Abstract
Background: Desert dust outbreaks and dust storms are the major source of particulate matter globally and pose a major threat to human health. We investigated the microorganisms transported with desert dust particles and evaluated their potential impact on human health. Methods: A systematic review of all reports on the association between non-anthropogenic desert dust pollution, dust microorganisms and human health is conducted. Results: In total, 51 articles were included in this review. The affected regions studied were Asia (32/51, 62.7%) followed by Europe (9/51, 17.6%), America (6/51, 11.8%), Africa (4/51, 7.8%) and Australia (1/51, 2.0%). The Sahara Desert was the most frequent source of dust, followed by Asian and American deserts. In 39/51 studies the dust-related microbiome was analyzed, while, in 12/51 reports, the association of desert dust with infectious disease outbreaks was examined. Pathogenic and opportunistic agents were isolated from dust in 24/39 (61.5%) and 29/39 (74.4%) of the studies, respectively. A significant association of dust events with infectious disease outbreaks was found in 10/12 (83.3%) reports. The infectious diseases that were mostly investigated with dust outbreaks were pneumonia, respiratory tract infections, COVID-19, pulmonary tuberculosis and coccidioidomycosis. Conclusions: Desert dust outbreaks are vehicles of a significant number of pathogenic or opportunistic microorganisms and limited data indicate an association between dust events and infectious disease outbreaks. Further research is required to strengthen the correlation between dust events and infectious diseases and subsequently guide preventive public health measures.
Keywords: airborne pathogens, climate change, desert dust, ecosystem and public health, global warming, pollution, microorganisms, infectious diseases, human health
1. Introduction
Climate change is one of the biggest health threats facing humanity. Dust outbreaks are part of the climate effects that affect air quality and ecosystems in general [1,2]. Recently, extreme climate phenomena and global warming have affected the frequency of dust and sandstorms [1,3]. Dust pollution is becoming more intense globally, as dry conditions allow the expansion of arid lands, which are the main source of dust [1,3].
Dust contains particulate matter (PM) that includes sand and decaying soil particles, minerals, pollutants and bioaerosols [1,2,4]. Among all the sources of non-anthropogenic pollution, desert dust outbreaks are the major cause of increased PM concentrations in the atmosphere [2]. Dust particles remain airborne for several weeks and travel across thousands of kilometers away from their source region, crossing oceans and continents and globally increasing dust concentrations [2,5].
Dust airborne particles are categorized based on their size to those less than 10 μm (PM10), less than 2.5 μm (PM2.5) and ultrafine particles (UFP, less than 100 nm) [4]. These particles are inhaled and deposited in airways, causing health problems [4]. Adverse health effects are mostly associated with PM less than 2.5 μm, particularly UFΡ, as these are absorbed by bronchial epithelial cells and trigger inflammatory responses and oxidative stress, increasing the risk for pulmonary and cardiovascular diseases [4,5,6]. Indeed, there has been increasing evidence that acute and/or chronic exposure to dust PM is associated with pulmonary and cardiovascular problems, as well as increased morbidity and mortality [5,6,7].
It has been recently recognized that PM is a considerable source of microorganisms [8]. Airborne dust particles that contain microorganisms are reasonably concerning as they facilitate transport over long distances around the globe, altering airborne microbiome and facilitating the spread of infectious agents [6,8]. Since air microbiome can directly impact human and ecosystem health, there are concerns on the type of microorganisms that are transported and their potential effects on human health. In this study, we systematically reviewed the literature and assembled information on the potential pathogens that can be transported in the atmosphere with dust particles, their origin and their impact on human health.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
This study was conducted in accordance with the “Preferred Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines [9]. A comprehensive search of Medline and Scopus databases was conducted, without language nor date/year restriction, to identify articles reporting on the association between non-anthropogenic pollution due to desert dust particles and infectious diseases. Various search strategies were used, opting for maximum sensitivity. The following algorithm was used: (desert dust) AND (bacteria OR fung * OR virus OR microorganism OR infection OR pathogen). The last search was updated on the 28th of May 2022. The title and abstract of the articles were screened for eligibility. Cited references were also reviewed to identify additional relevant studies. Two authors independently assessed full-text articles according to study selection criteria. In cases of unresolved disagreement during the screening process, a third author was consulted to reach consensus. Included studies were then reviewed in detail for data extraction.
All studies that examined the content of airborne microbial communities in desert dust particles or the association of desert dust and infectious diseases on humans or animals were included. Duplicate studies and studies in non-English language were excluded. Review articles, in vitro or animal experiments, studies reporting on effects in aqueous ecosystems or plants and studies without report on specific microorganisms were also excluded.
2.2. Data Extraction
From each eligible study, data extraction was performed in a standardized electronic form. Information on authors, year of publication, time and duration of the study, region affected, dust source, type of microorganisms detected in dust particles and associated infectious diseases, if any, were recorded.
3. Results
3.1. Search Results and Included Studies
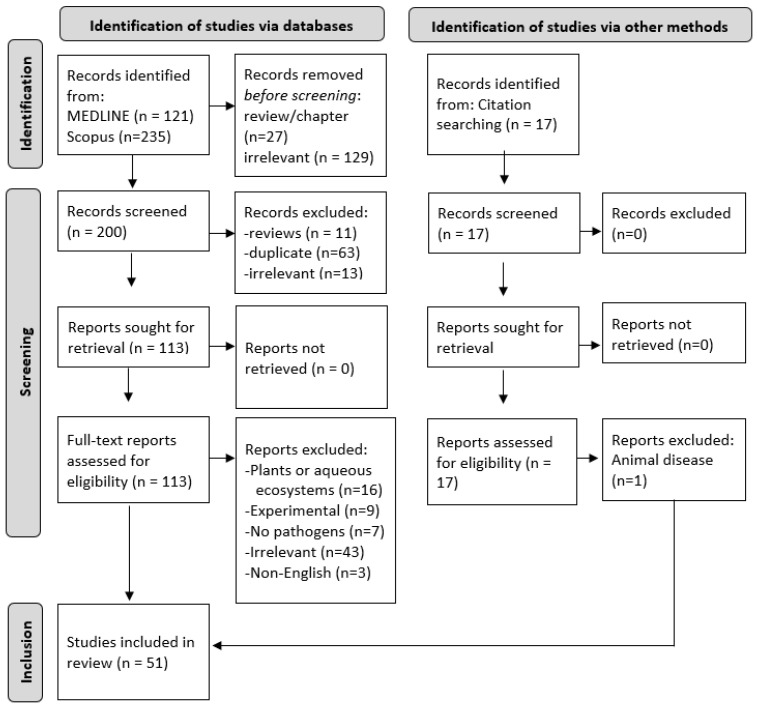
Figure 1 illustrates the PRISMA flowchart of study screening and selection. A total of 356 records were obtained from database searches, and 17 records were identified from citation searches. Finally, 51 studies were included in this review after the removal of review articles (n = 38), duplicate articles (n = 63), irrelevant studies (n = 185), articles in non-English language (n = 3) or studies that did not fulfill inclusion/exclusion criteria (n = 33) (Figure 1).
Figure 1.
PRISMA flow diagram of study selection. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
3.2. Characteristics of Included Studies
All studies were published between 2004 and 2021 [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. The affected regions that were studied were Asia (32/51, 62.7%), followed by Europe (9/51, 17.6%), America (6/51, 11.8%), Africa (4/51, 7.8%) and Australia (1/51, 2.0%). Among all included studies, 39/51 studies analyzed environmental samples and alteration in air microbiome, while in 12/51 studies, an association with dust storms and infectious diseases was performed. In experimental studies, the microorganisms were examined in air/aerosol samples (21/39, 53.8%), dust samples (10/30, 25.6%), soil/sand (6/39, 15.4%), or snow (2/39, 5.1%), collected after various dust storms in the period from 2004 to 2020. The Asian deserts were the more frequent source of dust in the included studies (22/51, 43.1%), followed by the Sahara/North Africa deserts (21/51, 41.2%), American deserts (6/51, 11.8%) and Arabian deserts (4/51, 7.8%).
3.3. Identified Microorganisms from Airborne Sampling
Isolated microorganisms with the potential to induce infections in humans are summarized in Table 1. Other microorganisms that have not been associated with human disease are summarized in Supplemental Table S1.
Table 1.
Pathogenic and opportunistic microorganisms transmitted via desert dust outbreaks globally.
| Region; Study | Dust Source | Sample | Potential Pathogenic or Opportunistic Microorganism |
|---|---|---|---|
| Africa | |||
| Chad, Cape Verde Islands; [25] | The Sahara | dust samples | Firmicutes-Bacillacea, Proteobacteria-Oxalobacteraceae, Microsporidia |
| Mali; [16] | The Sahara | atmospheric particle samples | B. cereus, E. coli, P. aeruginosa, F. nucleatum |
| Mali; [27] | The Sahara | air particles | Acinetobacter spp., Bacillus spp., Corynebacterium spp., Staphylococcus spp., Aspergillus spp. |
| Senegal; [26] | The Sahara | dust samples | Micrococcus spp., Bacillus spp., Kytococcus spp., Pseudomonas spp., Burkholderia spp., Brucella spp., S. aureus, Rhizobium radiobacter, Sphingomonas paucimibilis, Serratia plymuthica, Enterobacter cloacae, Aeromonas hydrophila, Serratia rubidaea |
| America | |||
| Chile; [28] | Atacama Desert | dust samples | Kocuria flava, Bacillus subtilis, Brachybacterium paraconglomeratum, Oceanobacillus oncorhynchi, Microbacterium barkeri, Bacillus sp., Microbacterium paraoxydans, Bacillus firmus, Aspergillus versicolor, Aspergillus nidulans |
| Mexico and New Mexico USA; [29] | Chihuahuan Desert | air (particular matter) and soil samples | Fusarium spp., Aspergillus spp. |
| Asia | |||
| China, South Korea; [30] | Asian desert dust | sand samples | Massilia spp., Planococcus spp. |
| Dunhuang, China; [51] | Taklimakan Desert | airborne dust | Staphylococcus spp., Pseudomonas spp. |
| Dunhuang, China; [52] | Asian desert dust, Gobi desert |
bioaerosoles | Bacillus spp., Staphylococcus spp. |
| China; [43] | Asian desert dust | air samples | Proteobacteria, Firmicutes, Bacillus spp. |
| South Korea; [37] | Asian desert dust | air samples | Prevotellaceae bacterium sp. |
| South Korea; [31] | Asian desert dust | air samples | Proteobacteria, Firmicutes |
| South Korea; [32] | Asian desert dust | air samples | Bacillus spp., Bacillus circulans; Sphingomonas starnbergensis |
| Korea; [44] | Asian desert dust | dust particulate matter | Bacillus subtilis |
| Korea; [45] | Asian desert dust | soil samples | Staphylococcus spp., Bacillus cereus |
| Taiwan; [33] | Asian desert dust | air samples | Influenza A virus |
| Taiwan; [50] | Asian desert dust | spore trap | Penicillium spp., Aspergillus spp. |
| Israel; [34] | The Sahara, Arabian deserts | aerosols | Enterobacteriaceae spp., Lactobacillus spp., Corynebacterium spp. |
| Israel; [36] | South Europe, North Africa | dust samples | α-Proteobacteria, Actinobacteria, β-Proteobacteria, Tremellomycetes |
| Israel; [49] | The Sahara | dust particles | Aspergillus fumigatus, A. niger, Penicillium chrysogenum |
| Lebanon; [35] | North African and Asian desert dust | dust rain samples | β-Proteobacteria, a-proteobacteria, Firmicutes, E-proteobacteria, γ-Proteobacteria |
| Japan; [47] | Asian desert dust | aerosole samples | α-Proteobacteria, β-Proteobacteria and γ-Proteobacteria |
| Japan; [39] | Asian desert dust | bioaerosols | Bacillus subtilis |
| Japan; [40] | Asian desert dust | air samples | Firmicutes (B. subtilis, B. pumilus), a-Proteobacteria |
| Japan; [53] | Gobi desert | dust samples | B. subtilis and B. licheniformis |
| Mongolia; [10] | Gobi Desert | air samples | α- Proteobacteria, β-Proteobacteria and γ-Proteobacteria |
| Mongolia; [48] | Gobi desert | soil samples | Bacillus spp., Staphylococcus spp. |
| Eastern Mediterranean; [41] | The Sahara Desert | air samples (particulate matter) | Micrococcus terreus |
| Iran; [42] | Arabian Deserts | air samples | Bacillus spp., Mycosporium |
| Kuwait and Iraq; [38] | Arid areas of Iraq and Kuwait | dust and soil samples | Mycobacterium spp., Brucella spp., Coxiella burnetii, Clostridium perfringens, Bacillus spp. |
| Iraq; [46] | Middle Eastern desert dust | air samples | Bacillus spp., Micrococcus spp., Streptomyces spp., Staphylococcus spp. |
| Europe | |||
| Spain; [54] | The Sahara Desert | soil samples | Alpha- and Betaproteobacteria, Actinobacteria, Bacteroidetes, Firmicutes |
| Greece; [56] | The Sahara Desert | air samples | Firmicutes |
| Swiss Alps; [22] | The Sahara Desert | snow samples | Oxalobacteriaceae, Neisseria spp., Streptococcus spp. |
| Italy; [57] | African desert dust | air samples (particulate matter) | Sphingobacterium multivorum, Clostridium cadaveris, S. aureus, Propionibacterium avidum, Propionibacterium acnes, Salmonella enterica, Providencia rettgeri, Acinetobacter lwoffi, Acinetobacter ursingii, Acinetobacter johnsonii, Enterobacter cloacae, Enterobacter asburiae, Enterobacter aerogenes, Enterobacter amnigenus, Enterobacter hormaechei |
| Italy; [58] | The Sahara Desert | snow samples | Bacillus spp., Aurebasidium, Periconia, Pleosporaceae |
| Italy; [55] | African desert dust | dust samples | Bacillus spp., Streptococcus spp., Lactococcus spp., Corynebacterium spp., Brevundimonas spp., Paracoccus spp., Sphingomonas spp., Aspergillus spp. |
| Australia | |||
| Australia; [59] | Australian desert dust | dust and rain samples | Bacillus spp., Pseudomonas spp. |
Pathogenic and opportunistic agents were isolated from dust-related microbiome in 24/39 (61.5%) and 29/39 (74.4%) of studies, respectively. The pathogenic microorganisms that were isolated in dust microbiome were S. aureus, Streptococcus spp., N. meningitidis, Enterobacteriaceae spp., P. aeruginosa, Brucella spp. and Coxiela burnetti, as well as Coccidioides spp. fungus and influenza A virus [10,16,22,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. The remaining studies reported opportunistic pathogens, mostly Bacillus spp., Aspergillus spp., etc.
3.4. Associated Infectious Diseases
Among the included studies, 12 examined the association of dust storms with infectious diseases (Table 2). A significant positive association was found in 10/12 (83.3%) of them. The infectious diseases positively associated with desert dust storms were mostly pneumonia and other respiratory tract infections, including COVID-19, pulmonary tuberculosis and coccidioidomycosis (Valley fever) [11,13,17,18,19,20,23,24,60,61]. Other infectious diseases that have been positively associated with dust storms are measles and meningococcal meningitis [12,21]. In two studies, no significant link was noted between dust storms and the infectious diseases studied, pneumonia [13] or coccidiomycosis [11].
Table 2.
Desert dust events associated with infectious disease outbreaks.
| Study | Region | Pathogens Studied | Association with Infectious Diseases |
|---|---|---|---|
| Bell, M.L., et al. [13] | Taiwan | - | Pneumonia (no significant association) |
| Cheng, M.F., et al. [60] | Taiwan | Pneumonia | |
| Comrie, A.C., et al. [11] | California, USA | Coccidioides spp. | Coccidioidomycosis (Valley fever) (no significant association) |
| Kang, J.H., et al. [61] | Taiwan | - | Pneumonia |
| Lauer, A., et al. [15] | California, USA | Coccidioides spp. | Coccidioidomycosis (Valley fever) |
| Lauer, A., et al. [17] | California, USA | Coccidioides spp. | Coccidioidomycosis (Valley fever) |
| Ma, Y., et al. [12] | China | Measles virus | Measles |
| Rohrer, M., et al. [19] | Spain | SARS-CoV-2 | COVID-19 |
| Tobías, A., et al. [21] | Spain | Neisseria meningitidis | Meningococcal meningitis |
| Tong, D.Q., et al. [18] | Southwestern USA | C. immitis, C. posadasii | Valley fever |
| Trianti, S.M., et al. [19] | Greece | - | Pneumonia, other respiratory tract infections |
| Wang, Y., et al. [23] | China | M. tuberculosis | Pulmonary tuberculosis |
4. Discussion
Over the last decades, desert dust outbreaks and sandstorms have been associated with an increase in the concentration of microorganisms in the atmosphere and with several disease outbreaks [4,6,12]. In the present study, we systematically appraised the available evidence to better characterize the microorganisms with pathogenic potential that can be transported with desert dust particles as well as the association of desert dust events with infectious disease outbreaks. Based on our findings, among the studied regions, the most affected ones were Europe and Asia and most of the dust—related microbes originate from large deserts, particularly Asian deserts and the Sahara. In case of the Sahara, these data are in accordance with the fact that approximately more than 100 tons of dust travel each year from the Sahara throughout the globe [6].
Dust events are characterized by significant alternations in the air microbiome [10]. Several of these dust-derived microorganisms are environmental microbes that are highly resistant to stress and capable of surviving under harsh environmental conditions, such as UV radiation, extreme temperatures, drought and a lack of nutrients [61,62]. These microorganisms are expected to preserve the potential to proliferate once they are transported in more friendly environmental conditions [62]. However, this is not always the case, as in several studies, microbes sensitive to extreme environmental conditions have been identified [26,38,57]. There is indeed considerable variation in the type of microorganisms detected in the included studies, as a result of the differences in study design. For example, the included studies differed substantially on the regions studied, the sampling methods and microbiological analysis used, which are issues that may severely affect the microbiome isolated. Additionally, several studies were focused on particular pathogens only, such as fungi or influenza A [33,50]. A better understanding of the factors influencing the detection and composition of these microbial communities is important to address questions related to the impart of desert events on infectious diseases.
Interestingly, apart from the environmental microorganisms, in the great majority of studies, either pathogenic or potentially pathogenic microorganisms were detected. Many of these pathogens, such as N. meningitidis, S. aureus, Enterobacteriaceae spp., Brucella spp., Coxiela burnetti and P. aeruginosa are major human pathogens that may pose an important human health risk. However, data from the studies included in this review may allow us to make only indirect associations with infectious diseases as no direct link was investigated. Notably, the fact that pathogens such as Brucella spp., Coxiella burnetti and Salmonella spp. have been detected in the samples raises the possibility of the existence of herds of domestic animals in the pathway of dust to the sampling site. Furthermore, it is also possible that desert dust may affect the health of animals as well as the number of animal reservoirs in the affected regions. However, further studies are required to investigate the impact of desert dust in animal health.
As far as means of transmission and distance are concerned, potential human pathogens were found in all size fractions of dust tested, including the smaller particles that are more likely to undergo long-distance transport and reach the distal airways via inhalation [16]. In addition, some of the isolated pathogens, such as Coxiella burnetti, are well-known for their ability to be transmitted via inhalation from long distances. Airborne particles may not only affect human health through inhalation; they may also end up in soil and water with rain and, thus, affect plants, animals and the entire ecosystem. Transmission via fomites or via expansion of animal reservoirs may also be plausible, yet it remains unproven.
While epidemiological studies over the last years have demonstrated that dust outbreaks are associated with severe respiratory and cardiovascular disease and increased risk of hospitalization and mortality, limited information exists on the role in promoting infectious disease outbreaks [63,64]. Based on our findings, limited data point towards a significant association of dust storms to infectious disease outbreaks. The most common infectious diseases associated with dust storms were respiratory tract infections, such as pneumonia, COVID-19, pulmonary tuberculosis, coccidioidomycosis and others. It is interesting that a potential association was also found with measles that, similarly to M. tuberculosis, is known to be transmitted via airborne aerosols [19]. In another study, Respiratory Syncytial virus infection has been associated with increased PM concentrations and haze; however, no data exist on the source of the haze; thus, correlations with desert dust cannot be made [65].
Interestingly, in another study, an increase in hospital admissions for meningococcal meningitis was noted in Barcelona one month after a Saharan dust intrusion [21]. Additionally, N. meningitidis was isolated in dust storm samples in several other experimental studies [22]. While other studies have also suggested an association of meningitis episodes with dust storms periods [66,67], it is not clear whether this association is direct or indirect. Based on Tobias et al., the incubation period for meningococcal meningitis is usually less than one week and it seems unlikely that a relationship with a delay of one month after a Saharan dust storm was direct [21]. In this case, dust events are likely to be correlated with other factors, such as changes in human behavior or the breach of protective mucosal barriers due to dryness that may then influence the risk of meningitis [21]. Indeed, we hypothesize that dust intrusion may affect the transmission of infectious diseases in an indirect way, via, for example, fomites for human pathogens, via expanding the animal reservoir (in case of zoonoses) or via affecting human behavior and/or the susceptibility of the host. Similar explanations are probably the case for the transmission of COVID-19, measles and coccidiomycosis in correlation with dust events.
5. Conclusions
To conclude, it appears that desert dust events and sandstorms are vehicles of a significant number of pathogenic or potentially pathogenic microorganisms. Data on their association to infectious disease outbreaks are limited but are suggestive, in general, of a positive association. Considering that climate change and global warming are expected to increase dust activities globally, more research is needed to investigate viability and pathogenic potential of dust microorganisms and assess the risk for human health. This information is crucial to properly guide preventive public health measures to develop a better strategy for the preparation, prevention and mitigation of the destructive effects of these events.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19116907/s1, Table S1: Other microorganisms found in dust storms globally.
Author Contributions
E.V. and G.R.; data curation, E.V. and M.A.; writing—original draft preparation, E.G.; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu Y., Wen B., Li S., Guo Y. Sand and dust storms in Asia: A call for global cooperation on climate change. Lancet Planet Health. 2021;5:e329–e330. doi: 10.1016/S2542-5196(21)00082-6. [DOI] [PubMed] [Google Scholar]
- 2.Sandstrom T., Forsberg B. Desert dust: An unrecognized source of dangerous air pollution? Epidemiology. 2008;19:808–809. doi: 10.1097/EDE.0b013e31818809e0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang P., Jeong J.H., Yoon J.H., Kim H., Wang S.S., Linderholm H.W., Fang K., Wu X., Chen D. Abrupt shift to hotter and drier climate over inner East Asia beyond the tipping point. Science. 2020;370:1095–1099. doi: 10.1126/science.abb3368. [DOI] [PubMed] [Google Scholar]
- 4.Yang J., Kim E.K., Park H.J., McDowell A., Kim Y.K. The impact of bacteria-derived ultrafine dust particles on pulmonary diseases. Exp. Mol. Med. 2020;52:338–347. doi: 10.1038/s12276-019-0367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dockery D.W. Health effects of particulate air pollution. Ann. Epidemiol. 2009;19:257–263. doi: 10.1016/j.annepidem.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin D.W. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 2007;20:459–477. doi: 10.1128/CMR.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aghababaeian H., Ostadtaghizadeh A., Ardalan A., Asgary A., Akbary M., Yekaninejad M.S., Stephens C. Global Health Impacts of Dust Storms: A Systematic Review. Environ. Health Insights. 2021 doi: 10.1177/11786302211018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy M. Dust clouds implicated in spread of infection. Lancet. 2001;358:478. doi: 10.1016/S0140-6736(01)05677-X. [DOI] [PubMed] [Google Scholar]
- 9.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Teezlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. (Engl. Ed.) 2021;74:790–799. doi: 10.1016/j.recesp.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Maki T., Kurosaki Y., Onishi K., Lee K.C., Pointing S.B., Jugder D., Yamanaka N., Hasegawa H., Shinoda M. Variations in the structure of airborne bacterial communities in Tsogt-Ovoo of Gobi desert area during dust events. Air Qual. Atmos. Health. 2017;10:249–260. doi: 10.1007/s11869-016-0430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comrie A.C. No Consistent Link Between Dust Storms and Valley Fever (Coccidioidomycosis) Geohealth. 2021;5:e2021GH000504. doi: 10.1029/2021GH000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y., Zhou J., Yang S., Zhao Y., Zheng X. Assessment for the impact of dust events on measles incidence in western China. Atmos. Environ. 2017;157:1–9. doi: 10.1016/j.atmosenv.2017.03.010. [DOI] [Google Scholar]
- 13.Bell M.L., Levy J.K., Lin Z. The effect of sandstorms and air pollution on cause-specific hospital admissions in Taipei, Taiwan. Occup. Environ. Med. 2008;65:104–111. doi: 10.1136/oem.2006.031500. [DOI] [PubMed] [Google Scholar]
- 14.Kang J.H., Keller J.J., Chen C.S., Lin H.C. Asian dust storm events are associated with an acute increase in pneumonia hospitalization. Ann. Epidemiol. 2012;22:257–263. doi: 10.1016/j.annepidem.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Lauer A., Etyemezian V., Nikolich G., Kloock C., Arzate A.F., Sadiq Batcha F., Kaur M., Garcia E., Mander J., Passaglia A.K. Valley Fever: Environmental Risk Factors and Exposure Pathways Deduced from Field Measurements in California. Int. J. Environ. Res. Public Health. 2020;17:5285. doi: 10.3390/ijerph17155285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern R.A., Mahmoudi N., Buckee C.O., Schartup A.T., Koutrakis P., Ferguson S.T., Wolfson J.M., Wofsy S.C., Daube B.C., Sunderland E.M. The Microbiome of Size-Fractionated Airborne Particles from the Sahara Region. Environ. Sci. Technol. 2021;55:1487–1496. doi: 10.1021/acs.est.0c06332. [DOI] [PubMed] [Google Scholar]
- 17.Lauer A., Lopez J., Abarca S., Bains J. Earthquake-Ridden Area in USA Contains Coccidioides, the Valley Fever Pathogen. Ecohealth. 2020;17:248–254. doi: 10.1007/s10393-020-01485-w. [DOI] [PubMed] [Google Scholar]
- 18.Tong D.Q., Wang J.X.L., Gill T.E., Lei H., Wang B. Intensified dust storm activity and Valley fever infection in the southwestern United States. Geophys. Res. Lett. 2017;44:4304–4312. doi: 10.1002/2017GL073524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohrer M., Flahault A., Stoffel M. Peaks of Fine Particulate Matter May Modulate the Spreading and Virulence of COVID-19. Earth Syst. Environ. 2020;4:789–796. doi: 10.1007/s41748-020-00184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trianti S.M., Samoli E., Rodopoulou S., Katsouyanni K., Papiris S.A., Karakatsani A. Desert dust outbreaks and respiratory morbidity in Athens, Greece. Environ. Health. 2017;16:7. doi: 10.1186/s12940-017-0281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobias A., Cayla J.A., Pey J., Alastuey A., Querol X. Are Saharan dust intrusions increasing the risk of meningococcal meningitis? Int. J. Infect. Dis. 2011;15:e503. doi: 10.1016/j.ijid.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Meola M., Lazzaro A., Zeyer J. Bacterial Composition and Survival on Sahara Dust Particles Transported to the European Alps. Front. Microbiol. 2015;6:1454. doi: 10.3389/fmicb.2015.01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Wang R., Ming J., Liu G., Chen T., Liu X., Liu H., Zhen Y., Cheng G. Effects of dust storm events on weekly clinic visits related to pulmonary tuberculosis disease in Minqin, China. Atmos. Environ. 2016;127:205–212. doi: 10.1016/j.atmosenv.2015.12.041. Greenwood. [DOI] [Google Scholar]
- 24.Gat D., Mazar Y., Cytryn E., Rudich Y. Origin-Dependent Variations in the Atmospheric Microbiome Community in Eastern Mediterranean Dust Storms. Environ. Sci. Technol. 2017;51:6709–6718. doi: 10.1021/acs.est.7b00362. [DOI] [PubMed] [Google Scholar]
- 25.Favet J., Lapanje A., Giongo A., Kennedy S., Aung Y.Y., Cattaneo A., Davis-Richardson A.G., Brown C.T., Kort R., Brumsack H.-J., et al. Microbial hitchhikers on intercontinental dust: Catching a lift in Chad. ISME J. 2013;7:850–867. doi: 10.1038/ismej.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marone A., Kane C.T., Mbengue M., Jenkins G.S., Niang D.N., Drame M.S., Gernand J.M. Characterization of Bacteria on Aerosols From Dust Events in Dakar, Senegal, West Africa. Geohealth. 2020;4:e2019GH000216. doi: 10.1029/2019GH000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kellogg C.A., Griffin D.W., Garrison V.H., Peak K.K., Royall N., Smith R.R., Shinn E.A. Characterization of Aerosolized Bacteria and Fungi From Desert Dust Events in Mali, West Africa. Aerobiologia. 2004;20:99–110. doi: 10.1023/B:AERO.0000032947.88335.bb. [DOI] [Google Scholar]
- 28.Azua-Bustos A., Gonzalez-Silva C., Fernandez-Martinez M.A., Arenas-Fajardo C., Fonseca R., Martin-Torres F.J., Fernández-Sampedro M., Fairén A.G., Zorzano M. Aeolian transport of viable microbial life across the Atacama Desert, Chile: Implications for Mars. Sci Rep. 2019;9:11024. doi: 10.1038/s41598-019-47394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Delgado A., Shukla M.K., DuBois D.W., Flores-Margez J.P., Hernandez Escamilla J.A., Olivas E. Microbial and size characterization of airborne particulate matter collected on sticky tapes along US-Mexico border. J. Environ. Sci. (China) 2017;53:207–216. doi: 10.1016/j.jes.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 30.An S., Sin H.H., DuBow M.S. Modification of atmospheric sand-associated bacterial communities during Asian sandstorms in China and South Korea. Heredity (Edinb.) 2015;114:460–467. doi: 10.1038/hdy.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cha S., Lee D., Jang J.H., Lim S., Yang D., Seo T. Alterations in the airborne bacterial community during Asian dust events occurring between February and March 2015 in South Korea. Sci. Rep. 2016;6:37271. doi: 10.1038/srep37271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cha S., Srinivasan S., Jang J.H., Lee D., Lim S., Kim K.S., Jheong W., Lee D.-W., Park E.-R., Chung H.-M., et al. Metagenomic Analysis of Airborne Bacterial Community and Diversity in Seoul, Korea, during December 2014, Asian Dust Event. PLoS ONE. 2017;12:e0170693. doi: 10.1371/journal.pone.0170693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen P.S., Tsai F.T., Lin C.K., Yang C.Y., Chan C.C., Young C.Y., Lee C.H. Ambient influenza and avian influenza virus during dust storm days and background days. Environ. Health Perspect. 2010;118:1211–1216. doi: 10.1289/ehp.0901782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gat D., Reicher N., Schechter S., Alayof M., Tarn M.D., Wyld B.V., Zimmermann R., Rudich Y. Size-Resolved Community Structure of Bacteria and Fungi Transported by Dust in the Middle East. Front. Microbiol. 2021;12:744117. doi: 10.3389/fmicb.2021.744117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itani G.N., Smith C.A. Dust Rains Deliver Diverse Assemblages of Microorganisms to the Eastern Mediterranean. Sci. Rep. 2016;6:22657. doi: 10.1038/srep22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katra I., Arotsker L., Krasnov H., Zaritsky A., Kushmaro A., Ben-Dov E. Richness and diversity in dust stormborne biomes at the southeast mediterranean. Sci. Rep. 2014;4:5265. doi: 10.1038/srep05265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S., Choi B., Yi S.M., Ko G. Characterization of microbial community during Asian dust events in Korea. Sci. Total Environ. 2009;407:5308–5314. doi: 10.1016/j.scitotenv.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 38.Leski T.A., Malanoski A.P., Gregory M.J., Lin B., Stenger D.A. Application of a broad-range resequencing array for detection of pathogens in desert dust samples from Kuwait and Iraq. Appl. Environ. Microbiol. 2011;77:4285–4292. doi: 10.1128/AEM.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maki T., Susuki S., Kobayashi F., Kakikawa M., Tobo Y., Yamada M., Higashi T., Matsuki A., Hong C., Hasegawa H., et al. Phylogenetic analysis of atmospheric halotolerant bacterial communities at high altitude in an Asian dust (KOSA) arrival region, Suzu City. Sci. Total Environ. 2010;408:4556–4562. doi: 10.1016/j.scitotenv.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Maki T., Puspitasari F., Hara K., Yamada M., Kobayashi F., Hasegawa H., Iwasaka Y. Variations in the structure of airborne bacterial communities in a downwind area during an Asian dust (Kosa) event. Sci. Total Environ. 2014;488:75–84. doi: 10.1016/j.scitotenv.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 41.Mazar Y., Cytryn E., Erel Y., Rudich Y. Effect of Dust Storms on the Atmospheric Microbiome in the Eastern Mediterranean. Environ. Sci. Technol. 2016;50:4194–4202. doi: 10.1021/acs.est.5b06348. [DOI] [PubMed] [Google Scholar]
- 42.Nourmoradi H., Moradnejadi K., Moghadam F.M., Khosravi B., Hemati L., Khoshniyat R., Kazembeigi F. The Effect of Dust Storm on the Microbial Quality of Ambient Air in Sanandaj: A City Located in the West of Iran. Glob. J. Health Sci. 2015;7:114–119. doi: 10.5539/gjhs.v7n7p114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamaguchi N., Park J., Kodama M., Ichijo T., Baba T., Nasu M. Changes in the airborne bacterial community in outdoor environments following Asian dust events. Microbes Environ. 2014;29:82–88. doi: 10.1264/jsme2.ME13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo M.-S., Shin M., Kim Y., Jang M., Choi Y.E., Park S.J., Choi J., Lee J., Park C. Development of electrochemical biosensor for detection of pathogenic microorganism in Asian dust events. Chemosphere. 2017;175:269–274. doi: 10.1016/j.chemosphere.2017.02.060. [DOI] [PubMed] [Google Scholar]
- 45.Sekhon S.S., Kim M., Um H.J., Kobayashi F., Iwasaka Y., Shi G., Chen B., Cho S.-J., Min J., Kim Y.H. Proteomic Analysis of Microbial Community Inhabiting Asian Dust Source Region. Clean Soil Air Water. 2016;44:25–28. doi: 10.1002/clen.201400044. [DOI] [Google Scholar]
- 46.Soleimani Z., Goudarzi G., Sorooshian A., Marzouni M.B., Maleki H. Impact of Middle Eastern dust storms on indoor and outdoor composition of bioaerosol. Atmos. Environ. 2016;138:135–143. doi: 10.1016/j.atmosenv.2016.05.023. [DOI] [Google Scholar]
- 47.Maki T., Hara K., Kobayashi F., Kurosaki Y., Kakikawa M., Matsuki A., Chen B., Shi G., Hasegawa H., Iwasaka Y. Vertical distribution of airborne bacterial communities in an Asian-dust downwind area, Noto Peninsula. Atmos. Environ. 2015;119:282–293. doi: 10.1016/j.atmosenv.2015.08.052. [DOI] [Google Scholar]
- 48.Hagiwara K., Tamaki M., Purevsuren T., Kenji B., Buho H. Distribution of Viable Bacteria in the Dust-Generating Natural Source Area of the Gobi Region, Mongolia. Atmosphere. 2020;11:893. doi: 10.3390/atmos11090893. [DOI] [Google Scholar]
- 49.Schlesinger P., Mamane Y., Grishkan I. Transport of microorganisms to Israel during Saharan dust events. Aerobiologia. 2006;22:259–273. doi: 10.1007/s10453-006-9038-7. [DOI] [Google Scholar]
- 50.Wu P.C., Tsai J.C., Li F.C., Lung S.C., Su H.J. Increased levels of ambient fungal spores in Taiwan are associated with dust events from China. Atmos. Environ. 2004;38:4879–4886. doi: 10.1016/j.atmosenv.2004.05.039. [DOI] [Google Scholar]
- 51.Kakikawa M., Kobayashi F., Maki T., Yamada M., Higashi T., Chen B., Shi G., Hong C., Tobo Y., Iwasaka Y. Dustborne microorganisms in the atmosphere over an Asian dust source region, Dunhuang. Air Qual Atmos Health. 2008;1:195–202. doi: 10.1007/s11869-008-0024-9. [DOI] [Google Scholar]
- 52.Maki T., Susuki S., Kobayashi F., Kakikawa M., Yamada M., Higashi T., Chen B., Shi G., Hong C., Tobo Y., et al. Phylogenetic diversity and vertical distribution of a halobacterial community in the atmosphere of an Asian dust (KOSA) source region, Dunhuang City. Air Qual Atmos Health. 2008;1:81–89. doi: 10.1007/s11869-008-0016-9. [DOI] [Google Scholar]
- 53.Hua N.P., Kobayashi F., Iwasaka Y., Shi G.Y., Naganuma T. Detailed identification of desert-originated bacteria carried by Asian dust storms to Japan. Aerobiologia. 2007;23:291–298. doi: 10.1007/s10453-007-9076-9. [DOI] [Google Scholar]
- 54.Batarberan A., Henley J., Fierer N., Casamayor E.O. Structure, inter-annual recurrence, and global-scale connectivity of airborne microbial communities. Sci. Total Environ. 2014;487:187–195. doi: 10.1016/j.scitotenv.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 55.Rosselli R., Fiamma M., Deligios M., Pintus G., Pellizzaro G., Canu A., Duce P., Squartini A., Muresu R., Cappuccinelli P. Microbial immigration across the Mediterranean via airborne dust. Sci. Rep. 2015;5:16306. doi: 10.1038/srep16306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spivey A. Dust storm fallout. Environ. Health Perspect. 2008;116:A128. doi: 10.1289/ehp.116-a128b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romano S., Di Salvo M., Rispoli G., Alifano P., Perrone M.R., Tala A. Airborne bacteria in the Central Mediterranean: Structure and role of meteorology and air mass transport. Sci. Total Environ. 2019;697:134020. doi: 10.1016/j.scitotenv.2019.134020. [DOI] [PubMed] [Google Scholar]
- 58.Weil T., De Filippo C., Albanese D., Donati C., Pindo M., Pavarini L., Carotenuto F., Pasqui M., Poto L., Gabrieli J., et al. Legal immigrants: Invasion of alien microbial communities during winter occurring desert dust storms. Microbiome. 2017;5:32. doi: 10.1186/s40168-017-0249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim N., Munday C.I., Allison G.E., O’Loingsigh T., De Deckker P., Tapper N.J. Microbiological and meteorological analysis of two Australian dust storms in April 2009. Sci. Total Environ. 2011;412:223–231. doi: 10.1016/j.scitotenv.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 60.Cheng M.F., Ho S.C., Chiu H.F., Wu T.N., Chen P.S., Yang C.Y. Consequences of exposure to Asian dust storm events on daily pneumonia hospital admissions in Taipei, Taiwan. J. Toxicol. Environ. Health. 2008;71:1295–1299. doi: 10.1080/15287390802114808. [DOI] [PubMed] [Google Scholar]
- 61.Makhalanyane T.P., Valverde A., Gunnigle E., Frossard A., Ramond J.B., Cowan D.A. Microbial ecology of hot desert edaphic systems. FEMS Microbiol. Rev. 2015;39:203–221. doi: 10.1093/femsre/fuu011. [DOI] [PubMed] [Google Scholar]
- 62.Dose K., Bieger-Dose A., Ernst B., Feister U., Gomez-Silva B., Klein A., Risi S., Stridde C. Survival of microorganisms under the extreme conditions of the Atacama Desert. Orig. Life Evol. Biosph. 2001;31:287–303. doi: 10.1023/A:1010788829265. [DOI] [PubMed] [Google Scholar]
- 63.Kwon H.J., Cho S.H., Chun Y., Lagarde F., Pershagen G. Effects of the Asian dust events on daily mortality in Seoul, Korea. Environ. Res. 2002;90:1–5. doi: 10.1006/enrs.2002.4377. [DOI] [PubMed] [Google Scholar]
- 64.Middleton N., Yiallouros P., Kleanthous S., Kolokotroni O., Schwartz J., Dockery D.W., Demokritou P., Koutrakis P. A 10-year time-series analysis of respiratory and cardiovascular morbidity in Nicosia, Cyprus: The effect of short-term changes in air pollution and dust storms. Environ. Health. 2008;7:39. doi: 10.1186/1476-069X-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye Q., Fu J.F., Mao J.H., Shang S.Q. Haze is a risk factor contributing to the rapid spread of respiratory syncytial virus in children. Environ. Sci. Pollut. Res. Int. 2016;23:20178–20185. doi: 10.1007/s11356-016-7228-6. [DOI] [PubMed] [Google Scholar]
- 66.Greenwood B.M., Blakebrough I.S., Bradley A.K., Wali S., Whittle H.C. Meningococcal disease and season in sub-Saharan Africa. Lancet. 1984;1:1339–1342. doi: 10.1016/S0140-6736(84)91830-0. [DOI] [PubMed] [Google Scholar]
- 67.Molesworth A.M., Cuevas L.E., Connor S.J., Morse A.P., Thomson M.C. Environmental risk and meningitis epidemics in Africa. Emerg. Infect. Dis. 2003;9:1287–1293. doi: 10.3201/eid0910.030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.