Abstract
Sources of Escherichia coli in a coastal waterway located in Ft. Lauderdale, Fla., were evaluated. The study consisted of an extensive program of field measurements designed to capture spatial and temporal variations in E. coli concentrations as well as experiments conducted under laboratory-controlled conditions. E. coli from environmental samples was enumerated by using a defined substrate technology (Colilert-18). Field sampling tasks included sampling the length of the North Fork to identify the river reach contributing high E. coli levels, autosampler experiments at two locations, and spatially intense sampling efforts at hot spots. Laboratory experiments were designed to simulate tidal conditions within the riverbank soils. The results showed that E. coli entered the river in a large pulse during storm conditions. After the storm, E. coli levels returned to baseline levels and varied in a cyclical pattern which correlated with tidal cycles. The highest concentrations were observed during high tide, whereas the lowest were observed at low tide. This peculiar pattern of E. coli concentrations between storm events was caused by the growth of E. coli within riverbank soils which were subsequently washed in during high tide. Laboratory analysis of soil collected from the riverbanks showed increases of several orders of magnitude in soil E. coli concentrations. The ability of E. coli to multiply in the soil was found to be a function of soil moisture content, presumably due to the ability of E. coli to outcompete predators in relatively dry soil. The importance of soil moisture in regulating the multiplication of E. coli was found to be critical in tidally influenced areas due to periodic wetting and drying of soils in contact with water bodies. Given the potential for growth in such systems, E. coli concentrations can be artificially elevated above that expected from fecal impacts alone. Such results challenge the use of E. coli as a suitable indicator of water quality in tidally influenced areas located within tropical and subtropical environments.
Identification of sources of Escherichia coli in water bodies of urban systems is confounded by the presence of multiple sources as well as by the many factors that influence the ultimate fate of the microbe once it is released into the environment. Some important factors that have been described in prior studies include temperature (2, 34), light (25), salinity (19, 33), rainfall (21, 32), predation (3, 4, 7, 22), available nutrients (20), and environmental pollutants (24). Given the complexities of environmental systems, the influence of each factor in regulating the survival and growth of E. coli is difficult to predict for different field settings, although empirical models have been developed for some situations (5, 26). When one is faced with a river that is experiencing elevated E. coli levels, identifying the cause(s) of the microbial contamination is thus a formidable challenge, since the concentration of E. coli observed in the river is a function of the sources active at that time, chemical and biological factors, and the changing hydro-climatologic conditions that influence the fate of the organisms once they are released. This paper summarizes the approach utilized to identify the source(s) of E. coli for one urban river system. The approach is easily applicable to other river systems, and the results obtained from this study are potentially applicable to other tidally influenced rivers located in tropical or subtropical environments. The primary objective of the present investigation was to identify the source(s) of E. coli in a river located in Ft. Lauderdale, Fla., such that appropriate measures could be taken to improve water quality to meet recreational standards (14, 35). Previous analysis had shown that recreational standards were exceeded in over 90% of the samples collected from the river (fecal coliform levels were >200 CFU/100 ml). Suspected sources of contamination at the outset of the present investigation included septic tank systems, sanitary sewers, wastewater from live-aboard boats, inflows to the river from upstream and downstream water bodies, and animal sources. The basic approach chosen for the present investigation was to intensely monitor E. coli concentrations in the field under various hydro-climatologic conditions. From the data gathered during spatially and temporally intense sampling, more-focused sampling and laboratory efforts were developed, which targeted and defined the ultimate source(s) of contamination.
MATERIALS AND METHODS
Site description.
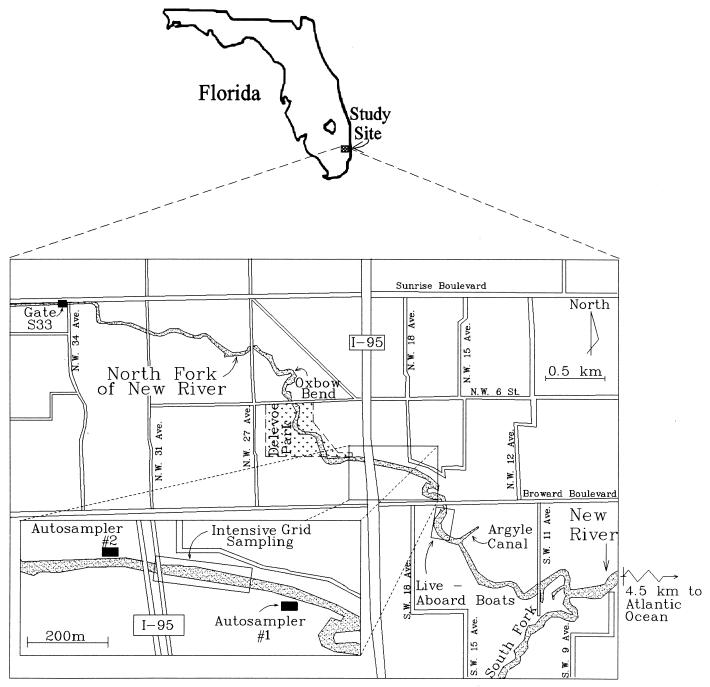
This investigation focused on the North Fork of the New River, which is located in a highly urbanized area of Ft. Lauderdale, Fla. Ft. Lauderdale, Fla., is characterized by a subtropical climate with an average yearly rainfall of 147 cm and an average temperature of 29.4°C. The North Fork is a 6-km tidally influenced tributary of the New River, which on its east end is defined by the split of the New River into its North and South Forks. A control structure, S33, defines its western boundary (Fig. 1). Conductivity measurements within the North Fork indicate that the river is brackish, with a maximum proportion of 10% seawater. A large portion of the North Fork is characterized by a natural meandering shoreline which is lined with dense vegetation. Seawalls are present in some areas and are especially prevalent on the river's east end. Live-aboard boats are found near the central portion of the river. Bird populations, especially ducks, are found throughout the length of the North Fork. Other animal populations, which include domesticated and wild animals, have been observed along the channel banks. The sanitary infrastructure is characterized by two distinct regions. The northern portion of the site (north of Broward Boulevard) is served primarily by sanitary sewers. This is in contrast to the areas to the south, which are served primarily by septic tanks.
FIG. 1.
Location of the North Fork of the New River.
Sampling strategy.
Sources of E. coli in the North Fork of the New River were investigated through five focused efforts. These efforts included (i) a series of quality control experiments aimed at establishing confidence limits of the analytical procedures and for evaluating the impact of an autosampler on sample quality, (ii) sampling the length of the North Fork to identify the river reach contributing high E. coli levels, (iii) autosampler experiments at two locations to determine temporal variations, (iv) spatially intense sampling efforts at hot spots, and (v) a laboratory effort aimed at further isolating suspected sources. Field sampling efforts included simultaneous measurements of relevant hydrologic parameters, including groundwater and canal elevations to determine the direction of lateral flows from the river, rainfall to determine storm water impacts, and tidal cycles to determine the effects of diurnal fluctuations in water levels. Furthermore, basic physicochemical parameters, including temperature, pH, conductivity (model 600R instrument; YSI, Yellow Springs, Ohio), and turbidity (model 40 instrument; Turner Designs, Sunnyvale, Calif.) were measured to establish correlations with E. coli concentrations.
Detection of E. coli.
E. coli from all environmental samples was enumerated by using a defined substrate technology (11–13) which is commercially known as Colilert-18 (IDEXX, Atlanta, Ga.). This technique has been shown to correlate very well with the traditional membrane filter and multiple-tube fermentation methods when used to test both freshwater (8, 10) and marine water (23). The method is based upon sample fluorescence under long-wavelength UV light when MUG (5-methylumbelliferyl-β-d-glucuronide) is metabolized by E. coli. The analytical method involved adding indicator nutrients containing 5-methylumbelliferyl-β-d-glucuronide to a 100-ml volume of water which included 10 ml of sample and 90 ml of sterile phosphate-buffered water prepared as described in Standard Methods for the Examination of Water and Wastewater (1). The solution was then placed in a distribution tray which separated the sample into a series of test wells. The trays were incubated for 18 h at 35.5°C, and wells that fluoresced were counted at the end of the incubation period to provide a most probable number (MPN) per 100 ml of water. All sets of analyses for E. coli determinations included blanks and replicate samples for quality control purposes.
Additional quality control tests conducted at the outset of the project included a split sampling effort with the local municipality to establish confidence limits for the analytical procedures and a set of experiments designed to determine the adequacy of using an autosampler for collecting samples for microbiological analysis. Split samples were collected from various rivers in Ft. Lauderdale. The municipality analyzed the samples in triplicate (each sample consisting of three dilutions) by using the traditional membrane filtration method (1). Samples analyzed at the University of Miami laboratory were analyzed in triplicate by using both the Colilert and membrane filter methods. Membrane filter and Colilert results were statistically the same for all samples containing fewer than 1,200 E. coli organisms per 100 ml. The 95% confidence limits of the analysis for the range of 100 to 300 E. coli organisms per 100 ml were ±100, and those for the range of 700 to 1,200 E. coli organisms per 100 ml were ±300. However, at much higher values (>10,000 E. coli organisms per 100 ml), the results from the Colilert method were significantly lower at the 95% confidence limits than the results from the membrane filter method (12,400 ± 2,600 for the Colilert method versus 16,700 ± 2,300 for membrane filter method). The lower values may be due to the specificity of the Colilert method.
Quality control tests needed for the autosampler experiments focused on evaluating the effect of an extended holding time (24 h) on E. coli concentrations and on estimating the impact of sample carryover from the autosampler inlet lines. The experiment designed to evaluate extended holding times consisted of collecting a 1-liter sample from the river and analyzing aliquots of this sample hourly by using Colilert-18 reagents over a 24-h period. The sample was kept inside a cooler on ice to simulate conditions inside an autosampler. The results indicated that the E. coli concentrations for the 24 samples ranged from 190 to 430 per 100 ml. The 95% confidence limits for the analysis were 340 ± 25 E. coli organisms per 100 ml. A slight trend was noted in the results, with concentrations slightly higher during the first 8 h of the experiment (400 ± 16) than during the remaining 16 h (310 ± 26). Carryover from the sampler lines was tested in the laboratory by collecting five different samples consisting of alternating sterile water and river water (average of 800 E. coli organisms per 100 ml) from the autosampler inlet. Sampler lines were rinsed with sterile deionized distilled water during two automated rinse cycles between sample collections. The results showed that carryover from the river water samples was 4 E. coli organisms per 100 ml. This quantity was small relative to the variability of the analytical procedure, and thus carryover from one sample to the other was considered insignificant.
Water and soil sampling.
To isolate the river reach contributing to E. coli contamination, the length of the North Fork was extensively sampled on six different occasions over a period of 1 year, which included different antecedent rain and tidal conditions. Samples were collected from a boat every 150 m along the 6-km length of the North Fork by submerging a presterilized pipette to a depth of 0.3 m below the surface and extracting a 10-ml sample. Each sample was immediately placed on ice inside a presterilized 100-ml bottle. Samples were analyzed within 4 h of collection. The results indicated that E. coli contamination is distributed throughout the length of the North Fork, with two notable hot spots: interstate highway 95 (I-95) and the Argyle Canal.
To further pinpoint sources of contamination, two autosamplers (models 6700 and 2700; ISCO, Lincoln, Nebr.) were installed near one of these hot spots, the I-95 site. Autosampler 1 was installed 300 m downstream of I-95, and autosampler 2 was installed 120 m upstream (Fig. 1). Autosamplers were installed on the bank of the river within locked enclosures. Lines were run between the autosampler and the river. The inlet side of the line consisted of a screen which was installed approximately 7 m into the river and 1 m above the bottom. The autosampler was fitted with 24 presterilized bottles which were maintained at 4°C throughout the sampling period. Samples were collected hourly for a period of 1 week, with sample retrieval and analysis every 24 h.
Further intense sampling efforts at the hot spots included sampling of storm sewers during dry conditions, intensive grid sampling of the water column, and soil sampling. Samples from storm sewers were collected from manholes and catchbasins by attaching a presterilized 1-liter bottle to a holding device fitted with a rope. The bottle was then lowered into the storm sewer, and a sample was retrieved. Intensive grid sampling of the water column involved setting up a five-by-seven grid at I-95, which cut the river into five channels across the width and seven rows along the length. Surface samples were collected in the same manner as during the full-length river sampling effort. In the three inner channels for all seven rows, samples were also taken at a 1-m depth, in order to obtain a vertical distribution of concentrations. A similar intensive grid sampling exercise was conducted at the Argyle Canal. At the Argyle Canal the grid consisted of three channels and seven rows.
Soil sampling efforts involved collecting 40 samples from the riverbanks at five different locations during low tide. At each location at least one sample was collected aseptically from the water's edge and at least four additional samples were collected from the banks in 6-in. intervals along a line perpendicular to the river. A total of five samples each were collected at 27th Avenue, which is a site characterized by clean water, and at Oxbow Bend, which is the discharge location of a large storm sewer outfall (Fig. 1). Ten samples each were collected along two transects at the north bank of I-95, the south bank of I-95, and the Argyle Canal. Moisture content and E. coli concentrations were determined for each sample. Analyses were by methods described by van Elsas and Smalla (36). In brief, the method involved aseptically scraping 1 to 2 spoonfuls of soil and placing the sample in a presterilized, preweighed Whirl Pak bag. An aliquot of this sample was removed for moisture content analysis (dried at 110°C for 16 h). Approximately 25 ml of sterile phosphate-buffered water was added to the bag with the remaining soil. The liquid and soil were mixed in the bag for 2 min, and the mixture was then filtered through a presterilized 28-μm-pore-size nylon filter. The filter was changed, and the steps were repeated until 100 ml of phosphate-buffered water was collected. The 100-ml water sample was then analyzed by using the Colilert-18 reagents as described above. The standard deviation for split soil samples was 40% of the average.
Laboratory evaluation of E. coli multiplication in soil.
The results of these earlier efforts supported the hypothesis that the source of E. coli brought to the water column was the soils along channel banks. Therefore, during the final effort of the study, soil samples were collected from the I-95 area and subjected to a set of laboratory experiments aimed at establishing whether E. coli was capable of growing in riverbank soils. The experiments consisted of collecting riverbank soils by aseptically scraping off the upper layer of soil from the north bank at I-95. Two soil samples were allowed to air dry. The first sample was dried to a moisture content of 0.8%, and the second was dried to a moisture content of 14%. A third soil sample was maintained wet (35% moisture content) by covering the sample with plastic. These moisture contents were chosen to simulate the range that would be observed along the banks of the river. Each sample was placed in a tray and then subjected to periodic wetting and drying in an effort to simulate tidal conditions in the river. The first sample analyzed was the sample that was air dried to a 0.8% moisture content. The initially wet sample was analyzed next, and then the soil sample that was air dried to a moisture content of 14% was analyzed. Wetting and drying cycles were simulated by submerging and removing each soil sample from a 20-liter fish tank filled with unsterilized water collected from the North Fork. The fish tank was kept in the dark inside an incubator maintained at 25°C. The wetting and drying schedule was as follows: 6 h wet, 6 h dry, 12 h wet, 6 h dry, 6 h wet, 12 h dry, etc.. This sequence was continued over a 4-day period. Four soil samples were collected for E. coli determinations during each wetting or drying cycle. Two samples were collected half an hour after the beginning of the cycle, and two were collected half an hour before the end of the cycle. E. coli was also measured immediately before the first wetting cycle to obtain an initial concentration in the water and soil. E. coli cells were enumerated by using Colilert-18 reagents. A negative control with presterilized water and presterilized soil was subjected to the same wetting and drying sequence described above. No E. coli growth was observed for the negative control. Fecal coliforms in samples were also enumerated, by using the standard membrane filter method (1), for the experiment conducted with the soil sample initially dried to 14% moisture.
RESULTS
River length sampling.
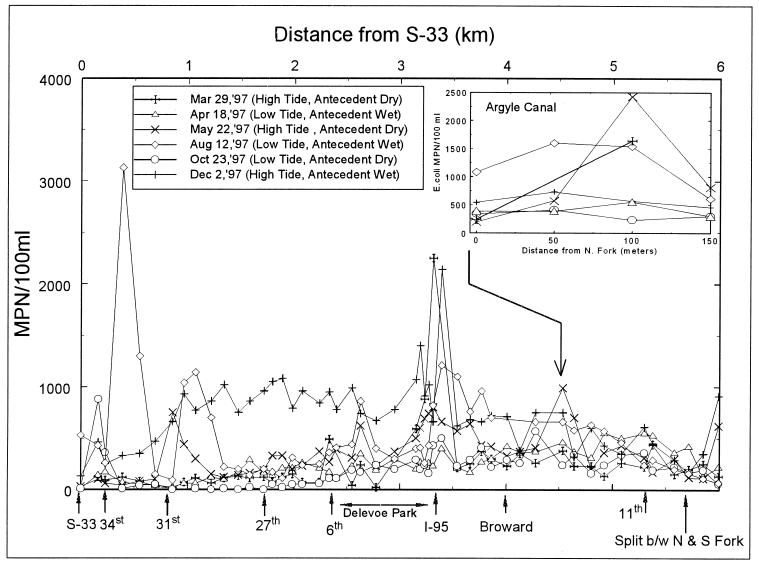
The results from full-length sampling of the North Fork (Fig. 2) indicate that E. coli baseline numbers were similar during five of the six sampling runs. During these runs, baseline E. coli numbers were below 500 MPN/100 ml on each end of the North Fork between 31st Avenue and Delevoe Park and between 11th Avenue and the junction with the South Fork. The primary exception to the low baseline levels corresponded to the December sampling trip, for which baseline values at each location almost doubled. The December samples were the only samples collected after a rain event and during high-tide conditions. These results suggest that E. coli entered the river during wet conditions and that during high tide the source is more pronounced. Stage measurements of the canal and groundwater during these tidal conditions indicated that groundwater contributions are strongest during low tide, thereby eliminating this water body, including contamination of groundwater from septic tanks and sanitary sewers, as a source of E. coli contamination. Although E. coli concentrations within the North Fork varied, the concentration observed between 11th Avenue and the junction with the South Fork were consistently low under all sampling conditions, indicating that the source of E. coli contamination to the North Fork does not come from the New River.
FIG. 2.
Results of river length sampling effort.
The data also show two areas with consistently elevated E. coli concentrations. These areas are located at I-95 and the Argyle Canal. These elevated levels are especially noticeable during high tide, with E. coli concentrations greater than 1,500 MPN/100 ml being recorded at both locations during two of the three high-tide sampling events. Other sporadic increases in E. coli levels were observed throughout this sampling effort. These increases were observed either on the west end of the North Fork, primarily during low-tide conditions, or at bridge crossings. The source at the west end is likely associated with two small canals that discharge to the North Fork immediately east of the S33 control structure. It is possible that these canals receive E. coli contamination and that during outgoing low tide E. coli may move from these small feeder canals toward the west end of the North Fork. It is suspected that the sporadic increases noted at bridge crossings, such as those noted at 31st and 34th Avenues, may be due to transient homeless populations living below the bridges.
Autosampler experiments.
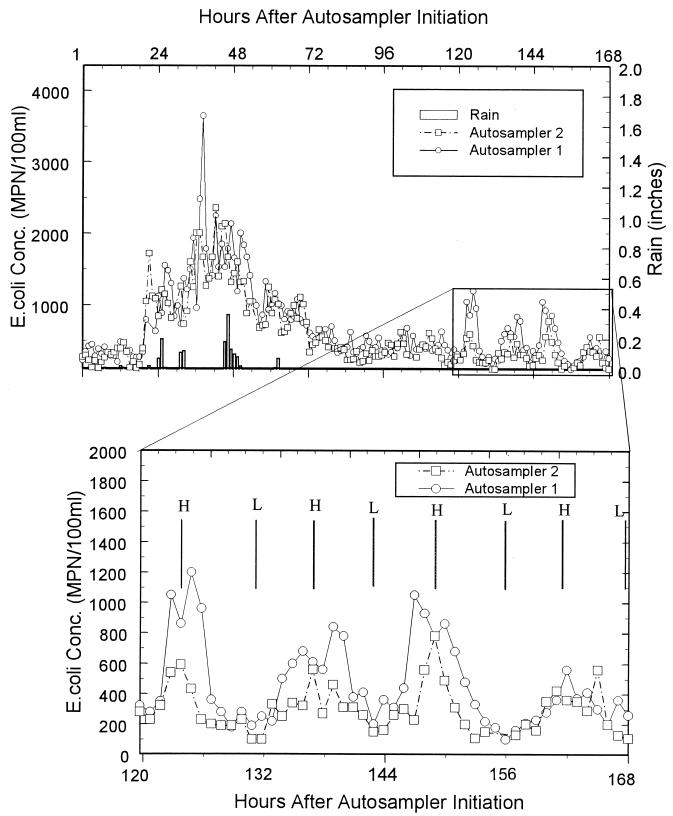
The results of the autosampler experiments (Fig. 3) show two distinct relationships between E. coli levels and hydrologic conditions. The first and most obvious relationship is the extremely high E. coli concentrations observed during periods of rainfall. The second trend occurred from h 120 to 168, where E. coli concentrations correlated with tidal cycles after a storm. During high tide, concentrations of E. coli were elevated, whereas the opposite was observed during low tide. It is interesting that the cyclical pattern in E. coli concentrations occured 2 days after the storm had ceased. The peaks during high tide were significantly higher than the values observed during the 2 days immediately after the storm.
FIG. 3.
Results of 1-week autosampler experiment. Conc., concentration; H, high tide; L, low tide.
Source-specific sampling effort.
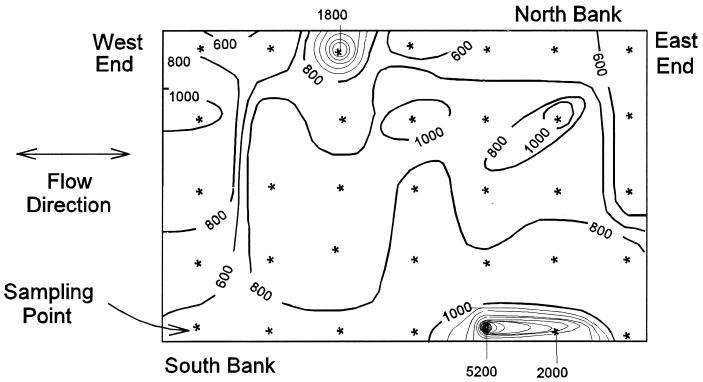
The storm sewer sampling effort showed that water within the sewers contained lower E. coli concentrations than river water, indicating that storm sewers are not a likely source between storms. Results of the intensive grid sampling at both I-95 and the Argyle Canal indicated that the highest E. coli concentrations were along the channel banks. This observation was consistent for both shallow and deep samples. For example, a maximum value of 5,200 E. coli organisms per 100 ml was observed in a shallow-water sample on the south bank near I-95, whereas concentrations of fewer than 1,000 E. coli organisms per 100 ml were observed toward the center of the river (Fig. 4). The results of the field soil sampling effort indicated that the clean-area control at 27th Avenue had the lowest level of E. coli, with 14 E. coli organisms per g of dry soil. This low concentration in soil is in contrast to the case for the other sampling sites, which had higher E. coli levels. The soil collected from the banks of the Argyle Canal contained 87 E. coli organisms per g of dry soil. The highest E. coli value was observed on the north bank of I-95, which contained 200 E. coli organisms per g of dry soil. This value is in contrast to the lower results obtained on the south bank (37 E. coli organisms per g of dry soil).
FIG. 4.
Results of intensive grid sampling effort at I-95 (contour interval, 200 E. coli organisms per 100 ml; shallow-water samples only).
Laboratory soil testing.
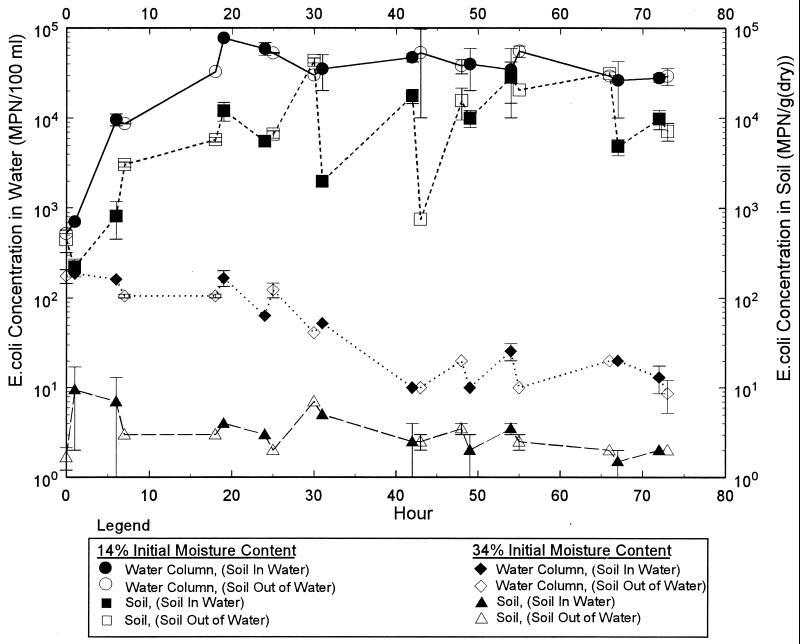
A comparison of the air-dried sample (initial moisture of 14%) with the sample that was maintained wet (initial moisture of 34%) shows a marked difference in growth characteristics (Fig. 5). At the beginning of the experiment, the water and soil E. coli concentrations in the air-dried sample (initial moisture of 14%) were 520 per 100 ml and 440 per g (dry weight), respectively. Six hours after the soil sample was lowered into the water, the concentrations in the water and soil rose to 812 per 100 ml and 9,600 per g (dry weight), respectively. During the next drying cycle, concentrations in the water and soil rose to values of 4.4 × 104 and 2.1 × 104 per 100 ml, respectively. After the first 24 h, the E. coli concentrations in water appeared to stabilize at roughly 4 × 105 E. coli organisms per 100 ml. The data for the water column are in contrast to the soil E. coli concentrations. After the rapid increase during the first 24 h, the soil E. coli concentrations would generally drop to lower values immediately after the soil was either lowered into or removed from the water. After lowering or removal, the E. coli concentrations generally increased until the soil setup was again changed. Apparently, once the E. coli concentrations reached values of greater than 103 per 100 ml, a change in soil moisture resulted in the loss of E. coli.
FIG. 5.
Results from laboratory experiments designed to simulate tidal conditions.
Experiments conducted with wet soil showed no pronounced increase in E. coli concentrations (Fig. 5); rather, a general decreasing trend was observed. The initial moisture content of the wet soil sample was 34%, with an E. coli concentration of 2 per g of dry soil. The initial E. coli concentration in the river water used for this experiment was 1.1 × 102 per 100 ml.
The results of the experiment conducted with the soil that was initially dried to 0.8% moisture indicated that when the initial soil moisture is decreased further, increases of 3 log units rather than 2 log units are observed for water E. coli concentrations. The E. coli concentrations in the water and soil at the beginning of this experiment were 200 per 100 ml and 15 per g (dry weight). During the next drying cycle (from h 6 to 12), concentrations in the water rose dramatically to values of 1.5 × 104 per 100 ml and soil values exceeded the limits of quantification. Efforts during the following cycles focused on changing sample dilutions such that E. coli concentrations were within the limit of quantification. The maximum concentrations recorded during this experiment were 1.2 × 105 per 100 ml for water and more than 4.8 × 104 per g (dry weight) for soil.
Microbe concentrations were within the same order of magnitude for samples analyzed by using both the Colilert-18 and membrane filter methods. In general, concentrations determined with the Colilert-18 reagents were lower than those with the membrane filter method during the first 48 h of the experiment; however, after the first 48 h, the reverse was noted. These data suggest that a fraction of the E. coli stressed during the drying period was non-culturable during the early part of the experiment. As the experiment progressed, significant quantities of other fecal coliform organisms were culturable.
The results of these experiments clearly showed that E. coli was capable of growing in soils collected from the North Fork, especially when subjected to periodic wetting and drying cycles. Apparently, the initial soil moisture of the sample plays a crucial role in the ability of E. coli to grow within the soils tested.
DISCUSSION
This study has shown that the soils along the riverbanks were the primary source of E. coli in the river between storm events. This finding is supported by the facts that intensive grid sampling showed the highest E. coli levels near channel banks, site-specific soil sampling showed that sites with elevated E. coli levels in the water were also characterized by high E. coli concentrations in the soil, and E. coli concentrations were observed to correlate with tidal cycles, with the highest concentrations observed during high tide. During high tide the river is at a higher stage, thereby causing the water column to come in contact with soils that were previously dried. Laboratory experiments confirmed that upon soil drying, E. coli is capable of multiplying by several orders of magnitude. Predation has been shown to play an important role in E. coli survival within natural systems (7, 20, 22) and soil moisture appears to be associated with predator survival. It is likely that E. coli can survive at a lower soil moisture than its predators. Therefore, upon soil drying, conditions are suitable for E. coli growth. It is hypothesized that the degree to which the soil is dried plays a critical role in regulating growth. It is likely that the outer fringes of the channel banks, which experience the most extreme drying conditions, dominate the contribution of E. coli to the water column.
Another curious aspect of the field data is the fact that the cyclical variations in E. coli concentrations in water were observed 2 days after the storm had ceased. If fecal deposition were the primary source of contamination, then why are there no peaks during these 2 days? The scenario where E. coli multiplies within the bank soils is more likely. One plausible explanation for this observation is that the storm flushes E. coli from the soil banks and that it takes E. coli roughly 2 days to increase to levels that affect the water column to a noticeable degree.
It is also likely that local conditions affect the degree to which E. coli is capable of multiplying to elevated levels. The banks along most of the North Fork of the New River are characterized by relatively steep channel embankments or retained by seawalls. The large shallow and shaded embankments along I-95 and the Argyle Canal distinguish these sites from the remaining portions of the North Fork. These conditions are conducive to E. coli growth, since light tends to inhibit growth (25) and the long, shallow embankments provide a large surface area where wetting and drying periodically occur. South Florida's climate, characterized by warm and humid conditions, also plays a likely role in E. coli growth in soil. Such growth has been documented in other studies conducted in tropical and subtropical regions of the world (6, 16, 28–30, 37, 38).
For the North Fork of the New River, the initial list of suspected E. coli sources has been narrowed as a result of this effort, and a new, unanticipated source, i.e., soils within the riverbanks, also was identified. Riverbank soils were found to be a significant source between storms, with the largest E. coli concentrations observed during high tide. High E. coli concentrations were also observed during storms. Such correlations with rainfall have been observed in other studies (21), some of which identified overland flow (9, 18) and sewerage overflows (27, 32) as potential contributors of E. coli. Likely sources of E. coli in the North Fork of the New River during storms therefore include direct runoff from the riverbanks, storm sewer inflows, and sanitary sewer overflows. The riverbank soils were found to contain E. coli, and these contaminated soils can be washed in by direct runoff. Another mechanism by which direct runoff acts as a source is by washing in fecal matter deposited by animals along the banks. It is also recognized that storm sewers contribute water to the river during storms, and this added flow can very possibly contain elevated E. coli concentrations. A sanitary sewer source is also a possibility, since storms can aggravate infiltration and inflows, thereby causing an indirect connection to the river through sewer overflows.
Additional study is recommended to further pinpoint the source of E. coli in the North Fork of the New River during storms. These additional studies should begin by capturing and analyzing storm sewer flows and direct runoff prior to their entering the river. A comprehensive study to investigate the growth patterns of E. coli within riverbank soils from the North Fork and other water bodies in climates similar to that of South Florida is further recommended. Efforts should focus on the impacts of periodic wetting and drying on the growth of E. coli and the impact of soil properties (e.g., clay versus sands [15, 17, 31]) on supporting regrowth of the microbe. If the results of such a comprehensive study confirm that E. coli is capable of multiplying in soils of tropical and subtropical regions to the point that it affects E. coli levels within the water column, perhaps the use of E. coli as an indicator organism for these areas is flawed and other, more novel indicators of human wastewater contamination should be considered for regulating water quality in such regions.
ACKNOWLEDGMENTS
Funding for this project was received from the city of Ft. Lauderdale.
We acknowledge the committee members and participants in Ft. Lauderdale's Blue Ribbon Committee meetings for their invaluable feedback throughout the course of this research.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. 19th ed. Washington, D.C.: American Public Health Association, Inc.; 1995. [Google Scholar]
- 2.Berry C, Lloyd B J, Colbourne J S. Effect of heat shock on recovery of Escherichia coli from drinking water. Water Sci Technol. 1991;24:85–88. [Google Scholar]
- 3.Bogosian G, Sammons L E, Morris P J L, O'Neil J P, Heitkamp M A, Weber D B. Death of the Escherichia coli K-12 strain W3110 in soil and water. Appl Environ Microbiol. 1996;62:4114–4120. doi: 10.1128/aem.62.11.4114-4120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brettar I, Höfle M G. Influence of ecosystematic factors on survival of Escherichia coli after large-scale release into lake water mesocosms. Appl Environ Microbiol. 1992;58:2201–2210. doi: 10.1128/aem.58.7.2201-2210.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canale R P, Auer M T, Owens E M, Heidtke T M, Effler S W. Modeling fecal coliform bacteria. II. Model development and application. Water Res. 1993;27:703–714. [Google Scholar]
- 6.Carillo M, Estrada E, Hazen T C. Survival and enumeration of the fecal indicators Bifidobacterium adolescentis and Escherichia coli in a tropical rain forest watershed. Appl Environ Microbiol. 1985;50:468–476. doi: 10.1128/aem.50.2.468-476.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao W L, Feng R L. Survival of genetically engineered Escherichia coli in natural soil and river water. J Appl Bacteriol. 1990;68:319–325. doi: 10.1111/j.1365-2672.1990.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 8.Clark D L, Milner B B, Stewart M H, Wolfe R L, Olson B H. Comparative study of commercial 4-methylumbelliferyl-β-d-glucuronide preparations with the Standard Methods membrane filtration fecal coliform test for the detection of Escherichia coli in water samples. Appl Environ Microbiol. 1991;57:1528–1534. doi: 10.1128/aem.57.5.1528-1534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyne M S, Gilfillen R A, Rhodes R W, Blevins R L. Soil and fecal coliform trapping by grass filter strips during simulated rain. J Soil Water Cons. 1995;50:405–408. [Google Scholar]
- 10.Eckner K F. Comparison of membrane filtration and multiple-tube fermentation by the Colilert and Enterolert methods for detection of waterborne coliform bacteria, Escherichia coli, and enterococci used in drinking water and bathing water quality monitoring in southern Sweden. Appl Environ Microbiol. 1998;64:3079–3083. doi: 10.1128/aem.64.8.3079-3083.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edberg S C, Allen M J, Smith D B the National Collaborative Study. National field evaluation of a defined substrate method for the simultaneous enumeration of total coliforms and Escherichia coli from drinking water: comparison with the multiple tube fermentation method. Appl Environ Microbiol. 1988;55:1003–1008. doi: 10.1128/aem.54.6.1595-1601.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edberg S C, Allen M J, Smith D B, Kriz N J. Enumeration of total coliforms and Escherichia coli from source water by the defined substrate technology. Appl Environ Microbiol. 1990;56:366–369. doi: 10.1128/aem.56.2.366-369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edberg S C, Allen M J, Smith D B. Defined substrate technology method for rapid and specific simultaneous enumeration of total coliforms and Escherichia coli from water: collaborative study. J Assoc Off Anal Chem. 1991;74:526–529. [PubMed] [Google Scholar]
- 14.Florida Department of Environmental Protection. Florida administrative code, surface water quality standards, 62-302. Tallahassee: Florida Department of Environmental Protection; 1996. [Google Scholar]
- 15.Gerba C, Wallis C, Mellnick J. Fate of wastewater bacteria and viruses in soil. J Irrig Drain. 1975;101:157–174. [Google Scholar]
- 16.Hardina C M, Fujioka R S. Soil: the environmental source of Escherichia coli and enterococci in Hawaii's streams. Environ Toxicol Water Quality. 1991;6:185–195. [Google Scholar]
- 17.Howell J M, Coyne M S, Cornelius P L. Effect of sediment particle size and temperature on fecal bacteria mortality rates and the fecal coliform/fecal streptococci ratio. J Environ Quality. 1996;25:1216–1220. [Google Scholar]
- 18.Hunter C, McDonald A, Beven K. Input of fecal coliform bacteria to an upland stream channel in the Yorkshire Dales. Water Resources Res. 1992;28:1869–1876. [Google Scholar]
- 19.Kator H, Rhodes M. Microbial and chemical indicators. In: Hackney C R, Pierson M D, editors. Environmental indicators of shellfish safety—1994. New York, N.Y: Chapman and Hall; 1994. pp. 30–91. [Google Scholar]
- 20.Korhonen L K, Martikainen P J. Survival of Escherichia coli and Campylobacter jejuni in untreated and filtered lake water. J Appl Bacteriol. 1991;71:379–382. doi: 10.1111/j.1365-2672.1991.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 21.Lipp E K, Rose J B, Vincent R, Kurz R C, Rodriguez-Palacios C. Assessment of the microbiological water quality of Charlotte Harbor, Florida. Technical Report. Brooksville: Southwest Florida Water Management District; 1999. [Google Scholar]
- 22.Mezrioui N, Baleux B, Troussellier M. A microcosm study of the survival of Escherichia coli and Salmonella typhimurium in brackish water. Water Res. 1995;29:459–465. [Google Scholar]
- 23.Palmer C J, Tsai Y-L, Lang A L. Evaluation of Colilert-marine water for detection of total coliforms and Escherichia coli in the marine environment. Appl Environ Microbiol. 1993;59:786–790. doi: 10.1128/aem.59.3.786-790.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathak S P, Bhattacherjee J W. Effect of pollutants on survival of Escherichia coli in microcosms of river water. Bull Environ Contam Toxicol. 1994;53:198–203. doi: 10.1007/BF00192033. [DOI] [PubMed] [Google Scholar]
- 25.Pommepuy M, Guillaud J F, Dupray E, Derrien A, LeGuyader F, Cormier M. Enteric bacteria survival factors. Water Sci Technol. 1992;25:93–103. [Google Scholar]
- 26.Presser K A, Ross T, Ratkowsky D A. Modelling the growth limits (growth/no growth interface) of Escherichia coli as a function of temperature, pH, lactic acid concentration, and water activity. Appl Environ Microbiol. 1998;64:1773–1779. doi: 10.1128/aem.64.5.1773-1779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richman M. Sewer separation lowers fecal coliform levels. Water Environ Technol. 1996;8:20–22. [Google Scholar]
- 28.Rivera S C, Hazen T C, Toranzos G A. Isolation of fecal coliforms from pristine sites in a tropical rain forest. Appl Environ Microbiol. 1988;54:513–517. doi: 10.1128/aem.54.2.513-517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roll B M, Fujioka R S. Sources of faecal indicator bacteria in a brackish, tropical stream and their impact on recreational water quality. Wat Sci Technol. 1997;35:179–186. [Google Scholar]
- 30.Santiago-Mercado J, Hazen T C. Comparison of four membrane filter methods for fecal coliform enumeration in tropical waters. Appl Environ Microbiol. 1987;53:2922–2928. doi: 10.1128/aem.53.12.2922-2928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherer B M, Miner J R, Moore J A, Buckhouse J C. Indicator bacteria survival in stream sediments. J Environ Quality. 1992;21:591–595. [Google Scholar]
- 32.Southwest Florida Water Management District and University of South Florida. Water quality assessment of the Pithlachascotee River following remediation programs. Technical Report. Brooksville: Southwest Florida Water Management District; 1997. [Google Scholar]
- 33.Tassoula E A. Growth possibilities of E. coli in natural waters. Int J Environ Studies. 1997;52:67–73. [Google Scholar]
- 34.Terzieva S I, McFeters G A. Survival and injury of Escherichia coli, Campylobacter jejuni, and Yersinia enterocolitica in stream water. Can J Microbiol. 1991;37:785–790. doi: 10.1139/m91-135. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Environmental Protection Agency. Quality criteria for water. EPA 440/9-76-023. U.S. Washington, D.C.: Environmental Protection Agency; 1976. [Google Scholar]
- 36.van Elsas J D, Smalla K. Methods for sampling soil microbes. In: Hurst C J, Knudsen G, McInerney M J, Stetzenbach L D, Walter M V, editors. Manual of environmental microbiology. Washington, D.C.: ASM Press; 1997. pp. 383–390. [Google Scholar]
- 37.Wright R C. The seasonality of bacterial quality of water in a tropical developing country (Sierra Leone) J Hyg Camb. 1986;96:75–82. doi: 10.1017/s0022172400062550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright R C. The survival patterns of selected faecal bacteria in tropical fresh waters. Epidemiol Infect. 1989;103:603–611. doi: 10.1017/s0950268800031009. [DOI] [PMC free article] [PubMed] [Google Scholar]