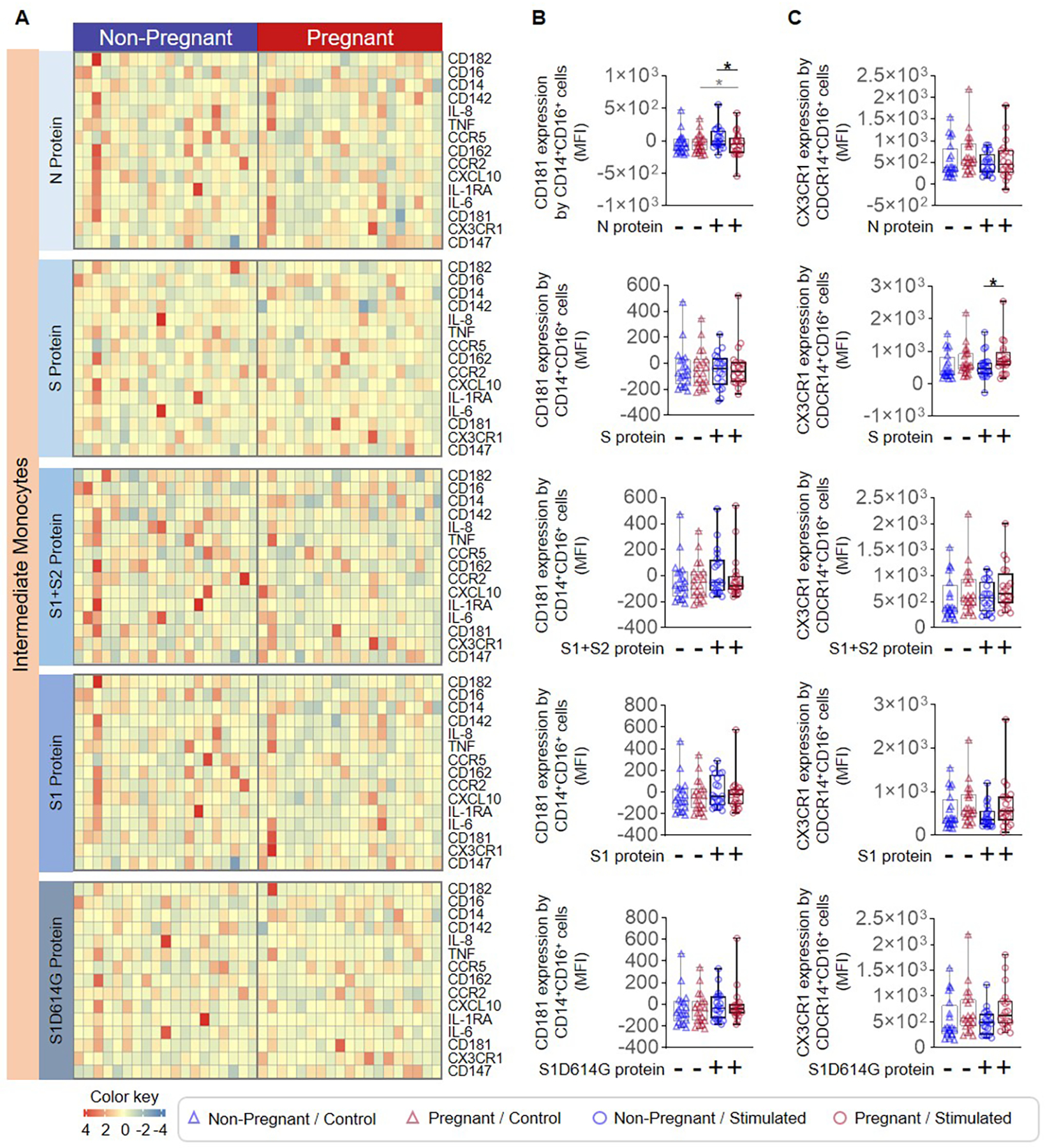
Peripheral blood samples were collected from non-pregnant (n = 20, indicated in blue) and pregnant (n = 20, indicated in red) women to isolate peripheral blood mononuclear cells (PBMCs) for in vitro stimulation with SARS-CoV-2 proteins. Flow cytometry was performed to determine the expression of activation markers by intermediate monocytes. (A) Heatmap representations showing the expression of activation markers by intermediate monocytes after stimulation with SARS-CoV-2 proteins or the mutant S1 variant S1D614G. Mean fluorescence intensity (MFI) of (B) CD181 expression and (C) CX3CR1 expression by intermediate monocytes from pregnant (red symbols) and non-pregnant (blue symbols) women in response to SARS-CoV-2 proteins (circles) or control (triangles). *p < 0.05. (+) Stimulated, (−) Control.
Figure 4. Classical monocyte response to SARS-CoV-2 proteins in pregnant and non-pregnant women.
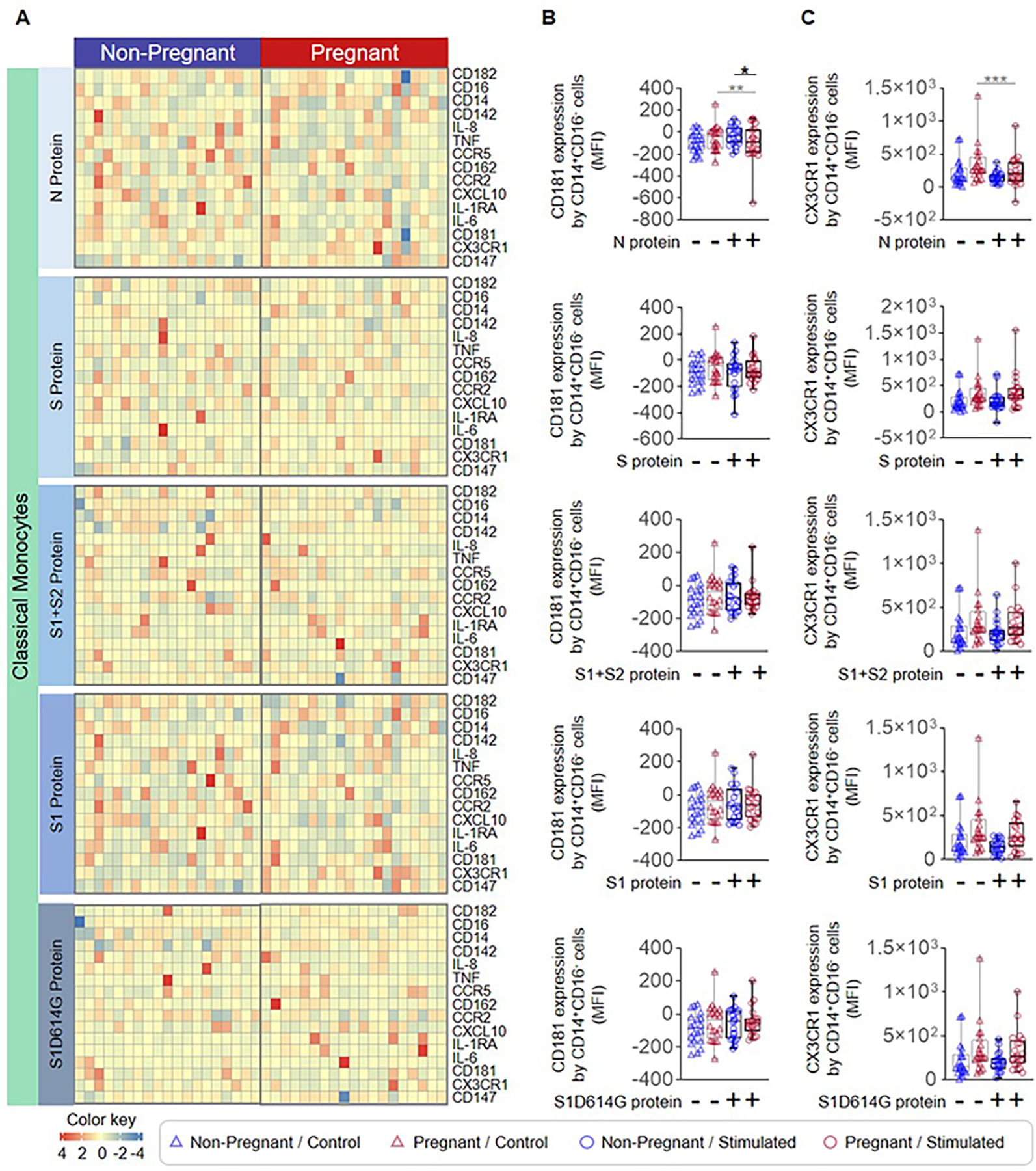
Peripheral blood samples were collected from non-pregnant (n = 20, indicated in blue) and pregnant (n = 20, indicated in red) women to isolate peripheral blood mononuclear cells (PBMCs) for in vitro stimulation with SARS-CoV-2 proteins. Flow cytometry was performed to determine the expression of activation markers by classical monocytes. (A) Heatmap representations showing the expression of activation markers by classical monocytes after stimulation with SARS-CoV-2 proteins or the mutant S1 variant S1D614G. Mean fluorescence intensity (MFI) of (B) CD181 expression and (C) CX3CR1 expression by classical monocytes from pregnant (red symbols) and non-pregnant (blue symbols) women in response to SARS-CoV-2 proteins (circles) or control (triangles). *p < 0.05; **p < 0.01; ***p < 0.001. (+) Stimulated, (−) Control.
To further refine our results, we performed stimulation of PBMCs using pools of peptides with coverage of the complete M protein, the complete N protein, the S1 domain of the S protein, and the functional domains of the S protein, respectively (Supplemental Fig. 2A). Stimulation with these peptide pools was capable of inducing an immune response in both pregnant and non-pregnant women (Extended Data). Yet, the heatmaps displayed in Supplemental Fig. 2B illustrate the minimal difference in monocyte phenotypes and cytokine production between pregnant and non-pregnant women in response to each of the peptide pools.
Taken together, the above results indicate that SARS-CoV-2 modulates monocyte activation in a specific manner during pregnancy. Furthermore, such pregnancy-specific monocyte responses differ between exposure to viral particles and full-length S and N proteins, indicating a tailored monocyte response during SARS-CoV-2 challenge.
Pregnancy mildly alters T-cell responses to SARS-CoV-2
T cells are the primary cellular component of the adaptive immune system and are essential mediators of host anti-viral responses (49). During pregnancy, the regulatory component of T-cell immunity is enhanced (33–36, 50–52), while conventional T helper functions are more tightly controlled (18, 53–56); thus, we reasoned that T-cell responses to SARS-CoV-2 antigens may differ between pregnant and non-pregnant women. PBMCs collected from non-pregnant and pregnant women were stimulated with SARS-CoV-2 particles as well as with the S and N proteins, the S1 and S2 subunits, S1D614G, and peptide pools (Fig. 5A), and the expression of multiple phenotypic, activation-associated, and functional markers by CD4+ and CD8+ T cells was determined (Fig. 5B and Supplemental Fig. 1B). T cells from non-pregnant and pregnant women showed minimal responsiveness toward SARS-CoV-2 proteins (Extended Data and Fig. 5C). A pregnancy-specific increase in the expression of CD40 ligand (CD40L), which is essential for mediating macrophage and B cell activation (57), was observed on CD4+ T cells in response to the S2 subunit, given that treatment with the S1+S2 protein induced such a change, but not the S1 subunit alone (Fig. 5D). CD4+ T cells also exhibited responsiveness to inert SARS-CoV-2 particles as well as peptide pools; yet, pregnancy-specific differences were not observed (Supplemental Fig. 3A&C and Extended Data). Similarly, CD8+ T cells also displayed a pregnancy-specific increase in CD40L expression in response to stimulation with the S2 subunit (Fig. 6A&B), while no other distinct pregnancy-related changes were observed in response to SARS-CoV-2 particles or peptide pools (Supplemental Fig. 3B&D and Extended Data). Together, these findings show that, while T-cell activation is observed in response to SARS-CoV-2 antigens that is independent of pregnancy status, pregnancy itself only drives subtle differences in T-cell activation.
Figure 5. CD4+ T-cell responses to SARS-CoV-2 antigens in pregnant and non-pregnant women.
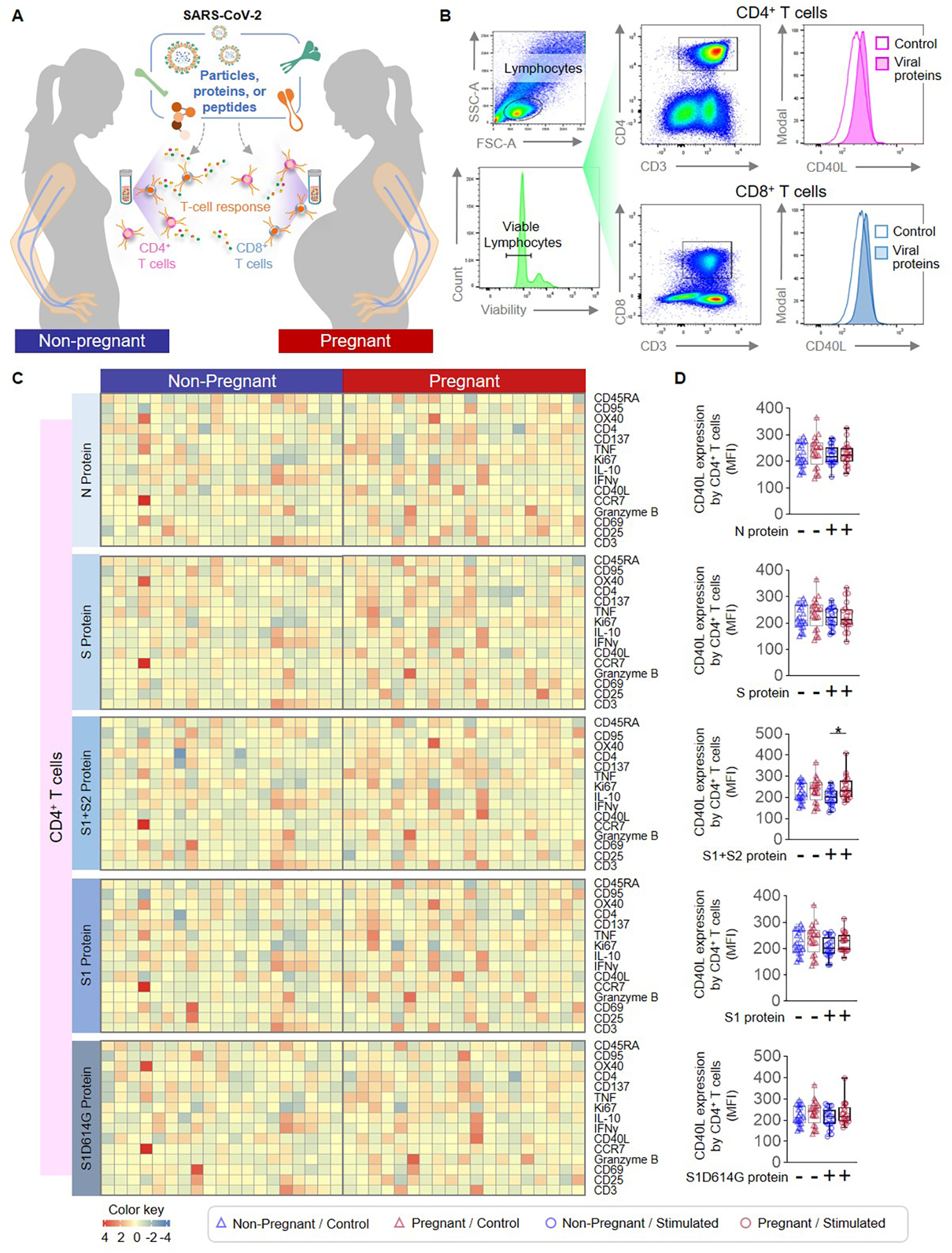
(A) Peripheral blood samples were collected from non-pregnant (n = 20, indicated in blue) and pregnant (n = 20, indicated in red) women to isolate peripheral blood mononuclear cells (PBMCs) for in vitro stimulation with SARS-CoV-2 particles, proteins or peptides. Flow cytometry was performed to determine the expression of activation markers by CD4+ and CD8+ T cells. (B) Representative gating strategy to identify the expression of activation markers on CD4+ and CD8+ T cells. (C) Heatmap representations showing the expression of activation markers by CD4+ T cells after stimulation with SARS-CoV-2 proteins or the mutant S1 variant S1D614G. (D) Mean fluorescence intensity (MFI) of CD40L expression by CD4+ T cells from pregnant (red symbols) and non-pregnant (blue symbols) women in response to SARS-CoV-2 proteins (circles) or control (triangles). *p < 0.05. (+) Stimulated, (−) Control.
Figure 6. CD8+ T-cell responses to SARS-CoV-2 proteins in pregnant and non-pregnant women.
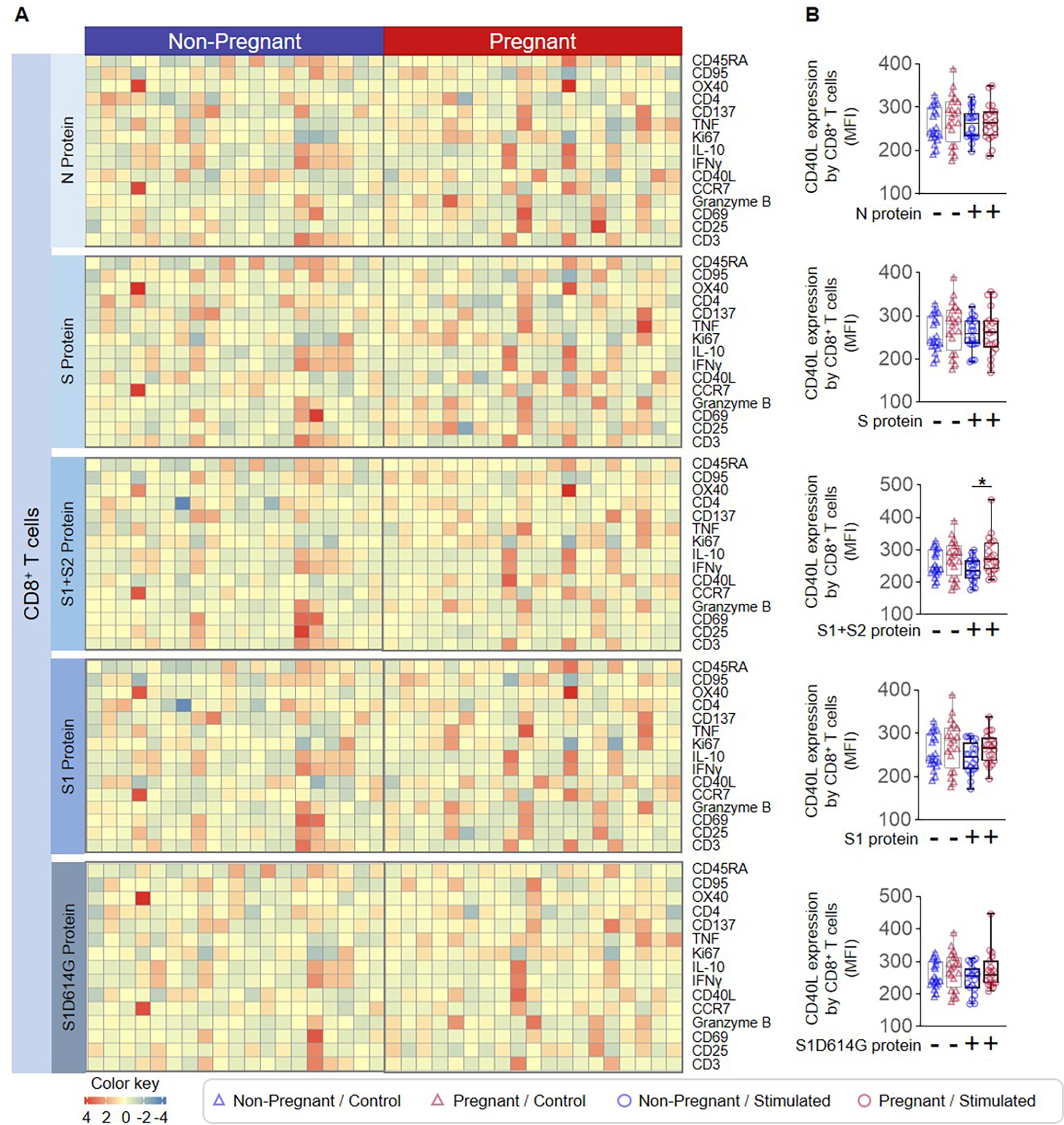
(A) Heatmap representations showing the expression of activation markers by CD8+ T cells after stimulation with SARS-CoV-2 proteins or the mutant S1 variant S1D614G. (B) Mean fluorescence intensity (MFI) of CD40L expression by CD8+ T cells from pregnant (red symbols) and non-pregnant (blue symbols) women in response to SARS-CoV-2 proteins (circles) or control (triangles). *p < 0.05. (+) Stimulated, (−) Control.
B-cell responses to SARS-CoV-2 proteins during pregnancy
The other major component of adaptive immunity is B cells, whose activation and class-switching are essential for the production of anti-SARS-CoV-2 IgM and IgG (58, 59). Therefore, we determined whether pregnancy-specific differences exist in B cell phenotypes in response to SARS-CoV-2 particles (Fig. 7A). We measured the expression of multiple phenotypic and activation-associated markers on peripheral B cells (Supplemental Fig. 1C and Fig. 7B) and noted a pregnancy-specific increase in the expression of CD22 coupled with decreased expression of CD24 in response to SARS-CoV-2 particle stimulation (Fig. 7C). We then examined whether changes in B-cell surface marker expression were translated to a shift in the proportions of circulating B-cell subsets. We examined the proportions of general B-cell populations as well as multiple subsets of these cells (Fig. 7D). Notably, pregnancy was associated with an increase in the proportions of peripheral CD23+CD19+CD20+ B cells (Fig. 7E) and IgM−IgD−CD19+CD20+CD27+ B cells (Fig. 7F) compared to non-pregnant women. Only pregnancy-derived IgG+CD19+CD20+CD27+ B cells were expanded upon SARS-CoV-2 particle stimulation, and thus the proportion of these cells was greater in pregnancy compared to the stimulated non-pregnant state (Fig. 7G). Peripheral CD23−CD19+CD20+ B cells from both non-pregnant and pregnant women were decreased in response to stimulation, and such a decrease was more pronounced in pregnant women (Fig. 7H). On the other hand, the CD24+CD38+CD19+CD20+ (Fig. 7I) and CD86+CD80+CD19+CD20+ B-cell (Fig. 7J) subsets were increased upon stimulation in both study groups, but this response was diminished in pregnant women compared to non-pregnant women. Interestingly, a stimulation-induced increase in the proportions of CD27+CD43−CD19+CD20+ (Fig. 7K) and CD27+IgD+CD19+CD20+ B cells (Fig. 7L) was only observed in non-pregnant individuals. Together, these data indicate that pregnancy is associated with altered proportions and phenotypes of peripheral B cells following challenge with SARS-CoV-2 particles and proteins/peptide pools.
Figure 7. B-cell responses to SARS-CoV-2 particles in pregnant and non-pregnant women.
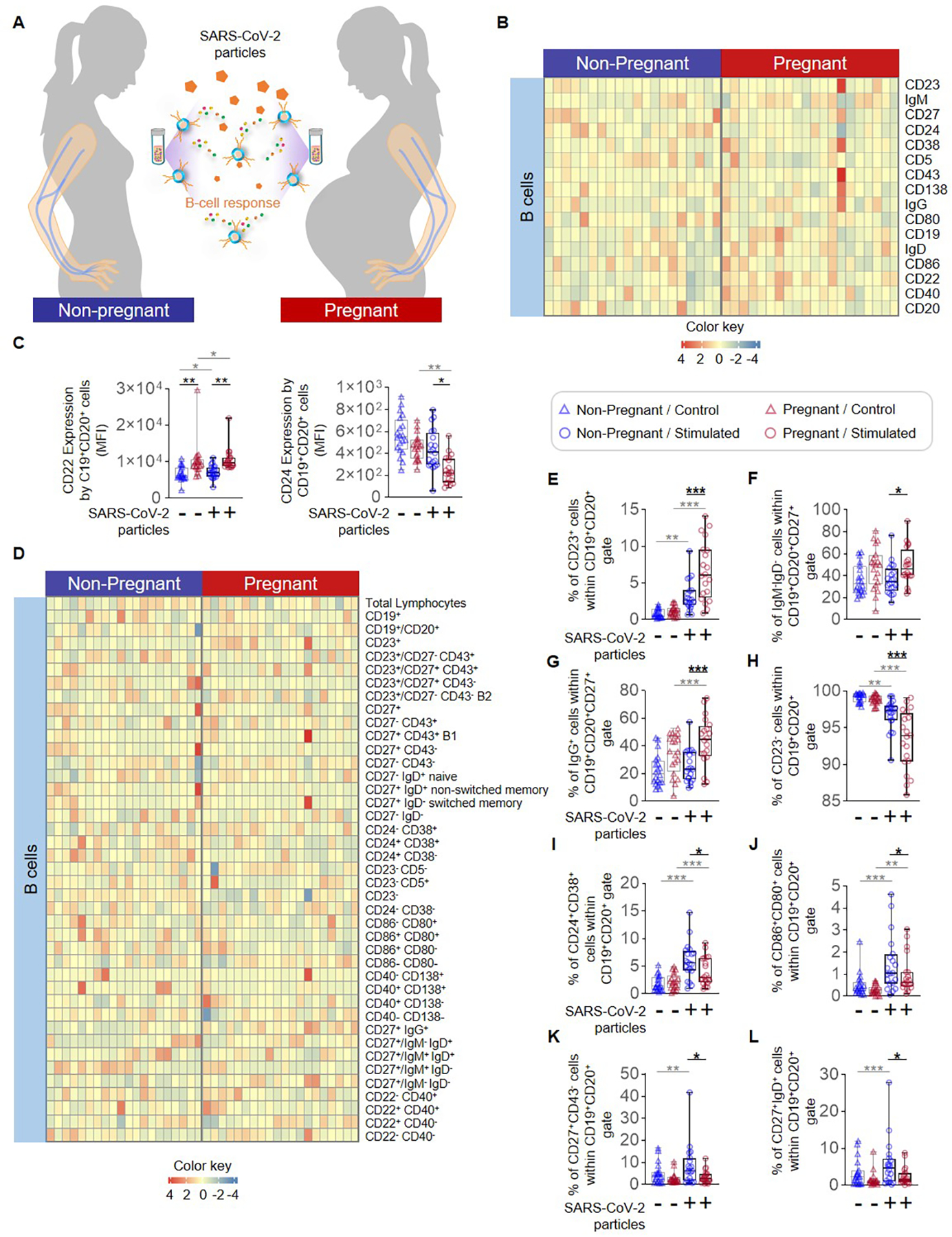
(A) Peripheral blood samples were collected from non-pregnant (n = 20, indicated in blue) and pregnant (n = 20, indicated in red) women to isolate peripheral blood mononuclear cells (PBMCs) for in vitro stimulation with SARS-CoV-2 particles. Flow cytometry was performed to identify evaluate surface marker expression by B-cell subsets. (B) Heatmap representation showing the expression of surface markers by B cells after stimulation with SARS-CoV-2 particles. (C) Mean fluorescence intensity (MFI) of CD22 (Left) and CD24 (Right) expression by B cells from pregnant (red symbols) and non-pregnant (blue symbols) women in response to SARS-CoV-2 particles (circles) or control medium (triangles). (D) Heatmap representation showing the proportions of B-cell subsets within the parent population after stimulation with SARS-CoV-2 particles. Proportions of (E) CD23+, (F) CD27+IgM−IgD−, (G) CD27+IgG+, (H) CD23−, (I) CD24+CD38+, (J) CD86+CD80+, (K) CD27+CD43−, and (L) CD27+IgD+ B cells from pregnant (red symbols) and non-pregnant (blue symbols) women in response to SARS-CoV-2 particles (circles) or control medium (triangles). *p < 0.05; **p < 0.01; ***p < 0.001. (+) Stimulated, (−) Control.
PBMC and whole blood cytokine responses to SARS-CoV-2 particles and proteins
Finally, we investigated differences in mediator release by PBMCs from non-pregnant and pregnant women in response to SARS-CoV-2 particles (Fig. 8A). Baseline production of the pro-apoptotic TRAIL by pregnancy-derived PBMCs was diminished compared to those from non-pregnant women (Fig. 8B). SARS-CoV-2 stimulation enhanced the release of multiple cytokines such as IFN-β, IFN-γ, TNF, IL-1β, IL-6, and IL-8 by PBMCs from both non-pregnant and pregnant women (Fig. 8B). However, upon stimulation, PBMCs from pregnant women showed diminished release of IFN-β and IL-8 compared to those from non-pregnant individuals (Fig. 8B), suggesting restrained specific cytokine response to SARS-CoV-2 exposure in pregnant women. Several measured mediators displayed no changes with stimulation in non-pregnant or pregnant women (Supplemental Fig. 4A) including Tissue Factor (TF), a major component of the coagulation cascade that has been implicated in COVID-19 pathogenesis (60).
Figure 8. Cytokine production by peripheral blood mononuclear cells (PBMCs) exposed to SARS-CoV-2 particles or whole-blood TruCulture® system containing S protein in pregnant and non-pregnant women.
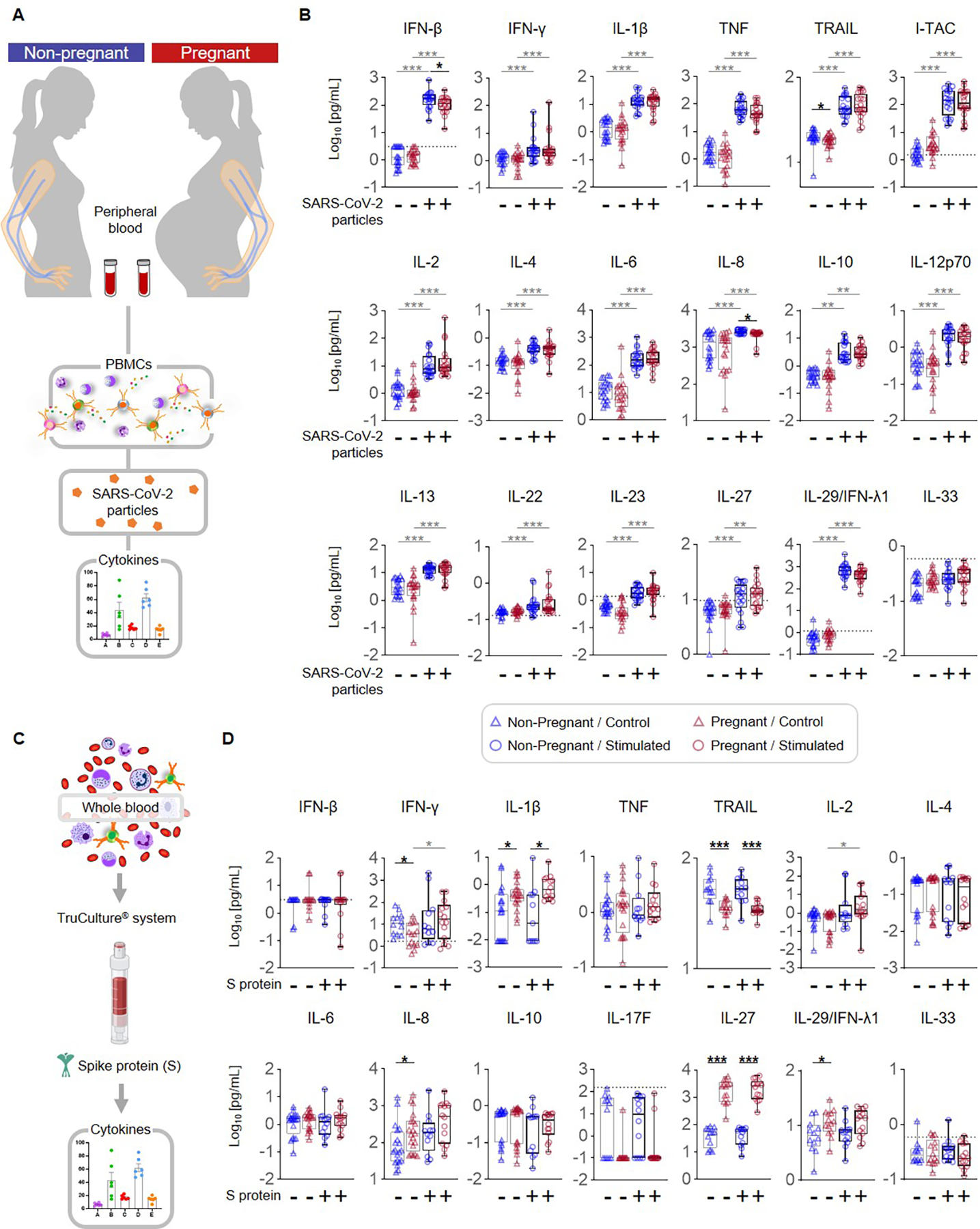
(A) Peripheral blood samples were collected from non-pregnant (n = 20, indicated in blue) and pregnant (n = 20, indicated in red) women to isolate PBMCs for in vitro stimulation with SARS-CoV-2 particles. Cytokine concentrations were then determined in culture supernatants. (B) Log10-transformed concentrations of IFN-β, IFN-γ, IL-1β, TNF, TRAIL, I-TAC, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-22, IL-23, IL-27, IL-29/IFN-λ1, and IL-33 in culture supernatants of PBMCs from non-pregnant (blue symbols) and pregnant (red symbols) women in response to SARS-CoV-2 particles (circles) or control medium (triangles). (C) Peripheral blood collected from non-pregnant (n = 14) and pregnant (n = 12) women was added to a whole-blood culture system (TruCulture®) containing SARS-CoV-2 S protein. After culture, supernatants were collected for cytokine determination. (D) Log10-transformed concentrations of IFN-β, IFN-γ, IL-1β, TNF, TRAIL, IL-2, IL-4, IL-6, IL-8, IL-10, IL-17F, IL-27, IL-29/IFN-λ1, and IL-33 in whole blood culture supernatants from pregnant (red symbols) and non-pregnant (blue symbols) women in response to S protein (circles) or control (triangles). *p < 0.05; **p < 0.01, ***p < 0.001. (+) Stimulated, (−) Control.
We also evaluated mediator release in response to the SARS-CoV-2 S protein using a whole blood TruCulture® system (42, 61) (Fig. 8C). The isolation of PBMCs is a useful technique for assaying the function of included immune cells such as monocytes/macrophages and lymphocytes. However, the isolation of PBMCs excludes other immune cells critical to the host response – neutrophils – as well as obscures the effects of intercellular signaling in the peripheral blood milieu. Therefore, assessing both PBMC and whole blood cultures can yield comprehensive insights into which cellular components are critical to the observed responses. Baseline whole blood levels of IFN-γ and TRAIL were reduced in pregnant women compared to non-pregnant (Fig. 8D), whereas the concentrations of IL-1β, IL-8, IL-27, and IL-29/IFN-λ1 were increased. Upon stimulation, a pregnancy-specific elevation of IL-1β and IL-27 concentrations was observed together with reduced TRAIL (Fig. 8D). Interestingly, an increased whole-blood IFN-γ and IL-2 response to S protein was only observed in pregnant women (Fig. 8D). Several mediators, including TF, showed no response to stimulation in non-pregnant or pregnant women (Supplemental Fig. 4B).
Collectively, these results suggest that the basal levels of specific cytokines (e.g., IFN-γ, IL-1β, IL-18, TRAIL, and IL-27) in the maternal circulation are altered by pregnancy status; however, the S protein alone does not induce a unique immune response. Yet, the release of IFN-β and IL-8 by maternal mononuclear cells was diminished upon SARS-CoV-2 particle challenge, indicating that pregnancy may imprint a differential cytokine response during coronavirus exposure.
DISCUSSION
Pregnancy itself is known to imprint specific immunological changes that can alter host responses to infection (37, 62), which is due in part to the dramatic physiologic changes and increasing energy demands that take place during this period (63). Mechanistic investigations of changes in cellular immune function during pregnancy have focused on differential responses to bacteria or their products, particularly endotoxin/LPS (37, 64, 65), given that bacterial infections are causally linked to pregnancy complications such as preterm birth (66–69). On the other hand, mechanistic exploration of differential responses to viral exposure or infection during pregnancy to complement the vast body of clinical investigations is lacking. Herein, we evaluated the differential cellular responses to SARS-CoV-2 particle and protein/peptide challenge in PBMCs and whole blood isolated from non-pregnant and pregnant women. Recent reports from the SARS-CoV-2 pandemic indicated a pregnancy-specific neutrophilia among both infected and non-infected women (70), as has also been observed in earlier studies unrelated to this virus (71). Moreover, neutrophils display a hyper-activated state during pregnancy (72), which could contribute to their elevated ROS production and potentially explain why stimulation with SARS-CoV-2 particles did not alter such function in the current study. Indeed, the elevated activation status/ROS production of neutrophils could result in increased COVID-19 severity in pregnant women (73), given that in silico analysis of peripheral neutrophils from non-pregnant cases of severe disease revealed upregulation of transcripts associated with oxidative stress (74). However, whether pregnancy-driven neutrophil activation is implicated in the severity of SARS-CoV-2 infection remains unclear.
Monocytes are primary participants in the host defense against viral infection (75). Under steady-state conditions, circulating classical, intermediate, and non-classical monocytes form a continuum of sequential transition, and interruption of this cycle during a systemic inflammatory response is counteracted by the early release of classical monocytes from the bone marrow (76). Consistently, significant monocyte expansion has been reported in cases of severe COVID-19 patients that were admitted to the intensive care unit (77, 78). The gene expression profiles of monocytes from cases of severe COVID-19 display highly dysregulated gene expression profiles (77, 79), which are indicative of enhanced cell activation and a migratory phenotype (79). Such intense alterations in monocyte phenotypes observed in COVID-19 patients are suggestive of emergency myelopoiesis (80) (i.e., the emergency activation of bone marrow hematopoiesis to accommodate demand for myeloid cells (81)). Notably, the predominant cellular infiltrate in bronchoalveolar fluid (BALF) obtained from COVID-19 patients is monocytes, and the abundance of these cells has been correlated with disease severity (82). In line with the abovementioned evidence, our current data support the accelerated transition and activation of monocytes in response to SARS-CoV-2 particles. Notably, monocytes derived from pregnant women showed elevated expression of CD16 in response to stimulation compared to those derived from non-pregnant women, suggesting enhanced activation and/or a more intensive transition process. Although monocytes from both pregnant and non-pregnant women displayed increased expression of activation markers in response to SARS-CoV-2 particles, the pregnancy-specific increase in CD16 expression could signify enhanced monocyte activation/transition that may contribute to the more severe disease progression observed in pregnant women (6–8, 83). Similarly, a prior study of pregnant women with mild COVID-19 noted a tendency for reduced classical monocytes and elevated intermediate monocytes compared to non-pregnant women (84), suggesting that a similar phenomenon is observed in women with severe COVID-19.
Interestingly, a previous investigation reported that the exposure of monocytes from healthy donors to SARS-CoV-2 N or S proteins resulted in increased expression IL6, IL1B, and IL10 as well as enhanced IL-6 release, suggesting the potential contribution of these cells to the uncontrolled production of inflammatory mediators observed in severe COVID-19 cases (85). Similarly, herein we showed that stimulation with SARS-CoV-2 proteins provokes an extensive in vitro inflammatory response in monocytes isolated from both pregnant and non-pregnant women. Notably, the effects of N protein exposure on monocytes have been shown to be more intense than those of the S protein (85), potentially due to the highly immunogenic nature of such protein (46). This is consistent with our data showing that monocyte responses were greatest upon exposure to the N protein followed by the S protein, regardless of pregnancy status. Furthermore, we observed pregnancy-specific alteration of surface expression of monocyte receptors such as CD181 (the IL-8 receptor) and CX3CR1 upon stimulation with the N and S proteins, respectively, suggesting that specific viral proteins could modulate monocyte chemotactic responses in pregnant women exposed to SARS-CoV-2.
Monocyte responses in pregnant and non-pregnant women were also evaluated in response to peptide pools corresponding to the SARS-CoV-2 N, S, and M proteins. Monocytes derived from both study groups showed extensive phenotypic changes in response to peptide exposure; yet, no inter-group differences in response were observed. A previous study, in which the authors investigated T-cell responses to HLA-DR- and HLA-A*24-restricted peptides in convalescent COVID-19 patients, identified epitopes with promiscuous or specific reactivity, respectively, that could govern long-term immunity (86). Therefore, the detection of specific SARS-CoV-2 peptides by memory T cells could be an important component of the immune response against secondary infection. However, in the current study, peripheral immune cells were isolated from pregnant and non-pregnant women and exposed to SARS-CoV-2 peptides in an in vitro setting, which may not elicit comparable responses to those induced by full-length proteins or viral particles.
SARS-CoV-2 infection is linked to lymphopenia, which includes reduced systemic proportions of T cells (87–89), and we have recently reported a similar phenomenon in pregnant women infected with SARS-CoV-2 (90). Such a phenomenon is reflected in the peripheral blood transcriptome of COVID-19 patients, in which upregulated transcripts were enriched for innate immune process while those downregulated included T-cell terms (87). Longitudinal immune profiling of severe COVID-19 patients demonstrated excessive T-cell activation, potentially indicating a compensatory increase in effector functionality to overcome diminished numbers (88, 89), which is consistent with the pregnancy-specific increase in T cell-associated cytokines such as IL-2 and IFNγ observed in the current study. These observations raise the critical question of how changes in the peripheral T-cell compartment induced by SARS-CoV-2 infection interact with those that occur naturally in pregnant women. During normal pregnancy, immunological adaptations include the specific modulation of T-cell signatures in the maternal circulation that increase throughout the third trimester and in normal labor at term (72, 91–93), which may reflect changes occurring in the T-cell compartment in the placental tissues (94). Indeed, we recently showed that the single-cell transcriptomic profiles of T cells in the chorioamniotic membranes from women with COVID-19 during pregnancy partially overlap with those of peripheral T cells from hospitalized COVID-19 patients (90). Therefore, immunological modulation by SARS-CoV-2 exposure is not limited to the subtle changes in peripheral T-cell phenotypes observed herein, but also extends to the maternal-fetal interface.
Extensive research has shown that COVID-19-induced lymphopenia includes a significant decrease in multiple subsets of circulating B cells with the exception of plasmablasts, which are increased in infected patients with SARS-CoV-2 and correlated with disease severity (87, 95–97). In the context of pregnancy, it has also been shown that the numbers and functions of B cells in the peripheral blood of pregnant women with COVID-19 differ from those of non-pregnant women with COVID-19 (98). Herein, we found that B-cell expression of CD22, which is a negative regulator of B-cell receptor (BCR) signaling (99), is increased in pregnant compared to non-pregnant women upon stimulation with SARS-CoV-2 particles. This is in line with a previous report showing the reduced expression of molecules associated with BCR signaling such as RAC2, CD79B, PTPRC, and BLNK in pregnant women with COVID-19 compared to non-pregnant patients (98). We also observed a pregnancy-specific reduction in the un-switched memory-like and transitional-like B-cell subsets, whereas switched memory-like B cells were more expanded in pregnant women upon stimulation. These results partially overlap with prior studies noting that the severity of COVID-19 was linked to reduced abundance of un-switched memory B cells (95), and may be indicative of an impaired B-cell response in pregnant women with SARS-CoV-2 infection. Memory B cells may undergo accelerated class switching in response to SARS-CoV-2 in pregnant women, which could explain the diminished proportions of un-switched memory-like B cells and elevated proportions of switched memory-like B cells compared to non-pregnant women. A limitation of our B-cell analyses is that we did not explore the immunoglobulin profiles of these cells in response to in vitro stimulation with SARS-CoV-2 particles or proteins. Yet, multiple investigations have focused on investigating in vivo B-cell responses in pregnant women infected with SARS-CoV-2 (39, 100, 101), therefore diminishing the need to evaluate this concept using viral particle challenge.
It is well documented that severe COVID-19 is characterized by the onset of a massive cytokine storm (102–104). Indeed, the systemic concentrations of pro-inflammatory cytokines have been utilized in the clinical setting to predict disease severity (105), and multiple proposed treatments for COVID-19 are directed towards mechanisms of cytokine release (104, 106). Here, we found that stimulation with SARS-CoV-2 particles increased the release of multiple cytokines by peripheral leukocytes from both pregnant and non-pregnant women, with the production of IFN-β and IL-8 being diminished in pregnancy. Interferons (IFNs) are a critical component of anti-viral defense (107, 108). Type I IFNs, including IFN-β, bind to their receptors (IFNAR) and induce the transcription of IFN-stimulated genes (ISGs), which restrict viral replication through different mechanisms (109, 110). A recent study reported no differences in the plasma concentrations of IFN-β between pregnant women who had recovered from SARS-CoV-2 infection and those with ongoing or recent infection (111); however, a direct comparison of systemic IFN-β levels between pregnant and non-pregnant women has not been made. Previous research has demonstrated the importance of the timing of the interferon response in relation to viral replication as a central determinant of the observed outcomes. While the early induction of type I IFN confers maximum protection, a delayed response will not only fail to control viral replication but can also lead to inflammation and tissue damage (110). Therefore, the current finding that IFN-β release in response to SARS-CoV-2 is diminished during pregnancy could indicate a delayed interferon response and thus contribute to a more severe phenotype in pregnant women.
Interleukin-8, also known as CXCL8, is a pro-inflammatory chemokine associated with neutrophil chemotaxis as well as with the activation of such innate immune cells (112). In the clinical setting, IL-8 has been demonstrated to play a role in the pathophysiology of obstetrical syndromes as evidenced by altered concentrations of this chemokine in the peripheral blood of women with preeclampsia (113, 114) as well as in the amniotic fluid of women who underwent preterm labor and birth (115, 116), where it is highly correlated with the presence of acute histologic chorioamnionitis (116). Indeed, IL-8 in cervical fluid has been utilized as a marker of intra-amniotic inflammation in a non-invasive point-of-care test to monitor women with preterm pre-labor rupture of membranes (PPROM) (117). In addition to perinatal complications, elevated amniotic fluid IL-8 concentrations are also associated with adverse neonatal outcomes such as bronchopulmonary dysplasia (118). Thus, modulation of systemic IL-8 concentrations by SARS-CoV-2 could be relevant for the care of infected pregnant women. In the current COVID-19 pandemic, IL-8 was shown to be increased in the peripheral blood from individuals with mild-moderate COVID-19 compared to healthy controls as well as in patients with severe COVID-19 compared to those presenting mild-moderate disease (119). A prior report did not find differences in systemic IL-8 concentrations between pregnant women who had recovered from SARS-CoV-2 infection and currently infected or convalescent pregnant women (111). Similarly, no differences were reported between pregnant and non-pregnant women with COVID-19 (70); however, only four patients were included in the latter study group. In contrast with the above studies, here we show differential release of IL-8 from peripheral leukocytes of pregnant and non-pregnant women in response to SARS-CoV-2 viral particle exposure. Such differences could be due in part to the altered neutrophil biology taking place during pregnancy, which is exacerbated by systemic inflammation (29). As pregnant women with COVID-19 are at increased risk of developing preeclampsia (7, 9), and the pathophysiology of preeclampsia involves endothelial dysfunction related with changes in IL-8 (113, 114), it is tempting to speculate that the altered SARS-CoV-2 particle-induced release of IL-8 from the PBMCs of pregnant women may contribute to the intravascular immune response implicated in the pathogenesis of preeclampsia.
The findings described herein must be interpreted with caution due to the in vitro nature of the study. Patients who self-reported as testing positive for SARS-CoV-2, having COVID-19, or receiving a vaccine were excluded from the study; yet, we were unable to rule out possible undiagnosed asymptomatic infection in these patients. Moreover, previous studies have indicated that T cells from patients with prior coronavirus infection were cross-reactive with SARS-CoV-2 epitopes (120). In the current study, the prior history of the patients was not available, and thus any potential contribution of T-cell cross-reactivity to the results reported herein could not be accounted for. Lastly, it is worth mentioning that there are potential confounding factors between pregnant and non-pregnant women that may influence the reported results, such as pregnancy-related medication use, dietary self-supplementation, and lifestyle changes, among others. Therefore, further in vivo and ex vivo investigations of the effects of pregnancy on the maternal immune response to SARS-CoV-2 are needed.
In summary, the in vitro model utilized herein allows for the evaluation of peripheral immune responses triggered by SARS-CoV-2 in pregnant and non-pregnant women. Overall, our current findings are consistent with those that were recently reported in our ex vivo investigation of pregnant women with COVID-19, the majority of which presented a mild form of the disease (90). However, our model does not account for critical changes taking place in maternal organs as well as those at the maternal-fetal interface, which have the largest impact on pregnancy outcome. Thus, different in vitro systems must be developed to fully simulate the immunological responses taking place in pregnant women with severe COVID-19, given that such patients are at greater risk of adverse perinatal consequences due to SARS-CoV-2 infection.
Supplementary Material
KEY POINTS.
SARS-CoV-2 particle/protein exposure induces monocyte activation in pregnancy
SARS-CoV-2 protein exposure mildly enhances T-cell activation during pregnancy
SARS-CoV-2 challenge triggers pregnancy-specific cytokine responses in the mother
ACKNOWLEDGMENTS
The authors thank Rona Wang from the Translational Science & Biomarkers Unit of the Perinatology Research Branch for carrying out some molecular assays, and George Schwenkel and Megan Faucett from the Clinical Laboratory of the Perinatology Research Branch for coordinating sample collection. The authors also thank the physicians, nurses, and research assistants from the Center for Advanced Obstetrical Care and Research, Intrapartum Unit, and the Perinatology Research Branch Clinical Laboratory for help with collecting samples.
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
This research was supported by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS) under Contract No. HHSN275201300006C (R.R.). This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health (N.G-L. and A.L.T.). R.R. has contributed to this work as part of his official duties as an employee of the United States Federal Government.
REFERENCES
- 1.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, and Tan W 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2021. COVID-19 Weekly Epidemiological Update
- 3.Centers for Disease Control. 2021. COVID-19 Data from the National Center for Health Statistics
- 4.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, and Zhang L 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng Y, Wu P, Lu W, Liu K, Ma K, Huang L, Cai J, Zhang H, Qin Y, Sun H, Ding W, Gui L, and Wu P 2020. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: A retrospective study of 168 severe patients. PLoS Pathog 16: e1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, Woodworth KR, Nahabedian JF 3rd, Azziz-Baumgartner E, Gilboa SM, Meaney-Delman D, Pregnancy CC-R, and Infant Linked Outcomes T 2020. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 69: 1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamieson DJ, and Rasmussen SA 2021. An Update on Coronavirus Disease 2019 (COVID-19) and Pregnancy. Am J Obstet Gynecol [DOI] [PMC free article] [PubMed]
- 8.Lokken EM, Huebner EM, Taylor GG, Hendrickson S, Vanderhoeven J, Kachikis A, Coler B, Walker CL, Sheng JS, Al-Haddad BJS, McCartney SA, Kretzer NM, Resnick R, Barnhart N, Schulte V, Bergam B, Ma KK, Albright C, Larios V, Kelley L, Larios V, Emhoff S, Rah J, Retzlaff K, Thomas C, Paek BW, Hsu RJ, Erickson A, Chang A, Mitchell T, Hwang JK, Erickson S, Delaney S, Archabald K, Kline CR, LaCourse SM, Adams Waldorf KM, and C.-i. P. C. Washington State. 2021. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol 225: 77 e71–77 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conde-Agudelo A, and Romero R 2021. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol [DOI] [PMC free article] [PubMed]
- 10.Lai J, Romero R, Tarca AL, Iliodromiti S, Rehal A, Banerjee A, Yu C, Peeva G, Palaniappan V, Tan L, Mehta M, and Nicolaides KH 2021. SARS-CoV-2 and the subsequent development of preeclampsia and preterm birth: evidence of a dose-response relationship supporting causality. Am J Obstet Gynecol [DOI] [PMC free article] [PubMed]
- 11.DeSisto CL, Wallace B, Simeone RM, Polen K, Ko JY, Meaney-Delman D, and Ellington SR 2021. Risk for Stillbirth Among Women With and Without COVID-19 at Delivery Hospitalization — United States, March 2020–September 2021. In MMWR Morb Mortal Wkly Rep CDC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kourtis AP, Read JS, and Jamieson DJ 2014. Pregnancy and infection. N Engl J Med 370: 2211–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, Ng PC, Lam PW, Ho LC, To WW, Lai ST, Yan WW, and Tan PY 2004. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 191: 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, Moore Z, Wiedeman C, Anand M, Tabony L, Nielsen CF, Waller K, Page S, Thompson JM, Avery C, Springs CB, Jones T, Williams JL, Newsome K, Finelli L, Jamieson DJ, and H. N. I. i. P. W. G. Pandemic. 2010. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 303: 1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfaraj SH, Al-Tawfiq JA, and Memish ZA 2019. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection during pregnancy: Report of two cases & review of the literature. J Microbiol Immunol Infect 52: 501–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazumder B, Almond D, Park K, Crimmins EM, and Finch CE 2010. Lingering prenatal effects of the 1918 influenza pandemic on cardiovascular disease. J Dev Orig Health Dis 1: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myrskyla M, Mehta NK, and Chang VW 2013. Early life exposure to the 1918 influenza pandemic and old-age mortality by cause of death. Am J Public Health 103: e83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erlebacher A 2013. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol 13: 23–33. [DOI] [PubMed] [Google Scholar]
- 19.La Rocca C, Carbone F, Longobardi S, and Matarese G 2014. The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett 162: 41–48. [DOI] [PubMed] [Google Scholar]
- 20.Ander SE, Diamond MS, and Coyne CB 2019. Immune responses at the maternal-fetal interface. Sci Immunol 4. [DOI] [PMC free article] [PubMed]
- 21.Arenas-Hernandez M, Romero R, Gershater M, Tao L, Xu Y, Garcia-Flores V, Pusod E, Miller D, Galaz J, Motomura K, Schwenkel G, Para R, and Gomez-Lopez N 2021. Specific innate immune cells uptake fetal antigen and display homeostatic phenotypes in the maternal circulation. J Leukoc Biol [DOI] [PMC free article] [PubMed]
- 22.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, and McCune JM 2008. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322: 1562–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frascoli M, Coniglio L, Witt R, Jeanty C, Fleck-Derderian S, Myers DE, Lee TH, Keating S, Busch MP, Norris PJ, Tang Q, Cruz G, Barcellos LF, Gomez-Lopez N, Romero R, and MacKenzie TC 2018. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez-Lopez N, Romero R, Xu Y, Miller D, Arenas-Hernandez M, Garcia-Flores V, Panaitescu B, Galaz J, Hsu CD, Para R, and Berry SM 2019. Fetal T Cell Activation in the Amniotic Cavity during Preterm Labor: A Potential Mechanism for a Subset of Idiopathic Preterm Birth. J Immunol 203: 1793–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clemens LE, Siiteri PK, and Stites DP 1979. Mechanism of immunosuppression of progesterone on maternal lymphocyte activation during pregnancy. J Immunol 122: 1978–1985. [PubMed] [Google Scholar]
- 26.Weinberg ED 1987. Pregnancy-associated immune suppression: risks and mechanisms. Microb Pathog 3: 393–397. [DOI] [PubMed] [Google Scholar]
- 27.Wegmann TG, Lin H, Guilbert L, and Mosmann TR 1993. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 14: 353–356. [DOI] [PubMed] [Google Scholar]
- 28.Sacks GP, Studena K, Sargent K, and Redman CW 1998. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 179: 80–86. [DOI] [PubMed] [Google Scholar]
- 29.Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, and Romero R 2001. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol 185: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 30.Kraus TA, Engel SM, Sperling RS, Kellerman L, Lo Y, Wallenstein S, Escribese MM, Garrido JL, Singh T, Loubeau M, and Moran TM 2012. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol 32: 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Care AS, Diener KR, Jasper MJ, Brown HM, Ingman WV, and Robertson SA 2013. Macrophages regulate corpus luteum development during embryo implantation in mice. J Clin Invest 123: 3472–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez-Lopez N, Garcia-Flores V, Chin PY, Groome HM, Bijland MT, Diener KR, Romero R, and Robertson SA 2021. Macrophages exert homeostatic actions in pregnancy to protect against preterm birth and fetal inflammatory injury. JCI Insight 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aluvihare VR, Kallikourdis M, and Betz AG 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 5: 266–271. [DOI] [PubMed] [Google Scholar]
- 34.Rowe JH, Ertelt JM, Xin L, and Way SS 2012. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, and Rudensky AY 2012. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Lopez N, Arenas-Hernandez M, Romero R, Miller D, Garcia-Flores V, Leng Y, Xu Y, Galaz J, Hassan SS, Hsu CD, Tse H, Sanchez-Torres C, Done B, and Tarca AL 2020. Regulatory T Cells Play a Role in a Subset of Idiopathic Preterm Labor/Birth and Adverse Neonatal Outcomes. Cell Rep 32: 107874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motomura K, Romero R, Tarca AL, Galaz J, Bhatti G, Done B, Arenas-Hernandez M, Levenson D, Slutsky R, Hsu CD, and Gomez-Lopez N 2020. Pregnancy-specific transcriptional changes upon endotoxin exposure in mice. J Perinat Med 48: 700–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atyeo C, DeRiso EA, Davis C, Bordt EA, De Guzman RM, Shook LL, Yonker LM, Fasano A, Akinwunmi B, Lauffenburger DA, Elovitz MA, Gray KJ, Edlow AG, and Alter G 2021. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and non-pregnant women. Sci Transl Med: eabi8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bordt EA, Shook LL, Atyeo C, Pullen KM, De Guzman RM, Meinsohn MC, Chauvin M, Fischinger S, Yockey LJ, James K, Lima R, Yonker LM, Fasano A, Brigida S, Bebell LM, Roberts DJ, Pepin D, Huh JR, Bilbo SD, Li JZ, Kaimal A, Schust DJ, Gray KJ, Lauffenburger D, Alter G, and Edlow AG 2021. Maternal SARS-CoV-2 infection elicits sexually dimorphic placental immune responses. Sci Transl Med 13: eabi7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ovies C, Semmes EC, and Coyne CB 2021. Pregnancy influences immune responses to SARS-CoV-2. Sci Transl Med 13: eabm2070. [DOI] [PubMed] [Google Scholar]
- 41.Patterson EI, Prince T, Anderson ER, Casas-Sanchez A, Smith SL, Cansado-Utrilla C, Solomon T, Griffiths MJ, Acosta-Serrano A, Turtle L, and Hughes GL 2020. Methods of Inactivation of SARS-CoV-2 for Downstream Biological Assays. J Infect Dis 222: 1462–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duffy D, Rouilly V, Libri V, Hasan M, Beitz B, David M, Urrutia A, Bisiaux A, Labrie ST, Dubois A, Boneca IG, Delval C, Thomas S, Rogge L, Schmolz M, Quintana-Murci L, Albert ML, and Milieu Interieur C 2014. Functional analysis via standardized whole-blood stimulation systems defines the boundaries of a healthy immune response to complex stimuli. Immunity 40: 436–450. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, and Lutz MB 2010. Nomenclature of monocytes and dendritic cells in blood. Blood 116: e74–80. [DOI] [PubMed] [Google Scholar]
- 44.Brian DA, and Baric RS 2005. Coronavirus genome structure and replication. Curr Top Microbiol Immunol 287: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang MY, Zhao R, Gao LJ, Gao XF, Wang DP, and Cao JM 2020. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front Cell Infect Microbiol 10: 587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arya R, Kumari S, Pandey B, Mistry H, Bihani SC, Das A, Prashar V, Gupta GD, Panicker L, and Kumar M 2021. Structural insights into SARS-CoV-2 proteins. J Mol Biol 433: 166725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, Hengartner N, Giorgi EE, Bhattacharya T, Foley B, Hastie KM, Parker MD, Partridge DG, Evans CM, Freeman TM, de Silva TI, McDanal C, Perez LG, Tang H, Moon-Walker A, Whelan SP, LaBranche CC, Saphire EO, and Montefiori DC 2020. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 182: 812–827.e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yurkovetskiy L, Wang X, Pascal KE, Tomkins-Tinch C, Nyalile TP, Wang Y, Baum A, Diehl WE, Dauphin A, Carbone C, Veinotte K, Egri SB, Schaffner SF, Lemieux JE, Munro JB, Rafique A, Barve A, Sabeti PC, Kyratsous CA, Dudkina NV, Shen K, and Luban J 2020. Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant. Cell 183: 739–751.e738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbas AK, and Janeway CA Jr. 2000. Immunology: improving on nature in the twenty-first century. Cell 100: 129–138. [DOI] [PubMed] [Google Scholar]
- 50.Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, and Volk HD 2005. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol 166: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson SA, Guerin LR, Moldenhauer LM, and Hayball JD 2009. Activating T regulatory cells for tolerance in early pregnancy - the contribution of seminal fluid. J Reprod Immunol 83: 109–116. [DOI] [PubMed] [Google Scholar]
- 52.Kahn DA, and Baltimore D 2010. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A 107: 9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, and Erlebacher A 2012. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 336: 1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Zwan A, Bi K, Norwitz ER, Crespo  C, Claas FHJ, Strominger JL, and Tilburgs T 2018. Mixed signature of activation and dysfunction allows human decidual CD8(+) T cells to provide both tolerance and immunity. Proc Natl Acad Sci U S A 115: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slutsky R, Romero R, Xu Y, Galaz J, Miller D, Done B, Tarca AL, Gregor S, Hassan SS, Leng Y, and Gomez-Lopez N 2019. Exhausted and Senescent T Cells at the Maternal-Fetal Interface in Preterm and Term Labor. J Immunol Res 2019: 3128010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller D, Gershater M, Slutsky R, Romero R, and Gomez-Lopez N 2020. Maternal and fetal T cells in term pregnancy and preterm labor. Cell Mol Immunol 17: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grewal IS, and Flavell RA 1998. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 16: 111–135. [DOI] [PubMed] [Google Scholar]
- 58.Baumgarth N, Nikolich-Zugich J, Lee FE, and Bhattacharya D 2020. Antibody Responses to SARS-CoV-2: Let’s Stick to Known Knowns. J Immunol 205: 2342–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quast I, and Tarlinton D 2021. B cell memory: understanding COVID-19. Immunity 54: 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, Righy C, Franco S, Souza TML, Kurtz P, Bozza FA, and Bozza PT 2020. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 136: 1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang S-A, Eisinger D, and LaBrie S 2020. A TruCulture Whole Blood Assay to Evaluate Annual Flu Vaccine Recall Responses. The Journal of Immunology 204: 245.214–245.214. [Google Scholar]
- 62.Abu-Raya B, Michalski C, Sadarangani M, and Lavoie PM 2020. Maternal Immunological Adaptation During Normal Pregnancy. Front Immunol 11: 575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tal R, Taylor HS, Burney RO, Mooney SB, and Giudice LC 2000. Endocrinology of Pregnancy. In Endotext Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, and Wilson DP, eds, South Dartmouth (MA). [Google Scholar]
- 64.Veenstra van Nieuwenhoven AL, Bouman A, Moes H, Heineman MJ, de Leij LF, Santema J, and Faas MM 2003. Endotoxin-induced cytokine production of monocytes of third-trimester pregnant women compared with women in the follicular phase of the menstrual cycle. Am J Obstet Gynecol 188: 1073–1077. [DOI] [PubMed] [Google Scholar]
- 65.Ziegler SM, Feldmann CN, Hagen SH, Richert L, Barkhausen T, Goletzke J, Jazbutyte V, Martrus G, Salzberger W, Renné T, Hecher K, Diemert A, Arck PC, and Altfeld M 2018. Innate immune responses to toll-like receptor stimulation are altered during the course of pregnancy. J Reprod Immunol 128: 30–37. [DOI] [PubMed] [Google Scholar]
- 66.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, and Novy MJ 1994. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 171: 1660–1667. [DOI] [PubMed] [Google Scholar]
- 67.Faro J, Romero R, Schwenkel G, Garcia-Flores V, Arenas-Hernandez M, Leng Y, Xu Y, Miller D, Hassan SS, and Gomez-Lopez N 2019. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome†. Biol Reprod 100: 1290–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coleman M, Orvis A, Wu TY, Dacanay M, Merillat S, Ogle J, Baldessari A, Kretzer NM, Munson J, Boros-Rausch AJ, Shynlova O, Lye S, Rajagopal L, and Adams Waldorf KM 2020. A Broad Spectrum Chemokine Inhibitor Prevents Preterm Labor but Not Microbial Invasion of the Amniotic Cavity or Neonatal Morbidity in a Non-human Primate Model. Front Immunol 11: 770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Motomura K, Romero R, Xu Y, Theis KR, Galaz J, Winters AD, Slutsky R, Garcia-Flores V, Zou C, Levenson D, Para R, Ahmad MM, Miller D, Hsu CD, and Gomez-Lopez N 2020. Intra-Amniotic Infection with Ureaplasma parvum Causes Preterm Birth and Neonatal Mortality That Are Prevented by Treatment with Clarithromycin. mBio 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen G, Liao Q, Ai J, Yang B, Bai H, Chen J, Liu F, Cao Y, Liu H, and Li K 2021. Immune Response to COVID-19 During Pregnancy. Front Immunol 12: 675476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lurie S, Rahamim E, Piper I, Golan A, and Sadan O 2008. Total and differential leukocyte counts percentiles in normal pregnancy. Eur J Obstet Gynecol Reprod Biol 136: 16–19. [DOI] [PubMed] [Google Scholar]
- 72.Aghaeepour N, Ganio EA, McIlwain D, Tsai AS, Tingle M, Van Gassen S, Gaudilliere DK, Baca Q, McNeil L, Okada R, Ghaemi MS, Furman D, Wong RJ, Winn VD, Druzin ML, El-Sayed YY, Quaintance C, Gibbs R, Darmstadt GL, Shaw GM, Stevenson DK, Tibshirani R, Nolan GP, Lewis DB, Angst MS, and Gaudilliere B 2017. An immune clock of human pregnancy. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laforge M, Elbim C, Frère C, Hémadi M, Massaad C, Nuss P, Benoliel JJ, and Becker C 2020. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol 20: 515–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saheb Sharif-Askari N, Saheb Sharif-Askari F, Mdkhana B, Hussain Alsayed HA, Alsafar H, Alrais ZF, Hamid Q, and Halwani R 2021. Upregulation of oxidative stress gene markers during SARS-COV-2 viral infection. Free Radic Biol Med 172: 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nikitina E, Larionova I, Choinzonov E, and Kzhyshkowska J 2018. Monocytes and Macrophages as Viral Targets and Reservoirs. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, Macallan D, and Yona S 2017. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med 214: 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wen W, Su W, Tang H, Le W, Zhang X, Zheng Y, Liu X, Xie L, Li J, Ye J, Dong L, Cui X, Miao Y, Wang D, Dong J, Xiao C, Chen W, and Wang H 2020. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang Y, Qian H, Dai T, Zhang T, Lai Y, Wang J, Liu Z, Chen T, He A, O’Dwyer M, and Hu J 2021. Frontline Science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J Leukoc Biol 109: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chilunda V, Martinez-Aguado P, Xia LC, Cheney L, Murphy A, Veksler V, Ruiz V, Calderon TM, and Berman JW 2021. Transcriptional Changes in CD16+ Monocytes May Contribute to the Pathogenesis of COVID-19. Front Immunol 12: 665773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mann ER, Menon M, Knight SB, Konkel JE, Jagger C, Shaw TN, Krishnan S, Rattray M, Ustianowski A, Bakerly ND, Dark P, Lord G, Simpson A, Felton T, Ho LP, Feldmann M, Grainger JR, and Hussell T 2020. Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci Immunol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manz MG, and Boettcher S 2014. Emergency granulopoiesis. Nat Rev Immunol 14: 302–314. [DOI] [PubMed] [Google Scholar]
- 82.Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, Liu L, Amit I, Zhang S, and Zhang Z 2020. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 26: 842–844. [DOI] [PubMed] [Google Scholar]
- 83.Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, Vecchiet J, Nappi L, Scambia G, Berghella V, and D’Antonio F 2020. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2: 100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Biasi S, Tartaro DL, Gibellini L, Paolini A, Quong A, Petes C, Awong G, Douglas S, Lin D, Nieto J, Galassi FM, Borella R, Fidanza L, Mattioli M, Leone C, Neri I, Meschiari M, Cicchetti L, Iannone A, Trenti T, Sarti M, Girardis M, Guaraldi G, Mussini C, Facchinetti F, and Cossarizza A 2021. Endogenous control of inflammation characterizes pregnant women with asymptomatic or paucisymptomatic SARS-CoV-2 infection. Nat Commun 12: 4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karwaciak I, Salkowska A, Karas K, Dastych J, and Ratajewski M 2021. Nucleocapsid and Spike Proteins of the Coronavirus SARS-CoV-2 Induce IL6 in Monocytes and Macrophages-Potential Implications for Cytokine Storm Syndrome. Vaccines (Basel) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bilich T, Nelde A, Heitmann JS, Maringer Y, Roerden M, Bauer J, Rieth J, Wacker M, Peter A, Horber S, Rachfalski D, Marklin M, Stevanovic S, Rammensee HG, Salih HR, and Walz JS 2021. T cell and antibody kinetics delineate SARS-CoV-2 peptides mediating long-term immune responses in COVID-19 convalescent individuals. Sci Transl Med 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bernardes JP, Mishra N, Tran F, Bahmer T, Best L, Blase JI, Bordoni D, Franzenburg J, Geisen U, Josephs-Spaulding J, Köhler P, Künstner A, Rosati E, Aschenbrenner AC, Bacher P, Baran N, Boysen T, Brandt B, Bruse N, Dörr J, Dräger A, Elke G, Ellinghaus D, Fischer J, Forster M, Franke A, Franzenburg S, Frey N, Friedrichs A, Fuß J, Glück A, Hamm J, Hinrichsen F, Hoeppner MP, Imm S, Junker R, Kaiser S, Kan YH, Knoll R, Lange C, Laue G, Lier C, Lindner M, Marinos G, Markewitz R, Nattermann J, Noth R, Pickkers P, Rabe KF, Renz A, Röcken C, Rupp J, Schaffarzyk A, Scheffold A, Schulte-Schrepping J, Schunk D, Skowasch D, Ulas T, Wandinger KP, Wittig M, Zimmermann J, Busch H, Hoyer BF, Kaleta C, Heyckendorf J, Kox M, Rybniker J, Schreiber S, Schultze JL, and Rosenstiel P 2020. Longitudinal Multi-omics Analyses Identify Responses of Megakaryocytes, Erythroid Cells, and Plasmablasts as Hallmarks of Severe COVID-19. Immunity 53: 1296–1314.e1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, Ellingson MK, Mao T, Oh JE, Israelow B, Takahashi T, Tokuyama M, Lu P, Venkataraman A, Park A, Mohanty S, Wang H, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Muenker MC, Fournier JB, Campbell M, Odio CD, Casanovas-Massana A, Herbst R, Shaw AC, Medzhitov R, Schulz WL, Grubaugh ND, Dela Cruz C, Farhadian S, Ko AI, Omer SB, and Iwasaki A 2020. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584: 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song JW, Zhang C, Fan X, Meng FP, Xu Z, Xia P, Cao WJ, Yang T, Dai XP, Wang SY, Xu RN, Jiang TJ, Li WG, Zhang DW, Zhao P, Shi M, Agrati C, Ippolito G, Maeurer M, Zumla A, Wang FS, and Zhang JY 2020. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun 11: 3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garcia-Flores V, Romero R, Xu Y, Theis KR, Arenas-Hernandez M, Miller D, Peyvandipour A, Bhatti G, Galaz J, Gershater M, Levenson D, Pusod E, Tao L, Kracht D, Florova V, Leng Y, Motomura K, Para R, Faucett M, Hsu CD, Zhang G, Tarca AL, Pique-Regi R, and Gomez-Lopez N 2022. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat Commun 13: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gomez-Lopez N, Romero R, Hassan SS, Bhatti G, Berry SM, Kusanovic JP, Pacora P, and Tarca AL 2019. The Cellular Transcriptome in the Maternal Circulation During Normal Pregnancy: A Longitudinal Study. Front Immunol 10: 2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tarca AL, Romero R, Xu Z, Gomez-Lopez N, Erez O, Hsu CD, Hassan SS, and Carey VJ 2019. Targeted expression profiling by RNA-Seq improves detection of cellular dynamics during pregnancy and identifies a role for T cells in term parturition. Sci Rep 9: 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han X, Ghaemi MS, Ando K, Peterson LS, Ganio EA, Tsai AS, Gaudilliere DK, Stelzer IA, Einhaus J, Bertrand B, Stanley N, Culos A, Tanada A, Hedou J, Tsai ES, Fallahzadeh R, Wong RJ, Judy AE, Winn VD, Druzin ML, Blumenfeld YJ, Hlatky MA, Quaintance CC, Gibbs RS, Carvalho B, Shaw GM, Stevenson DK, Angst MS, Aghaeepour N, and Gaudilliere B 2019. Differential Dynamics of the Maternal Immune System in Healthy Pregnancy and Preeclampsia. Front Immunol 10: 1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pique-Regi R, Romero R, Tarca AL, Sendler ED, Xu Y, Garcia-Flores V, Leng Y, Luca F, Hassan SS, and Gomez-Lopez N 2019. Single cell transcriptional signatures of the human placenta in term and preterm parturition. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sosa-Hernandez VA, Torres-Ruiz J, Cervantes-Diaz R, Romero-Ramirez S, Paez-Franco JC, Meza-Sanchez DE, Juarez-Vega G, Perez-Fragoso A, Ortiz-Navarrete V, Ponce-de-Leon A, Llorente L, Berron-Ruiz L, Mejia-Dominguez NR, Gomez-Martin D, and Maravillas-Montero JL 2020. B Cell Subsets as Severity-Associated Signatures in COVID-19 Patients. Front Immunol 11: 611004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martinez-Colon GJ, McKechnie JL, Ivison GT, Ranganath T, Vergara R, Hollis T, Simpson LJ, Grant P, Subramanian A, Rogers AJ, and Blish CA 2020. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med 26: 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, Alanio C, Kuri-Cervantes L, Pampena MB, D’Andrea K, Manne S, Chen Z, Huang YJ, Reilly JP, Weisman AR, Ittner CAG, Kuthuru O, Dougherty J, Nzingha K, Han N, Kim J, Pattekar A, Goodwin EC, Anderson EM, Weirick ME, Gouma S, Arevalo CP, Bolton MJ, Chen F, Lacey SF, Ramage H, Cherry S, Hensley SE, Apostolidis SA, Huang AC, Vella LA, Unit UPCP, Betts MR, Meyer NJ, and Wherry EJ 2020. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen G, Zhang Y, Zhang Y, Ai J, Yang B, Cui M, Liao Q, Chen H, Bai H, Shang D, Chen J, Sun C, Liu H, Liu F, Mao B, Sun G, Chen L, Lin JW, and Li K 2021. Differential immune responses in pregnant patients recovered from COVID-19. Signal Transduct Target Ther 6: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nitschke L, Carsetti R, Ocker B, Kohler G, and Lamers MC 1997. CD22 is a negative regulator of B-cell receptor signalling. Curr Biol 7: 133–143. [DOI] [PubMed] [Google Scholar]
- 100.Edlow AG, Li JZ, Collier AY, Atyeo C, James KE, Boatin AA, Gray KJ, Bordt EA, Shook LL, Yonker LM, Fasano A, Diouf K, Croul N, Devane S, Yockey LJ, Lima R, Shui J, Matute JD, Lerou PH, Akinwunmi BO, Schmidt A, Feldman J, Hauser BM, Caradonna TM, De la Flor D, D’Avino P, Regan J, Corry H, Coxen K, Fajnzylber J, Pepin D, Seaman MS, Barouch DH, Walker BD, Yu XG, Kaimal AJ, Roberts DJ, and Alter G 2020. Assessment of Maternal and Neonatal SARS-CoV-2 Viral Load, Transplacental Antibody Transfer, and Placental Pathology in Pregnancies During the COVID-19 Pandemic. JAMA Netw Open 3: e2030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Atyeo C, Pullen KM, Bordt EA, Fischinger S, Burke J, Michell A, Slein MD, Loos C, Shook LL, Boatin AA, Yockey LJ, Pepin D, Meinsohn MC, Nguyen NMP, Chauvin M, Roberts D, Goldfarb IT, Matute JD, James KE, Yonker LM, Bebell LM, Kaimal AJ, Gray KJ, Lauffenburger D, Edlow AG, and Alter G 2021. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 184: 628–642 e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pedersen SF, and Ho YC 2020. SARS-CoV-2: a storm is raging. J Clin Invest 130: 2202–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang J, Jiang M, Chen X, and Montaner LJ 2020. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol 108: 17–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moore JB, and June CH 2020. Cytokine release syndrome in severe COVID-19. Science 368: 473–474. [DOI] [PubMed] [Google Scholar]
- 105.Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, and Gnjatic S 2020. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 26: 1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, and Wei H 2020. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 117: 10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sadler AJ, and Williams BR 2008. Interferon-inducible antiviral effectors. Nat Rev Immunol 8: 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McNab F, Mayer-Barber K, Sher A, Wack A, and O’Garra A 2015. Type I interferons in infectious disease. Nat Rev Immunol 15: 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ivashkiv LB, and Donlin LT 2014. Regulation of type I interferon responses. Nat Rev Immunol 14: 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park A, and Iwasaki A 2020. Type I and Type III Interferons - Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe 27: 870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gee S, Chandiramani M, Seow J, Pollock E, Modestini C, Das A, Tree T, Doores KJ, Tribe RM, and Gibbons DL 2021. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat Immunol [DOI] [PubMed]
- 112.Holmes WE, Lee J, Kuang WJ, Rice GC, and Wood WI 1991. Structure and functional expression of a human interleukin-8 receptor. Science 253: 1278–1280. [DOI] [PubMed] [Google Scholar]
- 113.Walsh SW 2009. Plasma from preeclamptic women stimulates transendothelial migration of neutrophils. Reprod Sci 16: 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tosun M, Celik H, Avci B, Yavuz E, Alper T, and Malatyalioğlu E 2010. Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-alpha in normal pregnancies and in pregnancies complicated by preeclampsia. J Matern Fetal Neonatal Med 23: 880–886. [DOI] [PubMed] [Google Scholar]
- 115.Romero R, Ceska M, Avila C, Mazor M, Behnke E, and Lindley I 1991. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 165: 813–820. [DOI] [PubMed] [Google Scholar]
- 116.Cherouny PH, Pankuch GA, Romero R, Botti JJ, Kuhn DC, Demers LM, and Appelbaum PC 1993. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol 169: 1299–1303. [DOI] [PubMed] [Google Scholar]
- 117.Oh KJ, Lee J, Romero R, Park HS, Hong JS, and Yoon BH 2020. A new rapid bedside test to diagnose and monitor intraamniotic inflammation in preterm PROM using transcervically collected fluid. Am J Obstet Gynecol 223: 423 e421–423 e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, and Kim BI 1997. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 177: 825–830. [DOI] [PubMed] [Google Scholar]
- 119.Kesmez Can F, Özkurt Z, Öztürk N, and Sezen S 2021. Effect of IL-6, IL-8/CXCL8, IP-10/CXCL 10 levels on the severity in COVID 19 infection. Int J Clin Pract: e14970. [DOI] [PMC free article] [PubMed]
- 120.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, de Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S, and Sette A 2020. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 181: 1489–1501 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


