Abstract
Germination experiments with specific germination mutants of Bacillus subtilis, including a newly isolated mutant affected in pressure-induced germination, suggest that a pressure of 100 MPa triggers the germination cascades that are induced by the nutrient germinant alanine (Ala) and by a mixture of asparagine, glucose, fructose, and potassium ions (AGFK), by activating the receptors for alanine and asparagine, GerA and GerB, respectively. As opposed to germination at 100 MPa, germination at 600 MPa apparently shortcuts at least part of the Ala- and AGFK-induced germination pathways. Inhibitors of nutrient-induced germination (HgCl2 and Nα-P-tosyl-l-arginine methyl ester) also inhibit pressure-induced germination at 600 MPa, suggesting that germination at 600 MPa involves activation of a true physiological germination pathway and is therefore not merely a physico-chemical process in which water is forced into the spore protoplast.
The capacity to form endospores endows Bacillus subtilis with the ability to survive in adverse environmental conditions. However, the success of this survival strategy depends on the presence of an efficient mechanism for returning to the vegetative state under favorable conditions (14). A wide range of chemical and physical effectors can trigger germination of spores (5). In B. subtilis, the two best-known germination pathways are those induced by the chemical effectors alanine (Ala) and the combination of asparagine (Asn), glucose (Glu), fructose (Fru), and potassium ions (AGFK) (13, 14). While the first steps of the Ala- and AGFK-induced germination cascades are different, both cascades converge in a later stage. This has been demonstrated by the isolation of specific mutants defective in the Ala response (gerA), in the AGFK response (gerB and gerK), and in both responses (gerD). It was subsequently hypothesized that the gerA, gerB, and gerK gene products are receptors of the germinants Ala, Asn, and Glu, respectively (4, 12, 14, 18).
The gerA-encoded Ala receptor of B. subtilis is sufficient to trigger spore germination, while the gerB-encoded Asn receptor cannot trigger spore germination in absence of the Glu receptor (gerK) and Fru receptor (encoding gene unknown) (4, 12, 14, 18). Finally, Ala may also interact with the Asn receptor and induce germination, albeit with lower efficiency (12, 18).
The function of the gerD gene product is unclear but it must have an essential role in AGFK-induced germination since mutation of the gerD gene blocks the AGFK-induced germination. Although not essential, the gerD gene product is also involved in Ala-induced germination, since gerD mutants germinate very slowly in the presence of Ala (9, 13, 14, 19).
Little is known about the biochemical reactions involved in triggering spore germination, but activity of the major catabolic and anabolic pathways, and metabolic conversion of the germinant in particular, seems not to be required (10). Binding of the germinant to its receptor is believed to generate some sort of allosteric conformational change in this receptor (14, 20). The receptor itself or another protein could in turn act as an ion transporter or as an ion channel (14) to promote the efflux of Ca2+ and other ions and the influx of water in the spore, ultimately resulting in the activation of preformed, spore-specific degradative enzymes involved in cortex degradation (11, 14). Alternatively, the conformational change of the germinant receptor could activate these hydrolytic enzymes first, e.g. by proteolytic cleavage. The redistribution of ions and water in the spore protoplast would then be the consequence of cortex degradation.
As opposed to the mechanisms of nutrient-induced germination, the mechanisms of pressure-induced germination remain largely unknown. Based on the observation that some inhibitors of nutrient-induced germination also inhibited pressure-induced germination of Bacillus cereus and Bacillus megaterium, Gould and Sale (6) hypothesized that pressure-induced germination proceeds by activation of enzymes in the nutrient-induced germination cascade. Furthermore, it was observed that spores from which cations, including those in the core, have been exchanged with protons by exposure of the spores to a very acidic environment (so-called H-spores) are defective in pressure- and nutrient-induced germination (1, 15). In an earlier study, we demonstrated that spores germinated at a pressure of 100 MPa undergo a number of events similar to those during nutrient-induced germination and lose their resistance to various stresses to a similar degree as the latter. In contrast, spores germinated at 600 MPa retain their resistance to some stresses and do not initiate two key enzymatic reactions of nutrient-induced germination: the degradation of small, acid-soluble proteins and the rapid generation of ATP (21).
The objective of the present work was to investigate in greater detail the pathways of pressure-induced germination at 100 and 600 MPa and their relationship to the pathways of nutrient-induced germination in B. subtilis.
MATERIALS AND METHODS
Production of B. subtilis spore suspensions.
Strains of B. subtilis used in this study are listed in Table 1. To induce sporulation, cells from a −80°C glycerol stock culture were grown at 37°C in a humid atmosphere on the surface of nutrient agar CM3 (Oxoid, Basingstoke, United Kingdom) supplemented with 0.06 g of MgSO4 per liter and 0.25 g of KH2PO4 per liter. After 7 days, spores were harvested from the plates in sterile deionized water, washed twice, and were finally resuspended in sterile deionized water to a concentration of 107 to 108 spores ml−1, and the spores were kept at 4°C for up to 1 month.
TABLE 1.
Strains used in this study
| Strain | Relevant characteristics | Source |
|---|---|---|
| LMG7135 | B. subtilis Marburg | LMG culture collection, Gent, Belgium |
| LMM2021 | Derived from LMG7135 | This study |
| Deficient in germination at 100 MPa | ||
| 1604 | Derived from B. subtilis 168 | A. Moir |
| 1604AΔB | Derived from B. subtilis 168, gerAΔgerB | A. Moir |
| 1G7 | Derived from B. subtilis 168, gerA11 | Bacillus Genetic Stock Center, Columbus, Ohio |
| 1G8 | Derived from B. subtilis 168, gerB18 | Bacillus Genetic Stock Center |
| 1G9 | Derived from B. subtilis 168, gerD19 | Bacillus Genetic Stock Center |
| PY79 | Wild type | C. D. Webb |
Germination treatment.
For pressure-induced germination, aqueous spore suspensions were diluted twofold with 100 mM potassium phosphate buffer (pH 7) and were pressurized (100 or 600 MPa at 40°C) in heat-sealed sterile polyethylene bags in an 8-ml pressure vessel thermostatted with an external water jacket (Resato, Roden, The Netherlands). The compression rate was approximately 100 MPa/min, while decompression was immediate.
For nutrient-induced germination, aqueous spore suspensions were diluted twofold with germinant solution and were incubated for 5 h at 37°C. The germinant solutions used were 50 mM potassium phosphate buffer (pH 7) containing 10 mM l-Ala (Ala-induced germination) or 50 mM potassium phosphate buffer (pH 7) containing 10 mM l-Asn, 10 mM d-Glu, and 10 mM d-Fru (AGFK-induced germination).
To determine the degree of germination, treated and control spore suspensions were subjected to a heat treatment (80°C for 15 min) and were subsequently serially diluted, plated on the surface of tryptic soy agar (Oxoid), and were incubated for 24 h at 37°C. The heat treatment of 15 min at 80°C was found not to kill ungerminated native spores. Because the heat sensitivity of H-spores is higher than that of native spores (2), the heat treatment to determine the degree of germination of H-spores was 10 min at 60°C instead of 15 min at 80°C. The percentage of ungerminated spores was expressed as the ratio of the colony counts of the treated sample to the colony counts of the control sample multiplied by 100.
Selection of mutants affected in pressure-induced germination.
A selection procedure consisting of consecutive enrichment cycles was developed to isolate B. subtilis mutants defective in pressure-induced germination. Each enrichment cycle consisted of three steps. In the first step, spores were subjected to a pressure treatment (100 or 600 MPa at 40°C) to induce germination. In the second step, the pressure-germinated spores were killed by a heat treatment (80°C for 15 min). Only ungerminated spores survived this heat treatment. In the third step, the surviving spores were plated on nutrient agar and were allowed to germinate, grow, and sporulate again. These spores were then harvested and were subjected to a new enrichment cycle. In principle, this procedure should result in mutants with reduced pressure germination but which are still capable of germination on nutrient agar. A similar approach has allowed the isolation of highly pressure-resistant mutants of Escherichia coli MG1655 (7).
Preparation of H-spores.
Aqueous spore suspensions of B. subtilis PY79 were diluted fourfold with 1 M HCl. After 3 h of incubation at room temperature, the spore suspensions were centrifuged (2,650 × g for 15 min) and were resuspended in 50 mM phosphate buffer (pH 7). The successful formation of H-spores was confirmed by demonstrating their sensitivity at 80°C and resistance at 60°C (2). PY79 as a wild-type strain was used for the preparation of H-spore suspensions, because we have not succeeded in reproducibly isolating H-spores from strains LMG7135 and 1604.
Chemical inhibition of pressure-induced germination.
Aqueous spore suspensions, diluted fivefold with 50 mM phosphate buffer (pH 7) containing an inhibitor of nutrient-induced germination, were pressure treated (100 or 600 MPa at 40°C for 20 min). Inhibitors used were Nα-P-tosyl-l-arginine methyl ester (50 mM) (TAME) (3, 18), HgCl2 (3 mM) (8, 18), and phenol (0.2% [wt/vol]) (16).
To estimate the degree of germination inhibition exerted by an inhibitor, germination in the presence and absence of inhibitor was not measured by differential plating of heat-treated germinated and control spore suspensions as described above, but by measuring the decrease in optical density at 600 nm (OD600) that accompanies spore germination. This was necessary because the toxicity of HgCl2 precludes the formation of colonies. The OD600 of the spore suspension with and without an inhibitor before and after germination were measured, and the percentage of inhibition exerted by the inhibitor was then calculated as follows:
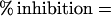 |
 |
Reproducibility of results.
The data presented are either means of three replicate experiments or from a single representative experiment.
RESULTS
Selection of B. subtilis mutants affected in pressure-induced germination.
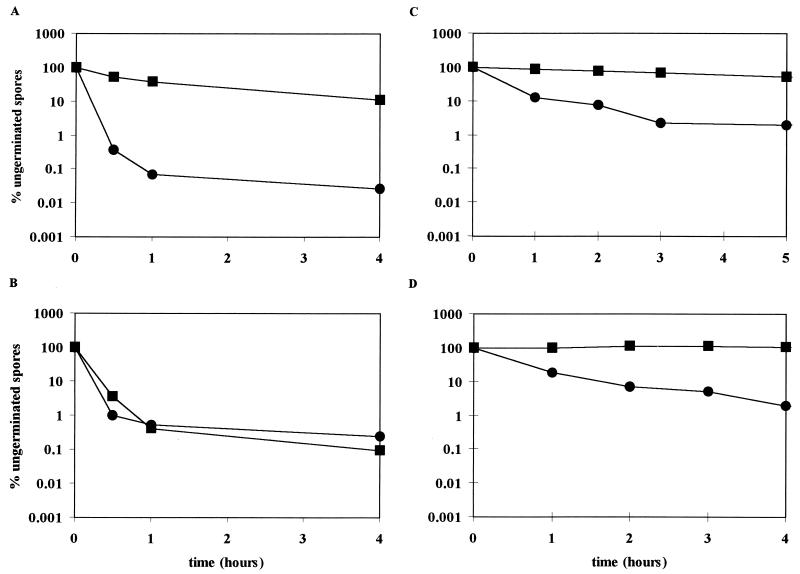
We attempted to select B. subtilis mutants showing reduced germination under pressure by using a specific enrichment procedure. After each enrichment cycle at 100 MPa, the resulting spores were found to be less susceptible to pressure-induced germination at 100 MPa. Therefore, the duration of the pressurization in the first step of the enrichment cycle was gradually increased in the consecutive enrichment steps in order to maintain a sufficient level of germination. Four selection rounds at 100 MPa resulted in mutant LMM2021, which germinated much slower at 100 MPa than the wild-type LMG7135 from which it was derived (Fig. 1A). The germination deficiency of this strain at 100 MPa was stable upon repeated subcultivation. Similar attempts were undertaken to produce a B. subtilis mutant that is unable to germinate at 600 MPa by using a pressure treatment of 600 MPa during the selection procedure. However, even after 10 enrichment cycles, these attempts remained unsuccessful.
FIG. 1.
Germination of spores of B. subtilis LMG7135 (wild type) (●) and LM2021 (pressure-selected mutant) (■) induced by 100 MPa (A), 600 MPa (B), Ala (C), or AGFK (D).
Effect of different germination stimuli on specific germination mutants.
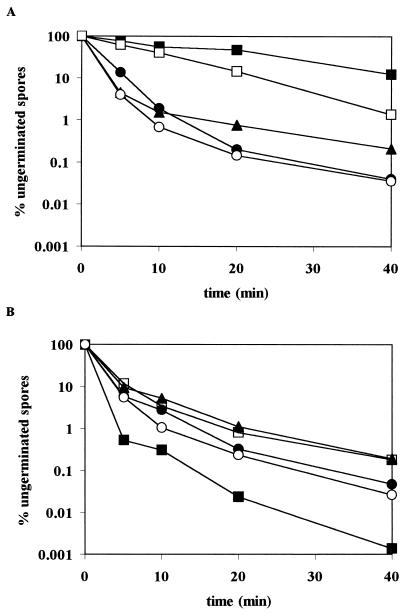
To investigate the relationship between the pathways of pressure-induced and nutrient-induced spore germination, the germination responses of the pressure-selected mutant LMM2021 (Fig. 1) and the nutrient-induced germination mutants gerA, gerB, gerD, and gerA gerB (Fig. 2) to both pressure and nutrient stimuli were compared.
FIG. 2.
Germination of spores of B. subtilis. Symbols: wild-type strain (●), gerA strain (▴), gerB strain (○), gerD strain (□), and gerA gerB strain (■) induced by 100 MPa (A) or 600 MPa (B).
Figure 1 shows that while spores of the selected mutant LMM2101 could not be induced to germinate at 100 MPa, they germinated at wild-type levels at 600 MPa. Interestingly, LMM2101 spores also did not germinate in the presence of Ala nor in the presence of AGFK.
All four nutrient-induced germination mutants germinated normally at 600 MPa or, in the case of the gerA gerB mutant, even better than the wild type (Fig. 2B). At 100 MPa, the gerA and gerB single mutants germinated normally, but the mutants that are affected in both Ala- and AGFK-induced germination (gerA gerB double mutant and gerD mutant) were also affected in pressure-induced germination at 100 MPa. Germination induced at 100 MPa was consistently more inhibited in the gerA gerB mutant than in the gerD mutant (Fig. 2A).
Germination response of H-spores.
The germination response of native and H-spores has been compared (Table 2). From the results, it was confirmed that the exchange of spore minerals by protons inhibits Ala-induced and AGFK-induced germination. Pressure-induced germination of H-spores was also completely inhibited at 100 MPa, but only slightly at 600 MPa.
TABLE 2.
Germination of native spores and H-spores of B. subtilis PY79
| Type | Germination with:
|
|||
|---|---|---|---|---|
| 100 MPaa | 600 MPaa | Alab | AGFKb | |
| Native spores | 0.050 | 0.013 | 0.81 | 0.48 |
| H-spores | 35 | 0.25 | 35 | 16 |
Percentage of ungerminated spores determined after 1 h of pressurization at 40°C.
Percentage of ungerminated spores determined after 5 h of incubation at 37°C.
Effect of inhibitors of nutrient-induced spore germination.
The effects of different inhibitors (TAME, HgCl2, and phenol) on nutrient- and pressure-induced germination at 100 and 600 MPa were compared (Table 3). Two inhibitors (HgCl2 and phenol) completely blocked both Ala- and AGFK-induced germination, and these were found to also completely block 100-MPa-induced germination. TAME inhibited only one of the two nutrient-dependent germination pathways completely, and it was found to exert only partial inhibition of 100-MPa-induced germination. Inhibition of germination at 600 MPa was either nearly complete (HgCl2), partial (TAME), or marginal (phenol).
TABLE 3.
Percentage inhibition of pressure-induced germination by OD600a
| Inhibitor | Germination with:
|
|||
|---|---|---|---|---|
| 100 MPa | 600 MPa | Ala | AGFK | |
| 3 mM HgCl2 | 120 ± 10 | 90 ± 18 | 97 ± 1 | 91 ± 4 |
| 50 mM TAME | 48 ± 22 | 41 ± 10 | 49 ± 17 | 101 ± 2 |
| 0.2% Phenol | 97 ± 18 | 10 ± 23 | 80 ± 12 | 97 ± 25 |
Values shown ± standard deviation.
DISCUSSION
Although it is well known that pressure may induce germination of bacterial spores, the factors affecting pressure-induced germination and its cellular and biochemical basis are poorly understood. In this study, it was investigated if the B. subtilis germination pathways triggered by nutrients (Ala and AGFK) and pressure share common steps. Two different pressure levels (100 and 600 MPa) were used throughout this study, because we have previously demonstrated some remarkable differences between germination at 100 and 600 MPa (21).
In a first step, we attempted to select mutants affected in pressure-induced germination. Mutants unable to germinate at 100 MPa could be readily obtained after sequential enrichment of the spores remaining ungerminated after treatment at 100 MPa. In contrast, up to 10 consecutive rounds of enrichment by the same procedure but at 600 MPa did not result in mutants affected in germination at 600 MPa. Interestingly, the mutant unable to germinate at 100 MPa germinated normally at 600 MPa, but was completely blocked in Ala- and AGFK-induced germination (Fig. 1). These observations suggest that the pathway of germination at 100 MPa shares one or more steps with Ala- and AGFK-induced germination. Previously, based on the observation that degradation of small, acid-soluble proteins and rapid ATP formation do not occur in spores germinated at 600 MPa, we proposed that germination at 600 MPa, as opposed to germination at 100 MPa (21), would be arrested at an early stage. These results additionally suggest that germination at 600 MPa involves a pathway or mechanism that is at least partly different from that followed at 100 MPa.
To further investigate the relationship between the pathways of pressure-induced and nutrient-induced germination, we analyzed the response of existing mutants affected in nutrient-induced germination to pressure treatments at 100 and 600 MPa. The observation that the nutrient-induced germination mutants gerA gerB and gerD are affected in pressure-induced germination at 100 MPa but not at 600 MPa (Fig. 2) supports the idea of a different pathway for germination at 100 and 600 MPa. Further, since it is widely accepted that gerA and gerB encode the receptors for Ala and Asn, respectively, it can be concluded from these results that treatment at 100 MPa can activate the germination cascade at the level of these receptors. The fact that the gerA and gerB mutants, in contrast to the gerA gerB mutant, germinate normally at 100 MPa indicates that both the Ala (gerA) and Asn (gerB) receptors are susceptible to activation at 100 MPa. In this way, a mutant that is deficient in only one of the nutrient-induced germination pathways will still germinate at 100 MPa. Possibly, low pressure activates the nutrient receptors by inducing conformational changes in their active sites. The fact that this activation occurs both in the Ala receptor and the Asn receptor can probably be explained by the high degree of homology between the sequences of the gerA and gerB operons, which are believed to have evolved by gene duplication and subsequent divergence from a common ancestral operon (4, 14). Germination at 600 MPa apparently shortcuts the Ala and AGFK germination pathways at least partly, since neither gerA or gerB, nor gerD, which is situated further downstream in the germination cascade, are required for germination at 600 MPa. B. subtilis spores contain a high concentration of cations (2, 17). It has been previously shown that the exchange of spore cations with protons not only reduces the heat resistance (2) but also the germination response of B. megaterium spores to pressure and nutrient germinants (1, 15). In the present study, we demonstrated that H-spores of B. subtilis are unable to germinate in Ala, in AGFK, or at 100 MPa (Table 2). Germination occurred at 600 MPa, although to a slightly lower extent than for the native spores. Therefore, the presence of spore cations appears to be important in the Ala-, AGFK-, and 100-MPa-induced germination cascades, while they have a minor role in the 600-MPa-induced germination cascade. Again, these observations support the existence of a different pathway for germination at 600 MPa.
A final experiment to characterize the germination pathways followed under different conditions examined the effect of specific inhibitors of germination. Gould and Sale (6) already observed that inhibitors of nutrient-induced germination also inhibited pressure-induced germination of B. cereus and Bacillus coagulans spores, but their experiments were done only at low pressure (at 40 and 60 MPa, respectively). In our work, we compared the effect of some known inhibitors of nutrient-induced germination (TAME, HgCl2, and phenol) on germination by Ala, AGFK, and 100 and 600 MPa of pressure (Table 3). The results from this experiment confirm and complement the conclusions from the experiments with the germination mutants. Inhibitors that block both the Ala and AGFK pathways (HgCl2 and phenol) also block the 100-MPa pathway. On the other hand, if the Ala and AGFK pathways are not completely blocked (TAME), then the 100-MPa pathway is not either. Of particular interest is the finding that two of the three inhibitors also inhibit germination at 600 MPa. This is the case for TAME (41% inhibition) and for HgCl2 (90% inhibition). Inhibition by these compounds is consistent with a role for specific enzymes in germination at 600 MPa. Germination at 600 MPa is, therefore, not merely a physico-chemical process in which water is forced into the spore protoplast, but involves activation of a true physiological germination pathway. At this time, our experiments do not allow us to conclude whether this 600-MPa pathway is really distinct from the 100-MPa pathway, or whether it corresponds to or overlaps with some lower part of the 100-MPa germination pathway downstream of gerD.
ACKNOWLEDGMENTS
This work was supported by grants from the Belgian Fund for Scientific Research (N.F.W.O. G.0395.98) and K.U. Leuven Onderzoeksfonds (OT/97/31). E.W. is a grant holder from the Flemish Institute for the promotion of Scientific-Technological Research (I.W.T.).
REFERENCES
- 1.Bender G R, Marquis R E. Sensitivity of various salt forms of Bacillus megaterium spores to the germination action of hydrostatic pressure. Can J Microbiol. 1982;28:643–649. [Google Scholar]
- 2.Bender G R, Marquis R E. Spore heat resistance and specific mineralization. Appl Environ Microbiol. 1985;50:1414–1421. doi: 10.1128/aem.50.6.1414-1421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boschwitz H, Gofshtein-Gandman L, Halvorson H O, Keynan A, Milner Y. The possible involvement of trypsin-like enzymes in germination of spores of Bacillus cereus T and Bacillus subtilis 168. J Gen Microbiol. 1991;137:1145–1153. doi: 10.1099/00221287-137-5-1145. [DOI] [PubMed] [Google Scholar]
- 4.Corfe B M, Sammons R L, Smith D A, Mauël C. The gerB region of the Bacillus subtilis 168 chromosome encodes a homologue of the gerA spore germination operon. Microbiology. 1994;140:471–478. doi: 10.1099/00221287-140-3-471. [DOI] [PubMed] [Google Scholar]
- 5.Gould G W. Germination. In: Gould G W, Hurst A, editors. The bacterial spore. London, England: Academic Press; 1969. pp. 397–444. [Google Scholar]
- 6.Gould G W, Sale A J H. Initiation of germination of bacterial spores by hydrostatic pressure. J Gen Microbiol. 1970;60:335–346. doi: 10.1099/00221287-60-3-335. [DOI] [PubMed] [Google Scholar]
- 7.Hauben K J A, Bartlett D H, Soontjens C C F, Cornelis K, Wuytack E Y, Michiels C W. Escherichia coli mutants resistant to inactivation by high hydrostatic pressure. Appl Environ Microbiol. 1997;63:945–950. doi: 10.1128/aem.63.3.945-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh L K, Vary J C. Germination and peptidoglycan solubilization in Bacillus megaterium spores. J Bacteriol. 1975;123:463–470. doi: 10.1128/jb.123.2.463-470.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irie R, Okamoto T, Fujita Y. Characterization and mapping of Bacillus subtilis gerD mutants. J Gen Appl Microbiol. 1986;32:303–315. [Google Scholar]
- 10.Johnstone K. The trigger mechanism of spore germination: current concepts. J Appl Bacteriol. 1994;76:17S–24S. doi: 10.1111/j.1365-2672.1994.tb04354.x. [DOI] [PubMed] [Google Scholar]
- 11.Keyan A. Spore structure and its relations to resistance, dormancy and germination. In: Chambliss G, Vary J C, editors. Spores VII. Washington, D.C.: American Society for Microbiology; 1978. pp. 43–53. [Google Scholar]
- 12.McCann K P, Robinson C, Sammons R L, Smith D A, Corfe B M. Alanine germination receptors of Bacillus subtilis. Lett Appl Microbiol. 1996;23:290–294. doi: 10.1111/j.1472-765x.1996.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 13.Moir A, Smith D A. The genetics of bacterial spore germination. Annu Rev Microbiol. 1990;44:531–553. doi: 10.1146/annurev.mi.44.100190.002531. [DOI] [PubMed] [Google Scholar]
- 14.Moir A, Kemp H E, Robinson C, Corfe B M. The genetic analysis of bacterial spore germination. J Appl Bacteriol. 1994;76:9S–16S. [PubMed] [Google Scholar]
- 15.Rode L J, Foster J W. Influence of exchangeable ions on germinability of bacterial spores. J Bacteriol. 1966;91:1582–1588. doi: 10.1128/jb.91.4.1582-1588.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell A D, Chopra I. Understanding antibacterial action and resistance. New York, N.Y: Ellis Horwood; 1990. [Google Scholar]
- 17.Setlow P. Mechanisms which contribute to the long-term survival of spores of Bacillus species. J Appl Bacteriol. 1994;76:49S–60S. doi: 10.1111/j.1365-2672.1994.tb04357.x. [DOI] [PubMed] [Google Scholar]
- 18.Venkatasubramanian P, Johnstone K. Biochemical analysis of germination mutants to characterize germinant receptors of Bacillus subtilis 1604 spores. J Gen Microbiol. 1993;139:1921–1926. doi: 10.1099/00221287-139-8-1921. [DOI] [PubMed] [Google Scholar]
- 19.Warburg R J, Moir A, Smith D A. Influence of alkali metal cations on the germination of spores of wild-type and gerD mutants of Bacillus subtilis. J Gen Microbiol. 1985;131:221–230. [Google Scholar]
- 20.Wolgamott G D, Durham N N. Initiation of spore germination in Bacillus cereus: a proposed allosteric receptor. Can J Microbiol. 1971;17:1043–1048. doi: 10.1139/m71-165. [DOI] [PubMed] [Google Scholar]
- 21.Wuytack E Y, Boven S, Michiels C W. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressure. Appl Environ Microbiol. 1998;64:3220–3224. doi: 10.1128/aem.64.9.3220-3224.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]