Abstract
Since December 2019, the 2019 coronavirus disease (COVID-19) outbreak has become a global pandemic. Understanding the role of environmental conditions is important in impeding the spread of COVID-19. Given that airborne spread and contact transmission are considered the main pathways for the spread of COVID-19, this narrative review first summarized the role of temperature and humidity in the airborne trajectory of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Meanwhile, we reviewed the persistence of the virus in aerosols and on inert surfaces and summarized how the persistence of SARS-CoV-2 is affected by temperature and humidity. We also examined the existing epidemiological evidence and addressed the limitations of these epidemiological studies. Although uncertainty remains, more evidence may support the idea that high temperature is slightly and negatively associated with COVID-19 growth, while the conclusion for humidity is still conflicting. Nonetheless, the spread of COVID-19 appears to have been controlled primarily by government interventions rather than environmental factors.
Keywords: SARS-CoV-2, Temperature, Humidity, Aerosol, Transmission
Graphical abstract
Highlights
-
•
The SARS-CoV-2 remained viable for a couple of hours in aerosols, comparing with longer stability on inert surfaces.
-
•
The temperature and humidity influenced the trajectory of SARS-CoV-2 transmission via aerosols.
-
•
More evidence may support that rising temperatures seem to be slightly and negatively associated with COVID-19 growth.
-
•
COVID-19 spread appears to have been controlled primarily by government interventions.
1. Introduction
Since December 2019, the 2019 coronavirus disease (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has become an unfolding global pandemic [1]. At the time of writing, many governments are urging a reopening of society to rescue the economy. To achieve this goal, it is essential to understand the key drivers of COVID-19 spread [2,3]. Vaccination and pharmacological intervention have been deemed the most important means to reduce/eliminate the spread of COVID-19. However, although the vaccination rate in the population is increasing, some governments are vacillating between the policies of ‘living with COVID-19’ and ‘COVID-zero’. Apart from the vaccination, existing studies have demonstrated that the spread of COVID-19 could be significantly shaped by a mixture of other factors, including social distancing, travel restrictions, environmental conditions, variants, and others [[4], [5], [6]]. Hence, to aid the fight against COVID-19, the relationship between the environmental conditions and COVID-19 spread has attracted mounting research attention.
Despite numerous studies, the evidence concerning the effect of weather on COVID-19 spread is still conflicting, probably because of considerable heterogeneities among data sources, methodology selection, study period, socio-economic status, and others. Although there have been several reviews and research studies on the associations between environmental factors and COVID-19 spread [[7], [8], [9], [10], [11], [12]], most only covered parts of the research area and failed to offer a comprehensive and full picture of how temperature/humidity drives the spread of COVID-19. For example, the World Meteorological Organization (WMO) released the first report on meteorological and air quality factors affecting the COVID-19 pandemic. However, this report mainly focused on epidemiological evidence [13]. In addition, environmental factors may influence the stability of the virus, the transmission pathway, and the reactions of the body’s receptors. To elucidate the research status of the critical role of temperature and humidity, the most important factors among weather conditions, in the spread of COVID-19, this narrative review has merged evidence from model simulation and experimental and epidemiological studies. In particular, we first summarized the role of temperature and humidity in the aerosol and contact transmission of SARS-CoV-2, which have been considered the major pathways of COVID-19 spread. Meanwhile, we discussed the effects of temperature and humidity on human antiviral defense, and the associated findings from epidemiological studies. This review promises to assist policy-making in the global battle against COVID-19.
2. The role of temperature and humidity in aerosol transmission
The stability of SARS-CoV-2 in aerosols: One precondition to support the airborne transmission of SARS-CoV-2 is that the virus can remain infectious in droplets generally longer than the time it takes for the droplets to travel from person to person [14]: the infectious titer of SARS-CoV-2 was demonstrated to decrease exponentially from 103.5 to 102.7 TCID50 (50% tissue-culture infectious dose) per liter of air after 3 h (21–23 °C and 65% relative humidity), with an estimated half-life of 1.1 h (95% confidence interval (CI): 0.64–2.64 h) [15] (Table 1). Furthermore, another study pointed out that SARS-CoV-2 could remain viable in respirable-sized aerosols for more than 16 h [16]. The SARS-CoV-2 variant in England was found to remain infectious for at least 1.5 h in experimental aerosols from artificial saliva and tissue culture mediums [17]. This study further illustrated that the SARS-CoV-2 was more susceptible at a higher relative humidity in tissue culture mediums, with the decay rate increasing from 0.91% per min (medium relative humidity: 40%–60%) to 2.27% per min (high relative humidity: 68%–88%) [17]. However, such findings were not observed for aerosolized SARS-CoV-2 from artificial saliva, where the decay rate was 75% lower at high relative humidity (1.59% per min at medium relative humidity and 0.40% per min at high relative humidity) [17].
Table 1.
The stability of SARS-CoV and MERS-CoV in aerosols.
| Virus strain | Temperature (°C) | Relative humidity | Inoculum | Stability (h) | T1/2 (h) | References |
|---|---|---|---|---|---|---|
| SARS-CoV-2 | 21–23 | 65% | 103.5 TCID50/L air | 15.8% survival at the end 3 h | 1.1 | [15] |
| SARS-CoV-1 | 104.3 TCID50/L air | 1.2 | ||||
| SARS-CoV-2 | 23 | 53% | NR | 16 | NR | [16] |
| SARS-CoV-2 England-2 variant |
19–22 | 40%–60% | (1.11–1.63) × 106 TCID50/mL aerosol of tissue culture mediums | 1.5 | 1.25 | [17] |
| 19–22 | 68%–88% | 0.5 | ||||
| 19–22 | 40%–60% | (0.77–2.28) × 106 TCID50/mL aerosol of artificial saliva | 0.7 | |||
| 19–22 | 68%–88% | 2.95 | ||||
| MERS-CoV | 25 | 79% | 105.5 TCID50/mL eluate | 63% at the end 1 h | NR | [10,18] |
| 38 | 24% | 4.7% at the end 1 h | ||||
| MERS-CoV | 20 | 40% | 106 TCID50/mL suspension | 93% viability at the end 10 min | NR | [58] |
| 20 | 70% | 11% at the end 10 min |
NR, not reported; TCID50, 50% tissue-culture infectious dose.
Compared with humidity, fewer studies have reported the relationship between temperature and SARS-CoV-2 stability in aerosols. However, existing evidence has demonstrated that MERS-CoV in aerosols was far less active in a hot and dry environment [18]. Similarly, HCV/229E coronavirus appears to survive for shorter periods in aerosols when temperature increases [19]. In particular, when the temperature increased from 6 to 20 °C, the half-life of HCV/229E declined from 34.5 h to 26.8 h at a relative humidity of 30%, from 102.5 h to 67.3 h at a relative humidity of 50%, and from 86.0 h to 3.3 h at a relative humidity of 80% [19], respectively. Although similar studies regarding SARS-CoV-2 Delta and Omicron variants have not yet been published at the time of writing, existing evidence may suggest that a high temperature in the aerosol leads to a decline in SARS-CoV-2 infectivity and further impedes the spread.
The stability of the virus in experimental aerosols has also been confirmed by field studies, as viable viruses in the air were detected in samples collected from hospitals worldwide [[20], [21], [22]]. A recent study collected air samples from eight groups of public and transportation locations: positive samples were detected in almost all regions, such as shopping malls (100%), subway trains (100%), airports (80%), buses (50%), and banks (30%). However, it failed to highlight that temperature was a determinant (the odds ratio was estimated to be 0.93, with a 95% CI of 0.72–1.17) for the presence of SARS-CoV-2 in the air [23]. A nationwide study in Turkey found that amounts of the virus in ambient particulate matter and humidity were both higher in Istanbul, Zonguldak, and Tekirdağ; however, the data were insufficient to address the association between humidity and virus exposure level in the air [24]. To date, field studies regarding how environmental conditions impact virus-laden aerosol concentrations remain scarce.
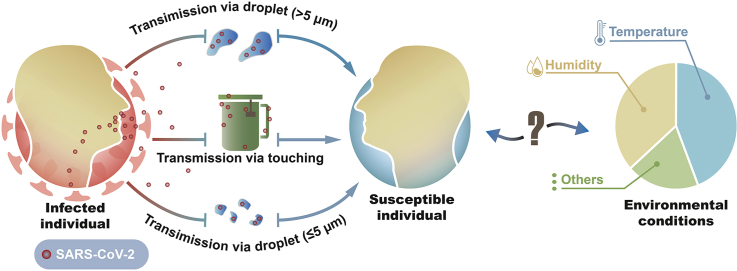
Temperature and humidity influence the SARS-CoV-2 trajectory in aerosols: Extensive evidence has suggested that one of the main pathways for COVID-19 spread appears to be airborne spread, including droplets (>5 μm) and droplet nuclei (≤5 μm) [25,26]. Hence, the trajectory of droplets and aerosols is crucial when addressing the transmission of SARS-CoV-2. The diameter is known to be an important parameter in the airflow patterns and trajectory of the virus from an infected source. In general, the larger droplets follow a ballistic trajectory regardless of flow in the gas phase, compared with the aerosols that are buoyant to a diverse degree within a turbulent gas cloud [7,27]. Apart from that, respiratory droplets are generated by various physical events, including talking, coughing, breathing, and sneezing [28,29], with initial speeds spanning from resulting in different distances at which droplets fall to the floor (Table 2). For example, sneezing was simulated to carry droplets larger than 100 μm and spread more than 2 m horizontally from infected persons, while droplets with a velocity of 1 m/s resulting from exhalation were found to fall to the floor within 1 m from the source [7].
Table 2.
The simulated spreading distance under various environmental conditions from a SARS-CoV-2 spreader in typical scenarios.
| Events | Size diameter (μm) | Velocity (m/s) | Temperature (°C) | Humidity | Horizontal distance (m) | Remark | References |
|---|---|---|---|---|---|---|---|
| Sneezing | 60–100 | 50 | 20 | 50% | about 5 | Using Wells evaporation-falling curve | [33] |
| Coughing | 45–80 | 10 | 20 | 50% | about 1.5–2 | ||
| 30 | 10 | 20 | 30% | 1.25–1.5 | |||
| 30 | 10 | 20 | 70% | about 2.25 | |||
| Speaking | 20 | 5 | 20 | 50% | about 1 | ||
| Breathing | 20 | 1 | 20 | 50% | <0.5 | ||
| Model assumptions | Size Distribution [109] | 4.1 | 30 | 20% | <1.8 | Under most regions proposed by the authors, the spreading distance may exceed 1.8 m | [31] |
| 30 | 60% | 1.8–2.4 | |||||
| 30 | 90% | >3.6 | |||||
| 20 | 20% | <1.8 | |||||
| 20 | 20% | about 1.8 | |||||
| 20 | 60% | >2.4 | |||||
| 20 | 90% | >3.6 | |||||
| Coughing | 60 | 10 | 25 | 35% | >2 | [110] | |
| Coughing | 100 (droplet size) | 10 | 30 | 84% | 6.6 | Outdoor, wind speed of 2 m/s | [111] |
| 0.8 | Outdoor, wind speed of 0 m/s | ||||||
| Coughing | 1000 (droplet size) | 1.3 | Outdoor, wind speed of 2 m/s | ||||
| Coughing | About 80–100 | 10 m/s at 0.1 s and decays to zero at 0.5 s | 25 | 60% | Probably <1 m | Considering evaporation | [30] |
| About 2–80 | 25 | 60% | Probably >1 m |
Model simulations have well demonstrated that the evaporation mechanism, particle size distribution, and exhaled profile characteristics are the key drivers of airborne transmission [30]. By altering the size distribution and evaporation process, the temperature and humidity can alter the shape and magnitude of the airborne trajectory [[31], [32], [33], [34]] (Table 2). Zhao et al. [31] suggested that droplets travel less than 1.8 m in a hot and dry environment. By contrast, in a low-temperature and high-humidity environment, the maximum distance of spread can be up to 3.6 m, and the number of aerosol particles also increases [31]. In a cold and damp environment, droplet sizes decrease due to the Kelvin effect, and finer droplets are capable of traveling further [35]. Notably, the reduction in the size of droplets may trigger a higher likelihood of deposition in human airways [36,37], which enhances infection risk. On the other hand, large droplets in conditions of low temperature and high humidity can be converted to numerous small droplets carrying fewer viruses, which are inclined to die out fast [38]. In addition, expelled droplets quickly lose water through evaporation. When humidity increases, the evaporation process becomes much slower because the higher relative humidity in the air has less potential to absorb water vapor [39]. In particular, droplets of about 60 μm appear more sensitive to relative humidity than those of other sizes [34]. However, when the air is dry, droplet nuclei may also be generated that are sufficiently fine to remain suspended in the air for a long time [40]. Regarding the temperature, low temperature in the environment provides less heat source for evaporation.
Moreover, Pal et al. [32] pointed out that most existing research has considered the droplet a pure water droplet, neglecting the formation and trajectories of aerosols. Given that the formation of crystals further delays the evaporation of droplets, hot and dry conditions appear to be more dangerous situations than previously speculated, and cold and humid circumstances may allow the droplets to travel up to 2–3 fold farther than previous estimations [32].
Generally, in flowing fluids in the respiratory tract, high viral loads reduce the minimum size of virus-laden respiratory secretions, indicating that more virus-laden aerosols are anticipated under conditions of high viral load [41]. Given that a relatively high viral load was found for Delta and Omicron [42], these variants of concerns (VOCs) are prone to cause quick and massive spread in enclosed spaces [43]. Furthermore, if these novel VOCs satisfy the four fundamental principles (asymptomatic host, high viral load, stability of viruses in the air, and binding affinity of viruses to human cells), associated rapid outbreaks of COVID-19 should be considered [41].
3. Contact transmission: SARS-CoV-2 on inert surfaces is affected by temperature and humidity
Besides airborne transmission, SARS-CoV-2 spread may occur indirectly via touching surfaces in the environment. High contact surfaces, including the touchscreens on electronic equipment, elevator push buttons, and handles of public utilities, potentially carry the virus from infected persons. An early experiment demonstrated that the transmission efficiency from the fingertip to the mouth was greater than 30% for bacteria and phages on fomites [44], suggesting that fomite transmission appears to be a highly efficient pathway [45].
Mounting studies have reported positive results for SARS-CoV-2 in environmental samples, including tables, handles, chairs, glasses, personal protective equipment [46], cell phones [20], room window ledges [20], light switches [47], and the food supply chain [48]. A recent review pointed out that hospitals and healthcare facilities with COVID-19 patients have the highest detection rates, yielding about 17.3% positive samples (n = 533) for SARS-CoV-2 RNA detected in 3077 surface samples [9]. Contrastingly, the rate of positive samples (n = 174) was 10.1% among samples collected in non-hospital settings (n = 1724) [9]. However, viable viruses have not been commonly confirmed in these environmental samples because viral infectivity assays were not always performed: they require propagation of SARS-CoV-2 in cell culture, which is only allowed in biosafety level 3 facilities [9].
The existence of SARS-CoV-2 in the environment has also been supported by its stability on a large array of inert surfaces, including metal, plastic, wood, paper, masks, and various other materials (Table 3). As summarized, the stability of SARS-CoV-2 ranges from <1 h to a couple of weeks. For example, on metal surfaces, the duration of SARS-CoV-2 stability was determined to be 4 h for copper and 72 h for stainless steel [15]. The low stability of SARS-CoV-2 on the copper surface may be due to the copper ions being able to aggregate virus particles [49]. Meanwhile, free copper ions may generate reactive oxygen species [50]. Active oxygen can attack the spike and envelope proteins and lipids of SARS-CoV-2, destroy the integrity of the virus, and inhibit its infection. A fall in temperature and humidity appears to strengthen the stability of the virus on stainless steel [51,52] (Table 3). Concerning plastic surfaces, an early study reported that infectivity lasted up to 3 days but had decayed at the end of the 4th day, under 40% relative humidity [15]. Later research reported that when the relative humidity increased to 65%, the duration of stability of SARS-CoV-2 increased to 4 days [51]. Notably, as displayed in Table 3, the stability of SARS-CoV-2 on non-porous surfaces appears to be similar to that of SARS-CoV-1.
Table 3.
The stability of SARS-CoV on different surfaces.
| Type of surface | Strain | T (°C) | Humidity | T1/2 (h) | S (h) | Time of 100% decay (h) | References |
|---|---|---|---|---|---|---|---|
| Plastic | SARS-CoV-2 | 21–23 | 40% | 6.8 | 72 | 96 | [15] |
| 22 | 65% | 11.4 | 96 | 168 | [51] | ||
| SARS-CoV-1 | 21–23 | 40% | 7.6 | 72 | 96 | [15] | |
| 21–25 | NR | NR | 96 | 120 | [50, 53] | ||
| 21–25 | NR | NR | 144 | 216 | [112] | ||
| 22–25 | 40%–50% | NR | 28 days | NR | [10, 57] | ||
| 33 | >95% | NR | 24 | NR | [10, 57] | ||
| 33 | 80%–89% | NR | 24 | NR | [10, 57] | ||
| 38 | >95% | NR | 24 | NR | [10, 57] | ||
| 38 | 80%–89% | NR | 24 | NR | [10, 57] | ||
| Stainless Steel | SARS-CoV-2 | 21–23 | 40% | 5.6 | 72 | 96 | [15] |
| 22 | 65% | 14.7 | 72 | 96 | [51] | ||
| 20 | 35%–40% | NR | 336 | 504 | [52] | ||
| SARS-CoV-1 | 21–23 | 40% | 4.2 | 48 | 72 | [15] | |
| Copper | SARS-CoV-2 | 21–23 | 40% | 0.8 | 4 | 8 | [15] |
| SARS-CoV-1 | 21–23 | 40% | 1.5 | 8 | 24 | [15] | |
| Glass | SARS-CoV-2 | 22 | 65% | 4.8 | 48 | 96 | [51] |
| SARS-CoV-1 | 21–25 | NR | NR | 96 | 120 | [50, 53] | |
| Cardboard | SARS-CoV-2 | 21–23 | 40% | 3.5 | 24 | 48 | [15] |
| SARS-CoV-1 | 21–23 | 40% | 0.6 | 8 | 24 | [15] | |
| Metal | SARS-CoV-1 | 21–25 | NR | NR | 120 | NR | [10, 53] |
| Mosaic | 21–25 | NR | NR | 72 | 96 | [10, 53] | |
| Paper | SARS-CoV-2 | 22 | 65% | NR | 0.5 | 3 | [51] |
| Paper (H)∗ | SARS-CoV-1 | 20 | NR | NR | NR | 24 | [113] |
| Paper (M) ∗ | 20 | NR | NR | NR | 3 | [113] | |
| Paper (L) ∗ | 20 | NR | NR | NR | <5 min | [113] | |
| Tissue paper | SARS-CoV-2 | 22 | 65% | NR | 0.5 | 3 | [51] |
| Press paper | SARS-CoV-1 | 21–25 | NR | NR | 96 | 120 | [50, 53] |
| Filter paper | 21–25 | NR | NR | 120 | NR | [50, 53] | |
| Wood | SARS-CoV-2 | 22 | 65% | NR | 6–24 | 24–48 | [51] |
| Wood boards | SARS-CoV-1 | 21–25 | NR | NR | 96 | 120 | [50, 53] |
| Cloth | SARS-CoV-2 | 22 | 65% | NR | 6–24 | 24–48 | [51] |
| Cloth | SARS-CoV-1 | 21–25 | NR | NR | 120 | NR | [50, 53] |
| Banknote | SARS-CoV-2 | 22 | 65% | 7.9 | 48 | 96 | [51] |
| Mask, inner layer | 22 | 65% | 9.9 | 96 | 168 | [51] | |
| Mask, outer layer | 22 | 65% | 23.9 | 168 | NR | [51] | |
| Plastic face shield | 20 | 35%–40% | NR | 504 | NR | [52] | |
| N95 mask | NR | 504 | NR | [52] | |||
| N100 mask | NR | 504 | NR | [52] | |||
| Nitrile gloves | NR | 168 | 336 | [52] | |||
| Chemical gloves | NR | 96 | 168 | [52] | |||
| Disposable gown (H) ∗ | SARS-CoV-1 | 20 | NR | NR | NR | 48 | [113] |
| Disposable gown (M) ∗ | 20 | NR | NR | NR | 24 | [113] | |
| Disposable gown (L) ∗ | 20 | NR | NR | NR | 1 | [113] | |
| Cotton | SARS-CoV-2 | 20 | 35%–40% | NR | 4 | 24 | [52] |
| Cotton gown (H) ∗ | SARS-CoV-1 | 20 | NR | NR | NR | 24 | [113] |
| Cotton gown (M) ∗ | 20 | NR | NR | NR | 1 | [113] | |
| Cotton gown (L) ∗ | 20 | NR | NR | NR | 5 min | [113] | |
| Tyvek | SARS-CoV-2 | 20 | 35%–40% | NR | 336 | 504 | [52] |
NR, not reported; ∗ H, high inoculation, 106 TCID50/mL; M, medium inoculation, 105 TCID50/mL; L, low inoculation, 104 TCID50/mL; T, temperature; S, stability.
Scientists have also investigated the stability of SARS-CoV-2 on porous surfaces. On facemasks, two studies reported that the SARS-CoV-2 could survive for 96–504 h, indicating that the infectious virus can possibly be recovered even after 21 days (Table 3). Publications have revealed relatively short durations of stability of SARS-CoV-2 on paper, banknotes, cards, woods, cloths, and cotton (Table 3). Although SARS-CoV-2 survived for less than 1 h on paper, the SARS-CoV-1 could remain viable on newspaper and filter paper for more than 96 h [50,53], illustrating some disparities between the two viruses.
It is well known that the protein coat of the virus may be impaired at high temperatures, and the virus, therefore, may lose its infectivity, which further validates the survivability of SARS-CoV-2 in response to temperature [10]. Shane et al. [45] found that the virus remained viable for up to four weeks on a paper note at 20 °C, while the virus survived for less than 48 h at 40 °C. This study also found a low half-life at higher temperatures on stainless steel and glass [45]. Using a simulated clinically relevant matrix with SARS-CoV-2 dried on nonporous surfaces at a room temperature of 24 °C, Jennifer et al. [54] noted that the virus half-life was 6.3–18.6 h, depending on the relative humidity. In contrast, the half-life reduced to 1.0–8.9 h when the temperature increased to 35 °C [54]. At room temperature (20–25 °C), Chan et al. [55] suggested that dried SARS-CoV-2 retained viability for a couple of days, with a prolonged survival duration of more than two weeks at 4 °C. By contrast, the virus lost its infectivity within 24 h when the temperature was set at 37 °C [55]. Another study verified the inverse association between SARS-CoV-2 stability and temperature by incubating fomites with SARS-CoV-2 at different temperatures. The long stability of the virus at low temperatures illustrates that more caution is required for low-temperature environments, for example, the food cold chain [56]. On the other hand, despite fewer studies being available on modification of the infectivity of SARS-CoV-2 by humidity, evidence from SARS-CoV-1 [57] and MERS [58] may indicate that viability would be impaired at extremely high relative humidity.
4. The effects of temperature and humidity on human antiviral defense
On infection, SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2) receptor of respiratory epithelial cells through the S protein, replicates in large quantities, and migrates to the lower respiratory tract to enter alveolar cells [59]. The rapid and sustained replication of SARS-CoV-2 resulting in alveolar cell death can elicit an intense immune response [60]. When type II alveolar cells die, they release specific inflammatory mediators and stimulate immune cell activation which secretes a large amount of interleukin 1 (IL-1), interleukin 6 (IL-6), tumor necrosis factor (TNF-α), and other pro-inflammatory factors, threatening to trigger a cytokine storm or cytokine release syndrome [61].
Human antiviral defenses may also be sensitive to temperature and humidity. Previous studies have pointed out that dry and cold conditions impair nasal and bronchial mucociliary clearance and further hinder the ability of the body to defend against inhaled virus-containing particles [13,62]. In general, the temperature of the air we breathe is below body temperature, causing heat loss in the nasal passages. It is well known that mucociliary clearance is the first line of defense against respiratory infection [63]. A reduction in temperature may slow metabolic activity, thus reducing both ciliary beat frequency and the rate of secretion of mucus, which further alters the rate of mucociliary clearance [63]. One report has demonstrated that the inhalation of dry air impairs innate antiviral defense and tissue repair [64].
The response of the immune system may also be associated with temperature. The expression of interferon-stimulated genes is vital to triggering an antiviral state [65], and findings from single-cell RNA sequencing have indicated that induction of type I interferon (IFN)—stimulated genes in response to viral infection was decreased in various cell types in the lungs of mice housed in low-humidity conditions [64]. Upon exposure to an environmental temperature of 36 °C, the mice reduced their food intake and showed a reduced adaptive immune response to influenza virus infection [66]. This may be because increased temperature inhibits virus-specific CD8+ T cell responses and antibody production after influenza infection [13,66]. In addition, airway cells with genetic deficiencies in RIG-I–like receptor or IFN receptor signaling support much higher levels of viral replication at 37 °C [67]. Another study demonstrated that, in the absence of IFNs, temperature-based rhinovirus amplification largely resulted from host cell antiviral restriction mechanisms operating more effectively at 37 °C than at lower temperatures [68].
5. Modifying effects of temperature and humidity on COVID-19 spread: Evidence from epidemiological studies
As summarized above, while the effect of humidity on COVID-19 spread remains controversial, hot temperatures may reduce COVID-19 spread by reducing the dispersal distance and impairing virus infectivity. Compared with summer, the spread of COVID-19 is anticipated to be higher in winter, and a recurrent outbreak of COVID-19, therefore, is highly probable.
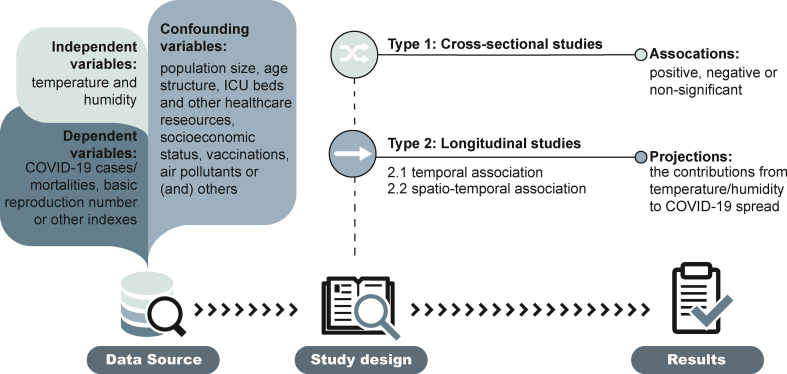
Therefore, direct epidemiological research on the alteration of COVID-19 spread by temperature and humidity has become a research focus, with a rapid increase in the number of associated publications. Fig. 1 illustrates the general research framework used to unveil the associations between COVID-19 spread and the two environmental conditions. Besides some descriptive studies, most studies can be divided into two types: 1) cross-sectional studies, in which the association was determined for the COVID-19 pandemic over a certain period in many areas, with statistics on the temperature, humidity, and other confounding variables; and 2) longitudinal studies, in which the associations of COVID-19 incidence or mortality were studied versus a daily measure of temperature and humidity. Furthermore, the longitudinal studies can be classified as: 1) purely temporal associations, either because the study was in a single region or a study performed in a single region was analyzed at the first stage, and a pooled analysis was implemented at the second stage; and 2) spatio–temporal associations, which simultaneously combined the temporal variables regarding temperature/weather and other spatial variables as a random term. Thus, the choice of model parameters is related to the study design. For instance, some confounding variables, including demographic and population factors that differ over regions but not at temporal scales, are capable of modifying the associations, as exemplified by how the age structures of European and North American population increased their COVID-related death rates [69]. An earlier study also pointed out that the study design does not significantly bias the findings estimated by previous studies [70].
Fig. 1.
The general study framework used to determine the associations between COVID-19 spread and temperature/humidity.
Given that the number of relevant publications is increasing sharply, this section mainly focuses on the findings of studies conducted at a global or national scale. Meanwhile, as the novel VOCs have spread rapidly worldwide, the findings from associated epidemiological studies are discussed in this section.
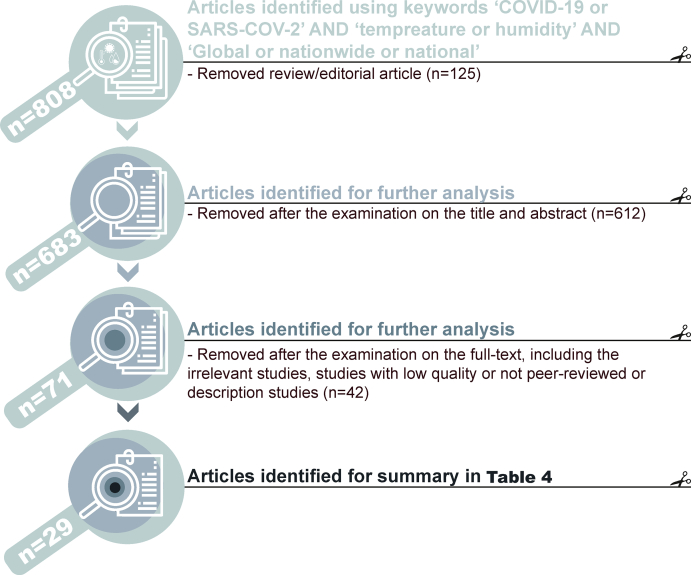
Associations between COVID-19 cases and temperature/humidity: Using the keywords of ‘COVID-19 or SARS-COV-2’ AND ‘temperature or humidity’ AND ‘Global or nationwide or national or worldwide’ on 19 March 2022, 29 remaining studies were tabulated in Table 4 after the examination of the titles, abstracts, full text and the removal of low-quality articles (Fig. 2).
Table 4.
Selected literature on global epidemiological analyses regarding COVID-19 spread and temperature/humidity.
| Region | Timespan | Meteorological variables | Outcome variable | Confounding variables | Study Type∗ | Inferences | References |
|---|---|---|---|---|---|---|---|
| 3739 global locations | December 12, 2019 to April 22, 2020 | Temperature, humidity, precipitation, snowfall, moon illumination, sunlight hours, ultraviolet index, cloud cover, wind speed and direction, pressure data | Effective reproduction number | Air pollutants, population density, and using models to control for estimating effective reproduction number | b | A moderate negative (coefficient: 0.037% with 95% CI of 0.019–0.054) relationship between the estimated reproduction number and temperatures above 25 °C was obtained | [3] |
| 277 global regions | December 1, 2019 to April 14, 2020 | Humidity, temperature, wind speed, and visibility data | Defined growth rate | Location, population, people aged over 65, area, life expectation, the number of hospital beds, GDP, and governmental policy | a | Temperature sensitivity of COVID-19 spread was only −2.7% (−5.2%–0%), indicating warm summer is unlikely to eliminate COVID-19 transmission naturally | [2] |
| 1236 regions | By the end of May 2020 | Daily mean temperature and humidity | New daily case and basic reproductive number | Gross regional product, population, government response, elevation and suspected population, working population, and school-age group | b | Temperature and relative humidity were negatively associated with the COVID-19 spread | [114] |
| 409 cities across 26 countries | January 1, 2020 to May 31, 2020 | Temperature, dewpoint temperature, surface solar radiation, downwards, precipitation, and wind | Effective reproduction number | Government response index, total population, population density, the elderly population (>65 years), and GDP and PM2.5 | a | Meteorological conditions influenced little COVID-19 spread. Population behavior and government interventions were more important drivers of transmission. | [78] |
| 202 locations in 8 countries | As of the early stage of the global pandemic | Daily temperature, humidity, and UV radiation | Basic reproductive number | – | b | Meteorological conditions did not statistically alter the COVID-19 spread | [72] |
| Global, first-level administrative division | As of March 17, 2020 | Daily temperature and humidity | Confirmed cases | Exposure day; the median age of the national population, population density, the capacity of the country to detect an emerging infectious disease | b | A negative association with COVID-19 incidence for temperatures of −15 °C and above was found | [76] |
| Global | Data on April 2, 2020 | Temperature | Proportion of cases | GDP, latitude, and longitude | a | The temperature may be negatively associated with both the proportions of COVID-19 cases/mortalities in the population | [75] |
| 50 cities | From at least 10 reported deaths in a country to March 10, 2020 | Mean temperature and humidity | Total number of cases | – | a | The distribution of substantial community outbreaks along restricted latitude, temperature, and humidity measurements was consistent with the behavior of a seasonal respiratory virus. | [115] |
| 144 geopolitical areas worldwide, excluding China, South Korea, Iran, and Italy | From at least 10 COVID-19 cases to March 20, 2020 |
Temperature and humidity | Epidemic growth rate | Elevation, GDP, health expenditure as a percent of GDP, life expectancy, rate of people aged over 65 years or older, the infectious disease vulnerability index, urban population density, number of flight passengers per capita, and closest distance to a country with an already established epidemic | a | COVID-19 spread was not associated with temperature but maybe weakly and negatively associated with humidity. | [77] |
| 249 countries | December 1, 2019, to March 30, 2020 | Daily data of precipitation, average temperature, maximum temperature, and minimum temperature | COVID-19 transmission and deaths | Exposure time, population density, and dummy month | b | The average temperature per country was negatively associated with the number of cases of SARS-CoV-2 infections | [116] |
| More than 200 countries | Counts on specific days | Monthly average temperature | COVID-19 cases | – | a | The temperature may be negatively associated with COVID-19 transmission | [73] |
| 166 countries, excluding China | As of March 27, 2020 | Temperature, humidity, wind speed | Daily new cases and deaths | The median age of the national population, population density, global health security, human development index, and exposure day | b | The temperature may be negatively related to COVID-19 transmission | [74] |
| Global, top 20 countries | January 22 to April 27, 2020 | Daily Temperature, dew/frost point, wind speed, precipitation, relative humidity, surface pressure | COVID-19 outcomes | – | b | High temperature and high relative humidity reduced the viability, stability, survival, and transmission of COVID-19, whereas low temperature prolonged the activation and infectivity of the virus | [117] |
| Global | The number of reported cases and deaths in April 2020 | Temperature and humidity | Reported positive cases and deaths | Human development index | a | Surface air temperature and Specific humidity do not have any statistically significant association with COVID-19 transmission, though there is a weak relationship between temperature and the pandemic’s mortality | [118] |
| Global | January to April, 2020 | Temperature, humidity, and UV | Growth rate | Population data | a | Without intervention, COVID-19 will decrease temporarily during summer, rebound by autumn, and peak next winter | [119] |
| Global, 209 countries | In the first 16 weeks of the infection | Climatic zone, temperature, solar irradiation, relative humidity, wind speed, surface pressure, precipitation | COVID-19 Incidence or prevalence | population size, land area | a | Climatic factors significantly influence the spread of SARS-CoV-2 | [120] |
| Global, 52 countries | December 31, 2019, to April 13, 2020 | Temperature, relative humidity, and solar radiation | Basic reproduction number | Global Health security index | a | A negative association is found between the incidence of COVID-19 and temperature. | [121] |
| Global, 206 regions/countries | January 8, 2020 to April 20, 2020. | Temperature, relative humidity, UV index, wind speed, cloud cover, precipitation, sea-level air pressure, and daytime length | newly reported COVID-19 | GDP per capita, and the Global Health Security Index | b | The positive association between COVID-19 and 14-day lagged temperature and a consistently higher rate of COVID-19 cases in absolute humidity of 5–10 g/m3 | [122] |
| 35 OECD countries and all US states | – | Ambient temperature, relative humidity, cumulative precipitation, and air pollution | COVID-19 mortality | Population size, population density, days of social distancing prior to first reported COVID-19 death, the Gini index as a measure of socioeconomic inequality, ICU beds, the prevalence of obesity, smoking prevalence, and proportion of the population older than 75 years. | a | A 1 °C increase in ambient temperature was associated with 6% lower COVID-19 mortality at 30 days following the first reported death | [96] |
| Global, 159 countries | January 22 to June 15, 2020 | Air temperature, specific humidity | Growth rate | Population density, size, and structure, per capita government health expenditure, and global airport connection | a | The role of environmental conditions on COVID-19 transmission is controversial. | [123] |
| Global, 188 countries | As of 31 December 2020 | Mean temperature, maximum temperature, minimum temperature, dew point temperature, precipitation, and wind speed | The number of daily new cases | Population density, government response stringency index, human development index, categorical variable for the country, and date | b | The mean temperature, wind speed, and relative humidity were negatively correlated with daily new cases of COVID-19, | [124] |
| Global | – | UV radiation, temperature, specific humidity, and precipitation | The daily growth rate of confirmed COVID-19 cases | Social and economic characteristics | a | Temperature and specific humidity cumulative effects are not statistically significant | [125] |
| 144 countries | At least 21 days of death until April 27, 2020. | Maximum temperature, relative humidity, and UV Index | COVID-19 mortality rates | Population structure, the number of hospital beds, governmental immigration restrictions, and GDP | a | The temperature has a negative association with the COVID-19 mortality rate. | [126] |
| 47 countries | February 22 to June 22, 2020 | Temperature, air pressure, and wind speed | Effective reproductive number | Human mobility, country, and date | b | A negative relationship between temperature and COVID-19 transmission rate | [127] |
| 615 cities | January to June 2020 | Temperature, pressure, humidity, dew, wind gust | Total number of COVID-19 confirmed cases | – | b | High ambient temperature and relative humidity have a mitigation effect on COVID-19. | [128] |
| 153 countries | January to May 2020 | Temperature, humidity, air pressure, wind speed, precipitation | Daily COVID-19 cases, basic reproductive number | Day, policy | b | The temperature was positively related to daily new cases at low temperature but negatively related to daily new cases at high temperature | [129] |
| 191 countries | Until January 22, 2021 | Temperature | Mortality rate | Daily test, political regime, urban population, GDP, air pollutant, and DALYs | a | Mean annual temperature showed a significant inverse association with the COVID-19 mortality rate | [130] |
| 150 countries | A minimum of 90 days since the first confirmed case | Temperature | Mortality rate | The probability of mortality due to respiratory disease, the stringency of government measures, countries’ hemisphere, DALYs, human development index, population density, and the proportion of people aged 65 and older | b | The increase in ambient temperature decreases the incidence of COVID-19 deaths | [131] |
| 58 countries, at least 30 daily cases as of July 29, 2020 | – | Temperature, precipitation | Basic reproduction number | Demographics, disease, economics, air pollution, habitat, health, and social factors | a | Temperature and humidity did not have strong relationships with R0 but were positive. | [132] |
∗ a, cross-sectional study; b, longitudinal study.
Fig. 2.
The framework to identify global analyses on the association between temperature/humidity and COVID-19 transmission in this study. n, sample size.
Based on global COVID-19 cases prior to 6 May 2020, an early descriptive study [71] noted that 60% of the confirmed cases were concentrated in regions where air temperature ranged from 5 to 15 °C, with a peak at 11.5 °C. Furthermore, approximately 74% of the cases occurred in places with absolute humidity in the range of 3–10 g/m3. The authors speculated that COVID-19 might spread cyclically, and outbreaks might recur in large cities in the mid-latitudes in autumn 2020 [71], as confirmed by later observations. When using various statistical approaches and data sources, conclusions on the correlations between daily confirmed COVID-19 cases and meteorological factors varied. For example, Pan et al. [72] employed wavelet coherency analysis for eight countries with 202 locations, finding no significant association between basic reproductive number and weather conditions. In contrast, by analyzing data between 25 March and 18 April 2020, a cross-sectional study [73] reported strong inverse correlations between monthly average environmental temperature and multiple COVID-19 indexes, such as total nationwide cases, active cases, and cases per million.
However, the two studies did not control the effects of confounding variables. Given that COVID-19 spread is strongly shaped by policy intervention, physical distancing, use of face masks, and some other factors, concerns were raised about the robustness of the results. After controlling influences from wind speed, nationwide population structure, the security index and exposure period, a longitudinal analysis in 166 countries concluded that both the temperature and relative humidity were negatively related to daily new cases [74]. In detail, each unit increase in temperature would significantly trigger a 3.08% (95% CI: 1.53%, 4.63%) reduction in daily new cases, while an increase in relative humidity resulted in a 0.85% (95% CI: 0.51%, 1.19%) decrease in daily new cases [74]. In addition, model assumptions on the lag structures did not significantly alter the results, suggesting that elevated temperature and humidity may partially influence the COVID-19 pandemic [74]. Various other cross-sectional studies, relying on daily confirmed or accumulated cases as dependent variables [75,76], have yielded homogeneous results. On the other hand, Jüni et al. [77] performed a prospective cohort study including 144 geopolitical areas worldwide and indicated that there was no association between COVID-19 growth and temperature but a weak negative effect for relative or absolute humidity.
In addition, some studies did not directly use daily cases as dependent factors. Generally, the authors first estimated the local effective reproduction number (Rt) or transmission rate and then linked these indexes to spatial and temporal variables. One merit of such studies is that these estimated indexes can better describe the growth of the COVID-19 pandemic, which promises to narrow the uncertainties raised by test coverage or incubation period. A cross-sectional analysis first evaluated the Rt in 409 cities in 26 countries [78]. After controlling the impacts from selected confounding parameters including the government response index, total population, population density, elderly population (>65 years), GDP, and particulate matter, a slight decrease of 0.087 (95% CI: 0.025, 0.148) in Rt caused by mean temperature was observed, as well as less evidence for relative humidity [78]. The authors concluded that population behavior and government interventions rather than meteorological conditions were the key drivers for COVID-19 spread [78]. In line with this study, Su et al. [6] found that the estimated transmission rate of COVID-19 at an early stage was slightly associated with temperature rather than humidity. An ancillary finding is that the proportion of people over 65 was a crucial driver of COVID-19 spread and those elderly people may face an approximately 2.5-fold higher infection risk than younger people [6]. By calculating the daily Rt at 3739 global locations, a recent global analysis considered the co-effects of air pollutants and weather conditions [3]. The authors stated that a moderate negative association between the estimated Rt and a temperature higher than 25 °C, whereas no obvious relationship was found for humidity, and weaker positive associations were found for sulfur dioxide and ozone [3]. Similarly, findings from Fontal et al. [79] supported the notion of a uniform summer recession of COVID-19 spread, indicating that COVID-19 may become a seasonal low-temperature infection. To date, it appears that the relationships between temperature and COVID-19 observed in most global analyses were negative, but associations for humidity remain unclear.
Besides global analysis, more studies have been carried out at national, provincial, or city scales, as summarized in a previous study [70]. Compared with global analysis, the spatial variations in studies with smaller scales may produce lower levels of bias when estimating the associations. In China, Fang et al. [80] found that the median value of Rt was 0.46 in the north, much higher than the 0.2 in southern cities. Meanwhile, the transmissibility of COVID-19 was negatively associated with temperature, and an increase in humidity from 40% to 75% would increase the spread of COVID-19 by 47% [80]. A modeling study also revealed that higher temperature was associated with decreased COVID-19 spread by a lag effect and concluded that a temperature increase in China made a key, but not determining, contribution to impeding the COVID-19 outbreak [81]. In 211 counties of 46 states in the USA, the mean Rt was estimated to be 5.7 in the first two weeks, and research showed that the Rt declined when the temperature was in the range of 0–11 °C and >20 °C, while the Rt was increased when the temperature was between 11 and 20 °C [82]. Similarly, another study revealed that cold and dry conditions might be moderately associated with increased SARS-CoV-2 transmissibility [83]. Research implemented in 15 countries in Europe estimated that a unitary increase in temperature can result in a 0.9% decline in COVID-19 transmission [84]. In England, Nottmeyer et al. [85] pointed out that dry and cold conditions may elevate the COVID-19 incidence. In Africa, relative humidity and temperature appeared to be inversely correlated with COVID-19 growth [86]. Similar results were observed in India [87], Bangladesh [88], Indonesia [89], Mexico [90], and Brazil [91]. To some extent, although the conclusions derived from local studies become increasingly heterogeneous, it appears that inverse associations between temperature and COVID-19 have been demonstrated by many studies, while relationships for humidity are controversial.
At the time of writing, only one publication has reported the association between temperature/humidity and the transmission of SARS-CoV-2 Delta or Omicron variants. In Sydney, Australia, an observational study suggested that humidity was negatively associated with SARS-CoV-2 Delta cases [92]. Furthermore, when the humidity was below 70%, a relationship between temperature and cases of the Delta variant was also apparent [92].
Associations between COVID-19 mortality rates and temperature/humidity: Wu et al. [74] illustrated that a unit increase in temperature might lead to a 1.19% (95% CI: 0.44%–1.95%) decrease in daily new deaths. Similarly, a study conducted in Nigeria yielded a significantly weak negative correlation between temperature and cumulative mortality [93]. A recent global analysis covering 149 countries reported a negative association between temperature and COVID-19 mortality in high-income economies [94]. Contrastingly, the diurnal daily temperature was positively related to COVID-19 in low- and middle-income countries [94]. In China, a longitudinal analysis addressed the non-linear association between temperature and COVID-19 mortality rates, and a unitary increase in temperature may decrease daily cumulative relative death risk by 12.3% (95% CI: 3.4%–20.4%) [95]. Research conducted in multiple countries suggested that a unitary increase in temperature triggered a 6% lower COVID-19 mortality [96]. Such combined evidence indicates that temperature may be inversely associated with the COVID-19 mortality rate. In addition to the notion that a high temperature may potentially reduce COVID-19 cases, another reason may be that the immune system functions differently under various ambient temperatures.
Compared with temperature, the associations between humidity and COVID-19 mortality have been studied to a lesser extent. A study in Wuhan suggested that humidity was negatively associated with daily death counts [97]. A worldwide study indicated that a 1% increase in humidity resulted in a 0.51% (95% CI: 0.34%–0.67%) decrease in daily new cases [74]. In Pakistan, the humidity was found to be insignificantly associated with daily death rates [98]. Thus, with respect to the associations for humidity, results from existing studies appear to be conflicting.
Data-related, methodological and other obstacles:Table 5 lists some common study limitations, which have also been well-reviewed previously [70,99]. In general, the study weaknesses in existing publications can be divided into three types: data, methodology, and others. Commonly, most studies have struggling with inconsistent data on COVID-19 cases, mortality rates, and other health-related indicators [13]. For example, all data used in models, including the independent, dependent, and confounding variables, should be treated with caution (Table 5). Using the daily confirmed cases as an example, insufficient test coverage at an early stage would reduce the number of positive samples. Hence, spatially varied test capability will bias the estimation in ecological studies. Similarly, the temporally varied test coverage has introduced uncertainties in time-series analysis. In addition, the incubation period of COVID-19 cases means that it is challenging but necessary to determine the lag effects of environmental factors. Another critical problem with the data is the quantification of confounding variables. Population behavior and government interventions have contributed significantly to impeding the COVID-19 outbreak, and these factors can therefore mask the effects of environmental conditions. Although effects for some confounding variables have already been evaluated [[100], [101], [102]], these results have not been well applied to the estimation of the associations between COVID-19 spread and environmental conditions.
Table 5.
Typical study weaknesses in research on the associations between COVID-19 spread and weather conditions.
| Issues | Details |
|---|---|
| Independent variables | The treatments of temperature and humidity varied, including daily measurements and aggregated averages. In addition, some studies considered the lag effects, while some did not. |
| Dependent variables |
|
| Confounding variables | Most ecological studies did not control the important confounding factors related to the COVID-19 spread. |
| Methodologies | Although some statistical models were utilized, the comparisons between these methods/models remain unclear. |
| Result interpretation | Sensitivity and uncertainty analysis was required in most studies. |
| Exposure period | Generally, a short study period was preferred, which is not able to account for long-term trends. |
| Research areal unit | Results may be quite different when studies are performed at the city, provincial, national, or global scale. |
| Other issues | Geographical variation in viral strains; cluster infections; the role of transmission in indoor environments, etc. |
With respect to the methodological obstacles, some studies did not follow standardized approaches for specific study designs. In existing publications, although correlation analysis, generalized additive models, Bayesian inference, meta-analysis, and some other statistical models were usually adopted, the preconditions for these approaches were seldom validated. In addition, most studies used empirical models rather than process-based models such as compartmental models that address interactions among susceptible, infectious, and recovered individuals within a population [13]. A weakness of an empirical model is that it only considers the fit to observed data without a priori hypotheses. Although it is difficult to quantify some parameters in a process-based model (for example, public health interventions), such an approach is useful for exploring transmission dynamics and epidemic trajectories.
To date, few studies have investigated transmission of SARS-CoV-2 in a cold chain-related environment, although several outbreaks in China were linked to food transported in the cold chain from abroad [[103], [104], [105]]. More than 10 provinces have detected SARS-CoV-2-positive samples collected from imported frozen food or its packaging [104]. As shown in Table 3, SARS-CoV-2 is relatively stable on plastic and stainless steel, where the virus has been reported to remain viable even after 72 h. Under a temperature of −20 °C, the titer of the infectious virus did not decline significantly [103]. An earlier study concluded that the infectivity of viruses in food might remain for up to 2 years during frozen storage and transport at −20 °C [104]. Hence, public health officials should be alerted to the infection risk of SARS-CoV-2 in contaminated refrigerated or frozen food.
6. The way forward: Possible research directions
Although research on the role of environmental conditions in the spread of COVID-19 has yielded some results, several critical questions remain unanswered. In particular, some research directions require special attention:
-
1)
The stability of SARS-CoV-2 variants and associated transmission, especially for VOCs or variants of interest. To date, a couple of variants have been identified whose properties, including spread, diagnostic tools, associated disease severity, therapeutics, and vaccine performance (https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/), are raising concerns for public health. For example, the Delta (B.1.617.2) variant, which first emerged in India, is now spreading rapidly worldwide and has even resulted in resurgence in nations with highly vaccinated populations [106]. The basic reproductive number (R0) of the Delta variant was estimated to reach 5.08, much higher than that of the ancestral SARS-CoV-2 [106]. Although there are available epidemiological studies on the spread of the VOCs, how environmental conditions influence the spread of these variants remains unclear. To date, there have been few studies on the stability of VOCs in aerosols or on inert surfaces.
-
2)
The study design or guidelines used in epidemiological studies. Owing to existing study limitations [70] (Table 5), most epidemiological research has failed to provide a robust scientific basis for the role of environmental conditions in COVID-19 spread. For example, do the modifying effects of temperature/humidity on different scales vary? Is it possible to use temperature and humidity as risk indicators for COVID-19 spread? What are the true interactive effects between temperature/humidity and other environmental factors? Meanwhile, the merits, demerits, and suitability of statistical methods should be compared and elucidated, and methodological guideline is highly encouraged.
-
3)
The role of environmental conditions in the spread of COVID-19 from the environment. High virus loads have been detected in wastewater, sediments, soils, and non-human animals [107,108]. These environmental mediums may become potential reservoirs for viruses. However, the possibility of the virus recycling from these pathways into the human population under suitable environmental conditions remains unclear.
-
4)
Adaptive strategies for COVID-19 control. To reduce or eliminate the COVID-19 risk requires multifaceted effort. The spread of COVID-19 appears to have been controlled primarily by government interventions rather than environmental factors. At this stage, when the vaccination rate in many populations is high, it is possible to consider how to control the spread of COVID-19 to achieve low or zero frequency and to reduce the cost of epidemic prevention derived from dynamic strategies, taking into account the combined effects of environmental conditions, non-pharmaceutical interventions and population behavior.
Declaration of competing interests
The authors have declared no conflicts of interest.
Acknowledgment
We thank the funding support by the National Natural Science Foundation of China (grant numbers 52091544 and 42041003), and thank the funding supported by Capital’s Funds for Health Improvement and Research (Nos. 2021-1G-2172 and 2022-1G-4231), the COVID-19 Emergency Funding (Nos. GWTX05 and SWJC05) from the National Institute of Environmental Health (NIEH) of China CDC.
Contributor Information
Ying Wang, Email: yingw@buaa.edu.cn.
Fengchang Wu, Email: wufengchang@vip.skleg.cn.
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su M., Peng S., Chen L., Wang B., Wang Y., Fan X., Dong Z. A warm summer is unlikely to stop transmission of COVID-19 naturally. GeoHealth. 2020 doi: 10.1029/2020GH000292. e2020GH000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu R., Rahmandad H., Gupta M., DiGennaro C., Ghaffarzadegan N., Amini H., Jalali M.S. Weather, air pollution, and SARS-CoV-2 transmission: a global analysis. Lancet Planet. Health. 2021;5(10):e671–e680. doi: 10.1016/S2542-5196(21)00202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon S., Joshi A.D., Lo C.-H., Drew D.A., Nguyen L.H., Guo C.-G., Ma W., Mehta R.S., Shebl F.M., Warner E.T., et al. Association of social distancing and face mask use with risk of COVID-19. Nat. Commun. 2021;12(1):1–10. doi: 10.1038/s41467-021-24115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J., Litvinova M., Wang W., Wang Y., Deng X., Chen X., Li M., Zheng W., Yi L., Chen X., et al. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect. Dis. 2020;20(7):793–802. doi: 10.1016/S1473-3099(20)30230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia Z., Lu Z. Modelling COVID-19 transmission: from data to intervention. Lancet Infect. Dis. 2020;20(7):757–758. doi: 10.1016/S1473-3099(20)30258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robles-Romero J.M., Conde-Guillén G., Safont-Montes J.C., García-Padilla F.M., Romero-Martín M. Behaviour of aerosols and their role in the transmission of SARS-CoV-2; a scoping review. Rev. Med. Virol. 2022;32(3) doi: 10.1002/rmv.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonçalves J., da Silva P.G., Reis L., Nascimento M.S.J., Koritnik T., Paragi M., Mesquita J.R. Surface contamination with SARS-CoV-2: a systematic review. Sci. Total Environ. 2021 doi: 10.1016/j.scitotenv.2021.149231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transbound. Emerg. Dis. 2021;68(2):296–312. doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shakil M.H., Munim Z.H., Tasnia M., Sarowar S. COVID-19 and the environment: a critical review and research agenda. Sci. Total Environ. 2020;745 doi: 10.1016/j.scitotenv.2020.141022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClymont H., Hu W. Weather variability and COVID-19 transmission: a review of recent research. Int. J. Environ. Res. Publ. Health. 2021;18(2):396. doi: 10.3390/ijerph18020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WMO First report of the WMO COVID-19 task team on meteorological and air quality (MAQ) factors affecting the COVID-19 pandemic. 2021. https://public.wmo.int/en/resources/library/first-report-of-wmo-covid-19-task-team-review-meteorological-and-air-quality Available:
- 14.Tang S., Mao Y., Jones R.M., Tan Q., Ji J.S., Li N., Shen J., Lv Y., Pan L., Ding P., et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 2020;144 doi: 10.1016/j.envint.2020.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fears A.C., Klimstra W.B., Duprex P., Hartman A., Weaver S.C., Plante K.S., Mirchandani D., Plante J.A., Aguilar P.V., Fernández D., et al. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg. Infect. Dis. 2020;26(9):2168. doi: 10.3201/eid2609.201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smither S.J., Eastaugh L.S., Findlay J.S., Lever M.S. Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerg. Microb. Infect. 2020;9(1):1415–1417. doi: 10.1080/22221751.2020.1777906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pyankov O.V., Bodnev S.A., Pyankova O.G., Agranovski I.E. Survival of aerosolized coronavirus in the ambient air. J. Aerosol Sci. 2018;115:158–163. doi: 10.1016/j.jaerosci.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ijaz M., Brunner A., Sattar S., Nair R.C., Johnson-Lussenburg C. Survival characteristics of airborne human coronavirus 229E. J. Gen. Virol. 1985;66(12):2743–2748. doi: 10.1099/0022-1317-66-12-2743. [DOI] [PubMed] [Google Scholar]
- 20.Santarpia J.L., Rivera D.N., Herrera V.L., Morwitzer M.J., Creager H.M., Santarpia G.W., Crown K.K., Brett-Major D.M., Schnaubelt E.R., Broadhurst M.J., et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan L., Ma B., Lai X., Han L., Cao P., Zhang J., Fu J., Zhou Q., Wei S., Wang Z., et al. Air and surface contamination by SARS-CoV-2 virus in a tertiary hospital in Wuhan, China. Int. J. Infect. Dis. 2020;99:3–7. doi: 10.1016/j.ijid.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niazi S., Groth R., Spann K., Johnson G.R. The role of respiratory droplet physicochemistry in limiting and promoting the airborne transmission of human coronaviruses: a critical review. Environ. Pollut. 2021;276 doi: 10.1016/j.envpol.2020.115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadei M., Mohebbi S.R., Hopke P.K., Shahsavani A., Bazzazpour S., Alipour M., Jafari A.J., Bandpey A.M., Zali A., Yarahmadi M., et al. Presence of SARS-CoV-2 in the air of public places and transportation. Atmos. Pollut. Res. 2021;12(3):302–306. doi: 10.1016/j.apr.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayalar O., Ari A., Konyalilar N., Dogan O., Can F., Sahin U.A., Gaga E.O., Kuzu S.L., Arı P.E., Odabası M. Existence of SARS-CoV-2 RNA on ambient particulate matter samples: a nationwide study in Turkey. Sci. Total Environ. 2021:147976. doi: 10.1016/j.scitotenv.2021.147976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang R., Li Y., Zhang A.L., Wang Y., Molina M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. U.S.A. 2020;117(26):14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization . vol. 73. 2020. Coronavirus Disease 2019 (COVID-19): Situation Report. [Google Scholar]
- 27.Bourouiba L., Dehandschoewercker E., Bush J.W. Violent expiratory events: on coughing and sneezing. J. Fluid Mech. 2014;745:537–563. [Google Scholar]
- 28.Stadnytskyi V., Anfinrud P., Bax A. Breathing, speaking, coughing or sneezing: what drives transmission of SARS-CoV-2? J. Intern. Med. 2021 doi: 10.1111/joim.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schijven J., Vermeulen L.C., Swart A., Meijer A., Duizer E., de Roda Husman A.M. Quantitative microbial risk assessment for airborne transmission of SARS-CoV-2 via breathing, speaking, singing, coughing, and sneezing. Environ. Health Perspect. 2021;129(4) doi: 10.1289/EHP7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H., Leong F.Y., Xu G., Kang C.W., Lim K.H., Tan B.H., Loo C.M. Airborne dispersion of droplets during coughing: a physical model of viral transmission. Sci. Rep. 2021;11(1):1–10. doi: 10.1038/s41598-021-84245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L., Qi Y., Luzzatto-Fegiz P., Cui Y., Zhu Y. COVID-19: effects of environmental conditions on the propagation of respiratory droplets. Nano Lett. 2020;20(10):7744–7750. doi: 10.1021/acs.nanolett.0c03331. [DOI] [PubMed] [Google Scholar]
- 32.Pal R., Sarkar S., Mukhopadhyay A. Influence of ambient conditions on evaporation and transport of respiratory droplets in indoor environment. Int. Commun. Heat Mass Tran. 2021;129 [Google Scholar]
- 33.Xie X., Li Y., Chwang A., Ho P., Seto W. How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17(3):211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 34.Pourfattah F., Wang L.-P., Deng W., Ma Y.-F., Hu L., Yang B. Challenges in simulating and modeling the airborne virus transmission: a state-of-the-art review. Phys. Fluids. 2021;33(10) doi: 10.1063/5.0061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang W., Marr L.C. Dynamics of airborne influenza A viruses indoors and dependence on humidity. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammer T., Gao H., Pan Z., Wang J. Relationship between aerosols exposure and lung deposition dose. Aerosol Air Qual. Res. 2020;20(5):1083–1093. [Google Scholar]
- 37.Wang H., Yin P., Fan W., Wang Y., Dong Z., Deng Q., Zhou M. Mortality risk associated with short-term exposure to particulate matter in China: estimating error and implication. Environ. Sci. Technol. 2021;19 doi: 10.1021/acs.est.0c05095. (55(2)) [DOI] [PubMed] [Google Scholar]
- 38.Mao N., An C., Guo L., Wang M., Guo L., Guo S., Long E. Transmission risk of infectious droplets in physical spreading process at different times: a review. Build. Environ. 2020 doi: 10.1016/j.buildenv.2020.107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X., Ou C., Yang H., Liu L., Song T., Kang M., Lin H., Hang J. Transmission of pathogen-laden expiratory droplets in a coach bus. J. Hazard Mater. 2020;397 doi: 10.1016/j.jhazmat.2020.122609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L., Li Y., Nielsen P.V., Wei J., Jensen R.L. Short-range airborne transmission of expiratory droplets between two people. Indoor Air. 2017;27(2):452–462. doi: 10.1111/ina.12314. [DOI] [PubMed] [Google Scholar]
- 41.Lee, B. U., Airborne transmission of the SARS-CoV-2 delta variant. Aerosol Air Qual. Res. 22, 210250.
- 42.Lee B.U. Why does the SARS-CoV-2 Delta VOC spread so rapidly? Universal conditions for the rapid spread of respiratory viruses, minimum viral loads for viral aerosol generation, effects of vaccination on viral aerosol generation, and viral aerosol clouds. Int. J. Environ. Res. Publ. Health. 2021;18(18):9804. doi: 10.3390/ijerph18189804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riediker M., Briceno-Ayala L., Ichihara G., Albani D., Poffet D., Tsai D.H., Iff S., Monn C. Higher viral load and infectivity increase risk of aerosol transmission for Delta and Omicron variants of SARS-CoV-2. Swiss Med. Wkly. 2022;1 doi: 10.4414/smw.2022.w30133. [DOI] [PubMed] [Google Scholar]
- 44.Rusin P., Maxwell S., Gerba C. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. J. Appl. Microbiol. 2002;93(4):585–592. doi: 10.1046/j.1365-2672.2002.01734.x. [DOI] [PubMed] [Google Scholar]
- 45.Riddell S., Goldie S., Hill A., Eagles D., Drew T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020;17(1):1–7. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcenac P., Park G.W., Duca L.M., Lewis N.M., Dietrich E.A., Barclay L., Tamin A., Harcourt J.L., Thornburg N.J., Rispens J., et al. Detection of SARS-CoV-2 on surfaces in households of persons with COVID-19. Int. J. Environ. Res. Publ. Health. 2021;18(15):8184. doi: 10.3390/ijerph18158184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W., He H., Zhu L., Liu G., Wu L. Food safety in post-COVID-19 pandemic: challenges and countermeasures. Biosensors. 2021;11(3):71. doi: 10.3390/bios11030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warnes S.L., Keevil C.W. Inactivation of norovirus on dry copper alloy surfaces. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0075017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azuma K., Yanagi U., Kagi N., Kim H., Ogata M., Hayashi M. Environmental factors involved in SARS-CoV-2 transmission: effect and role of indoor environmental quality in the strategy for COVID-19 infection control. Environ. Health Prev. Med. 2020;25(1):1–16. doi: 10.1186/s12199-020-00904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chin A.W., Chu J.T., Perera M.R., Hui K.P., Yen H.-L., Chan M.C., Peiris M., Poon L.L. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;1(1):e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kasloff S.B., Leung A., Strong J.E., Funk D., Cutts T. Stability of SARS-CoV-2 on critical personal protective equipment. Sci. Rep. 2021;11(1):1–7. doi: 10.1038/s41598-020-80098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan S.M., Zhao X.S., Wen R.F., Huang J.J., Pi G.H., Zhang S.X., Han J., Bi S.L., Ruan L., Dong X.P., et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irra-diation. Biomed. Environ. Sci. 2003;16:246–255. [PubMed] [Google Scholar]
- 54.Biryukov J., Boydston J.A., Dunning R.A., Yeager J.J., Wood S., Reese A.L., Ferris A., Miller D., Weaver W., Zeitouni N.E., et al. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. MSphere. 2020;5(4) doi: 10.1128/mSphere.00441-20. e00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan K.-H., Sridhar S., Zhang R.R., Chu H., Fung A.-F., Chan G., Chan J.-W., To K.-W., Hung I.-N., Cheng V.-C., et al. Factors affecting stability and infectivity of SARS-CoV-2. J. Hosp. Infect. 2020;106(2):226–231. doi: 10.1016/j.jhin.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He X., Liu X., Li P., Wang P., Cheng H., Li W., Li B., Liu T., Ma J. A multi-stage green barrier strategy for the control of global SARS-CoV-2 transmission via food cold chain. Engineering. 2021 doi: 10.1016/j.eng.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan K.-H., Peiris J.M., Lam S., Poon L., Yuen K., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011;2011 doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Doremalen N., Bushmaker T., Munster V. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18(38) doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- 59.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., Kritas S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34(2):327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 61.Song P., Li W., Xie J., Hou Y., You C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soane R., Carney A., Jones N., Frier M., Perkins A., Davis S., Illum L. The effect of the nasal cycle on mucociliary clearance. Clin. Otolaryngol. Allied Sci. 2001;26(1):9–15. doi: 10.1046/j.1365-2273.2001.00423.x. [DOI] [PubMed] [Google Scholar]
- 63.Eccles R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol. 2002;122(2):183–191. doi: 10.1080/00016480252814207. [DOI] [PubMed] [Google Scholar]
- 64.Kudo E., Song E., Yockey L.J., Rakib T., Wong P.W., Homer R.J., Iwasaki A. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc. Natl. Acad. Sci. U.S.A. 2019;116(22):10905–10910. doi: 10.1073/pnas.1902840116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ivashkiv L.B., Donlin L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014;14(1):36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moriyama M., Ichinohe T. High ambient temperature dampens adaptive immune responses to influenza A virus infection. Proc. Natl. Acad. Sci. U.S.A. 2019;116(8):3118–3125. doi: 10.1073/pnas.1815029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foxman E.F., Storer J.A., Fitzgerald M.E., Wasik B.R., Hou L., Zhao H., Turner P.E., Pyle A.M., Iwasaki A. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc. Natl. Acad. Sci. U.S.A. 2015;112(3):827–832. doi: 10.1073/pnas.1411030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foxman E.F., Storer J.A., Vanaja K., Levchenko A., Iwasaki A. Two interferon-independent double-stranded RNA-induced host defense strategies suppress the common cold virus at warm temperature. Proc. Natl. Acad. Sci. U.S.A. 2016;113(30):8496–8501. doi: 10.1073/pnas.1601942113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esteve A., Permanyer I., Boertien D., Vaupel J.W. National age and coresidence patterns shape COVID-19 vulnerability. Proc. Natl. Acad. Sci. U.S.A. 2020;117(28):16118–16120. doi: 10.1073/pnas.2008764117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong Z., Fan X., Wang J., Mao Y., Luo Y., Tang S. Data-related and methodological obstacles to determining associations between temperature and COVID-19 transmission. Environ. Res. Lett. 2021;16(3) [Google Scholar]
- 71.Huang Z., Huang J., Gu Q., Du P., Liang H., Dong Q. Optimal temperature zone for the dispersal of COVID-19. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.139487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan J., Yao Y., Liu Z., Meng X., Ji J.S., Qiu Y., Wang W., Zhang L., Wang W., Kan H. Warmer weather unlikely to reduce the COVID-19 transmission: an ecological study in 202 locations in 8 countries. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.142272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mandal C.C., Panwar M. Can the summer temperatures reduce COVID-19 cases? Publ. Health. 2020;185:72–79. doi: 10.1016/j.puhe.2020.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu Y., Jing W., Liu J., Ma Q., Yuan J., Wang Y., Du M., Liu M. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.139051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarmadi M., Marufi N., Moghaddam V.K. Association of COVID-19 global distribution and environmental and demographic factors: an updated three-month study. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyer A., Sadler R., Faverjon C., Cameron A.R., Bannister-Tyrrell M. Evidence that higher temperatures are associated with a marginally lower incidence of COVID-19 cases. Front. Public Health. 2020;8:367. doi: 10.3389/fpubh.2020.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jüni P., Rothenbühler M., Bobos P., Thorpe K.E., da Costa B.R., Fisman D.N., Slutsky A.S., Gesink D. Impact of climate and public health interventions on the COVID-19 pandemic: a prospective cohort study. CMAJ (Can. Med. Assoc. J.) 2020;192(21):566–573. doi: 10.1503/cmaj.200920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sera F., Armstrong B., Abbott S., Meakin S., O’Reilly K., von Borries R., Schneider R., Royé D., Hashizume M., Pascal M., et al. A cross-sectional analysis of meteorological factors and SARS-CoV-2 transmission in 409 cities across 26 countries. Nat. Commun. 2021;12(1):1–11. doi: 10.1038/s41467-021-25914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fontal A., Bouma M.J., San-José A., López L., Pascual M., Rodó X. Climatic signatures in the different COVID-19 pandemic waves across both hemispheres. Nature Computat. Sci. 2021;1(10):655–665. doi: 10.1038/s43588-021-00136-6. [DOI] [PubMed] [Google Scholar]
- 80.Fang L.Q., Zhang H.Y., Zhao H., Che T.L., Zhang A.R., Liu M.J., Shi W.Q., Guo J.P., Zhang Y., Liu W., et al. Meteorological conditions and nonpharmaceutical interventions jointly determined local transmissibility of COVID-19 in 41 Chinese cities: a retrospective observational study. The Lancet Regional Health-Western Pacific. 2020;2 doi: 10.1016/j.lanwpc.2020.100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ren M., Pei R., Jiangtulu B., Chen J., Xue T., Shen G., Yuan X., Li K., Lan C., Chen Z., et al. Contribution of temperature increase to restrain the transmission of COVID-19. Innovation. 2021;2(1) doi: 10.1016/j.xinn.2020.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rubin D., Huang J., Fisher B.T., Gasparrini A., Tam V., Song L., Wang X., Kaufman J., Fitzpatrick K., Jain A., et al. Association of social distancing, population density, and temperature with the instantaneous reproduction number of SARS-CoV-2 in counties across the United States. JAMA Netw. Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.16099. e2016099-e2016099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma Y., Pei S., Shaman J., Dubrow R., Chen K. Role of meteorological factors in the transmission of SARS-CoV-2 in the United States. Nat. Commun. 2021;12(1):1–9. doi: 10.1038/s41467-021-23866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ozyigit A. Disease & Health; Infection: 2020. Understanding Covid-19 Transmission: The Effect of Temperature and Health Behavior on Transmission Rates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nottmeyer L.N., Sera F. Influence of temperature, and of relative and absolute humidity on COVID-19 incidence in England-A multi-city time-series study. Environ. Res. 2021;196 doi: 10.1016/j.envres.2021.110977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adekunle I.A., Tella S.A., Oyesiku K.O., Oseni I.O. Spatio-temporal analysis of meteorological factors in abating the spread of COVID-19 in Africa. Heliyon. 2020 doi: 10.1016/j.heliyon.2020.e04749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar G., Kumar R. Diabetes & Metabolic Syndrome: Clinical Research & Reviews; 2020. A Correlation Study between Meteorological Parameters and COVID-19 Pandemic in Mumbai, India. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haque S.E., Rahman M. Association between temperature, humidity, and COVID-19 outbreaks in Bangladesh. Environ. Sci. Pol. 2020 doi: 10.1016/j.envsci.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rendana M. Impact of the wind conditions on COVID-19 pandemic: a new insight for direction of the spread of the virus. Urban Clim. 2020 doi: 10.1016/j.uclim.2020.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Méndez-Arriaga F. The temperature and regional climate effects on communitarian COVID-19 contagion in Mexico throughout phase 1. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.139560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosario D.K., Mutz Y.S., Bernardes P.C., Conte-Junior C.A. Relationship between COVID-19 and weather: case study in a tropical country. Int. J. Hyg Environ. Health. 2020;229 doi: 10.1016/j.ijheh.2020.113587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ward M.P., Liu Y., Xiao S., Zhang Z. Challenges in the control of COVID-19 outbreaks caused by the delta variant during periods of low humidity: an observational study in Sydney, Australia. Infect. Dis. Povert. 2021;10(1):1–10. doi: 10.1186/s40249-021-00926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ogaugwu C., Mogaji H., Ogaugwu E., Nebo U., Okoh H., Agbo S., Agbon A. Effect of weather on COVID-19 transmission and mortality in lagos, Nigeria. Scientifica. 2020:2020. doi: 10.1155/2020/2562641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rahman M., Islam M., Shimanto M.H., Ferdous J., Rahman A.A.-N.S., Sagor P.S., Chowdhury T. A global analysis on the effect of temperature, socio-economic and environmental factors on the spread and mortality rate of the COVID-19 pandemic. Environ. Dev. Sustain. 2021;23(6):9352–9366. doi: 10.1007/s10668-020-01028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu G., Zhu Y., Wang Z., Meng W., Wang X., Feng J., Li J., Xiao Y., Shi F., Wang S. The association between ambient temperature and mortality of the coronavirus disease 2019 (COVID-19) in Wuhan, China: a time-series analysis. BMC Publ. Health. 2021;21(1):1–10. doi: 10.1186/s12889-020-10131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Christophi C.A., Sotos-Prieto M., Lan F.-Y., Delgado-Velandia M., Efthymiou V., Gaviola G.C., Hadjivasilis A., Hsu Y.-T., Kyprianou A., Lidoriki I., et al. Ambient temperature and subsequent COVID-19 mortality in the OECD countries and individual United States. Sci. Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-87803-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma Y., Zhao Y., Liu J., He X., Wang B., Fu S., Yan J., Niu J., Zhou J., Luo B. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rehman Y., Rehman N. Association of climatic factors with COVID-19 in Pakistan. AIMS Public Health. 2020;7(4):854. doi: 10.3934/publichealth.2020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Villeneuve P.J., Goldberg M.S. Methodological considerations for epidemiological studies of air pollution and the SARS and COVID-19 coronavirus outbreaks. Environ. Health Perspect. 2020;128(9) doi: 10.1289/EHP7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J., El-harakeh A., Bognanni A., Lotfi T., Loeb M., et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou Y., Xu R., Hu D., Yue Y., Li Q., Xia J. Effects of human mobility restrictions on the spread of COVID-19 in Shenzhen, China: a modelling study using mobile phone data. The Lancet Digital Health. 2020;2(8):e417–e424. doi: 10.1016/S2589-7500(20)30165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davies N.G., Kucharski A.J., Eggo R.M., Gimma A., Edmunds W.J., Centre for the Mathematical Modelling of Infectious Diseases COVID-19 working group Effects of non-pharmaceutical interventions on COVID-19 cases, deaths, and demand for hospital services in the UK: a modelling study. Lancet Public Health. 2020 doi: 10.1016/S2468-2667(20)30133-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma H., Zhang J., Wang J., Qin Y., Chen C., Song Y., Wang L., Meng J., Mao L., Li F., et al. COVID-19 outbreak caused by contaminated packaging of imported cold-chain products—liaoning Province, China, July 2020. China CDC Weekly. 2021;3(21):441. doi: 10.46234/ccdcw2021.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu L.C., Quintela I., Lin C.H., Lin T.C., Lin C.H., Wu V.C., Lin C.S. A review of epidemic investigation on cold-chain food-mediated SARS-CoV-2 transmission and food safety consideration during COVID-19 pandemic. J. Food Saf. 2021;41(6) doi: 10.1111/jfs.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chi Y., Wang Q., Chen G., Zheng S. The long-term presence of SARS-CoV-2 on cold-chain food packaging surfaces indicates a new COVID-19 winter outbreak: a mini review. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.650493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Y., Rocklöv J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J. Trav. Med. 2021 doi: 10.1093/jtm/taab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods-A scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Palmer M.V., Martins M., Falkenberg S., Buckley A., Caserta L.C., Mitchell P.K., Cassmann E.D., Rollins A., Zylich N.C., Renshaw R.W., et al. Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2. J. Virol. 2021;95(11) doi: 10.1128/JVI.00083-21. e00083-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duguid J. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. Epidemiol. Infect. 1946;44(6):471–479. doi: 10.1017/s0022172400019288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ji Y., Qian H., Ye J., Zheng X. The impact of ambient humidity on the evaporation and dispersion of exhaled breathing droplets: a numerical investigation. J. Aerosol Sci. 2018;115:164–172. [Google Scholar]
- 111.Li H., Leong F.Y., Xu G., Ge Z., Kang C.W., Lim K.H. Dispersion of evaporating cough droplets in tropical outdoor environment. Phys. Fluids. 2020;32(11) doi: 10.1063/5.0026360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rabenau H., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005;194(1):1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lai M.Y., Cheng P.K., Lim W.W. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005;41(7):e67–e71. doi: 10.1086/433186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang C., Liao H., Strobl E., Li H., Li R., Jensen S.S., Zhang Y. The role of weather conditions in COVID-19 transmission: a study of a global panel of 1236 regions. J. Clean. Prod. 2021;292 doi: 10.1016/j.jclepro.2021.125987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sajadi M.M., Habibzadeh P., Vintzileos A., Shokouhi S., Miralles-Wilhelm F., Amoroso A. Temperature, humidity, and latitude analysis to estimate potential spread and seasonality of coronavirus disease 2019 (COVID-19) JAMA Netw. Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11834. e2011834-e2011834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sobral M.F.F., Duarte G.B., da Penha Sobral A.I.G., Marinho M.L.M., de Souza Melo A. Association between climate variables and global transmission oF SARS-CoV-2. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sarkodie S.A., Owusu P.A. Impact of meteorological factors on COVID-19 pandemic: evidence from top 20 countries with confirmed cases. Environ. Res. 2020:110101. doi: 10.1016/j.envres.2020.110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thazhathedath Hariharan H., Surendran A.T., Haridasan R.K., Venkitaraman S., Robert D., Narayanan S.P., Mammen P.C., Siddharth S.R., Kuriakose S.L. Global COVID-19 transmission and mortality—influence of human development, climate, and climate variability on early phase of the pandemic. GeoHealth. 2021;5(10) doi: 10.1029/2020GH000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Merow C., Urban M.C. Seasonality and uncertainty in global COVID-19 growth rates. Proc. Natl. Acad. Sci. U.S.A. 2020;117(44):27456–27464. doi: 10.1073/pnas.2008590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spada A., Tucci F.A., Ummarino A., Ciavarella P.P., Calà N., Troiano V., Caputo M., Ianzano R., Corbo S., de Biase M., et al. Structural equation modeling to shed light on the controversial role of climate on the spread of SARS-CoV-2. Sci. Rep. 2021;11(1):1–11. doi: 10.1038/s41598-021-87113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leichtweis B.G., de Faria Silva L., da Silva F.L., Peternelli L.A. How the global health security index and environment factor influence the spread of COVID-19: a country level analysis. One Health. 2021;12 doi: 10.1016/j.onehlt.2021.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Islam N., Bukhari Q., Jameel Y., Shabnam S., Erzurumluoglu A.M., Siddique M.A., Massaro J.M., D'Agostino Sr R.B. COVID-19 and climatic factors: a global analysis. Environ. Res. 2021;193 doi: 10.1016/j.envres.2020.110355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ficetola G.F., Rubolini D. Containment measures limit environmental effects on COVID-19 early outbreak dynamics. Sci. Total Environ. 2021;761 doi: 10.1016/j.scitotenv.2020.144432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yuan J., Wu Y., Jing W., Liu J., Du M., Wang Y., Liu M. Association between meteorological factors and daily new cases of COVID-19 in 188 countries: a time series analysis. Sci. Total Environ. 2021;780 doi: 10.1016/j.scitotenv.2021.146538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carleton T., Cornetet J., Huybers P., Meng K.C., Proctor J. Global evidence for ultraviolet radiation decreasing COVID-19 growth rates. Proc. Natl. Acad. Sci. U.S.A. 2021;118(1) doi: 10.1073/pnas.2012370118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Quilodrán C.S., Currat M., Montoya-Burgos J.I. Air temperature influences early Covid-19 outbreak as indicated by worldwide mortality. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shao W., Xie J., Zhu Y. Mediation by human mobility of the association between temperature and COVID-19 transmission rate. Environ. Res. 2021;194 doi: 10.1016/j.envres.2020.110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sarkodie S.A., Owusu P.A. Global effect of city-to-city air pollution, health conditions, climatic & socio-economic factors on COVID-19 pandemic. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu M., Li Z., Liu M., Zhu Y., Liu Y., Kuetche M.W.N., Wang J., Wang X., Liu X., Li X., et al. Association between temperature and COVID-19 transmission in 153 countries. Environ. Sci. Pollut. Res. 2022;29(11):16017–16027. doi: 10.1007/s11356-021-16666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Foo O., Hiu S., Teare D., Syed A.A., Razvi S. A global country-level analysis of the relationship between obesity and COVID-19 cases and mortality. Diabetes Obes. Metabol. 2021;23(12):2697–2706. doi: 10.1111/dom.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tapia-Muñoz T., González-Santa Cruz A., Clarke H., Morris W., Palmeiro-Silva Y., Allel K. COVID-19 attributed mortality and ambient temperature: a global ecological study using a two-stage regression model. Pathog. Glob. Health. 2022:1–11. doi: 10.1080/20477724.2021.2007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kong J.D., Tekwa E.W., Gignoux-Wolfsohn S.A. Social, economic, and environmental factors influencing the basic reproduction number of COVID-19 across countries. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252373. [DOI] [PMC free article] [PubMed] [Google Scholar]