Abstract
Objectives
Several studies have demonstrated an association between elevated cardiac biomarkers and adverse outcomes in patients with COVID-19. However, the prognostic and predictive capability of a multimarker panel in a prospectively collected, diverse “all-comers” COVID-19 population has not been fully elucidated.
Design & methods
We prospectively assessed high sensitivity cardiac troponin I (hsTnI), NT-pro B-type Natriuretic Peptide (NT-proBNP), Galectin-3 (Gal-3), and procalcitonin (PCT) in 4,282 serial samples from 358 patients admitted with symptomatic, RT-PCR confirmed SARS-CoV-2 infection. Outcomes examined were 30-day in-hospital mortality and requirement for intubation within 10 days.
Results
Baseline hsTnI had the highest AUC for predicting 30-day mortality (0.81; 95% CI, 0.73–0.88), followed by NT-proBNP (0.80; 0.74–0.86), PCT (0.77; 0.70–0.84), and Gal-3 (0.68; 0.60–0.76). HsTnI < 3.5 ng/L at baseline identified patients at low risk for in-hospital mortality (NPV 95.9%, sensitivity 97.3%) and 10-day intubation (NPV 90.4%, sensitivity 88.5%). Continuous, log-2 increases in troponin concentration were associated with reduced survival (p < 0.001) on Kaplan-Meier curves and increased risk of 30-day mortality: HR 1.26 (1.16–1.37) in univariate and 1.19 (1.03–1.4) in multivariate models. Time-varying doubling of concentrations of hsTnI and Gal-3 were associated with increased risk of 30-day mortality (adjusted HR 1.21, 1.06–1.4, and 1.92, 1.40–2.6).
Conclusion
HsTnI, NT-proBNP, Gal-3, and PCT are elevated at baseline in patients that have worse outcomes from COVID-19. HsTnI was the only independent predictor of 30-day mortality and intubation. Time-varying, doubling in hsTnI and Gal-3 further aided in prognostication of adverse outcomes. These results support the use of hsTnI for triaging patients with COVID-19.
Abbreviations: hs-TnI, High sensitivity troponin I; PCT, procalcitonin; NT, proBNP- N-terminal B-type natriuretic peptide; Gal-3, Galectin 3
Keywords: High sensitivity troponin, COVID-19, Biomarkers
1. Introduction
The pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused over 5 million deaths to date [1]. Concerns persist that healthcare systems in the US are not equipped long-term to deal with continued populations expected to need hospitalization, intubation, and intensive care admission. It is not known how best to triage patients so that resources are optimally allocated during this ongoing public health emergency and to date no risk stratification tool has been globally accepted to aid in identifying those at highest risk for requiring ventilation or mortality from COVID-19. Circulating cardiac biomarkers such as high sensitivity troponin I (hsTnI), Galectin-3 (Gal-3) and natriuretic peptides (NPs) are known to be effective in risk stratification for future major adverse cardiovascular events, among those with and without symptoms of cardiovascular disease [2], [3], [4] and have shown promise for this utility in COVID-19 patients [5], [6], [7].
Several studies have demonstrated worse outcomes among patients with SARS-CoV-2 infection and elevated cardiac biomarkers. An early study of 138 hospitalized patients found that patients admitted to the ICU had hsTnI more than twice as high as those that did not require ICU admission (11 vs 5.1 ng/L, p = 0.004) [8]. Another study of 416 consecutive COVID-19 patients demonstrated that almost 20% had cardiac injury, as defined by a hsTnI > 99th percentile; those with cardiac injury had a mortality rate > 10 times higher than those without (Hazard Ratio (HR) for death from time of symptom onset > 4) [9]. Similarly, a large study from the US demonstrated 2–3 times the risk of mortality when TnI concentrations exceeded 30 and 90 ng/L, respectively [10]. Notably, only one-quarter of patients in this study were African American or Asian and a contemporary TnI assay was employed, limiting applicability to all populations and the ability to accurately report below the 99th percentile. There is also evidence that the combination of natriuretic peptides and high sensitivity troponin may provide improved prognostic capability as opposed to either biomarker alone. In one study of 341 patients with COVID-19, elevations in both biomarkers conferred almost threefold risk for in-hospital mortality relative to non-elevated levels [11]. Studies have also demonstrated associations between natriuretic peptides, procalcitonin (PCT), and Gal-3 with worse outcomes in patients with SARS-CoV-2 infection [7], [12], [13]. However, limited data is available incorporating simultaneously measured biomarkers of myocardial injury, stress, fibrosis, and sepsis in a diverse, prospectively collected cohort. Most studies published to date are observational, involving limited racial and/or ethnic diversity and relying on retrospective examination of physician-ordered standard of care samples such that findings may not necessarily be applicable to all COVID-19 patients presenting to the Emergency Department (ED) setting.
Given the ongoing need for risk stratification in patients with COVID-19, we sought to determine the predictive and prognostic capability of a multimarker panel. Furthermore, we sought to establish if changes in circulating biomarkers could be used to risk stratify patients hospitalized with COVID-19.
2. Methods
2.1. Population and study design
This prospective observational study included adult patients admitted through the ED at Barnes Jewish Hospital between March 2020 and March 2021 with symptoms of COVID-19 and a real-time reverse transcriptase polymerase chain reaction (RT-PCR)-confirmed SARS-CoV-2 infection from an oropharyngeal swab. A total of 4,282 specimens from 358 patients were collected and analyzed. Conduct of this study was reviewed and approved by the Washington University Internal Review Board and it conformed with the principles of the Declaration of Helsinki. Those < 18 or ≥ 90 years of age, with a positive SARS-CoV-2 RT-PCR test within 30 days and/or direct admission or transfer from another hospital were excluded.
2.2. Specimen processing and biochemical analysis
Residual complete blood count (CBC) EDTA plasma specimens drawn in the ED and each day during admission were obtained and centrifuged at 1500 × g at 4˚C for 10 min. The separated plasma was aliquoted and stored at 4˚C for up to 3 days before storage at 80˚C until analysis. All testing was performed on the remnant EDTA plasma specimen. Previous studies have demonstrated stability for several days at 4˚C in separated plasma of these analytes [14], [15], [16], [17]. Specimens were then thawed, centrifuged at 1500 × g for 10 min, and immediately analyzed. Concentrations of hsTnI, NT-proBNP, Gal-3, and PCT were measured on an Abbott ARCHITECT i2000 automated chemistry analyzer according to the manufacturer's instructions (Abbott Diagnostics, Abbott Park, IL). The limit of quantification (LOQ) of the hsTnI assay is 3.5 ng/L, and the limit of detection (LOD) is 1.7 ng/L. The sex-specific 99th percentiles are 17 ng/L for females and 35 ng/L for males. Thresholds of 4 ng/L and 10 ng/L for females and 6 ng/L and 12 ng/L for males were also used for tiered risk stratification [3], [18]. For NT-proBNP, the LOQ is 5 pg/mL. Tiered thresholds used in the diagnosis of acute heart failure to rule out (<300 pg/mL or < 450 pg/mL if > 74 years of age), and rule-in (age < 50 years, >450 pg/mL; age 50–74, > 900 pg/ml; age > 74, > 1800 pg/mL) were used [19]. For Gal-3, tiered thresholds were used for low (<17.8 ng/mL), intermediate (17.9–25.9 ng/mL), and high risk (>25.9 ng/mL) [20].
2.3. Endpoints
Primary outcomes evaluated included 30-day mortality from ED presentation and respiratory failure requiring invasive ventilation within 10 days of ED presentation. If intubation was indicated but declined or if the patient had a “do not resuscitate” directive, the case was adjudicated as “required intubation”.
2.4. Data collection
All outcomes, demographics, data, and medical history were obtained using the electronic medical record (EMR, EPIC). Comorbidities assessed included body mass index (BMI), previous pulmonary embolism, diabetes, chronic obstructive pulmonary disease, previous cardiovascular disease, acute respiratory distress syndrome, chronic kidney injury, end-stage renal disease, and history of deep vein thrombosis. Transthoracic echocardiography was performed in 39 patients, but results were not included in multivariate models due to the low number of patients.
2.5. Statistical analysis
Baseline characteristics including age, sex, race, BMI (continuous), BMI category, days since symptom onset and 10-day intubation requirement were reported by 30-day mortality status. For continuous variables, mean and standard deviation were reported if the variable was approximately normally distributed, and median with minimum and maximum values were reported otherwise. For significance testing by 30-day mortality status, a t-test was used to compare groups if normally distributed and a Mann-Whitney test was used otherwise. For categorical variables, number and percentage were reported, and for significance testing, Pearson’s chi-squared test was used if all expected cell counts were > 5 and Fisher’s exact test was used otherwise.
To assess the relationship between baseline biomarker values and the endpoints of 30-day mortality and 10-day intubation, a univariate analysis was first performed by determining the area under the curve (AUC) for each biomarker with each endpoint. 95% confidence intervals (CIs) for AUC, sensitivity, and specificity were calculated by creating 2000 bootstrapped datasets and then taking the 2.5th and 97.5th percentiles, following the percentile bootstrapping method. For each biomarker, the cutoff corresponding to the Youden index is reported. Additionally, distribution plots were created by performing a logarithm base 2 transformation for each candidate biomarker and then plotting the distribution at baseline by the study endpoints.
Survival analysis was performed to further assess the relationship between biomarker concentrations and 30-day mortality and 10-day intubation. Kaplan-Meier curves were constructed for each endpoint, with patient risk categories determined by biomarker thresholds. The log rank test for differences in survival curves was performed. Univariate Cox proportional hazards models were estimated for baseline biomarker concentrations and sequentially collected concentrations to determine their association with each endpoint. The analysis with sequential samples incorporates all available samples for each patient by using a gap time approach, considering each interval between biomarker measurements and an increase of one log-transformed unit in the biomarker concentration. The biomarkers hsTnI, NT-proBNP, and Galectin-3 were transformed using logarithm base 2. A multivariable adjusted model for each endpoint, both baseline and sequentially, was constructed using the demographic and clinical characteristics of age, sex, race, BMI category, and symptom onset in days. Due to low sample size (n = 8), patients with Asian race were excluded from this multivariable analysis.
All hypothesis testing was 2-sided and used an alpha level of 0.05 to determine statistical significance. All analyses were performed using R version 4.0.5 [21].
3. Results
A total of 361 patients were screened and 358 patients (99.2%) met the inclusion criteria. Baseline characteristics are shown in Table 1 . 210 (58.7%) patients were male. The mean age of the population studied was 59.8 years (SD = 17.1). Within 30-days of ED presentation, 50 (14%) patients died. 93 (26%) patients required intubation, 76 (21%) of whom required intubation within 10-days of ED presentation. Among the 76 patients requiring intubation within 10-days, 36 (47.4%) died within 30-days of hospital admission. The mean (SD) number of days from symptom onset to ED presentation was 4.97 (5.90) for patients who survived and 3.78 (4.67) for those that died within 30-days. Of the 255 patients (71.2%) that identified as black, 31 (12.2%) died. There was no significant (p = 0.5243) difference in mortality rates between black and white patients.
Table 1.
Baseline characteristics by 30-Day Mortality.
| No (N = 308) | Yes (N = 50) | Overall (N = 358) | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 57.8 (16.7) | 72.1 (14.8) | 59.8 (17.1) |
| Median [Min, Max] | 61.0 [18.0, 92.0] | 72.5 [23.0, 98.0] | 63.0 [18.0, 98.0] |
| Sex | |||
| Female | 128 (41.6%) | 20 (40.0%) | 148 (41.3%) |
| Male | 180 (58.4%) | 30 (60.0%) | 210 (58.7%) |
| Race | |||
| White | 70 (22.7%) | 13 (26.0%) | 83 (23.2%) |
| Black | 224 (72.7%) | 31 (62.0%) | 255 (71.2%) |
| Asian | 6 (1.9%) | 2 (4.0%) | 8 (2.2%) |
| Missing | 8 (2.6%) | 4 (8.0%) | 12 (3.4%) |
| BMI | |||
| Mean (SD) | 30.0 (9.18) | 28.2 (7.94) | 29.8 (9.04) |
| Median [Min, Max] | 28.6 [15.0, 88.6] | 27.4 [14.9, 49.0] | 28.4 [14.9, 88.6] |
| Missing | 3 (1.0%) | 3 (6.0%) | 6 (1.7%) |
| BMI Category | |||
| Underweight | 11 (3.6%) | 5 (10.0%) | 16 (4.5%) |
| Normal | 83 (26.9%) | 14 (28.0%) | 97 (27.1%) |
| Overweight | 69 (22.4%) | 11 (22.0%) | 80 (22.3%) |
| Obese | 142 (46.1%) | 17 (34.0%) | 159 (44.4%) |
| Missing | 3 (1.0%) | 3 (6.0%) | 6 (1.7%) |
| Symptom Onset in Days | |||
| Mean (SD) | 4.97 (5.90) | 3.78 (4.67) | 4.80 (5.75) |
| Median [Min, Max] | 3.00 [0, 43.0] | 2.00 [0, 22.0] | 3.00 [0, 43.0] |
| 10-Day Intubation Required | |||
| No | 268 (87.0%) | 14 (28.0%) | 282 (78.8%) |
| Yes | 40 (13.0%) | 36 (72.0%) | 76 (21.2%) |
Median baseline troponin concentration in patients that died within 30-days of ED presentation was 50 ng/L (IQR 26–427) and in those that survived was 8 ng/L (4–20, Fig. 1 A). Three (6%) patients that died and 70 (23%) patients that survived had troponin concentrations below the LOQ (4 ng/L) at ED presentation. Similar results were observed in patients that died relative to those that survived for median NT-proBNP (died = 2019 pg/mL, IQR 568–6332, survived = 177 pg/mL, IQR 46–622), median Gal-3 (died = 55.5 ng/mL, IQR 41.2–87.0, survived = 39.0 ng/mL, IQR 25.4–58.5), and median PCT (died = 0.38 ng/mL, IQR 0.18–2.66, survived = 0.09 ng/mL, IQR 0.04–0.27) concentrations. Patients that required intubation within 10 days of admission had higher median hsTnI concentrations (intubated = 37 ng/L, IQR 9–101, non-intubated = 8 ng/L, IQR 3–22), NT-proBNP (intubated = 850 pg/mL, IQR 201–3028, non-intubated = 176 pg/mL, IQR 45–711), Gal-3 (intubated = 51.8 ng/mL, IQR 37.7–85.5, non-intubated = 38.1 ng/mL IQR 25.1–56.9), and PCT (intubated = 0.37 ng/mL IQR 0.16–1.30, non-intubated = 0.09 ng/mL IQR 0.04–0.23, Fig. 1 B). Of the 63 (20%) patients with hsTnI > 99th% URL that survived, 45 had comorbidities that could cause elevations in troponin. The most common comorbidities included previously diagnosed cardiovascular disease (30%), chronic kidney disease (30%) and end-stage renal disease (18%).
Fig. 1.
Baseline biomarker concentrations stratified by outcomes. hsTnI, NT-proBNP, Gal-3, and PCT concentrations at baseline stratified by A. 30-day mortality and B. requirement of intubation within 10-days of ED presentation.
HsTnI at baseline had the highest AUC for predicting 30-day mortality (Table 2 ) at 0.81 (95% CI, 0.73–0.88), followed by NT-proBNP (0.80, 0.74–0.86), PCT (0.77, 0.74–0.86), and Gal-3 (0.68, 0.60–0.76). AUCs for predicting 10-day intubation requirement were 0.71 (0.64–0.78) for hsTnI, 0.69 (0.62–0.76) for NT-proBNP, 0.66 (0.59–0.73) for Gal-3 and 0.75 (0.69–0.82) for PCT. A single hsTnI cutoff of 25 ng/L demonstrated a sensitivity of 0.76 (0.61–0.87) and specificity of 0.77 (0.44–0.86) for 30-day mortality. The hsTnI LOQ cutoff of < 3.5 ng/L at baseline identified a cohort of 70 patients (25%; NPV 95.9%, sensitivity 97.3%) and 66 patients (26%, NPV 90.4%, sensitivity 88.5%) for in-hospital mortality and 10-day intubation, respectively. Three patients that died within 30-days had baseline hsTnI < 4 ng/L, two of whom had advanced respiratory distress syndrome requiring intubation at ED presentation while one had a subsequent increase in hsTnI to 53 ng/L within two days of admission.
Table 2.
Performance of multimarker panel for 30-day mortality and 10-day intubation.
| Outcome | Biomarker | AUC | 95% CI | Youden Index | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| 30-day Mortality | hsTnI | 0.806 | (0.734,0.878) | 25 | 0.76 (0.61–0.77) | 0.77 (0.46–0.86) |
| NT-proBNP | 0.796 | (0.735,0.857) | 553 | 0.76 (0.6–0.89) | 0.71 (0.56–0.83) | |
| Gal-3 | 0.681 | (0.602,0.761) | 40.3 | 0.76 (0.61–0.89) | 0.53 (0.28–0.66) | |
| PCT | 0.769 | (0.7,0.838) | 0.15 | 0.84 (0.66–0.95) | 0.61 (0.38–0.71) | |
| 10-day Intubation | hsTnI | 0.712 | (0.642,0.783) | 27 | 0.60 (0.41–0.68) | 0.77 (0.6–0.86) |
| NT-proBNP | 0.69 | (0.624,0.757) | 137 | 0.83 (0.71–0.94) | 0.43 (0.33–0.54) | |
| Gal-3 | 0.663 | (0.592,0.733) | 47.7 | 0.57 (0.41–0.71) | 0.68 (0.54–0.77) | |
| PCT | 0.754 | (0.69,0.818) | 0.15 | 0.77 (0.63–0.87) | 0.63 (0.49–0.73) | |
Kaplan-Meier curves demonstrated markedly reduced survival probability in patients with baseline troponin > 99th % URL relative to those at or below 99th % (p < 0.0001, Fig. 2 A). Similar results were observed for 10-day intubation probability for baseline hsTnI (p < 0.0001, Fig. 2 B). Using hsTnI thresholds to stratify low, medium, and high risk demonstrated similar results for 30-day mortality (p = 0.003 Fig. S1 A), and 10-day intubation (p = 0.011, Fig. S1 B). Age specific NT-proBNP cutpoints for identifying acute heart failure were also highly predictive of 30-day mortality risk (p = 0.0007, Fig. S2 A) and 10-day intubation (p = 0.0027, Fig. S2 B). Baseline Gal-3 concentrations using tiered thresholds did not predict risk for 30-day mortality or 10-day intubation (Fig. S3 A-B).
Fig. 2.
Kaplan-Meier Curves for hsTnI by outcome. hsTnI concentration at baseline were stratified by results ≥ and < 99th percentile upper reference limit for A. 30-day mortality and B. requirement of intubation within 10-days of ED presentation.
Univariable Cox proportional hazard models demonstrated that baseline hsTnI concentrations were associated with increased risk of 30-day mortality (HR 1.26, 95% CI, 1.12–1.37, Table 3 ). This association was modestly enhanced with serial measurement of time-varying hsTnI concentrations throughout hospital admission (1.29, 1.18–1.41). Baseline NT-proBNP (1.22, 1.11–1.35), time-varying NT-proBNP (1.26, 1.14–1.38), baseline Gal-3 (1.56, 1.13–2.14), and time-varying Gal-3 (2.04, 1.56–2.69) concentrations were also associated with increased risk. Similarly, the HR for requiring intubation within 10-days of admission for hsTnI was 1.20 (1.11–1.30), for NT-proBNP was 1.12 (1.04–1.20), and for Gal-3 was 1.57 (1.23–2.01). The HR for all three biomarkers increased with serial measurements throughout admission (Table 3).
Table 3.
Univariate Cox-models for 30-day mortality and 10.
| Outcome | Biomarker | Fit | n | # Events | HR | 95% CI | p valie |
|---|---|---|---|---|---|---|---|
| 30-day Mortality | hsTnI log2 | baseline | 325 | 46 | 1.26 | (1.16–1.37) | 0 |
| hsTnI log2 | time-varying | 1410 | 47 | 1.29 | (1.18,1.41) | 0 | |
| NT-proBNP log2 | baseline | 316 | 45 | 1.22 | (1.11–1.35) | 0 | |
| NT-proBNP log2 | time-varying | 1402 | 47 | 1.26 | (1.14–1.38) | 0 | |
| Gal-3 log2 | baseline | 318 | 46 | 1.56 | (1.13–2.14) | 0.006 | |
| Gal-3 log2 | time-varying | 1404 | 47 | 2.04 | (1.56–2.69) | 0 | |
| PCT | baseline | 288 | 43 | 1.00 | (0.99–1.01) | 0.823 | |
| PCT | time-varying | 1369 | 46 | 1.00 | (1.0–1.01) | 0.349 | |
| 10-day Intubation | hsTnI log2 | baseline | 325 | 68 | 1.20 | (1.11–1.3) | 0 |
| hsTnI log2 | time-varying | 900 | 68 | 1.27 | (1.18–1.36) | 0 | |
| NT-proBNP log2 | baseline | 316 | 66 | 1.12 | (1.04–1.2) | 0.002 | |
| NT-proBNP log2 | time-varying | 892 | 67 | 1.16 | (1.08–1.29) | 0 | |
| Gal-3 log2 | baseline | 318 | 68 | 1.57 | (1.23–2.01) | 0 | |
| Gal-3 log2 | time-varying | 894 | 68 | 1.67 | (1.32–2.11) | 0 | |
| PCT | baseline | 288 | 61 | 1.00 | (0.99–1.01) | 0.57 | |
| PCT | time-varying | 863 | 63 | 1.00 | (0.99–1.01) | 0.336 | |
| Outcome | Biomarker | Fit | n | # Events | HR | 95% CI | p valie |
| 30-day Mortality | hsTnI log2 | baseline | 325 | 46 | 1.26 | (1.16–1.37) | 0 |
| hsTnI log2 | time-varying | 1410 | 47 | 1.29 | (1.18,1.41) | 0 | |
| NT-proBNP log2 | baseline | 316 | 45 | 1.22 | (1.11–1.35) | 0 | |
| NT-proBNP log2 | time-varying | 1402 | 47 | 1.26 | (1.14–1.38) | 0 | |
| Gal-3 log2 | baseline | 318 | 46 | 1.56 | (1.13–2.14) | 0.006 | |
| Gal-3 log2 | time-varying | 1404 | 47 | 2.04 | (1.56–2.69) | 0 | |
| PCT | baseline | 288 | 43 | 1.00 | (0.99–1.01) | 0.823 | |
| PCT | time-varying | 1369 | 46 | 1.00 | (1.0–1.01) | 0.349 | |
| 10-day Intubation | hsTnI log2 | baseline | 325 | 68 | 1.20 | (1.11–1.3) | 0 |
| hsTnI log2 | time-varying | 900 | 68 | 1.27 | (1.18–1.36) | 0 | |
| NT-proBNP log2 | baseline | 316 | 66 | 1.12 | (1.04–1.2) | 0.002 | |
| NT-proBNP log2 | time-varying | 892 | 67 | 1.16 | (1.08–1.29) | 0 | |
| Gal-3 log2 | baseline | 318 | 68 | 1.57 | (1.23–2.01) | 0 | |
| Gal-3 log2 | time-varying | 894 | 68 | 1.67 | (1.32–2.11) | 0 | |
| PCT | baseline | 288 | 61 | 1.00 | (0.99–1.01) | 0.57 | |
| PCT | time-varying | 863 | 63 | 1.00 | (0.99–1.01) | 0.336 |
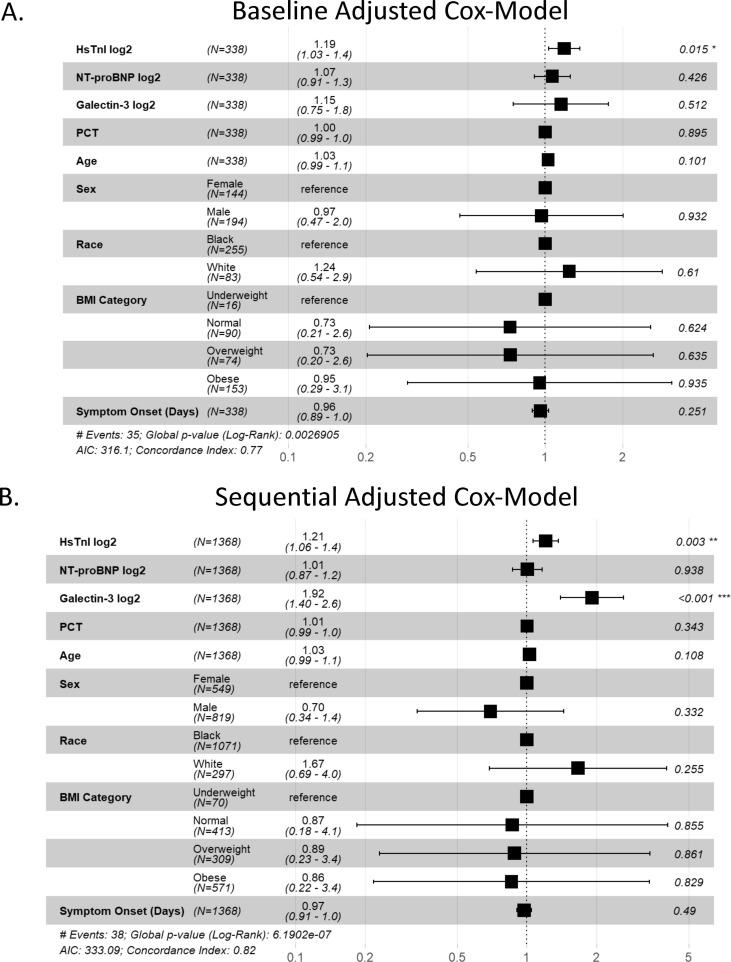
In multivariable models adjusted for all baseline biomarker concentrations, age, sex, race, BMI category, and symptom onset, hsTnI concentration at baseline was the only independent predictor of 30-day mortality (HR 1.19, 1.03–1.4, Fig. 3 A) and 10-day intubation requirement (HR 1.16, 1.02–1.3, Fig. 3 B). In a multivariable adjusted model including time-varying concentrations, time-varying hsTnI concentrations were associated with 30-day mortality (HR 1.21, 1.06–1.4), with a log-2 unit increase (doubling) in hsTnI associated with a 21% (6–40%) increased risk of 30-day mortality (Fig. S4 AC). Time-varying Gal-3 concentrations were also associated with 30-day mortality (HR 1.92, 1.4–2.6), with a log-2 unit increase in Gal-3 associated with a 92% (60–160%) increased mortality risk. Time-varying hsTnI was also associated with 24% (10–40%) increased risk of 10-day intubation (HR 1.24, 1.1–1.4, Fig. S4 B). There was no significant association between, sex, race, BMI, or days from symptom onset to presentation with 30-day mortality or 10-day intubation at baseline. In multivariable models, age was a significant predictor of 10-day intubation both at baseline (HR 1.04, 1.01–1.1) and using time-varying biomarker concentrations (HR 1.02, 1.01–1.05). Cox models were well-calibrated, with concordance indices of 0.77, 0.82, 0.73, and 0.74 for baseline and sequential models for 30-day mortality and baseline and sequential models for 10-day intubation, respectively.
Fig. 3.
Multivariate Cox Models for Predicting Adverse Outcomes. Adjusted models based on continuous multimarker concentrations, age, sex, race, BMI, and time from symptoms to presentation. Shown are A. baseline HR (95% CI), B. time-varying HR for predicting 30-day morality.
4. Discussion
In this study, we assessed the utility of cardiac, fibrosis, and sepsis biomarkers measured serially for prognostication of 30-day mortality and a requirement of intubation in patients symptomatic for COVID-19 presenting to the ED with RT-PCR confirmed SARS-CoV-2 infection. To date, most studies have assessed the utility of biomarkers for prognostication of COVID-19 using either a single biomarker or have relied on physician ordered standard of care testing [5], [22]. One recent Italian study demonstrated that the combination of NPs and hsTn identified patients at increased risk for in-hospital mortality, although the racial composition of this Italian cohort was not reported [11]. To our knowledge, this is the first study incorporating simultaneously measured biomarkers of myocardial injury, stress, fibrosis, and sepsis in a diverse (71% black), prospectively collected cohort with RT-PCR confirmed symptomatic COVID-19.
We found that increased hsTnI at ED presentation was the strongest independent predictor of 30-day mortality and requiring intubation in COVID-19 patients when adjusting for age, gender, race, and comorbidities. Interestingly, studies using baseline concentrations of hsTn as a predictor of mortality have demonstrated mixed outcomes. A previous study of 367 patients using an hsTnT assay demonstrated no significant association with short-term mortality [5]. In contrast, results from a study of 2,736 patients with a generation (contemporary) TnI assay measured within 24 h of admission for COVID-19 found a HR of 1.75 and 3.03 for patients with troponin between 30 and 90 ng/L and > 90 ng/L respectively [10]. A study from Italy using the same hsTnI assay used in this study demonstrated a HR of 9.0 for in-hospital mortality in patients with TnI > 99th % URL [23]. Here, we demonstrate a significant HR of 1.19 for baseline troponin concentration in our patient population. Differences between these studies are multifactorial but are likely due in part to ethnic and racial disparities. Previous studies enrolled patients based on physician-adjudicated need for troponin testing and concern for myocardial injury [5], [10], [22], [23]. Therefore, a strength of this study is that it was not biased to a patient population with a higher likelihood of myocardial injury, demonstrating hsTnI as a tool for risk stratification in all patients with symptomatic COVID-19. Another strength of this study was the diverse patient population (71% black, 23% white, and 2.2% Asian). This may also underlie differences between this study and others that primarily consisted of white [5], [23] or Asian populations [8], [9] and may improve transferability of results to hospitals with similar demographics across the US. Importantly, using the LOQ cutoff of < 3.5 ng/L, 27% of patients would have been ruled-out for mortality and 28% for intubation with > 90% NPV, aiding in the rapid triage and treatment of higher and lower risk patient populations. Importantly, only 3 patients that died within 30-days had baseline hsTnI < 3.5 ng/L (NPV of 95.9%), two of which had advanced respiratory distress syndrome requiring intubation at ED presentation implying that hs-cTnI was likely unnecessary for prognostication. These results imply that baseline troponin measurement in patients presenting with symptomatic COVID-19 is a useful tool for risk stratification and prognostication [5], [6], [22], [23], [24].
We also demonstrate that continuous monitoring through serial testing modestly improves the prognostic value of TnI measurement beyond baseline concentrations for predicting 30-day mortality and 10-day requirement for intubation. There are limited studies assessing cardiac biomarkers serially in hospitalized patient with COVID-19, and previous studies were observational, retrospective studies dependent on physician-ordered troponin testing. This likely resulted in a relatively sparse dataset, and the requirement to rely on maximum troponin as opposed to continuous monitoring using a gap time approach [5]. This likely underlies differences in our study with an adjusted HR of 1.21 relative to previous studies of 1.001 [5]. We previously demonstrated that patients with COVID-19 and a rise and/or fall in cTnI using a contemporary assay had worse outcomes [24]. Therefore, this study adds to the growing body of literature demonstrating the value of serial assessment of hsTnI in patients admitted with COVID-19; importantly, serial measurement of troponin may aid in risk stratification, particularly for those patients that present with baseline biomarkers that are only modestly elevated.
Contrary to other published studies, we found little added value of baseline NT-proBNP, Gal-3, or PCT. While NT-proBNP and Gal-3 were elevated at baseline in patients with worse outcomes, had similar AUCs for ROC analysis, and elevated HR on univariable analysis, neither were independent predictors of 30-day mortality or 10-day intubation in fully adjusted multivariable models. This implies that baseline NT-proBNP and Gal-3 concentrations in patients with worse outcomes are associated with other predictive factors of mortality such as elevated TnI. Interestingly, Gal-3 was the only other biomarker that was an independent predictor of 30-day mortality when measured sequentially. Gal-3 elevation most likely reflects acute inflammation in worsening disease, and has been postulated as a marker for therapy in acute fibrosis [25], [26]. Nonetheless, these results imply that serial Gal-3 testing may be useful for risk stratification in patients with COVID-19. To our knowledge, this is the first study demonstrating the potential utility of Gal-3 for serial risk stratification and further studies are needed to validate these findings.
There were several limitations associated with this study. Most specimens were from patients presenting prior to the widespread implementation of SARS-CoV-2 vaccines, which may limit the applicability of these study results to vaccinated populations. Furthermore, the infecting SARS-CoV-2 strain was unknown, and patients presented prior to the Delta and Omicron strains. Given that preliminary reports have demonstrated reduced disease severity and better outcomes in patients infected with the Omicron strain [27], these results may not be applicable as the pandemic continues or if SARS-CoV-2 becomes endemic. However, a previous study demonstrated better performance of troponin for predicating mortality in December 2020 vs March 2020 (HR of 1.8 vs 1.53), implying that these results may be transferable with less virulent strains and in the vaccine era [28]. Nonetheless, further studies are needed to address this. Another limitation was that physician adjudication of myocardial infarction and acute injury was not performed. However, previous studies have demonstrated that myocardial infarction is relatively rare in patients with COVID-19 [5]. Prospective studies are needed assessing how earlier risk stratification with hsTnI and Gal-3 would impact treatment or outcomes; identification of high-risk groups with cardiac biomarkers could lead to earlier escalation of therapies such as dexamethasone and tocilizumab that are typically reserved for patients requiring supplemental oxygen. A further limitation of this study is that established biomarkers for risk stratification of COVID-19 such as C-reactive protein and d-dimer were not assessed, limiting our ability to compare the utility of the multimarker panel used here relative to previously established tools [29], [30]. Notably, previous studies have demonstrated a matrix effect of EDTA plasma relative to lithium heparin using a Beckman hsTnI assay [31]. While not applicable to the Abbott method used here, it is an important caveat for generalizing these study results to other institutions. Finally, despite the diverse patient population in this study, we were not sufficiently powered to assess Asian race or Hispanic ethnicity in multivariate models. Further studies are needed in these patient populations to further assess risk, particularly using time-varying biomarkers.
In conclusion, we demonstrate that elevated hsTnI is an independent predictor for 30-day mortality and 10-day intubation in patients presenting to the ED for COVID-19. Serial hsTnI and Gal-3 determination further increased the HR for adverse outcomes. These results support the use of hsTnI for triaging patients with COVID-19.
Funding
This work was funded by Abbott Diagnostics.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinbiochem.2022.06.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.COVID-19 Map [Internet]. Johns Hopkins Coronavirus Resource Center. [cited 2022 Jan 15]. Available from: https://coronavirus.jhu.edu/map.html.
- 2.Than M.P., Pickering J.W., Sandoval Y., Shah A.S.V., Tsanas A., Apple F.S., Blankenberg S., Cullen L., Mueller C., Neumann J.T., Twerenbold R., Westermann D., Beshiri A., Mills N.L., George P.M., Richards A.M., Troughton R.W., Aldous S.J., Chapman A.R., Anand A., Greenslade J., Parsonage W., Boeddinghaus J., Wildi K., Nestelberger T., Badertscher P., Du S., Huang J., Smith S.W., Sörensen N.A., Ojeda F. Machine Learning to Predict the Likelihood of Acute Myocardial Infarction. Circulation. 2019;140(11):899–909. doi: 10.1161/CIRCULATIONAHA.119.041980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blankenberg S., Salomaa V., Makarova N., Ojeda F., Wild P., Lackner K.J., Jørgensen T., Thorand B., Peters A., Nauck M., Petersmann A., Vartiainen E., Veronesi G., Brambilla P., Costanzo S., Iacoviello L., Linden G., Yarnell J., Patterson C.C., Everett B.M., Ridker P.M., Kontto J., Schnabel R.B., Koenig W., Kee F., Zeller T., Kuulasmaa K. Troponin I and cardiovascular risk prediction in the general population: the BiomarCaRE consortium. Eur. Heart J. 2016;37(30):2428–2437. doi: 10.1093/eurheartj/ehw172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besler C., Lang D., Urban D., Rommel K.-P., von Roeder M., Fengler K., Blazek S., Kandolf R., Klingel K., Thiele H., Linke A., Schuler G., Adams V., Lurz P. American Heart Association; 2017. Plasma and Cardiac Galectin-3 in Patients With Heart Failure Reflects Both Inflammation and Fibrosis. Circulation: Heart Failure. [DOI] [PubMed] [Google Scholar]
- 5.De Michieli L., Ola O., Knott J.D., Akula A., Mehta R.A., Hodge D.O., et al. High-Sensitivity Cardiac Troponin T for the Detection of Myocardial Injury and Risk Stratification in COVID-19. Clin. Chem. 2021;67:1080–1089. doi: 10.1093/clinchem/hvab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandoval Y., Januzzi J.L., Jaffe A.S. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020;76(10):1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portacci A., Diaferia F., Santomasi C., Dragonieri S., Boniello E., Di Serio F., et al. Galectin-3 as prognostic biomarker in patients with COVID-19 acute respiratory failure. Respir. Med. 2021;187 doi: 10.1016/j.rmed.2021.106556. 106556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B.o., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi S., Qin M.u., Shen B.o., Cai Y., Liu T., Yang F., Gong W., Liu X.u., Liang J., Zhao Q., Huang H., Yang B.o., Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F., Zhao S., Somani S., Van Vleck T., Vaid A., Chaudhry F., De Freitas J.K., Fayad Z.A., Pinney S.P., Levin M., Charney A., Bagiella E., Narula J., Glicksberg B.S., Nadkarni G., Mancini D.M., Fuster V. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J. Am. Coll. Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iorio A., Lombardi C.M., Specchia C., Merlo M., Nuzzi V., Ferraro I., et al. Combined Role of Troponin and Natriuretic Peptides Measurements in Patients With Covid-19 (from the Cardio-COVID-Italy Multicenter Study) Am J Cardiol. 2022;S0002–9149(21):01204–1212. doi: 10.1016/j.amjcard.2021.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J.-b., Xu C., Zhang R.-B., Wu M., Pan C.-K., Li X.-J., Wang Q., Zeng F.-F., Zhu S. Associations of procalcitonin, C-reaction protein and neutrophil-to-lymphocyte ratio with mortality in hospitalized COVID-19 patients in China. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-72164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Donnell C., Ashland M.D., Vasti E.C., Lu Y., Chang A.Y., Wang P., Daniels L.B., de Lemos J.A., Morrow D.A., Rodriguez F., O’Brien C.G. N-Terminal Pro-B-Type Natriuretic Peptide as a Biomarker for the Severity and Outcomes With COVID-19 in a Nationwide Hospitalized Cohort. J Am Heart Assoc. 2021;10(24):e022913. doi: 10.1161/JAHA.121.022913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller T., Dieplinger B. Soluble ST2 and Galectin-3: What We Know and Don’t Know Analytically. EJIFCC. 2016;27:224–237. [PMC free article] [PubMed] [Google Scholar]
- 15.Dasgupta A., Chow L., Tso G., Nazareno L. Stability of NT-proBNP in Serum Specimens Collected in Becton Dickinson Vacutainer (SST) Tubes. Clin. Chem. 2003;49:958–960. doi: 10.1373/49.6.958. [DOI] [PubMed] [Google Scholar]
- 16.Michel M., Mestari F., Alkouri R., Atlan G., Dever S., Devilliers C., Imbert-Bismut F., Bonnefont-Rousselot D., Monneret D. High-Sensitivity Cardiac Troponin T: a Preanalytical Evaluation. Clin Lab. 2016;62 doi: 10.7754/clin.lab.2016.160123. [DOI] [PubMed] [Google Scholar]
- 17.Wu A.H.B., Shea E., Lu Q.T., Minyard J., Bui K., Hsu J.C.Y., et al. Short- and Long-Term Cardiac Troponin I Analyte Stability in Plasma and Serum from Healthy Volunteers by Use of an Ultrasensitive. Single-Molecule Counting Assay. Clin. Chem. 2009;55:2057–2059. doi: 10.1373/clinchem.2009.128611. [DOI] [PubMed] [Google Scholar]
- 18.Jia X., Sun W., Hoogeveen R.C., Nambi V., Matsushita K., Folsom A.R., Heiss G., Couper D.J., Solomon S.D., Boerwinkle E., Shah A., Selvin E., de Lemos J.A., Ballantyne C.M. High-Sensitivity Troponin I and Incident Coronary Events, Stroke, Heart Failure Hospitalization, and Mortality in the ARIC Study. Circulation. 2019;139(23):2642–2653. doi: 10.1161/CIRCULATIONAHA.118.038772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Januzzi J.L., Chen-Tournoux A.A., Christenson R.H., Doros G., Hollander J.E., Levy P.D., Nagurney J.T., Nowak R.M., Pang P.S., Patel D., Peacock W.F., Rivers E.J., Walters E.L., Gaggin H.K. N-Terminal Pro-B-Type Natriuretic Peptide in the Emergency Department: The ICON-RELOADED Study. J. Am. Coll. Cardiol. 2018;71(11):1191–1200. doi: 10.1016/j.jacc.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Mueller T., Gegenhuber A., Leitner I., Poelz W., Haltmayer M., Dieplinger B. Diagnostic and prognostic accuracy of galectin-3 and soluble ST2 for acute heart failure. Clin. Chim. Acta. 2016;463:158–164. doi: 10.1016/j.cca.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 21.R: The R Project for Statistical Computing [Internet]. [cited 2022 Feb 10]. Available from: https://www.r-project.org/.
- 22.Nuzzi V., Merlo M., Specchia C., Lombardi C.M., Carubelli V., Iorio A., Inciardi R.M., Bellasi A., Canale C., Camporotondo R., Catagnano F., Dalla Vecchia L.A., Giovinazzo S., Maccagni G., Mapelli M., Margonato D., Monzo L., Oriecuia C., Peveri G., Pozzi A., Provenzale G., Sarullo F., Tomasoni D., Ameri P., Gnecchi M., Leonardi S., Agostoni P., Carugo S., Danzi G.B., Guazzi M., La Rovere M.T., Mortara A., Piepoli M., Porto I., Volterrani M., Senni M., Metra M., Sinagra G. The prognostic value of serial troponin measurements in patients admitted for COVID-19. ESC Heart Fail. 2021;8(5):3504–3511. doi: 10.1002/ehf2.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Michieli L., Babuin L., Vigolo S., Berti De Marinis G., Lunardon A., Favretto F., Lobo R., Sandoval Y., Bryant S.C., Donato D., Plebani M., Vettor R., Iliceto S., Cianci V., Jaffe A.S. Using high sensitivity cardiac troponin values in patients with SARS-CoV-2 infection (COVID-19): The Padova experience. Clin. Biochem. 2021;90:8–14. doi: 10.1016/j.clinbiochem.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franks C.E., Scott M.G., Farnsworth C.W. Elevated Cardiac Troponin I Is Associated with Poor Outcomes in COVID-19 Patients at an Academic Medical Center in Midwestern USA. J. Appl. Lab. Med. 2020;5:1137–1139. doi: 10.1093/jalm/jfaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Revilla J., Deierborg T., Venero J.L., Boza-Serrano A. Hyperinflammation and Fibrosis in Severe COVID-19 Patients: Galectin-3, a Target Molecule to Consider. Front. Immunol. 2020;11:2069. doi: 10.3389/fimmu.2020.02069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caniglia J.L., Guda M.R., Asuthkar S., Tsung A.J., Velpula K.K. A potential role for Galectin-3 inhibitors in the treatment of COVID-19. PeerJ. 2020;8:e9392. doi: 10.7717/peerj.9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewnard JA, Hong VX, Patel MM, Kahn R, Lipsitch M, Tartof SY. Clinical outcomes among patients infected with Omicron (B.1.1.529) SARS-CoV-2 variant in southern California [Internet]. 2022 Jan page 2022.01.11.22269045. Available from: https://www.medrxiv.org/content/10.1101/2022.01.11.22269045v1.
- 28.Bienstock S.W., Tandon P., Govindarajulu U., Leibner E., Glicksberg B.S., Samtani R., Giustino G., Gidwani U., Kohli-Seth R., Goldman M.E. Impact of Myocardial Injury in Hospitalized Patients With COVID-19 in 2 Peak Time Periods. J. Am. Coll. Cardiol. 2021;78(14):1482–1483. doi: 10.1016/j.jacc.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smilowitz N.R., Nguy V., Aphinyanaphongs Y., Newman J.D., Xia Y., Reynolds H.R., et al. American Heart Association; 2021. Multiple Biomarker Approach to Risk Stratification in COVID-19. Circulation; pp. 1338–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smilowitz N.R., Kunichoff D., Garshick M., Shah B., Pillinger M., Hochman J.S., et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur. Heart J. 2021;42:2270–2279. doi: 10.1093/eurheartj/ehaa1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kavsak P.A., Malinowski P., Roy C., Clark L., Lamers S. Assessing matrix, interferences and comparability between the Abbott Diagnostics and the Beckman Coulter high-sensitivity cardiac troponin I assays. Clin. Chem. Lab. Med. 2018;56:1176–1181. doi: 10.1515/cclm-2017-1122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.