Abstract
Age‐related osteopenia is characterized by a negative balance between bone resorption and formation. The anti‐osteoporotic drug strontium ranelate was found to reduce bone resorption and to promote bone formation. Here, we investigated the implication of the calcium‐sensing receptor (CaSR) in the response to strontium ranelate using osteoblasts from CaSR knockout [CaSR−/−] and wild‐type [CaSR+/+] mice. We showed that calcium and strontium ranelates increased cell replication in [CaSR−/−] and [CaSR+/+] osteoblasts. Strontium ranelate rapidly increased ERK1/2 phosphorylation in [CaSR+/+] but not in [CaSR−/−] osteoblasts, indicating that strontium ranelate can act independent of the CaSR/ERK1/2 cascade to promote osteoblast replication. We also showed that strontium ranelate prevented cell apoptosis induced by serum deprivation or the pro‐inflammatory cytokines IL‐1β and TNF‐α in [CaSR−/−] and [CaSR+/+] osteoblasts, indicating that CaSR is not the only receptor involved in the protective effect of strontium ranelate on osteoblast apoptosis. Strontium ranelate activated the Akt pro‐survival pathway in [CaSR−/−] and [CaSR+/+] osteoblasts, and pharmacological inhibition of Akt abrogated the anti‐apoptotic effect of strontium ranelate. Furthermore, both the proliferative and anti‐apoptotic effects of strontium ranelate in [CaSR−/−] and [CaSR+/+] osteoblasts were abrogated by selective inhibition of COX‐2. The results provide genetic and biochemical evidence that the effects of strontium ranelate on osteoblast replication and survival involve ERK1/2 and Akt signalling and PGE2 production, independent of CaSR expression. The finding that CaSR‐dependent and CaSR‐independent pathways mediate the beneficial effects of strontium ranelate on osteoblasts, provides novel insight into the mechanism of action of this anti‐osteoporotic agent on osteoblastogenesis.
Keywords: strontium ranelate, osteoblasts, ERK1/2, Akt, calcium sensing receptor, replication, apoptosis
Introduction
Bone loss associated with aging, oestrogen deficiency, immobilization or glucocorticoid treatment is characterized by insufficient bone formation relative to resorption, resulting in negative bone balance, alterations of bone mass and increased incidence of fractures [1, 2]. The available anti‐osteoporotic drugs act by decreasing bone resorption or promoting bone formation, thereby resulting in increased bone mass and reduced fracture incidence in patients with osteoporosis [2]. The di‐strontium salt strontium ranelate is a new anti‐osteoporotic drug. In postmenopausal osteoporotic women, strontium ranelate (2 g/day) was found to reduce the risk of vertebral fracture, non‐vertebral fracture and hip fracture over 3 years [3, 4]. Strontium ranelate was reported to act by inhibiting bone resorption and promoting bone formation [5], thereby inducing a positive bone balance in experimental osteopenic models [6, 7] and in patients with postmenopausal osteoporosis [3, 4, 5, 8]. Previous studies indicate that strontium ranelate may act on osteoblasts to promote cell activity and differentiation [9, 10, 11, 12]. Notably, others and we showed that strontium ranelate can activate cell replication in rodent calvaria osteoblasts, resulting in stimulation of collagen synthesis [9, 13]. However, the cellular mechanisms involved in the effects of strontium ranelate in osteoblasts are not fully understood. One attractive hypothesis is that strontium may act in part by activating the seven‐transmembrane‐spanning extracellular calcium‐sensing receptor (CaSR), a member of the G‐protein coupled receptor family that responds to extracellular calcium, and other cations with different affinity [14]. The CaSR plays a non‐redundant role in the functions of the parathyroid gland and the kidney [15, 16]. The parathyroid CaSR homologue is expressed in bone cells [17, 18, 19] and thereby may play a role in osteoblasts [20, 21, 22]. In various cell types, activation of the CaSR by calcium activates the downstream ERK1/2 signalling pathway, leading to increased cell replication [23, 24]. This raises the possibility that strontium, an agonist of CaSR [25, 26], may enhance osteoblast replication through activation of CaSR and downstream ERK1/2 signalling pathway. Osteoblast survival is another important mechanism that controls osteoblastogenesis and bone formation [27, 28, 29]. Notably, increased osteoblast apoptosis has been implicated in age‐related and glucocorticoid‐induced osteoporosis [30]. Consistently, effective anti‐osteoporotic agents such as oestrogens and parathyroid hormone were found to decrease osteoblast apoptosis [31, 32]. In contrast, inflammatory cytokines such as IL‐1β and TNF‐α were found to induce osteoblast apoptosis [27, 33, 34], which may contribute to reduce the number of osteoblasts in postmenopausal osteoporosis [30].
In this study, we tested the hypothesis that strontium ranelate may act on osteoblast replication and survival through activation of the CaSR and determined the downstream signalling pathways in primary osteoblastic cells. To this goal, we investigated the implication of CaSR in the osteoblast response to strontium ranelate by a genetic approach, taking advantage of primary calvarial cells isolated from [CaSR+/+] and [CaSR−/−] mice.
Materials and methods
Animals
[CaSR+/+] and [CaSR−/−] mice were obtained from Drs. C. Seidman and J. Seidman [35]. Mice were generated by disrupting the murine CasR gene performed with homologous recombination, leading to a deletion of 20 base pairs following the insertion of a cassette containing the neomycine resistance gene in exon 4 [35]. Male and female heterozygous mice were used as the parental generation to obtain homozygous mice (CNRS facilities; Gif Sur Yvette, France). Mice were maintained in accordance with European guidelines. Because [CaSR−/−] mice are indistinguishable from littermates at birth, genotype determination was performed within 24 hrs after birth on DNA extracted from a tail sample. [CaSR+/+] and [CaSR−/−] newborn mice were then selected for isolation of calvarial osteoblasts.
Cell cultures and treatments
Calvaria were dissected from [CaSR+/+] and [CaSR−/−] mice as previously described [13]. Briefly, the brain was detached from the skull, the skin and periosteum were removed and the calvaria was dissected in small fragments prior to sequential digestions consisting five successive 20 min. collagenase digestions at 37°C. The pool of cells obtained from the first and second sequential type I collagenase digestions, enriched in pre‐osteoblastic cells, was used for the determination of DNA synthesis. The pool of the three following digestions consisted of osteoblastic cells, as assessed by alkaline phosphatase activity.
Strontium ranelate, as a mixture of strontium chloride (SrCl2. 6H2O) (Sigma, St Louis, MI, USA) and sodium ranelate (Technologie Servier, Orléans, France), was added to the culture medium at a molar ratio of 100 : 1, respectively, in order to reflect the ratio found in blood of postmenopausal osteoporotic patients treated with strontium ranelate [3, 4]. Strontium ranelate concentrations were expressed as Sr2+ concentrations. Type I collagenase, calcium chloride, wortmanin – a pharmacological inhibitor of PI3K signaling, rabbit polyclonal antibody raised against β‐actin and ‘NS‐398 – a selective inhibitor of COX‐2’, were purchased from Sigma. IL‐1β and TNF‐α were from Peprotech (Neuilly sur Seine, France). The mouse monoclonal antibody raised against GAPDH was from Abcam (Cambridge, UK). The rabbit polyclonal raised anti‐CaSR was from Affinity BioReagents (Golden, CO, USA). The rabbit polyclonal antibody raised against phospho‐Akt (p‐Akt) was from Cell Signalling (Ozyme, Saint Quentin Yvelines, France). The mouse monoclonal antibody raised against phospho‐ERK1/2 (p‐ERK1/2) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell replication analysis
Osteoblastic cells isolated from [CaSR+/+] and [CaSR−/−] mice were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 5% fetal calf serum (FCS). Pre‐confluent cells were treated with strontium ranelate at doses that promote osteoblastic cell replication [13], calcium chloride or their solvent (control). Time‐course studies were conducted at 24 and 48 hrs of treatment. In some experiments, the cells were treated with NS‐398 (0.1 μM) or the solvent (ethanol, <0.1%). Cell replication was measured using the BrdU ELISA assay (Roche, France) according to the manufacturer’s instructions. The experiments were repeated three to six times with at least six replicates per experiment.
Western blot and immunoprecipitation analyses
Osteoblastic cells isolated from [CaSR+/+] and [CaSR−/−] mice were seeded in DMEM supplemented with 10% FCS at a density of 30,000 cells/cm2. The day after, cells were washed twice with serum‐free medium, then incubated for further 24 hrs in 0.5% FCS containing medium, before treatment with or without calcium chloride or strontium ranelate at the indicated doses. Cell cultures were stopped after 10 or 30 min. incubation by snap freezing. Cells were lysed in 50 mM Tris/HCl pH 7.4, 150 mM NaCl, 10 mM MgCl2, 10% glycerol, 10 mM Na3VO4, 1% NP‐40, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin for 30 min. on ice, then scrapped and centrifuged for 10 min. at 12,000g at 4°C to remove insoluble materials. Protein concentrations in the supernatants were measured using the DC Protein assay (Bio‐Rad Laboratories, Marne La Coquette, France). Equal aliquots (50 μ g)) of cell lysates were resolved on 12% SDS‐PAGE and electrotransferred onto PVDF nitrocellulose membranes (Millipore Corporation, Bedford, USA). Filters were incubated at RT for 2 hrs in Tris‐Buffered Saline [50 mM Tris/HCl pH 7.4, 150 mM NaCl] containing 0.1% Tween‐20 and 0.5% bovine serum albumin (TBST/BSA), then overnight at 4°C on a shaker with specific primary antibodies in TBST/BSA (p‐ERK1/2, 1/1000; p‐Akt, 1/1000; or β‐actin, 1/2000). Following incubation with corresponding HRP‐conjugated secondary antibody (1/20,000 in TBST/BSA) and final washes, the signals were visualized with enhanced chemiluminescence Western blotting detection reagent (ECL, Amersham Biosciences, Piscataway, NJ, USA) and autoradiographic film (X‐OMAT‐AR, Eastman Kodak Company, Rochester, NY, USA). Densitometric analysis using ImageQuant software was performed following digital scanning (Agfa). Representative images of immunoblots are shown. Relative levels are expressed as treated over control ratio, after correction to the housekeeping protein and are representative of two to three independent experiments.
For analysis of the CaSR expression, immunoprecipitation analysis was performed with micro‐MACS protein A/G microbeads magnetic separation (Miltenyi Biotech Auburn CA, USA) according to manufacturer recommendations. Briefly, 300 μg of total protein were incubated for 30 min. on ice with 2 μg of the indicated antibody and 20 μl of protein A/G magnetically labelled. The magnetically labelled immune complex was passed over a microcolumn placed in a magnetic field. The complex bound was washed with lysis buffer, and immunoprecipitated protein was eluted from the column with SDS gel loading buffer ready for Western blot assay.
Cell apoptosis analysis
Cells were plated at a density of 30,000 cells/cm2 in DMEM supplemented with 5% FCS. In a first series of experiments, cells were incubated in DMEM supplemented with 5% FCS (control condition) or 1% FCS (apoptosis induction) for 24 hrs in the presence or absence of strontium ranelate at the indicated concentration. In a second series of experiments, cells were incubated in DMEM supplemented with 5% FCS and treated for 24 hrs with or without IL‐1β or TNF‐α at effective doses [33] in the presence or absence of strontium ranelate (5 mM). In some experiments, the cells were treated with NS‐398 (0.1 μM) or the solvent (ethanol, <0.1%). Cell apoptosis was evaluated using the Apopercentage assay kit (Biocolor), based on the alteration of cellular membrane induced in the early stage of apoptosis. Dye absorbance was read at 550 nm. The experiments were repeated three to six times with at least six replicates per experiment.
Caspase activity
To identify the signalling pathways involved in the response to strontium ranelate, cells were cultured in 1% FCS to induce apoptosis and treated with strontium ranelate (3 mM) or calcium chloride (3 mM) in the presence or absence of the specific PI3K inhibitor wortmanin (10 mM) for 24 hrs, and caspases 3, 6, 7 activity was determined as described [36]. Briefly, cellular extracts (50 μg) were incubated with 0.2 mM of Ac‐DEVD‐pNA (caspases‐3, ‐6, ‐7; Alexis Biochemicals, CA, USA) as substrate for various times at 37°C, in the presence or the absence of the specific caspase inhibitor Ac‐DEVD‐CHO (10 μM). The specific activity (nmol pNA/min/mg protein) was expressed as treated over control ratios. The experiments were repeated three times with two replicates per experiment.
To identify the signalling pathways involved in the response to strontium ranelate, cells were cultured in 1% FCS to induce apoptosis and treated with strontium ranelate (3 mM) or calcium (3 mM) in the presence or absence of the specific PI3K inhibitor wortmanin (10 mM) for 24 hrs, and caspases 3, 6, 7 activity was determined as described [36]. The experiments were repeated three times with at least six replicates per experiment.
Statistical analyses
All values are presented as the mean ± standard deviation (SD). Data were analysed with the unpaired two‐tailed Student’s t‐test as appropriate for the data set. A P value <0.05 was considered statistically significant.
Results
CaSR expression and mitogenic effect of strontium ranelate
We first checked the expression of CaSR in osteoblastic cells from [CaSR+/+] and [CaSR−/−] mice. As shown in Figure 1A, immunoprecipitation analysis showed that a protein with the expected size reacted with the CaSR antibody in [CaSR+/+] calvarial osteoblasts. In contrast, no detectable CaSR was found in [CaSR−/−] calvaria osteoblasts (Fig. 1A). In addition, to provide evidence for CaSR expression in murine primary calvaria osteoblasts, this validates the use of [CaSR−/−] osteoblasts to test the implication of the CaSR in the response to strontium ranelate. We then analysed whether calcium and strontium ranelate may induce osteoblastic cell replication via the CaSR. As shown in Figure 1B, calcium (1–3 mM) increased DNA synthesis in osteoblasts from [CaSR+/+] mice, as assessed by the BrdU assay. Similar effects were observed in osteoblasts from [CaSR−/−] mice (Fig. 1C). Interestingly, strontium ranelate (1–3 mM) also increased cell replication in osteoblasts from [CaSR+/+] and [CaSR−/−] mice (Fig. 1B and C). The positive effect of strontium ranelate on DNA synthesis was confirmed using tritiated thymidine incorporation into DNA (data not shown). This indicates that calcium and strontium ranelates enhance cell replication independent of CaSR expression in primary murine calvaria osteoblastic cells. To further validate these results, we analysed the kinetics of response to calcium and strontium ranelates in osteoblasts isolated from [CaSR+/+] and [CaSR−/−] mice. Calcium (3 mM) increased cell replication at 24 and 48 hrs in [CaSR+/+] osteoblasts (Fig. 1D), as well as in [CaSR−/−] osteoblasts (Fig. 1E), confirming that calcium can increase osteoblast replication independent of CaSR. Similarly, strontium ranelate (3 mM) increased cell replication at 24 and 48 hrs in osteoblasts isolated from [CaSR+/+] and [CaSR−/−] mice (Fig. 1D and E). These results provide genetic evidence that strontium ranelate can promote osteoblast replication independent of CaSR expression.
Figure 1.
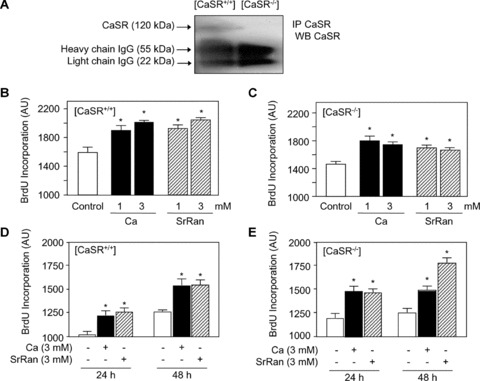
Calcium chloride and strontium ranelate (SrRan) stimulate osteoblast replication independent of CaSR expression. (A) Immunoprecipitation analysis showing expression of CaSR in [CaSR+/+] but not [CaSR−/−] osteoblasts isolated from newborn mice. Protein lysates were immunoprecipitated with anti‐CaSR, electrophoresed, reacted with CaSR antibody and immunoblots were probed with a peroxidase‐coupled specific secondary antibody. (B, C) Osteoblasts from [CaSR+/+] and [CaSR−/−] mice were treated with calcium chloride or SrRan at the indicated concentration for 24 hrs. (D, E) Time‐dependent increase in cell replication in [CaSR+/+] and [CaSR−/−] osteoblasts. After 24–48 hrs of incubation with calcium or SrRan, cells were pulsed with BrdU and processed by ELISA to determine BrdU incorporation. Results are expressed as mean ± SD (n= 6). * indicates a significant difference with untreated controls (P < 0.05).
ERK1/2 activation
In non‐skeletal cells, CaSR activation by calcium is known to increase ERK1/2 phosphorylation [23, 24]. Activation of ERK1/2‐MAPKs mediates cell replication in various cell types, including osteoblasts [37, 38]. We determined whether strontium ranelate may activate osteoblast replication by activating CaSR and downstream ERK1/2 pathway. To test this hypothesis, osteoblastic cells isolated from [CaSR+/+] and [CaSR−/−] mice were treated for 10 min. with strontium ranelate, and ERK1/2 phosphorylation was monitored. As shown in Figure 2A, strontium ranelate rapidly induced a dose‐dependent increase in ERK1/2 phosphorylation level in osteoblasts from [CaSR+/+] mice. Strontium ranelate increased phospho‐ERK1/2 by two‐fold in [CaSR−/−] osteoblasts, with a maximal effect observed at 5 mM (Fig. 2A). This effect was nearly similar to the effect of calcium (Fig. 2A). In marked contrast, we found that strontium ranelate, even at maximal concentration (10 mM), failed to increase ERK1/2 phosphorylation in [CaSR−/−] osteoblasts, whereas calcium had a positive effect (Fig. 2B). To discard a possible difference in time course of ERK1/2 phosphorylation in [CaSR+/+] and [CaSR−/−] osteoblasts, we performed a kinetic analysis. As shown in Figure 2C, the increased p‐ERK1/2 levels induced by strontium ranelate at 10 min. was maintained at 30 min. in [CaSR+/+] osteoblasts. In contrast, strontium ranelate had no effect on p‐ERK1/2 levels even after 30 min. of treatment in [CaSR−/−] mice (Fig. 2D). These results indicate that strontium ranelate can rapidly activate ERK1/2 phosphorylation in [CaSR+/+] osteoblasts but is ineffective in inducing ERK1/2 phosphorylation in [CaSR−/−] murine osteoblasts within this time frame.
Figure 2.
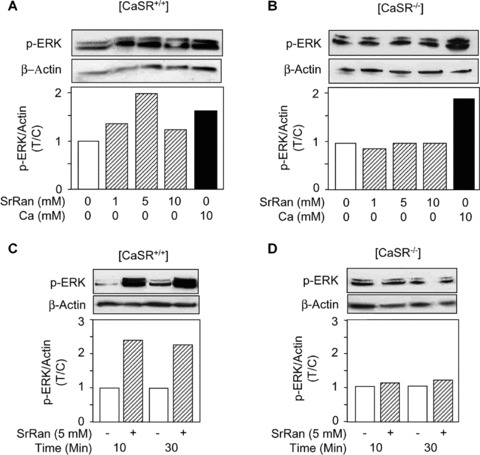
Strontium ranelate (SrRan) rapidly induces ERK1/2 phosphorylation in [CaSR+/+] but not [CaSR−/−] osteoblasts. (A, B) Osteoblasts from [CaSR+/+] and [CaSR−/−] mice were treated with SrRan or ‘calcium chloride’ at the indicated concentrations for 10 min., and ERK1/2 phosphorylation was determined by Western blot analysis. (C, D) Time‐course effect of SrRan on ERK1/2 phosphorylation in osteoblasts from [CaSR+/+] and [CaSR−/−] mice. The gels were scanned and the levels were corrected for loading using β‐actin. The data are expressed as treated over control ratio (T/C).
Cell apoptosis
We then analysed whether strontium ranelate may modulate osteoblast apoptosis through the CaSR. As shown in Figure 3A, serum deprivation (1% FCS) increased osteoblast apoptosis in [CaSR+/+] and [CaSR−/−] osteoblasts, as evaluated by the Apopercentage assay. Treatment with strontium ranelate (5–10 mM) reduced serum deprivation‐induced apoptosis in [CaSR+/+] and [CaSR−/−] osteoblasts (Fig. 3A and B). These results indicate that strontium ranelate can reduce osteoblast apoptosis induced by growth factor deprivation, independent of CaSR expression.
Figure 3.
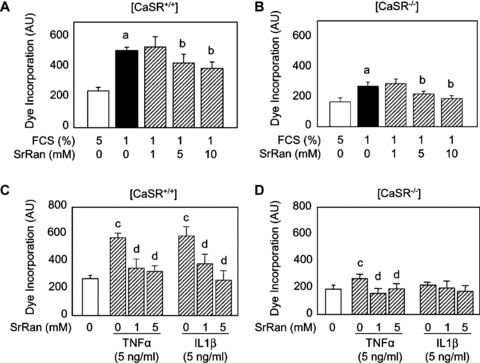
Strontium ranelate (SrRan) protects against apoptosis in osteoblasts. (A, B) Osteoblasts from [CaSR+/+] and [CaSR−/−] mice were cultured in DMEM supplemented with 5% foetal calf serum (FCS) or FCS‐deprived (1% FCS) medium and treated with SrRan at the indicated concentration for 24 hrs. (C, D) Osteoblasts from [CaSR+/+] and [CaSR−/−] mice cultured were treated with TNF‐α or IL‐1‐β (5 ng/ml) in the presence of SrRan at the indicated concentrations for 24 hrs. Cell apoptosis was evaluated by the Apopercentage assay. Results are expressed as mean ± SD (n= 6). a and b indicate a significant difference with the corresponding 5% and 1% FCS control groups, respectively. c and d indicate a significant difference with the corresponding untreated control groups, respectively (P < 0.05).
To further delineate the effect of strontium ranelate on osteoblast apoptosis, we induced apoptosis using inflammatory cytokines used at doses that promote osteoblast apoptosis [33]. As shown in Figure 3C and D, treatment with TN F‐α (5 ng/ml) induced apoptosis in [CaSR+/+] and [CaSR−/−] osteoblasts, although the effect was lower in the later cells. Treatment with IL‐1β (5 ng/ml) also increased osteoblast apoptosis in [CaSR+/+] osteoblasts (Fig. 3C). The addition of strontium ranelate (1–5 mM) abolished TNF‐α‐induced osteoblast apoptosis in [CaSR+/+] and [CaSR−/−] osteoblasts (Fig. 3C and D). Strontium ranelate (1–5 mM) also abolished IL‐1β‐induced apoptosis in [CaSR+/+] osteoblasts (Fig. 3D). These results indicate that strontium ranelate can protect against osteoblast apoptosis induced by these pro‐inflammatory cytokines.
Akt signalling
Others and we have previously shown that Akt is an important signalling pathway controlling osteoblast survival in vitro[39, 40] and in vivo[41, 42]. Here, we determined the implication of this important signalling pathway in the anti‐apoptotic effect of strontium ranelate in primary osteoblasts. Western blot analysis showed that strontium ranelate (1–10 mM) rapidly increased p‐Akt levels in [CaSR+/+] and [CaSR−/−] osteoblasts (Fig. 4A and B). This effect was sustained in [CaSR+/+] osteoblasts, as it was also observed at 30 min. of treatment (Fig. 4 C and D). These results indicate that strontium ranelate activates the pro‐survival pathway Akt in [CaSR+/+] and [CaSR−/−] osteoblasts.
Figure 4.
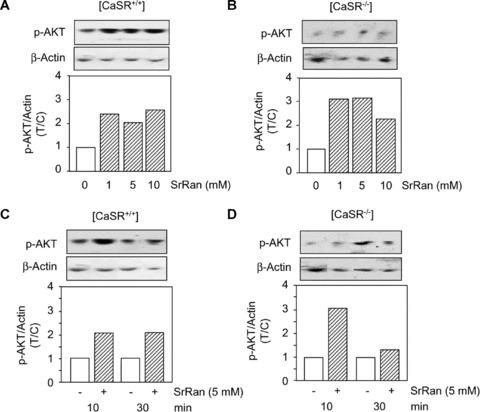
Strontium ranelate (SrRan) activates Akt signalling in osteoblasts. Osteoblasts from [CaSR+/+] and [CaSR−/−] mice were cultured in DMEM supplemented with 5% FCS for 24 hrs, then treated with or without increasing doses of SrRan at the indicated concentrations for 10 (A, B) or 30 min. (C, D). Phosphorylation level of Akt was evaluated by Western blot analysis, relative quantification was performed after correction for β‐actin content, and the results expressed as treated over control ratio (T/C).
Effector caspases activity
To further determine the role of strontium ranelate and CaSR in osteoblast survival, we determined the activity of effector caspases, which act downstream of signalling pathways to activate the apoptotic process [43]. As shown in Figure 5, serum deprivation (1% FCS) increased caspases 3, 6, 7 activity in both [CaSR+/+] and [CaSR−/−] osteoblasts compared with basal conditions (10% FCS). Treatment with calcium or strontium ranelate (3 mM) prevented in part the inducing effect of serum deprivation on effector caspases activity (Fig. 5A and B). These results indicate that strontium ranelate reduces osteoblast apoptosis induced by serum deprivation by reducing effector caspases activity, independent of CaSR expression.
Figure 5.
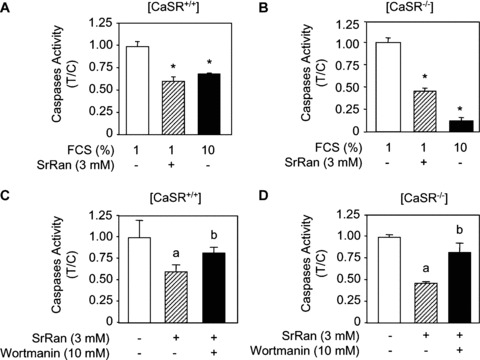
The anti‐apoptotic effect of strontium ranelate (SrRan) is mediated by PI3K Akt signalling in osteoblasts. (A, B) Osteoblasts from [CaSR+/+] and [CaSR−/−] mice were cultured in DMEM supplemented with 10% FCS or 1% FCS for 24 hrs and treated with or without SrRan for 24 hrs. Effector caspases activity was determined and the results are expressed as treated over control ratio (T/C) (mean ± SD). * indicates a significant difference with the 1% FCS control group (P<0.05). (C, D) Osteoblasts from [CaSR+/+] and [CaSR−/−] mice were cultured in DMEM supplemented with 1% FCS, for 24 hrs, treated with or without SrRan (3 mM) for 24 hrs in the presence or absence of the PI3K inhibitor wormanin (10 mM), and effector caspases activity was determined. Results are expressed as mean ± SD (n= 6). a and b indicate a significant difference with the 1% FCS and SrRan groups, respectively (P < 0.05).
The data shown above indicate that strontium ranelate can activate Akt, a major signalling pathway controlling osteoblast survival. To determine whether the pro‐survival effect of strontium ranelate may be mediated by PI3K Akt, [CaSR+/+] and [CaSR−/−] osteoblasts were treated with wortmanin, a specific PI3K pharmacological inhibitor. As shown in Figure 5C and D, strontium ranelate (3 mM) reduced effector caspases activity induced by serum deprivation in both [CaSR+/+] and [CaSR−/−] osteoblasts. This effect was partly prevented by wortmanin (Fig. 5 C and D). These results indicate that the anti‐apoptotic effect of strontium ranelate in primary osteoblasts is mediated, at least in part, by the pro‐survival PI3K Akt signalling pathway, and that this protecting effect can occur independent of CaSR expression.
Implication of PGE2
Previous studies have shown that prostaglandins can stimulate bone formation [54, 55]. Interestingly, strontium was found to induce COX‐2 expression and prostaglandin E2 (PGE2) production in murine calvarial osteoblasts through activation of ERK signalling [56]. Further studies showed that PGE2 is involved in strontium ranelate‐induced osteoblast differentiation of bone marrow stromal cells [57]. Here we investigated whether the mitogenic and anti‐apoptotic effects of strontium ranelate may be dependent on PGE2 production in [CaSR+/+] and [CaSR−/−] osteoblasts. To test this hypothesis, [CaSR+/+] and [CaSR−/−] osteoblasts were treated with NS‐398, a selective inhibitor of COX‐2, at a dose (0.1 μM) that was shown to abrogate PGE2 production [56]. As shown in Figure 6A and B, strontium ranelate increased cell proliferation in both [CaSR+/+] and [CaSR−/−] osteoblasts, and this effect was abrogaded by NS‐398. We also examined the role of PGE2 in the anti‐apoptotic effect of strontium ranelate in [CaSR+/+] and [CaSR−/−] osteoblasts. As shown in Figure 6 C and D, strontium ranelate reduced apoptosis induced by serum deprivation in both [CaSR+/+] and [CaSR−/−] osteoblasts, and this effect was prevented by the COX‐2 inhibitor NS‐398. These results suggest that the effects of strontium ranelate on cell replication and apoptosis are mediated, at least in part, by PGE2 production.
Figure 6.
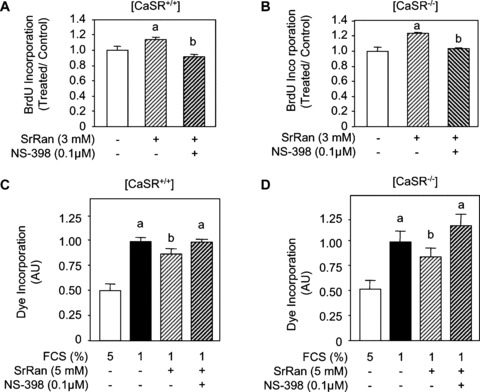
The effects of strontium ranelate (SrRan) in osteoblasts involve prostaglandins. (A, B) Osteoblasts from [CaSR+/+] and [CaSR−/−] mice were treated with SrRan (3 mM) for 24 hrs in the presence of the COX‐2 inhibitor NS‐398 (0.1 μM) or the solvent, cells were pulsed with BrdU and processed by ELISA to determine BrdU incorporation. Results are expressed as treated over control ratio (T/C) (mean ± SD; n= 6). a and b indicate a significant difference with untreated and SrRan‐treated cells, respectively (P < 0.05). (C, D) Osteoblasts from [CaSR+/+] and [CaSR−/−] mice were cultured in DMEM supplemented with 5% FCS (control) or 1% FCS for 24 hrs and treated with SrRan (3 mM) for 24 hrs in the presence of the COX‐2 inhibitor NS‐398 (0.1 μM) or the solvent, and cell apoptosis was evaluated by the Apopercentage assay. Results are expressed as treated over control ratio (T/C) (mean ± SD; n= 6). a, b and c indicate a significant difference with the 10% FCS, 1% FCS and SrRan‐treated groups, respectively (P < 0.05).
Overall, the present results indicate that strontium ranelate activates osteoblastic cell replication through CasR‐mediated ERK1/2 signalling and another unidentified cation‐sensing receptor. In addition, strontium ranelate induces anti‐apoptotic effects in osteoblasts through CaSR‐dependent and CaSR‐independent mechanisms involving the PI3K Akt pro‐survival signalling pathway (Fig. 7). Furthermore, the effects of strontium ranelate on osteoblast replication and survival appear to be dependent, at least in part, on prostaglandin production.
Figure 7.
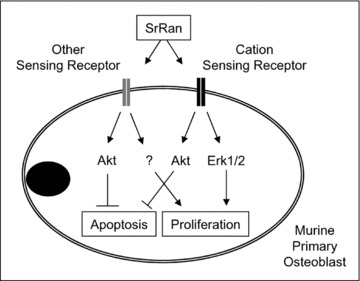
Proposed mechanisms by which strontium ranelate (SrRan) controls cell replication and protects against apoptosis in murine primary osteoblasts. SrRan activates cell replication in part through CasR‐mediated ERK1/2 signalling and another unidentified cation‐sensing receptor. In addition, SrRan induces anti‐apoptotic effects in murine osteoblasts through CaSR‐dependent and CaSR‐independent mechanisms involving the PI3K Akt pro‐survival pathway.
Discussion
The main findings of this study are that strontium ranelate activates osteoblast replication and exerts protective effects on osteoblast apoptosis via CaSR‐dependent‐ and CaSR‐independent mechanisms. Previous in vitro studies indicate that the CaSR can mediate mitogenic effects in primary osteoblasts [24]. However, we found that calcium at millimolar concentrations increased cell replication in primary osteoblasts from both [CaSR+/+] and [CaSR−/−] mice, indicating that calcium may promote cell replication independent of CaSR expression in primary osteoblasts. An important issue was then to determine the response to strontium in relation with CaSR in osteoblasts. We found that strontium ranelate activated cell replication in murine [CaSR+/+] osteoblasts, which supports the recent observation that the CaSR could mediate, in part, strontium ranelate‐induced cell replication in rat primary osteoblasts [44]. However, our data indicate that strontium ranelate can also promote osteoblast replication in the absence of CaSR, which provides genetic evidence that CaSR is not essential for the stimulatory effect of strontium ranelate on osteoblast cell replication. It can be argued that the effective strontium ranelate concentration used (1 mM) was higher than the actual circulating strontium concentrations in osteoporotic patients treated with strontium ranelate (0.12 mM) [3, 4]. However, as high concentrations of calcium (8–40 mM) have been found in the vicinity of bone resorbing cells [45], it is likely that high concentrations of strontium, a bone seeking agent that is mostly adsorbed at the calcium hydroxyapatite surface [46], are present in the local bone environment.
Another important issue was to determine the role of CaSR downstream signalling pathway involved in strontium ranelate‐induced replication in murine osteoblasts. Activation of ERK1/2 is known to play a major role in the induction of osteoblast replication [38]. Notably, ERK1/2‐MAPKs have been proposed to be implicated in the high calcium‐induced replication in fibroblasts and human osteoblastic cells [21, 22]. We showed here that strontium ranelate rapidly increased ERK1/2 phosphorylation in [CaSR+/+] osteoblasts, indicating that CaSR may be responsible, at least in part, for ERK1/2 activation by strontium ranelate in these cells. However, our finding that strontium ranelate increased cell replication despite failure to rapidly activate ERK1/2 in [CaSR−/−] osteoblasts strongly indicates that strontium ranelate can induce osteoblast replication independent of CaSR‐mediated ERK1/2 signalling. These results support the hypothesis that a functional receptor distinct from the parathyroid cell CaSR homologue could mediate the effect of strontium ranelate on cell replication in osteoblasts. We therefore propose that strontium ranelate can act, in addition to CaSR, through a molecular signal distinct from CaSR‐mediated ERK1/2 to activate pre‐osteoblast replication (Fig. 7). In support of this concept, recent data indicate that the response to cations in osteoblasts may be mediated by CaSR, as well as other cation‐sensitive receptors [47, 48, 49]. For example, very high doses of strontium (40 mM) were recently reported to activate ERK1/2 signalling in non‐skeletal (HEK293) cells transfected with GPRC6A, an extracellular cation‐sensing receptor with apparent lower affinity for calcium than CaSR [50]. Whether this receptor or another yet unidentified cation‐sensing receptor or channel may mediate the action of millimolar concentrations of strontium on cell replication in primary osteoblasts remains to be determined.
Age‐related or glucocorticoid‐induced osteoporosis is characterized by decreased number of active osteoblasts, which is related, in part, to increased osteoblast apoptosis [30]. This led to the concept that therapeutic prevention of osteoblast apoptosis may contribute to improve bone formation in osteoporosis [31, 32]. In non‐skeletal cell types, elevated extracellular calcium was recently reported to prevent apoptosis via the CaSR [51], by acting on mitochondrial pathways [52, 53]. We tested the hypothesis that strontium ranelate, in addition to promoting cell replication, may control cell apoptosis in osteoblasts via CaSR activation. Our findings clearly indicate that strontium ranelate can protect against serum deprivation‐induced apoptosis in osteoblasts. Furthermore, we show that the anti‐apoptotic effect of strontium ranelate in primary osteoblasts occurs independent of the CaSR, raising the possibility that another cation‐sensing receptor may mediate the anti‐apoptotic effect of strontium ranelate in these cells. Importantly, we found that strontium ranelate activated the Akt pro‐survival pathway in primary osteoblasts, which identifies one signalling pathway that may be involved in the anti‐apoptotic effect of strontium ranelate in osteoblasts. Our finding that pharmacological inhibition of PI3K Akt signalling reduced the strontium ranelate‐induced anti‐apoptotic effect further indicates that this signalling pathway is involved in the protective effect of strontium ranelate on osteoblast apoptosis. Interestingly, recent findings indicate that the anti‐apoptotic effects of PTH and Wnt3a protein, two major anabolic agents in bone, involve Akt signalling in cultured osteoblasts [31, 39]. Our finding that the protective effect of the anti‐osteoporotic agent strontium ranelate involves PI3K Akt survival signalling further points to Akt as an important anti‐apoptotic signalling pathway in osteoblasts.
Prostaglandins are potent regulators of bone cell functions [54]. Notably, PGE2 regulates osteoblast proliferation, differentiation and survival [55]. Previous studies have indicated that strontium ranelate increases COX‐2 expression and PGE2 production through ERK signalling [56], and that PGE2 induced by this compound is involved in the increased osteoblast differentiation of murine bone marrow stromal cells induced by strontium ranelate [57]. We showed here that the mitogenic and anti‐apoptotic effects of strontium ranelate in [CaSR+/+] and [CaSR−/−] osteoblasts involve PGE2 production, suggesting that the local production of PGE2 may mediate the positive effects of strontium ranelate on osteoblasts, independent of the CaSR.
Collectively, the present results bring novel insights into the regulation of osteoblastogenesis by strontium ranelate. Our data provide evidence that CaSR is not the only receptor involved in the stimulatory effects of strontium ranelate on osteoblasts. We also show that strontium ranelate protects against apoptosis in primary osteoblasts, which provides a novel mechanism by which strontium ranelate can promote osteoblastogenesis. Our data also indicate that strontium ranelate‐induced activation of osteoblast replication and survival is mediated, at least in part, by ERK1/2 and Akt signalling and ‘PGE2 production’, independent of CaSR expression. This provides genetic and biochemical evidence that CaSR‐dependent and CaSR‐independent modulation of osteoblast replication and survival may contribute to the anti‐osteoporotic effects of strontium ranelate.
Acknowledgements
We thank Professors C. Seidman and J. Seidman (Department of Genetics, Harvard Medical School, Boston, MA, USA) for allowing access to [CaSR−/−] mice. Financial support was provided in part by Institut de Recherches Servier (IRIS, Courbevoie, France) and Association Rhumatisme et Travail (Hopital Lariboisière, Paris, France), which were not involved in planning, performance, data analysis or manuscript writing.
References
- 1. Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest . 2005; 115: 3318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res . 2005; 20: 177–84. [DOI] [PubMed] [Google Scholar]
- 3. Meunier PJ, Roux C, Seeman E, et al . The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med . 2004; 350: 459–68. [DOI] [PubMed] [Google Scholar]
- 4. Reginster JY, Seeman E, De Vernejoul MC, et al . Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab . 2005; 90: 2816–22. [DOI] [PubMed] [Google Scholar]
- 5. Marie PJ. Strontium as therapy for osteoporosis. Curr Opin Pharmaco . 2005; 5: 633–6. [DOI] [PubMed] [Google Scholar]
- 6. Marie PJ, Hott M, Modrowski D, et al . An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen‐deficient rats. J Bone Miner Res . 1993; 8: 607–15. [DOI] [PubMed] [Google Scholar]
- 7. Hott H, Deloffre P, Tsouderos Y, et al . S12911–2 reduces bone loss induced by short‐term immobilization in rats. Bone 2003; 33: 115–23. [DOI] [PubMed] [Google Scholar]
- 8. Arlot ME, Jiang Y, Genant HK, et al . Histomorphometric and microCT analysis of bone biopsies from postmenopausal osteoporotic women treated with strontium ranelate. J Bone Miner Res . 2008; 23: 215–22. [DOI] [PubMed] [Google Scholar]
- 9. Barbara A, Delannoy P, Denis BG, et al . Normal matrix mineralization induced by strontium ranelate in MC3T3‐E1 osteogenic cells. Metabolism 2004; 53: 532–7. [DOI] [PubMed] [Google Scholar]
- 10. Bonnelye E, Chabadel A, Saltel F, et al . Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro . Bone 2008; 42: 129–38. [DOI] [PubMed] [Google Scholar]
- 11. Choudhary S, Halbout P, Alander C, et al . Strontium ranelate promotes osteoblastic differentiation and mineralization of murine bone marrow stromal cells: involvement of prostaglandins. J Bone Miner Res . 2007; 22: 1002–10. [DOI] [PubMed] [Google Scholar]
- 12. Zhu LL, Zaidi S, Peng Y, et al . Induction of a program gene expression during osteoblast differentiation with strontium ranelate. Biochem Biophys Res Commun . 2007; 355: 307–11. [DOI] [PubMed] [Google Scholar]
- 13. Canalis E, Hott M, Deloffre P, et al . The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro . Bone 1996; 18: 517–23. [DOI] [PubMed] [Google Scholar]
- 14. Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signalling. Physiol Rev . 2001; 81: 239–97. [DOI] [PubMed] [Google Scholar]
- 15. Brown EM. Clinical lessons from the calcium‐sensing receptor. Nat Clin Pract Endocrinol Metab . 2007; 3: 122–33. [DOI] [PubMed] [Google Scholar]
- 16. Chang W, Shoback D. Extracellular Ca2+‐sensing receptors–an overview. Cell Calcium . 2004; 35: 183–96. [DOI] [PubMed] [Google Scholar]
- 17. House MG, Kohlmeier L, Chattopadhyay N, et al . Expression of an extracellular calcium‐sensing receptor in human and mouse bone marrow cells. J Bone Miner Res . 1997; 12: 1959–70. [DOI] [PubMed] [Google Scholar]
- 18. Yamaguchi T, Kifor O, Chattopadhyay N, et al . Expression of extracellular calcium (Ca2+)‐sensing receptor in the clonal osteoblast‐like cell lines, UMR‐106 and SAOS‐2. Biochem Biophys Res Commun . 1998; 243: 753–7. [DOI] [PubMed] [Google Scholar]
- 19. Chang W, Tu C, Chen TH, et al . Expression and signal transduction of calcium‐sensing receptors in cartilage and bone. Endocrinology . 1999; 140: 5883–93. [DOI] [PubMed] [Google Scholar]
- 20. Yamaguchi T, Chattopadhyay N, Kifor O, et al . Mouse osteoblastic cell line (MC3T3‐E1) expresses extracellular calcium (Ca2+o)‐sensing receptor and its agonists stimulate chemotaxis and proliferation r of MC3T3‐E1 cells. J Bone Miner Res . 1998; 13: 1530–8. [DOI] [PubMed] [Google Scholar]
- 21. Yamaguchi T, Chattopadhyay N, Kifor O, et al . Activation of p42/44 and p38 mitogen‐activated protein kinases by extracellular calcium‐sensing receptor agonists induces mitogenic responses in the mouse osteoblastic MC3T3‐E1 cell line. Biochem Biophys Res Commun . 2000; 279: 363–8. [DOI] [PubMed] [Google Scholar]
- 22. Chattopadhyay N, Yano S, Tfelt‐Hansen J, et al . Mitogenic action of calcium‐sensing receptor on rat calvarial osteoblasts. Endocrinology 2004; 145: 3451–62. [DOI] [PubMed] [Google Scholar]
- 23. McNeil SE, Hobson SA, Nipper V, et al . Functional calcium‐sensing receptors in rat fibroblasts are required for activation of SRC kinase and mitogen‐activated protein kinase in response to extracellular calcium. J Biol Chem . 1998; 273: 1114–20. [DOI] [PubMed] [Google Scholar]
- 24. Kifor O, MacLeod RJ, Diaz R, et al . Regulation of MAP kinase by calcium‐sensing receptor in bovine parathyroid and CaR‐transfected HEK293 cells. Am J Physiol Renal Physiol . 2001; 280: F291–302. [DOI] [PubMed] [Google Scholar]
- 25. Brown EM. Is the calcium receptor a molecular target for the actions of strontium on bone Osteoporos Int. 2003; 14 Suppl 3: S25–34. [DOI] [PubMed] [Google Scholar]
- 26. Coulombe J, Faure H, Robin B, et al . In vitro effects of strontium ranelate on the extracellular calcium‐sensing receptor. Biochem Biophys Res Commun . 2004; 323: 1184–90. [DOI] [PubMed] [Google Scholar]
- 27. Jilka RL, Weinstein RS, Bellido T, et al . Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res . 1998; 13: 793–802. [DOI] [PubMed] [Google Scholar]
- 28. Xing L, Boyce BF. Regulation of apoptosis in osteoclasts and osteoblastic cells. Biochem Biophys Res Commun . 2005; 328: 709–20. [DOI] [PubMed] [Google Scholar]
- 29. Fromigué O, Modrowski D, Marie PJ. Apoptosis in membranous bone formation: role of fibroblast growth factor and bone morphogenetic protein signalling. Crit Rev Eukaryot Gene Expr . 2005; 15: 75–92. [DOI] [PubMed] [Google Scholar]
- 30. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev . 2000; 21: 115–37. [DOI] [PubMed] [Google Scholar]
- 31. Jilka RL, Weinstein RS, Bellido T, et al . Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest . 1999; 104: 439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kousteni S, Bellido T, Plotkin LI, et al . Nongenotropic, sex‐nonspecific signalling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 2001; 104: 719–30. [PubMed] [Google Scholar]
- 33. Tsuboi M, Kawakami A, Nakashima T, et al . Tumor necrosis factor‐alpha and interleukin‐1beta increase the Fas‐ mediated apoptosis of human osteoblasts. J Lab Clin Med . 1999; 134: 222–31. [DOI] [PubMed] [Google Scholar]
- 34. Hill PA, Tumber A, Meikle MC. Multiple extracellular signals promote osteoblast survival and apoptosis. Endocrinology 1997; 138: 3849–58. [DOI] [PubMed] [Google Scholar]
- 35. Ho C, Conner DA, Pollak MR, et al . A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet . 1995; 11: 389–94. [DOI] [PubMed] [Google Scholar]
- 36. Fromigué O, Haÿ E, Modrowski D, et al . RhoA GTPase inactivation by statins induces osteosarcoma cell apoptosis by inhibiting p42/p44‐MAPKs‐Bcl‐2 signalling independently of BMP‐2 and cell differentiation. Cell Death Differ . 2006; 13: 1845–56. [DOI] [PubMed] [Google Scholar]
- 37. Kaiser E, Chandrasekhar S. Distinct pathways of extracellular signal‐regulated kinase activation by growth factors, fibronectin and parathyroid hormone 1–34. Biochem Biophys Res Commun . 2003; 305: 573–8. [DOI] [PubMed] [Google Scholar]
- 38. Huang Z, Cheng SL, Slatopolsky E. Sustained activation of the extracellular signal‐regulated kinase pathway is required for extracellular calcium stimulation of human osteoblast proliferation. J Biol Chem 2001 ; 276: 21351–8. [DOI] [PubMed] [Google Scholar]
- 39. Almeida M, Han L, Bellido T, et al . Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta‐catenin‐dependent and ‐independent signalling cascades involving Src/ERK and phosphatidylinositol 3‐kinase/AKT. J Biol Chem . 2005; 280: 41342–51. [DOI] [PubMed] [Google Scholar]
- 40. Dufour C, Guenou H, Kaabeche K, et al . FGFR2‐Cbl interaction in lipid rafts triggers attenuation of PI3K/Akt signalling and osteoblast survival. Bone 2008; 42: 1032–9. [DOI] [PubMed] [Google Scholar]
- 41. Dufour C, Holy X, Marie PJ. Transforming growth factor‐beta prevents osteoblast apoptosis induced by skeletal unloading via PI3K/Akt, Bcl‐2, and phospho‐Bad signalling. Am J Physiol Endocrinol Metab . 2008; 294: E794–801. [DOI] [PubMed] [Google Scholar]
- 42. Kawamura N, Kugimiya F, Oshima Y, et al . Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS ONE. 2007; 2: e1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Green DR. Apoptotic pathways: ten minutes to dead. Cell 2005; 121: 671–4. [DOI] [PubMed] [Google Scholar]
- 44. Chattopadhyay N, Quinn SJ, Kifor O, et al . The calcium‐sensing receptor (CaR) is involved in strontium ranelate‐induced osteoblast proliferation Biochem Pharmacol . 2007; 74: 438–47. [DOI] [PubMed] [Google Scholar]
- 45. Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988; 175: 266–76. [DOI] [PubMed] [Google Scholar]
- 46. Dahl SG, Allain P, Marie PJ, et al . Incorporation and distribution of strontium in bone. Bone 2001; 28: 446–53. [DOI] [PubMed] [Google Scholar]
- 47. Quarles LD, Hartle JE 2nd, Siddhanti SR, et al . A distinct cation‐sensing mechanism in MC3T3‐E1 osteoblasts functionally related to the calcium receptor. J Bone Miner Res. 1997; 12: 393–402. [DOI] [PubMed] [Google Scholar]
- 48. Pi M, Garner SC, Flannery P, et al . Sensing of extracellular cations in CasR‐deficient osteoblasts. Evidence for a novel cation‐sensing mechanism. J Biol Chem . 2000; 275: 3256–63. [DOI] [PubMed] [Google Scholar]
- 49. Pi M, Quarles LD. A novel cation‐sensing mechanism in osteoblasts is a molecular target for strontium. J Bone Miner Res . 2004; 19: 862–9. [DOI] [PubMed] [Google Scholar]
- 50. Pi M, Faber P, Ekema G, et al . Identification of a novel extracellular cation‐sensing G‐protein‐coupled receptor. J Biol Chem . 2005; 280: 40201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lin KI, Chattopadhyay N, Bai M, et al . Elevated extracellular calcium can prevent apoptosis via the calcium‐sensing receptor. Biochem Biophys Res Commun . 1998; 249: 325–31. [DOI] [PubMed] [Google Scholar]
- 52. Clapham DE. Calcium signalling. Cell 2007; 131: 1047–55. 18083096 [Google Scholar]
- 53. Hajnóczky G, Saotome M, Csordás G, et al . Calcium signalling and mitochondrial motility. Novartis Found Symp . 2007; 287: 105–17. [DOI] [PubMed] [Google Scholar]
- 54. Raisz LG. Anabolic effects of prostaglandins. Clin Rev Bone Mineral Metab . 2006; 4: 123–8. [Google Scholar]
- 55. Pilbeam CC, Harrison JR, Raisz LG. Prostaglandins and bone metabolism. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology Vol. 2. NY : Academic Press; 2002. pp. 979–94. [Google Scholar]
- 56. Choudhary S, Wadhwa S, Raisz LG, et al . Extracellular calcium is a potent inducer of cyclo‐oxygenase‐2 in murine osteoblasts through an ERK signaling pathway. J Bone Miner Res . 2003; 18: 1813–24. [DOI] [PubMed] [Google Scholar]
- 57. Choudhary S, Halbout P, Alander C, et al . Strontium ranelate promotes osteoblastic differentiation and mineralization of murine bone marrow stromal cells: involvement of prostaglandins. J Bone Miner Res . 2007; 22: 1002–10. [DOI] [PubMed] [Google Scholar]


