Abstract
Mesenchymal stem cells (MSCs) have attracted attention for their potential use in regenerative medicine such as brain transplantation. As MSCs are considered to be hypoimmunogenic, transplanted MSCs should not trigger a strong host inflammatory response. To verify this hypothesis, we studied the brain immune response after transplantation of human or rat MSCs into the rat striatum and MSC fate at days 5, 14, 21 and 63 after transplantation. Flow cytometry analysis indicated that both MSCs express CD90 and human leucocyte antigen (MHC) class I, but no MHC class II molecules. They do not express CD45 or CD34 antigens. However, MSC phenotype varies with passage number. Human MSCs have mRNAs for interleukin (IL)‐6, IL‐8, IL‐12, tumour necrosis factor (TNF)‐α and TGF‐β1, whereas rat MSCs express IL‐6‐, IL‐10‐, IL‐12‐ and TGF‐β1‐mRNAs. The quantification shows higher levels of mRNAs for the anti‐inflammatory molecules IL‐6 and TGF‐β1 than for pro‐inflammatory cytokines IL‐8 and IL‐12; ELISA analysis showed no IL‐12 whereas TGF‐β1 and IL‐6 were detected. Transplant size did not significantly vary between 14 and 63 days after transplantation, indicating an absence of immune rejection of the grafts. Very few mast cells and moderate macrophage and microglial infiltrations, observed at day 5 remained stable until day 63 after transplantation in both rat and human MSC grafts. The observations of very few dendritic cells, T αβ‐cells, and no T γδ‐lymphocytes, all three being associated with Tp rejection in the brain, support the contention that MSCs are hypoimmunogenic. Our results suggest that MSCs are of great interest in regenerative medicine in a (xeno)transplantation setting.
Keywords: mesenchymal stem cells, allotransplantation, xenotransplantation, rat brain
Introduction
Mesenchymal stem cells (MSCs) are non‐haematopoetic, stromal cells. They have the capacity for unlimited self‐renewal, but they also retain the potential to differentiate into a variety of cell types [1] as they exhibit multi‐lineage differentiation capacity with the ability to give rise to diverse tissues, including bone, cartilage, adipose tissue, tendon and muscle. Originally, Friedenstein et al. isolated MSCs from the bone marrow (BM) and stroma of the spleen and thymus [2]. Subsequently BM aspirates were found to be the most accessible and enriched source of MSCs [3]. It has been estimated that MSC represent 0.0001–0.01% of the nucleated cells in adult human BM. MSCs derived from adult BM were first described as able to differentiate along three main pathways: osteoblastic, adipocytic and chondrocytic pathways [1]. Numerous studies with animal models have shown that MSCs may be useful in the repair of damaged bone/cartilage [4] and myocardial tissues [5].
Beside these basic potentialities, MSCs may also be able to acquire phenotypes of haematopoietic‐supportive cells [6] and muscle cells [7] suggesting that they are widely multipotent. Several studies showed that MSCs were not only restricted to mesoderm‐derived lineages but were also able to express neuronal and glial markers in vitro[8, 9, 10] and to differentiate into neurons [10]. However, the differentiation of MSCs into neural cells in vivo is rare and seems to depend on lesion conditions [11].
Because logistical (difficult availability of numerous human foetuses), immunological (need of immunosupressors), and ethical limitations (because of the use of human tissue) have complicated the use of foetal/embryonic cell sources for therapeutic transplantation in human [12], MSCs have gained considerable interest due to their potential use in transplantation therapy as they have been shown to restore deficits [13, 14] and provide growth factor support [brain derived neurotrophic factor (BDNF), nerve growth factor (NGF), extracellular matrix proteins; for review see [11]) in rodent models of neurological disease and injury. Therefore transplantation of MSCs (from the marrow and then expanded in vitro) could be very attractive in regenerative medicine by providing some trophic support to injured/neurodegenerative neurons and delaying/stopping the degenerative process and/or allow the surviving host cells in the vicinity of the MSC‐Tp to function more efficiently.
For a long time, the brain was considered immunologically privileged, but it is now evident that tissue grafted into the adult brain is subject to considerable immune surveillance [15]. Indeed, the transplantation procedure invariably leads to inflammation and to the initiation of effective mechanisms that coordinate innate and adaptive immune responses. The dynamic interactions between immune cells and the neural parenchyma enable the brain to respond vigorously to the grafted tissue. As a consequence, the majority of cell types transplanted to the adult brain, especially in the case of xenotransplantation, exhibit poor long‐term survival, in the absence of immunosupressors [16, 17].
However, in vitro and in vivo evidence has suggested that MSCs are not intrinsically immunogenic, do not stimulate alloreactivity, and exert suppressive effects on T‐cell proliferation, stimulation and mixed lymphocyte reactions [18]. Previous reports show that MSCs inhibit T [19] and natural killer (NK) [20] cell proliferation. They also prevent dendritic cell (DC) differentiation [21] and induce anergy of B cell [22]. MSCs affect several other immune cell functions, including T‐cell cytokine secretion and cytotoxicity [23], B‐cell maturation and antibody secretion, NK‐cell cytokine production and cytotoxicity and the activation and antigen presentation in DCs [24, 25].
Human MSCs express intermediate levels of human leucocyte antigen (HLA), or major histocompatibility complex (MHC) class I, and present MHC class II antigens when activated [26]. MSCs do not express co‐stimulatory molecules B7–1, B7–2, CD40, and CD40 ligand and, therefore, should not activate alloreactive T cells [27]. Other data suggest that they might interfere with production of B‐lineage cells [28]. Previous research has shown that MSCs have the ability to inhibit an immune response when co‐grafted with other cell types (e.g. haematopoietic stem cells [54]) which normally elicit an immune response [55]. Thus, if MSC transplantation is able to induce a weak host immune response, the use of co‐transplantation of MSCs with other cell types such as xenoneuroblasts that are more vulnerable to an immune response, could be a seducing alternative to the use of immunosupressors and/or the use human foetal tissue.
However, many questions remain regarding the fate and consequences of MSC transplantation in the brain. Specifically, the nature of the host immune response to transplanted MSCs, as well as the fate of MSCs once transplanted into the brain or the infiltration of mast cells. To address these questions and provide an assessment of the immune plasticity in the adult brain after adult stem cell transplant, human or rat MSCs were transplanted into the rat striatum and the inflammatory responses were observed at 14, 21 and 63 days after transplantation. In addition, the differentiation ability of MSCs into glial cells and neurons was investigated.
Materials and methods
Isolation and culture of MSC
Adult (8–12 week‐old) Sprague‐Dawley rat BM cells were obtained from femoral and tibial bones by aspiration. Adult human BM was aspirated from the head of human femoral bones collected following hip replacement surgery under the guidelines of Nantes University/County Hospital. Both types of BM were suspended in 10 ml of Dulbecco’s Modified Eagle’s Medium (DMEM: Sigma, Saint‐Quentin Fallavier, France). After plastic adherence selection, human and rat MSCs were cultured at 8000 cells/cm2 for four passages [29].
Generation of transgenic rats expressing green flourescent protein (GFP)
Recombinant lentiviruses, coding for eGFP under the transcriptional control of the ubiquitous phosphor glycerol kinase promoter were microinjected in the perivitelline space of zygotes from the Sprague‐Dawley strain of rat as described by Lois et al.[30]. A transgenic line expressing eGFP in several tissues including BM has been identified (manuscript in preparation) and MSCs from this line were cultured as described above.
Flow cytometry [31]
Intracellular staining
At 4 passages, MSCs were detached with 0.5% trypsin, washed and triturated to a single cell suspension in phosphate‐buffered‐saline/fetal calf serum/sodium azide (PFN) (0.1 M phosphate buffer saline [PBS], 2% fetal calf serum (FCS), 0.1% sodium azide). Cells were distributed into 96‐well, U‐bottomed microtitre plates (Nunc, Denmark), fixed in 4% paraformaldehyde for 20 min. on ice, washed and incubated in PBS 0.1 M /0.5% saponin for 20 min. on ice. After three washes in PBS/0.1% saponin, cells were incubated on ice for 30 min. with primary antibodies diluted in PBS containing 0.1% saponin.
For characterization of rat MSCs, we used monoclonal antibodies Fluourescein IsoThioCynate (FITC)‐CD90 (1:300, Pharmingen, San Diego, CA, USA), FITC‐CD45 (1:300, Pharmingen), FITC‐CD11b (1:500, Pharmingen). Rat MHC I molecules (RT1A) and rat MHC II molecules (RT1B) were, respectively, labelled with OX18 and OX6 (1:1000, Pharmingen). Glial Fibrillary Acidic Protein (GFAP) monoclonal antibodies (mAbs) (1:800); Sigma‐Aldrich (Saint‐Quentin Fallavier, France) were used for detection of astrocytes and TH ones to characterize the catecholaminergic phenotype (1:1000; Pel Freeze, Rogers, AR, USA).
Human monoclonal antibodies CD90 (1:300, Pharmingen), FITC‐CD45/PE‐CD34 (1:500, Pharmingen), anti‐HLA class I (1:1000, clone W6/32, ATCC), anti‐HLA DR (1:1000, clone L243: anti‐HLA‐DRB1 + DRB4 IgG‐2α, monomorphic), were used for the characterization of human MSCs.
Following an additional three washes, all cells were incubated for 30 min. with FITC‐antimouse IgG diluted 1/200 in PBS/0.1% saponin. The cells were then washed in PBS/0.1% saponin and resuspended in PFN prior to fluorescence‐activated cell sorting (FACS) analyses.
Cell surface antigen staining
Single cell suspensions of MSCs were prepared for intracellular staining and treated as described above, except that the saponin was omitted in all of the steps.
FACS analysis
Fluorescent signalling was assessed immediately after staining using a FACScalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), equipped with an argon laser with an emission wavelength of 488 nm. FITC was identified using a 530 band pass filter. The analysis was performed with CELLQUEST software (Becton Dickinson). A primary gate based on physical parameters (forward and side light scatter, FSC and SSC, respectively) was set to exclude dead cells or debris. The background level was estimated by omitting the primary antibody.
Additional analyses of the rat MSC phenotype were also performed at passages 12, 20 and 25 in order to characterize if the number of passages influences the phenotype of the MSCs. We focussed on CD90, CD45, CD44, CD11b as these four markers are used by most to phenotype MSCs and on nestin, a maker of neural stem/ progenitor cells.
mRNA analyses
mRNAs for pro‐inflammatory molecules such as IL‐2, IL‐4, IL‐5, IL‐8, IL‐12p25 and p40, IL‐16, IL 17, interferon (INF)‐γ, tumour necrosis factor (TNF)‐α and for anti‐inflammatory molecules, such as IL‐6, IL‐10, IL‐13, TGF‐β1 were investigated. Cytokines classification into the pro‐ or anti‐inflammatory groups was from Opal and DePalo [32].
MSCs were disassociated and homogenized using syringe and needle. Total RNA was then isolated using Trizol® reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA). Potential genomic DNA contamination was removed by treatment with Turbo™ DNase (Ambion Inc., Austin, TX, USA). RNA was quantified at 260 nm, using ND‐1000 UV‐Vis Spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). RNA integrity was controlled on agarose gel. cDNA was synthesized from 5 μg of RNA, using the Moloney murine leukaemia virus RT kit (Invitrogen, San Diego, CA, USA) as previously described [32] and diluted to a final concentration of 100 ng cDNA/μl.
Analyses of transcripts were performed by RT‐PCR using oligonucleotides for rat MSCs and TaqMan® Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) for human MSCs as described in Table 1. The hypoxanthine phosphoribosyltransferase (HPRT) gene was used as endogenous control gene in both groups of MSCs. For the human MSCs, amplification was performed on the ABI PRISM 7700 Sequence detector, using 1 μl of RT (100 ng cDNA), 1.25 μl of probes, and 12.75 μl TaqMan® (Applied Biosystems, Foster City, CA, USA) Universal PCR Master Mix 2× (AB) in a total volume of 25 μl. For the rat MSCs, amplification was performed on the Gene Amp PCR system 9700 (Applied Biosystems), using 1 μl of RT (100 ng cDNA) and the AmpliTaq Gold® DNA Polymerase (Applied Biosystems, AB), in a total volume of 25 μl. For human and rat MSCs, cycling condition was as followed: 10 min. at 95°C and 40 cycles of 15 sec. at 95°C and 1 min. at 60°C. The PCR products were resolved on agarose gels and stained with ethidium bromide.
Table 1.
Sequences of oligonucleotides and references of the probes
| Gene | Primer | Sequence | Gene | Primer | Sequence |
|---|---|---|---|---|---|
| Rat HPRT | Forward | TTCCTCCTCAGACCGCTTTT | Rat IL‐12p40 | Forward | TCATCAGGGACATCATCAAACC |
| Reverse | CTTATAGCCCCCCTTCAGCA | Reverse | CGAGGAACGCACCTTTCTG | ||
| Rat IL‐2 | Forward | CCTTGTCAACAGCGCACCC | Rat INF‐γ | Forward | TGGATGCTATGGAAGGAAAGA |
| Reverse | GCTTTGACAGATGGCTATCC | Reverse | GATTCTGGTGACAGCTGGTG | ||
| Rat IL‐4 | Forward | CGGTATCCACGGATGTAACG | Rat TGF‐β | Forward | CTCAACACCTGCACAGCTCC |
| Reverse | CGGTGCAGCTTCTCAGTGAG | Reverse | ACGATCATGTTGGACAACTGCT | ||
| Rat IL‐6 | Forward | GCAAGAGACTTCCAGCCAGTT | Rat IL‐13 | Forward | TATGGAGCGTGGACCTGACA |
| Reverse | CATCATCGCTGTTCATACAATCA | Reverse | GCGGAAAAGTTGCTTGGAGTA | ||
| Rat IL‐10 | Forward | TGCTATGTTGCCTGCTCTTACTG | Rat IL‐16 | Forward | TGAAGGGTCACTGCTTGTGG |
| Reverse | TCAAATGCTCCTTGATTTCTGG | Reverse | CTCTCGGCAGGAGTGTGACA | ||
| Rat IL‐12p35 | Forward | TGATGATGACCCTGTGCCTT | Rat IL‐17 | Forward | TGCTGTTGCTGCTACTGAACC |
| Reverse | GCATGGAGCAGGATACAGAGC | Reverse | AACTTCCCCTCAGCGTTGAC |
| Gene | TaqMan® Gene Expression Assays ID (Applied Biosystems) |
|---|---|
| Human HPRT | Hs99999909_m1 |
| Human IL‐2 | Hs00174114_m1 |
| Human IL‐4 | Hs00174122_m1 |
| Human IL‐5 | Hs00174200_m1 |
| Human IL‐6 | Hs00174131_m1 |
| Human IL‐8 | Hs00174103_m1 |
| Human IL‐10 | Hs00174086_m1 |
| Human IL‐12 | Hs00168405_m1 |
| Human INF‐γ | Hs00174143_m1 |
| Human TNF‐α | Hs00174128_m1 |
| Human TGF‐β1 | Hs00171257_m1 |
In addition, absolute quantification of gene expression by qPCR was realized on human MSCs for the transcripts detected by PCR and electrophoresis on agarose gel. After amplification of each mRNA by PCR with TaqMan® Gene Expression Assays (as previously described), the amplicons were gel extracted with NucleoSpin® Extract II (Macherey‐Nagel, GmbH & Co. KG, Düren, Germany) and used as cDNA standards. The concentration of the standards was evaluated by their absorbance with ND‐1000 UV‐Vis Spectrophotometer. The copy number of each standard was calculated using the Avogadro constant and the size of the amplicon. To generate the standard curves, the standards were serially diluted in 1:10 steps. Usually, a range from 109 to 102 copies was used. Three different samples of human MSC were tested and performed in duplicate. Then, amplification data were analysed with an ABI Prism Sequence Detection Software 2.1 (AB). The copy number of each cDNA in each sample was calculated by dint of the equation of the corresponding standard curve. Finally, the copy numbers were report per nanogram of cDNA (100 ng used per reaction).
Cytokine measurement
Supernatants were collected from human MSCs propagated for four passages and cytokine concentration assessed in duplicate by ELISA according to manufacturer’s recommendations. Human ELISA sets for IL‐6 (Biosource, Camarillo, CA, USA), IL‐12p40 (OptEIA™ set; BD PharMingen, Pharmingon, Le Pont de Claix, France) and TGF‐β1 (OptEIA™ set; BD PharMingen) were used.
MSC transplantation
Thirty‐seven male, non‐syngeneic Sprague‐Dawley rats (Charles River, Rouen, France; 200–250 g body weight at the beginning of the study) were used. They were housed at 22°C, under 12‐hr light/12‐hr dark conditions with ad libitum access to food and water. The care and treatment of the animals was approved by the University of Nantes Ethics Committee.
Before the fourth passage and the transplantation procedure, the non‐transgenic MSCs were incubated for 5 min. with Hoechst 33258 (5 μg/ml). After being anesthetized with an intraperitoneal (i.p.) injection of xylazine (10 mg/kg)/ketamine (75 mg/kg), the animals were transplanted (2 μl) with 400,000 eGFP rat MSCs [33], or Hoechst labelled [34], human MSCs (xenotransplantation) or rat MSCs (allotransplantation) into the left striatum, using a 10 μl Hamilton microsyringe placed, at the following coordinates, relative to Bregma: +0.7 mm AP; +2.8 mm ML; –5.8 mm and −5.4 mm DV (with tooth bar at −3.3 mm) [35]. Rats were sacrificed at 5, 14, 21 or 63 days after transplantation. The 37 animals were randomly assigned to one of six transplants (Tp) groups: human MSC‐Tp (5‐day survival, n= 3), vehicle only (culture medium; 5‐day survival, n= 3), rat MSC‐Tp (14‐day survival, n= 4; 21‐day survival, n= 5; 63‐day survival, n= 5) and human MSC‐Tp (14‐day survival, n= 4; 21‐day survival, n= 6; 63‐day survival, n= 7).
In order to investigate the early inflammation response, three animals with human MSC‐Tp and three animals with injection of the vehicle only were sacrificed at day 5 after transplantation.
Tissue processing
Rats were i.p. anesthetized with Xylazine (10 mg/kg)/Ketamine (75 mg/kg) and transcardially perfused with 0.9% saline, followed by fixation with 4% paraformaldehyde in 0.1 M PBS at pH 7.4. Brains were rapidly removed, immersed in the same fixative for 1 hr at 4°C, and stored in 15% sucrose in PBS for 24 hrs, and then transferred to 30% sucrose in 0.1 M PBS for cryopreservation. Serial coronal sections were cut on a cryostat (Leica, CM 3050) at a thickness of 16 μm, and were mounted on 0.5% gelatin‐coated Superfrost® slides (CML, France), and stored at –80°C until staining.
Investigation of the host inflammatory response
After blocking non‐specific sites with 10% normal goat serum (Sigma), sections were incubated in primary antibodies overnight. Immune cells were labelled with a series of mAbs obtained from the European Collection of Cell Culture (ECCC, Salisbury, UK). ED1 mAbs labelled macrophages and strongly activated microglia as compared to OX42 mAbs which labelled activated microglia. R7.3 mAbs detects the TCR‐αβ chain on T lymphocytes. V65 mAbs stained T γδ‐cells. OX62 mAbs labelled DCs and T γδ‐cells. OX1/OX30 mAbs identified the common leucocyte antigen CD45 (dilution 1:1). NeuN mAbs (Chemicon) and GFAP mAbs (Sigma) were used for detection of neurons and astrocytes, respectively. Details concerning the immunocytochemistry procedure have been published elsewhere [36].
For quantification of the inflammatory response to the transplantation, the immune cells types were counted at three different striatal levels using a Zeiss Axioshop 2 plus microscope at 20× magnification. The first section used was the one presenting the largest area of the transplant and the two others were 144 μm anterior and posterior to it. These three measurements were averaged and the final number was used as an individual value.
Identification of mast cells
Sections were mounted on gelatin‐coated slides and air‐dried for at least 4 hrs, prior to staining. Sections were emerged for 90 sec. in 0.05% aqueous solution of toluidine blue (C.I. 52040, Fluka 89640) acidified with HCL to pH 2.3, rinsed for 15 sec. in acidified distilled water of the same pH, then dehydrated in an ascending series of ethanol. Mast cells are easily differentiated from the pale blue background of the section, due to the purple metachromatic staining of their cytoplasmic granules [37].
Graft area
For each rat, the graft area was measured at the same three levels used for the quantification of inflammatory response in eGFP or Hoechst 33258 stained tissue. The area was quantified using Image J software (National Institutes of Health, Bethesda, MD, USA) and averaged.
Statistical analysis
The graft area was analysed using the Kruskall–Wallis test. When a significant interaction was observed (P < 0.05), the data were further analysed with the Dunn’s Multiple Comparison post hoc test using Prism© software (Prism Software Corporation, Orange County, CA, USA).
Concerning the quantification of the inflammatory response of the brain parenchyma to the MSC‐Tps, a two way anova was carried to evaluate potential differences between MSC types (human versus rat) and time between transplantation and sacrifice (21 versus 63 days after transplantation) in terms of inflammatory marker expression, specifically ED1, OX1‐OX30, OX 42, OX 62 and R7/3 in the event of main effect for cell type. Differences were considered significant when P < 0.05.
Results
Characterization of the human MSCs and rat MSCs
Results from flow cytometry analysis (Fig. 1A) indicated that, after four passages, human and rat MSCs express CD90 (Thy‐1), HLA/MHC class I molecules, but not HLA/MHC class II. Neither cell type expressed CD34 or CD45 antigens, which are specific of haematopoietic precursor cells and haematopoietic cells, respectively. Concerning rat MSCs, no expression of myeloid, neuronal, glial or vascular markers was observed (Fig. 1A). In addition these adult stem cells do not express the nestin antigen, which is used to label neural stem/progenitor cells.
Figure 1.
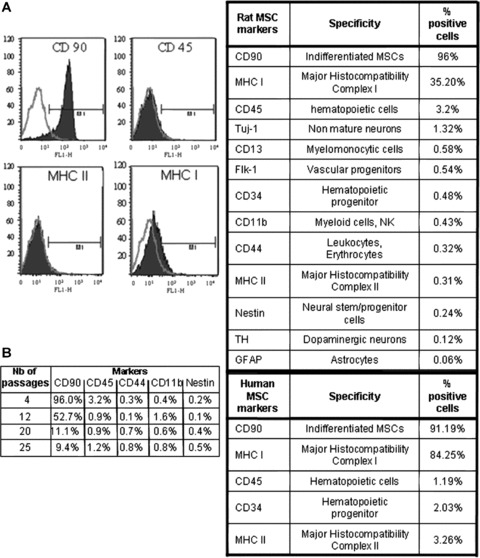
A. FACS analysis of rat and human MSC phenotypes before transplantation. Rat and human MSCs were positive for CD 90 which labels MSCs, and negative for CD34 and CD45 which labels haematopoietic precursors and haematopoietic cells, respectively. Rat MSCs express neither neural phenotypes nor neural stem cell/progenitor nestin antigens following four passages. Note: open green (light) curve = control; closed blue (dark) curve = sampled cells; M1 = region of positive cells. B. FACS analysis of rat MSC phenotype after 4, 12, 20 and 25 passages. Note that there is a strong decrease with passages of the CD90 phenotype. CD45 very low at passage 4 (3.2%) decreases to 1.2% at passage 25 whereas CD44, CD11b and nestin phenotypes remain very low.
The analysis of rat MSC phenotype shows that CD90 phenotype is mainly altered by the passages. FACS analysis after 4, 12, 20 and 25 passages show a very strong decrease with passages of the CD90 phenotype (from 96% to 9.4%). Although weak, the CD45 phenotype also decreases with passages from 3.2% to 1.2%. The three other phenotypes studied (CD44, CD11b and nestin) remain very low and quite stable from passage 4 to passage 25.
Concerning mRNA cytokine expressions, we used PCR for the rat MSCs and TaqMan® PCR analysis for human MSCs. mRNAs for pro‐inflammatory molecules such as IL‐2, IL‐4, IL‐5, IL‐8, IL‐12, IL‐16, IL 17, INF‐γ, TNF‐α, and for anti‐inflammatory molecules, such as IL‐6, IL‐10, IL‐13, TGF‐β1 were investigated.
Results indicated that human MSCs have mRNAs for IL‐6, IL‐8, IL‐12p40 and TNF‐α using the TaqMan® analysis (see Fig. 2A and B for gel representations), whereas rat MSCs express IL‐6, IL‐10, IL‐12 p35 and p40 and TGF‐β1 mRNAs (Fig. 2C and D).
Figure 2.
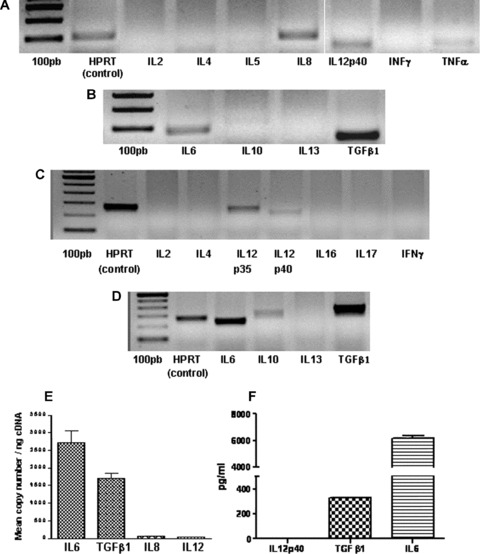
A and B, RT‐PCR analysis of human MSC mRNA, at passage 4, using TaqMan® probes. Pro‐inflammatory molecules tested: IL2, IL4, IL5, IL8, IL12p40, INFγ and TNFα (A); Anti‐inflammatory molecules tested: IL6, IL10, IL13 and TGFβ1 (B). C and D, RT‐PCR analysis of rat MSCs at passage 4 with primers. Pro‐inflammatory molecules tested: IL2, IL4, IL12p35 & p40, IL16, IL17, INFγ (C); Anti‐inflammatory molecules tested: IL6, IL10, IL13 and TGFβ1 (D). E. Gene expression of cytokines in human MSCs. Gene expression absolute quantifications were realized by qPCR for anti‐inflammatory cytokines (IL6, TGFβ1) and pro‐inflammatory cytokines (IL8, IL12) with standards curves (data not shown). Experiments were performed on 3 different samples in duplicates. Human MSCs are characterized by 2717 and 1707 IL6 and TGFβ1 transcripts per ng of cDNA respectively as compared to 54.80 and 42.80 IL8 and IL12 transcripts per ng of cDNA, respectively. There are about a 50‐fold and a 30‐fold more IL6 and TGFβ1 transcripts than IL8 and IL12 transcripts, respectively. F. Supernatants were collected from human MSCs propagated for 4 passages and analysed by ELISA to determine levels of IL‐6, IL12p40 and TGFβ1. Data presented are representative of 2 independent experiments. No pro‐inflammatory IL12p40 presence was detected as compared to the 2 anti‐inflammatory cytokines TGFβ1 and IL6.
In addition to transcript detections, we also quantified the expression of transcripts in human MSCs with qPCR and absolute quantification with standards curves (data not shown) for the two anti‐inflammatory cytokines detected (IL‐6 and TGF‐β1) and for two pro‐inflammatory cytokines (IL‐8 and IL‐12). Our quantification shows that MSCs express more than 50‐ and 30‐fold, the transcripts of IL‐6 and TGF‐β1 than the transcripts of IL‐8 and IL‐12, respectively.
Concerning cytokine levels, the ELISA analysis performed on human MSCs showed no presence of the pro‐inflammatory cytokine IL‐12p40 but the presence of the two anti‐inflammatory cytokines TGF‐β1 and IL‐6 was visualized, with 15‐fold more IL‐6 than TGF‐β1.
The absence of IL‐12p40 cytokine suggests a post‐transcriptional regulation (as IL‐12p40 transcripts were detected) that further experiments could elucidate.
Survival and migration of MSCs in the adult brain
In order to locate the transplanted MSCs in the striatum, these cells were marked with two different reporters prior to transplantation, depending on whether or not they were transgenic. Before transplantation, over 80% of the MSCS from the eGFP rats expressed eGFP (FACS analysis, manuscript in preparation). Using Hoechst labelling, 100% of the both human and wild‐type MSCs were visualized. The survival and localization of transplanted MSCs was initially assessed at 14 days after transplantation. In all rats, the donor cells were identified using fluorescence (Fig. 3A) or using an eGFP‐specific antibody (Fig. 3B). Similar results were observed with Hoechst‐labelled MSCs (Fig. 3C). No evidence migration of either human MSCs or rat MSCs was found. In addition, at 14 days after transplantation, no evidence of glial‐ (using GFAP Abs; Fig. 3D) or neuronal‐ (using NeuN Abs; Fig 3F) phenotype within the transplant was seen. No colocalization of merging Hoechst‐stained MSCs with GFAP‐ (Fig. 3D and E) or NeuN‐ (Fig. 3F and G) labelled cells was observed, implying that none of the transplanted MSCs differentiated into neural cells and that none of the Hoechst dye was transferred from MSCs to host cells. Observation of sections stained for Hoechst at 21 and 63 days after transplantation (Fig. 4A and B) indicate that both rat and human MSCs were still present in the implantation site. The transplant size was not significantly different between the rat MSC‐Tp group and the human MSC‐Tp group at 14 and 63 days after transplantation (Fig. 5A). However, a significant increase in graft size between rat MSC‐Tp and human MSC‐Tp groups was observed at day 21 (Dunn’s test, P < 0.01; Fig. 5A).
Figure 3.
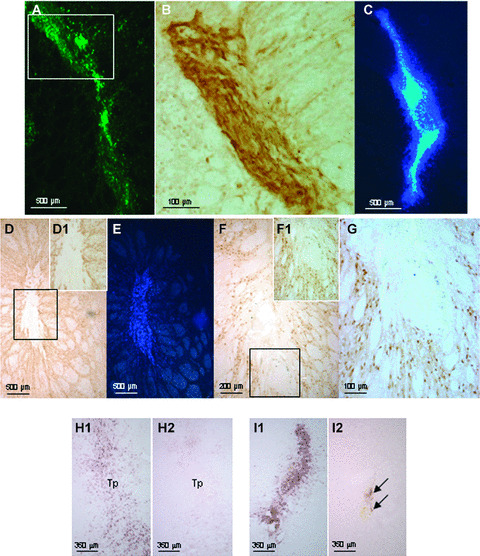
Transplantation of rat eGFP‐labelled MSCs were detected at 14 days under fluorescence (A) and using Abs anti‐eGFP (B, DAB detection) and Hoechst 33258‐labelled‐human MSCs (C). Within the striatum, astrocytes, revealed by anti‐GFAP Abs (D), did not colocalized with the Hoechst 33258‐labelled‐MSCs at 14 days post‐transplantation (E). Also note that the GFAP staining is very weak implying that MSC‐Tp does not trigger a strong astrocytic response. Striatal neurons detected by anti‐NeuN Abs at 14 days post‐transplantation (F) did not colocalized with the Hoechst 33258‐labelled‐MSCs (G). Note: D‐G are pictures taken from the same brain section, with the inserts D1 and F1 at higher magnification within the areas of the black squares in D and F, respectively. Five days after human MSC transplantation (Tp), ED1‐positive cells were visible within the implantation site (H1). Quite similar staining was observed when the vehicle only was injected in the striatum (I1). A slight infiltration of T αβ lymphocytes was observed in the implantation site only after vehicle injection (arrows, H2 as compared to I2). All sections are 144 μm apart).
Figure 4.
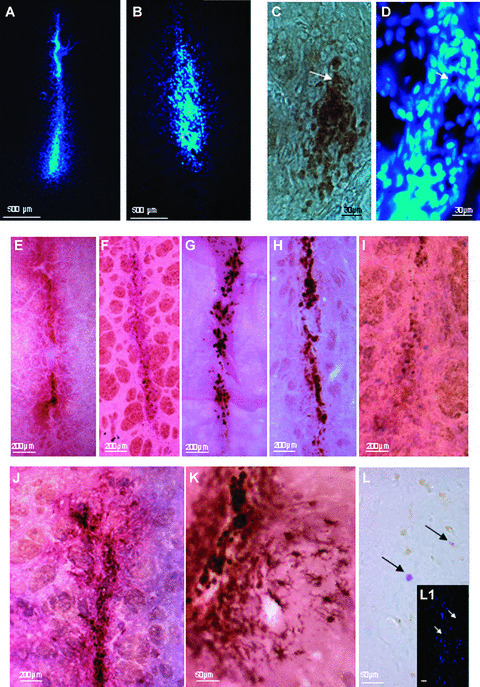
Hoechst 33258‐labelled‐MSCs are detected in rat (A) and human (B) grafts at 63 days post‐ implantation. Macrophage/ strongly activated microglia revealed by ED1 Abs at 63 days post implantation (C) did not colocalize with the Hoechst 33258‐labelled‐MSCs (D). Note: C and D are from the same brain section. Macrophage/ strongly activated immunostaining (ED1 Abs) at 21 days (E) and 63 days (F) after allotransplantation and at 21 days (G) and 63 days (H) after xenotransplantation, revealed macrophage/strongly activated microglia infiltration in the implantation site. Detection of T αβ lymphocytes with R7/3 antibodies at 21 days after human MSC transplantation (I) shows a reduced number of T lymphocytes. In J, activated microglia (OX42 Abs) 21 days after xenotransplantation. Higher magnification of activated microglia 63 days post‐xenotransplantation (K). Very few mast cells were visualized at 21 days after xenotransplantation (L, black arrows pointing at 2 mast cells). Note: L1 is the same section using Hoechst 33258 staining (white arrows pointing at the location of the 2 mast cells in L, but unlike the transplanted MSCs, these cells are not Hoechst 33258‐positive.
Figure 5.
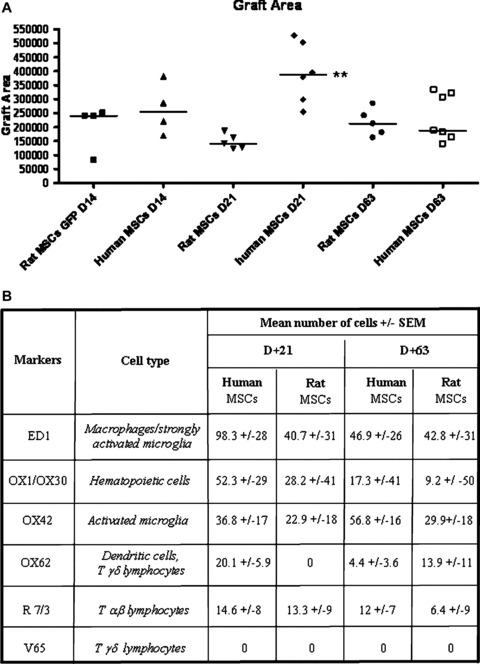
A) Graft areas (in μm2) were quantified for human‐ or rat‐ MSC‐Tp at post‐transplant days 14‐, 21‐ and 63. Tp size remains stable from14 to 63 days. The straight line represents the median, the other dots, individual values. Human MSC‐Tp are significantly larger than rat‐MSC‐Tp at day 21 (**, Dunn’s test, P < 0.01). B) Quantification of the immune cells types in the implantation site at 21‐ (D+21) and 63‐ (D+63) days post‐transplantation. No significant differences were observed between human‐ and rat‐MSC‐Tp conditions suggesting a similar host inflammatory pattern for these 2 types of MSCs within host parenchyma.
Transplantation of MSCs and inflammatory response
Five days after human MSC transplantation, ED1+ cells (i.e. macrophages/strongly activated microglia) were visible within the implantation site (Fig. 3H1). However, quite similar staining was observed when the vehicle only was injected in the striatum (Fig. 3I1). Interestingly, a slight infiltration of T αβ‐lymphocytes was observed in the implantation site only after vehicle injection (Fig. 3, H2 as compared to I2).
At 14 days after transplantation of rat MSCs, ED1+ cells (macrophages/strongly activated microglia) were still detected in the implantation site (not shown). This infiltration remained quite stable up to day 63 days after transplantation (Fig. 4E and F) as no significant difference was observed in the number of ED1+ cells between 21 and 63 days (Fig. 5B). Similar results were obtained after human MSC‐Tps (Figs. 4G and H and 5B). In addition, no significant difference in macrophage/strongly activated microglia infiltration was observed after rat or human MSC transplantation. The presence of activated microglia visualized using OX42 Abs was stable between days 21 and 63 for rat MSC‐Tp group and not significantly different between 21 and 63 days in the case of human MSC transplantation (Figs. 4J and K and 5B). OX1/OX30 mAbs, targeting the common leucocyte antigen CD45, revealed haematopoietic cell infiltration at a level similar in intensity to the macrophage response after allo‐ or xenotransplantation (Fig. 5B). More precisely, very few DCs (none in the case of rat MSC‐Tps at 21 days after transplantation), using combined OX62 and V65 stains, labelling DCs/T γδ‐lymphocytes and T γδ‐lymphocytes, respectively, and T αβ‐lymphocytes (Figs. 4E and 5B) were observed in the grafts at the 21‐ and 63‐day time for either type of Tps (using OX62 and R 7/3 stainings; Fig. 4J and K). Using V65 antibodies, T γδ‐lymphocytes were never found in either rat MSC or human MSC transplantation at 14, 21 and 63 days after transplantation (Fig. 5B). Very few mast cells between 0 and 2 maximum per section, were observed after allotransplantation (data not shown) or xenotransplantation (Fig. 4L). Most of the mast cell were degranulated (granule extrusion consisting in dispersed metachromatic material outside the cells). Mast cells are known to play a role in the inflammatory process [36].
Sections labelled to identify inflammation were observed under both light and fluorescence microscopy in order to visualize Hoechst‐labelled MSCs. This analysis failed to reveal any colocalization between inflammatory makers and the transplanted MSCs (see Fig. 4C and D, using ED1 and Hoechst stainings).
Discussion
In order to study the brain’s inflammatory response following allo‐ or xenotransplantation of MSCs, rat or human MSCs were transplanted into the rat striatum, respectively. Five days after human MSC transplantation or vehicle injection, macrophages/strongly activated microglia were visible within the implantation site. These results suggest that most of this striatal infiltration was induced by the implantation technique we used (i.e. use of a needle and cells suspended in culture medium). Interestingly, the slight infiltration of T αβ‐lymphocytes only observed after vehicle injection suggests that MSCs inhibited/delayed lymphocyte infiltration of the implantation area.
Although an inflammatory response of moderate intensity consisting of macrophages, activated microglia and very few lymphocytes and mast cells, both rat and human MSC‐Tps were able to survive for at least 63 days into the rat striatum. No significant difference in the intensity of the inflammatory response was triggered by the two types of MSCs. The presence of human MSCs at 63 days after implantation is of particular importance as it has been shown that porcine neuroblasts are rejected within 1 month after transplantation into the rat striatum [34]. The analysis of the graft area did not significantly vary between 14 and 63 days after transplantation suggesting that no immune rejection of the grafts had begun. In agreement, the quantification at 21 and 63 days showed that the inflammatory response remained stable. In vitro observations of human and rat MSCs showed that human MSCs have a larger cell body area than rat MSCs which could, therefore, explain the differences in graft size between the two species at day 21 after transplantation.
Allogenic rejection is traditionally mediated by an adaptive cell response that plays a role in the presentation of the antigens by specialized cells and the clonal selection which selectively activates the populations of T lymphocytes [38]. This response uses mainly the T‐cytotoxic lymphocytes [16, 39]. Our results show only limited T‐lymphocyte infiltration at both 21 and 63 days after transplantation of rat as well as human MSCs, implying that the immune response is not due to a cellular type response, but rather, corresponds to an inflammatory reaction. In support of the hypothesis, human and rat MSCs do not express class II MHC excluding them as antigen presenting cells to T CD4+ lymphocytes. We cannot rule out that host antigen‐presenting cells could engulf the transplanted MSCs and would therefore be able to stimulate T CD4+ cells to recognize MSCs. However, as few antigen‐presenting cells and even less T lymphocytes were observed within the implantation site in our investigation, this mechanism must be very weak. In addition, MSCs do not express factors of co‐stimulation like CD40L, CD40 and CD86, which are essential for induction of a immune response and, thus, an effective response of T lymphocytes [26]. However, both human and rat MSCs express class I MHC molecules enabling them to avoid a NK cell response [40]. MSCs also decrease the maturation of DC [21] which plays a key role in the humoral and cellular immune response [41]. It appears that MSCs interfere with the maturation of these cells including the concomitant increase in antigenicity, thus inducing a tolerance and, once again, reducing the cellular response of T cells [42].
The inflammatory response in the brain is mainly coordinated by the macrophages and the activated microglia [43]. Although we mainly observed a macrophage response associated with microglial activation, human and rat MSCs were found in the implantation site of all animals at 63 days after implantation. As such, our observations suggest that MSCs are able to reduce the immune response of the brain that occurs after both allo‐ and xenotransplantation. It has been noticed that MSCs secrete many cytokines which play a very important part in the intensity of the immune response. Our results have shown that rat MSCs have mRNAs, for pro‐inflammatory molecules IL‐12p40, whereas human MSCs express IL‐8‐, IL‐12p40‐, TNF‐α‐mRNAs. However, we showed that mRNAs for anti‐inflammatory molecules (IL‐6, IL 10 and TGF‐β1) are expressed by both MSC types with higher levels of IL‐6‐ and TGF‐β1‐mRNAs (as compared to IL‐8 and IL‐12p40 mRNA pro‐inflammatory cytokine levels). Concerning cytokine levels studied in human MSCs, no pro‐inflammatory IL‐12p40 was detected as compared to the two anti‐inflammatory molecules TGF‐β1 and IL‐6. These later results could, in part, explain the weakening of the host inflammatory response following MSC transplantation. In addition, IL‐10, by inhibiting IL‐12 [44], induces a reduction of T‐cell proliferation and a diminution of the synthesis of the pro‐inflammatory IFN‐γ by T and NK cells. IL‐10 also modulates the production by T cells of pro‐inflammatory molecules, like TNF‐α and IL‐1β[45]. In addition, MSCs produce the hepatocyte growth factor, which contributes to the formation of an immunosuppressive environment [46]. These data emphasize the hypoimmunogenic functions of MSCs and could explain the survival of our MSCs after allo‐ or xenotransplantation.
A recent study by Coyne and colleagues [47] contradicts our results, as well as those in previous findings [12, 13, 14, 29] that demonstrate long‐term survival of transplanted MSCs in brain parenchyma. As in the current study, Coyne et al. used MSCs from eGFP‐transfected rats that were transplanted into the rat striatum, but contrary to our findings, they observed graft rejection as early as 14 days. We used the human phosphoglycerokinase as a promoter, and Coynes et al. used a composite chicken‐actin promoter. Differences in the promoters used to drive the reporter gene might have contributed to the apparent lack of survival reported in the Coynes study where silencing of the promoter might have lead to an apparent loss of gene expression in the transplanted cells thus resulting in an interpretation of cell loss. Another plausible hypothesis to explain the absence of surviving MSCs in Coyne et al.[47] study was that the rat MSCs used in their study underwent 10 to 15 cellular passages whereas the number of MSCs passages used in the current study, and those from other studies showing long‐term viability of transplanted MSCs [12, 13, 14, 29], was approximately four passages. We have shown that rat MSCs can modify their phenotype with passages and therefore their intrinsic properties could vary according to the number of cell passages as suggested by others [48]. We demonstrated that there was a strong decrease in the CD90 phenotype by almost 2‐fold at passage 12 and about 10‐fold after 25 passages. As CD90 is a GlycosylPhosphatidyl Inositol‐anchored membrane glycoprotein of the immunoglobulin superfamily involved in signal transduction, the decrease of CD90 expression could explain, at least in part, the difference in immunogenic properties of MSCs between the present study, which use only 4 passages, and the Coyne et al. study, which used 10+ passages.
Some authors have shown that MSCs have the ability to differentiate into macrophages after intravenous injection [49]. However, using light microscopy with ED1 and fluorescence with Hoechst 33258, we observed no colocalization between ED1+ cells and MSCs, indicating that grafted MSCs did not differentiate into macrophages and that there was no transfer of the cellular staining of Hoechst to the macrophages, nor to any of the host neurons or glial cells. In addition, the transplanted MSCs failed to express neuronal or glial phenotypes.
Mast cells can increase in number rapidly in the adult brain in response to altered physiological conditions and affect brain immunity [50] mostly through the release of serotonin [51]. Serotonin is involved in neuroimmunomodulation processing [52] as it induces the production of chemotactic factors, modulates delayed‐type hypersensitivity responses, activates T and NK cells, and controls the macrophage‐dependant natural immunity [52]. In our study, mast cells were observed very sparsely within the striatum at both 14 and 21 days after transplantation. These data suggest that they were not implicated in the brain inflammatory response following allo‐ or xenotransplantation of rat or human MSCs. However, because no mast cells were seen in control, non transplanted rats (data not shown), we suggested that the few mast cells we did observe were attracted from the blood by trophic factors, such as BDNF and NGF [53], that are known to be released by MSCs [54].
Our findings suggest that transplanted MSCs could induce a positive environment that is beneficial to cell survival within the rat striatum. The MSCs appear to decrease the general activity of the immunogenic cells, such as DCs, macrophages and T lymphocytes, which translates into reducing the probability of cell rejection after transplantation. An intriguing application of these findings may be the use of co‐transplantation of MSCs with other cell types that may be more vulnerable to an immune response. Xenotransplantation of porcine foetal neuroblasts to overcome striatal dopamine or GABA decreases in patients with Parkinson’s or Huntington’s disease, respectively, seems a viable alternative to allotransplantion of human foetal donor tissue, especially because the latter is complicated by both practical and ethical issues [55]. However, it has been shown that xenotransplantation induces a severe immune response leading to the graft rejection [35, 36, 55]. For example, foetal porcine xenoneuroblasts can trigger a severe lymphocyte response [35, 36] which might be mitigated with co‐transplantation of MSCs. Therefore, MSCs, as being hypoimmunogenic, could increase graft survival without the use of immunosupressors when co‐transplanted with pig brain tissue. Further investigations will tell if co‐transplantation of MSCs with xenoneuroblasts have the potential to delay graft rejection. Whether or not this type of application proves effective, our present results indicate that both allo‐ and xeno‐ intrastriatal transplantation of MSCs, when passaged only four times, can survive for at least 63 days, decrease T‐cell infiltration, and prevent or forestall a significant immune response. Although MSCs do not transdifferentiate in neurons or in astrocytes after transplantation in the non‐lesioned rat brain, our data suggest that MSCs can be of great interest in regenerative medicine using a transplantation setting.
Acknowledgements
This work was supported by funding provided by grants from L’Association Huntington France (to L.L.), the Field Neurosciences Institute (to G.L.D.) and La Fondation Progreffe (to INSERM UMR 643).
References
- 1. Pittenger MF, Mackay AM, Beck SC, et al . Multilineage potential of adult human mesenchymal stem cells. Science . 1999; 284: 143–7. [DOI] [PubMed] [Google Scholar]
- 2. Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol . 1976; 4: 267–74. [PubMed] [Google Scholar]
- 3. Tuli R, Tuli S, Nandi S, et al . Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells . 2003; 21: 681–93. [DOI] [PubMed] [Google Scholar]
- 4. Noel D, Djouad F, Jorgense C. Regenerative medicine through mesenchymal stem cells for bone and cartilage repair. Curr Opin Investig Drugs . 2002; 3: 1000–4. [PubMed] [Google Scholar]
- 5. Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res . 2004; 95: 9–20. [DOI] [PubMed] [Google Scholar]
- 6. Dennis JE, Carbillet JP, Caplan AI, et al . The STRO‐1+ marrow cell population is multipotential. Cells Tissues Organs . 2002; 170: 73–82. [DOI] [PubMed] [Google Scholar]
- 7. Makino S, Fukuda K, Miyoshi S, et al . Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest . 1999; 103: 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woodbury D, Schwarz EJ, Prockop DJ, et al . Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res . 2000; 61: 364–70. [DOI] [PubMed] [Google Scholar]
- 9. Sanchez‐Ramos J, Song S, Cardozo‐Pelaez F, et al . Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol . 2000; 164: 247–56. [DOI] [PubMed] [Google Scholar]
- 10. Hung SC, Cheng H, Pan CY, et al . In vitro differentiation of size‐sieved stem cells into electrically active neural cells. Stem Cells . 2002; 20: 522–9. [DOI] [PubMed] [Google Scholar]
- 11. Dunbar GL, Sandstrom MI, Rossignol J, et al . Neurotrophic enhancers as therapy for behavioral deficits in rodent models of Huntington’s disease: use of gangliosides, substituted pyrimidines, and mesenchymal stem cells. Behav Cogn Neurosci Rev . 2006; 5: 63–9. [DOI] [PubMed] [Google Scholar]
- 12. Svendsen C. Adult versus embryonic stem cells: which is the way forward Trends Neurosci. 2000; 23: 450. [DOI] [PubMed] [Google Scholar]
- 13. Lescaudron L, Unni D, Dunbar GL. Autologous adult bone marrow stem cell transplantation in an animal model of huntington’s disease: behavioral and morphological outcomes. Int J Neurosci . 2003; 113: 945–56. [DOI] [PubMed] [Google Scholar]
- 14. Dunbar GL, Oh‐Lee J, Lescaudron L. Use of bone marrow stem cells as therapy for behavioral deficits in rodent models of Huntington’s disease. In: Davis CD and Sanberg PR, editors. Contemporary neuroscience: cell therapy for brain repair. Totowa , NJ : Humana Press Inc.; 2005. pp. 117–138. [Google Scholar]
- 15. Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia . 2001; 36: 118–24. [DOI] [PubMed] [Google Scholar]
- 16. Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx . 2004; 1: 472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Widner H, Brundin P. Immunological aspects of grafting in the mammalian central nervous system. A review and speculative synthesis. Brain Res . 1988; 472: 287–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le Blanc K, Tammik C, Rosendahl K, et al . HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol . 2003; 31: 890–6. [DOI] [PubMed] [Google Scholar]
- 19. Glennie S, Soeiro I, Dyson PJ, et al . Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood . 2005; 105: 2821–7. [DOI] [PubMed] [Google Scholar]
- 20. Spaggiari GM, Capobianco A, Becchetti S, et al . Mesenchymal stem cell‐natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL‐2‐induced NK‐cell proliferation. Blood . 2006; 107: 1484–90. [DOI] [PubMed] [Google Scholar]
- 21. Ramasamy R, Fazekasova H, Lam EW, et al . Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation . 2007; 83: 71–6. [DOI] [PubMed] [Google Scholar]
- 22. Corcione A, Benvenuto F, Ferretti E, et al . Human mesenchymal stem cells modulate B‐cell functions. Blood . 2006; 107: 367–72. [DOI] [PubMed] [Google Scholar]
- 23. Krampera M, Glennie S, Dyson J, et al . Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen‐specific T cells to their cognate peptide. Blood . 2003; 101: 3722–9. [DOI] [PubMed] [Google Scholar]
- 24. Le Blanc K, Rasmusson I, Sundberg B, et al . Treatment of severe acute graft‐versus‐host disease with third party haploidentical mesenchymal stem cells. Lancet . 2004; 363: 1439–41. [DOI] [PubMed] [Google Scholar]
- 25. Beyth S, Borovsky Z, Mevorach D, et al . Human mesenchymal stem cells alter antigen‐presenting cell maturation and induce T‐cell unresponsiveness. Blood . 2005; 105: 2214–9. [DOI] [PubMed] [Google Scholar]
- 26. Majumdar MK, Keane‐Moore M, Buyaner D. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003; 10: 228–41. [DOI] [PubMed] [Google Scholar]
- 27. Tse WT, Pendleton JD, Beyer WM, et al . Suppression of allogeneic T‐cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation . 2003; 75: 389–97. [DOI] [PubMed] [Google Scholar]
- 28. Gray Parkin K, Stephan RP, Apilado RG, et al . Expression of CD28 by bone marrow stromal cells and its involvement in B lymphopoiesis. J Immunol . 2002; 169: 2292–302. [DOI] [PubMed] [Google Scholar]
- 29. Azizi SA, Stokes D, Augelli BJ, et al . Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats–similarities to astrocyte grafts. Proc Natl Acad Sci USA . 1998; 95: 3908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lois C, Hong EJ, Pease S, et al . Germline transmission and tissue‐specific expression of transgenes delivered by lentiviral vectors. Science . 2002; 295: 868–72. [DOI] [PubMed] [Google Scholar]
- 31. Sergent‐Tanguy S, Michel DC, Neveu I, et al . Long‐lasting coexpression of nestin and glial fibrillary acidic protein in primary cultures of astroglial cells with a major participation of nestin(+)/GFAP(−) cells in cell proliferation. J Neurosci Res . 2006; 83: 1515–24. [DOI] [PubMed] [Google Scholar]
- 32. Opal SM, DePalo VA. Anti‐inflammatory cytokines. Chest. 2000; 117; 1162–72. [DOI] [PubMed] [Google Scholar]
- 33. David A, Coupel‐Clauce H, Chetritt J, et al . Anti‐adenovirus immune responses in rats are enhanced by interleukin 4 but not interleukin 10 produced by recombinant adenovirus. Hum Gene Ther . 1998; 9: 1755–68. [DOI] [PubMed] [Google Scholar]
- 34. Lescaudron L, Fulop Z, Sutton RL, et al . Behavioral and morphological consequences of primary astrocytes transplanted into the rat cortex immediately after nucleus basalis ibotenic lesion. Int J Neurosci . 2001; 106: 63–85. [DOI] [PubMed] [Google Scholar]
- 35. Remy S, Canova C, Daguin‐Nerriere V. Different mechanisms mediate the rejection of porcine neurons and endothelial cells transplanted into the rat brain. Xenotransplantation . 2001; 8: 136–48. [DOI] [PubMed] [Google Scholar]
- 36. Michel DC, Nerriere‐Daguin V, Josien R, et al . Dendritic cell recruitment following xenografting of pig fetal mesencephalic cells into the rat brain. Exp Neurol . 2006; 202: 76–84. [DOI] [PubMed] [Google Scholar]
- 37. Dubayle D, Serviere J, Menetrey D. Evidence for serotonin influencing the thalamic infiltration of mast cells in rat. J Neuroimmunol . 2005; 159: 20–30. [DOI] [PubMed] [Google Scholar]
- 38. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood . 2005; 105: 1815–22. [DOI] [PubMed] [Google Scholar]
- 39. Lawrence JM, Morris RJ, Wilson DJ, et al . Mechanisms of allograft rejection in the rat brain. Neuroscience . 1990; 37: 431–62. [DOI] [PubMed] [Google Scholar]
- 40. Ruggeri L, Capanni M, Martelli MF, et al . Cellular therapy: exploiting NK cell alloreactivity in transplantation. Curr Opin Hematol . 2001; 8: 355–9. [DOI] [PubMed] [Google Scholar]
- 41. Guinan EC, Gribben JG, Boussiotis VA, et al . Pivotal role of the B7:CD28 pathway in transplantation tolerance and tumor immunity. Blood . 1994; 84: 3261–82. [PubMed] [Google Scholar]
- 42. Jiang XX, Zhang Y, Liu B, et al . Human mesenchymal stem cells inhibit differentiation and function of monocyte‐derived dendritic cells. Blood . 2005; 105: 4120–6. [DOI] [PubMed] [Google Scholar]
- 43. Perry VH, Bell MD, Brown HC, et al . Inflammation in the nervous system. Curr Opin Neurobiol . 1995; 5: 636–41. [DOI] [PubMed] [Google Scholar]
- 44. Rasmusson I, Ringden O, Sundberg B, et al . Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005; 305: 33–41. [DOI] [PubMed] [Google Scholar]
- 45. Voll RE, Herrmann M, Roth EA, et al . Immunosup‐pressive effects of apoptotic cells. Nature . 1997; 390: 350–1. [DOI] [PubMed] [Google Scholar]
- 46. Kuroiwa T, Kakishita E, Hamano T. Hepatocyte growth factor ameliorates acute graft‐versus‐host disease and promotes hematopoietic function. J Clin Invest . 2001; 107: 1365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coyne TM, Marcus AJ, Woodbury D, et al . Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells . 2006; 24: 2483–92. [DOI] [PubMed] [Google Scholar]
- 48. Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA . 2003; 100: 11917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mezey E, Chandross KJ, Harta G, et al . Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science . 2000; 290: 1779–82. [DOI] [PubMed] [Google Scholar]
- 50. Silverman AJ, 1 Anne K, Sutherland AK, et al . Mast Cells Migrate from Blood to Brain. J Neuroscience , 2000, 20: 401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Menétrey D, Dubayle D. A one‐step dual‐labeling method for antigen detection in mast cells. Histochem Cell Biol . 2003; 120: 435–42. [DOI] [PubMed] [Google Scholar]
- 52. Mössner R, Lesch KP. Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav Immun . 1998; 12: 249–71. [DOI] [PubMed] [Google Scholar]
- 53. Florenzano F, Bentivoglio M. Degranulation, density, and distribution of mast cells in the rat thalamus: a light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J Comp Neurol . 2000; 424: 651–69. [PubMed] [Google Scholar]
- 54. Li Y, Chen J, Chen XG, et al . Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology . 2002; 59: 514–23. [DOI] [PubMed] [Google Scholar]
- 55. Koopmans J, de Haan A, Bruin E, et al . Porcine fetal ventral mesencephalic cells are targets for primed xenoreactive human T cells. Cell Transplant . 2006; 15: 381–7. [DOI] [PubMed] [Google Scholar]


