Abstract
This systematic review identifies, evaluates, and summarises the findings of all relevant individual studies on the prevalence of BRCA mutation (BRCAm) in endometrial cancer patients and the incidence of endometrial cancer in BRCAm women patients. Consequently, the benefits and limits of a prophylactic hysterectomy at the time of the risk-reducing salpingo-oophorectomy are analysed and discussed. A systematic literature search was performed in the databases of PubMed, Cochrane, and Web of Science until May 2022; 13 studies met the eligibility criteria. Overall, 1613 endometrial cancer patients from 11 cohorts were tested for BRCA1/2 mutation. BRCA1/2m were identified in 4.3% of women with endometrial cancer (70/1613). BRCA1m was the most represented (71.4%) pathogenic variant. Alongside, a total of 209 BRCAm carriers from 14 studies were diagnosed with endometrial cancer. Only 5 out of 14 studies found a correlation between BRCAm and an increased risk of endometrial cancer. Nevertheless, two studies found a statistical difference only for BRCA1m women. The present systematic review does not provide strong evidence in favour of performing routine hysterectomy at the time of risk-reducing salpingo-oophorectomy; however, it provides epidemiological data that can be useful for counselling patients in order to offer a tailored approach.
Keywords: endometrial cancer, BRCA1, BRCA2, risk-reducing salpingo-oophorectomy, hysterectomy
1. Introduction
Breast cancer gene 1, located on the long arm (q) of chromosome 17, and the BRCA 2 gene, located on the long arm of chromosome 13, are both autosomal dominant tumour suppressor genes involved in DNA damage repair before cell replication. The lifetime risk of breast and ovarian cancer increases for those carrying a pathogenic variant of breast cancer gene 1 (BRCA1) or breast cancer gene 2 (BRCA2) by 40–80% and 11–40%, respectively [1]. In order to reduce the lifetime risk of breast, ovarian, and fallopian tube cancer, NCCN guidelines (National Comprehensive Cancer Network) currently consider a risk-reducing mastectomy (RRM) and recommend salpingo-oophorectomy (RRSO) in women with BRCA mutations (pathogenic variants) (BRCAm) [2]. RRSO is associated with a 42% and 94% reduced risk of developing breast and ovarian cancer in BRCAm carriers, respectively [3], and a 60% reduced all-cause mortality [4].
BRCAm carriers are exposed to a higher risk of other less frequent cancers such as fallopian tube cancer and primary peritoneal cancer [5], pancreatic cancer [6], prostate, and gastric cancer [7].
The risk of uterine cancer in BRCAm women and, consequently, its role as part of the BRCA mutated syndrome is still debated. The analogies between uterine, mainly serous carcinoma and serous ovarian carcinoma, have led to the investigation of potential common pathogenetic features, as well as hereditary causes. Positive family history is noted in approximately 10% of cases of endometrial cancer, suggesting an inherited predisposition, even if the precise genes pattern involved are largely unknown [8].
Uterine serous carcinoma, representing less than 10% of all endometrial cancers, is an aggressive histologic subtype with a poor prognosis. It accounts for about 25% of the entire endometrial cancer mortality, with an overall survival rate at 5 years of 18–27% due to frequent advanced disease at diagnosis and a high rate of distant recurrences even in patients with early-stage disease [9,10]
Some authors [11,12,13] confirmed a higher risk of endometrial cancer in BRCAm women, especially for uterine serous cancer in BRCA1m, while others [14,15] did not support this correlation.
Hence, while RRSO is a well-established procedure for women with BRCAm at 35–40 years of age for BRCA1m and at 40–45 years for BRCA2m [2], prophylactic hysterectomy at the time of RRSO is still a matter of debate [16]. If women carrying BRCA1m are confirmed to be at an increased risk for serous or serous-like endometrial cancer, this should be considered when counselling a patient with regards to the risks and benefits of the addition of hysterectomy at the time of RRSO.
This systematic review aims to assess the risk of endometrial cancer and examine the benefits and limits of a prophylactic hysterectomy in BRCAm women, analysing both the prevalence of BRCAm in endometrial cancer patients and the incidence of endometrial cancer found in BRCAm patients undergoing RRSO with hysterectomy.
2. Material and Methods
2.1. Information Sources
A systematic literature search was performed in the databases of PubMed, Cochrane, and Web of Science until May 2022. No beginning date limit or language restrictions were used. The review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [17].
2.2. Search Strategy
The search terms consisted of “BRCA” and “endometrial cancer” or “uterine cancer”. Reference lists of identified systematic reviews and included studies were manually screened for any other eligible studies.
2.3. Study Selection
Titles and abstracts were screened. Articles reporting the incidence of endometrial cancer in BRCAm women or the prevalence of BRCAm in patients affected by endometrial cancer were obtained in full for further evaluation. Studies were excluded if they were case reports, editorials, reviews, or short communications because they did not provide sufficient information to assess the methodological quality.
Title and abstract screening, as well as full-text screening, were performed independently and simultaneously by two authors (SB and MLG) based on pre-defined criteria. All dissents were resolved by consensus.
2.4. Data Extraction
For each eligible article, information was collected concerning the first author, year of publication, country of origin, study period, design of the study, the total number of patients, mean or median age, genotyping testing method (including different BRCA deletions and other genes investigated), number of endometrial cancers and uterine serous carcinoma, FIGO stage, previous Tamoxifen use, history of breast cancer, and type of BRCAm. Median follow-up was expressed in years or women-years. Standard Incidence Ratios (SIR) were reported for assessing endometrial cancer risk in BRCAm women when available.
2.5. Quality Assessment
The evaluation of the risk of bias in estimates of the comparative effectiveness (harm or benefit) of interventions from the included studies was performed with the “Risk Of Bias In Non-randomized Studies-of Interventions” (ROBINS-I) tool [18].
3. Results
3.1. Literature Search
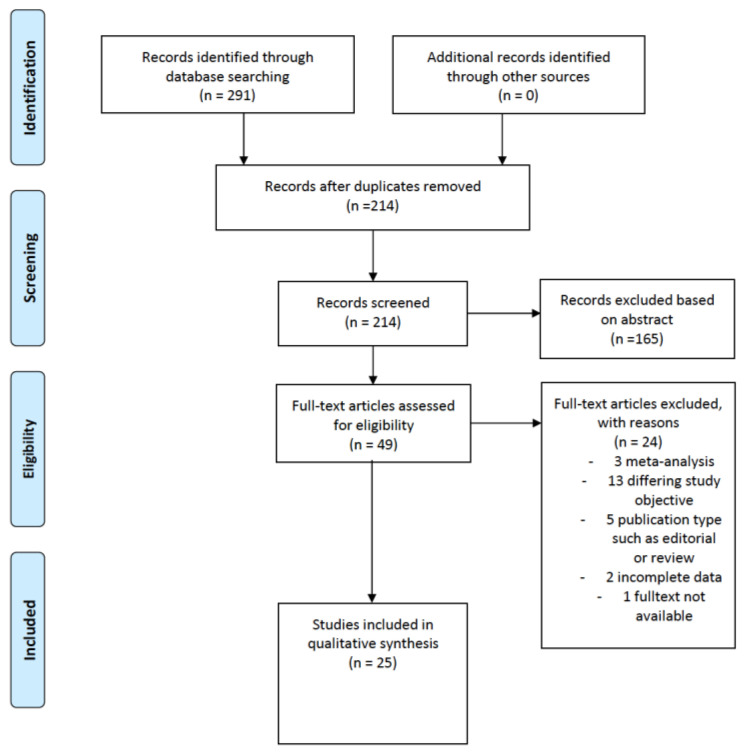
Overall, a total of 291 records were identified. After removing 77 duplicates, 214 manuscripts were screened, and 165 were excluded based on the abstract. A full text was obtained for 48 of 49 records. At the end of the screening process, 24 full-text articles were included in the systematic review. All papers were in English.
Titles/abstracts were screened according to the inclusion and exclusion criteria. Most manuscripts were excluded during the screening process due to differing study objectives (n = 13), publication types such as editorial or review (n = 8), and incomplete data (n = 2).
Details about the literature search results are reported in Figure 1. A total of 11/25 included studies that assessed the prevalence of BRCAm in patients affected by endometrial cancer [8,19,20,21,22,23,24,25,26,27,28], and 14/25 included studies that evaluated the incidence of endometrial cancer in BRCAm [11,12,13,14,15,29,30,31,32,33,34,35,36,37].
Figure 1.
PRISMA flow chart of study identification.
3.2. Patients Characteristics of the Included Studies
Overall, the total number of patients analysed in this systematic review was 37,286.
The number of patients ranged between 20 and 628 in the included studies concerning the incidence of BRCAm in patients with endometrial cancer and between 315 and 14,621 in the included studies concerning the incidence of endometrial cancer in BRCAm patients.
The mean/median age of patients ranged between 20 and 72 years; most of the included patients had a FIGO stage I, and median follow-up ranged between 1.5–9 years and 1.779–59.199 women-years.
3.3. Methodological Aspects of the Included Studies
A total of 13 observational retrospective cohort studies, 3 retrospective case-control studies, 7 observational prospective cohort studies, 1 prospective case-control study, and 1 longitudinal cohort study were included in this systematic review. Of these, 10 were multicenter-based studies. Additionally, four studies included only Jewish women, and one study included only patients with hereditary endometrial cancer (Lynch syndrome and hereditary breast-ovarian cancer). The main characteristics of eligible studies on the prevalence of BRCAm in patients with endometrial cancer and on the incidence of endometrial cancer in BRCAm patients are shown in Table 1 and Table 2, respectively. The risk of bias assessment is reported in Table 3.
Table 1.
Characteristics of the included studies on the prevalence of BRCAm in patients with endometrial cancer.
| Authors | Publication Year | Country | Time Period | Study Type | Study Group |
|---|---|---|---|---|---|
| Niederacher et al. [19] | 1998 | Germany | 1980–1994 | Retrospective case-control | EC |
| Goshen et al. [20] | 2000 | Canada | 1996–2006 | Retrospective multicenter cohort | USC |
| Levine et al. [21] | 2001 | Israel | 1986–1998 | Retrospective cohort | Jewish patients, EC |
| Lavie et al. [22] | 2004 | Israel | 1999–2002 | Retrospective multicenter cohort | USC |
| Biron-Shental et al. [23] | 2006 | Israel | 1997–2003 | Retrospective cohort | Jewish patients, USC |
| Barak et al. [8] | 2010 | Israel | 1982–2008 | Retrospective and prospective cohort | Jewish patients, EC |
| Bruchim et al. [24] | 2010 | Israel | 1997–2007 | Retrospective cohort | Jewish patients, USC |
| Pennington et al. [25] | 2013 | USA | NA | Retrospective cohort | USC |
| Mahdi et al. [26] | 2015 | USA | NA | Retrospective cohort | USC or ovarian serous carcinoma |
| Kadan et al. [27] | 2018 | Israel, Arabia | 1993–2014 | Retrospective multicenter cohort | USC |
| Vietri et al. [28] | 2021 | Italy | NA | NA | Hereditary EC (LS and HBOC) |
BRCAm: breast cancer gene mutation, EC: endometrial cancer, USC: uterine serous carcinoma, LS: Lynch Syndrome, HBOC: Hereditary Breast and Ovarian Cancer syndrome, NA: not available.
Table 2.
Characteristics of the included studies on the incidence of endometrial cancer in BRCAm patients.
| Authors | Publication Year | Country | Time Period | Study Type | Study Group |
|---|---|---|---|---|---|
| Thompson et al. [29] | 2002 | Western Europe and North America | 1960–2002 | Retrospective multicenter cohort | BRCAm |
| Beiner et al. [30] | 2007 | North America, Europe and Israel | NA | Prospective multicenter cohort | BRCAm |
| Reitsma et al. [14] | 2012 | The Netherlands | 1996–2012 | Prospective cohort | BRCAm, RRSO |
| Segev et al. [31] | 2013 | Canada, Italy, USA, Austria, Poland, Norway | NA | Prospective multicenter case-control | BRCAm |
| Casey et al. [13] | 2015 | USA | 1959–2013 | Retrospective cohort | BRCAm with invasive gynecologic and/or peritoneal cancers |
| Segev et al. [32] | 2015 | North America, Europe and Israel | NA | Retrospective multicenter case-control | BRCAm |
| Shu et al. [12] | 2016 | USA, UK | 1995–2011 | Prospective multicenter cohort | BRCAm, RRSO |
| Zakhour et al. [33] | 2016 | USA | 2000–2014 | Prospective cohort | BRCAm, RRSO |
| Bogani et al. [34] | 2017 | Italy | 2014–2017 | Prospective cohort | BRCAm or significant family history of breast/ovarian cancer, RRSO ± hysterectomy |
| Lee et al. [35] | 2017 | Australia, New Zealand |
NA | Prospective multicenter cohort | BRCAm |
| Minig et al. [36] | 2018 | Spain | 2010–2017 | Retrospective multicenter cohort |
BRCAm, RRSO |
| Saule et al. [15] | 2018 | France | 1996–2016 | Prospective cohort | BRCAm, RRSO |
| Laitman et al. [11] | 2019 | Israel | 1998–2016 | Retrospective case-control | BRCAm |
| Kitson et al. [37] | 2020 | UK | 1991–2017 | Retrospective cohort | BRCAm |
BRCAm: breast cancer gene 1/2 mutation, RRSO: risk-reducing salpingo-oophorectomy, NA: not available.
Table 3.
Quality assessment of individual study.
| Author | Bias due to Confounding | Bias in Selection of Partecipants | Bias Due to Missing Data | Bias in Classification of Interventions |
Bias in Measurement of Outcomes | Bias in Selection of the Results | Overall |
|---|---|---|---|---|---|---|---|
| Niederacher et al., 1998 [19] | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Goshen et al., 2000 [20] | Serious | Serious | Serious | Moderate | Moderate | Serious | Serious |
| Levine et al., 2001 [21] | Moderate | Serious | Moderate | Moderate | Moderate | Low | Serious |
| Thompson et al., 2002 [29] | Moderate | Serious | Serious | Moderate | Moderate | Moderate | Serious |
| Lavie et al., 2004 [22] | Moderate | Serious | Moderate | Moderate | Low | Low | Serious |
| Biron-Shental et al., 2006 [23] | Serious | Moderate | Moderate | Moderate | Moderate | Low | Serious |
| Beiner et al., 2007 [30] | Low | Low | Low | Moderate | Moderate | Moderate | Moderate |
| Bruchim et al., 2010 [24] | Moderate | Low | Moderate | Moderate | Moderate | Serious | Serious |
| Barak et al., 2010 [8] | Moderate | Serious | Serious | Moderate | Low | Moderate | Serious |
| Reitsma et al., 2012 [14] | Low | Moderate | Low | Low | Moderate | Moderate | Moderate |
| Pennington et al., 2013 [25] | Moderate | Serious | Serious | Low | Low | Low | Serious |
| Segev et al., 2013 [31] | Low | Low | Low | Moderate | Moderate | Moderate | Moderate |
| Casey et al., 2015 [13] | Serious | Moderate | Moderate | Moderate | Moderate | Moderate | Serious |
| Mahdi et al., 2015 [26] | Moderate | Serious | Serious | Moderate | Low | Moderate | Serious |
| Segev et al., 2015 [32] | Moderate | Serious | Moderate | Moderate | Serious | Moderate | Serious |
| Zakhour et al., 2016 [33] | Moderate | Low | Moderate | Moderate | Moderate | Moderate | Moderate |
| Shu et al., 2016 [12] | Low | Moderate | Low | Moderate | Serious | Moderate | Serious |
| Lee et al., 2017 [35] | Moderate | Low | Low | Moderate | Serious | Moderate | Serious |
| Bogani et al., 2017 [34] | Low | Low | Low | Moderate | Moderate | Moderate | Moderate |
| Mining et al., 2018 [36] | Moderate | Serious | Serious | Moderate | Serious | Serious | Serious |
| Saule et al., 2018 [15] | Low | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Laitman et al., 2018 [11] | Moderate | Serious | Serious | Moderate | Serious | Serious | Serious |
| Kadan et al., 2018 [27] | Moderate | Moderate | Moderate | Low | Moderate | Low | Moderate |
| Kitson et al., 2020 [37] | Moderate | Moderate | Moderate | Low | Moderate | Moderate | Moderate |
| Vietri et al., 2021 [28] | Low | Low | Low | Low | Low | Moderate | Moderate |
3.4. Main Findings
A total of 1613 endometrial cancer patients from 11 studies were tested for BRCA1/2 pathological variants [8,19,20,21,22,23,24,25,26,27,28]. Uterine serous carcinoma was diagnosed in 1129 patients. Of note, the diagnosis of uterine serous carcinoma was one of the inclusion criteria for 972 patients out of 1129. The notion of previous breast cancer was reported in 6 out of 10 studies; in particular, 47 out of 344 (13.7%) women with endometrial cancer had a personal history of breast malignancy. Only one paper reported the use of hormone therapy before endometrial cancer diagnosis [23]. BRCA1/2m were identified in 70 women with endometrial cancer out of 1613 (4.3%). Notably, BRCA1m represented 71.4% of BRCA pathological variants found in endometrial cancer patients (50/70).
A significant difference in the increased risk of endometrial cancer among BRCAm patients was found by Beiner [30] [SIR = 5.3 (p = 0.0011)], Saule [15] [SIR = 32.2 (95% CI, 11.5–116.4, p < 0.001)], and Thompson [29] [SIR = 2.65 (1.69–4.16, p < 0.001)]. However, two other studies confirmed this difference only for BRCA1m women [11,31]. Notably, Laitman et al. registered 14 cases of endometrial cancer among 2627 Jewish patients included in their study, assessing an increased overall rate of uterine cancer of almost 4-fold [SIR = 3.98 (95% CI, 2.174–6.673)] [11]. In a sub-analysis, this risk was significantly augmented for BRCA1 patients [SIR = 5.236 (95% CI, 2.659–9.382, p < 0.001)] but not for BRCA2 patients [SIR = 2.339 (95% CI, 0.743–5.642, p = 0.124)] [11]. Segev et al. confirmed the same results [BRCA1 = SIR 1.91 (95% CI, 1.06–3.19, p = 0.03), BRCA2 = SIR 1.75 (95% CI, 0.55–4.23, p = 0.2)] [31]. In contrast, Kitson et al. found no significant increased risk for endometrial cancer in the 2609 included in their study for both BRCA1/2m [SIR = 1.70 (95% CI, 0.74–3.33)] [37] as well as Goshen et al., even if they did not consider 2 of the 3 BRCA1 mutations examined in many other studies [20].
BRCA1/2 testing was generally performed by traditional Sanger sequencing. The next-generation sequencing (NGS) technique was otherwise adopted in 3 studies out of 24 [11,19,25].
The main findings of the included studies on the prevalence of BRCAm in patients with endometrial cancer are reported in Table 4.
Table 4.
Main findings of the included studies on the prevalence of BRCAm in patients with endometrial cancer.
| Author | Total Patients | Age, yr [Mean ± SD/Median (Range)] † | Genotyping | Total EC | EC Histopathology (n. of Patients; %) | USC | EC with Previous Breast Cancer | EC in Patients Using Tamoxifen | Positive Family History of Breast Cancer | Number of BRCA Mutated Patients | EC with BRCAm (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | BRCA Mutation | Other Genes Tested | FIGO Stage | Grade | BRCA 1 | BRCA 2 | Tot | |||||||||
| Barak et al. [8] | 289 | 63 ± 12 | Traditional Sanger | BRCA1 (185delAG, 5383InsC, Tyr978X) BRCA2 (6174delT, 8765delAG) |
- | 289 | In situ (2, 0.7%) I (234, 81%) II (24, 8%) III (25, 9%) IV (4, 1%) |
In situ (2, 0.7%) I (168, 58%) II (50, 17%) III (69, 24%) |
34 | NA | NA | NA | 4 | 1 | 5 | 1.7 |
| Biron-Shental et al. [23] | 22 | 72 (56–79) | Traditional Sanger | BRCA1 (185delAG, 5382insC) BRCA2 (6174delT) |
- | 22 | I-II (9, 41%) III-IV (13; 59%) |
NA | 22 | 7 | NA | 7 | 3 | 3 | 6 | 22.7 |
| Bruchim et al. [24] | 31 | 72 (47–87) | Traditional Sanger | BRCA1 (185delAG, 5382insC) BRCA2 (617delT) |
- | 31 | I-II (16, 52%) III-IV (15; 48%) |
NA | 31 | 7 | 6 | 5 | 4 | 4 | 8 | 25.8 |
| Goshen et al. [20] | 56 | NA | Traditional Sanger | BRCA1 (185del AG, 5382insC, dup(ex13)) BRCA2 (6174delT) |
- | 56 | I (27; 48%) II (6, 11%) III (13, 23%) IV (6, 11%) NA (4, 7%) |
NA | 56 | 6 | NA | 6 | 0 | 0 | 0 | 0 |
| Kadan et al. [27] | 64 | 66 ± 9.7 ** | Traditional Sanger | BRCA1 (185delAG, 5382insC) BRCA2 (6174delT) |
- | 64 | I (32; 50%) II (3, 5%) III (12, 19%) IV (16, 25%) NA (1, 1%) |
NA | 64 | 18 | NA | NA | 9 | 5 | 14 | 21.9 |
| Lavie et al. [22] | 20 | 72 (56–91) |
Traditional Sanger | BRCA1 (185delAG, 5382insC) BRCA2 (6174delT) |
- | 20 | I (NA, 30%) II (NA, 15%) III (NA; 40%) IV (NA, 15%) |
NA | 20 | 7 | NA | 7 | 4 | 0 | 4 | 20 |
| Levine et al. [21] | 199 | 66 ± 11 | Traditional Sanger | BRCA1 (185delAG, 5382insC) BRCA2 (6174delT) |
- | 199 | I (144; 72%) II (17, 9%) III (22, 11%) IV (14, 7%) NA (2, 1%) |
1 (73, 37%) 2 (70, 35%) 3 (53, 27%) NA (3, 1%) |
17 | NA | NA | NA | 1 | 2 | 3 | 1.5 |
| Mahdi et al. [26] | 241/628 * | 68 (44–94) | NGS | NA | ABL1, AKT1, ALK, APC, ATM, BRAF, CDH1, CSF1R, CTNNB1, EGFR, ERBB2, ERBB4, FBXW7, FGFR1, FGFR2, FLT3, GNA11, GNAS, GNAQ, HNF1A, HRAS, IDH1, JAK2, JAK3, KDR (VEGFR2), KIT, KRAS, MET, MLH1, MPL, NOTCH1, NPM1, NRAS, PDGFR, PIK3CA, PTEN, PTPN11, RB1, RET, SMAD4, SMARCB1,SMO, STK11, TP53, VHL |
628 | NA | NA | 628 | NA | NA | NA | 3 | 2 | 5 | 0.8 |
| Niederacher et al. [19] | 113 | NA | Traditional Sanger | BRCA1-D17S855 | TP53- AFM051, TCRD, ESR, D11S35, D16S511 | 113 | I (69; 61%) II (18, 16%) III (15, 13%) IV (11, 10%) |
1 (52, 46%) 2 (30, 27%) 3 (21, 19%) NA (10, 8%) |
106 | NA | NA | NA | 13 | 0 | 13 | 11.5 |
| Pennington et al. [25] | 151 | 68 | NGS | NA | APC ATM BAP1 BARD1 BMPR1A BRIP1 BUB1B CDH1 CDK4 CDKN2A CHEK2 KIT MLH1 MRE11A MSH2 (.EPCAM) MSH6 MUTYH NBN PALB2 PMS2 TEN (.KILLIN) RAD50 RAD51C RET SMAD4 STK11 TP53 VHL |
151 | I (61; 40%) II (16, 11%) III (34, 23%) IV (38, 25%) NA (2, 1%) |
NA | 151 | 2 | NA | 22 | 3 | 0 | 3 | 2 |
| Vietri et al. [28] | 40 | 35 (20–54) *** | NGS | MLH1, MSH2 | 40 | NA | NA | NA | NA | NA | 6 | 3 | 9 | 22.5 | ||
USC: uterine serous carcinoma; BRCAm breast cancer gene mutated patient, EC endometrial cancer, NA not available. † Data are expressed in mean or median as reported in each study. * Mahdi et al. included 5936 patients, of whom 5335 were affected by an ovarian serous carcinoma and 628 were affected by endometrial cancer, of which 241 were tested with NGS. ** Mean age of BRCA mutation carrier group. *** Mean age in EC patients.
A total of 209 BRCAm carriers from 14 studies diagnosed with BRCA1/2m were diagnosed with endometrial cancer [11,12,13,14,15,29,30,31,32,33,34,35,36,37]. A total of 9 studies out of 14 calculated the standardised incidence ratio (SIR) for the risk of uterine cancer in BRCAm women, dividing the total number of observed cases by the total number of expected cases [11,12,14,15,29,30,31,35,37]. Five authors found a statistical difference in the risk of endometrial cancer for BRCAm patients [11,15,29,30,31]. Nevertheless, two studies found a statistical difference only for BRCA1m women [11,31]. One study demonstrated an increased risk of developing aggressive and serous-like endometrial cancer, especially in BRCA1 mutation carriers [11]. The main findings of the included studies on the incidence of endometrial cancer in BRCAm patients are reported in Table 5.
Table 5.
Main findings of the included studies on the incidence of endometrial cancer in BRCAm patients.
| Author | Total Patients | Age, yr [Mean ± SD/Median (Range)] † | Total EC | EC Histopathology (n. of Patients; %) | EC with Previous Breast Cancer | EC in Patients Using Tamoxifen/Tot Patients Using Tamoxifen | History of Breast Cancer | Number of BRCA Mutated Patients | EC with BRCAm | Follow-Up [Mean/Median (Range), yr] or Women-Years (Median) | EC Risk in BRCAm (SIR [95% CI, p]) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FIGO Stage | Grade | BRCA 1 (BRCA1mEC/EC) | BRCA 2 (BRCA2mEC/EC) | Tot | |||||||||||
| Beiner et al. [30] | 857 | 54 (45–70) | 6 | I (4, 66%) II (1, 17%) NA (1, 17%) |
1 (4, 66%) 2 (1 (17%) 3 (1, 17%) |
5 | 0 | 4/226 | 551 | 619 (4/6) | 236 (2/6) | 857 | 6 | 3.3 (0.01–9.6) | 5.3 (p = 0.0011) |
| Casey et al. [13] | 101 | NA | 8 | NA | 1 (2, 25%) 2 (2, 25%) 3 (3, 38%) NA (1, 12%) |
6 | 3 | 2/8 | 6/8 | 89 (7/8) | 12 (1/8) | 101 | 8 | NA | NA |
| Kitson et al. [37] | 2609 | 20 (20–32) | 14 | I (5, 36%) II (1, 7%) III (2, 14%) NA (6, 43%) |
NA | NA | NA | NA | NA | 1350 (7/14) | 1259 (7/14) | 2609 | 14 | 59,199 (23.8) women years | 1.70 (0.74–3.33) |
| Laitman et al. [11] | 2627 | 43 ± 7.7 | 14 | NA | NA | 7 | 5 | 2/178 | 1240 | 1746 (10/14) | 1367 (4/14) | 2627 | 14 | 32.744 † women years 20.468 †† women years |
USC *** 14.29 (4.64–33.34, p < 0.001) Sarcoma *** 37.74 (10.28–96.62, p < 0.001) BRCA1 5.236 (2.659–9.382, p < 0.001) BRCA2 2.339 (0.743–5.642, p = 0.124) |
| Lee et al. [35] | 828 | 43 (34–52) | 5 | I (3, 60%) II (2, 40%) |
1 (2, 40%) 2 (1, 20%) 3 (2, 40%) |
3 | 0 | 3/160 | 419 | 438 (3/5) | 390 (2/5) | 828 | 5 | 9.0 | 2.45 (95% CI: 0.80–5.72, p = 0.11) BRCA1 2.87 (95% CI 0.59–8.43, p = 0.18) BRCA2 2.01 (95% CI 0.24–7.30, p = 0.52) |
| Minig et al. [36] | 359 | 49 ± 9.0 | 1 | I (1, 100%) | NA | 1 | 1 | NA | 225 | 223 (NA) | 141 (NA) | 359 | 1 | 2.4 (0.3–7.7) | NA |
| Reitsman et al. [14] | 315 | 43 (30–71) | 2 | I (2, 100%) | NA | 1 | 0 | 0/19 | 118 | 201 (1/2) | 114 (1/2) | 315 | 2 | 6 (0– 27) | 2.13 (0.24–7.69; p = 0.27) |
| Saule et al. [15] | 369 | BRCA1 47 ± 1.3 BRCA 2 53 ± 6.8 |
2 | IV (2, 100%) | NA | 0 | 2 | 0/5 | 0 | 238 (2/2) | 131 (0/2) | 369 | 2 | 1779 woman-years | 32.2 (11.5–116.4, p < 0.001) |
| Segev et al. [31] | 4456 | 43 | 17 | NA (17, 100%) | 1 (5, 29%) 2 (1, 6%) NA (11, 65%) |
10 | 1 | 8/697 | 1837 | 3536 (13/17) | 920 (4/17) | 4456 | 17 | 5.7 | 1.87, (1.13–2.94, p = 0.01) BRCA1 1.91 (1.06–3.19, p = 0.03) BRCA2 1.75 (0.55–4.23, p = 0.2) |
| Shu et al. [12] | 1083 | 46 (41–53) | 8 | I (5, 63%) II (2, 25%) III (1, 12%) |
NA | 4 | 5 | 3/273 | 727 | 630 (5/8) | 456 (3/8) | 1083 | 8 | 5.1 (3.0–8.4) | 1.9 (0.8–3.7, p = 0.09) |
| Thompson et al. [29] | 7106 ** | NA | 47 | NA | NA | NA | NA | NA | 1928 | 2245 (11/11) | 0 | 2245 | 11 | NA | 2.65 (1.69–4.16, p < 0.001) |
| Segev et al. [32] | 14,621 | 52 (23–67) **** | 83 | NA | NA | 46 | NA | 17/76 | 394/46 | 951 (62/83) | 76 (21/83) | 1027 | 83 | NA | NA |
| Zakhour et al. [33] | 257 | 46 (28–79) | 1 | II (1, 100%) | 3 (1, 100%) | NA | 0 | NA | 110 | 153 | 103 (1/1) | 257 | 1 | NA | NA |
| Bogani et al. [34] | 85 | 47 ± 8.2 | 1 | NA | NA | 1 | 0 | NA | 60 | 32 (1/1) | 25 | 57 | 1 | 1.5 ± 0.4 | NA |
BRCAm breast cancer gene mutated patient, EC endometrial cancer, USC uterine serous carcinoma, NA not available, BRCA1mEC breast cancer gene. One mutated patient with EC, BRCA2mEC breast cancer gene 2 mutated patient with EC. † women-years of follow up in BRCAm group. †† women-years of follow up in non-BRCA group. ** Thompson et al. included 11,847 patients, of whom 7106 were women. *** Risk in carriers group. **** Median age for cases.
4. Discussion
In this systematic review, we summarised all the studies that tested endometrial cancer patients for BRCAm and the incidence of endometrial cancer in BRCAm women. Controversial data have been found in the literature, and the correlation between BRCA mutations and uterine cancer is still debated. If a clear correlation were to be demonstrated, hysterectomy should systematically be added to bilateral salpingo-oophorectomy as a risk-reducing surgery. Shu et al. investigated the role of concomitant hysterectomy during RRSO in BRCAm patients to reduce the risk of uterine cancer and found that even though the overall risk for uterine cancer after RRSO was not increased, the risk for uterine serous cancer was increased in BRCA1m patients [12].
The Royal Australian and New Zealand College of Obstetricians and Gynaecologists classifies laparoscopic surgeries in 6 levels of complexity: 1. Diagnostic laparoscopy, 2. Salpingo-oophorectomy; 3. Laparoscopic-assisted vaginal hysterectomy; 4. Excision of ASRM stage 3 endometriosis and laparoscopic hysterectomy; 5. Laparoscopic myomectomy, excision of stage IV endometriosis; 6. excision of stage IV endometriosis necessitating bowel or urological resection, retroperitoneal lymphadenectomy, sacrocolpopexis [38]. Hence, adding hysterectomy to the risk-reducing procedures increases the degree of complexity of the procedure. This may reflect on various aspects. Firstly, a procedure that can nowadays be performed by virtually every gynaecologist may require a gynaecologist with more advanced surgical skills. Secondly, the addition of the hysterectomy to the bilateral salpingo-oophorectomy will increase operating room (OR) time and estimated blood loss, leading to an increase in direct costs linked to the length of hospital stay, medications required, OR time, etc. and indirect costs due to a loss of workdays related to the longer recovery period. Also, since the complication rate is usually related to the complexity of the procedure, a larger number of complications has to be expected. This aspect is of particular relevance when evaluating the risk-benefit balance of a prophylactic measure which is offered to women who are affected with a medical condition (BRCAm) but not with an illness. Usually, in experienced hands, the risk of iatrogenic lesions during a laparoscopic hysterectomy without additional complexity (e.g., endometriosis, fibroids, and adhesions) is very small. Furthermore, the long-term consequences of the hysterectomy need to be put into the equation, as well. A history of hysterectomy is associated with a slight increase in the risk of developing pelvic organ prolapse. In nulliparous women, who lack the most important risk factor for pelvic organ prolapse, namely having given vaginal birth, a history of hysterectomy increases the risk of developing pelvic organ prolapse by 60%. However, this increase in risk has a small clinical impact as it increases the risk from 12.7 to 20.5 per 100,000 risk years [39].
Another open question is how to process the endometrial lining of the uterus in case of risk-reducing surgery. Occult high grade serous ovarian cancer is identified in 6–17% of BRCAm carriers undergoing a risk-reducing salpingo-oophorectomy [40,41]. Nowadays, the pathological analysis of the tubes removed for risk-reducing surgery in BRCAm carriers is substantially different and more thorough as compared to when the tubes are removed secondary to another indication. In the first case, multiple sections of the ovaries and tubes should be performed to look for occult carcinoma using a specific protocol for patients at high risk of occult malignancy [40,41,42,43]. This labour-intense analysis increases the detection rate of occult ovarian or fallopian tube cancer in BRCAm women seven-fold [41]. Serous endometrial intraepithelial carcinoma (SEIC), a malignant lesion associated with p53 mutation in a background of atrophic endometrium, has been postulated to be a precursor of uterine serous carcinoma [44,45]. SEIC has been identified in 40–89% of patients diagnosed with serous endometrial cancer [46,47,48,49]. Furthermore, concordant genetic mutations have been demonstrated in both components of SEIC and serous endometrial cancer [50]. SEIC lesions may be focal and small, making the histological diagnosis and an extensive sampling of the uterus necessary to identify an invasive component [51]. This is of utmost importance since an extrauterine spread of disease in the absence of myometrial invasion has been described [51,52,53,54].
The advantages of a concomitant hysterectomy at the time of RRSO should also be taken into account for the management of menopause symptoms after surgery in BRCAm patients. Premature menopause in young women is one of the most important secondary effects of RRSO, leading to an increased risk of cardiovascular disease, bone mineral loss, and cognitive dysfunctions [55]. Many authors in the last decade have investigated the role and risks of hormone replacement therapy (HRT) in BRCAm women, and a distinction should be made between estrogen-only and combined estrogen and progestin HRT [56]. For women under 45 years of age who underwent RRSO, Kostopoulos et al. recorded a statistically significant protective effect on breast cancer of an estrogen-only HRT with an 18% risk reduction per year of treatment (95% CI, 0.69–0.97) [57]. On the contrary, a combined estrogen-progestin HRT confers a non-significative increase in breast cancer risk of 14% (95% CI 0.90–1.46) [57]. A striking pro-oncogenic role on mammary epithelial cells has been demonstrated for progesterone in a murine model [58].
In BRCA2m cancer-free women who do not want to undergo a prophylactic mastectomy, a chemoprevention strategy with Tamoxifen can be offered to reduce the incidence of breast cancer by 62% [59]. However, Tamoxifen is associated with a 2–3 fold increase in uterine malignancies [60,61,62]; therefore, hysterectomy at the time of the RRSO may be an option to avoid this risk. This option can also be considered for women with a BRCA1/2 mutation who underwent a mastectomy for breast cancer and who are taking Tamoxifen as adjuvant therapy.
5. Conclusions
This systemic review aims to provide clinicians with all recent data necessary for clear and exhaustive counselling about the benefits and risks of hysterectomy at the time of RRSO for BRCAm patients. As of now, data supporting the need to perform a hysterectomy at the time of RRSO are inconclusive, so a routine removal of the uterus should not be performed. However, this information should be discussed with the patient in order to offer a tailored approach.
Even if, to date, no guidelines recommend performing a hysterectomy as a risk-reducing procedure for HBOC syndrome, potential complications and costs of the surgical procedure (bleeding, infection, organ lesions, and vaginal cuff dehiscence) should be individually balanced with the potential increased risk of uterine cancer in this population and the reduced risks associated with an estrogen-only HRT.
Author Contributions
Conceived the idea: M.L.G.; Designed the manuscript and the analysis: M.L.G.; Reviewed the literature: S.B. and M.L.G.; Drafted the paper: S.B., I.C. and A.A.F.; Tables: S.B.; Risk of bias assessment: V.D.D. and I.C.; Editing and Revision: M.L.G. and V.D.D.; Final revision: A.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the nature of the study (systematic review).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kuchenbaecker K.B., Hopper J.L., Barnes D.R., Phillips K.-A., Mooij T.M., Roos-Blom M.-J., Jervis S., Van Leeuwen F.E., Milne R.L., Andrieu N., et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 2.Daly M.B., Pal T., Berry M.P., Buys S.S., Dickson P., Domchek S.M., Elkhanany A., Friedman S., Goggins M., Hutton M.L., et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19:77–102. doi: 10.6004/jnccn.2021.0001. [DOI] [PubMed] [Google Scholar]
- 3.Eleje G.U., Eke A.C., Ezebialu I.U., Ikechebelu J.I., Ugwu E.O., Okonkwo O.O. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst. Rev. 2018;8:CD012464. doi: 10.1002/14651858.CD012464.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domchek S.M., Friebel T.M., Singer C.F., Evans D.G., Lynch H.T., Isaacs C., Garber J.E., Neuhausen S.L., Matloff E., Eeles R., et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finch A., Beiner M., Lubinski J., Lynch H.T., Moller P., Rosen B., Murphy J., Ghadirian P., Friedman E., Foulkes W.D., et al. Hereditary Ovarian Cancer Clinical Study Group. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA. 2006;296:185–192. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 6.Hu C., Hart S.N., Polley E.C., Gnanaolivu R., Shimelis H., Lee K.Y., Lilyquist J., Na J., Moore R., Antwi S.O., et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA. 2018;319:2401–2409. doi: 10.1001/jama.2018.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanagh H., Rogers K.M. The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers. Hered. Cancer Clin. Pract. 2015;13:16. doi: 10.1186/s13053-015-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barak F., Milgrom R., Laitman Y., Gemer O., Rabinovich A., Piura B., Anteby E., Baruch G.B., Korach J., Friedman E. The rate of the predominant Jewish mutations in the BRCA1, BRCA2, MSH2 and MSH6 genes in unselected Jewish endometrial cancer patients. Gynecol. Oncol. 2010;119:511–515. doi: 10.1016/j.ygyno.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg P., Blom R., Högberg T., Simonsen E. Death rate and recurrence pattern among 841 clinical stage I endometrial cancer patients with special reference to uterine papillary serous carcinoma. Gynecol. Oncol. 1993;51:311–315. doi: 10.1006/gyno.1993.1296. [DOI] [PubMed] [Google Scholar]
- 10.de Boer S.M., Powell M.E., Mileshkin L., Katsaros D., Bessette P., Haie-Meder C., Ottevanger P.B., Ledermann J.A., Khaw P., Colombo A. PORTEC study group. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laitman Y., Michaelson-Cohen R., Levi E., Chen-Shtoyerman R., Reish O., Josefsberg Ben-Yehoshua S., Bernstein-Molho R., Keinan-Boker L., Rosengarten O., Silverman B.G., et al. Uterine cancer in Jewish Israeli BRCA1/2 mutation carriers. Cancer. 2019;125:698–703. doi: 10.1002/cncr.31842. [DOI] [PubMed] [Google Scholar]
- 12.Shu C.A., Pike M.C., Jotwani A.R., Friebel T.M., Soslow R.A., Levine D.A., Nathanson K.L., Konner J.A., Arnold A.G., Bogomolniy F., et al. Uterine Cancer After Risk-Reducing Salpingo-oophorectomy Without Hysterectomy in Women With BRCA Mutations. JAMA Oncol. 2016;2:1434–1440. doi: 10.1001/jamaoncol.2016.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey M.J., Bewtra C., Lynch H.T., Snyder C.L., Stacey M. Endometrial cancers in mutation carriers from hereditary breast ovarian cancer syndrome kindreds: Report from the Creighton University Hereditary Cancer Registry with review of the implications. Int. J. Gynecol. Cancer. 2015;25:650–656. doi: 10.1097/IGC.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 14.Reitsma W., Mourits M.J., de Bock G.H., Hollema H. Endometrium is not the primary site of origin of pelvic high-grade serous carcinoma in BRCA1 or BRCA2 mutation carriers. Mod. Pathol. 2013;26:572–578. doi: 10.1038/modpathol.2012.169. [DOI] [PubMed] [Google Scholar]
- 15.Saule C., Mouret-Fourme E., Briaux A., Becette V., Rouzier R., Houdayer C., Stoppa-Lyonnet D. Risk of Serous Endometrial Carcinoma in Women with Pathogenic BRCA1/2 Variant After Risk-Reducing Salpingo-Oophorectomy. J. Natl. Cancer Inst. 2018;110:213–215. doi: 10.1093/jnci/djx159. [DOI] [PubMed] [Google Scholar]
- 16.Nahshon C., Segev Y., Gemer O., Bar Noy T., Schmidt M., Ostrovsky L., Lavie O. Should the risk for uterine cancer influence decision making for prophylactic hysterectomy in BRCA1/2 mutated patients—a systematic review and meta-analysis. Gynecol. Oncol. 2021;160:755–762. doi: 10.1016/j.ygyno.2020.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niederacher D., An H.X., Camrath S., Dominik S.I., Göhring U.J., Oertel A., Grass M., Hantschmann P., Lordnejad M.R., Beckmann M.W. Loss of heterozygosity of BRCA1, TP53 and TCRD markers analysed in sporadic endometrial cancer. Eur. J. Cancer. 1998;34:1770–1776. doi: 10.1016/S0959-8049(98)00270-6. [DOI] [PubMed] [Google Scholar]
- 20.Goshen R., Chu W., Elit L., Pal T., Hakimi J., Ackerman I., Fyles A., Mitchell M., Narod S.A. Is uterine papillary serous adenocarcinoma a manifestation of the hereditary breast-ovarian cancer syndrome? Gynecol. Oncol. 2000;79:477–481. doi: 10.1006/gyno.2000.6003. [DOI] [PubMed] [Google Scholar]
- 21.Levine D.A., Lin O., Barakat R.R., Robson M.E., McDermott D., Cohen L., Satagopan J., Offit K., Boyd J. Risk of endometrial carcinoma associated with BRCA mutation. Gynecol. Oncol. 2001;80:395–398. doi: 10.1006/gyno.2000.6082. [DOI] [PubMed] [Google Scholar]
- 22.Lavie O., Hornreich G., Ben-Arie A., Rennert G., Cohen Y., Keidar R., Sagi S., Lahad E.L., Auslander R., Beller U. BRCA germline mutations in Jewish women with uterine serous papillary carcinoma. Gynecol. Oncol. 2004;92:521–524. doi: 10.1016/j.ygyno.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Biron-Shental T., Drucker L., Altaras M., Bernheim J., Fishman A. High incidence of BRCA1-2 germline mutations, previous breast cancer and familial cancer history in Jewish patients with uterine serous papillary carcinoma. Eur. J. Surg. Oncol. 2006;32:1097–1100. doi: 10.1016/j.ejso.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Bruchim I., Amichay K., Kidron D., Attias Z., Biron-Shental T., Drucker L., Friedman E., Werner H., Fishman A. BRCA1/2 germline mutations in Jewish patients with uterine serous carcinoma. Int. J. Gynecol. Cancer. 2010;20:1148–1153. doi: 10.1111/IGC.0b013e3181ef622d. [DOI] [PubMed] [Google Scholar]
- 25.Pennington K.P., Walsh T., Lee M., Pennil C., Novetsky A.P., Agnew K.J., Thornton A., Garcia R., Mutch D., King M.-C., et al. BRCA1, TP53, and CHEK2 germline mutations in uterine serous carcinoma. Cancer. 2013;119:332–338. doi: 10.1002/cncr.27720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahdi H., Xiu J., Reddy S.K., DeBernardo R. Alteration in PI3K/mTOR, MAPK pathways and Her2 expression/amplification is more frequent in uterine serous carcinoma than ovarian serous carcinoma. J. Surg. Oncol. 2015;112:188–194. doi: 10.1002/jso.23993. [DOI] [PubMed] [Google Scholar]
- 27.Kadan Y., Raviv O., Segev Y., Lavie O., Bruchim I., Fishman A., Michaelson R., Beller U., Helpman L. Impact of BRCA mutations on outcomes among patients with serous endometrial cancer. Int. J. Gynaecol. Obstet. 2018;142:91–96. doi: 10.1002/ijgo.12486. [DOI] [PubMed] [Google Scholar]
- 28.Vietri M.T., D’Elia G., Caliendo G., Casamassimi A., Federico A., Passariello L., Cioffi M., Molinari A.M. Prevalence of mutations in BRCA and MMR genes in patients affected with hereditary endometrial cancer. Med. Oncol. 2021;38:13. doi: 10.1007/s12032-021-01454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson D., Easton D.F., Breast Cancer Linkage Consortium Cancer Incidence in BRCA1 mutation carriers. J. Natl. Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 30.Beiner M.E., Finch A., Rosen B., Lubinski J., Moller P., Ghadirian P., Lynch H.T., Friedman E., Sun P., Narod S.A. Hereditary Ovarian Cancer Clinical Study Group. The risk of endometrial cancer in women with BRCA1 and BRCA2 mutations. A prospective study. Gynecol. Oncol. 2007;104:7–10. doi: 10.1016/j.ygyno.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Segev Y., Iqbal J., Lubinski J., Gronwald J., Lynch H.T., Moller P., Ghadirian P., Rosen B., Tung N., Kim-Sing C., et al. Hereditary Breast Cancer Study Group. The incidence of endometrial cancer in women with BRCA1 and BRCA2 mutations: An international prospective cohort study. Gynecol. Oncol. 2013;130:127–131. doi: 10.1016/j.ygyno.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 32.Segev Y., Rosen B., Lubinski J., Gronwald J., Lynch H.T., Moller P., Kim-Sing C., Ghadirian P., Karlan B., Eng C., et al. Hereditary Breast Cancer Study Group. Risk factors for endometrial cancer among women with a BRCA1 or BRCA2 mutation: A case control study. Fam. Cancer. 2015;14:383–391. doi: 10.1007/s10689-015-9798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zakhour M., Danovitch Y., Lester J., Rimel B.J., Walsh C.S., Li A.J., Karlan B.Y., Cass I. Occult and subsequent cancer incidence following risk-reducing surgery in BRCA mutation carriers. Gynecol. Oncol. 2016;143:231–235. doi: 10.1016/j.ygyno.2016.08.336. [DOI] [PubMed] [Google Scholar]
- 34.Bogani G., Tagliabue E., Signorelli M., Chiappa V., Carcangiu M.L., Paolini B., Casarin J., Scaffa C., Gennaro M., Martinelli F., et al. Assessing the Risk of Occult Cancer and 30-day Morbidity in Women Undergoing Risk-reducing Surgery: A Prospective Experience. J. Minim. Invasive Gynecol. 2017;24:837–842. doi: 10.1016/j.jmig.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Lee Y.C., Milne R.L., Lheureux S., Friedlander M., McLachlan S.A., Martin K.L., Bernardini M.Q., Smith C., Picken S., Nesci S., et al. Risk of uterine cancer for BRCA1 and BRCA2 mutation carriers. Eur. J. Cancer. 2017;84:114–120. doi: 10.1016/j.ejca.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Kitson S.J., Bafligil C., Ryan N.A.J., Lalloo F., Woodward E.R., Clayton R.D., Edmondson R.J., Bolton J., Crosbie E.J., Evans D.G. BRCA1 and BRCA2 pathogenic variant carriers and endometrial cancer risk: A cohort study. Eur. J. Cancer. 2020;136:169–175. doi: 10.1016/j.ejca.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.RANZCOG (Royal Australian and New Zealand College of Obstetricians & Gynaecologists) Guidelines for Performing Gynaecological Endoscopic Procedures. 2019. [(accessed on 4 May 2022)]. Available online: https://ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG-MEDIA/Women%27s%20Health/Statement%20and%20guidelines/Clinical%20-%20Training/Guidelines-for-performing-gynaecological-endoscopic-procedures-(C-Trg-2).pdf?ext=.pdf.
- 38.Husby K.R., Gradel K.O., Klarskov N. Pelvic organ prolapse following hysterectomy on benign indication: A nationwide, nulliparous cohort study. Am. J. Obstet. Gynecol. 2022;226:386.e1–386.e9. doi: 10.1016/j.ajog.2021.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Callahan M.J., Crum C.P., Medeiros F., Kindelberger D.W., Elvin J.A., Garber J.E., Feltmate C.M., Berkowitz R.S., Muto M.G. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J. Clin. Oncol. 2007;25:3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 40.Powell C.B., Kenley E., Chen L.M., Crawford B., McLennan J., Zaloudek C., Komaromy M., Beattie M., Ziegler J. Risk-reducing salpingo-oophorectomy in BRCA mutation carriers: Role of serial sectioning in the detection of occult malignancy. J. Clin. Oncol. 2005;23:127–132. doi: 10.1200/JCO.2005.04.109. [DOI] [PubMed] [Google Scholar]
- 41.Eisen A., Rebbeck T.R., Wood W.C., Weber B.L. Prophylactic surgery in women with a hereditary predisposition to breast and ovarian cancer. J. Clin. Oncol. 2000;18:1980–1995. doi: 10.1200/JCO.2000.18.9.1980. [DOI] [PubMed] [Google Scholar]
- 42.Leeper K., Garcia R., Swisher E., Goff B., Greer B., Paley P. Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol. Oncol. 2002;87:52–56. doi: 10.1006/gyno.2002.6779. [DOI] [PubMed] [Google Scholar]
- 43.Sherman M.E., Bitterman P., Rosenshein N.B., Delgado G., Kurman R.J. Uterine serous carcinoma. A morphologically diverse neoplasm with unifying clinicopathologic features. Am. J. Surg. Pathol. 1992;16:600–610. doi: 10.1097/00000478-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Ambros R.A., Sherman M.E., Zahn C.M., Bitterman P., Kurman R.J. Endometrial intraepithelial carcinoma: A distinctive lesion specifically associated with tumors displaying serous differentiation. Hum. Pathol. 1995;26:1260–1267. doi: 10.1016/0046-8177(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 45.Yadav S., Agarwal A., Mokal S., Menon S., Rekhi B., Deodhar K. Serous endometrial intraepithelial carcinoma: A clinico-pathological study of 48 cases and its association with endometrial polyps—A tertiary care oncology centre experience. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021;264:168–172. doi: 10.1016/j.ejogrb.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Tolcher M.C., Swisher E.M., Medeiros F., Lima J.F., Hilderbrand J.L., Donovan J.L., Garcia R.L., Cliby W.A., Dowdy S.C. Characterisation of precursor lesions in the endometrium and fallopian tube epithelium of early-stage uterine serous carcinoma. Int. J. Gynecol. Pathol. 2015;34:57–64. doi: 10.1097/PGP.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherman M.E., Bur M.E., Kurman R.J. p53 in endometrial cancer and its putative precursors: Evidence for diverse pathways of tumorigenesis. Hum. Pathol. 1995;26:1268–1274. doi: 10.1016/0046-8177(95)90204-X. [DOI] [PubMed] [Google Scholar]
- 48.Kurman R.J., Ellenson L.H. Blaustein’s Pathology of the Female Genital Tract. 7th ed. Springer; Boston, MA, USA: 2011. [Google Scholar]
- 49.Kawata M., Miyoshi A., Fujikawa E., Kanao S., Takeda M., Mimura M., Nagamatsu M., Yokoi T. Serous Endometrial Intraepithelial Carcinoma: Case Report and Literature Review. J. Clin. Gynecol. Obstet. 2017;6:49–52. doi: 10.14740/jcgo446w. [DOI] [Google Scholar]
- 50.Baergen R.N., Warren C.D., Isacson C., Ellenson L.H. Early uterine serous carcinoma: Clonal origin of extrauterine disease. Int. J. Gynecol. Pathol. 2001;20:214–219. doi: 10.1097/00004347-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Yan Z., Hui P. Minimal uterine serous carcinoma with extrauterine tumor of identical morphology: An immunohistochemical study of 13 cases. Appl. Immunohistochem. Mol. Morphol. 2010;18:75–79. doi: 10.1097/PAI.0b013e3181b1d10e. [DOI] [PubMed] [Google Scholar]
- 52.Furuya M., Sato T., Tanaka R., Yamamoto M., Yokota N.R., Miyagi E. Metachronous serous endometrial intraepithelial carcinoma and serous peritoneal carcinoma: Analysis of probable independent lesions. Diagn. Pathol. 2016;11:130. doi: 10.1186/s13000-016-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawano K., Ushijima K., Yokomine M., Fukui A., Ijichi M., Kamura T. A case of minimal uterine serous carcinoma with distant lymph node metastasis without peritoneal dissemination. J. Gynecol. Oncol. 2011;22:53–56. doi: 10.3802/jgo.2011.22.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guidozzi F. Hormone therapy after prophylactic risk-reducing bilateral salpingo-oophorectomy in women who have BRCA gene mutation. Climacteric. 2016;19:419–422. doi: 10.1080/13697137.2016.1209396. [DOI] [PubMed] [Google Scholar]
- 55.Gasparri M.L., Taghavi K., Fiacco E., Zuber V., Di Micco R., Gazzetta G., Valentini A., Mueller M.D., Papadia A., Gentilini O.D. Risk-Reducing Bilateral Salpingo-Oophorectomy for BRCA Mutation Carriers and Hormonal Replacement Therapy: If It Should Rain, Better a Drizzle than a Storm. Medicina. 2019;55:415. doi: 10.3390/medicina55080415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kotsopoulos J., Gronwald J., Karlan B.Y., Huzarski T., Tung N., Moller P., Armel S., Lynch H.T., Senter L., Eisen A., et al. Singer CF, Foulkes WD, Jacobson MR, Sun P, Lubinski J, Narod SA.; Hereditary Breast Cancer Clinical Study Group. Hormone Replacement Therapy After Oophorectomy and Breast Cancer Risk Among BRCA1 Mutation Carriers. JAMA Oncol. 2018;4:1059–1065. doi: 10.1001/jamaoncol.2018.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poole A.J., Li Y., Kim Y., Lin S.C., Lee W.H., Lee E.Y. Prevention of BRCA1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science. 2006;314:1467–1470. doi: 10.1126/science.1130471. [DOI] [PubMed] [Google Scholar]
- 58.King M.C., Wieand S., Hale K., Lee M., Walsh T., Owens K., Tait J., Ford L., Dunn B.K., Costantino J., et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286:2251–2256. doi: 10.1001/jama.286.18.2251. [DOI] [PubMed] [Google Scholar]
- 59.Committee Opinion No. 601: Tamoxifen and uterine cancer. Obstet. Gynecol. 2014;123:1394–1397. doi: 10.1097/01.AOG.0000450757.18294.cf. [DOI] [PubMed] [Google Scholar]
- 60.Cohen I., Altaras M.M., Shapira J., Tepper R., Rosen D.J., Cordoba M., Zalel Y., Figer A., Yigael D., Beyth Y. Time-dependent effect of tamoxifen therapy on endometrial pathology in asymptomatic postmenopausal breast cancer patients. Int. J. Gynecol. Pathol. 1996;15:152–157. doi: 10.1097/00004347-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Machado F., Rodríguez J.R., León J.P., Rodríguez J.R., Parrilla J.J., Abad L. Tamoxifen and endometrial cancer. Is screening necessary? A review of the literature. Eur. J. Gynaecol. Oncol. 2005;26:257–265. [PubMed] [Google Scholar]
- 62.Fisher B., Costantino J.P., Redmond C.K., Fisher E.R., Wickerham D.L., Cronin W.M. Endometrial cancer in tamoxifen-treated breast cancer patients: Findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J. Natl. Cancer Inst. 1994;86:527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]